Abstract
Urinary tract infections (UTIs) are among the most common bacterial infections in the United States and are a major driver of antibiotic use, both appropriate and inappropriate, across healthcare settings. Novel UTI diagnostics are a strategy that might enable better UTI treatment. Members of the Antibacterial Resistance Leadership Group Laboratory Center and the Infectious Diseases Society of America Diagnostics Committee convened to envision ideal future UTI diagnostics, with a view towards improving delivery of healthcare, patient outcomes and experiences, and antibiotic use, addressing which types of UTI diagnostics are needed and how companies might approach development of novel UTI diagnostics.
Keywords: urinary tract infection, UTI, laboratory diagnosis, diagnostics
Urinary tract infections are among the most common bacterial infections in the United States and are a major driver of antibiotic use, both appropriate and inappropriate, across healthcare settings.
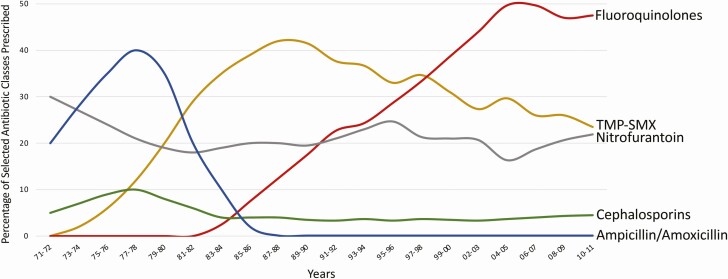
Urinary tract infections (UTIs) are among the most common bacterial infections in the United States and are a major driver of antibiotic use, both appropriate and inappropriate, across healthcare settings. UTI treatment has become complex because of antibacterial resistance; one-quarter of urinary tract isolates of Escherichia coli in the United States in 2017 were resistant to fluoroquinolones and one-third to trimethoprim-sulfamethoxazole [1], agents with historically predictable activity against E coli. As a result, more broad-spectrum antibiotics are being used to treat UTIs, contributing to selection of further antibiotic resistance (Figure 1). This also exposes patients to adverse consequences, such as allergies, side effects, Clostridioides difficile infection, and microbiome disturbances [2]. Compounding the situation, many patients receive unnecessary antibiotics for abnormal urinalyses (eg, pyuria, bacteriuria) [3] or positive urine cultures (asymptomatic bacteriuria/bladder colonization) [4] in the setting of nonspecific symptoms (eg, fatigue) [5], without true UTI. Treatment directed at UTIs when no treatment is needed, alongside treatment with unnecessarily broad-spectrum antibiotics, are fueling antibiotic resistance, which is “one of the biggest public health challenges of our time” [6].
Figure 1.
Trends in antimicrobial therapy for acute uncomplicated cystitis [47–49]. Widespread use of the β-lactams, amoxicillin, and cephalexin, as standard therapy for urinary tract infections in the 1970s and 1980s was followed by emergence of resistance and treatment failure. These were replaced by trimethoprim-sulfamethoxazole as the empiric therapy of choice in the 1980s, which was again followed by increasing antimicrobial resistance and treatment failure. Fluoroquinolones eventually replaced trimethoprim-sulfamethoxazole as the preferred empiric therapy in the 1990s, again followed by increasing antimicrobial resistance and treatment failure.
Diagnostic tests for UTIs have remained largely unchanged over the past half-century. Urine culture is the most common microbiologic test performed in the outpatient setting and remains the gold standard test for diagnosis of UTI despite its relatively long time to results, limited specificity for UTI requiring treatment, and bias toward isolation of classical uropathogens. 16S ribosomal RNA gene sequencing studies have revealed a lower urinary tract microbiome that is not detectable by routine culture methods [7]. Whether microbiome dysbiosis is associated with UTI is an area of active research. In addition, viable but nonculturable uropathogenic E coli (missed by standard laboratory evaluation) has been proposed as a potential cause of recurrent UTI in some cases [8]. Urinalysis for evaluation of pyuria (various cutoffs used [eg, >10 white blood cells/mm3 [9]]) is also an imperfect test with limited positive predictive value [10]. Alternatively, a normal urinalysis can be useful for excluding UTI as the cause of symptoms in otherwise healthy adults [11]. Other tests used to diagnose UTIs include dipstick leukocyte esterase testing, other urine biochemical tests (eg, testing for nitrites), and urine Gram stain (read by a machine or person), with none being ideal.
Innovations in diagnostic testing for other infectious diseases, such as pneumonia, bloodstream infection, gastroenteritis, and most recently, coronavirus disease 2019, are being delivered at rapid rates. Many diagnostic companies have indicated an interest in developing improved UTI tests. Although certainly needed, defining the parameters of what will be most helpful to clinical decision-making without inadvertently leading to unnecessary antibiotic use is challenging. With this in mind, members of the Antibacterial Resistance Leadership Group (ARLG) Laboratory Center and the Infectious Diseases Society of America (IDSA) Diagnostics Committee convened to envision ideal future UTI diagnostics, with a view toward improving delivery of healthcare, patient outcomes and experiences (quality of life), and antibiotic use.
WHICH UTI DIAGNOSTICS ARE NEEDED?
The reason to diagnose UTIs is to inform appropriate treatment. Specifically, UTI treatment active against the infecting bacterium, based on predicted or actual results of in vitro susceptibility testing should be administered if there is a clinical indication for treatment. The goals of treatment are symptomatic relief and prevention of clinical worsening. Ideally, treatment should be oral, inexpensive, low in toxicity, and have the lowest possible potential of negatively affecting the patient’s endogenous microbiota. Members of the ARLG/IDSA writing group applied a 3-step approach to UTI diagnosis to define the steps at which future rapid UTI diagnostics could be useful: (1) Does the patient have a UTI, and if yes, (2) what is the pathogen, and (3) with what should the patient be treated? Each of these 3 steps is discussed next.
Step 1: Does the Patient Have a UTI?
Current diagnostic strategies rely heavily on the presence of characteristic symptoms. According to US Food and Drug Administration (FDA) guidance for UTI therapeutics, there are 2 types of UTIs, uncomplicated and complicated [9, 12]. However, the term UTI encompasses several clinical syndromes, with different associated symptoms, affecting diverse patient types, having a variety of microbiologic etiologies, and treated in different ways (Table 1). These multiple UTI syndromes are not always easily distinguished. For example, there is a continuum from asymptomatic bladder colonization to symptomatic bladder infection [13]. Acute uncomplicated cystitis is a common indication for antibiotic prescription to otherwise healthy community-dwelling women. Despite its name, diagnosing it is not always “uncomplicated.” According to FDA guidance, acute uncomplicated cystitis in women is characterized by at least 2 of the following: dysuria, urinary frequency, urinary urgency, and/or suprapubic pain, plus evidence of pyuria, without fever or costovertebral angle pain [12]. Relying heavily on the presence of symptoms for diagnosis can be problematic because symptom ascertainment can be challenging and several noninfectious and infectious (eg, sexually transmitted infection) conditions (Table 2) can present with similar symptoms to UTIs.
Table 1.
Urinary Tract Infection Syndromes a
| Clinical Definition | Microbiologic Definition | Incidence/Prevalenceb | Populations Affected | Typical Pathogens (in Decreasing Order of Commonality) | References | |
|---|---|---|---|---|---|---|
| Adults | ||||||
| Asymptomatic bacteriuria | Incidental detection of bacteriuria in patient without clinical signs or symptoms attributable to UTI | High-level growth (≥105 CFU/mL) of 1 or more species of bacteria from urine in patient without clinical symptoms of UTI (irrespective of pyuria) | See Table 3 | Varies | □ Any species, including uropathogens | [16, 27, 28] |
| Acute uncomplicated cystitis | New-onset lower urinary tract symptoms with evidence of infectionc | Pyuria plus significant growth of a uropathogen (ie, ≥103–105 CFU/mL) from urine cultured | Incidence: 11% | Premenopausal women with normal urinary tract anatomy |
□ Escherichia coli (75%–95%) □ Klebsiella pneumoniae □ Staphylococcus saprophyticus □ Enterococcus faecalis□ Streptococcusagalactiae □ Proteus mirabilis □ Pseudomonas aeruginosa □ Staphylococcus aureus □ Candida species |
[14, 24, 29–31] |
| Acute complicated UTI | UTI with signs or symptoms of pyelonephritis or systemic infection OR UTI in patient with structural or functional urinary tract abnormality or significant immunocompromise |
See acute uncomplicated cystitis | Incidence: 1% | Varies; premenopausal women or other population |
□ E coli
□ Enterococcus species □ K pneumoniae □ Candida species □ S aureus □ P mirabilis □ P aeruginosa □ S agalactiae |
[14, 32–34] |
| Pyelonephritis | See also acute complicated UTI. UTI with signs or symptoms of upper urinary tract involvemente | See acute uncomplicated cystitis | Incidence: 0.2% in women; 0.03%–0.05% in men | Varies; premenopausal women or other population | Same as acute complicated UTI | [30, 35] |
| Recurrent uncomplicated cystitis | ≥2 UTI in 6 months OR ≥3 UTI in 1 year |
See acute uncomplicated cystitis | Incidence: 27%–44% recurrence in 1 year following initial UTI | Premenopausal women with normal urinary tract anatomy | Same as acute uncomplicated cystitis | [30, 36, 37] |
| Catheter-associated UTI | Clinical and laboratory findings compatible with UTI in patient with indwelling bladder catheter and no alternative cause of symptoms or infectionf | Pyuriag plus significant growth of a uropathogen (≥105 CFU/mL) from urine cultured | Incidence: 3–8 infections per 1000 catheter-days |
Hospitalized or nonhospitalized patient with indwelling urinary catheter | Same as acute complicated UTI | [38–40] |
| Postmenopausal infectious cystourethritis and UTI | Acute lower urinary tract symptoms greater than baseline with evidence of infectionc | See acute uncomplicated cystitis | Prevalence: 9%–12% |
Postmenopausal women | Same as acute uncomplicated cystitis; see text | [29] |
| Children | ||||||
| Neonatal bacteriuria and UTI | Clinical and laboratory findings compatible with UTI and no alternative source of infectionh | Significant growth of a uropathogen (ie, ≥103–105 CFU/mL) from of appropriately collected urine specimen (pyuria not required)d | Prevalence: 7%–15% among febrile neonates | Infants ≤30 days of age | Same as acute complicated UTI in adults | [41, 42] |
| UTI in infants and young children | Clinical and laboratory findings compatible with UTI and no alternative source of infectionh | Pyuria plus significant growth of a uropathogen (≥5 × 104 CFU/mL catheter-collected urine) from culture of appropriately collected urine specimend | Prevalence: 5%–7% in febrile infants and young children depending on age | Infants and young children 2–24 months of age | Same as acute complicated UTI in adults | [43, 44] |
| Acute cystitis in children and adolescents | Clinical and laboratory findings compatible with UTI and no alternative source of infectionh | Pyuria plus significant growth of a uropathogen (≥105 CFU/Ml clean catch urine; ≥5 × 104 CFU/Ml catheter-collected urine) from urine cultured | Prevalence: 8% | Children and adolescents ≥2 years old | Same as acute uncomplicated cystitis in adults | [43, 44] |
Abbreviations: CFU, colony-forming unit; UTI, urinary tract infection.
aExcludes other genitourinary infections such as urethritis, prostatitis, epididymo-orchitis, and UTIs in renal transplant recipients and those with ileal loops.
bIncidence (per year) or prevalence based on available data.
cLower urinary tract symptoms include dysuria, increased urinary frequency or urgency, suprapubic pain or tenderness, and hematuria.
dQuantitative thresholds for significant growth vary by urine collection method, population, UTI type, organism, and institution/laboratory; the level of evidence for various thresholds is generally low. For example, Hooton et al. suggest detection of ≥100 (102) E coli CFU/mL from midstream urine is sufficient to confirm acute uncomplicated UTI in healthy premenopausal women with acute lower urinary tract symptoms. However, most clinical laboratories use higher thresholds for pathogen identification and reporting (eg, ≥103–105 CFU/mL for nonsterile urine samples [eg, midstream void]; ≥103 CFU/mL for “sterile” urine samples [in and out catheter, suprapubic aspirate]).
eUpper urinary tract signs/symptoms include flank pain, fever, and costovertebral angle tenderness.
fIndwelling urinary catheters can cause lower urinary tract symptoms, pyuria, and bacteriuria without UTI.
gPyuria has a high negative predictive value but low positive predictive value in catheter-associated UTI.
hPediatric UTI definitions have a lower threshold for testing and diagnosis because of inability of patients to verbalize and greater need to rely on nonspecific clinical findings, such as abdominal pain or fever.
Table 2.
Other Causes of Lower Urinary Tract Symptoms
| Diagnosis | Predominant Symptom(s) |
|---|---|
| Interstitial cystitis/bladder pain syndrome | Pain, pressure, discomfort |
| Overactive bladder | Urgency frequency, nocturia |
| Bladder or urethral cancer | Pain, hematuria |
| Benign pelvic mass(es) | Pressure, pain |
| Bladder stone or other foreign object | Pain, discomfort |
| Urethral diverticulum | Pain, discomfort, postvoid dribbling |
| Neurologic dysfunction, outlet obstruction | Pain, urinary retention |
| Sexually transmitted infection | Dysuria, vaginal discharge |
| Candidal vulvovaginitis | Dysuria, vaginal discharge, itching |
| Atrophic vaginitis | Dysuria, vaginal discharge, pain, itching, burning |
| Bacterial vaginosis | Dysuria, vaginal discharge, pain, itching, burning |
Complicated UTIs involve at least 2 of the following: chills, rigors, or warmth associated with fever; flank or pelvic pain; nausea or vomiting; dysuria, urinary frequency, or urinary urgency; and/or costovertebral angle pain or tenderness. The presence of a complicating host factor, such as a functional or anatomical abnormality of the urinary tract or catherization can also be used to define a UTI as complicated. By the FDA’s definition, all pyelonephritis cases and UTIs in men are considered complicated [9]. In practice, correctly diagnosing the patient’s status given the UTI continuum requires a certain level of sophistication, considering symptoms and signs, patient factors, and results of diagnostic tests.
E coli is the most common causative agent of both uncomplicated and complicated UTIs. For uncomplicated UTIs, other causative agents (in order of prevalence) are Klebsiella pneumoniae, Staphylococcus saprophyticus, Enterococcus faecalis, Streptococcus agalactiae, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida species, and for complicated UTIs are Enterococcus species, K pneumoniae, Candida species, S aureus, P mirabilis, P aeruginosa, and S agalactiae [14].
Those developing novel UTI diagnostics will face the challenge that detection of a potential uropathogen in urine does not mean the patient has a UTI that needs treatment; instead, the patient may have asymptomatic bacteriuria or a compromised specimen contaminated by commensal urogenital microbiota. Furthermore, some women with uncomplicated cystitis may have resolution of symptoms without antibiotics [15]. Asymptomatic bacteriuria is more prevalent than UTI requiring treatment (Table 3), and unfortunately, no available test (including tests focused on bacteria or pyuria) distinguishes it from symptomatic UTI requiring treatment [13, 16]. The writing group recognized that in some special populations (eg, spinal cord injury, patients who cannot report symptoms because of altered consciousness, those with atypical symptoms, those with indwelling catheters), symptoms cannot be accurately assessed, making it a challenge to know whether or not bacteria in their urine warrant treatment. Even in populations where clinical history taking is more straightforward, avoiding treatment of a positive urinalysis or urine culture when there is no indication for treatment can be challenging. A novel test that focuses on detection of bacteria, without considering whether a UTI needing treatment is present, may only recapitulate a known downside of culture—that is, positivity in cases of asymptomatic bacteriuria—perpetuating overuse of unnecessary antibiotics. Although improvements in diagnostic stewardship will help, the writing group considers a test that supports improved diagnostic approaches to distinguish asymptomatic bacteriuria from UTI needing treatment to be a high priority.
Table 3.
Prevalence of Asymptomatic Bacteriuria in Adults
| Population | Prevalence |
|---|---|
| Patients with long-term indwelling catheters [19] | 100% |
| Patients with spinal cord injury and intermittent catheterization [19] | 23%–89% |
| Patients with short-term indwelling catheters [19] | 9%–23% |
| Diabetic women [19] | 9%–27% |
| Women in long-term care facilities [19] | 25%–50% |
| Men in long-term care facilities [19] | 15%–40% |
| Healthy premenopausal women [16, 19, 45] | 1%–5% |
| Patients on hospital admission [46] | 8% |
Novel diagnostic tests addressing whether a UTI is present would likely focus on either of the 2 elements necessary for infection: the host or the pathogen. Host-focused diagnostics could identify a biomarker or panel of biomarkers that determine whether a UTI is present. Whether measured in urine or other body fluids such as blood, a host-based test would need to be sufficiently specific to discriminate infection from asymptomatic bacteriuria. Host-focused diagnostics are particularly appealing because they eliminate the guesswork of differentiating “colonization” from infection. More familiar to clinicians are the many pathogen-directed strategies that could determine if a potential pathogen is present. One consideration would be a rapid screen to rule out the possibility of UTI, for example by quickly identifying urine specimens in which cultures would be negative or reveal mixed microbiota. In a recent study performed in an outpatient setting, 21% of 1260 patients with negative urine cultures or colony counts below the laboratory’s cutoff level were treated for UTI. Whether faster availability of “negative” results might have abrogated unnecessary treatment is unknown. “Reflex” urine culture protocols, whereby culture is only performed if urinalysis is suggestive of a UTI, have similarly been implemented to enable antibiotic stewardship initiatives. Available evidence suggests that such approaches may help to safely reduce unnecessary antibiotic prescriptions for asymptomatic bacteruria [17].
Current diagnostics are generally applied to the various types of UTIs in a “1-size-fits” all fashion. Ideal novel diagnostics may be different for each scenario (eg, focusing on the most common organism-types involved rather than trying to target a broader array of potential uropathogens). Such an approach would, however, need to be carefully executed to avoid overtreating asymptomatic bacteriuria. In addition, special consideration should be given to patients with long-term catheterization, a clinical scenario where UTIs are more likely to be polymicrobial in nature and potentially involve a different array of organisms, including Providencia stuartii and Morganella morganii [18].
Urine culture is recommended for complicated UTIs. Alternatively, culture may not be routinely performed for acute uncomplicated cystitis [19], on the premise that such infections can be treated with recommended first-line recommended antibiotics. The assumption that uncomplicated UTIs are caused by susceptible bacteria, however, is becoming out of step with current uropathogen resistance patterns in many practice settings. If the practice of not routinely using urine cultures for acute uncomplicated cystitis is in place, this may limit uptake and development of novel diagnostic tests intended to recapitulate results of cultures.
Step 2: What Is the Pathogen?
As mentioned, urine culture is the gold standard test for diagnosis of UTI. A downside of culture (discussed previously) is that it does not differentiate UTI requiring treatment from asymptomatic bacteriuria. As a result of asymptomatic bacteriuria, the specificity of urine culture (and presumably by proxy any test that recapitulates its results), varies across patient populations, ranging from 80%–90% in healthy outpatients and approximating 0% in patients with chronic indwelling catheters [10] (Table 3). Another downside is that results may yield mixed microbiota because of contamination with vaginal, epidermal, and/or perineal microbiota [20]; rates of contaminated urine cultures may be especially high in asymptomatic pregnant women [21]. A recent study showed that 55% of urine cultures collected in primary care clinics were contaminated (ie, mixed microbiota, non-uropathogens, or ≥3 bacteria detected), and that 1 in 5 patients with contaminated urine cultures was treated with antibiotics [22]. Urine cultures may conversely be falsely negative in patients treated with antibiotics, with fastidious microorganisms or with low microbial abundance.
For clinical treatment trials of uncomplicated UTIs, the FDA recommends that a single bacterial species be isolated in pure culture at ≥105 colony-forming unit (CFU)/mL [9]. However, this commonly used criterion for defining significant bacteria was established for women with acute pyelonephritis and may not be applicable to all populations [23]. Patients may have symptomatic UTI with lower colony counts; uropathogens at ≥102 CFU/mL may be significant for certain bacteria [24], patients receiving antibiotics and in men. Microbial quantification may also be needed with new UTI diagnostics, and actionable quantities may differ depending on the microorganism and UTI type. Regardless of the challenges presented, novel pathogen identification tests may be clinically helpful. Tests that yield substantially faster results than culture would be particularly important because urine culture and susceptibility results are typically returned well into a course of treatment (eg, 2–3 days later).
There was discussion as to whether the second step (ie, what is the pathogen?) is necessary if antibacterial susceptibility testing, which comprises the third step, can be performed by other means. That is, if a patient has been determined to have a UTI, it might be acceptable to use a novel diagnostic test to determine which treatment should be used, foregoing identification of the pathogen (or pathogens) involved. In most cases, antibiotic susceptibility is more important than pathogen identification for clinicians to decide on treatment since it has direct bearing on the choice of antibiotic. Members of the writing group did not resolve whether pathogen identification could be entirely circumvented. An advantage to identifying the pathogen is that this information is available to guide treatment if the patient subsequently presents with an infection that could reasonably have originated in the urinary tract (such as sepsis). Organism identification linked to antimicrobial susceptibility results also enables tracking of resistance rates in key pathogens, which is essential for public health surveillance and hospital epidemiology.
Step 3: With What Should the Patient Be Treated?
Antibacterial susceptibility testing (AST) is used to guide selection of antibacterial therapy. The main downside of AST, as currently performed, is turnaround time. Consequently, AST does not currently feature prominently in the initial management of UTIs, especially in outpatient or emergency department settings. This is because a decision about antibiotic choice is typically made at the time the UTI is diagnosed. By the time AST results are available, the patient is often days into therapy. At this point, deescalation in the outpatient setting is impractical. Because of the emergence of antibiotic resistance, UTIs are increasingly caused by resistant pathogens (eg, extended spectrum β-lactamase producing E coli). As a result, patients may not receive active therapy upfront, necessitating additional follow-up and delaying initiation of effective treatment. New UTI diagnostics should yield rapid, actionable results that are available to guide collaborative patient-provider discussions and personalized therapeutic decisions before an antibiotic prescription is filled. Ideally, results would be available at the point of encounter, within 20 minutes (although ideal timeframes remain to be determined) of specimen collection. This would likely mean near-to-care testing (ie, likely not in a centralized laboratory because of the associated transportation time). Although acceptable turnaround times may be longer for hospitalized patients and those in the emergency department requiring hospitalization, the same principle should apply; that is, results should be available before an antibiotic is prescribed for patients without sepsis.
In addition to providing rapid, onsite guidance about which antibiotics to use, rapid susceptibility tests could facilitate the use of older, narrow-spectrum, or inexpensive antibiotics in settings in which they have been abandoned because of unpredictable susceptibility. Examples of such antibiotics that could be used with supportive AST data are trimethoprim-sulfamethoxazole, cephalexin, and other oral cephalosporins, fosfomycin and nitrofurantoin. Tests that enable avoidance of antibiotics of higher toxicity (eg, fluoroquinolones) unless absolutely needed will likely offer clinical benefit and should be prioritized. This could be accomplished by rapid phenotypic or genotypic tests, with the ideal approach remaining to be determined.
OTHER CONSIDERATIONS FOR DEVELOPMENT OF RAPID UTI DIAGNOSTICS
The ARLG/IDSA writing group recognized potential barriers to implementation of novel UTI diagnostics. Factors that may affect uptake of new tests for UTI include workflow considerations, desire for proof of clinical utility, overlap with sexually transmitted infections, and definition of screening questions to guide appropriate use of novel UTI diagnostics. These issues are addressed point-by-point below, along with a listing of additional potential implementation barriers.
Workflow Considerations
Sites at which UTI evaluation/testing is occurring today need to be considered when designing novel diagnostics; these include primary care sites, urgent care centers, long-term care facilities, retail pharmacies, and student health clinics. Many have established workflows that might need redesign to incorporate novel diagnostics, whether performed at the point of care or in a central laboratory. Real-world data as to which diagnostics are being used in such settings today would be helpful. In settings where there is little current laboratory support beyond urine dipstick testing, implementation of novel diagnostics may be challenging, especially if the main goal is reducing empiric use of antibiotics. Workflow considerations may differ by UTI type (eg, acute uncomplicated cystitis versus pyelonephritis). Although novel UTI diagnostics may not be relevant for every patient, they may create opportunities for practice changes that could render them useful.
Demonstration of Clinical Utility
A novel diagnostic that rapidly defines the microbiology of acute uncomplicated cystitis and effective antimicrobials for the organism(s) present would provide faster and/or more information than available today; the benefit of such a diagnostic may need to be demonstrated especially if the test adds cost and/or logistical complexity to care. The ARLG/IDSA writing group offered the following thoughts as to how a novel UTI test might be evaluated. Test performance could be evaluated in the specific practice type and patient population in which the test is anticipated to be used and consider false-positive as well as false-negative rates and their implications. An example for assessing value of a new diagnostic approach (beyond or possibly incorporated into what is needed for regulatory approval) would be to test all women who present for primary care (for any reason) with a novel UTI diagnostic, and define results in the context of positive/contaminated urine cultures, presence/absence of UTI-type symptoms, and whether clinical judgment would have classified them as having a UTI or not. This same process could be repeated with other patient groups, such as men, older community-dwelling adults, adult residents of long-term care facilities, children, pregnant women, and patients with indwelling urinary catheters, to understand test performance in varied populations.
Overlap With Sexually Transmitted Infections
Patients with UTI symptoms, including some with pyuria, may have a sexually transmitted infection. A urine test that offers the possibility to simultaneously test for UTI and sexually transmitted infections, such as Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, and Mycoplasma genitalium, might be valuable in the context of women with symptoms of acute uncomplicated cystitis. Resistance testing for N gonorrhoeae and M genitalium are additional considerations. Genital herpes simplex virus infection may also yield an overlapping clinical presentation with UTI.
Screening Questions to Guide Appropriate Testing With Novel UTI Diagnostics
A need to define appropriate screening questions to guide proper testing with novel UTI diagnostics (including home self-testing) and antibiotic prescribing for the various settings where UTIs are diagnosed and the various types of UTIs was identified.
Additional Potential Barriers
Other potential challenges to adoption of novel UTI diagnostics will likely include cost, reimbursement, and the need to familiarize both healthcare providers and patients with the new test’s performance characteristics and limitations.
HOW SHOULD COMPANIES APPROACH NOVEL UTI DIAGNOSTICS?
A synopsis of possible new UTI diagnostics and considerations for development/implementation is presented in Table 4. As with all modern diagnostics, rapid, inexpensive, and accurate tests are needed. Although there is a need for improvement in UTI diagnosis, new tests should strive to go beyond more rapid identification of organisms present (or not) and AST, though these may also have value [25, 26]. A strategy to accurately detect UTI and to differentiate it from bacteriuria that does not require therapy, sample contamination, noninfectious processes, and sexually transmitted infections (which could be simultaneously diagnosed) would theoretically substantially advance clinical practice and improve antibiotic prescribing tailored to the individual. However, such a test is not immediately possible given the lack of biomarkers to distinguish between UTI and self-limited or asymptomatic bacteriuria. Host response approaches could be leveraged to make this distinction. If a UTI is present, a strategy that leads to ideal initial treatment is needed, either with an older antibiotic if a susceptible bacterium is present, or an appropriate more advanced generation antibiotic if a resistant pathogen is detected. It is likely that clinical workflows will need to be modified to realize the value of such novel UTI diagnostics. Patient care settings where novel diagnostics will have the most impact need definition. The voice of the patient, which has historically been underappreciated, should also be considered in the development of strategies for implementing novel UTI diagnostics, as should the goal of increasing appropriate and reducing inappropriate antibiotic use. Ultimately, the medical and diagnostics communities should work together to understand how novel tests for UTI will bring value to patients, their providers, and the community at large.
Table 4.
Considerations for Novel UTI Diagnostics
| Possible tests |
| Rapid screen to rule out UTI |
| Test that distinguishes asymptomatic bacteriuria from UTI needing treatment |
| Quick (ideally <20 minutes) test that recapitulates culture and susceptibility results (possibly with microbial quantification) |
| Rapid susceptibility test which enables up-front use of older, less expensive, or narrow-spectrum antibiotics |
| Simultaneous test for Neisseria gonorrhoeae (including antibiotic resistance), Chlamydia trachomatis, Trichomonas vaginalis, and Mycoplasma genitalium (including antibiotic resistance) when clinically indicated |
| Considerations |
| Urine cultures are not routinely obtained for acute uncomplicated cystitis—this practice may limit uptake of novel diagnostic tests that recapitulate culture results |
| Need actionable, rapid results, available before antibiotic prescription |
| May need healthcare workflow redesign |
| Need outcomes studies showing value of new diagnostics in improving patient satisfaction, appropriate use of antibiotics, etc. |
Abbreviation: UTI, urinary tract infection.
Notes
Acknowledgements. Generation of the content reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI104681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the United State Government.
Potential conflicts of interest. L.M. reports consulting fees from BioRad and Hitachi Chemical and payment or honoraria from Thermofisher. R.P. reports support for the present manuscript from ARLG; grants or contracts from ContraFect, TenNor Therapeutics Limited, and Biofire; consulting fees from Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, CARB-X, Qvella- monies are paid to Mayo Clinic; relationship with Adaptive Phage Therapeutics (Mayo Clinic and she); consultant to Netflix; patent on Bordetella pertussis/parapertussis polymerase chain reaction issued, patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and patent on an anti-biofilm substance issued; ASM Past President: Chair, Governance Committee; and receipt of an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. C.R.P. reports support for the study from the National Institutes of Health (NIH) ARLG Diagnostics Subcommittee as an unpaid member; payment or honoraria from Ferring Pharmaceuticals for an educational lecture about Clostridioides difficile diagnostics as part of “Ask the expert” series; and leadership or fiduciary role for being a member of American Board of Pathology Test Development and Advisory Committee. L.G. reports grants or contracts made to institution from NIH, Agency for Healthcare Research and Quality (AHRQ), VA Health Services Research & Development, and investigator-initiated research grant by Rebiotix Inc. B.T. reports US federal funding for the study from NIH, AHRQ, Centers for Disease Control and Prevention, and VA Health Services Research and Development. E.L.T. reports consulting fees from Predigen Inc. and patents for Host based molecular signatures of human infection with severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019), Transcriptional Signature for Candidemia, Gene Expression Signatures Useful to Predict or Diagnose Sepsis and Methods of Using the Same, an miRNA host response signature accurately discriminates acute respiratory infection etiologies, Methods to Diagnose and Treat Acute Respiratory Infections, Biomarkers for the Molecular Classification of Bacterial Infection; stock or stock options from Predigen Inc.; and is a cofounder of Predigen Inc. S.B.D. reports consulting fees from Sysmex, serving as Member, Diagnostics Committee, for IDSA, and stock and stock options from Qvella, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One 2019; 14:e0220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butler AM, Durkin MJ, Keller MR, Ma Y, Powderly WG, Olsen MA. Association of adverse events with antibiotic treatment for urinary tract infection. Clin Infect Dis 2021. doi: 10.1093/cid/ciab637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khawcharoenporn T, Vasoo S, Ward E, Singh K. Abnormal urinalysis finding triggered antibiotic prescription for asymptomatic bacteriuria in the ED. Am J Emerg Med 2011; 29:828–30. [DOI] [PubMed] [Google Scholar]
- 4. Sloane PD, Kistler CE, Reed D, Weber DJ, Ward K, Zimmerman S. Urine culture testing in community nursing homes: gateway to antibiotic overprescribing. Infect Control Hosp Epidemiol 2017; 38:524–31. [DOI] [PubMed] [Google Scholar]
- 5. Pallin DJ, Ronan C, Montazeri K, et al. Urinalysis in acute care of adults: pitfalls in testing and interpreting results. Open Forum Infect Dis 2014; 1:ofu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Diseasae Control and Prevention. Antibiotic resistance threats in the United States, 2019. 2019. [Google Scholar]
- 7. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 2014; 52:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev 2010; 34:415–25. [DOI] [PubMed] [Google Scholar]
- 9. United States Food and Drug Administration. Complicated urinary tract infections: developing drugs for treatment guidance for industry. 2018. [Google Scholar]
- 10. Chan-Tack KM, Trautner BW, Morgan DJ. The varying specificity of urine cultures in different populations. Infect Control Hosp Epidemiol 2020; 41:489–91. [DOI] [PubMed] [Google Scholar]
- 11. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med 1983; 75:53–8. [DOI] [PubMed] [Google Scholar]
- 12. United States Food and Drug Administration. Uncomplicated urinary tract infections: developing drugs for treatment guidance for industry. 2019. [Google Scholar]
- 13. Trautner BW. Urinary tract infections as a continuum: implications for diagnostic and antibiotic stewardship. Clin Infect Dis 2021; 72:1339–41. [DOI] [PubMed] [Google Scholar]
- 14. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bleidorn J, Gágyor I, Kochen MM, Wegscheider K, Hummers-Pradier E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?–results of a randomized controlled pilot trial. BMC Med 2010; 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hooton TM, Roberts PL, Stapleton AE. Asymptomatic bacteriuria and pyuria in premenopausal women. Clin Infect Dis 2021; 72:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee ALH, Leung ECM, Lee MKP, Lai RWM. Diagnostic stewardship programme for urine culture: impact on antimicrobial prescription in a multi-centre cohort. J Hosp Infect 2021; 108:81–9. [DOI] [PubMed] [Google Scholar]
- 18. Nicolle LE. Catheter-related urinary tract infection. Drugs Aging 2005; 22:627–39. [DOI] [PubMed] [Google Scholar]
- 19. Colgan R, Nicolle LE, McGlone A, Hooton TM. Asymptomatic bacteriuria in adults. Am Fam Physician 2006; 74:985–90. [PubMed] [Google Scholar]
- 20. Bekeris LG, Jones BA, Walsh MK, Wagar EA. Urine culture contamination: a College of American Pathologists Q-Probes study of 127 laboratories. Arch Pathol Lab Med 2008; 132:913–7. [DOI] [PubMed] [Google Scholar]
- 21. O’Leary BD, Armstrong FM, Byrne S, Talento AF, O’Coigligh S. The prevalence of positive urine dipstick testing and urine culture in the asymptomatic pregnant woman: a cross-sectional study. Eur J Obstet Gynecol Reprod Biol 2020; 253:103–7. [DOI] [PubMed] [Google Scholar]
- 22. Grigoryan L, Matas J, Hansen M, et al., eds. Optimizing urine collection represents an important stewardship opportunity in primary care. SHEA; Spring 2021. Abstract. [Google Scholar]
- 23. Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis 2004; 38:1150–8. [DOI] [PubMed] [Google Scholar]
- 24. Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med 2013; 369:1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toosky MN, Grunwald JT, Pala D, et al. A rapid, point-of-care antibiotic susceptibility test for urinary tract infections. J Med Microbiol 2020; 69:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pilmis B, Jiang O, Thy M, et al. Clinical impact of rapid susceptibility testing on Mueller-Hinton Rapid-SIR directly from urine specimens. Eur J Clin Microbiol Infect Dis 2020; 39:1373–7. [DOI] [PubMed] [Google Scholar]
- 27. Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–54. [DOI] [PubMed] [Google Scholar]
- 28. Rowe TA, Juthani-Mehta M. Urinary tract infection in older adults. Aging Health 2013; 9. doi: 10.2217/ahe.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 2000; 10:509–15. [DOI] [PubMed] [Google Scholar]
- 30. Kranz J, Schmidt S, Lebert C, et al. The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients: part 1. Urol Int 2018; 100:263–70. [DOI] [PubMed] [Google Scholar]
- 31. Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 32. Nicolle LE; AMMI Canada Guidelines Committee*. . Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol 2005; 16:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anger J, Lee U, Ackerman AL, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol 2019; 202:282–9. [DOI] [PubMed] [Google Scholar]
- 34. Carreno JJ, Tam IM, Meyers JL, Esterberg E, Candrilli SD, Lodise TP Jr. Corrigendum to: longitudinal, nationwide, cohort study to assess incidence, outcomes, and costs associated with complicated urinary tract infection. Open Forum Infect Dis 2020; 7:ofz536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Czaja CA, Scholes D, Hooton TM, Stamm WE. Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis 2007; 45:273–80. [DOI] [PubMed] [Google Scholar]
- 36. Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health 1990; 80:331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikäheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 1996; 22:91–9. [DOI] [PubMed] [Google Scholar]
- 38. Hooton TM, Bradley SF, Cardenas DD, et al. ; Infectious Diseases Society of America. . Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:625–63. [DOI] [PubMed] [Google Scholar]
- 39. Edwards JR, Peterson KD, Andrus ML, et al. ; NHSN Facilities. . National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control 2007; 35:290–301. [DOI] [PubMed] [Google Scholar]
- 40. Kwon JH, Fausone MK, Du H, Robicsek A, Peterson LR. Impact of laboratory-reported urine culture colony counts on the diagnosis and treatment of urinary tract infection for hospitalized patients. Am J Clin Pathol 2012; 137:778–84. [DOI] [PubMed] [Google Scholar]
- 41. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr 2011; 158:91–4. [DOI] [PubMed] [Google Scholar]
- 42. Lin DS, Huang SH, Lin CC, et al. Urinary tract infection in febrile infants younger than eight weeks of Age. Pediatrics 2000; 105:E20. [DOI] [PubMed] [Google Scholar]
- 43. Subcommittee on Urinary Tract Infection Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011; 128:595–610. [DOI] [PubMed] [Google Scholar]
- 44. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008; 27:302–8. [DOI] [PubMed] [Google Scholar]
- 45. Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000; 343:992–7. [DOI] [PubMed] [Google Scholar]
- 46. Trevino SE, Henderson JP, Wu J, Cass C, Marschall J. Prevalence of asymptomatic bacteriuria in hospitalized patients. Infect Control Hosp Epidemiol 2016; 37:749–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med 2002; 162:41–7. [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. Outpatient antibiotic prescribing practices for uncomplicated urinary tract infection in women in the United States, 2002-2011. Open Forum Infect Dis 2016; 3:ofw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nickel JC. Management of urinary tract infections: historical perspective and current strategies: part 2–modern management. J Urol 2005; 173:27–32. [DOI] [PubMed] [Google Scholar]