Abstract
Erenumab is a monoclonal antibody targeting the calcitonin gene-related peptide (CGRP) receptor suitable for episodic and chronic migraine prevention. Randomized clinical trials proved the superiority of erenumab to placebo in a strictly selected population, while real-world studies confirmed treatment efficacy in more severe forms of disease – most patients suffered from chronic migraine with medication overuse headache, had prior treatment failures, and long disease duration. According to guidelines, anti-CGRP pathway monoclonal antibodies should be reserved to patients who failed or have contraindication to several classes of preventive treatments. However, their ease of use, tolerability and efficacy make these monoclonal antibodies ideally suitable for most patients with migraine; cost-effectiveness needs to be considered when looking at expanding current prescription criteria. Also, data from open label extensions of randomized control trials confirmed sustained benefits of prolonged treatment up to 5 consecutive years without significant risk of adverse events. Further studies will provide insights on optimal treatment duration to achieve migraine remission and predictors of treatment response. In the present work, we aimed at reviewing design and results of the main studies on erenumab and discussing treatment use in the current migraine prevention scenario; we also summarized the main ongoing research projects and provided clinical perspectives for the future.
Keywords: erenumab, migraine, real-world, randomized clinical trials, RCTs
Introduction
Migraine is a primary headache disorder affecting 14.4% of the global population, mostly females, and ranks third among the causes of disability-adjusted life-years according to the Global Burden of Disease.1 Migraine prevention aims at reducing the high disability impact on patients’ life and has remarkably evolved since the identification of the role of Calcitonin Gene-related Peptide (CGRP) and trigeminovascular system in the genesis of migraine pain.2 CGRP became the ideal target of monoclonal antibodies (mAbs) and small antagonizing molecules called gepants,3 which inhibit the CGRP pathway.
Among mAbs acting on the CGRP pathway, erenumab (AMG334) was the first developed in 2012 and commercialized in the United States in 2018,4 and is the sole targeting the CGRP receptor, while fremanezumab, galcanezumab and eptinezumab target the soluble CGRP. Real-world evidence is depicting the use of mAbs in clinical practice and providing information on patients excluded by randomized clinical trials (RCTs). We aimed at reviewing the most relevant RCTs and real-world data on erenumab and suggesting future clinical and research perspectives, such as the need for redefining patient selection criteria and treatment duration, identification of predictors of treatment response, or the combination of mAbs with other treatments acting on the CGRP pathway. We searched PubMed and Clinicaltrails.gov databases using keywords and MeSH (Medical Subject Headings) terms “erenumab” and “migraine”; results were limited to articles in English language.
Erenumab Mechanisms of Action and Pharmacological Characteristics
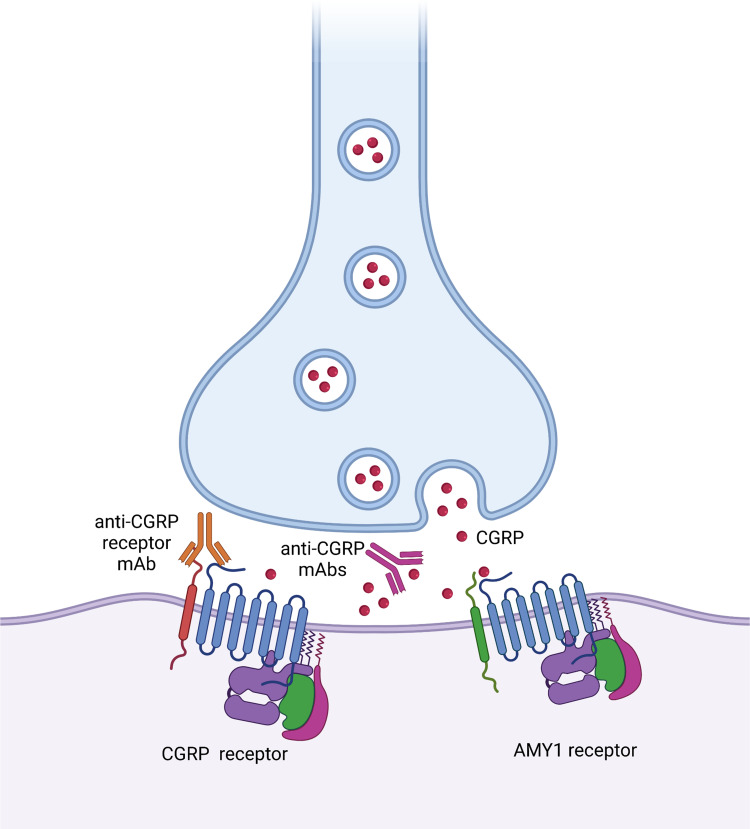
Erenumab is the sole fully human mAb IgG2 targeting a fusion protein of the extracellular domains of the CGRP receptor.5,6 Different targets – soluble CGRP and CGRP receptor – might explain slightly different mechanisms of actions of the available mAbs. Besides, migraines occurring under prevention with anti-CGRP receptor mAb might be due to CGRP binding to other receptors (ie AMY1) with a structure similar to CGRP receptor, while migraine occurring under prevention with anti-CGRP mAbs might be due to other peptides binding to the CGRP receptor7 (Figure 1). Preclinical evidence proved the importance of CGRP in migraine pathogenesis due to its role in pain transmission, vasodilation and neurogenic inflammation.2 CGRP receptor is expressed by Aδ-type fibers, C-type fibers, smooth muscular cells, and mast cells.3,6 During a migraine attack, signals form the CNS structures – dorsal pons, hypothalamus and thalamus2,8,9 – activate the trigeminovascular system leading to the release of CGRP from C-type terminations of sensory pain fibers of the trigeminal ganglion and trigeminal nerves; from trigeminal structures, pain signals run back to the CNS, where the attack started, perpetuating a vicious cycle.3,6,9
Figure 1.
Mechanisms of action of monoclonal antibody (mAb) targeting Calcintonine Gene-related peptide (CGRP) receptor (erenumab) and antibodies targeting the soluble CGRP at synaptic level and representation of CGRP receptor and (amylin) AMY1 receptor. Created with BioRender.com.
Phase 2 and Phase 3 RCTs proved the efficacy of erenumab 70 mg and 140 mg in patients with migraine (18–65 years); thus, those are the two doses commercially available for migraine prevention. Studies also demonstrated that the drug reaches its peak serum concentration in approximately 6 days, has a 28-day half-life and two elimination phases according to its concentration: at lower concentrations the elimination is predominantly through saturable binding to target; at higher and clinically relevant concentrations the elimination is largely through a non-specific, non-saturable proteolytic pathway. Renal or hepatic impairment does not affect pharmacokinetics.10,11
The main concern for using mAbs acting on the CGRP pathway is cardiovascular safety, due to the expression of CGRP receptor on smooth muscular cells of arteries and the inhibition of physiological vasodilation mediated by the CGRP-CGRP receptor interaction.12 At cardiac level the inhibition of vasodilation might cause a reduced coronary reserve,12 at a systemic level it might lead to arterial hypertension.13 Several preclinical studies proved that the treatment exerts its effect mainly on distal coronary arteries, thus not representing a risk for patients without coronary artery disease.12,14,15
Evidence from Randomized Clinical Trials and Real-World Studies
Inclusion criteria of RCTs and real-world studies remarkably differ (Figure 1) with possible consequences on efficacy outcomes. In the following paragraphs we summarized the main design of studies: inclusion criteria, baseline characteristics, efficacy and safety outcomes.
Overview of Randomized, Placebo-Controlled Clinical Trials
Study Design, Inclusion Criteria and Baseline Characteristics
Inclusion criteria were similar across phase 2 and 3 RCTs: age 18–65; a diagnosis of episodic migraine (ie 4–14 monthly headache days (MHDs)); freedom from other preventives for at least 2 months prior to baseline and 4 months in case of onabotulinumtoxinA. Relevant exclusion criteria were an onset of migraine at the age of 50, and, in most studies, failure of 2 or 3 different categories of preventatives.16–19 The RCTs of Goasby et al (STRIVE) and Reuter et al (LIBERTY) included patients with a history of prior treatment failures, who represented the subgroup population of post-hoc analyses.20,21 The sole studies evaluating the efficacy of erenumab in patients with chronic migraine were the RCT of Tepper et al (NCT02066415)18 and its open label extensions and post-hoc analyses22–26
Researchers assessed treatment efficacy as reduction in monthly migraine days (MMDs), reduction in monthly acute medication days (AMDs), and response rates defined as the 30-50-75-100% reduction in MMDs from baseline.16–19,27,28 Most trials also evaluated patient reported outcomes, such as Migraine Disability Assessment (MIDAS), Headache Impact Test (HIT-6) and, more rarely, Migraine-Specific Quality of Life Questionnaire (MSQ) and Migraine Physical Function Impact Diary (MPFID).16–19,28,29 All RCTs further reported proportion and type of adverse events (AEs), severe adverse events (SAEs) and AEs leading to treatment withdrawal.16–19,27,28
Double-blind treatment duration was between 12 and 24 weeks (corresponding to 3–6 drug administrations),16–19,27,28 while open label extension duration was 52, 64 weeks (corresponding to 13 and 16 drug administrations respectively) and 5 years (corresponding to 65 drug administrations).20,29,30 The doses of erenumab evaluated were 7 mg, 21 mg, 28 mg, 70 mg and 140 mg.16–19,27,28
RCT populations had a mean age ranging from 40 to 44 years, with most patients being females, mean disease duration ranged from 20.7 to 22 years, baseline mean MMDs from 8.1 to 18.2 days, and baseline AMDs from 3.2 to 9.7 days; 33–60% patients were naïve from previous preventive treatments, while the remaining reported 1 to 2 prior treatment failures16–19,27,28 (Table 1).
Table 1.
Main Baseline Characteristics and Outcomes of the Randomized Clinical Trials
| Study, year, NCT | Country |
DB follow-up, weeks |
Disease duration, mean years ± SD, treatment Vs placebo | Sample size, treatment Vs placebo | Migraine form | Mean age ± SD | Female sex, % |
MMDS/MHDs difference from baseline, treatment Vs placebo |
AMDs difference from baseline, treatment Vs placebo |
| Sun et al 2016 NCT0195257416 |
North America and Europe | 12 | 19.0 ± 11.4 7 mg, 20.1 ± 12.5 21 mg, 21.5 ± 1.7 70 mg, 20·7 ±11·5 |
108 7 mg; 108 21 mg; 107 70 mg, 160 |
EM | 40.3±10.9 7 mg, 39.9±12.3 21 mg, 42.6 ±9.9 70 mg, 41.4±10·0 |
81 7 mg, 81 21 mg, 77 70 mg, 83 |
2.2 ± 0.4 7 mg; 2.4 ± 0.4 21 mg; 3.4 ± 0.4 70 mg; 2.3 ± 0.3 |
1.2 70 mg |
| Sakai et al 2019 NCT0263045917 |
Japan | 24 | NA | 67 28 mg, 135 70 mg, 137 140 mg, 136 |
EM | 45 (21-61) 28 mg, 43 (22-57) 70 mg, 44 (20-64) 140 mg, 45 (23-64) |
82.1 28 mg, 85.2 70 mg, 81.8 140 mg, 86.8 |
1.19 (1.9-0.4) 28 mg 2.25 (3-1) 70 mg, 1.83 (2.3-1.3) 140 mg, 0.06 (0.4-0.5) |
0.19 (0.8–0.43) 28 mg, 1.07 (1.8-0.8) 70 mg, 1.16 (1.6-0.7) 140 mg, 0.8 (0.4-1.3) |
| Goadsby et al 2017 NCT0245674027 (STRIVE) |
North America, Europe and Turkey | 24 | NA | 317 70 mg, 319 140 mg, 319 |
EM | 41.1±11.3 70 mg, 40.4±11.1 140 mg, 41.3±11.2 |
84.5 70 mg, 85.3 140 mg, 85.9 |
3.2±0.2 70 mg, 3.7±0.2 140 mg, 1.8±0.2 |
1.1 ± 0.1 70 mg, 1.6 ± 0.1 140 mg, 0.2 ± 0.1 |
| Tepper et al 2017 NCT0206641518 |
North America and Europe | 12 | 20.7 ±12.8 70 mg, 21.9 ±11.8 140 mg 22.2 ±12.6 |
191 70 mg, 190 140 mg 286 |
CM | 41.4±1.3 70 mg, 42.9±11.1 140 mg, 42.1±11.3 |
87 70 mg, 84 140 mg, 79 |
6.6±0.4 70 mg, 6.6±0.4 140 mg, 4.2±0.4 |
3.5 (0.3) 70 mg, 4.1 (0.3) 140 mg, 1.6 (0.2) |
| Dodick et al 2018 NCT0248358519 (ARISE) |
North America and Europe | 12 | 22 ±13 70 mg 20 ± 12 |
286 70 mg 291 |
EM | 42 ± 11 70 mg, 42 ±12 |
85.7 70 mg, 84.9 |
2.9 ± 0.2 70 mg, 18 ± 0.2 |
1.2 ± 0.1 70 mg, 0.6 ± 0.1 |
| Reuter et al 2018 NCT0309683428(28) (LIBERTY) |
Europe and Australia | 12 | NA | 121 140 mg 125 |
EM | 44.2 ± 10.6 140 mg 44.6 ± 10.5 |
80 140 mg, 81 |
1.8 ±0.4 140 mg, 0.2 ± 0.4 |
1.3 ± 0.2 140 mg, 0.5 ± 0.3 |
Abbreviations: AMDs, monthly acute medication days; CM, chronic migraine; DB, double blind; EM, episodic migraine; MMDs, monthly migraine days; MHD, monthly headache days; NA, not available; SD, standard deviation.
Efficacy Outcomes
RCTs demonstrated the superiority of erenumab to placebo in reducing MMDs, AMDs and patient reported outcomes at 12 weeks of treatment; mean MMDs reduction ranged from −1.8 to −6.6 days; AMDs from −1.2 to −4.1 days; 50% response rate from 27.2 to 50%. Erenumab 7, 21 and 28 mg did not achieve significant results compared with placebo, thus they are currently not available for migraine prevention16–19,27,28 (Table 1 and Figure 2A).
Figure 2.
(A) 12-week reduction in monthly migraine days (MMDs) from baseline in placebo-controlled randomized trials. (B) 12-week reduction in monthly migraine days (MMDs) from baseline in real-world studies. Data were derived from MMDs at baseline and MMDs at week 12 of treatment provided by the studies.
Long-term results from open label extensions of RCTs confirmed the sustained efficacy of erenumab in migraine prevention even at 5230 and 64 weeks,31 and 5 years.20 At 52 weeks the mean MMDs reduction was −4.2± 0.2 days with the 70 mg dose and −4.6±0.2 days with the 140 mg dose;30 at 5 years −5.3±0.3 days with the 70 mg dose.29 Similarly, at 52 weeks mean AMDs reduction was −1.8±0.1 days with the 70 mg dose and −2.0±0.2 days with the 140 mg dose,30 and at 5 years −4.4 ±0.3 days with the 70 mg.29 Response rates slightly improved and kept stable during the open label extensions: at 52 weeks 50-75-100% response rates were 61.0%, 38.5%, and 19.8% respectively with the 70 mg dose, and 64.9%, 40.8%, and 21.2% with the 140 mg dose,30 at 5 years 71.0%, 47.1%, 35.5% with 70 mg dose;29 the 64-week open label extension from the LIBERTY trial showed that the 50% response rate for the overall population increased until weeks 37–40 and then stabilized throughout the latter part of the study period.31 Even improvements in patient reported outcomes kept steady throughout the open label extension periods.20,29,30
Post hoc analyses provided results on the efficacy of erenumab according to patients’ response rate, disease characteristics (ie prior treatment failures and menstrual migraine), and consequences of treatment discontinuation. The study of Brandes et al (NCT01066415) provided data on possible clinical benefits in treatment responders: at 3 months, patients with 50–75% response rate in the erenumab group were significantly higher than in the placebo group and reported the greatest reduction in MMDs and AMDs compared to the overall population.26 Patients with prior treatment failures registered a higher response to the treatment in terms of MMDs reduction and 50–75% response rate as compared to treatment-naïve patients, who, conversely, had a higher placebo response.21 Notably, a >50% response rate was higher with erenumab 70 mg compared with 140 mg in those without previous treatment failures.21 Erenumab proved its superiority to placebo even in females with self-reported menstrual related migraine with results consistent to the overall RCT population and independent from concomitant hormonal treatment,32 and in reverting medication overuse headache.24 mAbs discontinuation (including erenumab) after a 1-year treatment led to a disease rebound, consisting in increased MMDs and AMDs, up to levels still below baseline at 12 weeks from the last dose.33
Most of open label extension evaluated the efficacy of the 140 mg dose; the sole comparing both doses proved that erenumab 140 mg remained superior to the 70 mg dose even at 52 weeks.30
Head-to-Head Comparison
Up to date, only one RCT compared the efficacy of erenumab (70 and 140 mg) with an oral preventative, topiramate (titration up to 100 mg/daily). This double-dummy Phase 4 RCT proved the superiority of erenumab to the comparator in tolerability and efficacy in naïve patients or in those with contraindication to or failure of 1–3 prior preventive treatments. Patients in the topiramate group discontinued the treatment more frequently than in the erenumab group (38.9% vs 10.6%, P<0.001); compared with those in the topiramate group, patients in the erenumab group reported a greater >50% response rate (55.4% vs 31.2%, P<0.001), reduction in MMDs during month 4–6 (–5.86 Vs–4.02, P<0.001) and improvement in quality-of-life measures. Proportion of AEs was lower in erenumab compared with topiramate group (55.4% vs 81.2%); most common AEs were paresthesia, disturbance in attention, fatigue and nausea in the topiramate group and fatigue, nausea, disturbance in attention and dizziness in the erenumab group.34
Overview of Real-World Studies
Study Design, Inclusion Criteria and Baseline Characteristics
Inclusion criteria and study design varied across real-world studies reproducing a scenario similar to daily clinical practice. Overall, median age was higher in real-world studies than in RCTs; chronic migraine was the most frequent form of disease, often in comorbidity with medication overuse headache; patients reported several treatment failures or contraindications as suggested by the European and American Guidelines on the use of mAbs;35,36 many patients were on concomitant oral preventative treatments37–56 or on onabotulinumtoxinA.43,56
Researchers assessed treatment efficacy as mean reduction in MMDs, AMDs, and pain intensity measured as a Numerical Rating Score (NRS score), response rate measured as 30-50-75-100% reduction in MHDs/MMDs from baseline. Some studies also evaluated patient reported outcomes.37–56
Treatment duration varied across the studies, from 2 to 12 months, while the adopted doses of erenumab were those commercially available (ie 70 and 140 mg every 4 weeks).37–56
Population of real-world studies had a mean age ranging from 43 to 53 years, mostly female, a mean disease duration from 5 to 33 years, proportion of medication overuse from 19 to 100%, mean MMDs/MHDs from 9.4 to 26 days, AMDs from 10 to 20 days, NRS score from 6.8 to 10. Patients included in real-world studies had prior treatment failures ranging from 3.6 to 6.9; the high proportion of previous treatment failures was due to reimbursement criteria; specifically, many countries required several prior treatment failures (up to 5 prior failures including onabotulinumtoxinA) to grant access to erenumab37–56 (Table 2).
Table 2.
Main Baseline Characteristics and Outcomes of the Real-World Studies
| Study, Year | Country | Months of Follow-Up | Disease Duration, Mean Years ± SD | Sample Size | Migraine Forms, % of Patients | Mean Age ± SD | Female Sex, % | Medication Overuse (% of Patients) | Mean Prior Treatment Failures ±SD/(IQR) | MMDS/MHDs Difference from Baseline at Week 12 | AMDs Difference From Baseline at Week 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barbanti et al, 201937 | Italy | 2 | 5.13±1.8 EM, 5.4±2.6 CM |
78 | 16.7 EM, 84 CM |
47 ±14.4 EM, 47.1±10 CM |
71 | 61.5 EM, 84.6 CM | 29.1±15.3 EM, 30.2±11.7 CM |
NA | NA |
| Raffaelli et al, 202056 | Germany | 3 | NA | 139 | 100 CM | 53.4±10.2 | 83.5 | NA | 3.6±1.2 plus BoNTA | 4.5* | 6.3* |
| Lambru et al, 202038 | UK | 6 | 13±11.9 | 162 | 100 CM | 46±13 | 83.3 | 54 | 8.4±3.6 | 6* | 8.2* |
| Russo et al, 202039 | Italy | 6 | 33.1±1.2 | 70 | 100 CM | 46.9±1.4 | 78.6 | 91.4 | 4.7±0.3 | 9.7* | NA |
| Ornello et al, 202040 | Italy | 6 | 28.2±13.3 | 89 | 5.6 EM, 94.4 CM |
46.8±11.2 | 87.6 | 71.9 | 3.23 | 12* | 8* |
| Scheffler et al, 202041 | Germany | 3 | NA | 100 | 26 EM, 74 CM |
52.9±9.1 EM 45.8±12.9 CM |
88.4 EM 91.2 CM |
66 CM | NA | 2.5* | 3.6* |
| Robblee et al, 202055 | US | 6 | NA | 101 | 5.9 EM, 94.1 CM |
49 (18–80) | 89.1 | 18.8 | NA | 8.4 ±5.8–11.1 | NA |
| Ranieri et al, 202044 | Italy | 12 | NA | 30 | 30 EM, 70 CM |
44±11.5 | 90 | 28.5 | NA | 7.7* | 6* |
| Pensato et al, 202045 | Italy | 3 | 18.9±10.1 | 39 | 100 CM | 49.8±8.3 | 64 | 100 | 13 (11–16) | 13* | 12* |
| Matteo et al, 202053 | Italy | 6 | 33.6±11.8 | 159 | 23.3 EM 76.7 CM |
50.23±9.4 | 75 | 91 CM | 6.9±2.7 | 4.8* | 11.7* |
| Della Valle et al, 202054 | Italy | 10 | NA | 40 | 45 EM 55 CM |
45.2±7.4 | 75 | 52.5 | NA | NA | NA |
| Barbanti et al 2020 (EARLY)46 | Italy | 3 | 28.2±12.4 EM, 30.7 ± 12.2 CM | 372 | 103 EM, 269 CM |
47.5±11.4 EM, 48.4±10.2 CM | 71.8 EM, 78.1 CM | 37.9 EM, 85.1 CM | 3.4 ± 2 EM, 5.1 ± 2.7 CM |
4.5 ± 4.1 EM, 9.3±9.1 CM |
7 EM, 12 CM |
| Eghtesadi et al, 202149 | Canada | 12 | 32.9 ± 11.4 | 18 | 33.3 EM 66.7 CM |
48.7±7 | 83.3 | 58.3 CM | 4.3±1.3 | 5.2±6.9 | 5.4±7.0 |
| Cainazzo et al, 202150 | Italy | 12 | 33.17 ± 11.57 | 81 | 100 CM | 49.58±9.52 | 80.2 | 100 | 5.75±3.17 | 6* | 6.8* |
| Storch et al, 202151 | Germany | 6 | NA | 82 | 42.7 EM, 57.3 CM |
51.1±10.5 | 83 | 53.9 | 4.49±1.33 | 5.24±5.64 | NA |
| de Vries Lentsch, 202152 | Netherlands | 6 | NA | 100 | 54 EM 46 CM |
43±12 | 85 | NA | 5.0±1.0 | 3.8* | NA |
Abbreviations: AMDs, monthly acute medication days; BoNTA, botulinum toxin A; CM, chronic migraine; EM, episodic migraine; IQR, interquartile range; MMDs, monthly migraine days; MHD, monthly headache days; NA, not available; SD, standard deviation; US, United States; asterisk marks data calculated.
Outcomes of Effectiveness
Real-world studies confirmed the effectiveness of erenumab in reducing MMDs, AMDs and in improving patient reported outcomes. At 3 and 6 months MMDs reduction ranged respectively from -2 to −9.7 and from −6 to −14.3 days; AMDs from −3 to −8.2 and from −3.2 to −8 days; NRS score from −0.5 to −3.75 and from -0.7 to -3; 50% response rate from 20 to 76.6% and from 10 to 63%, 75% response rate from 5 to 32.9% and from 16.3 to 38.4%; 100% response rate from 1 to 10%37–56 and was 9.3% at 6 months in one study55 (Table 2 and Figure 2B). In real-world setting 11%-74% patients required dose escalation suggesting a higher effectiveness of the 140 mg. A real-world study comparing the two doses proved a higher effectiveness of the 140 mg dose in reducing the MHDs and HIT-6 score, a greater severity of disease in those receiving the higher dose might have affected results.50 A range from 25 to 71.9% patients ceased from medication overuse;37–56 one study demonstrated that results were similar in patients undergoing a detoxification process before starting erenumab and in those who did not.50 A proportion ranging from 22–83% converted from chronic to episodic migraine;38,39,41,47 a subgroup analysis from a real-world study showed a higher rate of conversion among difficult-to-treat patients with a long history of chronic migraine and multiple prior preventive treatment failures, including onabotulinumtoxinA.57
A study evaluated the effectiveness of erenumab in migraine prevention of patients with menstrually-related migraine. Overall, the treatment was effective, but there was no difference in response rate depending on menstrual cycle phase (ie menstrual, premenstrual or non-menstrual days) suggesting that menstruation remains a trigger for migraine occurrence.58
Prospective real-world studies providing data on erenumab discontinuation after 1-year treatment demonstrated that most patients rapidly rebound (ie register increased MMDS, AMDs and NRS scores) at week 8–1259 and 13–16 from the last erenumab injection even faster than in open label extensions.33 In one study, 68.7% patients did not restart treatment during the follow-up due to maintenance of effectiveness; most of them were >50% responders.59 A retrospective real-world study reported similar results on disease rebound at 3 months from treatment discontinuation after a 1-year treatment with different anti-CGRP mAbs.60
Safety
Erenumab showed overall good safety and tolerability profiles: RCTs and real-world studies reported similar proportion of AEs ranging from 44 to 70% and 7.8 to 70% respectively. The type of AEs was similar across the different studies apart from constipation, which was more frequent in real-world studies. Overall, most common (>2%) AEs related to the use of erenumab were site injection pain, upper respiratory infections, nausea, constipation, and back pain (Figure 3). SAEs were rare and mostly unrelated to the drugs.16–19,22,27,28 Safety data from open label extension proved the long-term tolerability of erenumab with proportions of AEs and SAEs similar to double blind phases.29–31
Figure 3.
Most common adverse events occurring during the use of erenumab. Created with BioRender.com.
Treatment Discontinuation
The tolerability of treatment might explain the low discontinuation rates of RCTs and real-world studies, which ranged from 3 to 11% 16–19,27,28 and from 0 to 40%38–42,44,46,50,51,55,56,61 respectively. Proportion of patients who discontinued treatment was similar across placebo and erenumab arm and greatly varied across real-world studies because of different definition of treatment failure and duration of study period. In few cases the reason for discontinuation was an adverse event. Overall, proportion of erenumab discontinuation is similar to onabotulinumtoxinA, but remarkably lower than topiramate,34,62 which showed a lower tolerability profile.
Patient Profiles
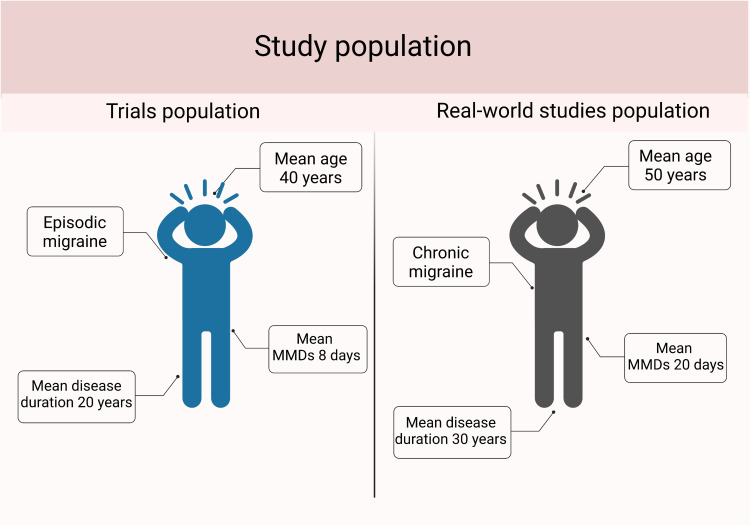
Evidence from RCTs and real-world studies proved benefits of erenumab in patients with episodic and chronic migraine. RCT populations only represent a subset of migraineurs: subgroup and post-hoc analysis provided insights on the efficacy of erenumab in those with 1–4 prior treatment failures, with chronic migraine and medication overuse headache, further included in real-world studies.63 RCTs focused on patients undergoing washout from other preventatives, pointing out the innovative potential of this class of treatment, which ensures high efficacy rates, patients’ compliance and satisfaction, and an optimal tolerability profile. Compared with RCTs, real-world populations were older, with a higher prevalence of chronic migraine and medication overuse, longer disease history, and higher number of prior treatment failures (Figure 4), thus, more similar to a clinical setting. Relevant differences in patients’ baseline characteristics might justify the better results of real-world studies than RCTs in terms of frequency of migraine attacks, consumption of acute medications and disability.
Figure 4.
Comparison between overall patients’ characteristics of randomized controlled trials and real-world studies.
Abbreviation: MMDs-monthly migraine days. Created with BioRender.com.
Placebo effect - efficacy of an agent related to the positive expectation of patients - and nocebo effect - negative outcomes of an agent due to patients’ negative expectation - might have also affected results of real-world studies. Data from RCTs highlighted that, among patients with chronic migraine, those with medication overuse tended to have a lower placebo response,24 suggesting that patients with more severe forms of disease had lower expectations on treatment efficacy. A recent meta-analysis pooling data from RCTs on anti-CGRP mAbs, topiramate, and onabotulinumtoxinA showed that placebo effect was more frequent in patients with episodic migraine than chronic migraine treated with mAbs, while nocebo effect did not significantly differ between the groups. Nocebo effect in RCTs on anti-CGRP mAbs was similar to onobotulinumtoxinA and less intense than topiramate for episodic migraine prevention.64 That information is relevant for treatment choice in clinical practice as it suggests that starting anti-CGRP treatment before migraine chronification might reduce negative expectations on treatment efficacy.
RCTs and real-world studies proved that anti-CGRP mAbs have a modest wearing-off effect - symptom worsening just before the following treatment administration due to drug low levels at the end of its half-life55 - which is lower than onabotulinumtoxinA.65,66 Clinical experience suggests that efficacy of anti-CGRP mAbs persists throughout the entire treatment period and, in some cases, even after treatment discontinuation.59 Data from real-world showed a delayed response to these treatments recommending a treatment duration of 3–6 months before declaring treatment failure due to non-response.40,49,52 Both persistent effect and delayed response might result from modulation of central neural circuits, which should be further investigated through functional neuroimaging and neurophysiology. A persistent and beneficial remodulation of dysfunctional neural circuits67 might also prevent disease chronification68 and encourage a prompt treatment with anti-CGRP mAbs even in patients with less severe forms of disease.
Taken together, results from RCTs and real-world studies confirmed the efficacy of erenumab in a wide group of patients and raised questions on patient selection. The main issue on expanding the use of anti-CGRP treatments is the high cost of anti-CGRP mAbs, thus, the European and American guidelines recommended them only to those without other suitable or equally effective therapeutic options.35,36 Recent cost-effectiveness analyses provided different results: a Swedish study showed that erenumab is cost-effective in patients with both episodic and chronic migraine, provided that they have 2 or more prior treatment failures;69 a Greek study proved that erenumab cost-effectiveness is comparable to onaboulinumtoxinA in patients with chronic migraine;70 a study performed in the US proved a greater cost-effectiveness of erenumab than onabotulinumtoxinA and hypothesized that treatment might be cost-effective even in episodic migraine prevention when considering loss of work productivity.71 All studies were region-restricted, none performed a wider analysis including different countries and, thus, did not considered possible local differences in treatment and healthcare costs. Cost-effectiveness was estimated from RCTs data, where patients with more severe forms of disease were underrepresented; wider cost-effectiveness analyses including data from real-world might provide a different scenario enlarging the group of patients, who might most benefit from the treatment.69–71
Safety
MAbs acting on the CGRP pathway showed a greater safety profile than oral preventives. Researchers extensively studied mAbs for the risk of cardiovascular adverse events; however, no safety concerns emerged, even after 5 years of treatment.29 Arterial hypertension is another relevant vascular adverse event, whose connection with erenumab is yet to be proved: a recent analysis of RCT data showed that the proportion of patients developing this condition was <1%, similar to the placebo groups of RCTs, and not relevant in a post-marketing setting;72 conversely, a retrospective descriptive study reported 61 cases of new-onset arterial hypertension shortly after treatment start.13 We would need larger post-marketing analyses evaluating connections and frequency of arterial hypertension occurring during erenumab treatment as well as comparative studies with the other anti-CGRP mAbs.
A causal relationship between erenumab and cerebrovascular accidents is still uncertain. Preclinical evidence showed that anti-CGRP treatments such as gepants worsened ischemic stroke (ie volume of the lesions, severity of neurological deficits) clinical and imaging outcomes due to collateral dysfunction in mice;73 a study evaluating cerebral blood flow velocities in humans did not reveal differences between patients with migraine receiving erenumab and healthy controls.74 RCTs did not reported ischemic stroke among the AEs and SAEs causally related to erenumab and excluded patients with a history of myocardial infarction and ischemic stroke as suggested by drug regulative agencies.11 Few cases of ischemic stroke occurred in real-world setting, erenumab might have played a role but was not the sole cause of the event.43,75
An AE strictly linked to erenumab is constipation due to antagonism of the gastrointestinal CGRP receptor. Constipation is usually mild; however, in some patients might be serious or even predispose to surgical complications.76 Notably, in real-world studies constipation was reported in higher proportions (up to 76.7%) compared with RCTs (less than 5.1%).
The high tolerability of erenumab, related to its specific action on migraine mechanisms, makes it a suitable therapeutic option for patients with contraindications to other migraine preventatives. Because anti-CGRP mAbs do not act as immunomodulatory agents, they might be combined with immunomodulatory treatments. So far, the sole evidence of a similar combination is a case report on treatment of erenumab with immunoglobulin for myasthenia gravis;63 we expect more data to become available in the next years as the use of immunomodulatory and immunosuppressive mAbs is increasing in several neurological conditions, including multiple sclerosis, autoimmune and paraneoplastic disorders, and inflammatory polyneuropathies.24
Dose
Erenumab and eptinezumab are the sole anti-CGRP mAbs allowing dose escalation as the two other mAbs commercially available have fixed administration schedules – galcanezumab has an initial loading dose of 240 mg followed by 120 mg monthly and fremanezumab has the 225 mg monthly or the 675 mg quarterly dose. The availability of erenumab in two doses allows different treatment approaches tailored on patients’ characteristics; dose escalation can be adopted in patients with poor response to the 70 mg dose, while treatment start with the highest dose can be adopted in hard-to-treat patients with multiple prior preventive treatment failures or medication overuse.77 RCTs suggested a higher efficacy of the 140 mg over the 70 mg dose, despite not directly comparing the two doses;77 real-world studies showed that 29–37% of patients with unsatisfactory response (<30% decrease in MHDs/MMDs) reported a >30% response rate after dose escalation.38,39 Indirect comparisons between the two doses confirmed the superiority of 140 mg to 70 mg without an increase in AEs.50,78 Patients’ variable responses to the two erenumab doses might reflect an interindividual affinity of CGRP pathway for anti-CGRP treatments. As RCTs proved the safety of erenumab 140 mg as a maximum dose, we do not know if higher doses could be tolerable and more effective.
Criteria and timing for dose escalation are yet to be defined: in real-world studies, clinicians allow mainly those with <30% response rate to escalate the dose in a wide timespan – from week 4 of up to week 12. Future studies might provide a clear clinical guidance on dose escalation maximizing treatment efficacy and cost-effectiveness.
Predictors of Treatment Response
Predictors of treatment response might help patient selection and advising clinical decision on when to start or discontinue the treatment with an anti-CGRP mAb. Most studies focused on predictors of negative or incomplete response to the treatment such as medication overuse, higher acute medications intake, higher number of prior preventive treatment failures,39,40,79 allodynia48 and psychiatric comorbidities.46 Few other studies identified predictors of positive response to the treatment such as triptans,80 unilateral localization of pain, allodynia, osmophobia, unilateral autonomic symptoms, and dopaminergic symptoms (ie yawning, somnolence, nausea, vomiting, mood changes, which are epiphenomena of a reduced inhibition of neuronal discharge in trigemino-cervical complex exerted by A11 dopaminergic cells).46 Further data from real-world studies will identify new clinical predictors of response and of sustained response after treatment discontinuation leading to a more accurate patient selection and optimal treatment duration. Assessing migraine biomarkers will also facilitate the identification of responders to migraine treatments, including erenumab.
The Effect of Erenumab After Discontinuation: Is It a Disease-Modifying Drug?
If the possible disease-modifying role of anti-CGRP should be further investigated, the treatment duration to obtain a sustained clinical remission is even more uncertain. The European guidelines for the use of mAbs targeting the CGRP pathway are based on RCTs evidence and recommend a 6–12-month treatment.81 Some national health authorities imposed a period of treatment stop between yearly cycles. Evidence on effects of treatment discontinuation after 52 weeks (ie 13 administrations) is poor, but showed a disease rebound in a relevant proportion of patients since the first 4 weeks of washout.33,59,82 Notably, a small proportion of patients retained treatment benefits for more than 4 weeks.59 Migraine, and mostly chronic migraine, is characterized by sensitization to pain, which implies complex rearrangements of several networks of the central nervous system.83,84 It is likely that prolonged treatment with erenumab can indeed lead to reversal in those connectivity rearrangements; however, one year of treatment might not be sufficient to ensure that effect. A further possible explanation to sustained benefits continuing after treatment discontinuation is the possible patients’ adoption of “healthier habits”, which might positively impact headache frequency such as a good sleep quality.
Open label extensions of RCTs proved that treatment efficacy was stable even at 1 or 5 years without any increase in the number of AEs. The good tolerability of anti-CGRP mAbs is a remarkable advantage over oral preventatives, which are generally administered in short cycles of 3–6 months to limit adverse events.85 This advantage allows long-term treatments that can provide a significant advantage to patients and potentially demodulate the sensitization to pain of central nervous structures.86 The definition of treatment duration sufficient for a prolonged disease remission after treatment discontinuation might significantly reduce patients’ burden, social and economic costs of disease.
Future Treatment Perspectives
Despite the high efficacy of erenumab, as well as the other anti-CGRP mAbs, there are still partial responders, who register a decrease of the overall disease-related disability and improved response to acute treatments. Causes of poor and no response to these highly-effective treatments are still under investigation: other central pathogenic pathways might be predominant in some patients, or blockade of the CGRP pathway might be insufficient in others, who, thus, might benefit from a combined treatment acting on the same pathway.61
In case of insufficient response to anti-CGRP pathway mAbs and predominance of central pathways in disease, the combination of mAbs with oral preventatives might improve treatment efficacy. Most real-world studies allowed concurrent oral preventatives, but none compared the effectiveness of add-on treatment with single anti-CGRP receptor treatment. Further studies might provide data on the effectiveness of a similar add-on approach and on subset of patients, who might most benefit from it. A further therapeutic option in patients with an incomplete response to erenumab might be the add-on of another anti-CGRP mAb or gepants, which can be used both as preventive and symptomatic treatments. A case report showed the efficacy of the combined treatment with erenumab as preventative and Rimegepant 75 mg (at most daily) as symptomatic treatment in two patients: rimegepant might enhance an incomplete blockade of CGRP receptor exerted by erenumab87 responsible for breakthrough attacks. Rimegepant 75 mg proved to be safe and tolerable even when combined with erenumab, fremanezumab and galcanezumab in a larger cohort of patients.88 Similarly, a Phase 1 RCT proved that ubrogepant 100 mg has similar pharmacokinetics and pharmacodynamics alone and in combination with erenumab or galcanezumab as a symptomatic treatment.89 Data available on the combination of different treatments acting on the same pathway are scarce but suggest that there is a rationale for a similar combination without safety concerns.
The rationale for the combination of an anti-CGRP mAb with an anti-CGRP receptor mAb is the possible binding of CGRP to AMY 1 receptor and the AMY ligand to CGRP receptor, which explains an incomplete blockade of the pathway exerted by only one type of mAbs.61 No study to date has investigated a similar approach, which might be difficult to achieve due to the high cost of the treatments and due to vascular safety concerns. Conversely, few data are available on the efficacy of antibody switch from erenumab to galcanezumab or fremanezumab in patients with a poor response to erenumab.90,91 A retrospective case series allowed antibody switch after 3 erenumab administrations in those with a <30% response rate and showed that 32% patients achieved the >30% response rate and 12% the 50% response rate. Patients also reported a significant reduction in median MHDs but no in AMDs.90 Smaller case series reported similar results, proving antibody switch as a suitable therapeutic approach in hard-to treat patients.92,93 The small sample size and the retrospective design limited these works, whose results should be confirmed by larger prospective real-world studies and double blind RCTs. Future evidence might lead to the recognition of antibody switch as a therapeutic option for poor responders; so far, local health authorities only allow it in case of AEs leading to treatment discontinuation or difficulties in the use of the autoinjector.
A recent consensus suggests the combination of onabotulinumtoxinA with anti-CGRP mAbs as a possible therapeutic strategy in patients with refractory migraine or a suboptimal response to the single treatment.94 Some case series proved the efficacy of this combined treatment in reducing MHDs/MMDs95,96 and headache severity97 with no safety concerns thanks to the optimal tolerability and safety profiles of the treatments.95–98 Both the treatments act on the CGRP pathway: mAbs inhibit CGRP binding to its receptor on Aδ-fibers, onabotulinumtoxinA exerts its action on C-type fiber with still unknown mechanisms; thus, their concomitant use might enhance the blockade of the pathway. However, the prohibitive cost of both treatments makes the reimbursement from National Health Services significantly challenging.
Ongoing Studies
We expect that ongoing studies will cover some “grey areas” on erenumab efficacy and mechanisms of action. RCTs are evaluating efficacy and safety of the treatment in subgroup of patients, including pediatric populations (NCT03836040, NCT03832998), Asian population (DRAGON), patients suffering from chronic migraine and medication overuse headache (NCT03971071); a study will focus on the efficacy of erenumab in terms of pain intensity reduction (EMBRACE), while a further study will compare erenumab to oral preventatives in terms of sustained response (NCT03927144) (Table 3).
Table 3.
Ongoing Phase 3 and 4 Randomized Controlled Studies
| NCT | Country | Comparator | Aim | Primary Endpoint | Sample Size, N of Patients | Migraine Form | Age, Years |
|---|---|---|---|---|---|---|---|
| NCT03927144 | US | Oral preventatives | Superiority of erenumab to oral preventatives | Treatment completion with a >50% response rate at month 12 | 621 | EM | >18 |
| NCT04252742 (EMBRACE) | US | Placebo | Treatment benefit on duration of moderate headache | Change in mean monthly hours of at least moderate headache pain intensity (11-point NRS scale) | 576 | High frequency EM | >18 |
| NCT03867201 (DRAGON) | China | Placebo | Efficacy and safety of erenumab in Asian patients with chronic migraine | Changes in MMDs during the last 4 weeks of a 12-week treatment | 557 | CM | 18–65 |
|
NCT03836040 NCT03832998 |
US | Placebo | Efficacy and safety of erenumab in children and adolescents | Changes in MMDs | 456 EM 286 CM |
EM CM |
6–12 12–18 |
| NCT04452929 | Denmark | Placebo | Effect of erenumab in calcitonin-gene related peptide and cilostazol human experimental models of migraine | Incidence of migraine-like attacks | 72 | EM/CM | 18–60 |
| NCT03971071 | US | Placebo | Efficacy and safety of erenumab in patients with chronic migraine and medication overuse | Number of participants with absence of Medication Overuse Headache at month 6 | 687 | CM | >18 |
Abbreviations: CM, chronic migraine; EM, episodic migraine; MMDs, monthly migraine days; NRS, numerical rating scale; US, United States.
Open-label studies are evaluating erenumab efficacy and safety in the long term (NCT04084314), exploring biomolecular markers (NCT04361721, NCT04659226) and genetic predictors of response (INTERROGATE), evaluating neuroimaging changes related to the treatment (NCT03773562), and investigating disease pathogenetic mechanisms in relation to CGRP binding - clinical response to erenumab in patients treated with injection of CGRP or cilostazol to trigger migraine (NCT04452929, NCT04452929) (Table 4).
Table 4.
Ongoing Open-Label Studies
| NCT | Country | Aim | Primary Endpoint | Sample Size, N of Patients | Migraine Form | Age, Years |
|---|---|---|---|---|---|---|
| NCT03773562 | US | Changes in brain function and structure under erenumab treatment | Changes in Pain-Induced Brain Activations and Brain Functional Connectivity (MRI) | 50 | EM | 18–65 |
| NCT04361721 | Italy | Impact of erenumab treatment on neurophysiological, biomolecular and psychological aspects | Spinal sensitization measured as TST of the nociceptive withdrawal reflex | 40 | CM | 18–65 |
| NCT04592952 | Denmark | Relationships between clinical response to erenumab and response to intravenous infusion of calcitonin CGRP | Headache diaries (migraine days, headache days, number of days with use of acute migraine medication, number of days with aura) | 400 | EM/CM | 18–50 |
|
NCT04265755 INTERROGATE |
Denmark, Iceland | Relationships between clinical response to erenumab and genetic biomarkers | >50% response rate in those with SNPs contributing to the migraine polygenic risk score | 2000 | EM/CM | >18 |
| NCT04659226 | Italy | Expression profiles of microRNAs before and after erenumab treatment | Changes in microRNA serum concentration | 40 | EM | 25–50 |
| NCT04084314 | Germany | Long-term safety and tolerability (128 weeks) of erenumab treatment | Adverse events | 699 | EM/CM | 18–60 |
Abbreviations: CM, chronic migraine; CGRP, Calcitonin gene-related peptide; EM, episodic migraine; MRI, magnetic resonance imaging; N, number of patients; SNP, single nucleotide polymorphism; TST, temporal summation threshold; US, United States.
All the aforementioned studies will enrich clinical knowledge on erenumab. In detail, the new RCTs might confirm results of real-world studies in those with more severe forms of disease (ie chronic migraine, medication overuse headache) and provide evidence on those younger than 18 years old. Data on genetic and biomolecular factors related to treatment response and disease pathogenesis will improve treatment decision and patient selection leading to a patient’s tailored therapy.
The Place of Erenumab in Migraine Prevention
The abovementioned cost-effectiveness considerations led to some limitations to erenumab prescription and reimbursement in clinical practice. Erenumab, together with the other anti-CGRP mAbs, is currently one of the last resources of migraine prevention; it can be used in patients with episodic migraine after the failure of several oral drugs and in those with chronic migraine after failure of oral drugs and onabotulinumtoxinA.94,99 However, this position of erenumab in the landscape of migraine treatment could change in the future. anti-CGRP mAbs demonstrated a high efficacy in patients with episodic migraine and could prevent chronification; therefore, their use in an earlier stage of migraine progression, before failing several preventive drugs, could maximize their efficacy and provide a higher benefit to patients, thus ultimately leading to a reduction in long-term healthcare costs. mAbs act fast, during the first weeks or even the first days after their start;100 the efficacy of mAbs can be assessed during the first 3–6 months of use, thus leading to the possibility of relatively early treatment switch in case of unsatisfactory response. It should also be mentioned that new migraine-specific preventive treatments, ie gepants, are entering the market and will be increasingly used in the next years. For those reasons, it is likely that the use of migraine-specific preventatives such as erenumab will spread from the most severe to milder forms of migraine, considering their largely positive long-term impact on healthcare resource utilization and lost productivity.
How Will the Use of Erenumab Contribute to Migraine Research
Several shortcomings in the clinical management of migraine reflect the lack of knowledge on disease pathogenesis. The comprehension of the key role of CGRP in migraine pain was fundamental for the development of mAbs targeting the CGRP pathway. Similarly, the understanding of mechanisms underlying the lack of treatment response, disease rebound after treatment discontinuation and chronification might lead to new therapeutic strategies and to a better selection of candidates for high effective treatments as erenumab. Results from ongoing and future head-to-head trials comparing combination of anti-CGRP treatments with single therapy as well as studies evaluating long-term effects of prolonged treatment on disease modulation will complement the existing knowledge .
A further development for research will be to evaluate the events occurring in the central nervous system during treatment with erenumab. Despite the large dimensions of mAbs do not allow them to cross the blood-brain barrier, they might exert a retrograde effect on some mechanisms occurring in the brain, such as cortical spreading depression (CSD) which is the supposed mechanism originating migraine aura. Clinical data on the specific response of aura to erenumab are not available, while functional studies might assess the possible central effects of erenumab (NCT03977649). There is suggestion that erenumab has indeed central effects, such a decrease in hypothalamic activation;101 further studies will provide novel data and generate new hypotheses on the action of the drug, ultimately contributing to the understanding of migraine mechanisms.
Conclusions
Erenumab was the first mAb targeting CGRP pathway commercially available; real-world studies confirmed RCTs data on treatment efficacy even in patients with more severe forms of disease. The high tolerability, safety, and ease of use make erenumab a treatment ideally suitable for a wide group of patients, but the high cost imposes strict reimbursement criteria. Real-world studies identified some clinical predictors of treatment response which might lead in the future to a therapy tailored on patients’ characteristics, especially if combined with biomarkers, thus maximizing cost-effectiveness. Further studies might provide insights on the mechanisms underlying delayed, sustained, and poor response to anti-CGRP mAbs, redefining treatment duration and providing a rationale to the combination of treatments acting on the same pathway (ie gepants, onabotulinumtoxinA, a different mAb) or to antibody switch. We expect interesting results from the ongoing studies on efficacy and safety of erenumab in new group of patients (ie children and adolescents) and on biomolecular and genetic markers of treatment response.
Take-Home Messages
Migraine is a disabling disease mainly affecting young women. Rationale of preventives is to reduce disease-related disability. In the last decades, preclinical studies proved the key role of the Calcitonin Gene-related peptide (CGRP) in migraine pain genesis allowing to develop migraine-specific preventatives.
Erenumab was the first monoclonal antibody (mAb) targeting the CGRP pathway released for migraine prevention, and the sole antagonizing CGRP receptor. Randomized clinical trials (RCTs) proved the superiority of the erenumab to placebo, and a recent double-dummy RCT proved the superiority of treatment to topiramate.
Real-world studies proved the effectiveness of erenumab in a clinical setting, thus, in patients with more severe forms of migraine according to disease duration, number of prior treatment failures, and prevalence of chronic form of disease. Erenumab proved to have optimal safety and tolerability profiles. Most common adverse events occurring both in RCTs and real-world studies were site injection pain, upper respiratory infections, nausea, constipation, and back pain. The main concerns were arterial hypertension, cardiovascular and cerebrovascular events due to treatment inhibition of CGRP receptor on smooth cells of vessels, but few cases had been reported, whose connection with the treatment is still debated.
In the future, criteria for erenumab prescription might be broader because of treatment efficacy both in patients with severe and mild forms of disease. Also, treatment duration might be redefined according to recent data of RCTs open-label extensions and real-world studies on treatment discontinuation. European and American guidelines suggest a 6 to 12-month treatment; treatment keeps its safety and efficacy even longer, but national authorities may impose yearly stops due economic reasons. Recent data showed that some patients keep treatment benefit even weeks after its discontinuation, while other registered a quick disease rebound. The identification of predictors of treatment response and sustained response might improve cost-effectiveness and pave the way to a tailor-made treatment.
A deep comprehension of mechanisms underlying insufficient response to erenumab might lead to future therapeutic strategies as the combination of different mAbs targeting soluble CGRP and its receptor, or mAbs and treatments active on the CGRP pathway as onabotulinumtoxinA and gepants.
Studies investigating neuroimaging, biomolecular and genetic markers of treatment response are ongoing, as well as others evaluating erenumab safety and efficacy in pediatric population. Further research interests will be mechanisms underlying chronification and insufficient treatment response, and aura response to treatments acting on the CGRP pathway, which might provide relevant insights on disease pathogenesis.
Disclosure
Prof. Simona Sacco reports grants and/or personal fees from Allergan/AbbVie, Novartis, Lilly, Teva, Lundbeck, NovoNordisk, AstraZeneca, and Uriach, during the conduct of the study. Dr. Raffaele Ornello reports personal fees and/or non-financial support from Novartis, Teva, Eli Lilly, and Allergan/AbbVie, outside the submitted work. Dr. Eleonora De Matteis has no disclosure to report. The authors report no other conflicts of interest in this work.
References
- 1.Deuschl G, Beghi E, Fazekas F, et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5(10):e551–e67. doi: 10.1016/S2468-2667(20)30190-0 [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Matteis E, Guglielmetti M, Ornello R, Spuntarelli V, Martelletti P, Sacco S. Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother. 2020;20(6):627–641. doi: 10.1080/14737175.2020.1772758 [DOI] [PubMed] [Google Scholar]
- 4.Markham A. Erenumab: first global approval. Drugs. 2018;78(11):1157–1161. doi: 10.1007/s40265-018-0944-0 [DOI] [PubMed] [Google Scholar]
- 5.Shi L, Lehto SG, Zhu DX, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther. 2016;356(1):223–231. doi: 10.1124/jpet.115.227793 [DOI] [PubMed] [Google Scholar]
- 6.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–350. doi: 10.1038/s41582-018-0003-1 [DOI] [PubMed] [Google Scholar]
- 7.Yuan H, Lauritsen CG, Kaiser EA, Silberstein SD. CGRP monoclonal antibodies for migraine: rationale and progress. BioDrugs. 2017;31(6):487–501. doi: 10.1007/s40259-017-0250-5 [DOI] [PubMed] [Google Scholar]
- 8.Barbanti P, Fofi L, Aurilia C, Egeo G. Does the migraine attack start in the cortex and is the cortex critical in the migraine process? Neurol Sci. 2019;40(Suppl 1):31–37. doi: 10.1007/s10072-019-03838-y [DOI] [PubMed] [Google Scholar]
- 9.Goadsby PJ, Holland PR. An update: pathophysiology of migraine. Neurol Clin. 2019;37(4):651–671. doi: 10.1016/j.ncl.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 10.Stoker K, Baker DE. Erenumab-aooe. Hosp Pharm. 2018;53(6):363–368. doi: 10.1177/0018578718797295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicine Agencies. Aimovig, INN-erenumab; 2020.
- 12.Rubio-Beltrán E, Labastida-Ramírez A, Haanes KA, et al. Characterisation of vasodilatory responses in the presence of the CGRP receptor antibody erenumab in human isolated arteries. Cephalalgia. 2019;39(14):1735–1744. doi: 10.1177/0333102419863027 [DOI] [PubMed] [Google Scholar]
- 13.Saely S, Croteau D, Jawidzik L, Brinker A, Kortepeter C. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61(1):202–208. doi: 10.1111/head.14051 [DOI] [PubMed] [Google Scholar]
- 14.Kudrow D, Pascual J, Winner PK, et al. Vascular safety of erenumab for migraine prevention. Neurology. 2020;94(5):e497–e510. doi: 10.1212/WNL.0000000000008743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, Villalón CM. Side effects associated with current and prospective antimigraine pharmacotherapies. Expert Opin Drug Metab Toxicol. 2018;14(1):25–41. doi: 10.1080/17425255.2018.1416097 [DOI] [PubMed] [Google Scholar]
- 16.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382–390. doi: 10.1016/S1474-4422(16)00019-3 [DOI] [PubMed] [Google Scholar]
- 17.Sakai F, Takeshima T, Tatsuoka Y, et al. A randomized Phase 2 study of erenumab for the prevention of episodic migraine in Japanese adults. Headache. 2019;59(10):1731–1742. doi: 10.1111/head.13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–434. doi: 10.1016/S1474-4422(17)30083-2 [DOI] [PubMed] [Google Scholar]
- 19.Dodick DW, Ashina M, Brandes JL, et al. ARISE: a Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037. doi: 10.1177/0333102418759786 [DOI] [PubMed] [Google Scholar]
- 20.Lanteri-Minet M, Goadsby PJ, Reuter U, et al. Effect of erenumab on functional outcomes in patients with episodic migraine in whom 2–4 preventives were not useful: results from the LIBERTY study. J Neurol Neurosurg Psychiatry. 2021;92(5):466–472. doi: 10.1136/jnnp-2020-324396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goadsby PJ, Paemeleire K, Broessner G, et al. Efficacy and safety of erenumab (AMG334) in episodic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2019;39(7):817–826. doi: 10.1177/0333102419835459 [DOI] [PubMed] [Google Scholar]
- 22.Ashina M, Tepper S, Brandes JL, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2018;38(10):1611–1621. doi: 10.1177/0333102418788347 [DOI] [PubMed] [Google Scholar]
- 23.Lipton RB, Tepper SJ, Reuter U, et al. Erenumab in chronic migraine: patient-reported outcomes in a randomized double-blind study. Neurology. 2019;92(19):e2250–e60. doi: 10.1212/WNL.0000000000007452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tepper SJ, Diener HC, Ashina M, et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology. 2019;92(20):e2309–e20. doi: 10.1212/WNL.0000000000007497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tepper SJ, Ashina M, Reuter U, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia. 2020;40(6):543–553. doi: 10.1177/0333102420912726 [DOI] [PubMed] [Google Scholar]
- 26.Brandes JL, Diener HC, Dolezil D, et al. The spectrum of response to erenumab in patients with chronic migraine and subgroup analysis of patients achieving ≥50%, ≥75%, and 100% response. Cephalalgia. 2020;40(1):28–38. doi: 10.1177/0333102419894559 [DOI] [PubMed] [Google Scholar]
- 27.Goadsby PJ, Reuter U, Hallström Y, et al. A Controlled Trial of Erenumab for Episodic Migraine. N Engl J Med. 2017;377(22):2123–2132. doi: 10.1056/NEJMoa1705848 [DOI] [PubMed] [Google Scholar]
- 28.Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–2287. doi: 10.1016/S0140-6736(18)32534-0 [DOI] [PubMed] [Google Scholar]
- 29.Ashina M, Goadsby PJ, Reuter U, et al. Long-term efficacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28(5):1716–1725. doi: 10.1111/ene.14715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goadsby PJ, Reuter U, Hallström Y, et al. One-year sustained efficacy of erenumab in episodic migraine: results of the STRIVE study. Neurology. 2020;95(5):e469–e79. doi: 10.1212/WNL.0000000000010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goadsby PJ, Reuter U, Lanteri-Minet M, et al. Long-term efficacy and safety of erenumab: results from 64 weeks of the LIBERTY Study. Neurology. 2021;96(22):e2724–e2735. doi: 10.1212/WNL.0000000000012029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlovic JM, Paemeleire K, Göbel H, et al. Efficacy and safety of erenumab in women with a history of menstrual migraine. J Headache Pain. 2020;21(1):95. doi: 10.1186/s10194-020-01167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raffaelli B, Mussetto V, Israel H, Neeb L, Reuter U. Erenumab and galcanezumab in chronic migraine prevention: effects after treatment termination. J Headache Pain. 2019;20(1):66. doi: 10.1186/s10194-019-1018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter U, Ehrlich M, Gendolla A, et al. Erenumab versus topiramate for the prevention of migraine - A randomised, double-blind, active-controlled phase 4 trial. Cephalalgia. 2021;4:3331024211053571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6. doi: 10.1186/s10194-018-0955-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1–18. [DOI] [PubMed] [Google Scholar]
- 37.Barbanti P, Aurilia C, Egeo G, Fofi L. Erenumab: from scientific evidence to clinical practice-the first Italian real-life data. Neurol Sci. 2019;40(Suppl 1):177–179. doi: 10.1007/s10072-019-03839-x [DOI] [PubMed] [Google Scholar]
- 38.Lambru G, Hill B, Murphy M, Tylova I, Andreou AP. A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21(1):61. doi: 10.1186/s10194-020-01127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo A, Silvestro M, Scotto Di Clemente F, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain. 2020;21(1):69. doi: 10.1186/s10194-020-01143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ornello R, Casalena A, Frattale I, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32. doi: 10.1186/s10194-020-01102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheffler A, Messel O, Wurthmann S, et al. Erenumab in highly therapy-refractory migraine patients: first German real-world evidence. J Headache Pain. 2020;21(1):84. doi: 10.1186/s10194-020-01151-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanaan S, Hettie G, Loder E, Burch R. Real-world effectiveness and tolerability of erenumab: a retrospective cohort study. Cephalalgia. 2020;40(13):1511–1522. doi: 10.1177/0333102420946725 [DOI] [PubMed] [Google Scholar]
- 43.Robbins L. Special report: CGRP monoclonal antibodies for chronic migraine. Practical Pain Managemen. 2020;19:45–52. [Google Scholar]
- 44.Ranieri A, Alfieri G, Napolitano M, et al. One year experience with erenumab: real-life data in 30 consecutive patients. Neurol Sci. 2020;41(Suppl 2):505–506. doi: 10.1007/s10072-020-04677-y [DOI] [PubMed] [Google Scholar]
- 45.Pensato U, Favoni V, Pascazio A, et al. Erenumab efficacy in highly resistant chronic migraine: a real-life study. Neurol Sci. 2020;41:457–459. doi: 10.1007/s10072-020-04658-1 [DOI] [PubMed] [Google Scholar]
- 46.Barbanti P, Aurilia C, Egeo G, et al. Erenumab in the prevention of high-frequency episodic and chronic migraine: erenumab in Real Life in Italy (EARLY), the first Italian multicenter, prospective real-life study. Headache. 2021;61(2):363–372. doi: 10.1111/head.14032 [DOI] [PubMed] [Google Scholar]
- 47.Barbanti P, Aurilia C, Cevoli S, et al. Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: results of the EARLY 2 study. Headache. 2021;61:1351–1363. doi: 10.1111/head.14194 [DOI] [PubMed] [Google Scholar]
- 48.Pensato U, Baraldi C, Favoni V, et al. Real-life assessment of erenumab in refractory chronic migraine with medication overuse headache. Neurol Sci. 2021;43:1273–1280. [DOI] [PubMed] [Google Scholar]
- 49.Eghtesadi M, Leroux E, Pagé G. Real-life response to erenumab in a therapy-resistant case series of migraine patients from the Province of Québec, Eastern Canada. Clin Drug Investig. 2021;41(8):733–739. doi: 10.1007/s40261-021-01059-w [DOI] [PubMed] [Google Scholar]
- 50.Cainazzo MM, Baraldi C, Ferrari A, Lo Castro F, Pani L, Guerzoni S. Erenumab for the preventive treatment of chronic migraine complicated with medication overuse headache: an observational, retrospective, 12-month real-life study. Neurol Sci. 2021;42(10):4193–4202. doi: 10.1007/s10072-021-05105-5 [DOI] [PubMed] [Google Scholar]
- 51.Storch P, Burow P, Möller B, et al. Pooled retrospective analysis of 70 mg erenumab in episodic and chronic migraine: a two tertiary headache centers experience during clinical practice. Acta Neurol Belg. 2021. doi: 10.1007/s13760-021-01770-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Vries Lentsch S, Verhagen IE, van den Hoek TC, MaassenVanDenBrink A, Terwindt GM. Treatment with the monoclonal calcitonin gene-related peptide receptor antibody erenumab: a real-life study. Eur J Neurol. 2021;28(12):4194–4203. doi: 10.1111/ene.15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matteo E, Favoni V, Pascazio A, et al. Erenumab in 159 high frequency and chronic migraine patients: real-life results from the Bologna Headache Center. Neurol Sci. 2020;41(Suppl 2):483–484. doi: 10.1007/s10072-020-04667-0 [DOI] [PubMed] [Google Scholar]
- 54.Valle ED, Di Falco M, Mancioli A, Corbetta S, La Spina I. Efficacy and safety of erenumab in the real-life setting of S. Antonio Abate Hospital’s Headache Center (Gallarate). Neurol Sci. 2020;41(Suppl 2):465. doi: 10.1007/s10072-020-04752-4 [DOI] [PubMed] [Google Scholar]
- 55.Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real-world patient experience with erenumab for the preventive treatment of migraine. Headache. 2020;60(9):2014–2025. doi: 10.1111/head.13951 [DOI] [PubMed] [Google Scholar]
- 56.Raffaelli B, Kalantzis R, Mecklenburg J, et al. Erenumab in chronic migraine patients who previously failed five first-line oral prophylactics and onabotulinumtoxina: a Dual-Center Retrospective Observational Study. Front Neurol. 2020;11:417. doi: 10.3389/fneur.2020.00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ornello R, Casalena A, Frattale I, et al. Conversion from chronic to episodic migraine in patients treated with erenumab: real-life data from an Italian region. J Headache Pain. 2020;21(1):102. doi: 10.1186/s10194-020-01171-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ornello R, Frattale I, Caponnetto V, De Matteis E, Pistoia F, Sacco S. Menstrual headache in women with chronic migraine treated with erenumab: an observational case series. Brain Sci. 2021;11(3):370. doi: 10.3390/brainsci11030370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Matteis E, Affaitati G, Frattale I, et al. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci. 2021;42(8):3297–3303. doi: 10.1007/s10072-020-05022-z [DOI] [PubMed] [Google Scholar]
- 60.Gantenbein AR, Agosti R, Gobbi C, et al. Impact on monthly migraine days of discontinuing anti-CGRP antibodies after one year of treatment - A real-life cohort study. Cephalalgia. 2021;41(11–12):1181–1186. doi: 10.1177/03331024211014616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Vries T, MaassenVanDenBrink A. CGRP-targeted antibodies in difficult-to-treat migraine. Nat Rev Neurol. 2019;15(12):688–689. doi: 10.1038/s41582-019-0275-0 [DOI] [PubMed] [Google Scholar]
- 62.Rothrock JF, Adams AM, Lipton RB, et al. FORWARD Study: evaluating the comparative effectiveness of onabotulinumtoxina and topiramate for headache prevention in adults with chronic migraine. Headache. 2019;59(10):1700–1713. doi: 10.1111/head.13653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiseo C, Ornello R, Pistoia F, Sacco S. How to integrate monoclonal antibodies targeting the calcitonin gene-related peptide or its receptor in daily clinical practice. J Headache Pain. 2019;20(1):49. doi: 10.1186/s10194-019-1000-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kokoti L, Drellia K, Papadopoulos D, Mitsikostas DD. Placebo and nocebo phenomena in anti- CGRP monoclonal antibody trials for migraine prevention: a meta-analysis. J Neurol. 2020;267(4):1158–1170. doi: 10.1007/s00415-019-09673-7 [DOI] [PubMed] [Google Scholar]
- 65.Ruscheweyh R, Athwal B, Gryglas-Dworak A, et al. Wear-off of onabotulinumtoxina effect over the treatment interval in chronic migraine: a retrospective chart review with analysis of headache diaries. Headache. 2020;60(8):1673–1682. doi: 10.1111/head.13925 [DOI] [PubMed] [Google Scholar]
- 66.Pak AT, Üstün İ, Sengul Y. Botulinum toxin type A wear-off phenomenon in chronic migraine patients: how long does the maximum efficiency last? Arq Neuropsiquiatr. 2021;79(10):886–890. doi: 10.1590/0004-282x-anp-2020-0542 [DOI] [PubMed] [Google Scholar]
- 67.Martelletti P, Edvinsson L, Ashina M. Shaping the future of migraine targeting calcitonin-gene-related-peptide with the Disease-Modifying Migraine Drugs (DMMDs). J Headache Pain. 2019;20(1):60. doi: 10.1186/s10194-019-1009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torres-Ferrús M, Ursitti F, Alpuente A, et al. From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. 2020;21(1):42. doi: 10.1186/s10194-020-01111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahon R, Lang A, Vo P, et al. Cost-effectiveness of erenumab for the preventive treatment of migraine in patients with prior treatment failures in Sweden. Pharmacoeconomics. 2021;39(3):357–372. doi: 10.1007/s40273-020-00996-2 [DOI] [PubMed] [Google Scholar]
- 70.Giannouchos TV, Mitsikostas DD, Ohsfeldt RL, Vozikis A, Koufopoulou P. Cost-effectiveness analysis of erenumab versus OnabotulinumtoxinA for patients with chronic migraine attacks in Greece. Clin Drug Investig. 2019;39(10):979–990. doi: 10.1007/s40261-019-00827-z [DOI] [PubMed] [Google Scholar]
- 71.Sussman M, Benner J, Neumann P, Menzin J. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: results from the US societal and payer perspectives. Cephalalgia. 2018;38(10):1644–1657. doi: 10.1177/0333102418796842 [DOI] [PubMed] [Google Scholar]
- 72.Dodick DW, Tepper SJ, Ailani J, et al. Risk of hypertension in erenumab-treated patients with migraine: analyses of clinical trial and postmarketing data. Headache. 2021;61(9):1411–1420. doi: 10.1111/head.14208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mulder IA, Li M, de Vries T, et al. Anti-migraine calcitonin gene-related peptide receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol. 2020;88(4):771–784. doi: 10.1002/ana.25831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Altamura C, Viticchi G, Fallacara A, et al. Erenumab does not alter cerebral hemodynamics and endothelial function in migraine without aura. Cephalalgia. 2021;41(1):90–98. doi: 10.1177/0333102420956692 [DOI] [PubMed] [Google Scholar]
- 75.Aradi S, Kaiser E, Cucchiara B. Ischemic stroke associated with calcitonin gene-related peptide inhibitor therapy for migraine: a Case Report. J Stroke Cerebrovasc Dis. 2019;28(10):104286. doi: 10.1016/j.jstrokecerebrovasdis.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 76.Frattale I, Ornello R, Pistoia F, Caponnetto V, Colangeli E, Sacco S. Paralytic ileus after planned abdominal surgery in a patient on treatment with erenumab. Intern Emerg Med. 2020;16:227–228. doi: 10.1007/s11739-020-02407-y [DOI] [PubMed] [Google Scholar]
- 77.Ornello R, Tiseo C, Frattale I, et al. The appropriate dosing of erenumab for migraine prevention after multiple preventive treatment failures: a critical appraisal. J Headache Pain. 2019;20(1):99. doi: 10.1186/s10194-019-1054-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, He Q, Wen D, Ma L, You C. Efficacy and safety of erenumab in migraine prevention: evidences from direct and indirect comparisons. Neurol Sci. 2021;5:1–8. [DOI] [PubMed] [Google Scholar]
- 79.Baraldi C, Castro FL, Cainazzo MM, Pani L, Guerzoni S. Predictors of response to erenumab after 12 months of treatment. Brain Behav. 2021;11(8):e2260. doi: 10.1002/brb3.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frattale I, Caponnetto V, Casalena A, et al. Association between response to triptans and response to erenumab: real-life data. J Headache Pain. 2021;22(1):1. doi: 10.1186/s10194-020-01213-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sacco S, Braschinsky M, Ducros A, et al. European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine & Headache Alliance (EMHA). J Headache Pain. 2020;21(1):76. doi: 10.1186/s10194-020-01130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raffaelli B, Terhart M, Overeem LH, et al. Migraine evolution after the cessation of CGRP(-receptor) antibody prophylaxis: a prospective, longitudinal cohort study. Cephalalgia;2021. 3331024211046617. doi: 10.1177/03331024211046617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46(Suppl 4):S182–91. doi: 10.1111/j.1526-4610.2006.00602.x [DOI] [PubMed] [Google Scholar]
- 84.May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–464. doi: 10.1038/nrneurol.2016.93 [DOI] [PubMed] [Google Scholar]
- 85.Ornello R, Sacco S. The paradigm shift in long-term treatments for migraine prevention. Eur J Neurol. 2021;28(5):1439–1440. doi: 10.1111/ene.14768 [DOI] [PubMed] [Google Scholar]
- 86.de Tommaso M, Vecchio E, Quitadamo SG, et al. Pain-related brain connectivity changes in migraine: a narrative review and proof of concept about possible novel treatments interference. Brain Sci. 2021;11(2):234. doi: 10.3390/brainsci11020234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mullin K, Kudrow D, Croop R, et al. Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology. 2020;94(20):e2121–e5. doi: 10.1212/WNL.0000000000008944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berman G, Croop R, Kudrow D, et al. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. 2020;60(8):1734–1742. doi: 10.1111/head.13930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jakate A, Blumenfeld AM, Boinpally R, et al. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: a randomized phase 1b drug-drug interaction study. Headache. 2021;61(4):642–652. doi: 10.1111/head.14095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Overeem LH, Peikert A, Hofacker MD, et al. Effect of antibody switch in non-responders to a CGRP receptor antibody treatment in migraine: a multi-center retrospective cohort study. Cephalalgia;2021. 3331024211048765. doi: 10.1177/03331024211048765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ziegeler C, May A. Non-responders to treatment with antibodies to the CGRP-receptor may profit from a switch of antibody class. Headache. 2020;60(2):469–470. doi: 10.1111/head.13729 [DOI] [PubMed] [Google Scholar]
- 92.Briceño-Casado MDP, Gil-Sierra MD, De-la-calle-riaguas B. Switching of monoclonal antibodies against calcitonin gene-related peptide in chronic migraine in clinical practice: a case series. Eur J Hosp Pharm. 2021;ejhpharm-2021-002946. doi: 10.1136/ejhpharm-2021-002946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patier Ruiz I, Sánchez-Rubio Ferrández J, Cárcamo Fonfría A, Molina García T. Early experiences in switching between monoclonal antibodies in patients with nonresponsive migraine in Spain: a Case Series. Eur Neurol. 2021;1–4. doi: 10.1159/000518899 [DOI] [PubMed] [Google Scholar]
- 94.Sacco S, Russo A, Geppetti P, et al. What is changing in chronic migraine treatment? An algorithm for onabotulinumtoxinA treatment by the Italian chronic migraine group. Expert Rev Neurother. 2020;20(12):1275–1286. doi: 10.1080/14737175.2020.1825077 [DOI] [PubMed] [Google Scholar]
- 95.Armanious M, Khalil N, Lu Y, Jimenez-Sanders R. Erenumab and onabotulinumtoxina combination therapy for the prevention of intractable chronic migraine without aura: a retrospective analysis. J Pain Palliat Care Pharmacother. 2021;35(1):1–6. doi: 10.1080/15360288.2020.1829249 [DOI] [PubMed] [Google Scholar]
- 96.Silvestro M, Tessitore A, Scotto Di Clemente F, Battista G, Tedeschi G, Russo A. Additive interaction between Onabotulinumtoxin-A and erenumab in patients with refractory migraine. Front Neurol. 2021;12:656294. doi: 10.3389/fneur.2021.656294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toni T, Tamanaha R, Newman B, et al. Effectiveness of dual migraine therapy with CGRP inhibitors and onabotulinumtoxinA injections: case series. Neurol Sci. 2021;42(12):5373–5376. doi: 10.1007/s10072-021-05547-x [DOI] [PubMed] [Google Scholar]
- 98.Clemow DB, Johnson KW, Hochstetler HM, Ossipov MH, Hake AM, Blumenfeld AM. Lasmiditan mechanism of action - review of a selective 5-HT(1F) agonist. J Headache Pain. 2020;21(1):71. doi: 10.1186/s10194-020-01132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.NICE guidelines.Management of migraine (with or without aura); 2021; https://www.nice.org.uk/guidance/conditions-and-diseases/neurological-conditions/headaches.
- 100.Schwedt T, Reuter U, Tepper S, et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain. 2018;19(1):92. doi: 10.1186/s10194-018-0923-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ziegeler C, Mehnert J, Asmussen K, May A. Central effects of erenumab in migraine patients: an event-related functional imaging study. Neurology. 2020;95(20):e2794–e802. doi: 10.1212/WNL.0000000000010740 [DOI] [PubMed] [Google Scholar]