Abstract
OBJECTIVE:
To compare sustained virologic response 12 weeks post-treatment completion (SVR12) and patient characteristics for older versus younger patients with chronic hepatitis C virus infection (HCV) receiving direct-acting antiviral (DAA) agent therapy.
METHODS:
This retrospective cohort study included patients with chronic HCV who received DAA therapy, between 2015 and 2018, in the largest health system in Rhode Island (N=154). Patient characteristics, comorbid diagnoses, and SVR12 status were compared between older (aged ≥60 years) and younger (<60 years) adults using chi-squared tests.
RESULTS:
Overall, 94.1% (95% CI: 90.4–97.8) achieved SVR12; response rates were 91.8% (95% CI: 84.9–98.6) for older adults and 95.6% (95% CI: 91.5–99.8) for younger adults (p=0.51).
CONCLUSIONS:
Our findings refute the historical notion that older adults were a “difficult-to-treat” subpopulation for whom clinicians should expect less treatment success. This is no longer the case with DAA therapy.
Keywords: hepatitis C, chronic, direct-acting antiviral agents, older adults, sustained virologic response
INTRODUCTION
Hepatitis C virus (HCV) currently affects 71 million people globally and 4.1 million people in the United States (U.S.).1,2 Between 75 and 85% of all acute HCV infections develop into chronic HCV infections, which have an estimated domestic prevalence of 1.0%.2 Chronic HCV can take decades to develop, as it is a slow, progressive scarring of liver tissue, often culminating intrahepatic and extrahepatic disease due to long-term systemic inflammation.3 Since the progression from acute to chronic HCV is typically asymptomatic, adults aged 60 years and older may have been unknowingly infected with HCV for decades, and as a result, are at a higher risk for HCV-associated complications compared to younger adults.3 In fact, natural history models predict that the largest burden of complications from HCV infections will fall on those aged 60 years and older.4
The advent of novel interferon-free, direct-acting antiviral (DAA) agent regimens have decreased the rate of adverse events and improved the rate of achieving cure for older adults receiving HCV therapy.3,5,6 Current American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA) guidelines recommend all patients with chronic HCV receive DAA-based therapy, regardless of age, unless treatment is unlikely to improve life expectancy.7 However, clinical studies reporting the efficacy and tolerability of DAA therapy enrolled a limited proportion of patients aged ≥ 60 years.3 Since clinicians rely on clinical trial data to make real-world decisions in treating patients, this less robust clinical trial data for older adults can affect a provider’s willingness to treat patients with HCV in this population.
While older adults experience less drug-drug interactions (DDIs) and adverse events on DAA regimens compared to interferon-based regimens, these events may still occur and discourage clinicians from pursuing guideline-concordant treatment.6,8 Older adults are at a particular risk for experiencing DDIs with DAA therapy compared to younger adults as older adults are more susceptible to polypharmacy, corresponding to having more comorbidities.8–10 Some clinicians may therefore perceive older patients with chronic HCV as a “difficult-to-treat” subpopulation, for whom less treatment success should be expected.11,12
Pharmacists may help overcome these challenges. Pharmacists are positioned to address many drug-related problems that older adults may be at a higher risk for, such as DDIs, side effects, and medication nonadherence that are typically of concern with patients starting DAA therapy. Given the critical role pharmacists may play in HCV treatment, our study examined patients receiving DAA therapy at clinics that include clinical pharmacists in the management of HCV.
We examined SVR12 rates and the burden of 19 comorbid medical, psychiatric, and substance use conditions for older versus younger adults in a cohort of patients with chronic HCV receiving DAA therapy at pharmacist-involved clinics. We hypothesized that older adults would be less likely to achieve SVR12 compared to younger adults due to clinicians’ historical perception that older adults are a “difficult-to-treat” subpopulation and a subsequent reluctance to pursue guideline-concordant HCV management.
METHODS
Study Design and Data Source
This was a retrospective cohort study using existing data on adult patients (age ≥18 years) diagnosed with chronic HCV who initiated treatment at one of two pharmacist-involved clinics within a single health system in Providence, Rhode Island, between January 1, 2015 and June 30, 2018. In both clinics, pharmacists educated patients with chronic HCV and monitored their care throughout DAA treatment. Additional details have been previously published.13
All data were collected from the patient’s electronic health record (EHR) by two researchers working together to identify patients, extract information, and confirm eligibility. Data were recorded using a standardized abstraction instrument. Patients were included in the analysis if it was their first treatment at the clinic and they were receiving DAA-based therapy with or without ribavirin. For patients who were prescribed a course of treatment more than once at the same clinic, only their first treatment regimen was included. Patients co-infected with human immunodeficiency virus (HIV) were treated at a separate immunology clinic and were not included in this study. There were no additional exclusion criteria applied to maximize generalizability. The Lifespan Institutional Review Board (IRB) determined this study to be exempt from IRB review.
Age and Covariates
Age was dichotomized using a cutoff of 60 years. Younger adults were those aged <60 years of age and older adults were those aged ≥60 at the time of clinic enrollment. The age threshold of ≥60 for defining older adults was selected to concord with related research.4,11,14
Additional variables determined from the literature to be related to achieving HCV cure were extracted from the EHR for each patient. These covariates were gender, race, income level, insurance type, HCV genotype, presence of cirrhosis, presence of decompensation, prior history of HCV treatment, type of DAA medication, use of ribavirin, length of treatment course (<24 weeks and ≥24 weeks), and risk factors for HCV infection, including patient-reported history of injection drug use, snorting drugs, tobacco use, male-to-male sexual encounters, blood transfusions prior to 1992, and incarceration.3,6,13 Insurance type was dichotomized to private insurance and public insurance, with public insurance including patients who had Medicaid, Medicare or a combination of both Medicaid and Medicare listed as their primary insurance provider.
Covariates for comorbid medical diagnoses determined from the literature to be prevalent comorbidities in patients with HCV included chronic pain, hepatitis B virus, hypertension, coronary artery disease, chronic heart failure, chronic kidney disease, chronic pulmonary disease, and diabetes.3,6,14,15 Comorbid psychological diagnoses included depression, anxiety, bipolar disorder, post-traumatic stress disorder, and schizophrenia.3,14,15 Current or past history of substance use disorders included alcohol, amphetamine, benzodiazepine, cannabis, cocaine, and opioid use disorders.14–16 A dichotomous variable was created to investigate the burden of comorbid diagnoses in the cohort. The two levels were “patient was diagnosed with at least one comorbid condition” and “patient was not diagnosed with any comorbid condition.”
Sustained Virologic Response (SVR12)
SVR12 for HCV therapy was defined as sustained virologic response, or a non-detectable HCV RNA viral load, 12 weeks following completion of treatment. Patients achieving SVR12 are deemed to be cured of HCV.7 Both intention-to-treat (ITT) and modified intention-to-treat (mITT) SVR12 rates were reported for the cohort. Only patients who completed their full prescribed course of DAA therapy and had a reported SVR12 status were included in the analysis of patient characteristics.
Statistical Analysis
Results were reported first for the entire cohort and then stratified by younger versus older adults. Chi-squared tests were used to compare older and younger adults, and report p-values and 95% confidence intervals (CIs). Data analyses were conducted using R version 3.4.1 (R Core Team; Vienna, Austria).17
RESULTS
Study Cohort
There were 162 patients with chronic HCV who initiated treatment at a pharmacist-involved clinic between January 1, 2015 and June 30, 2018. Of those, 154 patients initiated and completed treatment at those clinics within the same time frame. Considering only the 154 patients with chronic HCV who initiated and completed treatment, the mean age of the cohort was 55. The cohort was predominantly male (53.2%), white (63.6%), had a prior or current history of smoking (79.2%) and had public insurance (82.4%). Table 1 describes demographics overall and stratified by age.
Table 1.
Characteristics of hepatitis C virus patients stratified by age group (N=154)
| Overall (N=154) | Younger Adults <60 years (n=93) | Older Adults ≥60 years (n=61) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 55 (9.6) | 50 (7.6) | 64 (5.0) | -- |
| Female, n (%) | 72 (46.7) | 42 (45.1) | 30 (49.1) | 0.74 |
| Race, n (%) | 0.48 | |||
| White | 98 (63.7) | 62 (66.7) | 36 (59.1) | |
| Black | 32 (20.7) | 29 (20.4) | 13 (21.3) | |
| Other/Unspecified | 24 (15.6) | 12 (12.9) | 12 (19.6) | |
| Hispanic, n (%) | 29 (18.8) | 19 (20.4) | 10 (16.3) | 0.67 |
| Annual household income, n (%) | 0.41 | |||
| Tertile 1 ($30,711–40,455) | 57 (37.1) | 37 (39.8) | 20 (32.8) | |
| Tertile 2 ($40,456–55,632) | 44 (28.5) | 23 (24.8) | 21 (34.4) | |
| Tertile 3 ($55,632–117,408) | 53 (34.4) | 33 (35.4) | 20 (32.8) | |
| Public insurance, n (%) | 127 (82.4) | 79 (84.9) | 48 (78.6) | 0.43 |
| Ever smoker, n (%) | 122 (79.2) | 72 (77.4) | 50 (81.9) | 0.63 |
| Factors related to HCV exposure, n (%) | ||||
| History of intravenous illicit drug use | 77 (50.0) | 51 (54.8) | 26 (42.6) | 0.18 |
| History of snorting drugs | 67 (43.5) | 42 (45.1) | 25 (40.9) | 0.72 |
| History of high-risk sexual activity | 26 (16.8) | 15 (16.1) | 11 (18.0) | 0.92 |
| Blood transfusion prior to 1992 | 39 (25.3) | 20 (21.5) | 19 (31.1) | 0.24 |
| Incarceration | 47 (30.5) | 33 (35.4) | 14 (22.9) | 0.14 |
Chi-squared tests used to determine the relationship between categorical variables.
P values are comparing younger adults to older adults.
The overall ITT SVR12 rate was 90.7% (95% CI: 86.3–95.2). For those who completed treatment, the mITT SVR12 rate was 94.2% (95% CI: 90.4–97.8) (Table 2). Prior to beginning treatment, over half of the cohort was diagnosed with liver cirrhosis (51.9%) and 12.9% showed signs of decompensated cirrhosis. Most patients completed a DAA treatment course that was less than 24 weeks (80.5%), using ledipasvir/sofosbuvir (75.3%) without ribavirin (77.2%).
Table 2.
Characteristics of the treatment regimen received, hepatitis C virus, and comorbid diagnoses among study participants stratified by age group (N=154)
| Overall (N=154) | Younger Adults <60 years (n=93) | Older Adults ≥60 years (n=61) | P-valuea,b,c | |
|---|---|---|---|---|
| Hepatitis C Virus | ||||
| Genotype 1, n (%) | 123 (79.8) | 73 (78.4) | 50 (81.9) | 0.74 |
| Presence of liver cirrhosis, n (%) | 80 (51.9) | 48 (51.6) | 32 (52.4) | 1 |
| Presence of decompensated cirrhosis, n (%) | 20 (12.9) | 12 (12.9) | 8 (13.1) | 1 |
| Prior history of treatment, n (%) | 46 (29.8) | 27 (29.0) | 19 (31.1) | 0.91 |
| Treatment Regimen for Hepatitis C Virus | ||||
| Length of treatment, n (%) | 0.87 | |||
| < 24 weeks | 124 (80.5) | 74 (79.6) | 50 (82.0) | |
| 24 weeks | 30 (19.5) | 19 (20.4) | 11 (18.0) | |
| Type of DAA medication, n (%) | 0.55 | |||
| Ledipasvir/sofosbuvir | 116 (75.3) | 68 (73.1) | 48 (78.7) | |
| Other | 38 (24.7) | 25 (26.9) | 13 (21.3) | |
| Use of ribavirin, n (%) | 35 (22.7) | 17 (18.2) | 18 (29.5) | 0.15 |
| SVR12 achievedd, n (%) | 145 (94.1) | 89 (95.6) | 56 (91.8) | 0.51 |
| Comorbid Diagnoses | ||||
| Comorbid Medical Diagnoses, n (%) | ||||
| Chronic pain | 55 (35.7) | 32 (34.4) | 23 (37.7) | 0.80 |
| Hepatitis B virus | 6(3.8) | 5 (5.3) | 1 (1.6) | 0.4 |
| Hypertension | 86 (55.8) | 42 (45.1) | 44 (72.1) | 0.001** |
| Coronary artery disease | 8 (5.1) | 5 (5.3) | 3 (4.9) | 1 |
| Chronic heart failure | 5(3.2) | 3 (3.2) | 2 (3.2) | 1 |
| Chronic kidney disease | 11 (7.1) | 5 (5.3) | 6 (9.8) | 0.46 |
| Chronic pulmonary disease | 31 (20.1) | 21 (22.5) | 10 (16.3) | 0.46 |
| Diabetes | 46 (29.8) | 26 (27.9) | 20 (32.7) | 0.64 |
| Comorbid Psychiatric Diagnoses, n (%) | ||||
| Depression | 74 (48.0) | 51 (54.8) | 23 (37.7) | 0.05 |
| Anxiety | 48 (31.1) | 34 (36.5) | 14 (22.9) | 0.10 |
| Bipolar disorder | 12 (7.7) | 9 (9.6) | 3 (4.9) | 0.44 |
| Post-traumatic stress disorder | 10 (6.4) | 8 (8.6) | 2 (3.2) | 0.32 |
| Schizophrenia | 8 (5.1) | 6 (6.4) | 2 (3.2) | 0.61 |
| History of Comorbid Substance Use Disorder, n (%) | ||||
| Alcohol use disorder | 37 (24.0) | 24 (25.8) | 13 (21.3) | 0.65 |
| Amphetamine use disorder | 1 (0.6) | 1 (1.0) | 0 (0.0) | 1 |
| Benzodiazepine use disorder | 3 (1.9) | 1 (1.0) | 2 (3.2) | 0.71 |
| Cannabis use disorder | 2 (1.2) | 1 (1.0) | 1 (1.6) | 1 |
| Cocaine use disorder | 13 (8.4) | 8 (8.6) | 5 (8.1) | 1 |
| Opioid use disorder | 26 (16.8) | 19 (20.4) | 7 (11.4) | 0.21 |
Chi-squared tests used to determine the relationship between categorical variables.
P values are comparing younger adults to older adults.
**P value <0.01
Modified intention-to-treat SVR12, includes only patients who completed treatment and had a SVR12 reported
Looking at the distribution of comorbid conditions, 35.7% of the cohort was diagnosed with chronic pain, 55.8% had hypertension, 20.1% had chronic pulmonary disease, 29.8% had diabetes, 48.0% had depression, and 31.1% had anxiety. Close to a quarter of patients had a current or past history of alcohol use disorder (24.0%) and slightly less had a current or past history of opioid use disorder (16.8%).
Comparing Older and Younger Adults
Of the 162 patients with chronic HCV who initiated treatment, 99 patients were <60 years old and 63 patients were ≥60 years old. Considering only the 154 patients who initiated and completed treatment, 93 patients were <60 years old and 61 patients were ≥60 years old. Neither demographic characteristics nor factors related to HCV exposure were distributed differently between age groups.
The ITT SVR12 rate for older adults was 90.5% (95% CI: 83.2–97.7) and the mITT SVR12 rate was 91.8% (95% CI: 84.9–98.6), compared to ITT SVR12 rate for younger adults of 90.9% (95% CI: 85.2–96.5) and mITT SVR12 rate of 95.6% (95% CI: 91.5–99.8). The SVR12 rates did not differ significantly between age groups (p=0.51) (Table 2). Characteristics related to the patients’ HCV diagnosis and DAA treatment regimen did not vary significantly between older and younger adults in this cohort.
Figure 1 displays the burden of comorbid diagnoses stratified by age group. Older adults had a greater burden of comorbid diagnoses, with 93.4% (95% CI: 87.2–99.6) of older patients diagnosed with at least one comorbid illness, compared to 87.1% (95% CI: 80.2–93.9) of younger adult patients who were diagnosed with at least one comorbid illness (p=0.32). Only hypertension was markedly different between older and younger adults (Table 2). More older adults were diagnosed with hypertension (72.1%, 95% CI: 60.8–83.3) compared to younger adults (45.1%, 95% CI: 35.0–55.2) (p=0.001).
Figure 1.
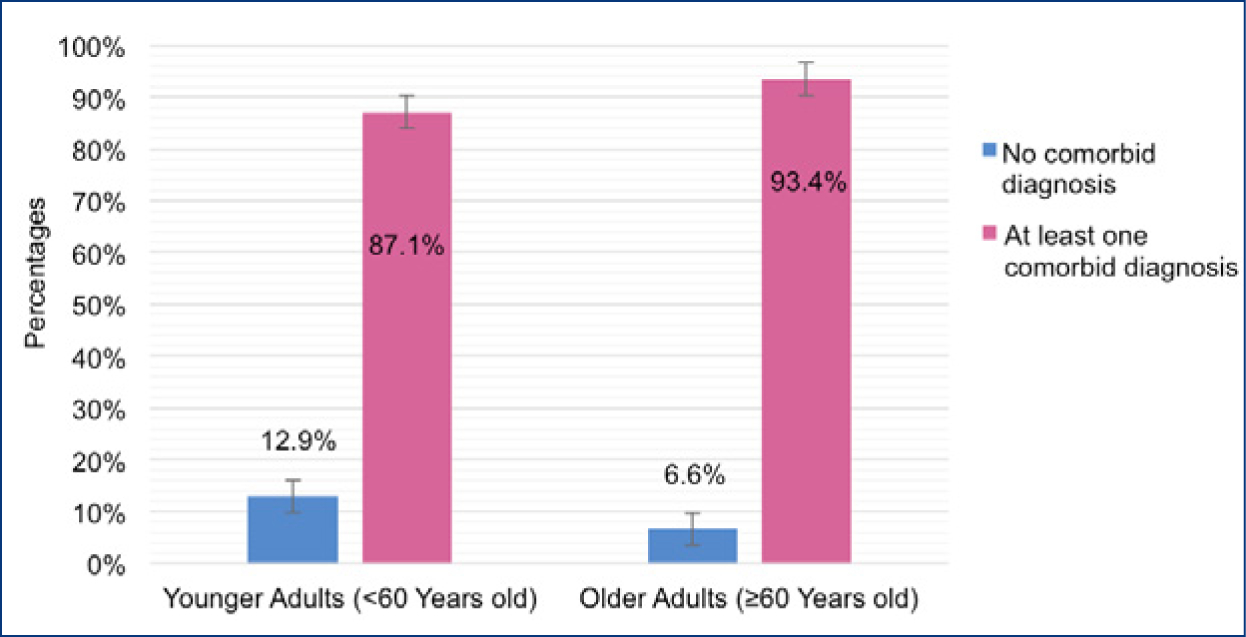
Bar plot displaying the burden of comorbid conditions among patients with hepatitis C virus infection (HCV) stratified by age group (N=154)
Comorbid conditions include chronic pain, hepatitis B virus, hypertension, coronary artery disease, chronic heart failure, chronic kidney disease, chronic pulmonary disease, diabetes, depression, anxiety, bipolar disorder, post-traumatic stress disorder, schizophrenia, alcohol use disorder, amphetamine use disorder, benzodiazepine use disorder, cannabis use disorder, cocaine use disorder, and opioid use disorder.
DISCUSSION
Among patients treated at pharmacist-involved HCV clinics, SVR12 rates did not significantly differ between age groups, suggesting that patients with chronic HCV, regardless of age or comorbid conditions, can attain SVR12 with DAA therapy. Overall, 94.2% achieved SVR12 and were cured of HCV. Although older adults in this cohort did have a slightly lower SVR12 rate compared to younger adults, the difference between the two age groups was not statistically significant.
The SVR12 rates observed are consistent with those found in the literature for similar patient populations receiving DAA therapy. Su et al. grouped patients with HCV into six age categories and reported SVR12 rates greater than 90% for patients in the age categories of 60–64, 65–69, 70–74, and 75 years and older.14 Vermehren et al. and Jhaveri et al. compared patients with HCV <65 years old to those ≥65 years old and found SVR12 rates between 91 to 99% for younger adults and SVR12 rates between 97 to 100% for older adults.5,8 Most recently, Pan et al. found no significant difference in achieving HCV cure between patients with HCV <65 years old and those ≥65 years old.18 Combined with our study, these findings suggest that age alone does not adversely impact achieving SVR12 through DAA therapy.
Since older adults are more likely to experience DDIs with DAA therapy as a result of having a greater burden of comorbidities and medications to treat them, it is important to include comorbid conditions and medication use in evaluations of DAA therapy.8–10 Prior literature examining SVR12 for DAA therapies included only a few comorbid conditions. Of those, to our knowledge, no study examined more than six diagnoses.6,14,16,18 Our study included 19 diagnoses for comorbid conditions (eight medical, five psychiatric, and six substance use disorders), which were specifically selected for their prevalence as comorbidities in patients with HCV.14,16 Older adults in this cohort shared a greater burden of having at least one comorbid diagnosis compared to younger adults, although this difference between age groups was not statistically significant. Only one comorbid condition, hypertension, was distributed differently between the younger and older adult age groups, with more older adults having with hypertension. This finding is consistent with older adults in both the general and HCV population, as the prevalence of hypertension increases with age.18,19 Older adults shared a greater burden of having at least one comorbid diagnosis, but both age groups achieved SVR12 at a similar rate. This indicates that the comorbid conditions evaluated in this study are not expected to significantly affect the ability of an older patient with chronic HCV to be cured of HCV.
Although not considered a comorbid condition in analysis, nearly 80% of our cohort reported a past or current history of smoking. The rate of tobacco use among patients with HCV is estimated to be three times higher than the rate of tobacco use among patients without HCV.20 This is likely linked to the fact that cardiovascular diseases are more prevalent among patients infected with HCV compared with the general public. Additionally, patients with HCV are more likely to die from cardiovascular and respiratory causes than liver-related causes, which further supports the importance of tobacco cessation counseling of patients being treated for HCV.20
Interestingly, the percent of patients with cirrhosis in our cohort was higher than expected, with over half diagnosed with cirrhosis at the start of the study. The World Health Organization estimates that between 15 and 30% of patients with HCV develop cirrhosis.1 This discrepancy is likely due to the fact that at the time that this study was conducted, many insurers in Rhode Island, both private and public, reserved coverage of DAA therapies to patients with stage three or stage four fibrosis. Since then, restrictions based on fibrosis score have largely been removed and patients with less severe fibrosis are able to access HCV treatment.
In our cohort, the distribution of patients with liver cirrhosis and decompensated cirrhosis were similar across both age groups. However, due to a longer duration of infection, older adults more often present with advanced stages of fibrosis compared to younger adults.3,18 Current HCV guidelines recommend treatment for all patients with HCV, unless they have a short life expectancy (less than 12 months) and are unlikely to receive benefit from therapy.7 Although older adults often present at a later stage of liver fibrosis, this does not correlate with their ability to respond to HCV treatment. The efficacy and tolerability of DAA therapy indicates older adults with HCV no longer need to be viewed as a “difficult-to-treat” population. However, there is some urgency regarding when to start therapy for an older patient with chronic HCV, as older adults do have a greater risk for HCV-related intrahepatic and extrahepatic disease.3 Given the similar distributions of HCV-related liver complications and the greater than 90% SVR12 rate in this cohort, it does not appear that older adults, who are who are at the highest-risk for HCV-related liver morbidity and mortality, should be denied treatment based on stage of liver disease.
Our finding that patients with chronic HCV are not limited by age or comorbid conditions in achieving HCV cure must be interpreted in light of some limitations. Given the retrospective chart review nature of this study, some comorbid diagnoses may be missing or misclassified. However, it is unlikely that missingness or misclassification would be differential by age. Additionally, this study employed data from a single health system, so results may not generalize to other institutions with markedly different patient populations.
CONCLUSIONS
Our findings suggest that HCV cure is possible for both younger and older patients with chronic HCV and is not limited by a patient’s age or the comorbid illnesses evaluated in this study. Although there were some differences between age groups, none of these differences are expected to influence the ability of a patient with chronic HCV to respond to HCV treatment and be cured of HCV. Given the longer duration of infection in older patients with HCV and the efficacy and tolerability of DAA therapy, it is imperative to treat older patients, who are at the highest-risk for HCV-related liver morbidity and mortality.
Acknowledgments
Prior presentation:
This work was presented as a Master Student Poster for Brown University’s School of Public Health Research Day, April 4, 2019.
Funding:
Drs. Zullo and Beaudoin are supported by a grant from the National Institute on Aging (R21AG061632). The data collection for this study was originally supported by a research grant from the American Society of Health-System Pharmacists (ASHP) Research and Education Foundation.
Footnotes
Conflict of Interest: Dr. Zullo is a U.S. Government employee; the views expressed in this article are those of the author and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Institutional Review Board Approval: The Lifespan Institutional Review Board (IRB) determined this study to be exempt from IRB review.
Contributor Information
Alyssa K. Greenwood Francis, School of Public Health, Brown University, Providence, RI..
Francesca L. Beaudoin, Associate Professor of Emergency Medicine and Health Services, Policy, and Practice, The Alpert Medical School of Brown University, Providence, RI..
Safiya S. Naidjate, Clinical Pharmacist Specialist, Ambulatory Care, Lifespan Corporation, Rhode Island Hospital, Providence, RI..
Christine Berard-Collins, Director, Department of Pharmacy, Rhode Island Hospital; Clinical Pharmacist Specialist, Ambulatory Care, Lifespan Corporation, Rhode Island Hospital, Providence, RI..
Andrew R. Zullo, Assistant Professor of Health Services, Policy, and Practice and Epidemiology, School of Public Health, Brown University; Research Fellow, Center of Innovation in Long-Term Services and Supports, Providence Veterans Affairs Medical Center; Clinical Pharmacist Specialist, Department of Pharmacy, Rhode Island Hospital, Providence, RI..
References
- 1.World Health Organization. Hepatitis C.; 2019. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed March 18, 2020.
- 2.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013–2016: Hepatology. Hepatology. 2019;69(3):1020–1031. doi: 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid M, Price JC, Tien PC. Hepatitis C Virus Infection in the Older Patient. Infect Dis Clin North Am. 2017;31(4):827–838. doi: 10.1016/j.idc.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis GL, Alter MJ, El–Serag H, Poynard T, Jennings LW. Aging of Hepatitis C Virus (HCV)-Infected Persons in the United States: A Multiple Cohort Model of HCV Prevalence and Disease Progression. Gastroenterology. 2010;138(2):513–521.e6. doi: 10.1053/j.gastro.2009.09.067 [DOI] [PubMed] [Google Scholar]
- 5.Jhaveri MA, Manne V, Kowdley KV. Chronic Hepatitis C in Elderly Patients: Current Evidence with Direct-Acting Antivirals. Drugs Aging. 2018;35(2):117–122. doi: 10.1007/s40266-017-0515-1 [DOI] [PubMed] [Google Scholar]
- 6.Mazzarelli C, Considine A, Childs K, et al. Efficacy and Tolerability of Direct-Acting Antivirals for Hepatitis C in Older Adults: Direct-acting antivirals in older adults. J Am Geriatr Soc. 2018;66(7):1339–1345. doi: 10.1111/jgs.15392 [DOI] [PubMed] [Google Scholar]
- 7.American Association for the Study of Liver Diseases and Infectious Diseases Society of America. When and in Whom to Initiate HCV Therapy. November 2019. https://www.hcvguidelines.org/evaluate/when-whom.
- 8.Vermehren J, Peiffer K-H, Welsch C, et al. The efficacy and safety of direct acting antiviral treatment and clinical significance of drug-drug interactions in elderly patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2016;44(8):856–865. doi: 10.1111/apt.13769 [DOI] [PubMed] [Google Scholar]
- 9.Cooper CL, Galanakis C, Donelle J, et al. HCV-infected individuals have higher prevalence of comorbidity and multimorbidity: a retrospective cohort study. BMC Infect Dis. 2019;19(1):712. doi: 10.1186/s12879-019-4315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nobili A, Garattini S, Mannucci PM. Multiple Diseases and Polypharmacy in the Elderly: Challenges for the Internist of the Third Millennium. J Comorbidity. 2011;1(1):28–44. doi: 10.15256/joc.2011.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–1482.e5. doi: 10.1053/j.gastro.2015.07.056 [DOI] [PubMed] [Google Scholar]
- 12.Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 13.Naidjate S, Zullo A, Dapaah-Afriyie R, et al. Comparative effectiveness of pharmacist care delivery models for hepatitis C clinics. Am J Health Syst Pharm. March 2019. doi: 10.1093/ajhp/zxz034 [DOI] [PubMed] [Google Scholar]
- 14.Su F, Beste LA, Green PK, Berry K, Ioannou GN. Direct-acting antivirals are effective for chronic hepatitis C treatment in elderly patients: a real-world study of 17 487 patients. Eur J Gastroenterol Hepatol. 2017;29(6):686–693. doi: 10.1097/MEG.0000000000000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15(1):31. doi: 10.1186/s12911-015-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yek C, de la Flor C, Marshall J, et al. Effectiveness of direct-acting antiviral therapy for hepatitis C in difficult-to-treat patients in a safety-net health system: a retrospective cohort study. BMC Med. 2017;15(1). doi: 10.1186/s12916-017-0969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.r-project.org/. [Google Scholar]
- 18.Pan CQ, Gayam V, Rabinovich C, et al. Efficacy of Direct-Acting Antivirals for Chronic Hepatitis C in a Large Cohort of Older Adults in the United States. J Am Geriatr Soc. 2020;68(2):379–387. doi: 10.1111/jgs.16206 [DOI] [PubMed] [Google Scholar]
- 19.Fryar CD, Zhang G. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. 2017;(289):8. [PubMed] [Google Scholar]
- 20.Kim RS, Weinberger AH, Chander G, Sulkowski MS, Norton B, Shuter J. Cigarette Smoking in Persons Living with Hepatitis C: The National Health and Nutrition Examination Survey (NHANES), 1999–2014. Am J Med. 2018;131(6):669–675. doi: 10.1016/j.amjmed.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]


