Abstract
Question
How effective and safe is telerehabilitation for people with COVID-19 and post-COVID-19 conditions?
Design
Systematic review of randomised trials.
Participants
People with COVID-19 and post-COVID-19 conditions.
Intervention
Any type of telerehabilitation.
Outcome measures
Satisfaction, quality of life, adverse events, adherence to telerehabilitation, dyspnoea, functional performance, readmissions, mortality, pulmonary function and level of independence.
Results
Database searches retrieved 2,962 records, of which six trials with 323 participants were included in the review. Breathing exercises delivered via telerehabilitation improved 6-minute walk distance (MD 101 m, 95% CI 61 to 141; two studies), 30-second sit-to-stand test performance (MD 2.2 repetitions, 95% CI 1.5 to 2.8; two studies), Multidimensional Dyspnoea-12 questionnaire scores (MD –6, 95% CI –7 to –5; two studies) and perceived effort on the 0-to-10 Borg scale (MD –2.8, 95% CI –3.3 to –2.3; two studies), with low certainty of evidence. Exercise delivered via telerehabilitation improved 6-minute walk distance (MD 62 m, 95% CI 42 to 82, four studies), 30-second sit-to-stand test performance (MD 2.0 repetitions, 95% CI 1.3 to 2.7; two studies) and Multidimensional Dyspnoea-12 scores (MD –1.8, 95% CI –2.5 to –1.1; one study), with low certainty of evidence. Adverse events were almost all mild or moderate and occurred with similar frequency in the telerehabilitation group (median 0 per participant, IQR 0 to 2.75) as in the control group (median 0 per participant, IQR 0 to 2); Hodges-Lehmann median difference 0 (95% CI 0 to 0), with low certainty of evidence.
Conclusion
Telerehabilitation may improve functional capacity, dyspnoea, performance and physical components of quality of life and does not substantially increase adverse events.
Registration
PROSPERO CRD42021271049.
key words: COVID-19, Systematic review, Patient safety, Telerehabilitation, Physical therapy
Introduction
A new strain of coronavirus emerged in late December 2019 in Wuhan city, Hubei province, China.1 With an increasing number of infected people and severe cases of pneumonia, severe acute respiratory coronavirus-2 (SARS-CoV-2) caused the World Health Organization to officially declare coronavirus disease (COVID-19) a pandemic in February 2020. Since then, COVID-19 has infected more than 462 million people, ending more than 6.05 million lives around the world.2
The practice of ‘social distancing’ has helped to prevent new infections with SARS-CoV-2 and the spread the COVID-19.3 However, the need to maintain social distancing has dramatically reduced the number of face-to-face healthcare appointments, surgeries and other health procedures. In this context, the pandemic has certainly strained healthcare systems by causing high rates of hospitalisation and deaths globally.4
Up to one-third of people hospitalised for COVID-19 have reported persistent impairment in cognitive function, dyspnoea and/or fatigue at discharge, requiring continued rehabilitation due to the high level of disability after COVID-19 infection.5 , 6 Thus, the use of technologies such as online video and phone communication instead of in-person contact has been encouraged to promote and enable healthcare follow-ups and consultations, opening new horizons for delivering clinical appointments through the introduction of telehealth and telerehabilitation.7, 8, 9, 10
Telerehabilitation enables interventions to be delivered through a range of synchronous/real-time (eg, videoconference) and asynchronous/store-forward (eg, digital images) consultation formats.11 , 12 Prior to and especially in the pandemic context, a growing number of professionals started to assess and treat patients remotely.8 , 13 Telerehabilitation has been recommended to allow physiotherapists to treat patients remotely;10 however, telehealth technology can fail to be implemented in clinical care, with non-use and discontinued use by patients for many different reasons (primarily barriers related to the user, the intervention or the context).14 This review aimed to systematically review the safety and effectiveness of telerehabilitation interventions in people with COVID-19 and post-COVID-19 conditions.
Therefore, the specific research question for the systematic review was:
How effective and safe is telerehabilitation for people with COVID-19 and post-COVID-19 conditions?
Method
This systematic review followed the methodological recommendations of the Cochrane Collaboration Handbook and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.15 , 16
Identification and selection of trials
The literature search strategy was initially devised for MEDLINE (via PubMed) and then adapted for use in the other databases. There were no restrictions to any specific language, date or type of publication. The electronic searches were performed in five databases: Cochrane Central Register of Controlled Trials (CENTRAL) via Wiley, Medical Literature Analysis and Retrieval System online (MEDLINE) via PubMed, EMBASE via Elsevier, Latin American and the Caribbean Literature in Health Sciences (LILACS) via Virtual Health Library, and Living Overview of Evidence (LOVE) database, which includes the available evidence on COVID-19 from a total of 41 databases from their inception to 13 March 2022. The detailed search strategies are presented in Appendix 1 on the eAddenda. Furthermore, a search was performed on the ClinicalTrials.gov registry website to find ongoing and unpublished trials.
The eligibility criteria were determined using the Patient/Population–Intervention–Comparison/Comparator–Outcome (PICO) acronym.17 The inclusion criteria are shown in Box 1 . The exclusion criteria were: telehealth interventions for monitoring symptoms or physiological parameters only (ie, telemonitoring) and studies comparing different types of telerehabilitation.
Box 1. Inclusion criteria.
Design
-
•
Randomised controlled trial
Participants
-
•
People aged ≥ 18 years
-
•
Participants who have had COVID-19 and post-COVID-19 conditions, with diagnosis confirmed by reverse transcription-polymerase chain reaction (RT-PCR) test for SARS-CoV-218
Intervention
-
•
Any type of telerehabilitation (ie, delivery of rehabilitation services at a distance, using telecommunications technology to deliver it)19
Primary outcomes
-
•
Satisfaction
-
•
Quality of life
-
•
Adverse events
Secondary outcomes
-
•
Adherence to the intervention or completion of (tele)rehabilitation, according to the criteria of the original trialists
-
•
Dyspnoea
-
•
Physical performance (ie, 30-second sit-to-stand test)
-
•
Physical function (eg, muscle strength assessed by any validated measure, 6-minute walking test)
-
•
Readmissions
-
•
Mortality
-
•
Respiratory function (ie, spirometry)
-
•
Level of independence (ie, Barthel index)
Comparisons
-
•
Telerehabilitation was compared with face-to-face treatments
-
•
Telerehabilitation versus no treatment or usual care
Two reviewers (AGSV, CGM) independently screened the titles and abstracts retrieved by the searches. If an article appeared to be potentially relevant from its title and abstract, it was retrieved as a full-text article and assessed as to whether it fulfilled the eligibility criteria. If a consensus could not be reached, a third reviewer (ACPNP) was consulted to solve potential disagreements regarding the included articles. The reviewers identified and excluded duplicates and collated multiple reports of the same study so that each study was included and analysed only once in the review.
Assessment of characteristics of trials
A pre-defined data collection form was used for collecting study characteristics and outcome data. Data and study characteristics from all included studies were extracted independently by two authors (AGSV, BMSPG) and checked by a third author (ACPNP). To characterise and assess the similarity of the participants among the included trials, the following data were extracted from each trial: eligibility criteria, sample size, age, comorbidities and country of recruitment. To characterise the experimental interventions, details were extracted on intervention content, session frequency, program duration and delivery format. To characterise the control interventions, details were extracted on the intervention content, where applicable. The range of outcome measures and their assessment time points were also extracted.
Risk of bias
Assessment of the risk of bias of individual studies was performed as recommended by the Cochrane Collaboration Handbook.16 The Risk of Bias 2.0 tool20 was used to evaluate the risk of bias in five domains: bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported result. Each domain was categorised as ‘low risk of bias’, ‘some concerns’ or ‘high risk of bias’. A third reviewer was involved if a consensus could not be reached (RKN).
Data analysis
This review analysed dichotomous outcomes as risk ratios (RR) with 95% CIs and reported continuous outcomes as mean differences (MD) with 95% CI. Whenever possible, change-from-baseline values were used to estimate between-group differences. For data from included trials that were clinically homogeneous, a pooled quantitative synthesis was performed using a random effects model to account for between-trial heterogeneity. The I2 statistic was used to report the heterogeneity.21 Where trials examined effects of multiple interventions, participants from each arm were included in separate meta-analyses. Where the trials were clinically heterogeneous, they were synthesised narratively, as it would not have been appropriate to combine the results in a meta-analysis.22 When data were not presented as mean and SD of the change from baseline, the recommendations in Chapter 6 of the Cochrane Handbook (Version 6.3) were used to calculate them. Subgroup analyses were planned based on participants’ age, weight and disease severity. If the review was able to pool more than 10 studies, we intended to evaluate publication bias. All analyses were performed using Cochrane softwarea.
Assessment of certainty of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to measure and summarise the overall current evidence of each outcome.23 The GRADE system consists of five items: study limitations (risk of bias), inconsistency of results (heterogeneity), indirectness of evidence, imprecision of the effect estimates and reporting bias. The quality of the evidence was classified as high, moderate, low or very low, in relation to the studies that contributed data for the main prespecified outcomes. All analyses were performed using GRADEpro GDT software.24
Results
Compliance with the review protocol
The review was conducted according to the registered protocol. Although subgroup analyses were planned, none of the pooled studies assessed patients with different age, weight or disease severity. Although it was planned to evaluate publication bias if it was possible to pool more than 10 studies, all of the meta-analyses included fewer than 10 studies.
Flow of trials through the review
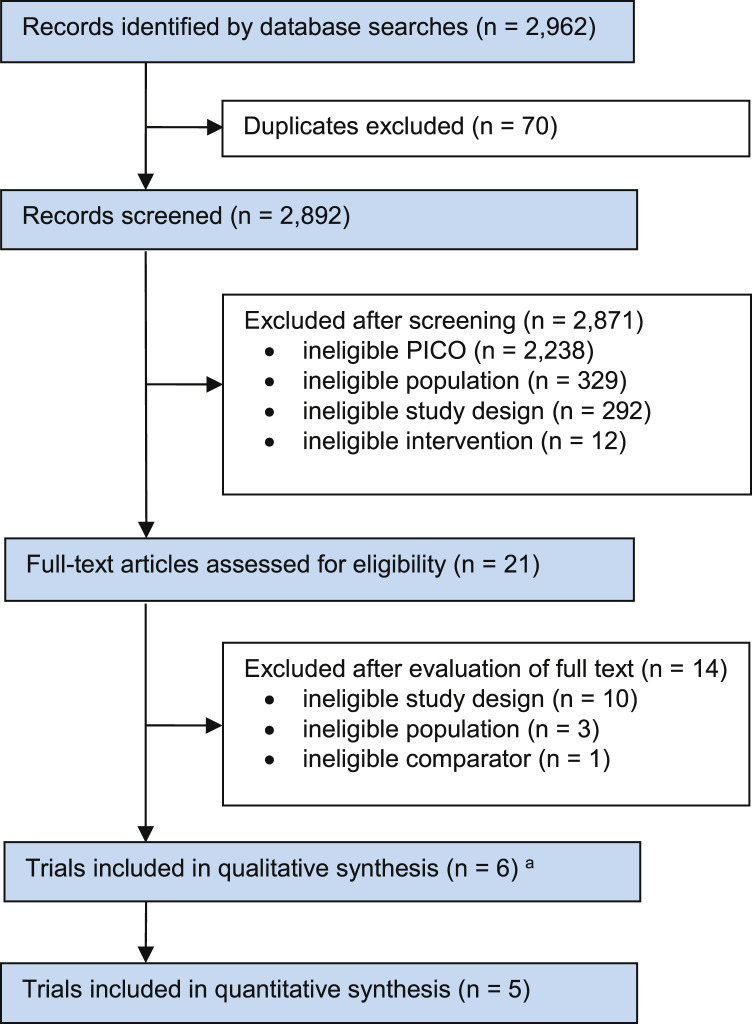
The electronic search strategy identified a total of 2,962 records from the selected databases. After screening titles, abstracts and reference lists, 21 potentially relevant records underwent full-text review. Of these, six randomised controlled trials (reported across seven articles) were included in this review.25, 26, 27, 28, 29, 30, 31 The 14 studies that did not meet the eligibility criteria were excluded due to ineligible study design (n = 10), ineligible population (n = 3) and ineligible comparator (n = 1) (Figure 1 ). Twenty-five ongoing studies were also identified: three on the databases and 22 on the ClinicalTrials.gov registry website; see Appendix 2 on the eAddenda for a summary of the ongoing trials.
Figure 1.
Flow of trials through the review.
a One trial was reported in two articles.
PICO = Patient Intervention Comparator Outcome.
Characteristics of included trials
Detailed characteristics of the included studies are shown in Table 1 .
Table 1.
Characteristics of the included trials (n = 6).
| Study and country | Participants | Intervention |
Outcome measures | |
|---|---|---|---|---|
| Exp | Con | |||
| Gonzalez-Gerez et al (2021) Spain |
n = 38 Age (y) = Exp 41 (SD 10), Con 40 (SD 13) Eligibility: mild-to-moderate COVID-19 symptoms in the acute stage Comorbidities: NR |
Breathing exercise program, delivered via a website Group exercise, one session/d, 7 d/wk, 1 wk |
No intervention | Adverse events Dyspnoea (MD12) Physical function (6MWT) Performance (30STST) Adjustments to the intensity of exercise (Borg scale) Timing: 0, 7 days |
| Li et al (2021) China |
n = 119 Age (y) = Exp 49 (SD 11), Con 52 (SD 11) Eligibility: discharged after hospitalisation for COVID-19, mMRC dyspnoea scores 2 to 3 Comorbidities: Exp = heart disease (3%), HT (14%), diabetes (14%), obesity (15%), lung disease (7%), other (27%); Con = heart disease (12%), HT (30%), diabetes (15%), obesity (13%), lung disease (5%), other (20%) |
Breathing control and thoracic expansion, aerobic exercise and lower limb muscle strength exercises, delivered via ‘RehabApp’ smartphone app and monitored with a telemetry device; three to four sessions/wk, 6 wks | Short educational instructions at baseline | Quality of life (SF-12) Adverse events Functional capacity (6MWT) Dyspnoea (mMRC) Performance (static squat test) Respiratory function (spirometry) Timing: 0, 6, 28 wks |
| Pehlivan et al (2021) Turkey |
n = 21 Age (y) = Exp 48, Con 44 Eligibility: discharged after hospitalisation for COVID-19 Comorbidities: NR |
Breathing exercises, active breathing techniques, lower and upper limb exercises, walking and wall squat exercises, delivered as a synchronised exercise program via videoconferencing; three sessions/wk, 6 wks | Educational material about COVID-19 and basic exercises that could be done at home | Performance (30STST) Performance (standing balance, eight-step walking speed and five sit-ups) Dyspnoea (mMRC) Fatigue (VASF) Timing: 0, 6 weeks |
| Rodriguez-Blanco et al (2021) Spain |
n = 36 Age (y) = Exp 39 (SD 12), Con 41 (SD 12) Eligibility: positive SARS-CoV-2 test in prior 40 d and in home isolation Comorbidities: NR |
Exercises of resistance and strength, delivered via a website; one session/d, 7 d/wk, 1 wk | No intervention | Physical function (6MWT) Performance (30STST) Adjustments to the intensity of exercise (Borg scale) Timing: 0, 7 days |
| Amaral et al (2022) Brazil |
n = 32 Age (y) = Exp 52 (SD 10), Con 53 (SD 12) Eligibility: discharged after hospitalisation for COVID-19 Comorbidities: Exp = HT (42%), diabetes (33%), obesity (67%), dyslipidaemia (8%), respiratory disease (8%), other (25%); Con = HT (55%), diabetes (5%), cardiovascular disease (10%), obesity (65%), dyslipidaemia (10%), respiratory disease (10%), hypothyroidism (5%), other (20%) |
Resistance and aerobic exercises program, delivered via smartphone guidance, supplementary material and website Resistance exercise three sessions/wk and aerobic exercise five sessions/wk, for 12 wks |
No intervention | Functional capacity (6MWT) Performance (FTSTS) Performance (grip strength) Respiratory function (FEV1/FVC) Timing: 0, 12 wks |
| Rodriguez-Blanco et al (2022) Spain |
n = 77 Age (y) = Exp 1 35 (SD 12), Exp 2 42 (SD 10), Con 42 (SD 12) Eligibility: acute COVID-19 and in home isolation Comorbidities: NR |
Exp 1 = strengthening exercise program delivered via a website; one session/d, 7 d/wk, 2 wks | No intervention | Fatigue (VASF) Dyspnoea (MD12) Physical function (6MWT) Performance (30STST) Adjustments to the intensity of exercise (Borg scale) Timing: 0, 14 days |
| Exp 2 = breathing exercise program delivered via a website; one session/d, 7 d/wk, 2 wks | ||||
Con = control group, COVID-19 = coronavirus disease, Exp = experimental group, FEV1/FVC = ratio of forced expiratory volume in the first second to forced vital capacity, FTSTS = five-times sit to stand, HR = heart rate, HT = hypertension, MD12 = multidimensional dyspnoea-12, mMRC = modified Medical Research Council dyspnoea scale, NR = not reported, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, SF-12 = short-form health survey-12, VASF = visual analogue scale fatigue, 6MWT = 6-minute walking test, 30STST = 30-second sit-to-stand test.
Rodriguez-Blanco et al contributed two of the included trials.25 , 26 In their trial published in 2021, a 7-day strength and resistance exercise program was compared with no intervention.25 The experimental group received instructions on how to perform the exercises through a website and a daily text message was sent asking about the exercises and as a follow-up method to improve adherence. Additionally, contact to reinforce the exercise program was made by a physiotherapist at least twice during the intervention duration through telematic control by videoconference. All outcomes were assessed through video calls on the first and seventh days. In their trial published in 2022, they compared three groups: breathing exercises, strengthening exercises and control (no intervention).26 The experimental groups received instructions about the interventions on the first day and evaluators maintained daily communication with participants via a smartphone software applicationb as a reminder during the treatment period and also to improve adherence. The assessments were conducted through videoconference at baseline and on day 14.
Three trials investigated telerehabilitation exercise programs in COVID-19 survivors after hospital discharge. In Li et al,27 a 6-week telerehabilitation program was compared with control (ie, short educational instruction) for COVID-19 survivors after hospital discharge. The program consisted of home-based exercises with sessions of 40 to 60 minutes by using a smartphone application. Participants were instructed to monitor and record oxygen saturation before and after exercise with a finger pulse oximeter. Telemetry was used to monitor the participants’ heart rate during the exercises. Besides providing instructions on telemetry adjustments and exercise program execution, the smartphone application also sent notifications to remind participants to start the exercise. In addition, consultations were carried out via smartphone or voice calls on a smartphone communication appc every week and participants could also send their feedback using the application. The authors reported an unexpected change in assessment location during the research execution, which delayed the final assessment by 4 weeks.
In the trial by Pehlivan et al,28 a 6-week program – including breathing exercises, lower and upper limb exercises, walking and wall squat exercises – was compared with no intervention in COVID-19 survivors after hospital discharge. Participants in the experimental group were instructed to complete the exercise sessions three times per week through synchronised videoconference. Participants in the control group received educational material with information about the disease and some basic exercises that could be performed at home. All participants were assessed by video calls and there was no face-to-face meeting.
In the trial by Amaral et al,29 a 12-week tele-supervised home-based exercise training program was compared with no intervention in COVID-19 survivors after discharge from hospital. The assessments were performed in a face-to-face meeting in a controlled room. At the baseline evaluations, the experimental group participants received instructions on how to properly execute each proposed exercise. Supplementary material containing exercise instructions was also sent by smartphone applicationc immediately after the instructional session for the experimental group. The experimental intervention lasted for 12 weeks and consisted of both resistance exercise (twice a week) and aerobic exercise (five times a week) on separate days. The authors reported no adverse events during the trial.
Only Rodriguez-Blanco et al (2022)26 reported technology-related issues. One of the participants reported a problem with the telerehabilitation device, which could not be resolved, and decided not to participate. Other technological issues, such as loss of connection or difficulty in handling the platform, were not reported in the included articles.
In the trial by Gonzales-Gerez et al,30 a breathing exercise program delivered through telerehabilitation was compared with a control group in participants in the acute phase of COVID-19. In this trial30, the experimental group was instructed to perform a program of 10 breathing exercises for 7 days. They were contacted during the intervention protocol twice by videoconference and also received one message per day, which was intended to encourage them to adhere to the program. No technological issues were reported in this study.
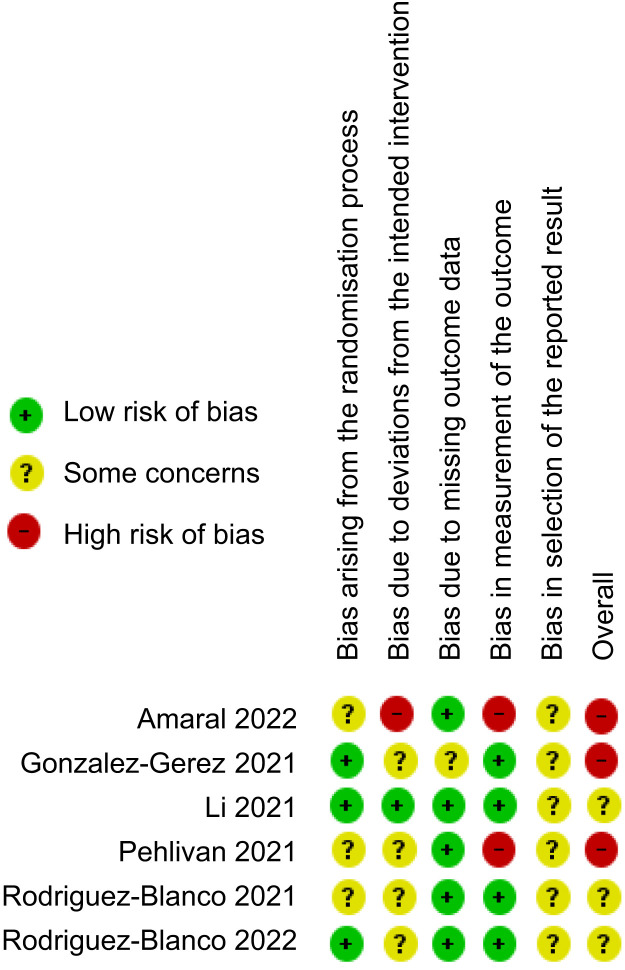
Risk of bias
The risk of bias did not differ among the outcomes within each included trial so the details of judgements for each domain of risk of bias are presented by trial in Figure 2 . The trial by Li et al27 indicated concerns of bias in only one domain: selective outcome reporting. The trial by Gonzales-Gerez et al30 was judged as having a high risk of bias, as there were concerns in several domains: deviations from the intended interventions, missing outcome data and selective outcome reporting.30 Gonzales-Gerez et al also did not perform an intention-to-treat analysis and the trial protocol was registered after commencing data collection.30 The trial by Pehlivan et al28 was judged as having a high risk of bias, as there were concerns in multiple domains (the randomisation process, deviations from the intended interventions and selection of the reported result) and a high risk of bias in measurement of the outcome. The 2021 trial by Rodriguez-Blanco et al25 presented concerns of bias in the randomisation process, deviations from the intended interventions and failure to present the results of one of the variables pre-specified in the analysis plan. The trial by Amaral et al29 was judged as having a high risk of bias, as there were concerns in two domains (the randomisation process due the difference in the baseline characteristics, and selection of the report result because the authors did not plan an intention-to-treat analysis) and a high risk in the domain of deviations from the intended interventions owing the published result not being completely in accordance with a pre-specified analysis. The 2022 trial by Rodriguez-Blanco et al26 was judged as having some concerns of bias in the domain of deviations from the intended interventions. The authors were unable to mask the treatment from participants and therapists due to the nature of the interventions.
Figure 2.
Risk of bias of the included studies assessed using the Cochrane Risk of Bias 2.0 Tool.
Effects of intervention
Breathing exercises via telerehabilitation versus no rehabilitation
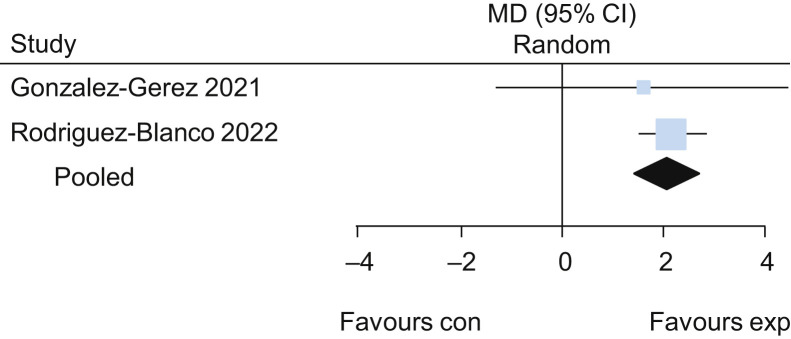
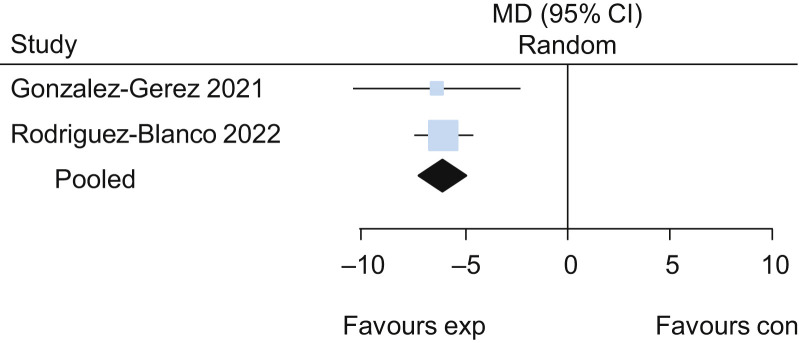
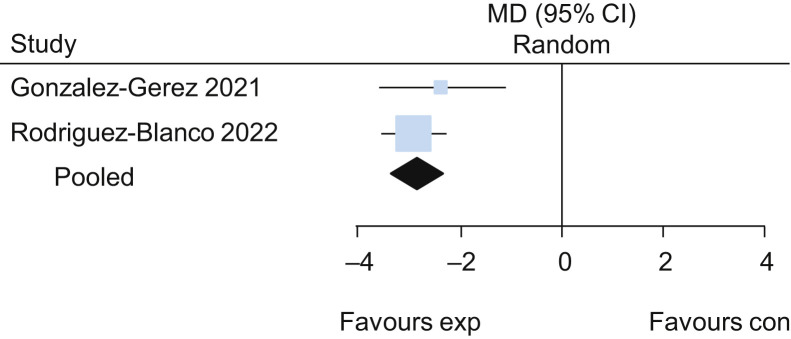
Two trials evaluated the effects of breathing exercises in participants in the acute phase of COVID-19.26 , 30 The pooled results of these trials suggested that breathing exercises delivered via telerehabilitation led to a higher functional capacity assessed by the 6-minute walk test (MD 101 m, 95% CI 61 to 141; two studies) (Figure 3 ) when compared with no intervention, with low certainty of evidence. Participants who received breathing exercises via telerehabilitation also had better performance on the 30-second sit-to-stand test (MD 2.2 repetitions, 95% CI 1.5 to 2.8; two studies) (Figure 4 ), lower dyspnoea on the Multidimensional Dyspnoea-12 (MD –6, 95% CI –7 to –5; two studies) (Figure 5 ) and lower perceived effort on the Borg scale (MD –2.8, 95% CI –3.3 to –2.3; two studies) (Figure 6 ), each with low certainty of evidence. For detailed forest plots, see Figures 7 to 10 on the eAddenda. The published reports of these trials did not mention the occurrence of adverse events. A summary of these findings is presented in Table 2 .
Figure 3.
Detailed forest plot of the mean difference (95% CI) in effect of breathing exercises delivered via telerehabilitation for 2 weeks compared with no rehabilitation on functional capacity assessed by the 6-minute walk test (m), based on pooled data from two trials.
Figure 4.
Mean difference (95% CI) in effect of breathing exercises delivered via telerehabilitation for 2 weeks compared with no rehabilitation on performance assessed by the 30-second sit-to-stand test (repetitions), based on pooled data from two trials.
Figure 5.
Mean difference (95% CI) in effect of breathing exercises delivered via telerehabilitation for 2 weeks compared with no rehabilitation on dyspnoea assessed by Multidimensional Dyspnoea-12, based on pooled data from two trials.
Figure 6.
Mean difference (95% CI) in effect of breathing exercises delivered via telerehabilitation for 2 weeks compared with no rehabilitation on perceived effort assessed by Borg scale, based on pooled data from two trials.
Table 2.
Summary of the findings in relation to breathing exercises via telerehabilitation.
| Breathing exercises compared with no intervention for COVID-19 | ||||||
|---|---|---|---|---|---|---|
| Patient or population: COVID-19 | Setting: home-based | Intervention: breathing exercises | Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effectsa (95% CI) |
Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with breathing exercises | |||||
| Functional capacity at 2 wks: 6-minute walk test | The mean change in functional capacity at 2 wks ranged from –3.23 to 6 m | MD 101 m higher (61 higher to 141 higher) | – | 89 (2) | ⊕⊕○○ Lowb,c |
Breathing exercises may slightly increase functional capacity at 2 wks. |
| Performance at 2 wks: 30-second sit-to-stand | The mean change in performance at 2 wks ranged from –0.59 to –0.31 repetitions | MD 2.2 repetitions higher (1.5 higher to 2.8 higher) | – | 89 (2) | ⊕⊕○○ Lowb,c |
Breathing exercises may slightly increase performance at 2 wks. |
| Dyspnoea at 2 wks: Multidimensional Dyspnoea-12 | The mean change in dyspnoea at 2 wks ranged from 0.05 to 0.32 | MD 6 lower (7 lower to 5 lower) | – | 89 (2) | ⊕⊕○○ Lowb,c |
Breathing exercises may slightly reduce dyspnoea at 2 wks. |
| Perceived effort at 2 wks: Borg scale | The mean change in perceived effort at 2 wks ranged from –0.32 to 0.14 | MD 2.8 lower (3.3 lower to 2.3 lower) | – | 89 (2) | ⊕⊕○○ Lowb,c |
Breathing exercises may result in a slight reduction in perceived effort at 2 wks. |
| GRADE Working Group grades of evidence: High certainty: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded one level due to serious risk of bias (some concerns due to deviations from intended interventions and selection of the reported result).
Downgraded one level due to serious imprecision (few participants and studies).
Exercise via telerehabilitation versus no rehabilitation
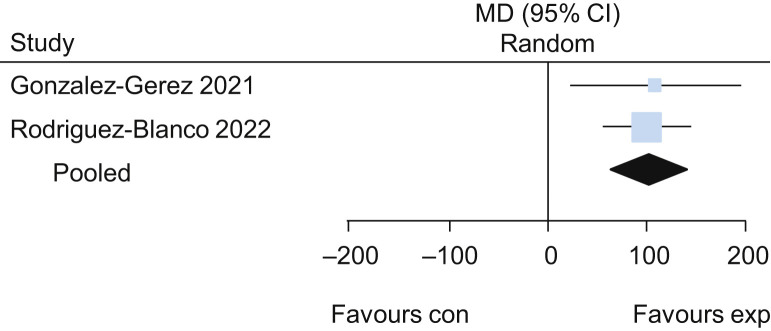
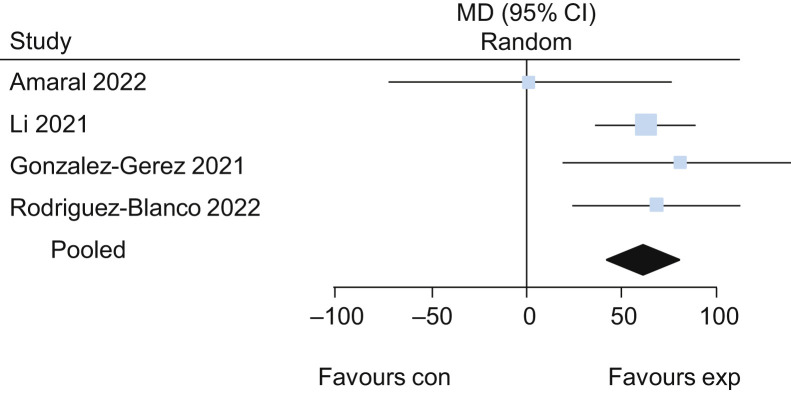
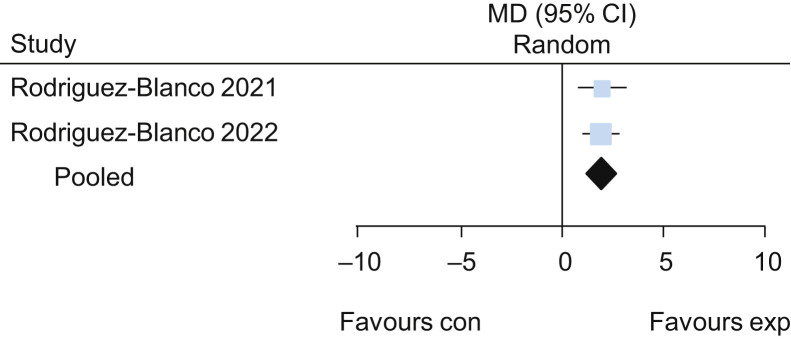
Five trials25, 26, 27, 28, 29 evaluated the effects of exercise delivered via telerehabilitation versus no exercise. The pooled results of these trials suggested that exercise delivered via telerehabilitation may lead to a higher functional capacity assessed by the 6-minute walk test (MD 62 m, 95% CI 42 to 82, four studies) (Figure 11 ) when compared with no exercise, with low certainty of evidence. Participants who received exercise via telerehabilitation also had improved lower limb performance assessed by 30-second sit-to-stand test (MD 2.0 repetitions, 95% CI 1.3 to 2.7; two studies) (Figure 12 ) and lower dyspnoea assessed by Multidimensional Dyspnoea-12 (MD –1.8, 95% CI –2.5 to –1.1; one study26), each with low certainty of evidence. For detailed forest plots, see Figures 13 and 14 on the eAddenda. A summary of the findings is presented in Table 3 .
Figure 11.
Mean difference (95% CI) in effect of exercises delivered via telerehabilitation for 12 weeks compared with no rehabilitation on functional capacity assessed by the 6-minute walk test (m), based on pooled data from four trials.
Figure 12.
Mean difference (95% CI) in effect of breathing exercises delivered via telerehabilitation for 2 weeks compared with no rehabilitation on the 30-second sit-to-stand test (repetitions), based on pooled data from two trials.
Table 3.
Summary of the findings in relation to exercise via telerehabilitation.
| Exercise program compared with no rehabilitation for COVID-19 | ||||||
|---|---|---|---|---|---|---|
| Patient or population: COVID-19 | Setting: home-based | Intervention: exercise program | Comparison: no rehabilitation | ||||||
| Outcomes | Anticipated absolute effectsa (95% CI) |
Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no rehabilitation | Risk with exercise program | |||||
| Functional capacity at 12 wks | The mean change in functional capacity at 12 wks ranged from –3.22 to 18 m | MD 62 m higher (42 higher to 82 higher) | – | 228 (4) | ⊕⊕○○ Lowb,c |
Exercise program may increase functional capacity at 12 wks slightly. |
| Performance at 6 wks: 30-second sit-to-stand | The mean change in performance at 6 wks ranged from –0.59 to –0.55 repetitions | MD 2 repetitions higher (1.3 higher to 2.7 higher) | – | 84 (2) | ⊕⊕○○ Lowb,c |
Exercise program may increase performance at 6 wks slightly. |
| Dyspnoea at 2 wks: Multidimensional Dyspnoea-12 | The mean change in dyspnoea at 2 wks was 0.318 | MD 1.8 lower (2.5 lower to 1.1 lower) | – | 48 (1) | ⊕⊕○○ Lowb,c |
Exercise program may reduce dyspnoea at 12 wks slightly. |
| Free of dyspnoea at 12 wks | 617/1,000 | 907/1,000 (728 to 1,000) | RR 1.47 (1.18 to 1.82) | 112 (1) | ⊕⊕○○ Lowb,d |
Exercise program may increase the likelihood of being dyspnoea-free at 12 wks. |
| Pulmonary function at 6 wks: FEV1/FVC | The mean change in pulmonary function at 6 wks was 0.01 | MD 0.03 higher (0.03 lower to 0.09 higher) | – | 107 (1) | ⊕○○○ Very lowb,e |
Exercise program effect on pulmonary function at 6 wks remain very uncertain. |
| Lower limb muscle strength at 6 wks | The mean change in lower limb muscle strength at 6 wks was 7.98 | MD 21.37 higher (12.47 higher to 30.27 higher) | – | 112 (1) | ⊕⊕○○ Lowb,c |
Exercise program may increase lower limb muscle strength at 6 wks. |
| Quality of life (physical component) at 6 wks | The mean change in quality of life (physical component) at 6 wks was 3.84 | MD 3.97 higher (1.26 higher to 6.68 higher) | – | 112 (1) | ⊕⊕○○ Lowb,c |
Exercise program may increase quality of life (physical component) at 6 wks. |
| Quality of life (mental component) at 6 wks | The mean change in quality of life (mental component) at 6 wks was 4.17 | MD 1.98 higher (1.7 lower to 5.66 higher) | – | 112 (1) | ⊕○○○ Very lowb,e |
Exercise program effects on quality of life (mental component) at 6 wks remain very uncertain. |
| GRADE Working Group grades of evidence: High certainty: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded one level due to methodological limitations.
Downgraded one level due to serious imprecision (few participants).
Downgraded one level due to serious imprecision (few events).
Downgraded two levels due to very serious imprecision (few participants and large confidence interval).
It was decided not to include Amaral et al29 data in the meta-analysis because they performed the five times sit-to-stand test, and this test uses a different scale and has a different direction of the effect compared with the other included studies. This review was unable to estimate the treatment effects or to perform a meta-analysis with Pehlivan et al28 data because the authors provided incomplete information about the units of outcome measurement; they also did not provide a trial registration number so additional information about statistical analysis was unavailable. The exercise program’s effects on pulmonary function assessed by FEV1/FVC remain very uncertain (MD 0.03, 95% CI –0.03 to 0.09; one study27, very low certainty of evidence). Amaral et al29 did not report the measure of variability for the spirometry results and it was not possible to include the result in a meta-analysis.
The results of one trial also suggested that exercise delivered via telerehabilitation increases the likelihood of being free of dyspnoea at 12 weeks (RR 1.47, 95% CI 1.18 to 1.82; one study27), with low certainty of evidence; and improves the physical component of quality of life assessed by 12-Item Short-form Health Survey (SF-12) (MD 3.97, 95% CI 1.26 to 6.68; one study27), with low certainty of evidence. The effect of exercise delivered via telerehabilitation on the mental component of quality of life was unclear (MD 1.98, 95% CI –1.70 to 5.66; one study27). A summary of these findings is presented in Table 3.
Regarding safety, only one trial27 formally evaluated the incidence of adverse events. Among the 174 events that were reported, 78 were in the control group and 96 were in the telerehabilitation group. Only one adverse event was classified as moderately severe (stomach ulcers, control group), the others were very mild to moderate. The events comprised chest tightness, weakness, cough, reduced muscle strength, sputum discharge, dizziness, chest and back pain. Adverse events occurred with similar frequency in the telerehabilitation group (median 0 per participant, IQR 0 to 2.75) as in the control group (median 0 per participant, IQR 0 to 2); Hodges-Lehmann median difference 0 (95% CI 0 to 0), with low certainty of evidence. In the 2021 trial by Rodriguez-Blanco et al,25 one hospitalisation was reported at the end of intervention; it was in the control group. In the 2022 trial by Rodriguez-Blanco et al,26 two hospitalisations were reported; both were in the control group.
Discussion
It is believed that this is the first study to systematically review, with high methodological rigor, the safety and effectiveness of telerehabilitation in patients with COVID-19 and post-COVID-19 conditions. The present systematic review found that an exercise program delivered via telerehabilitation may improve functional capacity, lower limb performance, dyspnoea and physical components of quality of life compared with no exercise. It appears to be safe, with similar incidence of adverse events between the experimental and control groups. The adverse events that occurred were generally mild or moderate, and included chest tightness, weakness, cough, reduced muscle strength, sputum discharge, dizziness, and chest and back pain. Additionally, breathing exercises delivered via telerehabilitation may improve functional capacity, lower limb performance and dyspnoea compared with not performing breathing exercises.
Despite the fact that telerehabilitation has been encouraged around the world during the pandemic, there is a lack of strong scientific evidence published to support this practice with COVID-19 and post-COVID-19 conditions. There are only six currently published randomised trials on the effects of telerehabilitation in patients with COVID-19 and post-COVID-19, in which several methodological issues were identified. Three of the included trials were classified as being at high risk of bias and the other three trials were judged as having some concerns about the risk of bias. Important methodological issues were found, such as the absence of information about the randomisation process, lack of pre-specified protocols, bias due to deviations from intended interventions and bias due to missing outcome data. The small sample size and the eligibility criteria may also limit the generalisation of results to other groups of patients, such as those with mild and moderate symptoms combined with previous underlying disease and comorbidities.
The six included trials presented heterogeneity in participants' demographic and clinical characteristics, stage of disease and telerehabilitation delivery method. The different types of telemonitoring options (eg, mobile phone, WeChat voice calls, text messages, videoconference and YouTube) may have influenced the telerehabilitation outcomes. It is important to note that the pandemic increased the challenge for researchers and participants to conduct clinical trials, especially due to the strict infection control measures and economic downturn. Moreover, the accelerated regulations aiming at rapidly introducing remotely delivered interventions were accompanied by poor guidance for implementation and insufficient professional training.10 While field hospitals were built to support the growing need for hospitalisation, rehabilitation centre supply did not change or even decreased initially.32 This is reflected in one of the most important limitations of Li’s study:27 although several resources were incorporated, an unexpected change in the assessment location delayed the final assessment by 4 weeks. In another trial,26 the total planned time of the interventions was also impacted due to the quarantine period, from 3 to 2 weeks. Thus, there were some difficulties in managing telerehabilitation studies in patients with COVID-19 or post-COVID-19 conditions that went beyond symptoms alone. Saaei et al33 identified, through a questionnaire given to 228 physiotherapists, that teaching exercises virtually, as well as sharing exercises and educational materials across multiple platforms, are the most challenging issues of virtual care. In addition, patients' technological literacy levels were another challenge that was reported.33 Rodriguez-Blanco et al (2021),25 Pehlivan et al,28 Amaral et al,29 Li et al27 and Gonzales-Perez et al30 did not report any difficulty such as the one reported by Saaei et al.33
Approximately 90% of hospitalised patients with COVID-19 experience post-acute sequelae of COVID-19.34 , 35 Thus, even patients who have not needed hospitalisation may experience different levels of respiratory and functional impairment after the acute phase of the disease, indicating the relevance of physical and respiratory rehabilitation.34, 35, 36 The most frequently reported symptom was dyspnoea, followed by fatigue and exercise intolerance.34, 35, 36 Therefore, physiotherapy treatment delivered by telerehabilitation could be an excellent therapeutic alternative for promoting early intervention to re-establish the pre-infection respiratory and functional status, especially in the context of social isolation imposed by the pandemic. Telerehabilitation can also increase patient adherence, due to its convenience and accessibility.37 , 38
This review suggests that telerehabilitation improves outcomes compared with no rehabilitation. Nevertheless, the results must be evaluated with caution due to the small sample size and few events that occurred for some outcomes in the included trials. The best telerehabilitation platform to be used during the COVID-19 pandemic is still undergoing remodelling due to technological advances.33 It is important to assess whether patients can easily handle the different forms of delivery of telerehabilitation in order to increase the adherence, accessibility, interactivity and flexibility of this form of delivery—as observed in telerehabilitation studies on patients with chronic lung disease39 and cardiovascular diseases.40 Several barriers can limit the safe use of telerehabilitation by healthcare institutions, including: data privacy, patient safety and reimbursement. Patients also face barriers to adequate participation in telerehabilitation, including: lack of internet devices at home, poor home internet connection, and factors such as age, cognition and educational level.41
Despite the limitations, telerehabilitation is able to reach more patients, including those with difficulty accessing a healthcare centre and those isolating at home due to COVID-19. However, it is extremely important that trained professionals with a view to patient safety can identify the right time to adjust the intensive exercise program and to stop the activity. Overall, the small number of studies and the different forms of telerehabilitation, with few participants, impose limitations as to the strength of the evidence provided by this review. Mixed delivery models with in-person and remote elements, and different exercise prescriptions might be investigated to evaluate, in a more robust way, the effect of telerehabilitation in patients with COVID-19 and post-COVID-19 conditions.
Future studies are required with higher methodological quality, larger sample sizes and other relevant outcomes such as satisfaction, level of functional independence, costs and mortality. These studies may help verify the safety and effectiveness of telerehabilitation in view of the risk of clinical worsening and necessity of hospitalisation in patients with COVID-19 or low tolerance due to sequelae after COVID-19. Twenty-five ongoing studies were identified in this review. There are many research opportunities in multiple domains that can be used to improve remote care and its outcomes, and to promote the science that supports telerehabilitation.
In conclusion, exercise programs delivered via telerehabilitation may improve functional capacity, lower limb performance, dyspnoea and the physical component of quality of life compared with no rehabilitation in patients with COVID-19 in the acute phase and in people with post-COVID-19 conditions. It appears to be safe, with similar median number of adverse events per participant between the experimental and control groups. The adverse events that occurred were generally mild or moderate, and telerehabilitation did not increase readmissions to hospital. Furthermore, breathing exercises delivered via telerehabilitation in patients in the acute phase of COVID-19 versus no rehabilitation may improve functional capacity, lower limb performance and dyspnoea compared with no rehabilitation. Future primary studies are needed to confirm the effectiveness and safety of telerehabilitation at different stages of COVID-19 and the individualisation of exercises according to disease stage.
What was already known on this topic: Many people who are hospitalised for COVID-19 report persistent impairment in cognitive function, dyspnoea and/or fatigue at hospital discharge. Use of telehealth technology to provide rehabilitation to people with COVID-19 or post-COVID conditions conforms with infection control policies.
What this study adds: Telerehabilitation consisting of breathing exercises and/or general exercise improves functional capacity, physical performance and dyspnoea in people with COVID-19 or post-COVID conditions.
Acknowledgements
The authors thank Helena Spalic for proofreading this manuscript.
Data sharing: The authors will make all relevant data available upon reasonable request.
Provenance: Not invited. Peer reviewed.
Footnotes
Footnotes:a Review Manager V.5.4.1, The Nordic Cochrane Centre, Copenhagen, Denmark.
b WhatsApp, Meta, Menlo Park, USA.
c WeChat, Tencent, Shenzhen, China.
eAddenda: Figures 7, 8, 9, 10, 13 and 14 and Appendices 1 and 2 can be found online at https://doi.org/10.1016/j.jphys.2022.03.011
Ethics approval: Not applicable.
Competing interests: Nil.
Source(s) ofsupport: Nil.
Appendix
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int
- 3.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes L.G., Saragiotto B.T. To what extent can telerehabilitation help patients in low- and middle-income countries? Braz J Phys Ther. 2021;25:481–483. doi: 10.1016/j.bjpt.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantas L.O., Barreto R.P.G., Ferreira C.H.J. Digital physical therapy in the COVID-19 pandemic. Braz J Phys Ther. 2020;24:381–383. doi: 10.1016/j.bjpt.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisk M., Livingstone A., Pit S.W. Telehealth in the Context of COVID-19: Changing Perspectives in Australia, the United Kingdom, and the United States. J Med Internet Res. 2020;22 doi: 10.2196/19264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fioratti I., Fernandes L.G., Reis F.J., Saragiotto B.T. Strategies for a safe and assertive telerehabilitation practice. Braz J Phys Ther. 2021;25:113–116. doi: 10.1016/j.bjpt.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pramuka M., van Roosmalen L. Telerehabilitation technologies: accessibility and usability. Int J Telerehabil. 2009;1:85–98. doi: 10.5195/ijt.2009.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galea M.D. Telemedicine in Rehabilitation. Phys Med Rehabil Clin N Am. 2019;30:473–483. doi: 10.1016/j.pmr.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Peretti A., Amenta F., Tayebati S.K., Nittari G., Mahdi S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil Assist Technol. 2017;4:e7. doi: 10.2196/rehab.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinhardt G., Schwarz P.E., Harst L. Non-use of telemedicine: A scoping review. Health Informatics J. 2021;27 doi: 10.1177/14604582211043147. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Altman D.G. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Thomas J. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 17.Thomas J, Kneale D, McKenzie JE, Brennan SE, Bhaumik S. Determining the scope of the review and the questions it will address. Cochrane Handbook for Systematic Reviews of Interventions. Published online 2019:13-31. https://doi.org/10.1002/9781119536604.ch2
- 18.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang R., Bruning J., Morris N., Mandrusiak A., Russell T. A systematic review of the effects of telerehabilitation in patients with cardiopulmonary diseases. J Cardiopulm Rehabil Prev. 2015;35:380–389. doi: 10.1097/HCR.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Savović J., Page M.J., lbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook
- 22.McKenzie JE, Brennan SE. Synthesizing and presenting findings using other methods. Cochrane Handbook for Systematic Reviews of Interventions. Published online 2019:321-347. https://doi.org/10.1002/9781119536604.ch12
- 23.Schünemann H., Brożek J., Guyatt G., Oxman A., editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. guidelinedevelopment.org/handbook Available from. [Google Scholar]
- 24.Lukaschek K., Frank M., Gensichen J., Halfter K., Schneider A. [Short screener for suicidal behaviour in primary care - a systematic review] MMW Fortschr Med. 2021;163:9–18. doi: 10.1007/s15006-021-0507-2. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Blanco C., Gonzalez-Gerez J.J., Bernal-Utrera C., Anarte-Lazo E., Perez-Ale M., Saavedra-Hernandez M. Short-Term Effects of a Conditioning Telerehabilitation Program in Confined Patients Affected by COVID-19 in the Acute Phase. A Pilot Randomized Controlled Trial. Medicina. 2021;57:7. doi: 10.3390/medicina57070684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Blanco C., Bernal-Utrera C., Anarte-Lazo E., Saavedra-Hernandez M., De-La-Barrera-Aranda E., Serrera-Figallo M.A., et al. Breathing exercises versus strength exercises through telerehabilitation in coronavirus disease 2019 patients in the acute phase: A randomized controlled trial. Clin Rehabil. 2022;36:486–497. doi: 10.1177/02692155211061221. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Xia W, Zhan C, Liu S, Yin Z, Wang J, et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax. Published online 2021:thoraxjnl - 2021. https://doi.org/10.1136/thoraxjnl-2021-217382 [DOI] [PMC free article] [PubMed]
- 28.Pehlivan E., Gayretli Altan S., Palalı İ., Turan D., Çınarka H., Çetinkaya E. COVID 19 Hastalarında Telerehabilitasyon Egzersiz Programının Etkinliği Performans Testleri ile Değerlendirilebilir mi? Pilot Çalışma. Sağlık Profesyonelleri Araştırma Dergisi. 2021;3:1–7. [Google Scholar]
- 29.Amaral VT, Viana AA, Heubel AD, Linares SN, Martinelli B, Witzler PH, et al. Cardiovascular, respiratory and functional effects of tele-supervised home-based exercise training in individuals recovering from COVID-19 hospitalization: A randomized clinical trial. medRxiv.https://doi.org/10.1101/2022.01.24.22269745
- 30.Gonzalez-Gerez J.J., Saavedra-Hernandez M., Anarte-Lazo E., Bernal-Utrera C., Perez-Ale M., Rodriguez-Blanco C. Short-Term Effects of a Respiratory Telerehabilitation Program in Confined COVID-19 Patients in the Acute Phase: A Pilot Study. Int J Environ Res Public Health. 2021;18:14. doi: 10.3390/ijerph18147511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Xia W, Zhan C, Liu S, Yin Z, Wang J, et al. Effectiveness of a telerehabilitation program for COVID-19 survivors (TERECO) on exercise capacity, pulmonary function, lower limb muscle strength, and quality of life: a randomized controlled trial. medRxiv.https://doi.org/10.1101/2021.03.08.21253007
- 32.Houchen-Wolloff L., Steiner M.C. Pulmonary rehabilitation at a time of social distancing: prime time for tele-rehabilitation? Thorax. 2020;75:446–447. doi: 10.1136/thoraxjnl-2020-214788. [DOI] [PubMed] [Google Scholar]
- 33.Saaei F., Klappa S.G. Rethinking Telerehabilitation: Attitudes of Physical Therapists and Patients. J Patient Exp. 2021;8 doi: 10.1177/23743735211034335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paneroni M, Vitacca M, Bernocchi P, Bertacchini L, Scalvini S. Feasibility of tele-rehabilitation in survivors of COVID-19 pneumonia. Pulmonology. Published online 14 April 2021. https://doi.org/10.1016/j.pulmoe.2021.03.009 [DOI] [PMC free article] [PubMed]
- 35.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaVergne S.M., Stromberg S., Baxter B.A., Webb T.L., Dutt T.S., Berry K., et al. A longitudinal SARS-CoV-2 biorepository for COVID-19 survivors with and without post-acute sequelae. BMC Infect Dis. 2021;21:677. doi: 10.1186/s12879-021-06359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werneke M.W., Deutscher D., Grigsby D., Tucker C.A., Mioduski J.E., Hayes D. Telerehabilitation During the COVID-19 Pandemic in Outpatient Rehabilitation Settings: A Descriptive Study. Phys Ther. 2021;101 doi: 10.1093/ptj/pzab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng X., Su Y., Hu Z., Sun X., Li X., Dolansky M.A., et al. Home-based telehealth exercise training program in Chinese patients with heart failure: A randomized controlled trial. Medicine. 2018;97 doi: 10.1097/MD.0000000000012069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox N.S., Dal Corso S., Hansen H., McDonald C.F., Hill C.J., Zanaboni P., et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;1 doi: 10.1002/14651858.CD013040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandran HJ, Jiang Y, Tam WWS, Yeo TJ, Wang W. Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: a systematic review and meta-analysis. Eur J Prev Cardiol. Published online 13 July 2021. https://doi.org/10.1093/eurjpc/zwab106 [DOI] [PMC free article] [PubMed]
- 41.Milani G., Demattè G., Ferioli M., Dallagà G., Lavezzi S., Basaglia N., et al. Telerehabilitation in Italy During the COVID-19 Lockdown: A Feasibility and Acceptability Study. Int J Telerehabil. 2021;13:e6334. doi: 10.5195/ijt.2021.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- www.clinicaltrials.gov/search ClinicalTrials.gov.
- www.prisma-statement.org PRISMA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.