Abstract
Purpose
Cardiorespiratory fitness (CRF) plays an essential role in health outcomes and quality of life. However, it is often not assessed nor estimated. Objective CRF assessment is costly, labour intensive and not widely available. Patient-reported outcome measures estimate CRF more cost-efficiently, but current questionnaires lack accuracy. The aim of this study is to develop a new self-reported questionnaire to estimate CRF.
Materials and Methods
The FitMáx©-questionnaire, consisting of only three questions assessing walking, stair climbing, and cycling capacity, was compared with the commonly used Duke Activity Status Index (DASI) and Veterans Specific Activity Questionnaire (VSAQ). These questionnaires were compared to peak oxygen uptake (VO2peak) as measured with cardiopulmonary exercise testing. This study included 759 cardiac, pulmonary and oncologic patients and healthy persons aged 18‒90.
Results
FitMáx© strongly correlated (r = 0.94 (0.92‒0.95) SEE = 4.14 mL∙kg−1∙min−1) with measured VO2peak. Bias between predicted and measured VO2peak was −0.24 (−9.23‒8.75; 95% limits of agreement) mL·kg−1·min−1. The FitMáx© scored superiorly on correlation and SEE compared with the DASI and VSAQ, r = 0.75 (0.68‒0.80) SEE = 4.62 mL∙kg−1∙min−1 and r = 0.87 (0.83‒0.90) SEE = 6.75 mL∙kg−1∙min−1, respectively.
Conclusion
FitMáx© is a valid and accessible questionnaire to estimate CRF expressed as VO2peak in clinical practice and shows substantial improvement compared to currently used questionnaires.
Keywords: cardiorespiratory fitness, sports medicine, rehabilitation, self-reported questionnaire, cardiopulmonary exercise testing
Introduction
Cardiorespiratory fitness (CRF), commonly defined as peak oxygen uptake (VO2peak), is considered a vital sign, and holds an essential role in health outcomes and quality of life.1–3 Low CRF is associated with all-cause mortality.4–8 Enhancement of CRF leads to improvements in quality of life, and diminishes disease-related symptoms. Increasing VO2peak by only 3.5 mL∙kg−1∙min−1 has been associated with a substantial 8‒35% survival benefit in various populations.1,2,9 Given the importance of CRF in healthcare, and the fact that it is often amenable for improvement with exercise training, surprisingly it is often not assessed, nor estimated in most clinical situations.
Cardiorespiratory fitness can be objectively determined with cardiopulmonary exercise testing (CPET) in which the maximum amount of energy obtained by aerobic metabolism, or VO2peak is assessed.10–13 Clinical CPET is increasingly used to diagnose heart and lung diseases, since it also determines the cause(s) of exercise limitation. In many clinical cases, assessing and monitoring the exercise capacity of diseased patients (during treatment), is more important than to quantify the exact exercise limitation.14 Unfortunately, CPET is a costly, labour intensive, and not widely available test which leads to limited applicability.15,16
An alternative way to assess CRF is use of self-reported questionnaires.14 The Duke Activity Status Index (DASI) and Veterans Specific Activity Questionnaire (VSAQ) are such questionnaires.17,18 The DASI, reached a correlation of r=0.81 with VO2peak measured by CPET when taken by healthcare providers. In case of self-report however, DASI reached a correlation of r=0.58 in the same study. The standard error of the estimate (SEE) for the DASI was not reported.17 The VSAQ estimates the metabolic equivalent of a task (MET) and reached a correlation of r=0.82 with maximal MET achieved on CPET with a SEE of 1.43 MET (5.0 mL·kg−1·min−1). A major drawback of the VSAQ is the use of activities, such as basketball and skiing, which are not practiced globally.19
The capacity to perform daily activities like walking, stair climbing and bicycling are directly related to CRF.20–25 We developed the FitMáx© questionnaire, hereafter written as FitMáx, based upon these three activities to give a more accurate estimate of CRF, which is widely applicable in healthy persons and patients. The aim of this study is to validate the FitMáx as self-reported questionnaire to estimate CRF.
Materials and Methods
Setting
Data was collected prospectively from March 2018 until March 2020 in Máxima Medical Center (Máxima MC). This is a large Dutch non-academic teaching hospital with expertise in sports medicine and exercise physiology embedded in healthcare for cardiac, pulmonary and oncologic patients.26–29 Approximately 20 CPETs are performed every week for diagnostic or scientific purposes, and as part of (p)rehabilitation programmes. The current study complied with the Declaration of Helsinki and the authorized Medical Research Ethics Committee of Máxima MC issued a “non-WMO acknowledgement” (reference number N18.051).30 The study was registered as NL8568 in the Netherlands Trial Register.
Study Population
Before data collection started, the FitMáx was tested and improved in a pilot study (n=20) via cognitive walkthrough. After this pilot study, minor adjustments were made and FitMáx was applied as self-reported questionnaire in the current study. Subjects aged ≥18 years, scheduled for CPET for Medical reasons or as part of a health check, were asked to participate on the day of the appointment prior to CPET. They received the study information letter, informed consent form and the FitMáx, VSAQ and DASI questionnaires. Since CRF can change over time, we have chosen to include subjects in the current study only if documents were completed within 6 weeks (<42 days) prior to or after performing CPET. Maximal exercise effort was considered to be achieved if one of the following criteria was met i) a breathing reserve <15% (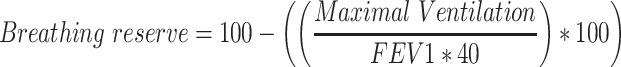 ) ii) percentage of age related predicted maximal heart rate and age related Respiratory Exchange Ratio (RER) or iii) arterialized lactate level at maximal exercise >4 mmol/L.10,31–34 Subjects were excluded when CPET was terminated submaximal by the physician due to eg uncontrolled arrhythmia or syncope, or if the FitMáx was incomplete. All participants have given written informed consent to inclusion of CPET and questionnaire data, which were fully anonymized.
) ii) percentage of age related predicted maximal heart rate and age related Respiratory Exchange Ratio (RER) or iii) arterialized lactate level at maximal exercise >4 mmol/L.10,31–34 Subjects were excluded when CPET was terminated submaximal by the physician due to eg uncontrolled arrhythmia or syncope, or if the FitMáx was incomplete. All participants have given written informed consent to inclusion of CPET and questionnaire data, which were fully anonymized.
Cardiopulmonary Exercise Testing
In this study cycle ergometer CPET was used to measure VO2peak, which was defined as the averaged peak oxygen uptake in the last 30 seconds of CPET. The VO2peak is often used interchangeably with maximum oxygen uptake (VO2max), however to determine VO2max, a plateau in VO2 uptake despite increasing work load is necessary.35 In clinical practice, this plateau is often not reached, which makes VO2peak the preferred measure to express CRF.36,37 According to previous studies, cycle ergometer CPET is likely to underestimate VO2peak compared to treadmill CPET.38,39 However, for safety concerns, to prevent measurement errors and because of the clinical infrastructure in the Máxima MC, we used cycle ergometer CPET as gold standard. All CPETs were supervised by sports physicians or a human movement scientist, and conducted according to international standards.40–42 Prior to CPET, maximum attainable workload (in Watts) was estimated based on patient characteristics and subjective physical exercise capacity. Based on estimated maximum workload, a ramp protocol was used in which the subject was expected to reach maximum load within approximately 8–12 minutes.41,42 The person who supervised CPET was blinded for the results of the questionnaires which, if not blinded, could have biased the choice of exercise protocol. The tests were performed in a temperature-humidity controlled room. A 12-lead electrocardiogram was continuously recorded during rest, warm-up, exercise, and at least three minutes after maximal exercise (suction electrode KISS, GE Healthcare, Chicago, USA or Custo, CustoMed, GmbH, Ottobrunn, Germany). Gas exchange variables were measured breath-by-breath (Vyntus CPX, CareFusion, Hochberg, Germany or MetaLyzer 3B, Cortex, Leipzig, Germany). The metabolic cart was calibrated prior to testing by using a known gas concentration and automatic volume calibration.
Questionnaires
The FitMáx consists of three single-answer questions about the maximum capacity of daily life activities that are all frequently performed by the general Dutch population. A strength of this questionnaire is the use of three different activities in increasing intensity. This enables subjects to estimate their capacity on each activity separately, instead of using several activities in one question. Maximum walking capacity was chosen as a measure of CRF, since the distance walked during a six-minute walk test is strongly associated with VO2peak in patients with severely reduced functional capacity.20,21 Maximum stair climbing capacity was chosen because previous studies indicate that the risk of perioperative pulmonary complications can be estimated with a stair climbing test.22–24 Lastly, maximum cycling capacity was used, since Dutch people often cycle in daily life and exercise testing is often performed on cycle ergometers to measure CRF.25 We tried to draft distinguishable answer options that are unequivocal. The final FitMáx consists of a 1‒14 scale for walking, 1–11 scale for stair climbing and 1–12 scale for cycling. The Dutch version of the FitMáx was translated into English, using forward backward translation according to the procedure described in the guidelines for cross-cultural adaptation.43 The FitMáx is available in the Supplementary File.
To assess the ability of subjects to complete the FitMáx, three extra questions with a scale 1‒10 were used for walking, stair climbing and cycling capacity separately, in which 1 indicates “I cannot estimate properly” and 10 indicates “I can estimate properly”. These questions were added in a later phase of the study, and therefore results of n=243 participants are described.
Beside the FitMáx, subjects were asked to complete the VSAQ from the beginning of the study. The VSAQ was developed to estimate the maximal MET value in American veterans, which was then used in a formula to estimate the CRF expressed in VO2peak.18 To expand the comparison with other physical fitness questionnaires, we also added the DASI to the validation study in April 2019. The DASI was developed to estimate aerobic capacity in cardiovascular patients and used to monitor rehabilitation progress.17 To enable direct comparison, results of all questionnaires were converted into VO2peak in mL·kg−1·min−1, following the guidelines of these questionnaires.17,18
Statistical Analysis
To evaluate the criterion validity of the FitMáx, cycle ergometer CPET was used as gold standard measure for CRF. Additionally, FitMáx was compared with DASI and VSAQ in the same population to evaluate the construct validity. To determine the internal consistency of the FitMáx we calculated the Cronbach’s Alpha.44 Normality of the study data was assessed by visually evaluating histograms and calculation of skewness and kurtosis values.45 Descriptive statistics for subjects’ characteristics are reported as mean and SD in case of normal distribution, and median and interquartile range (IQR) otherwise. For continuous variables, unpaired Student’s t-tests were used to evaluate differences between groups. If the assumption of a normal distribution was not met, the Wilcoxon rank sum test was used instead. The Chi-squared test was used for categorical variables.
For the estimation of CRF via the FitMáx scores, linear regression was chosen after exploratory data analysis. The steps of the FitMáx are ordered by definition. For the analysis, each step of the FitMáx was replaced by the mean VO2peak of all values from subjects’ self-reported scores. These values were then used as regression variable in the model, together with significantly associated dependent variables. To avoid overfitting, we used the sample function in R to randomly create two subgroups: 70% of the subjects as training set and the remaining 30% as testing set.46 The training set was used to select the best-fitting linear regression model. We used stepwise regression to retain the variables that are most relevant for the prediction of CRF. We performed stepwise selection with 10-fold cross-validation with 100 repeats, retaining 20% of the data at each loop for validation. The residuals of the chosen model were examined on bias and heteroscedasticity (studentized Breusch-Pagan (Koenker-Bassett) test).47,48
The final model was cross-validated on the testing set. Since no outliers were identified, the Pearson correlation coefficient (r) was used to evaluate the linear relationship between measured VO2peak and VO2peak estimated by FitMáx, VSAQ and DASI.49 Additionally, the coefficient of determination (R2) and SEE were calculated. While the correlation is not able to assess absolute agreement, the Intraclass Correlation Coefficient (ICC) was calculated.50 Bland Altman plots were used to determine whether mean differences between estimated and measured VO2peak, with corresponding limits of agreement (LoA), are dependent on the size of the measured VO2peak values.
The same methods were used to estimate the VO2peak from the three FitMáx questions separately, and also from the FitMáx with walking and stair climbing only. All computations were implemented in R (R-version 4.0).46 A p-value of <0.05 was considered statistically significant.
Results
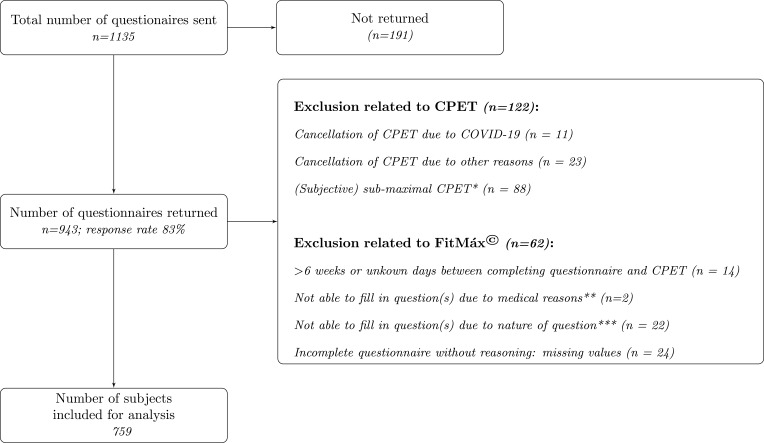
A total of 759 subjects (560 men and 199 women) who performed CPET and completed the FitMáx were included for analysis (see Figure 1). From the study population, 159 participants performed CPET as part of a health check and 600 participants for medical reasons. Since the DASI was added in a later phase of the study, it was received by 581 subjects, and completed by 517 subjects. The training set consisted of 531 subjects and the testing set of 228 subjects.
Figure 1.
Flow diagram of participant selection. * Subjects with evidently submaximal performance on the CPET (ie not achieving volitional maximal effort), due to medical contraindications for maximal testing or measurement errors; ** Medical reasons were restrictions given by cardiologist and use of stair lift; *** eg never cycled before, use of electric bike.
Abbreviations: CPET, cardiopulmonary exercise testing; COVID-19, coronavirus disease 2019; IC, informed consent.
In the testing set, subjects’ age ranged from 19–90 years with a VO2peak from 9.6‒71.4 mL∙kg−1∙min−1, whereas in the training group the subjects’ age ranged from 18–88 years with a VO2peak from 7.5‒67.2 mL∙kg−1∙min−1. Variables relevant to interpret CPET results include height, bodyweight, lung function, Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, use of beta-blockers and reason for CPET. No significant differences were found between testing and training set (Table 1). The patients’ ability to complete the FitMáx on a scale from 1–10, showed a median of 7 (IQR 8–9) for all three questions.
Table 1.
Participant Characteristics in the Training and Testing Set, Displayed Separately
| Variable | Training Set (70%; n=531) | Testing Set (30%; n=228) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| n | 390 | 141 | 170 | 58 |
| Anthropometric data | ||||
| Age (yr) | 59 (49‒68) | 57 (46‒69) | 60 (49‒67) | 61 (49‒71) |
| Height (cm) | 180 (175‒184) | 165 (160‒172) | 180 (174‒184) | 164 (160‒168) |
| Weight (kg) | 82.4 (74.4‒91.1) | 70.4 (61.8‒82.3) | 82.7 (75.0‒89.9) | 67.2 (60.5‒75.9) |
| BMI (kg∙m−2), | 25.4 (23.6‒28.1) | 25.8 (22.3‒29.8) | 25.4 (23.5‒27.8) | 25.0 (22.7‒27.9) |
| FEV1 (L) | 3.6 (2.9‒4.3) | 2.5 (1.8‒3.0) | 3.6 (2.9‒4.3) | 2.4 (1.7‒3.1) |
| FVC (L) | 4.7 (3.9‒5.4) | 3.1 (2.5‒3.8) | 4.7 (3.8‒5.5) | 3.0 (2.4‒3.8) |
| COPD, GOLD classification | ||||
| None | 355 (91.0%) | 121 (85.8%) | 158 (92.9%) | 49 (84.5%) |
| GOLD I | 10 (2.6%) | 1 (0.7%) | 2 (1.2%) | 7 (12.1%) |
| GOLD II | 17 (4.4%) | 13 (9.2%) | 5 (2.9%) | 2 (3.4%) |
| GOLD III | 5 (1.3%) | 5 (3.5%) | 5 (2.9%) | 0 (0%) |
| GOLD IV | 3 (0.8%) | 1 (0.7%) | 0 (0%) | 0 (0%) |
| Use of β-blocker | ||||
| Yes, n (%) | 86 (22.1%) | 31 (22.0%) | 34 (20.0%) | 6 (10.3%) |
| No, n (%) | 304 (77.9%) | 110 (78.0%) | 136 (80.0%) | 52 (89.7%) |
| CPET data | ||||
| Reason CPET/Department | ||||
| Health check, n (%) | 88 (22.6%) | 15 (10.6%) | 45 (26.5%) | 11 (19.0%) |
| Cardiac, n (%) | 208 (53.3%) | 43 (30.5%) | 84 (49.4%) | 7 (12.1%) |
| Pulmonary, n (%) | 84 (21.5%) | 71 (50.4%) | 34 (20.0%) | 34 (58.6%) |
| Oncologic, n (%) | 8 (2.1%) | 10 (7.1%) | 7 (4.1%) | 6 (10.3%) |
| Other reason, n (%) | 2 (0.5%) | 2 (1.4%) | 0 (0%) | 0 (0%) |
| Maximal workload (W) | 266 (161‒347) | 123 (79‒180) | 251 (130‒350) | 113 (76‒161) |
| Exercise time (min) | 9.4 (8.5‒10.3) | 9.0 (7.8–10.5) | 9.7 (8.2‒10.6) | 9.6 (8.1‒10.8) |
| VO2peak (mL∙kg−1∙min−1) | 34.6 (21.9‒43.3) | 20.8 (16.3‒27.9) | 32.3 (20.0‒43.3) | 22.0 (17.1‒26.7) |
| VO2peak reference* (mL∙kg−1∙min−1) | 33.1 (29.2‒37.4) | 22.5 (17.7‒27.9) | 33.8 (28.3‒38.3) | 21.6 (18.4‒27.6) |
| HRpeak (beat∙min−1) | 158 (139‒172) | 151 (129‒171) | 162 (138‒176) | 160 (137‒171) |
| RER (VCO2/VO2) | 1.2 (1.1‒1.2) | 1.1 (1.1‒1.2) | 1.2 (1.1‒1.2) | 1.1 (1.1‒1.2) |
| Questionnaire data | ||||
| VO2peak DASI (mL∙kg−1∙min−1)*** | 34.6 (25.4‒34.6)** | 25.0 (20.2‒33.8) | 34.6 (24.5‒34.6)** | 28.0 (20.1‒34.6) |
| VO2peak VSAQ (mL∙kg−1∙min−1) | 31.3 (17.6‒39.3) | 18.6 (12.6‒25.7) | 33.5 (16.2‒40.3) | 16.9 (13.6‒23.1) |
| VO2peak FitMáx (mL∙kg−1∙min−1) | 34.3 (21.7‒43.6) | 21.6 (17.9‒26.8) | ||
| Δ Time CPET and questionnaire (days) | 1 (0‒8) | 0 (0‒7) | 1 (0‒6) | 1 (0‒5) |
Notes: Results are displayed as n (%) or median (IQR). No statistical significant differences were found between the testing and training set. Missing information, number of subjects: FEV1, 9; FVC, 9; Estimated VO2peak DASI, 242; VSAQ, 6 (in training set). *The prediction model for VO2peak of the Fitness Registry and Importance of Exercise National Database (FRIENDS) is recommended by the American College of Sports Medicine as international reference value.9,39 **DASI shows a ceiling effect in the study results. ***DASI results were collected in a subset of the total study population (n=517).
Abbreviations: cm, centimeters; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FRIENDS, Fitness Registry and Importance of Exercise National Database; FVC, forced vital capacity; HR, heart rate; kg, kilograms; kg∙m−2, kilograms per square meter; L, liters; min, minutes; mL, milliliters; n, number of subjects; yr, years; W, watts.
Development Prediction Model
Age (whole years), sex and BMI (rounded to two decimals) proved to be significantly associated with VO2peak and were included in the final model, together with the weighted scores of the FitMáx (p<0.05) (see Table 2). Homoscedasticity was not rejected by the studentized Breusch-Pagan (Koenker-Basset) test (p-value=0.76). The Cronbach’s Alpha of the three FitMáx questions was 0.93 (0.93‒0.94) in the total study sample.
Table 2.
Linear Model FitMáx Model Fit. Standard Error, t-Values and p-values are Reported for All Variables Included in the Model
| Term | Standard Error | Coefficient t-Value | p-value |
|---|---|---|---|
| (Intercept) | 3.559 | 3.989 | <0.001 |
| Sex | 0.508 | −2.704 | 0.007 |
| Age | 0.045 | −0.943 | 0.346 |
| BMI | 0.052 | −6.945 | <0.001 |
| Walk | 0.085 | 4.681 | <0.001 |
| Stair climbing | 0.040 | 6.123 | <0.001 |
| Cycling | 0.040 | 12.548 | <0.001 |
| Walking*Age | 0.001 | −2.788 | 0.006 |
Note: * Sex is 0 for male and 1 for female, age in whole years and BMI in kg∙m−2 with two decimals.
Abbreviations: Walk, maximum walking capacity score of the FitMáx (1–14); stair climbing, maximum stair climbing capacity score of the FitMáx (1–11); cycling, maximum cycling capacity score of the FitMáx (1–12).
Validation Prediction Model
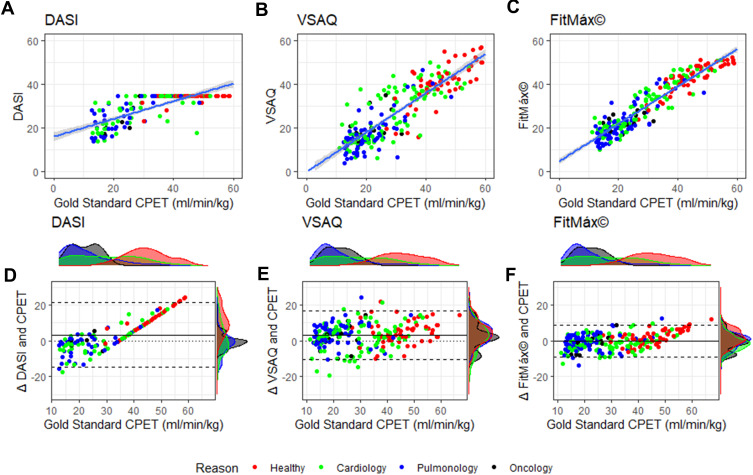
Correlation of VO2peak estimated by FitMáx with measured VO2peak was higher r=0.94 (0.92‒0.95) than the correlation of DASI r=0.75 (0.68‒0.80) and VSAQ r=0.87 (0.83‒0.90) (Figure 2A–C). Moreover, SEE and bias with LoA were smaller for the FitMáx and the R2 and ICC were higher compared to the same values for DASI and VSAQ corrected for the smaller complete subset of the DASI (Table 3). Bias of the FitMáx was −0.24 mL·kg−1·min−1, which is smaller than the DASI (3.32 mL·kg−1·min−1) and VSAQ (3.44 mL·kg−1·min−1). Also, results of predicting VO2peak with the three FitMáx questions separately, and the combination of the walking and stair climbing capacity are presented in Table 3. The estimated VO2peak based on walking and stair climbing capacity reached a correlation of r=0.90 (0.88‒0.92) with VO2peak measured by CPET. Although the values of correlation are comparable, SEE and LoA of the FitMáx including all three questions are smaller than the combination of walking and stair climbing only.
Figure 2.
(A–C) Scatterplots with identity line (ie perfect prediction) for FitMáx, DASI and VSAQ; (D–F) Bland Altman plots for DASI, VSAQ and FitMáx, above and on the right side of the axis histograms are plotted per reason of the CPET. The colors indicate the reason of the CPET visit. The dashed lines represent the limits of agreement, from −1.96 SD to +1.96 SD. The solid line represents bias and the dotted line represents the zero bias line.
Abbreviations: CPET, cardiopulmonary exercise testing; DASI, Duke Activity Status Index; min, minutes; mL, milliliters; kg, kilograms; VSAQ, Veterans Specific Activity Questionnaire.
Table 3.
Statistics Validation of the Prediction Model Including Walking, Stair Climbing and Cycling Capacity Separately
| Model | n | Bias (lb ‒ ub) | r (lb ‒ ub) | R2 | SEE | ICC (lb – ub) |
|---|---|---|---|---|---|---|
| LM FitMáx | 228 | −0.24 (−9.23–8.75) | 0.94 (0.92–0.95) | 0.88 | 4.14 | 0.93 (0.91–0.95) |
| LM walk | 228 | −0.19 (−12.37–12.00) | 0.88 (0.85–0.91) | 0.78 | 5.40 | 0.87 (0.84–0.90) |
| LM stair climb | 228 | −0.66 (−12.41–11.08) | 0.89 (0.86–0.92) | 0.79 | 5.25 | 0.88 (0.85–0.91) |
| LM cycle | 228 | −0.14 (−9.86–9.57) | 0.93 (0.91–0.94) | 0.86 | 4.49 | 0.92 (0.90–0.94) |
| LM walk + stair climb | 228 | −0.42 (−11.55–10.71) | 0.90 (0.88–0.92) | 0.82 | 5.05 | 0.90 (0.87–0.92) |
| VSAQ | 228 | 3.44 (−10.11–16.98) | 0.87 (0.83–0.90) | 0.75 | 6.75 | 0.87 (0.83–0.90) |
| LM FitMáx* | 150 | −0.32 (−9.13–8.48) | 0.94 (0.92–0.95) | 0.88 | 4.01 | 0.94 (0.91–0.95) |
| DASI | 150 | 3.32 (−14.81–21.44) | 0.75 (0.68–0.80) | 0.56 | 4.62 | 0.62 (0.51–0.71) |
Note: *Corrected for missing values of the DASI in the testing set.
Abbreviations: LM, linear model; lb, lower bound; ub, upper bound.
Bland Altman analysis shows the agreement between measured and estimated VO2peak by the FitMáx (LoA −9.23–8.75) to be independent of the VO2peak value measured by CPET and superior to DASI and VSAQ (Figure 2D–F). Density plots per indication of CPET are displayed above and on the right side of the axis. The density plots on the y-axis of the FitMáx indicate that most of the results from the subjects are within the 95% LoA.
Discussion
Cardiorespiratory fitness (CRF) is a paramount aspect of health, but currently often overlooked in clinical medicine.2–6 We developed the FitMáx containing only three single-answer questions to estimate CRF. The VO2peak estimated by the FitMáx showed a strong correlation (r=0.94) and an almost perfect agreement (ICC=0.93) with VO2peak objectively measured by CPET. The FitMáx showed a SEE=4.14 mL·kg−1·min−1 in the current study population. The validation was performed in a heterogeneous population of both healthy subjects and patients with variety in age and a wide CRF range. Therefore, FitMáx proves to be applicable in young fit individuals, elderly and patients.
In our study population, FitMáx showed superior results compared with the DASI and VSAQ, which are currently most often used in healthcare to estimate CRF.17,18 The FitMáx shows a good performance in a wide range of CRF and only has a ceiling effect in subjects with a very high CRF, in contrast to DASI.17,51 A possible explanation of these findings could be that activities included in the VSAQ and DASI are more difficult to recognize for the Dutch population, compared to the activities included in the FitMáx.19 Moreover, the FitMáx© enables subjects to rate their capacity for three activities separately on an increasing scale, instead of using several activities in one question. The ability to complete the FitMáx in the current study was rated on a scale from 1–10 with a median and interquartile range of 7 (8–9).
Other Instruments
More recently, the CLINIMEX aerobic fitness questionnaire (C-AFQ) was developed, as a questionnaire-based prediction model for CRF.52 Despite a high correlation with CPET (r=0.91), C-AFQ leaves considerable inaccuracy in the estimation of VO2max (SEE=5.39 mL·kg−1·min−1). Compared to the original C-AFQ study, correlation, SEE and bias with LoA are better for the FitMáx in the current study population.52 Moreover, an interview was used to complete the C-AFQ in the validation study, this could have led to a high correlation (r=0.91). This phenomenon is also seen in the validation of the DASI.17
Bradshaw et al developed a questionnaire-based prediction model, and reached a correlation of r=0.91 with CPET and SEE=3.63 mL·kg−1·min−1 in the original research.53 However, the population in the Research of Bradshaw et al was small (100 participants) and had a relatively good CRF (mean VO2max 39.96 mL·kg−1·min−1 ±9.54).53 This potentially leads to limited applicability in clinical cases, as CRF is often less high in patients.
Finally, the HUNT study developed a prediction model to estimate VO2peak that included age, physical activity, waist circumference, and resting heart rate.54 This approach is currently advised by the American Heart Association.2 However, applicability is limited to settings where such measurements are available. Correlation, SEE and bias reported in the HUNT study were worse than in the current FitMáx study, but results were obtained on study-specific populations only.54 Therefore, to draw conclusions on clinometric properties, the mentioned questionnaires should be compared within the same study population.
Strengths of Current Study
The strength of our study lies in direct comparison of the ability of self-reported questionnaires to estimate VO2peak, with VO2peak objectively measured by CPET, in a diverse population of healthy persons and patients with a wide range of CRF.
Limitations of Current Study
The study population consisted of mainly male subjects (74%). Analyses on sex showed that FitMáx was able to estimate CRF accurately in both men and women. Nevertheless, to enhance the interpretability of the results for female subjects, more data should be collected. No cross-cultural validations of VSAQ and DASI were available in Dutch, so the translation of the questionnaires was done by the researchers. For international use, the question about the maximum cycling capacity may be a limitation in the applicability of the FitMáx. However, estimated VO2peak based on the FitMáx without the maximum cycling capacity still reached a correlation of r=0.90 (0.88‒0.92) with measured VO2peak. Despite the high correlation, the LoA are still relatively large indicating that discrepancies between patients’ self-reported and measured exercise capacity occur.55 Also the FitMáx estimates CRF, but does not diagnose the underlying limitation. FitMáx should not be considered as a full replacement for CPET, but rather a complementary tool to be used in settings where exercise testing is unavailable.
Clinical Relevance of FitMáx
In the current study no physician or healthcare provider was involved in completing the FitMáx. Also DASI and VSAQ were evaluated as strictly self-reported measures for CRF. For the Dutch population, the three questions of FitMáx are easy to relate to, and their sex, age, and BMI are usually known or easy to assess. For healthcare professionals, there is a well-known, single result of the questionnaire: VO2peak. This is clinically applicable as an estimate to stratify the risk of patients with low CRF, who may benefit from more extensive diagnostic exercise testing or to discern the optimal protocol for clinical exercise testing. On the other hand the results of the FitMáx can be used to measure and monitor the effects of an exercise intervention or rehabilitation programme.
Implications for the Future
To enhance clinical applicability of the FitMáx, future studies will focus on the ability to monitor changes in CRF over time and comparison with other clinical exercise tests. This would be especially useful in oncologic patients who participate in rehabilitation programmes or surgical patients in (p)rehabilitation programmes. It may also contribute in the risk assessment before surgery. We also intend to add a question about daily activities to further improve the accuracy and applicability of the FitMáx.
To enable healthcare professionals and researchers in using the FitMáx questionnaire, we have developed an online platform (www.fitmaxquestionnaire.com), where we invite researchers to collaborate with us to further improve and validate the questionnaire. The online platform provides up-to-date information about the questionnaire and research projects. In addition, a technical interface has been developed to implement the FitMáx into third party applications. More information about our research group, hospital and FitMáx can be found on https://www.mmc.nl/english/fitmax/.
Conclusion
Cardiorespiratory fitness is of paramount importance in healthcare, given its substantial relation to survival and quality of life. To tailor treatment and exercise interventions, it is important to measure or estimate CRF. The FitMáx consists of only three questions and is a simple tool to estimate CRF accurately.
Acknowledgments
We would like to thank J. Dieleman, L. Bacas, A. den Bresser and J. Schellekens for their contribution to this research. Moreover, we would like to thanks J. van der Elsen, S. Bell, J. de Koning, T. Buscop and M. van Nieuwburg for helping with the forward backward translation method to provide the questionnaire in English.
Funding Statement
This work was partially funded by the Stichting SOS and by the National Foundation Against Cancer (NFtK).
Abbreviations
BMI, Body Mass Index; C-AFQ, CLINIMEX Aerobic Fitness Questionnaire; COPD, Chronic Obstructive Pulmonary Disease; CPET, Cardiopulmonary Exercise Testing; CRF, Cardiorespiratory Fitness; DASI, Duke Activity Status Index; FRIENDS, Fitness Registry and Importance of Exercise National Database; GOLD, Global initiative for chronic Obstructive Lung Disease; ICC, Intraclass Correlation Coefficient; IQR, Interquartile Range; MET, Metabolic Equivalent of Task; Máxima MC, Máxima Medical Center; r, Pearson correlation coefficient; R2, Coefficient of determination; RER, Respiratory Exchange Ratio; SEE, Standard Error of the Estimate; VO2max, maximum oxygen uptake; VO2peak, peak oxygen uptake; VSAQ, Veterans Specific Activity Questionnaire.
Data Sharing Statement
More information on the data gathered, the analysis used, and calculations used in the model is available upon reasonable request. The use of FitMáx is free when used from the website or when incorporated in studies that help us to further validate the questionnaire in different settings and populations. In addition, there is a commercial licensed model available for usage of the questionnaire in different settings. More information can be found on www.fitmaxquestionnaire.com.
Ethics Approval
The authorized Medical Research Ethics Committee of Máxima Medical Center issued a “non WMO acknowledgement” for this study (reference number N18.051). All subjects provided written and/or oral consent for participation in compliance with the declaration of Helsinki.
Disclosure
Miss Renske Meijer reports grants from Stichting SOS, grants from National Foundation Against Cancer (NFtK), during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. [DOI] [PubMed] [Google Scholar]
- 3.Evaristo S, Moreira C, Lopes L, et al. Muscular fitness and cardiorespiratory fitness are associated with health-related quality of life: results from labmed physical activity study. J Exerc Sci Fit. 2019;17(2):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imboden MT, Harber MP, Whaley MH, et al. Cardiorespiratory fitness and mortality in healthy men and women. J Am Coll Cardiol. 2018;72(19):2283–2292. [DOI] [PubMed] [Google Scholar]
- 5.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. [DOI] [PubMed] [Google Scholar]
- 6.Carbone S, Kim Y, Kachur S, et al. Peak oxygen consumption achieved at the end of cardiac rehabilitation predicts long-term survival in patients with coronary heart disease. Eur Heart J. 2021. doi: 10.1093/ehjqcco/qcab032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewert R, Obst A, Mühle A, et al. Value of cardiopulmonary exercise testing in the prognosis assessment of chronic obstructive pulmonary disease patients: a retrospective, multicentre cohort study. Respiration. 2021; 101(4):353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orimoloye OA, Kambhampati S, Hicks AJ 3rd, et al. Higher cardiorespiratory fitness predicts long-term survival in patients with heart failure and preserved ejection fraction: the Henry Ford Exercise Testing (FIT) Project. Arch Med Sci. 2019;15(2):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross RM. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. [DOI] [PubMed] [Google Scholar]
- 11.Riebe D, Ehrman JK; American College of Sports M. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia: Wolters Kluwer; 2018. English [Google Scholar]
- 12.Mezzani A, Agostoni P, Cohen-Solal A, et al. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009;16(3):249–267. [DOI] [PubMed] [Google Scholar]
- 13.Thompson PD, Arena R, Riebe D, et al. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12(4):215–217. [DOI] [PubMed] [Google Scholar]
- 14.Qiu S, Cai X, Sun Z, et al. Is estimated cardiorespiratory fitness an effective predictor for cardiovascular and all-cause mortality? A meta-analysis. Atherosclerosis. 2021;330:22–28. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier L, Kervio G, Doutreleau S, et al. The medical value and cost-effectiveness of an exercise test for sport preparticipation evaluation in asymptomatic middle-aged white male and female athletes. Arch Cardiovasc Dis. 2017;110(3):149–156. [DOI] [PubMed] [Google Scholar]
- 16.Shah SJ, Rehman A, Shaukat MHS, et al. Cost-effectiveness of exercise stress testing performed as part of executive health examinations. Ir J Med Sci. 2017;186(2):281–284. [DOI] [PubMed] [Google Scholar]
- 17.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64(10):651–654. [DOI] [PubMed] [Google Scholar]
- 18.Myers J, Do D, Herbert W, et al. A nomogram to predict exercise capacity from a specific activity questionnaire and clinical data. Am J Cardiol. 1994;73(8):591–596. [DOI] [PubMed] [Google Scholar]
- 19.Kojima S, Wang DH, Tokumori K, et al. Practicality of veterans specific activity questionnaire in evaluation of exercise capacity of community-dwelling Japanese elderly. Environ Health Prev Med. 2006;11(6):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mänttäri A, Suni J, Sievänen H, et al. Six-minute walk test: a tool for predicting maximal aerobic power (VO2 max) in healthy adults. Clin Physiol Funct Imaging. 2018;38(6):1038–1045. [DOI] [PubMed] [Google Scholar]
- 21.Dourado VZ, Nishiaka RK, Simões MSMP, et al. Classification of cardiorespiratory fitness using the six-minute walk test in adults: comparison with cardiopulmonary exercise testing. Pulmonology. 2021;27(6):500–508. [DOI] [PubMed] [Google Scholar]
- 22.Biccard BM. Relationship between the inability to climb two flights of stairs and outcome after major non-cardiac surgery: implications for the pre-operative assessment of functional capacity. Anaesthesia. 2005;60(6):588–593. [DOI] [PubMed] [Google Scholar]
- 23.Girish M, Trayner E Jr, Dammann O, et al. Symptom-limited stair climbing as a predictor of postoperative cardiopulmonary complications after high-risk surgery. Chest. 2001;120(4):1147–1151. [DOI] [PubMed] [Google Scholar]
- 24.Salahuddin N, Fatimi S, Salahuddin N, et al. Predicting postoperative cardio-pulmonary complications by a test of stair climbing. J Coll Physicians Surg Pak. 2005;15(12):761–764. [PubMed] [Google Scholar]
- 25.Smith TB, Stonell C, Purkayastha S, et al. Cardiopulmonary exercise testing as a risk assessment method in non cardio-pulmonary surgery: a systematic review. Anaesthesia. 2009;64(8):883–893. [DOI] [PubMed] [Google Scholar]
- 26.Kemps HM, Thijssen EJ, Schep G, et al. Evaluation of two methods for continuous cardiac output assessment during exercise in chronic heart failure patients. J Appl Physiol. 2008;105(6):1822–1829. [DOI] [PubMed] [Google Scholar]
- 27.Sleutjes BT, Kemps HM, Thijssen EJ, et al. The reliability of continuous measurement of mixed venous oxygen saturation during exercise in patients with chronic heart failure. Eur J Appl Physiol. 2008;102(4):493–496. [DOI] [PubMed] [Google Scholar]
- 28.van Wetering CR, Hoogendoorn M, Mol SJ, et al. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax. 2010;65(1):7–13. [DOI] [PubMed] [Google Scholar]
- 29.van Rooijen S, Carli F, Dalton S, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rickham PP. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. Br Med J. 1964;2(5402):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner J, Niemeyer M, Infanger D, et al. New data-based cutoffs for maximal exercise criteria across the lifespan. Med Sci Sports Exerc. 2020;52(9):1915–1923. [DOI] [PubMed] [Google Scholar]
- 32.Keteyian SJ, Kitzman D, Zannad F, et al. Predicting maximal HR in heart failure patients on β-blockade therapy. Med Sci Sports Exerc. 2012;44(3):371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brawner CA, Ehrman JK, Schairer JR, et al. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am Heart J. 2004;148(5):910–914. [DOI] [PubMed] [Google Scholar]
- 34.Schmid A, Schilter D, Fengels I, et al. Design and validation of an interpretative strategy for cardiopulmonary exercise tests. Respirology. 2007;12(6):916–923. [DOI] [PubMed] [Google Scholar]
- 35.Guazzi EM, Adams V, Conraads V, et al.; Committee W, EACPR. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012;33(23):2917–2927. [DOI] [PubMed] [Google Scholar]
- 36.Takken T, Bongers BC, van Brussel M, et al. Cardiopulmonary exercise testing in pediatrics. Ann Am Thorac Soc. 2017. Jul;14(Supplement_1):S123–s128. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training. Circulation. 2013;128(8):873–934. [DOI] [PubMed] [Google Scholar]
- 38.Mazaheri R, Sadeghian M, Nazarieh M, et al. Performance of heart failure patients with severely reduced ejection fraction during cardiopulmonary exercise testing on treadmill and cycle ergometer; similarities and differences. Int J Environ Res Public Health. 2021;18(24):12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaminsky LA, Arena R, Myers J, et al. Updated reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the Fitness Registry and the Importance of Exercise National Database (FRIEND). Mayo Clinic Proceedings. 2022;97(2):285–293. [DOI] [PubMed] [Google Scholar]
- 40.ERS Task Force on Standardization of Clinical Exercise Testing. European Respiratory Society. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. Eur Respir J. 1997;10(11):2662–2689. [DOI] [PubMed] [Google Scholar]
- 41.Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor FG ACSM’s sports medicine: a comprehensive review. Wolters Kluwer Health; 2012. [Google Scholar]
- 43.Beaton DE, Bombardier C, Guillemin F, et al. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25(24):3186–3191. [DOI] [PubMed] [Google Scholar]
- 44.Taber KS. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48(6):1273–1296. [Google Scholar]
- 45.D’Agostino RB, Belanger A. A suggestion for using powerful and informative tests of normality. Am Stat. 1990;44(4):316–321. [Google Scholar]
- 46.Team RC. R: A Language and Environment for Statistical Computing. 4.0. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 47.Breusch TS, Pagan AR, Simple A. Test for heteroscedasticity and random coefficient variation. Econometrica. 1979;47(5):1287–1294. [Google Scholar]
- 48.Koenker R, Bassett G. Robust tests for heteroscedasticity based on regression quantiles. Econometrica. 1982;50(1):43–61. [Google Scholar]
- 49.Chok NS Pearson’s versus Spearman’s and Kendall’s correlation coefficients for continuous data [Master’s Thesis]: University of Pittsburgh; 2010. [Google Scholar]
- 50.Morgan CJ, Aban I. Methods for evaluating the agreement between diagnostic tests. J Nucl Cardiol. 2016;23(3):511–513. [DOI] [PubMed] [Google Scholar]
- 51.Khan S, Nagarwala R, Shyam A, et al. Validation of Duke activity status index questionnaire to determine functional capacity in young healthy nonexercising individuals. Physiotherapy. 2019;13(1):14–17. [Google Scholar]
- 52.Araújo CGS, Castro CL, Franca JF, et al. CLINIMEX aerobic fitness questionnaire: proposal and validation. Int J Cardiovasc Sci. 2019;32:331–342. [Google Scholar]
- 53.Bradshaw DI, George JD, Hyde A, et al. An accurate VO2max nonexercise regression model for 18–65-year-old adults. Res Q Exerc Sport. 2005;76(4):426–432. [DOI] [PubMed] [Google Scholar]
- 54.Nes BM, Janszky I, Vatten LJ, et al. Estimating V·O 2peak from a nonexercise prediction model: the HUNT Study, Norway. Med Sci Sports Exerc. 2011;43(11):2024–2030. [DOI] [PubMed] [Google Scholar]
- 55.Stokes JW, Wanderer JP, McEvoy MD. Significant discrepancies exist between clinician assessment and patient self-assessment of functional capacity by validated scoring tools during preoperative evaluation. Perioper Med. 2016;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]