Abstract
Traumatic brain injury has been described as the signature affliction of recent military conflicts and repetitive TBIs, particularly associated with military and athletic activities, typically result in more severe clinical effects. The majority of TBIs are mild, but they can result in long term cognitive deficits for which there is no effective treatment. One of the most significant deficits observed in TBI patients is memory loss, which suggests that TBI can induce pathological changes within the hippocampus. tert-butylhydroquinone (tBHQ) and pioglitazone activate the Nrf2 and PPAR-γ transcription factors, respectively, and both have been shown to be neuroprotective in model systems. We examined the morphological changes within the hippocampus following repetitive mild TBI and simultaneous treatment with both factors. We utilized a closed head injury mouse model with five injuries over 5 weeks. Our results showed marked morphological changes among the dendrites and dendritic spines of the neurons of the dentate gyrus of the hippocampus. We observed decreases in overall dendritic length, as well as in the quantity and density of dendritic spines. Our treatment partially ameliorated these effects, suggesting that the Nrf2 and PPAR-γ transcription factors may be important targets for future drug development in the treatment of TBI in humans.
Keywords: Mild traumatic brain injury, Transcription factors, Mouse models, TBHQ, Pioglitazone, Dendritic arbor, Sholl analysis
1. Introduction
Brain trauma represents one of the most significant causes of disability and death in the United States, accounting for approximately 30% of all injury-related deaths (Faul et al., 2010). Approximately 2.5 million TBI-related hospital visits occur each year in the United States (Peterson et al., 2015). Military personnel, in particular, are at an increased risk. In fact, among US military forces alone, there have been over 384,000 brain injuries sustained since 2000 (Defense and Veterans Brain Injury Center, 2018). Along with military personnel, athletes are at a particular risk for repeated injuries, which has gained increasing public interest in recent years (Hoge et al., 2008; Powell and Barber-Foss, 1999). Mild TBIs are most common, however, up to 10% of mild TBI patients can suffer long-term complications for which there is no effective treatment (Gardner and Yaffe, 2015; Iverson, 2005; McCrea et al., 2003; Schneiderman et al., 2008; Terrio et al., 2009).
The development of a treatment to protect the brain from secondary damage caused by the neuroinflammation and oxidative stress, which follows the initial mechanical injury, could involve targeting transcription factor signaling. Activation of Peroxisome proliferator-activated receptor-gamma (PPAR-γ) by its agonist, oral insulin drug pioglitazone, has been shown to reduce neuroinflammation and neuronal damage following brain injury (Deng et al., 2020; Liu et al., 2017; Sauerbeck et al., 2011). Even a single post-injury dose of pioglitazone can reduce cortical oxidative damage and microglial responses (Pilipovic et al., 2015). Another transcription factor of interest is Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which is activated by tertbutylhydroquinone (tBHQ), a common food additive. We have shown previously that activation of the Nrf2 pathway with tBHQ following brain injury leads to improved cognitive performance in mouse models (Saykally et al., 2012). While both of these compounds have been shown to be neuroprotective individually, simultaneous activation of both pathways may provide a synergistic effect that leads to further improved outcomes following brain injury. We have shown previously that a combination treatment of both pioglitazone and tBHQ results in improved recognition memory following repeated closed head injuries in mice (Ratliff et al., 2020b).
Among the long-term complications of mild TBI, perhaps the most pervasive is memory impairment (Nicholl and LaFrance, 2009; Rutland-Brown et al., 2006). The hippocampus plays an integral role in the formation of new memories and has been previously shown to be susceptible to mechanical injury which may contribute to the memory impairment observed in TBI patients (Hicks et al., 1993). As such, it is important to carefully investigate changes within the hippocampus following injury and in the search for potential neuroprotective treatments. Our mouse model utilizes a repeated closed head injury to recapitulate the impact forces, pathology, and cognitive deficits observed in human TBI patients (Zohar et al., 2003). This model has been extensively tested and has been shown to cause both molecular changes and changes to the morphology of individual neurons and glial cells (Saykally et al., 2012; Saykally et al., 2018; Tashlykov et al., 2007; Tweedie et al., 2007). In this study, we have investigated the morphological changes of neurons within the hippocampus following repeated injury and the impact of post-injury treatment with tBHQ and pioglitazone. Our results suggest that, in addition to the cognitive improvements reported previously (Ratliff et al., 2020b), simultaneous activation of the Nrf2 and PPAR-γ transcription factors is able to ameliorate some of the potentially harmful morphological changes observed within the hippocampus following injury.
2. Results
2.1. Dendritic arbor analysis
Neurons from mice 8 weeks post-injury or sham were analyzed via Golgi staining. Mice from the TBI group showed significant loss of the dendritic arbor within the dentate gyrus of the hippocampus (Fig. 2A). Total dendritic length was significantly reduced in the TBI group receiving only vehicle treatment, with no significant difference between sham mice and tBHQ plus pioglitazone treated mice (Fig. 2B). Dendritic arbor complexity was analyzed within the dentate gyrus using Sholl Analysis, as previously described (Diamond et al., 2006; Shim et al., 2013; Shingo et al., 2015). Sholl analysis identifies the mean amount and distribution of the dendritic arbor (Sholl, 1953). Concentric circles, referred to as “shells”, are superimposed over the Golgi stained neurons. The radius of the first shell was 30 μm from the cell soma and subsequent shells increased by 30 μm increments up to 240 μm away from the soma. Interactions are measured by the number of times a dendrite intersects with a shell. The number of interactions at 150 μm and 180 μm away from the soma was reduced in mice receiving TBI and vehicle treatment, though this was not statistically significant. No difference was observed between sham animals and those receiving the combination treatment (Fig. 2C).
Fig. 2.

Dendritic Arbor. (A) Neurons of the dentate gyrus of the hippocampus were Golgi stained from mice receiving either a sham injury with vehicle treatment, repeated TBI with vehicle treatment, or repeated TBI with combination treatment. (B) Total dendritic length was analyzed and found to be significantly reduced in TBI vehicle mice when compared to sham (p = 0.01). (C) Sholl analysis was performed to analyze dendritic complexity. TBI vehicle mice showed a slight reduction in dendritic intersections at 150 μm and 180 μm from the soma when compared to sham, however, this was not statistically significant.
3. Dendritic spine analysis
Dendritic Spines of the neurons of the Golgi stained neurons were also analyzed (Fig. 3A). In TBI mice receiving only vehicle, there was a significant reduction in total dendritic spines (Fig. 3B) and dendritic spine density (per μm) (Fig. 3C) among the analyzed neurons of the dentate gyrus of the hippocampus when compared to sham mice. Treated mice had significantly increased total dendritic spines and spine density, compared to vehicle treated mice, but still remained significantly reduced from sham levels. Upon investigation of the location of dendritic spines relative to the soma, it was found that the there was a significant reduction in dendritic spine density in TBI mice receiving vehicle was 90–180 μm away from the soma when compared to sham mice. Treated mice also showed a significant reduction in spine density in this region when compared to sham mice and while there was a slight trend toward increased density over vehicle treated TBI mice, this was not significant (Fig. 3B). Next, spine density was analyzed by branch order. A first-order branch emanates directly from the soma. The point where this branch splits into two branches is the first-order branch point and the resultant branches are second-order branches. The point at which these branches split is the second-order branch point and the new branches are third-order branches. This pattern continues until each branch reaches an endpoint. At branch orders 2 through 6, spine density was significantly reduced in TBI vehicle mice when compared to sham. At branch orders 3 through 5, TBI treated mice showed significantly higher spine density when compared to TBI treated mice. However, Sham mice still had significantly higher spine density than both TBI groups (Fig. 3E).
Fig. 3.
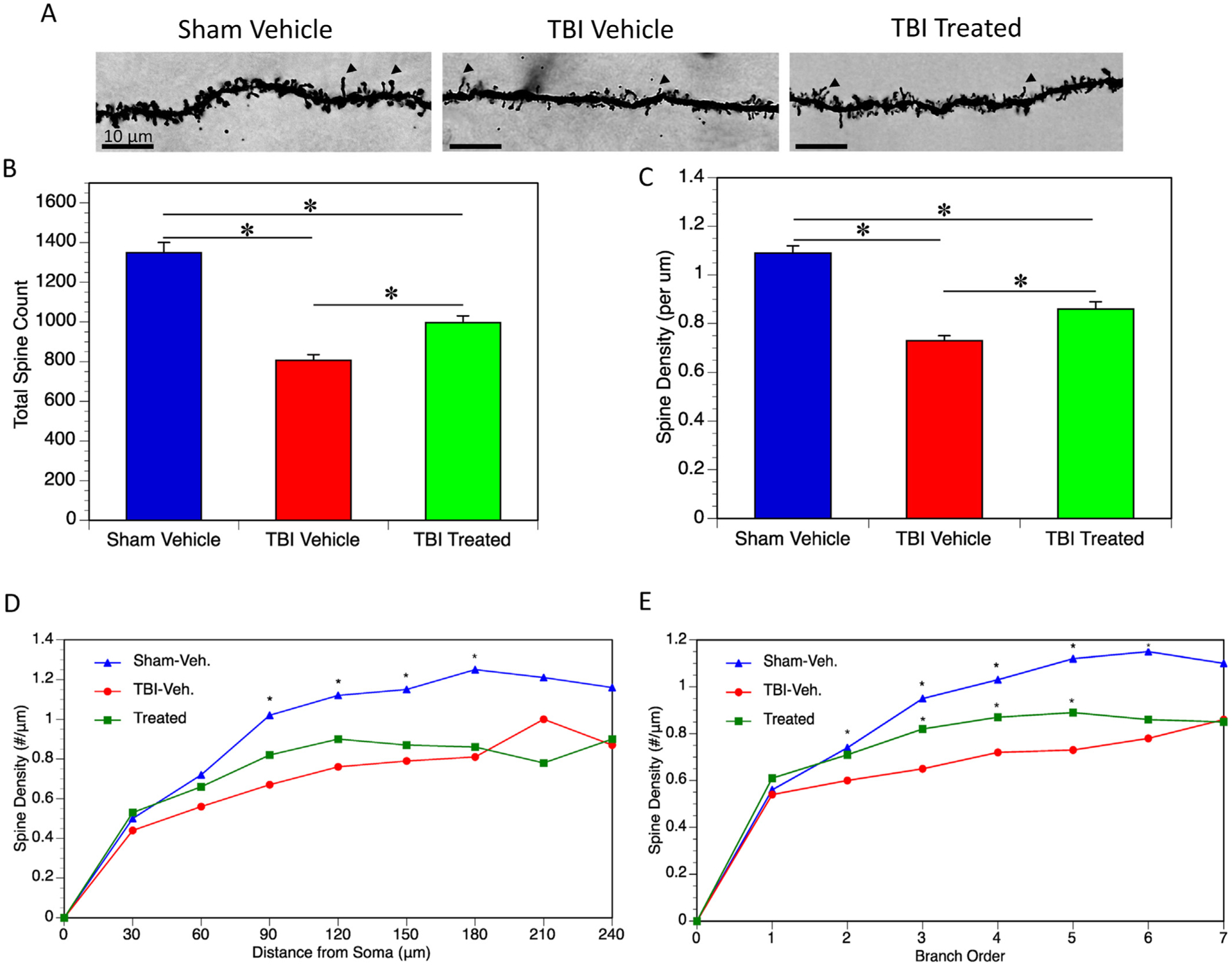
Dendritic Spines. (A) Dendritic spines of Golgi stained neurons of the dentate gyrus of the hippocampus were analyzed. Arrows indicate examples of dendritic spines. (B) Total spines among the 5 neurons analyzed per mouse was quantified. Total spines were significantly reduced in TBI vehicle mice when compared to sham (p < 0.0001). Total spines were increased in TBI treated mice compared to TBI vehicle mice (p = 0.0021), however there was still a significant reduction in total spines when compared to sham (p < 0.0001). (C) The same trend was observed in spine density (per μm). Both TBI groups showed a reduction in spine density compared to sham (both p < 0.0001), while TBI treated mice did have significantly higher spine densities compared to TBI vehicle mice (p = 0.0016). (D) Spine density was quantified based on distance from the soma. TBI vehicle mice had significantly reduced spine density 90–180 μm from the soma when compared to sham mice (p < 0.0001 for all distances). TBI treated mice had a slightly higher trend than TBI vehicle mice, however, this was not statistically significant. (E) Spine density was quantified based on branch order. TBI vehicle mice had significantly reduced spine density at orders 2 through 6 when compared to sham mice (p = 0.046 for the second branch order, p < 0.0001 for the 3rd – 6th branch orders). TBI treated mice showed some increase in spine density over TBI vehicle mice at the 3rd (p = 0.011), 4th (p = 0.029), and 5th (p = 0.018) branch orders.
4. Discussion
Our closed head injury model investigated the morphological changes which occur within the hippocampus following repeated mild injury and the potential protective effects of a combination treatment which activates Nrf2 and PPAR-γ transcription factors simultaneously. Mice received 5 mild injuries, each 1 week apart, followed by treatment via intraperitoneal injection 30 min post-injury. We have shown previously that this experimental paradigm is capable of created marked changes in gene expression, as well as changes in recognition memory, which persist 8 weeks post-injury and are prevented with treatment (Ratliff et al., 2020b). In this study, we see changes in the dendritic arbor and dendritic spines of hippocampal neurons within the ipsilateral dentate gyrus, which also persist 8 weeks post-injury. Treatment with activators of Nrf2 and PPAR-γ appear to partially or totally ameliorate these changes. This is suggestive that our treatment may be effective at protecting against the morphological and subsequent cognitive changes observed following repeated injury.
Dendritic arbor complexity was evaluated by Sholl analysis and spine densities were also measured in the amygdala and the hippocampus after TBI and treatments in rats and mice (Carlson et al., 2014; Hoffman et al., 2017). In rats following 1,7, and 28 days post fluid percussion injury, neuron dendritic complexity proximal to the soma was shown to increase at all post injury time points (Hoffman et al., 2017). In mice, overexpression of pioglitazone responsive insulin-like growth factor (IGF1), was shown to enhance hippocampal neurogenesis and restore arbor complexity after controlled cortical impact (Carlson et al., 2014).
There are similarities in the results of this study using a closed head injury model to other TBI models. In a previous study using a blast TBI model, we observed similar decreases in dendritic length within the hippocampus at 3 days post-injury to what we saw at 8 weeks post-injury (Ratliff et al., 2020a). In a rat controlled cortical impact (CCI) model, reductions in both dendritic length and number of branch points were observed 1 month after injury, which the largest reductions occurring in the hippocampus (Casella et al., 2014). Similar results have been observed 7 days post injury in rats using a fluid percussion model (Thomas et al., 2018). In the current study, we also observed marked reductions in both dendritic spine quantity and density, which were seen in a previous study at 24 h post-injury using CCI (Winston et al., 2013). Another study using CCI observed that reductions in dendritic length, branching, and Sholl intersections were observed within the dentate gyrus and that these morphological changes were accompanied by spatial memory deficits (Semple et al., 2017).
Overall, our results show a decrease in complexity and distribution of the dendritic arbor following injury, which can be partially prevented though activation of the Nrf2 and PPAR-γ transcription factors. These results from our closed head injury model, along with previously observed behavioral data (Ratliff et al., 2020b), may provide an important connection between morphological changes within the brain following TBI and associated behavioral and cognitive changes. Our results may suggest a reduction in connectivity within the hippocampal region. In a previous study, changes in branch point morphology within the hippocampus directly impacted the ability of neurons to generate, propagate, and time action potentials (Ferrante et al., 2013). Losses in neuronal connectivity have been shown to be a major contributor to the symptoms observed after injury (Rafols et al., 2007; Scheff et al., 2005; Semchenko et al., 2006). We have seen decreased complexity and distribution of the dendritic arbor in the hippocampus which suggests a morphological change which may account for the cognitive deficits we’ve observed previously, as well as in brain-injured humans (McDowell et al., 1997; Nicholl and LaFrance, 2009; Ratliff et al., 2020b; Rutland-Brown et al., 2006; Stuss et al., 1985). Our treatment results suggest that the Nrf2 and PPAR-γ transcription factors may represent important targets for the treatment of TBI to prevent long term morphological changes which may ultimately improve the symptoms, such as memory deficits, which have the greatest impact on quality of life for veterans and all TBI patients.
5. Experimental procedure
5.1. Animals
The animal protocol was approved by the Bay Pines VA Institutional Animal Care and Use Committees (IACUC) and performed in accordance with all institutional, agency, and governmental Animal Welfare Regulations.
Male C57BL6/J mice at 5 weeks of age were obtained from Jackson Laboratories (Bar Harbor, ME). They were housed 3–4 per cage in a 22 °C ± 0.5 °C temperature-controlled environment with a 12 h light/dark cycle. Food and water were available ad libitum. All mice were allowed to acclimate to the facility for one week prior to experimentation.
6. Closed head injury
A concussive closed head injury weight drop model, described previously (Zohar et al., 2003), was used to induce a diffuse mild traumatic brain injury (mTBI). Mice were first anesthetized using isoflurane and placed on a soft sponge, which allows for rotation of the head, better replicating the rotational forces of a human brain injury (Milman et al., 2005). A 13 mm tube attached to a solid stand was then placed over the head, spanning the right hemisphere caudal to the eye and rostral to the ear. A 30 g (first four injuries) or 50 g (final injury) cylindrical weight was then dropped 80 cm through the length of the tube to deliver a mild TBI. In sham mice, anesthesia was used and mice were placed onto the sponge, but no weight was dropped. This procedure has been demonstrated to cause a mild injury (Tashlykov et al., 2007), and was repeated five times with each injury occurring one week apart (Fig. 1). Mice were monitored in a separate chamber while recovering from anesthesia before being returned to regular housing. No noticeable differences in recovery were noted between groups or over the course of the experiment. There were 8 mice per experimental group.
Fig. 1.
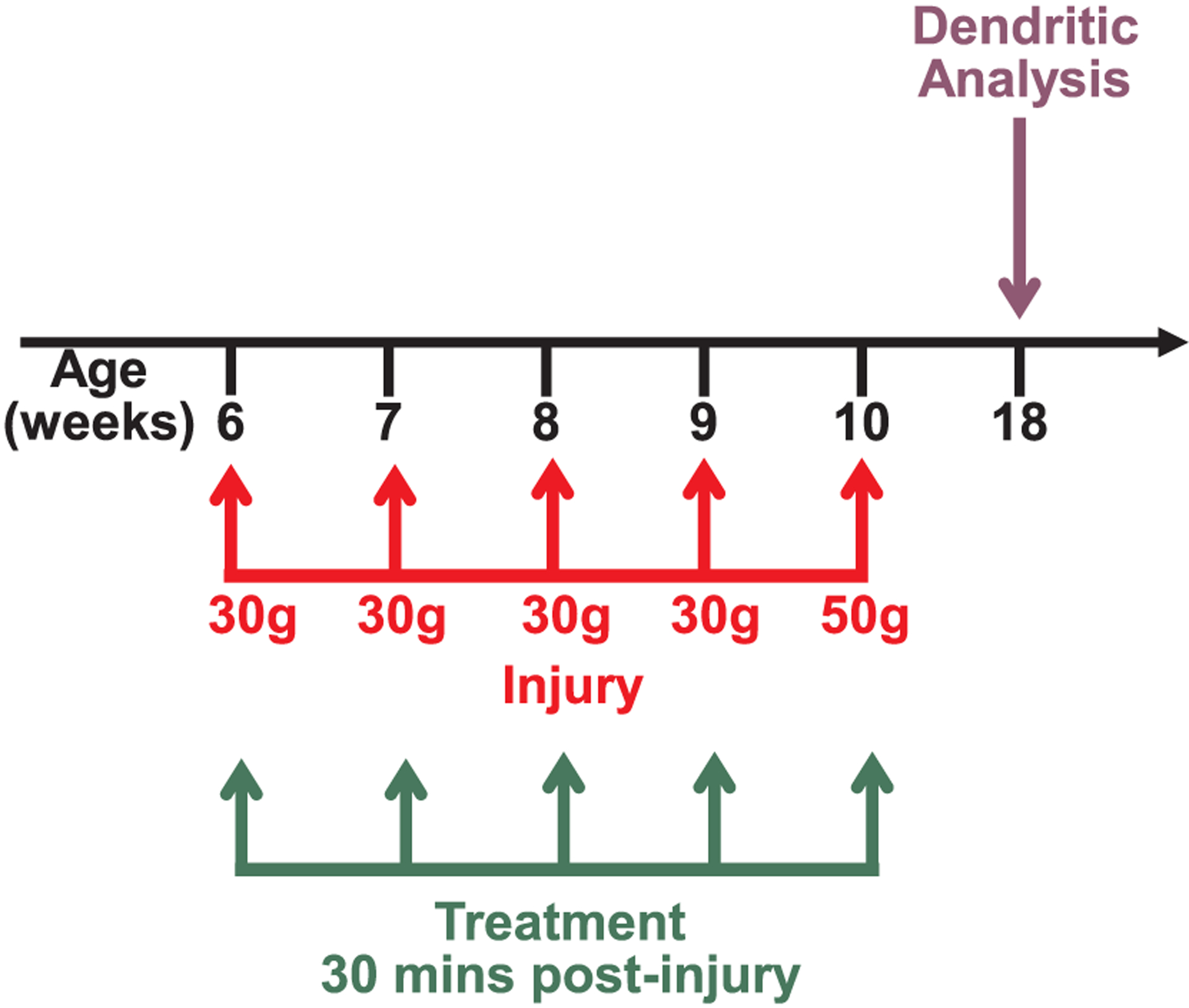
Experimental timeline. Mice in the TBI group received 5 closed head injuries once per week. Treatment or vehicle was administered via intraperitoneal injection 30 min following each injury. Eight weeks after the last injury, brains were harvested for dendritic analysis.
7. Combination treatment
Approximately 30 min after each of the 5 injuries (or sham), mice were treated with a combination treatment containing 33.4 mg/kg tertbutylhydroquinone (tBHQ) and 3 mg/kg pioglitazone or a 5% DMSO in saline vehicle via intraperitoneal injection. Total injection volume ranged from 133.6 μL to 233.8 μL and was calculated based on mouse body weight. Injection solutions were made fresh on each day of injection.
8. Golgi staining
Eight weeks following the final injury and treatment, brains were prepared for analysis using the FD Rapid Golgi Stain kit (Neurodigitech, San Diego, CA) according to the manufacturer’s instructions. Mice were euthanized via cervical dislocation and brains were carefully removed. Brains were briefly rinsed with distilled water to remove any excess blood. Each brain was fully immersed in impregnation solution. The following day, brains were transferred to fresh impregnation solution and allowed to incubate in the dark at room temperature with occasional swirling of the tubes 3 times per week. After 14 days, the brains were transferred to Solution C. The brains were then packaged and shipped to Neurodigitech for analysis. The five highest quality brains from each experimental group were selected for analysis. All analyses were performed blindly. The sections containing the dentate gyrus of the hippocampus were analyzed. The sections were chosen and analyzed using a stereology-based software (Neurolucida, v10, Microbrightfield, Williston, VT, USA) and a Zeiss Axioplan 2 image microscope with an Optronics MicroFire CCD (1600 × 1200) digital camera, motorized X, Y, and Z-focus for high-resolution image acquisition and digital quantitation. Neurons that were completely impregnated with no soma overlap with neighboring neurons and which were not truncated by the sectioning were selected for analysis. Quantitative analysis was performed using the NeuroExplorer program (Microbrightfield, Williston, VT, USA). Neurodigitech’s methods have been described previously (Wu et al., 2004).
9. Statistics
Group comparisons were performed by 2-way ANOVA with post-hoc testing conducted with Tukey’s multiple comparisons test. Mean values are depicted ± standard deviation and with p < 0.05 indicating significance.
HIGHLIGHTS.
Repeated TBI decreases total dendritic length in the hippocampus.
Repeated TBI reduces dendritic spine quantity and density in hippocampus.
Activating Nrf2 and PPAR-γ after injury protects against some neuronal changes.
Acknowledgments
We thank Andrea Smith for expert animal assistance. We also thank Courtney Smith and Tanya Michaels for experimental assistance. This study was supported by the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development (I01RX001520)), the Assistant Secretary of Defense for Health Affairs through the Congressionally Directed Gulf War Illness Research Program (W81XWH-16-1-0626), the Florida Department of Health James and Esther King Biomedical Research Program (4KB14), The Bay Pines Foundation, and the Veterans Bio-Medical Research Institute.
Footnotes
CRediT authorship contribution statement
Whitney A. Ratliff: Investigation, Formal analysis, Writing - original draft. Vedad Delic: Formal analysis, Writing - review & editing. Chaim G. Pick: Formal analysis, Writing - review & editing. Bruce A. Citron: Investigation, Formal analysis, Funding acquisition, Writing - review & editing.
Author disclosure statement
No competing interests exist. The contents do not represent the views of the Department of Veterans Affairs or the United States Government and the opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
References
- Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, Saatman KE, 2014. Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J. Neuropathol. Exp. Neurol 73, 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella EM, Thomas TC, Vanino DL, Fellows-Mayle W, Lifshitz J, Card JP, Adelson PD, 2014. Traumatic brain injury alters long-term hippocampal neuron morphology in juvenile, but not immature, rats. Childs Nerv. Syst 30, 1333–1342. [DOI] [PubMed] [Google Scholar]
- Defense and Veterans Brain Injury Center, 2018. DOD Worldwide Numbers for TBI, in: Defense D.o. (Ed.), Washington, DC, pp. 1–5. [Google Scholar]
- Deng Y, Jiang X, Deng X, Chen H, Xu J, Zhang Z, Liu G, Yong Z, Yuan C, Sun X, Wang C, 2020. Pioglitazone ameliorates neuronal damage after traumatic brain injury via the PPARgamma/NF-kappaB/IL-6 signaling pathway. Genes Diseases 7, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF, 2006. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus 16, 571–576. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG, 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta, GA. [Google Scholar]
- Ferrante M, Migliore M, Ascoli GA, 2013. Functional impact of dendritic branch-point morphology. J. Neurosci 33, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K, 2015. Epidemiology of mild traumatic brain injury and neuro-degenerative disease. Mol. Cellular Neurosci 66, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RR, Smith DH, Lowenstein DH, Saint Marie R, McIntosh TK, 1993. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J. Neurotrauma 10, 405–414. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Paode PR, May HG, Ortiz JB, Kemmou S, Lifshitz J, Conrad CD, Currier Thomas T, 2017. Early and persistent dendritic hypertrophy in the baso-lateral amygdala following experimental diffuse traumatic brain injury. J. Neurotrauma 34, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA, 2008. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med 358, 453–463. [DOI] [PubMed] [Google Scholar]
- Iverson GL, 2005. Outcome from mild traumatic brain injury. Curr. Opin. Psychiatry 18, 301–317. [DOI] [PubMed] [Google Scholar]
- Liu M, Bachstetter AD, Cass WA, Lifshitz J, Bing G, 2017. Pioglitazone attenuates neuroinflammation and promotes dopaminergic neuronal survival in the nigrostriatal system of rats after diffuse brain injury. J. Neurotrauma 34, 414–422. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP, 2003. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 290, 2556–2563. [DOI] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D’Esposito M, 1997. Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia 35, 1341–1353. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG, 2005. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J. Neurotrauma 22, 1003–1010. [DOI] [PubMed] [Google Scholar]
- Nicholl J, LaFrance WC Jr., 2009. Neuropsychiatric sequelae of traumatic brain injury. Semin. Neurol 29, 247–255. [DOI] [PubMed] [Google Scholar]
- Peterson A, Xu L, Daugherty J, Breiding MJ, 2015. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta, GA. [Google Scholar]
- Pilipovic K, Zupan Z, Dolenec P, Mrsic-Pelcic J, Zupan G, 2015. A single dose of PPARgamma agonist pioglitazone reduces cortical oxidative damage and microglial reaction following lateral fluid percussion brain injury in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 59, 8–20. [DOI] [PubMed] [Google Scholar]
- Powell JW, Barber-Foss KD, 1999. Traumatic brain injury in high school athletes. JAMA 282, 958–963. [DOI] [PubMed] [Google Scholar]
- Rafols JA, Morgan R, Kallakuri S, Kreipke CW, 2007. Extent of nerve cell injury in Marmarou’s model compared to other brain trauma models. Neurol. Res 29, 348–355. [DOI] [PubMed] [Google Scholar]
- Ratliff WA, Mervis RF, Citron BA, Schwartz B, Rubovitch V, Schreiber S, Pick CG, 2020a. Effect of mild blast-induced TBI on dendritic architecture of the cortex and hippocampus in the mouse. Sci. Rep 10, 2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff WA, Qubty D, Delic V, Pick CG, Citron B, 2020b. Repetitive mild traumatic brain injury and transcription factor modulation. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL, 2006. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil 21, 544–548. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG, 2011. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol 227, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykally JN, Rachmany L, Hatic H, Shaer A, Rubovitch V, Pick CG, Citron BA, 2012. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience 223, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykally JN, Ratliff WA, Keeley K, Pick CG, Mervis RF, Citron BA, 2018. Repetitive mild closed head injury alters protein expression and dendritic complexity in a mouse model. J. Neurotrauma 35, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S, Price D, Hicks R, Baldwin S, Robinson S, Brackney C, 2005. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J. Neurotrauma 22, 719–732. [DOI] [PubMed] [Google Scholar]
- Schneiderman AI, Braver ER, Kang HK, 2008. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol 167, 1446–1452. [DOI] [PubMed] [Google Scholar]
- Semchenko V, Bogolepov N, Stepanov S, Maksimishin S, Khizhnyak A, 2006. Synaptic plasticity of the neocortex of white rats with diffuse-focal brain injuries. Neurosci. Behav. Physiol 36, 613–618. [DOI] [PubMed] [Google Scholar]
- Semple BD, Dixit S, Shultz SR, Boon WC, O’Brien TJ, 2017. Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury. Behav. Brain Res 319, 48–62. [DOI] [PubMed] [Google Scholar]
- Shim SS, Hammonds MD, Mervis RF, 2013. Four weeks lithium treatment alters neuronal dendrites in the rat hippocampus. Int. J. Neuropsychopharmacol 16, 1373–1382. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Mervis RF, Kanabayashi T, Kito S, Murase T, 2015. The dendrites of granule cell layer neurons are the primary injury sites in the “Brain Diabetes” rat. Behav. Brain Res 280, 78–83. [DOI] [PubMed] [Google Scholar]
- Sholl DA, 1953. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat 87, 387–406. [PMC free article] [PubMed] [Google Scholar]
- Stuss D, Ely P, Hugenholtz H, Richard M, LaRochelle S, Poirier C, Bell I, 1985. Subtle neuropsychological deficits in patients with good recovery after closed head injury. Neurosurgery 17, 41–47. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Gazit V, Zohar O, Schreiber S, Pick CG, 2007. Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Res. 1130, 197–205. [DOI] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D, 2009. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil 24, 14–23. [DOI] [PubMed] [Google Scholar]
- Thomas TC, Ogle SB, Rumney BM, May HG, Adelson PD, Lifshitz J, 2018. Does time heal all wounds? Experimental diffuse traumatic brain injury results in persisting histopathology in the thalamus. Behav. Brain Res 340, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, Pistell PJ, Lahiri DK, Hoffer BJ, Wang Y, Pick CG, Greig NH, 2007. Apoptotic and behavioral sequelae of mild brain trauma in mice. J. Neurosci. Res 85, 805–815. [DOI] [PubMed] [Google Scholar]
- Winston CN, Chellappa D, Wilkins T, Barton DJ, Washington PM, Loane DJ, Zapple DN, Burns MP, 2013. Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. J. Neurotrauma 30, 1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Chawla F, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE, 2004. Selective vulnerability of dentate granule cells prior to amyloid deposition in PDAPP mice: digital morphometric analyses. Proc. Natl. Acad. Sci. U. S. A 101, 7141–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG, 2003. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience 118, 949–955. [DOI] [PubMed] [Google Scholar]


