Abstract
Background
Inaccessibility of medicines in low- and middle-income countries is a frequent challenge. Yet it is typically assumed that high-income countries have complete access to the full arsenal of medicines. This study tests this assumption for new antibacterials, which are saved as a last resort in order to prevent the development of resistance, resulting in insufficient revenues to offset costs. Prior studies report only regulatory approval, missing the important lag that occurs between approval and commercial launch, although some antibiotics never launch in some countries.
Methods
We identified all antibacterials approved and launched in the G7 and 7 other high-income countries in Europe for the decade beginning 1 January 2010, using quantitative methods to explore associations.
Results
Eighteen new antibacterials were identified. The majority were accessible in only 3 countries (United States, United Kingdom, and Sweden), with the remaining 11 high-income countries having access to less than half of them. European marketing authorization did not lead to automatic European access, as 14 of the antibacterials were approved by the European Medicines Agency but many fewer were commercially launched. There was no significant difference in access between “innovative” and “noninnovative” antibacterials. Median annual sales in the first launched market (generally the United States) for these 18 antibiotics were low, $16.2M.
Conclusions
Patient access to new antibacterials is limited in some high-income countries including Canada, Japan, France, Germany, Italy, and Spain. With low expected sales, companies may have decided to delay or forego commercialization due to expectations of insufficient profitability.
Keywords: access, antibacterial, approval, commercial launch
Antibacterials approved since 2010 are not being commercially launched promptly in many high-income countries, even after regulatory approval. With low expected sales, companies may have decided to delay or forego commercialization due to expectations of insufficient profitability.
In 2010, the Infectious Diseases Society of America called for 10 new antibiotics by 2020 [1]. This goal was achieved in terms of the number of drug approvals, but actual patient access requires commercial launches in many countries, which itself requires sustainable commercial markets. Prior work has described limited access to new antibacterials in low- and middle-income countries (LMICs), in part due to the inability of many to afford these drugs [2]. This study examines patient access for new antibacterials in the G7 and 7 other high-income countries in Europe, to better understand other barriers to patient access to effective antibacterial therapy.
DATA AND METHODS
We included all new molecular entity (NME) antibiotics (drugs with a J01 code from the Anatomical Therapeutic Chemical [ATC] classification maintained by the World Health Organization [WHO]) that were approved by either the United States (US) Food and Drug Administration (FDA), the European Medicines Agency (EMA), Health Canada, or the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) for the decade from 1 January 2010 until 31 December 2019. We also included fidaxomicin, classified as an A07 antidiarrheal drug for the treatment of Clostridioides difficile–associated infection (CDI), and bezlotoxumab, classified as J06 for recurrent CDI, which is generally associated with prior antibiotic use. We excluded generics, topical drugs, drugs with other ATC classifications, and combination drugs without any NME component.
For each such drug (referred to hereinafter as an “antibacterial”), we recorded the approval dates, International Nonproprietary Name, brand name, current sponsor, route of administration, and various regulatory designations in the countries studied. This information was collected as of 31 December 2020 from government websites (Supplementary Materials) as well as filings with the US Securities and Exchange Commission, and company press releases. Sponsors are categorized as “large” if they employ 250 or more full-time equivalent employees; otherwise, “small or medium enterprises” (SMEs).
We collected commercial launch (first commercial sale) dates in the 7 members of the G7 (Canada, France, Germany, Italy, Japan, the United Kingdom [UK], and the US) and 7 other high-income countries in Europe, based on data availability: Croatia, Denmark, Greece, Norway, Romania, Spain, and Sweden. Date of commercial launch in each country was obtained from the sponsor. If sponsor data were not available, the earliest reported commercial launch date from public or commercial databases was used.
We collected possible indicators of the potential clinical importance of each drug. From the WHO, we determined whether each drug was on the Essential Medicines List (EML) and its placement on the “Access, Watch, and Reserve” (AWaRe) list of antibacterials [3, 4]. We also used the assessments of innovation from the WHO Antibacterial Agents in Clinical Development reports [5, 6]. From the Pew Charitable Trusts, we determined whether the antibacterial was considered by Pew to be innovative [7]. Both Sweden and National Health England are piloting postapproval incentives for antibacterials, so we tracked which drugs have been selected as of 31 December 2020.
Sales data in the US for the trailing 12-month period ending June 2020 were obtained from Needham & Co. analyst Alan Carr [8]. Sponsors were noted if, subsequent to first regulatory approval, they filed for bankruptcy in their country of domicile or, if publicly traded, had market capitalization lower than $300 million. For the full data set and additional methods, see the Supplementary Materials.
We analyzed the data using comparative descriptive and inferential statistics. We calculated median launch lags by drug and country (including EMA as a country for this purpose). Launch lag is the time from first regulatory approval in the US, European Union, Japan, or Canada to the date of commercial launch in a given country. For second and subsequent regulatory approvals, we calculated the time from approval in the first country to the date of approval and, separately, commercial launch in the studied country. For antibacterials not yet approved or commercially launched in any of the other studied countries, we reported the time from first regulatory approval to the study end date of 31 December 2020 as “lag to date.”
To examine median launch lag between innovative and noninnovative drugs among the 11 antibacterials launched in 2 or more countries in our sample, we tested the difference in median launch lag for statistical significance using the related-samples Wilcoxon signed-rank test. Antibacterials were defined as “innovative” if designated as “innovative” or “possibly innovative” by WHO or as “novel” by the Pew Charitable Trusts. The same analysis was performed for drugs on the EML, as a proxy for global clinical need, and difference in median launch lag between listing and nonlisting in the EML was assessed for significance. We also tested the difference in median launch lag by sponsor characteristics, proxied by SME status using the Wilcoxon test.
To assess how the burden of drug resistance, as a determinant of demand in countries, is associated with median launch lags, we performed a bivariate correlation using Spearman rho (rs), with P = .01 (2-tailed) for all 14 countries. The burden was proxied by the Drug Resistance Index (DRI) [9]. For Japan with no data in the DRI, we used the values in the dataset from Sweden, given Japan’s comparable record against drug resistance [10]. We also assessed the correlation between median launch lag outside the US and the 12-month trailing sales in the US for antibacterials with multiple launches by Spearman rho.
RESULTS
Characteristics of Drugs Studied
Eighteen antibacterials met the inclusion criteria (16 J01 antibiotics and 2 products for CDI, bezlotoxumab and fidaxomicin) as shown in Table 1.
Table 1.
Approval and Commercial Launch in 14 High-Income Countries of New Molecular Entity Antibacterials First Approved by the United States Food and Drug Administration, European Medicines Agency, Japanese Pharmaceuticals and Medical Devices Agency, or Health Canada, 2010–2019
| INN | First Approval | US | EMA | UK | Sweden | France | Germany | Italy | Norway | Spain | Greece | Romania | Croatia | Denmark | Japan | Canada | Launches |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefiderocol | 14 Nov 2019 | 102 | 23 Apr 2020 (161) | Yes (306) | Yes (413) | No | No | No | No | No | No | No | No | No | No | No | 3 |
| Lascufloxacin | 20 Sep 2019 | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes (103) | No | 1 |
| Lefamulin | 19 Aug 2019 | 21 | 27 Jul 2020 (343) | No | No | No | No | No | No | No | No | No | No | No | No | No | 1 |
| Imipenem-cilastatin/relebactam | 16 Jul 2019 | 321 | 13 Feb 2020 (212) | Yes (382) | Yes (382) | No | No | No | Yes (290) | No | No | No | No | No | No | No | 4 |
| Omadacycline | 2 Oct 2018 | 122 | No | No | No | No | No | No | No | No | No | No | No | No | No | No | 1 |
| Sarecycline | 1 Oct 2018 | 92 | No | No | No | No | No | No | No | No | No | No | No | No | No | No | 1 |
| Eravacycline | 27 Aug 2018 | 35 | 20 Sep 2018 (24) | No | No | No | No | No | No | No | No | No | No | No | No | No | 1 |
| Plazomicin | 25 Jun 2018 | 6 | No | No | No | No | No | No | No | No | No | No | No | No | No | No | 1 |
| Meropenem/vaborbactam | 29 Aug 2017 | 33 | 20 Nov 2018 (448) | Yes (815) | Yes (1037) | Yes (1064) | No | No | No | No | No | No | No | No | No | No | 4 |
| Delafloxacin | 19 Jun 2017 | 196 | 16 Dec 2019 (910) | Yes (1121) | No | No | No | No | No | No | No | No | No | No | No | No | 2 |
| Bezlotoxumab | 21 Oct 2016 | 115 | 18 Jan 2017 (89) | Yes (174) | Yes (131) | Yes (1045) | Yes (527) | Yes (618) | Yes (206) | Yes (557) | No | No | No | No | Yes (413) | No | 9 |
| Ceftazidime/avibactam | 25 Feb 2015 | 35 | 23 Jun 2016 (484) | Yes (748) | Yes (827) | Yes (1967) | Yes (720) | Yes (1049) | Yes (310) | Yes (999) | Yes (980) | Yes (933) | Yes (1184) | Yes (841) | No | No | 12 |
| Ceftolozane/tazobactam | 14 Dec 2014 | 49 | 18 Sep 2015 (278) | Yes (352) | Yes (383) | Yes (598) | Yes (322) | Yes (657) | Yes (383) | Yes (443) | Yes (383) | Yes (808) | Yes (657) | Yes (352) | Yes (1630) | Yes (291) | 14 |
| Oritavancin | 6 Aug 2014 | 56 | 18 Mar 2015 (224) | No | No | No | No | No | No | No | No | No | No | No | No | No | 1 |
| Tedizolid | 20 Jun 2014 | 10 | 23 Mar 2015 (276) | Yes (315) | Yes (438) | Yes (577) | Yes (276) | Yes (1046) | Yes (390) | Yes (294) | Yes (681) | Yes (742) | No | Yes (276) | Yes (1432) | No | 12 |
| Dalbavancin | 23 May 2014 | 39 | 19 Feb 2015 (272) | Yes (914) | Yes (1279) | Yes (1097) | Yes (918) | Yes (740) | No | Yes (619) | Yes (954) | Yes (862) | Yes (923) | No | No | No | 10 |
| Fidaxomicin | 27 May 2011 | 35 | 5 Dec 2011 (192) | Yes (371) | Yes (371) | Yes (542) | Yes (585) | Yes (889) | Yes (385) | Yes (554) | Yes (432) | Yes (797) | Yes (1711) | Yes (371) | Yes (2651) | Yes (1648) | 14 |
| Ceftaroline | 29 Oct 2010 | 64 | 22 Aug 2012 (663) | Yes (726) | Yes (764) | Yes (844) | Yes (717) | Yes (1007) | Yes (755) | Yes (1162) | Yes (1315) | Yes (795) | Yes (2764) | Yes (734) | No | No | 12 |
| No. approved or launched | 18 | 17 | 14 | 11 | 10 | 8 | 7 | 7 | 7 | 7 | 6 | 6 | 5 | 5 | 5 | 2 |
No = not commercially launched, except in the EMA column where No indicates not approved by EMA. Number indicates lag from first approval to commercial launch, in days, except in the EMA column where number is the lag from first approval to EMA approval, in days. The US was the country for all first approvals and first commercial launches, with the exception of lascufloxacin, approved and launched only in Japan.
Abbreviations: EMA, European Medicines Agency; INN, International Nonproprietary Name; UK, United Kingdom; US, United States.
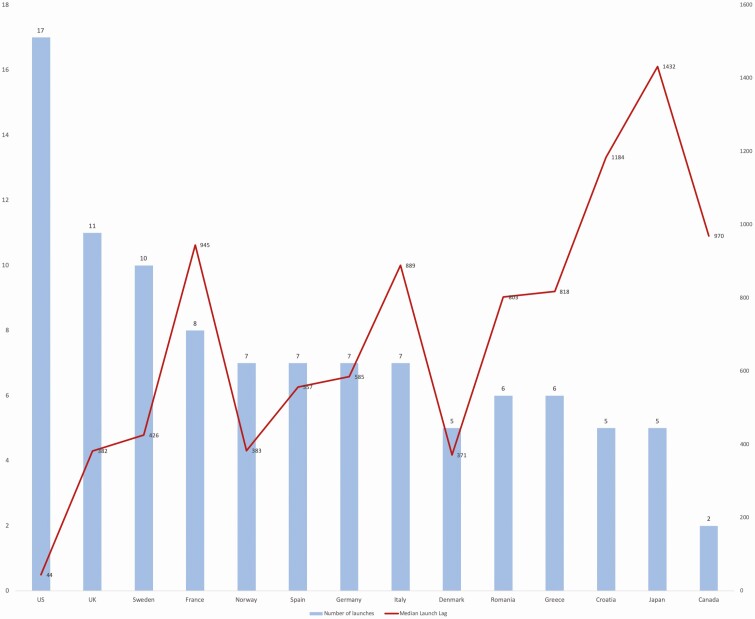
In all but 3 of the countries studied, the majority have not been commercially launched as of 31 December 2020. Canada had the fewest launched antibacterials. Only 4 antibacterials (22%) are commercially launched in all European countries studied. Only 2 antibacterials (fidaxomicin and ceftolozane/tazobactam), or 11% of the 18 antibacterials, have been commercially launched in all 14 high-income countries studied. Median launch lags are presented in Figure 1.
Figure 1.
Median launch lag, in days (right axis) and number of launches (left axis) in 14 high-income countries for new molecular entity antibacterials first approved by the United States Food and Drug Administration, European Medicines Agency, Japanese Pharmaceuticals and Medical Devices Agency, or Health Canada, 2010–2019. Abbreviations: UK, United Kingdom; US, United States.
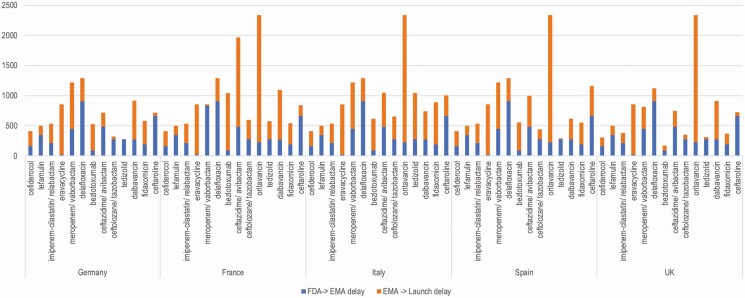
Granting of marketing authorization did not necessarily lead to commercial launch, as can be seen clearly in the 5 largest drug markets in Europe, where much of the launch lag has occurred after regulatory approval for the drug (Figure 2). For 2 of the 4 antibacterials lacking EMA approval (plazomicin and omadacycline), pending EMA market authorization approvals were voluntarily rescinded by the sponsors due to poor economic prospects for sales in Europe. The sponsor of plazomicin (Cipla Europe NV, successor to Achaogen after the bankruptcy sale) cited the cost of the required approval and postapproval work making the product “financially and commercially unviable with the limited indication that was to be accepted” [11]. For omadacycline, the sponsor (Paratek Ireland Ltd) was similarly pragmatic: “The insistence for a second CAP [community-acquired pneumonia] study to support approval for this indication in EU has significantly changed the value proposition” and therefore “all partner discussions have now been discontinued” [12]. Paratek predicted that European patient access to omadacycline would be delayed by 5 years.
Figure 2.
Delay in days before and after European Medicines Agency (EMA) approval for 13 recently approved antibacterials with approvals by both the United States Food and Drug Administration and EMA, in 5 high-income European countries. Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration; UK, United Kingdom.
Four antibacterials were deemed “innovative” (lefamulin, meropenem/vaborbactam, ceftazidime/avibactam, and bezlotoxumab) and 1 “possibly innovative” (cefiderocol), but most are not available in a majority of these countries.
Eight (44%) of the drugs are on the 2020 WHO EML (ceftaroline, delafloxacin, ceftolozane/tazobactam, omadacycline, eravacycline, plazomicin, meropenem/vaborbactam, and ceftazidime/avibactam) [3]. Eravacycline, omadacycline, and plazomicin are not launched in any of the countries studied other than the US. Delafloxacin is launched in 2 of the countries studied (UK and US). Ceftaroline is launched in 12 of the countries studied (US, plus all of the European countries studied, but not Canada or Japan).
Seven of the 18 drugs (39%) were sponsored by SMEs, accounting for 6 of the 11 drugs (55%) approved since 1 January 2016. Drugs sponsored by SMEs are being launched more slowly in Europe, Canada, and Japan. Five of the 7 SME drugs have not been launched outside the US. The other 2 SME drugs (delafloxacin and meropenem/vaborbactam) have only been launched in the UK, the US, France, and Sweden (delafloxacin only in the UK).
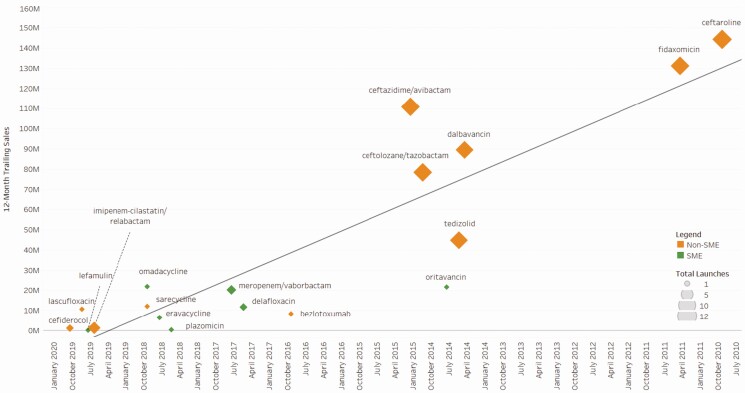
We report commercial data for these antibacterials drugs (Figure 3). In the US, commercial sales for all but 3 of these drugs (ceftazidime/avibactam, fidaxomicin, and ceftaroline) are below $100 million for the most recent 12-month trailing period, and none of the 18 drugs exceeded $150 million in sales despite having been on the US market for up to 10 years (ceftaroline). The last 12-month sales in the first approval market for all 18 drugs are a median of $16.2 million and a cumulative sum of $714.3 million. Sponsors for 4 drugs filed for bankruptcy after first regulatory approval and sponsors for 3 other drugs had market capitalizations less than $300 million.
Figure 3.
United States trailing 12-month sales for antibacterials approved 2010–2019, by launch date, sponsor size, and number of high-income country commercial launches, with linear trend. Abbreviation: SME, small or medium enterprise.
Analysis of Median Launch Lag by Selected Characteristics
Of the 11 antibacterials with launches in >1 country, we found a significant difference in median launch lag (P = .043) between listing and nonlisting on the EML. Unexpectedly, antibacterials listed on the EML had longer median launch lags. The 5 multiple-launch EML drugs (ceftaroline, meropenem/vaborbactam, ceftazidime/avibactam, ceftolozane/tazobactam, delafloxacin) had a median launch lag of 780 days, compared to 414 days for the 6 nonlisted multiple-launch drugs (fidaxomicin, dalbavancin, tedizolid, imipenem/cilastatin/relebactam, cefiderocol, and bezlotoxumab) (Supplementary Materials).
In contrast, there was no significant difference in median launch lags between noninnovative (584 days) and “innovative” and “possibly innovative” (650 days) antibacterials (P = .465; Supplementary Materials).
Over the sample of the 14 selected countries, there was a very weak, but not significant, association between DRI and median launch lag (rs = 0.037, P = .899) and between the number of launches and DRI (rs = 0.06, n = 13, P = .873) (Supplementary Materials).
While there was a moderate correlation between US 12-month trailing sales and median launch lag outside the US, this was not statistically significant (rs = 0.409, n = 11, P = .212) (Supplementary Materials).
Discussion
While the innovation crisis in antibiotics is well-known [13–15], we describe serious limitations on commercial launch and therefore patient access in high-income countries. Most new antibacterials approved since 1 January 2010 are not commercially available to patients in many high-income countries due to a combination of delayed marketing authorization submission and approval, delayed commercial launch after marketing approval, and market withdrawals and delays due to bankruptcy. Regulatory approval is clearly not the only barrier, given the long commercial launch lags in many European countries despite EMA authorization, and in Canada, after Health Canada authorization. With low expected sales, companies may have decided to delay or forego commercialization due to expectations of insufficient profitability.
Antibacterials are generally approved based upon noninferiority clinical trial designs, meaning that the new drug is shown to be not inferior to a comparator, typically an older generic antibacterial [16]. In many European countries, this trial design automatically adjusts the reimbursement amount to the same amount as the comparator drug. Even if a new antibacterial demonstrates improved efficacy after commercialization, it is difficult to have the unit price increased. Knowing this, companies take these regulations into consideration when determining their commercialization strategies. Other constraints on revenues include payment for hospital antibiotics through diagnostic-related groups (which do not account for higher-priced antibacterials) [17], governments requiring significant price reductions [18], and the impact of appropriate stewardship, which lowers sales volumes. For the 2 sponsors that withdrew marketing applications from EMA, costs from required approval and postapproval work outweighed the limited prospects for net revenues.
Commercial drug innovation is supported by sales during the patent term [19]. For antibacterials, sales in the US are currently insufficient to sustain innovation [13–15, 20, 21]. Delayed or abandoned commercial launches in other high-income countries result in a large share of the patent term outside of the US yielding little or no revenues. Even within the first launch market of choice (the US for 17 of 18 drugs [94%]), the trailing 12-month sales for the entire sector were low: a cumulative sum of $714.3 million, which means that the entire antibacterial branded market—all antibacterials approved for the decade beginning 1 January 2010—was valued at less than a single blockbuster drug. Median sales of $16.2 million are insufficient to cover ongoing commercialization costs, including manufacturing, regulatory, medical affairs, and postapproval commitments, with no opportunity to recover sunk research and development (R&D) costs. This economic situation explains why sponsors for 7 of the 18 products suffered either bankruptcy or market capitalizations below the sunk cost of R&D, with the bulk of this economic damage coming since April 2019. Relative sales success in the US did not predict commercial launch in the other high-income countries studied, suggesting that these markets are segmented by national characteristics driving commercial prospect for sales, but these national characteristics do not include the level of drug resistance, measured by the DRI, or national health expenditures (data in Supplementary Materials).
For all of these drugs, careful stewardship, while essential for public health, may be a contributing cause of lower sales. For the antibacterials categorized on the WHO AWaRe list, all but 1 was placed on the most restrictive “Reserve” category, primarily saving the drug for future patients. While a laudable public health imperative, the economic impact on the companies is clearly apparent. In light of modern stewardship practices [4, 22, 23], it is very difficult for companies selling newly launched Reserve antibacterials to earn sufficient revenues from volume of sales alone.
An alternative explanation for low sales and delayed launches could be inadequate antibacterial innovation in the past decade, with these drugs selling poorly because they simply are not clinically important drugs. But we find poor sales and delayed patient access even for the antibacterials labeled as novel by the Pew Charitable Trusts or innovative by the WHO, as well as those placed on the Essential Medicines List by the WHO.
Canada and Japan present interesting case studies. Both are members of the G7, but they trail all other high-income countries studied for commercial launches of antibacterials, at rates exceeding prior studies of Canadian and Japanese drug lags. Japan was traditionally a strong launch market for antibiotics [24, 25], with significant leadership from Japanese companies. For example, data from 1999 to 2007 found that the Japanese lag for anti-infectives was only 13.6 months [24]. Canadian data from 2000–2011 reported much shorter lags than what we report here [25]. In this study, a number of less wealthy European countries such as Greece, Romania, and Spain have seen greater numbers of commercial antibacterial launches than Canada and Japan. Plausible explanations include the consolidated marketing approval process through the EMA, as compared to the need to separately apply to Health Canada and the Japanese PMDA and the need for additional clinical studies in Japan [24, 25]. With Brexit on 31 December 2020, there is an opportunity to study the future impact of the new process for marketing approvals in the UK.
Sweden has launched a program to encourage companies to commercially launch antibacterials in Sweden [26]; this program has already led to 3 new commercial launches (cefiderocol, imipenem-cilastatin/relebactam, and meropenem/vaborbactam). This single program has moved Sweden from 7 to 10 launched drugs, moving to sole possession of third place in the high-income countries studied, behind only the US and the UK. Sweden’s program is therefore a highly successful access initiative. Sweden’s program is explicitly not an innovation incentive, that is, it is not attempting to provide a return on investment for the R&D costs of new antibacterials. It guarantees annual revenues of approximately US$475 000 per drug to enhance patient access in Sweden. The program’s design is elegant in its simplicity and could be scaled up to also provide an innovation incentive proportional to Sweden’s economic stature, or indeed any other country so inclined. The UK pilot, subscribing for 2 innovative drugs (ceftazidime/avibactam and cefiderocol), is explicitly designed as an innovation incentive [27].
The fruit of a decade of antibacterial innovation has little financial value today if value is driven only by the frequency of current use, and obviously clinical impact is absent for patients in countries where the product is not commercially available. To address this, reimbursement must begin to recognize the preparedness value of having antibacterials on hand before they are needed. Preparedness value can be thought of as analogous to the value of having a fire extinguisher or entire fire department [28]: Benefits of novel antibacterials can then be seen based on the “STEDI” attributes of spectrum of coverage, transmission interruption, enablement of medical care, diversity of antibiotic choice, and insurance against pandemic spread [29, 30].
As preparedness value is most fundamentally a function of offering novel utility, we also must reorient the R&D community toward truly novel antibacterials and hence more clinically important drugs. Intrinsically useful properties can be defined well in advance of registration [28], and delinked pull approaches such as the Pioneering Antimicrobial Subscriptions To End Up Surging Resistance Act of 2020 (PASTEUR) Act and the UK antibiotic pilot propose rewards scaled to demonstration of high-value attributes [27, 31]. The European Union has also committed to creating a delinked antibiotic pilot in 2021 [32].
While this study did not assess the commercialization of new antibacterials in LMICs, our findings do not bode well for these countries, given low profitability expectations [2]. Delinked pull incentives may be required to persuade sponsors to serve these markets, even on a nonprofit basis.
In conclusion, patient access to new antibacterials is limited, not just in LMICs, as previously reported, but also in high-income countries such as Canada, Japan, and many European countries. Companies appear to eschew antibacterial markets not offering attractive commercial prospects, which are almost all markets currently. If truly innovative antibacterials, like those identified by WHO and Pew, cannot find profitable markets, antibacterial innovation is in serious jeopardy, which reinforces calls for new economic incentives, delinked from unit sales.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Potential conflicts of interest. K. O. reports being a principal investigator and executive director of CARB-X, a global nonprofit supporting antibacterial innovation that has received grants from the governments of the United States, United Kingdom, and Germany; Wellcome Trust and Bill & Melinda Gates Foundation, outside the submitted work. C. A. has received grants from the Norwegian Research Council (research grant number 300867), European Commission (research grant number 761296), and Global AMR R&D Hub, all paid to the Norwegian Institute of Public Health, outside the submitted work. J. R. reports being a chief medical officer and director at F2G, editor-in-chief at AMR.Solutions, operating partner and consultant at Advent Life Sciences, and Adjunct Professor of Medicine at McGovern Medical School; has received grant support from the Wellcome Trust; serves on scientific advisory boards of Bugworks Research, Basilea Pharmaceutica, Forge Therapeutics, Novo Holdings, and Roche Pharma Research & Early Development; has received consulting fees from Phico Therapeutics, ABAC Therapeutics, Polyphor, Heptares Therapeutics., Gangagen., Meiji Seika Pharma, Basilea Pharmaceutica International., Allecra Therapeutics GmbH, Forge Therapeutics, SinSa Labs, AtoxBio, Peptilogics, F. Hoffmann-LaRoche, Novo Holdings, Innocoll, Vedanta, Progenity, Nosopharm SA, Roivant Sciences, Shionogi, GlaxoSmithKline, and Pfizer Pharmaceuticals; and is or has been a shareholder in AstraZeneca Pharmaceuticals, F2G, Advent Life Sciences, Zikani Therapeutics, and Bugworks Research. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Infectious Diseases Society of America. The 10×’20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis 2010; 50:1081–3. [DOI] [PubMed] [Google Scholar]
- 2. Kållberg C, Årdal C, Salvesen Blix H, et al. . Introduction and geographic availability of new antibiotics approved between 1999 and 2014. PLoS One 2018; 13:e0205166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO model lists of essential medicines. 2020. Available at: https://www.who.int/medicines/publications/essentialmedicines/en/. Accessed 24 October 2020.
- 4. World Health Organization. Adopt AWaRe: handle antibiotics with care. 2020. Available at: https://adoptaware.org/. Accessed 24 October 2020.
- 5. World Health Organization. Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline, including tuberculosis. 2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/258965/WHO-EMP-IAU-2017.11-eng.pdf. Accessed 15 January 2020.
- 6. World Health Organization. 2019 antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/330420/9789240000193-eng.pdf. Accessed 15 January 2020.
- 7. Pew Charitable Trusts. Antibiotics currently in clinical development. 2020. Available at: https://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2014/antibiotics-currently-in-clinical-development. Accessed 24 October 2020.
- 8. Carr A. Antibiotic and antifungal update: August 2020.2020. Available at: https://needham.bluematrix.com/sellside/EmailDocViewer?encrypt=0cab5f90-fe35-4809-b7d5-1ce40d49dc12&mime=pdf&co=needham&id=acarr@needhamco.com&source=library. Accessed 24 October 2020.
- 9. Center for Disease Dynamics, Economics, and Policy. Drug resistance index. 2021. Available at: https://resistancemap.cddep.org/DRI.php. Accessed 24 March 2021.
- 10. AMR One Health Surveillance Committee. Nippon AMR One Health report (NAOR) 2018. 2018. Available at: https://www.mhlw.go.jp/content/10900000/000530087.pdf. Accessed 24 March 2021.
- 11. European Medicines Agency. Zemdri: withdrawal of the marketing authorisation application. 2020. Available at: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/zemdri. Accessed 24 October 2020.
- 12. European Medicines Agency. Nuzyra: withdrawal of the marketing authorisation application. 2020. Available at: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/nuzyra. Accessed 24 October 2020.
- 13. Sertkaya A, Eyraud J, Birkenbach A, et al. . Analytical framework for examining the value of antibacterial products. Report to US DHHS. United States Department of Health and Human Services; 2014. Available at: http://aspe.hhs.gov/sp/reports/2014/antibacterials/rpt_antibacterials.cfm. Accessed 17 June 2014. [Google Scholar]
- 14. Ardal C, Findlay D, Savic M, et al. . DRIVE-AB report: revitalizing the antibiotic pipeline: stimulating innovation while driving sustainable use and global access. 2018. Available at: http://drive-ab.eu/wp-content/uploads/2018/01/CHHJ5467-Drive-AB-Main-Report-180319-WEB.pdf. Accessed 6 October 2019.
- 15. Review on Antimicrobial Resistance. Tackling drug-resistant infections globally: final report and recommendations. 2016. Available at: http://amr-review.org/. Accessed 14 January 2020.
- 16. Rex JH, Fernandez Lynch H, Cohen IG, Darrow JJ, Outterson K. Designing development programs for non-traditional antibacterial agents. Nat Commun 2019; 10:3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Outterson K. A shot in the arm for new antibiotics. Nat Biotechnol 2019; 37:1110–2. [DOI] [PubMed] [Google Scholar]
- 18. Yfantopoulos JN, Chantzaras A. Drug policy in Greece. Value Health Reg Issues 2018; 16:66–73. [DOI] [PubMed] [Google Scholar]
- 19. Outterson K. Pharmaceutical arbitrage: balancing access and innovation in international prescription drug markets. Yale J Health Policy Law Ethics 2005; 5:193–291. [PubMed] [Google Scholar]
- 20. Årdal C, Røttingen JA, Opalska A, Van Hengel AJ, Larsen J. Pull incentives for antibacterial drug development: an analysis by the transatlantic task force on antimicrobial resistance. Clin Infect Dis 2017; 65:1378–82. [DOI] [PubMed] [Google Scholar]
- 21. Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria. Recommendations for incentivizing the development of vaccines, diagnostics, and therapeutics to combat antibiotic-resistance. 2017. Available at: https://www.hhs.gov/ash/advisory-committees/paccarb/reports-and-recommendations/index.html. Accessed 24 October 2020.
- 22. Clancy CJ, Nguyen MH. It’s worse than we thought: the US market for novel gram-negative antibiotics. Lancet Infect Dis 2020; 20:1009–10. [DOI] [PubMed] [Google Scholar]
- 23. Strich JR, Warner S, Lai YL, et al. . Needs assessment for novel gram-negative antibiotics in US hospitals: a retrospective cohort study. Lancet Infect Dis 2020; 20:1172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsuji K, Tsutani K. Approval of new drugs 1999-2007: comparison of the US, the EU and Japan situations. J Clin Pharm Ther 2010; 35:289–301. [DOI] [PubMed] [Google Scholar]
- 25. Shajarizadeh A, Hollis A. Delays in the submission of new drugs in Canada. CMAJ 2015; 187:E47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Public Health Agency of Sweden. Availability of antibiotics. 2019. Available at: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/antibiotics-and-antimicrobial-resistance/availability-of-antibiotics/. Accessed 6 February 2020.
- 27. Gov.UK. World-first scheme underway to tackle AMR and protect UK patients. 2020. Available at: https://www.gov.uk/government/news/world-first-scheme-underway-to-tackle-amr-and-protect-uk-patients. Accessed 24 October 2020.
- 28. Rex JH, Outterson K. Antibiotic reimbursement in a sales-delinked model: context and a benchmark-based global approach. Lancet Infect Dis 2016; 16:500–5. [DOI] [PubMed] [Google Scholar]
- 29. Rothery C, Woods B, Schmitt L, Claxton K, Palmer S, Sculpher M. Framework for value assessment of new antimicrobials.2018. Available at: http://www.eepru.org.uk/wp-content/uploads/2017/11/eepru-report-amr-oct-2018-059.pdf. Accessed 29 January 2019.
- 30. Outterson K, Rex JH. Evaluating for-profit public benefit corporations as an additional structure for antibiotic development and commercialization. Transl Res 2020; 220:182–90. [DOI] [PubMed] [Google Scholar]
- 31. Outterson K, Rex JH. PASTEUR Act (re)introduced: a delinked pull award advances in the US!2020. Available at: https://amr.solutions/2020/09/30/pasteur-act-reintroduced-a-delinked-pull-award-advances-in-the-us/. Accessed 24 October 2020.
- 32. European Commission. A pharmaceutical strategy for Europe. 2020. Available at: https://ec.europa.eu/health/human-use/strategy_en. Accessed 20 December 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.