Summary
Background
Multiple vaccine candidates against COVID-19 are currently being evaluated. We evaluate the safety and immunogenicity protein of a novel SARS-CoV-2 virus receptor-binding domain (RBD) vaccine.
Methods
A phase 1-2, randomised, double-blind, placebo-controlled trial was carried out in “Saturnino Lora” Hospital, Santiago de Cuba, Cuba. Subjects (healthy or those with controlled chronic diseases) aged between 19 and 80 years, who gave written informed consent were eligible. Subjects were randomly assigned (1:1:1, in blocks) to three groups: placebo, 25 µg and 50 µg RBD vaccine (Abdala). The product was administered intramuscularly, 0·5 mL in the deltoid region. During the first phase, two immunization schedules were studied: 0-14-28 days (short) and 0-28-56 days (long). In phase 2, only the short schedule was evaluated. The organoleptic characteristics and presentations of vaccine and placebo were identical. All participants (subjects, clinical researchers, statisticians, laboratory technicians, and monitors) remained masked during the study period. The main endpoints were safety and the proportion of subjects with seroconversion of anti-RBD IgG antibodies, analysed by intention to treat and per protocol, respectively. The trial is registered with the Cuban Public Registry of Clinical Trials, RPCEC00000346.
Findings
Between Dec 7, 2020, and Feb 9, 2021, 792 subjects were included; 132 (66 in each vaccination schedule, divided into 22 for each group) in phase 1, and 660 (220 in each group plus 66 from the short scheme of phase 1) in phase 2. The product was well tolerated. No severe adverse events were reported. During phase 1, the incidence of adverse events in the 25 µg, 50 µg, and placebo arms for the short schedule were 6/22 (27·3%), 6/22 (27·3%), 3/22 (13·6%), respectively, and for the long schedule were 8/22 (36·4%), 9/22 (40·9%), 4/22 (18·2%), respectively. In phase 2, adverse reactions were reported by 53/242 (21·9%), 75/242 (31·0%) and 41/242 (16·9%) participants in the 25 µg, 50 µg, and placebo group, respectively. Adverse reactions were minimal, mostly mild, and from the injection site, which resolved in the first 24-48 hours. In phase 1, seroconversion at day 56 was seen in 95·2% of the participants (20/21) in the 50 μg group, 81% (17/21) in the 25 μg group, and none in the placebo group (0/22). For the long schedule, seroconversion at day 70 was seen in 100% of the participants (21/21) in the 50 μg group, 94·7% (18/19) in the 25 μg group, and none in the placebo group (0/22). In phase 2, seroconversion of anti-RBD IgG antibodies at day 56 was seen in 89·2% of the participants in the 50 μg group (214/240; 95% CI 84·5-92·82), 77·7% in the 25 μg group (185/238; 72·0-82·9) and 4·6% in the placebo group (11/239; 2·3-8·1). Compared with the placebo arm, the differences in the proportion of participants with seroconversion were 73·1% (95% CI 66·8-79·5) and 84·6% (79·4-89·7) in the 25 μg and 50 μg groups, respectively. The seroconversion rate in the 50 μg group was significantly higher than in the 25 μg group (p=0·0012).
Interpretation
The Abdala vaccine was safe, well tolerated, and induced humoral immune responses against SARS-CoV-2. These results, in the context of the emergency COVID-19 pandemic, support the 50 μg dose, applied in a 0-14-28 days schedule, for further clinical trials to confirm vaccine efficacy.
Funding
Centre for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba.
Key Words: Abdala vaccine, COVID-19, SARS-CoV-2, Randomised clinical trial
Research in context.
Evidence before this study
We searched PubMed website on February 21, 2022, for published research articles, with no language or date restrictions, using the search terms “COVID-19″ “Vaccine″ and “Pichia pastoris″. We found 13 articles related with the expression of RBD protein of SARS-CoV-2 using this expression system and its evaluation in animal models, but none related with results on clinical trials.
Added value of this study
This is the first in human study of Abdala´s vaccine candidate against COVID-19 based on a SARS-CoV-2 recombinant spike RBD protein produced in the yeast Pichia pastoris. We carried out a Phase 1-2 clinical trial where two different immunization schedules (0-14-28 days and 0-28-56 days) and two strengths of the antigen were assessed. The study provides safety and immunogenicity data obtained in adults from 19 to 80 years of age. The vaccine was well tolerated and no short-term safety concerns were raised. High anti-RBD IgG immune responses were elicited as well as neutralizing antibodies against live virus.
Implications of all the available evidence
Various COVID-19 vaccines have been developed or are under clinical evaluation using different technological platforms. The positive results presented here support the further evaluation of a three-dose immunization schedule (0-14-28 days) with this vaccine candidate in a Phase 3 efficacy trial.
Alt-text: Unlabelled box
Introduction
The global pandemic of the new 2019 coronavirus disease (COVID-19) caused by the SARS-CoV-2 started in Wuhan, China in December 2019, and from then on has spread throughout the world.1,2
The clinical spectrum of a SARS–CoV-2 infection ranges from the absence of symptoms (asymptomatic infection) or mild respiratory symptoms to severe acute respiratory illness and death. Initially, it manifests mainly as fever, but sometimes only chills and respiratory symptoms occur due to mild dry cough and gradual dyspnea, in addition to fatigue and even diarrhea. In severe cases, the disease can progress rapidly, causing acute respiratory distress syndrome, pneumonia, septic shock, irreversible metabolic acidosis, multiple organ failure, and clotting disorders, among other complications. The prognosis varies from recovery in most cases to torpid evolution and death.2,3
Vaccines are urgently needed to mitigate the consequences of this pandemic and protect humanity from future epidemics caused by this virus. In this sense, clinical trials with multiple vaccine candidates, with accelerated designs and overlapping of the traditional phases of clinical research, are currently carried out worldwide, without breaching Good Clinical Practices (GCP).4 Obtaining safe and effective preventive vaccines, as well as implementing them with broad global coverage, would be the fastest and safest strategy to manage this terrible pandemic.5
At CIGB work has been made on several vaccine candidates using platforms already known to this institution and also considering the state-of-the-art of research around COVID-19, especially the immunological aspects necessary for the development of vaccines against this infection. One of these vaccine candidates was Abdala, based on the recombinant RBD subunit of the spike protein produced in Pichia pastoris yeast, adjuvanted to alumina.
The aim of the present work was to evaluate the safety and immunogenicity of the Abdala vaccine, administered intramuscularly in different strengths and schedules, for specific active immunization against SARS-CoV-2 infection in adults between 19 and 80 years of age.
Methods
Study design
A randomised, adaptive, double blind, placebo-controlled, phase 1-2 clinical trial was carried out in “Saturnino Lora” Hospital, Santiago de Cuba.
The trial was conducted in medical wards and certified areas for the vaccination process. The participating researchers were specialists in internal medicine and intensive care, and the vaccination under study was administered by specialized nurses. The protocol followed the Declaration of Helsinki guidelines and was evaluated by the Ethics and Review Committee of the Provincial Hospital "Saturnino Lora" in Santiago de Cuba (clinical site participating in the trial), who granted ethical approval of the study. This Institutional Review Boards was made up of highly qualified medical specialists not linked to the study, as well as a member of the community. This committee followed up on the research ensuring the protection of the rights, safety and well-being of the subjects involved in the study. In addition, the Cuban Centre for State Control of Drugs, Medical Devices and Equipment approved the start of the clinical trial after considering the scientific, methodological and ethical aspects. The manuscript adheres to CONSORT reporting guidelines.
Participants
Subjects aged between 19 and 54 years (for phase 1; healthy adults) and between 19 and 80 years (for phase 2; healthy adults or with comorbidities, compensated), who gave their written, informed consent to participate, were enrolled. Exclusion criteria were: virological diagnosis by RT-PCR of infection to SARS-CoV-2, contact or suspect of COVID-19, subjects at high risk of exposure to SARS-CoV-2 infection (health workers in 1st line of medical care), acute infection in the last 15 days, chronic or autoimmune or endocrine-metabolic diseases decompensated at the time of inclusion, subject treated in the last three months or with any medical condition that requires an immunomodulator, steroid (except topical or inhaled) or cytostatic during the study. Individuals with body mass index ≤18 or ≥35 Kg/m2, tattoos in both deltoid regions, administration of any research product in the last three months, allergy to thiomersal or any other component of the medicament, pregnancy or breastfeeding, and mental disorders, were also excluded.
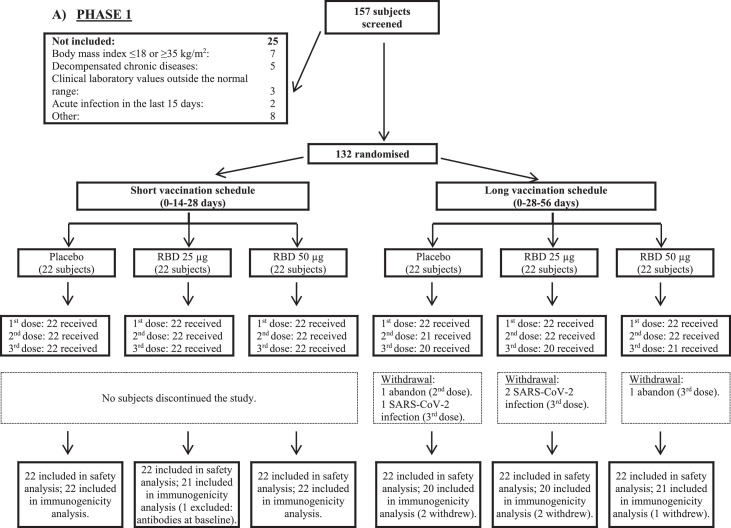
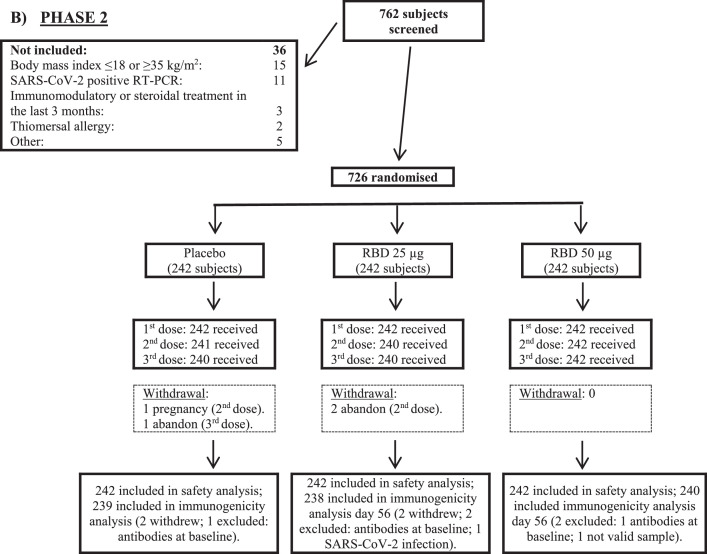
Participants for immunological evaluations are also shown in Figure 1. In phase 2, results of the same groups from phase 1 trial were combined and analyzed together. Neutralizing antibodies (Nab) were only measured in the subset of individuals with >30% of inhibition of RBD-ACE-2 (angiotensin-converting enzyme 2). Additionally, in Phase 2, due to constrains on laboratory capacity, not all the samples that fulfill that condition were evaluated (only 182). For that reason, the decision was made to evaluate the first samples that fulfill the initial criterion in a ratio 2.5:1 regarding the number of samples from 50 μg and 25 μg group, respectively (131 and 51 samples for each group).
Figure 1.
Trial profile. RBD: receptor binding domain.
Randomisation and masking
The subjects included were randomly distributed (1:1:1) to 3 groups: I) placebo; II) 25 µg RBD and III) 50 µg RBD. Randomisation was carried out in the supply group of the Clinical Research Direction of the CIGB, in blocks of 12 or 6 individuals (phase 1 and 2, respectively), by means of a computerised random number generator. The site received the product in such blocks, in masked vials in order to prevent their identification, labelled with each subject´s number. The organoleptic characteristics and presentations of vaccine and placebo were identical. Therefore, the decision to accept or reject a participant was made, and informed consent was obtained from the participant, in ignorance of the assignment in the sequence. All participants (investigators, subjects, and monitors) were kept blinded during all the study performance and data management. Statistical analyses were done without knowledge of the groups´ identity. This was known after the analyses were concluded.
Procedures
The product was applied intramuscularly, 0·5 mL in the deltoid region. During the first phase, two immunization schedules were studied: short (0-14-28 days) and long (0-28-56 days). In phase 2, only the short scheme (0-14-28 days), selected during the interim analysis, was evaluated. Concomitant treatment was not anticipated.
The adverse events (type, duration, severity, outcome, and causality relationship) were carefully registered. The severity of the adverse events was classified in three levels: (a) mild, if no therapy was necessary; (b) moderate, if a specific treatment was needed, and (c) severe, when hospitalisation or its prolongation was required, the reaction was life-threatening or contributed to patient's death. A qualitative assessment was used to classify the causal relationship as definite, probable, possible or doubtful.6 Adverse reactions associated with vaccination were especially sought (pain at the injection site, erythema, induration, headache, fever, among others).
In phase 1 trial, blood samples were collected in all groups at day 0, before the application of the first dose (baseline), and at several points according to the corresponding vaccination schedule at days 42 and 56 for the short schedule (0-14-28 days) and at days 56 and 70 for the long schedule (0-28-56 days) to determine the level of RBD-specific IgG antibodies, in terms of seroconversion rates and geometric mean of the titers (GMT), the percentage of inhibition of RBD-ACE-2 binding in terms of proportions and means (95% CI) and levels of Nab to live SARS-CoV-2 in terms of proportions an GMT. In the phase 2 trial, blood samples were taken at time 0 and at days 42 and 56 (14 and 28 days after the third dose).
IgG antibodies were quantified by UMELISA SARS-CoV-2 anti-RBD (Immunoassay Centre, Havana).7 Titers are given in arbitrary units per millilitre (AU/mL) with a cut-off value of 1·95. The percentage of inhibition to RBD-ACE-2 binding was determined using and in-house virus neutralization test (CIGB, Havana). Results are given in inhibition percentage. The assay threshold for positivity was 30%. Nab titers were detected by a standard virus microneutralization assay using live SARS-CoV-2 (CUT2010-2025/Cuba/2020 strain)8 carried out at Civilian Defense Scientific Research Centre, Cuba. Viral neutralizing titers were calculated as the highest serum dilution with an optical density (OD) higher that the cut-off value (calculated as the average of the OD of the cell control wells divided by two). Results are given in Nab titer ID50. The sequence of the RBD antigen used in the vaccine and in the analytical systems is identical to that of the ancestral SARS-CoV-2 Wuhan-Hu-1 strain (NCBI Acc. No.YP_009724390).
Outcomes
For the phase 1 trial, the primary outcome was the safety of the candidate (the non-occurrence of serious adverse events with a causal relationship attributable to the research product in no more than 5% of the subjects) and the secondary outcome was immunogenicity (proportion of subjects with seroconversion of anti-RBD IgG antibodies to SARS-CoV-2). During phase 2, the main outcome was seroconversion day 56, define as at least a four-fold increase of antibody titers over baseline. Secondary immunogenic endpoints were safety, percentage of inhibition to RBD-ACE-2 binding as well as the neutralizing antibodies to live SARS-CoV-2.
Statistical analysis
A sample size of 20 subjects, computed using the PASS software (www.ncss.com), was required to estimate a two sided exact Clopper-Pearson 95% confidence interval for the rate of related serious adverse events, assuming a rate of related serious adverse events of less than 5%, with type I and II errors of 0·05 and 0·20, respectively. Considering 10% dropouts, the final sample size was 22 subjects per group (132 volunteers) for phase 1. To estimate a 95% Pearson's chi-squared confidence interval for difference of proportions, with a lower limit greater than 30%, under the assumptions of a true difference of proportions between seroconversion rates in treated and control of 50%, a proportion of individuals with an immune response in control group in the range 1-5%, power of 80% and 10% dropouts, the final sample size during the second stage of the study was 242 individuals per group (726 subjects in total, including the 66 participants from phase 1 assigned to the short immunization schedule).
Statistical analyses were done with R version 3.6.2. The immunogenicity analyses were done in the per-protocol population, refer to participants who fulfilled the inclusion and exclusion criteria, who completed the course of vaccination schedule and had valid immunogenicity results both before immunization and the indicated days after vaccination as defined in the study protocol. Individuals, who had anti-RBD IgG antibodies at the baseline time determination, before the first dose of the product was applied, were excluded from the immunogenicity analyses so that all study groups started from the same condition and the effect of the primary immunization schedule could be clearly assessed. The percentage of individuals with serious or severe adverse events related to the research product was estimated (during the period of execution of the clinical trial) and the 95% confidence interval was calculated as confirmatory analysis for phase 1 primary endpoint. For phase 2 primary endpoint, the confidence interval of the difference with respect to the control of the proportion of participants with seroconversion of anti-RBD IgG antibodies was estimated for each treatment arm at day 56. We used the Pearson χ² test for the analysis of categorical outcomes. We calculated 95% confidence intervals (CI) for all categorical outcomes using the Clopper-Pearson method. GMTs were calculated as the mean of the assay results after the logarithmic transformation was made; we then exponentiated the mean to express results on the original scale. Two-sided 95% CI were obtained by performing logarithmic transformations of titers, calculating the 95% confidence interval with reference to Student's t-distribution, and then exponentiating the limits of the confidence intervals. We used the Kruskal-Wallis test as a non-parametric alternative for ANOVA to compare the geometric means of antibody titers and the means of the percentage of inhibition across the three arms to compare the log-transformed antibody titer. As a complement to this test, once the null hypothesis is rejected, multiple comparison tests with Bonferroni's correction were performed to adjust by the number of comparisons. Hypothesis testing was two-sided and we considered p values of less than 0·05 to be significant. We used the Student's t-test to compare the mean of two samples. Mann Whitney U test was used as a nonparametric alternative to Student's t-test. Pearson's linear correlations were also calculated to assess the association between responses on different assays by time of evaluation and study group. To assess safety, adverse events were tabulated and plotted by dose and study group. In phase 2, all analysis were performed globally and by age cohorts: 19 to 54 years and 55 to 80 years.
An independent data monitoring committee consisted of one independent statistician, pathologist, clinician, epidemiologist and immunologist was established before commencement of the study. Safety data were assessed and reviewed by the committee to ensure the toxicity criteria of phase 1 were not met and allow the further proceeding of the clinical trial.
This study was registered with Cuban Public Clinical Trial Registry, RPCEC00000346.
Role of the funding source
The funder of the study had a role in study design, data interpretation, and writing of the report, but had no role in data collection or data analysis.
Results
From 07 December 2020 to 09 February 2021 a total of 792 subjects were included out of 919 that were screened. Their disposition is shown in Figure 1. During phase 1, 132 subjects were included, randomly distributed into two vaccination schedules (0-14-28 and 0-28-56 days) and three study groups for each schedule (placebo and two RBD strengths: 25 μg and 50 μg). After an interim analysis of the results and given the complex epidemiological situation resulting from the COVID-19 pandemic, it was strategically decided to continue towards phase 2 of the trial with the three study groups of the short vaccination schedule (0-14-28 days). In this sense, phase 2 included 660 new individuals, to which were added the 66 subjects evaluated in a similar vaccination scheme during the first stage (726 subjects in total; 242 in each study group). All volunteers completed the vaccination schedule (three doses), except for five individuals assigned to the long schedule in phase 1 (2 in the placebo and RBD 25 μg groups, and 1 in the 50 μg group of the vaccine candidate) and four subjects during phase 2 (two in the placebo and lower strength vaccine groups).
Table 1 shows the demographic and baseline characteristics of the subjects. Most of them were males, 19 to 80 years-old, with the ethnic distribution of the Cuban population in the south eastern region of the country. No relevant imbalances can be seen.
Table 1.
Demographic characteristics of the participants in the Abdala trial at enrollment.
| Variable | Short schedule (0-14-28 days) |
Long schedule (0-28-56 days) |
Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | RBD 25 μg | RBD 50 μg | Subtotal | Placebo | RBD 25 μg | RBD 50 μg | Subtotal | |||
| PHASE 1 (19-54 years) | ||||||||||
| N | 22 | 22 | 22 | 66 (50·0) | 22 | 22 | 22 | 66 (50.0) | 132 (100) | |
| Sex – no. (%) | Female | 11 (50·0) | 7 (31·8) | 9 (40·9) | 27 (40·9) | 10 (45·5) | 12 (54·5) | 11 (50·0) | 33 (50·0) | 60 (45·5) |
| Male | 11 (50·0) | 15 (68·2) | 13 (59·1) | 39 (59·1) | 12 (54·5) | 10 (45·5) | 11 (50·0) | 33 (50·0) | 72 (55·5) | |
| Age | years | 37·9 ± 10·0 | 40·7 ± 7·3 | 44·4 ± 9·2 | 41·0 ± 9·2 | 41·1 ± 9·4 | 39·9 ± 9·4 | 44·2 ± 8·3 | 41·7 ± 9·1 | 41·3 ± 9·1 |
| Ethnicity – no. (%) |
White | 4 (18·2) | 4 (18·2) | 7 (31·8) | 15 (22·7) | 7 (31·8) | 3 (13·6) | 2 (9·1) | 12 (18·2) | 27 (20·5) |
| Black | 11 (50·0) | 2 (9·1) | 6 (27·3) | 19 (28·8) | 6 (27·3) | 4 (18·2) | 8 (36·4) | 18 (27·3) | 37 (28·0) | |
| Mestizo | 7 (31·8) | 16 (72·7) | 9 (40·9) | 32 (48·5) | 9 (40·9) | 15 (68·2) | 12 (54·5) | 36 (54·5) | 68 (51·5) | |
| BMI | Kg/m2 | 25·2 ± 4·0 | 26·5 ± 4·7 | 25·5 ± 3·3 | 25·7 ± 4·0 | 24·4 ± 3·3 | 25·9 ± 3·3 | 26·3 ± 4·4 | 25·5 ± 3·7 | 25·6 ± 3·9 |
| PHASE 2 (19-80 years; short schedule: 0-14-28 days) | ||||||||||
| Age group: 19 - 54 years | Age group: 55 - 80 years | |||||||||
| N | 153 | 149 | 151 | 453 (62·4) | 89 | 93 | 91 | 273 (37·6) | 726 (100) | |
| Sex – no. (%) | Female | 76 (49·7) | 80 (53·7) | 76 (50·3) | 232 (51·2) | 28 (31·5) | 40 (43·0) | 37 (40·7) | 105 (38·5) | 337 (46·4) |
| Male | 77 (50·3) | 69 (46·3) | 75 (49·7) | 221 (48·8) | 61 (68·5) | 53 (57·0) | 54 (59·3) | 168 (61·5) | 389 (53·6) | |
| Age | years | 35·7 ± 12·0 | 35·6 ± 11·4 | 30·1 ± 12·5 | 35·0 ± 12·0 | 65·7 ± 8·0 | 63·2 ± 7·5 | 64·0 ± 7·3 | 64·3 ± 7·2 | 46·5 ± 17·3 |
| Ethnicity – no. (%) |
White | 53 (34·6) | 63 (42·3) | 51 (33·8) | 167 (36·9) | 28 (31·5) | 24 (25·8) | 35 (38·4) | 87 (31·9) | 254 (35·0) |
| Black | 34 (22·2) | 20 (13·4) | 17 (11·3) | 71 (15·7) | 25 (28·1) | 17 (18·3) | 16 (17·6) | 58 (21·2) | 129 (17·8) | |
| Mestizo | 66 (43·1) | 66 (44·3) | 83 (55·0) | 215 (47·5) | 36 (40·4) | 52 (55·9) | 40 (44·0) | 128 (46·9) | 343 (47·2) | |
| BMI | Kg/m2 | 25·1 ± 4·2 | 25·2 ± 4·1 | 24·7 ± 4·1 | 25·0 ± 4·1 | 25·9 ± 4·0 | 27·2 ± 4·4 | 26·6 ± 4·4 | 26·6 ± 4·3 | 25·6 ± 4·3 |
Plus-minus values are means ± SD.
BMI: Body-mass index (is the weight in kilograms divided by the square of the height in meters. The calculation was based on the weight and height measured at the time of screening). RBD: receptor binding domain.
The product was well tolerated. The product was well tolerated. No severe adverse events were reported (0/22 for each arm in phase 1; 0/242 for each arm in phase 2) and there were no withdrawals for this cause. In the phase 1 trial, the overall incidence of adverse reactions was 6/22 (27·3%) participants in the 25 and 50 μg groups, respectively, and 3/22 (13·6%) in the placebo group in the short vaccination schedule (0-14-28 days); and 8/22 (36·4%) in the 25 μg group, 9/22 (40·9%) in the 50 μg group, and 4/22 (18·2%) in the placebo group in the long vaccination schedule (0-28-56 days). During phase 2, adverse reactions were reported by 53/242 (21·9%) subjects in the 25 μg group, 75/242 (31·0%) in the 50 μg group, and 41/242 (16·9%) in the placebo group, all under the short vaccination schedule.
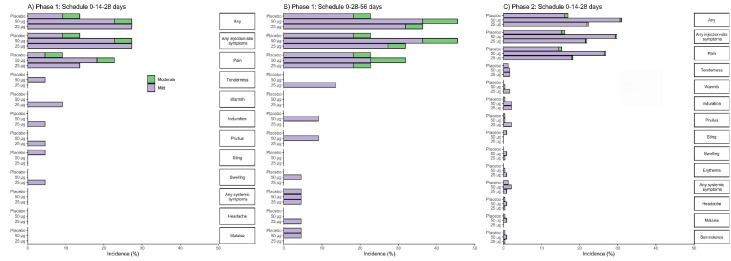
In the two phases of the clinical trial, overall reactogenicity was largely absent or mild in most reports, and the following doses were neither withheld nor delayed due to reactogenicity (Figure 2). After the first vaccination, local and systemic reactogenicity it was absent or decreased with the application of subsequent doses. Most of the adverse reactions resolved spontaneously in the first 24-48 hours without medication. There were no clinically relevant laboratory abnormalities associated with vaccination.
Figure 2.
Percentage of participants in each phase of the trial according to the occurrence of adverse reactions, by group and vaccination schedule.
The percentage of participants in each study group (RBD 25 µg, RBD 50 µg, Placebo) with adverse reactions according to the maximum FDA (Food and Drug Administration) toxicity grade (mild or moderate) from first dose up to 14 days after third dose is plotted by signs or symptoms. Participants who reported 0 events make up the remainder of the 100%. Panel A: phase 1 (schedule 0-14-28 days); Panel B: phase 1 (schedule 0-28-56 days); Panel C: phase 2 (schedule 0-14-28 days).
The percentage of participants in each study group (RBD 25 µg, RBD 50 µg, Placebo) with adverse reactions according to the maximum FDA (Food and Drug Administration) toxicity grade (mild or moderate) from first dose up to 14 days after third dose is plotted by signs or symptoms. Participants who reported 0 events make up the remainder of the 100%. Panel A: phase 1 (schedule 0-14-28 days); Panel B: phase 1 (schedule 0-28-56 days); Panel C: phase 2 (schedule 0-14-28 days).
In phase 1, seroconversion rates of anti-RBD IgG for the short schedule (0-14-28 days), measured at day 42, were 81% for the 25 μg group and 95·5% for the 50 μg group (p=0·19). At day 56 (28 days after the third dose) there were 81% and 95·2% for the 25 μg and 50 μg group respectively (p=0·21). The results for the long schedule (0-28-56 days) at day 56 were 55% for the 25 μg group and 57·1% for the 50 μg group (p=1·0) that increased, at day 70, to 94·7% and 100% for the 25 μg and 50 μg group (p=0·96) (Table 2). None of the participants in the placebo groups seroconverted. For both strengths of RBD/vaccination schedules high seroconversion rates were obtained.
Table 2.
Seroconversion rates for anti-RBD IgG and proportion of individuals with inhibition to RBD-ACE2 binding and neutralizing antibodies to SARS-CoV-2.
| Phase 1 | Placebo | RBD 25 μg | RBD 50 μg | p value* |
|---|---|---|---|---|
| Schedule 0-14-28 days | ||||
| Seroconversion of anti-RBD IgG (%) | ||||
| Day 42 | 0/22 (0%; 0-15·4) |
17/21 (81%; 58·1-94·6) |
21/22 (95·5%; 77·2-99·9) |
0·19 |
| Day 56 | 0/22 (0% 0-15·4) |
17/21 (81%; 58·1-94·6) |
20/21 (95·2 %; 76·2-99·9) |
0·21 |
| Inhibition to RBD-ACE2 binding | ||||
| Day 42 | 2/22 (9·1%; 1·1-29·2) |
11/21 (52·4%; 29·8-74·3) |
19/22 (86·4%; 65·1-97·1) |
0·036 |
| Day 56 | 7/22 (31·8% 13·9-54·9) |
14/21 (66·7%; 43·0-85·4) |
19/21 (90·5%; 69·6-98·8) |
0·13 |
| Neutralizing antibodies to live SARS-CoV-2 | ||||
| Day 42 | - | 4/8 (50%;15·7-84·3) |
18/19 (94·7%; 74-99·9) |
0·029 |
| Day 56 | - | 8/10 (80%; 44·4-97·5) |
18/19 (94·7%; 74-99·9) |
0·550 |
| Schedule 0-28-56 days | ||||
| Seroconversion of anti-RBD IgG (%) | ||||
| Day 56 | 0/20 (0%; 0-16·8) |
11/20 (55%; 31·5-76·9) |
12/21 (57·1%; 34-78·2) |
1·00 |
| Day 70 | 0/20 (0%; 0-16·8) |
18/19 (94·7%; 74-99·9) |
21/21 (100%; 84-100) |
0·96 |
| Inhibition to RBD-ACE2 binding | ||||
| Day 56 | 5/20 (25%; 8·7-49·1) |
12/20 (60%; 36·1-80·9) |
15/21 (71·4%; 47·8-88·7) |
0·66 |
| Day 70 | 9/20 (45% 23·1-68·5) |
17/19 (89·5% 66·9-98·7) |
20/21 (95·2%; 76·2-99·9) |
0·93 |
| Neutralizing antibodies to live SARS-CoV-2 | ||||
| Day 56 | - | 5/5 (100%; 47·8-100) |
7/7 (100%; 59·0-100) |
- |
| Day 70 | - | 15/16 (93·8%; 69·8-99·8) |
19/20 (95%, 75·1-99·9) |
0·999 |
RBD: receptor binding domain. Data are n/N (%; 95% CI). Days 42 and 56, refers to 14 and 28 days after the third dose of the 0-14-28 days vaccination schedule and days 56 and 70, refers to 28 days after second dose and 14 days after the third dose of the 0-28-56 days vaccination schedule, respectively. * p values are for comparisons between 25 μg and 50 μg groups.
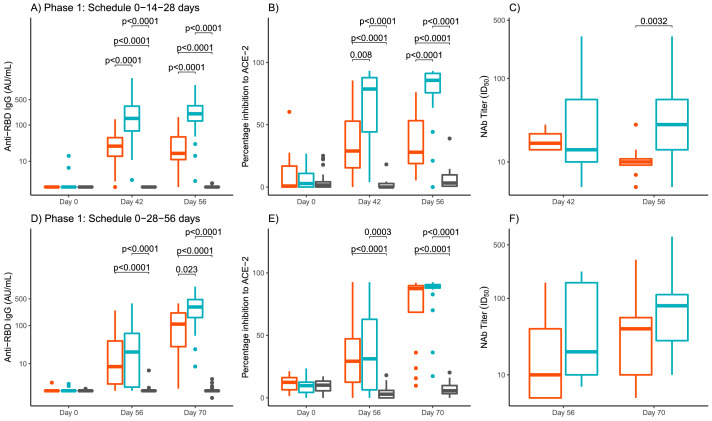
The results of GMTs of anti-RBD IgG are presented in Figure 3 and Supplementary material, Table 1. At baseline, GMTs in all the participants were at the lower limit of quantitation (1·95). For the short schedule (Figure 3A) by day 42, GMT had increased to 22·35 for the 25 μg group and to 131·20 for the 50 μg group. At day 56, GMT for the 25 μg group were 22·23 and GMT had further increased to 155·18 for the 50 μg group. Higher GMTs were found at 42 and 56 days for the 50 μg group, with significant differences between GMTs of 25 μg and 50 μg groups (p<0·0001). In the placebo groups GMTs remained as in the baseline evaluation and significant differences with both 25 μg and 50 μg groups were found (Figure 3A). For the long schedule (Figure 3D) the results after only two administered doses (day 56) were 12·21 for the 25 μg group and 16·11 for the 50 μg group. At day 70, GMT had further increased for both dose levels, 72·99 for the 25 μg group and 221·43 for the 50 μg group (p=0·023) (Figure 3D). For both 25 μg and 50 μg groups, statistical differences with placebo were found at 56 and 70 days for the 50 μg group (Figure 3D).
Figure 3.
Quantitative variables of immunogenicity in the three study groups by schedule and days.
Panels A and C shown anti-RBD IgG antibody titers for short and long schedules, respectively. Panels B and E shown inhibition of RBD-ACE-2 binding, for short and long schedules, respectively. Panels C and F shown Nab titers, for short and long schedules, respectively. Study groups are represented by colors, red for RBD 25 µg, blue for RBD 50 µg and gray for placebo. The boxes and horizontal bars indicate interquartile range (IQR) and median, respectively. The whisker's end points are the maximum and minimum values below or above the median ± 1•5 times the IQR. Points represent possible outliers. The braces contain the results of the Mann Whitney U multiple comparison tests with Bonferroni correction in cases where more than two groups appear (graphs A,B,D,E), and the Mann Whitney U tests in cases where only two groups appear (panels C and F). Only p values for significant differences are shown. Schedules: 0-14-28 days (short) and 0-28-56 days (long). RBD: receptor binding domain. ACE-2: angiotensin-converting enzyme. AU/mL: arbitrary units per mL. Nab: Neutralizing antibody titers.
The proportion of individuals with positive inhibition of RBD-ACE-2 binding for the short schedule at day 42 was 52·4% for the 25 μg group and 86·4% for the 50 μg group (p=0·036), increasing to 66·7% and 90·5% at day 56 for the corresponding (p=0·13) (Table 2). The proportion of individuals with positive inhibition of RBD-ACE-2 binding for the short schedule at day 42 was 52·4% for the 25 μg group and 86·4% for the 50 μg group p=0·036), increasing to 66·7% and 90·5% at day 56 for the corresponding (p=0·13) (Table 2). The mean of the percentage of inhibition of RBD-ACE-2 binding for both schedules are presented in Figure 3 and Supplementary material Table 1. For the short schedule at baseline were very low 8·83% for the 25 μg group and 5·73% for the 50 μg group, with no differences among the groups including placebo (Figure 3B). At day 42, the mean had increased for both dose levels, for 25 μg group was 36·48% and 65·84% for the 50 μg group. By day 56, the results were 36·60% for the 25 μg group and a further increase was observed for the 50 μg group, 75·71%. For both 42 and 56 days significant differences were obtained among the three groups of study (p=0·008 and p<0·0001, respectively), with highest mean values for 50 μg group. (Figure 3B). For the long schedule, the baseline results were also very low 11·65% for the 25 μg group and 9·38% for the 50 μg group (Figure 3E). At day 56, after only two applied doses, the percentage if inhibition had increased for both dose levels, 34·28% for the 25 μg group and 37·56% for the 50 μg group, with no significant differences between them. By day 70, the mean had a further increase, 72·32% for the 25 μg group and 82·66% for the 50 μg group, but only significant differences with placebo were found (Figure 3E).
We also measured Nab titers against live-SARS-CoV-2 in serum samples of participants. For the short schedule, the proportion of individuals with Nab titers, at day 42, was 50% for the 25 μg group and 94·73% for the 50 μg group (p=0·029). At day 56, the percentages were 80% for the 25 μg group and 94·7% for the 50 μg group (p=0·55) (Table 2). For the long schedule, by day 56, 100% of individuals had neutralizing antibodies for both 25 μg and 50 μg group, and at day 70, 93·8% and 95·0% for 25 μg and 50 μg group, respectively (p=0·99) (Table 2). GMT values are presented in Figure 3 and Supplementary material Table 1. For the short schedule at day 42 were 18·20 for the 25 μg group and 22·80 for the 50 μg group (p=0·86). At day 56, GMT for the 25 μg group was 10·40 and 31·53 for the 50 μg group (p=0·0032) (Figure 3C). For the long schedule, the GMTs by day 56 were 17·41 and 31·09 for the 25 μg and 50 μg group, respectively (p=0·37) and at day 70, the 34·63 for the 25 μg group and 71·31 for the 50 μg group (p=0·097) (Figure 3F).
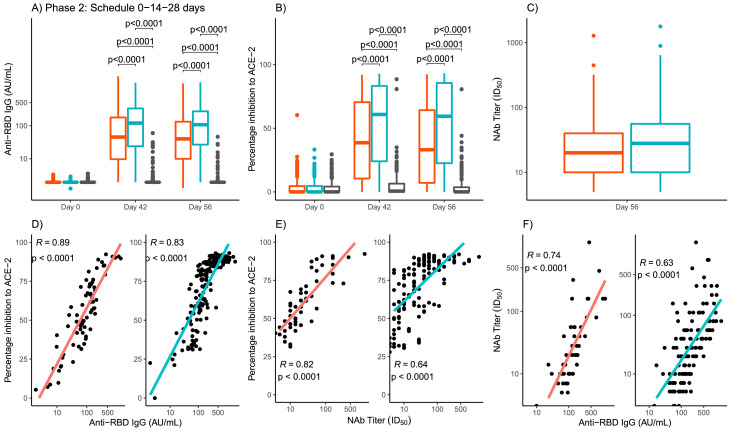
In phase 2, blood samples for the evaluation of immune response were taken at time 0 and at days 42 and 56 (14 and 28 days after the third dose). All the results were analyzed globally (Table 3 and Figure 4) and by age groups: 19 to 54 years and 55 to 80 years. Age-stratified results can be found in Supplementary material Table 2 and Figure 1. Seroconversion rates of anti RBD-IgG at day 42 were, 79·5% and 89·6% for the 25 μg and 50 μg group, respectively (p=0·0032) and at day 56, were very similar with 77·7% and 89·2% for the 25 μg and 50 μg group, respectively (p=0·0012). In the case of placebo group a small number of participants (less than 5%) seroconverted. The difference with respect to the placebo arm of the proportion of participants with seroconversion of anti-RBD IgG antibodies at day 56 was higher than 50% for all treatments arms, globally and for each age cohort. Globally, the differences were 73·1% (95% CI 66·8-79·5) and 84·6% (79·4-89·7), for the 25 μg and 50 μg, respectively. By day 42 and 56, GMT had increased for both 25 μg and 50 μg groups (39·62 and 93) and (36·66 and 80·36) respectively, with higher increases in the 50 μg group and significant differences with the 25 μg group for both evaluation days (p<0·0001) (Figure 4A).
Table 3.
Global immunological results by study groups at 42 and 56 days (Phase 2).
| Group | Placebo | RBD 25 μg | RBD 50 μg | p-value* |
|---|---|---|---|---|
| Seroconversion of anti-RBD IgG % (95% CI) | ||||
| Day 42 | 8 / 239 3·3% (1·5-6·5) |
190 / 239 79·5% (73·8-84·4) |
216 / 241 89·6% (85·1-93·2) |
0·0032 |
| Day 56 | 11 / 239 4·6% (2·3-8·1) |
185 / 238 77·7% (72·0-82·9) |
214 / 240 89·2% (84·5-92·82) |
0·0012 |
| Geometric mean titers of RBD-IgG antibodies (95% CI) | ||||
| Day 42 | 2·21 (2·08-2·35) | 39·62 (31·30-50·14) | 93·46 (73·96-118·10) | <0·0001 |
| Day 56 | 2·13 (2·01-2·26) | 36·66 (27·84-43·17) | 80·36 (63·97-100·93) | <0·0001 |
| Inhibition to RBD-ACE2 binding % (95% CI) | ||||
| Day 42 | 11 / 239 4·6% (2·3-8·1) |
137 / 239 57·3% (50·8-63·7) |
176 / 241 73·0% (67·0-78·5) |
0·0004 |
| Day 56 | 13 / 239 5·4% (2·9-9·1) |
132 / 238 55·5% (48·9–61·9) |
173 / 240 72·1% (65·9-77·7) |
0·0002 |
| Inhibition to RBD-ACE2 binding Media (95% CI) | ||||
| Day 42 | 5·00 (3·64-6·36) | 41·15 (37·09-45·21) | 53·52 (49·29-57·74) | <0·0001 |
| Day 56 | 3·82 (2·68-4·96) | 36·80 (32·78-40·82) | 53·40 (49·05-57·74) | <0·0001 |
| Neutralizing antibodies against live SARS-CoV-2 (95% CI) | ||||
| Day 56 | - | 58 / 61 95·1% (86·3-99·9) |
146 / 150 97·3% (93·3-99·3) |
0·69 |
| Geometric mean of neutralizing antibody titers against live SARS-CoV-2 (95% CI) | ||||
| Day 56 | - | 24·12 (17·56-33·14) | 30·81 (25·14-33·75) | 0·19 |
RBD: receptor binding domain. ACE-2: angiotensin-converting enzyme. ±SD: plus minus standard deviation. Data is n/N (%; 95% CI) for seroconversion rates of anti-RBD IgG, proportion of individuals with inhibition to RBD-ACE2 binding and neutralizing antibodies to SARS-CoV-2. Geometric mean titers are shown with 95% CI. Inhibition to RBD-ACE-2 are shown in means ± Standard deviation and 95% CI. Days 42 and 56 refers to 14 and 28 days, respectively, after the third dose of the 0-14-28 days vaccination schedule. *p-values correspond to comparisons between 25 μg and 50 μg groups.
Figure 4.
Phase 2 quantitative variables of global immunogenicity by study groups and days.
Panel A: anti-RBD IgG antibody titers. Panel B: Inhibition of RBD-ACE-2 binding. Panel C: Neutralizing antibody titers. The study groups are represented by colors, red for RBD 25 µg, blue for RBD 50 µg and gray for placebo. The boxes and horizontal bars indicate interquartile range (IQR) and the median, respectively. The whisker's end points are the maximum and minimum values below or above the median ± 1•5 times the IQR. Points represent possible outliers. The braces contain the results of the Mann Whitney U multiple comparison tests with Bonferroni correction. For the viral neutralization variable, Student's t tests were used to compare geometric means. Only p values for significant differences are shown. The bottom row shows the scatter graphs and the line adjusted by least squares by study group (red for RBD 25 µg, blue for RBD 50 µg) of the values corresponding to day 56. In each case, Pearson's correlation coefficient and the associated p-value are included. RBD: receptor binding domain. ACE-2: angiotensin-converting enzyme. AU/mL: arbitrary units per mL. Nab: Neutralizing antibody titers.
The proportion of individuals with positive inhibition of RBD-ACE-2 binding at day 42 was of 57·3% and 73·0% for 25 μg and 50 μg groups, respectively, (p=0·0004) and at day 56, 55·5% and 72·1% (p=0·0002) (Table 3). The mean of the percentage of inhibition of RBD-ACE-2 binding showed an increase at 42 and 56 days, with respect to baseline levels for both 25 μg and 50 μg groups. At 42 and 56 days, the mean of the percentage of inhibition in the group of 50 μg were significantly higher than the obtained for the 25 μg group (53·52 vs. 41·15, p<0·0001). This significant difference was maintained at day 56 (Figure 4B).
The proportion of individuals with Nab titers against live-SARS-CoV-2 was measured only at day 56. The results were similar for both 25 μg and 50 μg groups, 95·1% and 97·3%, respectively, (p=0·69) (Table 3). GMT of neutralizing antibodies were very similar for 25 μg and 50 μg groups (24·12 and 30·81) without significant differences between them (p=0.19) (Table 3, Figure 4C).
Correlation coefficients were calculated with global data of phase 2 trial, for both 25 μg and 50 μg groups. The correlation coefficient between anti RBD-IgG and the percentage of inhibition to ACE-2 was 0·89 (95% CI 0·82-0·93) for the 25 μg GMT of group and 0·83 (0·77-0·87) for the 50 μg group (Figure 4D). The correlation coefficient between Nab titers to live SARS-CoV-2 and the percentage of inhibition RBD-ACE-2 binding was 0·82 (0·70-0·89) for the 25 μg group and 0·64 (0·52-0·73) for the 50 μg group (Figure 4E). The correlation coefficient between anti RBD-IgG and Nab titers to live SARS-CoV-2 was 0·74 (0·58-0·84) for the 25 μg group and 0·63 (0·51-0·72) for the 50 μg group (Figure 4F). Strong and significant correlations were observed in all the cases, for both 25 μg and 50 μg groups.
Discussion
Several vaccine candidates has been developed and found safe and effective against COVID-19. The S protein RBD of SARS-CoV-2 has been the target antigen using different technological platforms such as mRNA, adenovirus vector, inactivated virus and subunit vaccines.9,10
This work reports for the first time the safety and immunogenicity of the Abdala vaccine based in the subunit RBD protein of SARS-CoV-2 in a randomised, double-blind, placebo-controlled trial. The trial performed well, without deviations and a minimum of dropouts. This was facilitated by the fact that vaccination was short lasting and inclusion could be completed easily. Randomisation and blinding assured that bias was minimal. Therefore, the internal validity of the study was adequate.
In phase 1 trial two different immunization schedules were evaluated (0-14-28 and 0-28-56 days). In our approach to develop COVID-19 vaccine it was foreseen that the immunization schedule would be of three doses, based on CIGB's previous experience with its recombinant hepatitis B vaccine, produced for more than 30 years, whose vaccine antigen is an also a recombinant protein obtained from the same technological platform that uses the SARS-CoV-2 RBD antigen of the Abdala vaccine under study in this trial. This strategy is not the most frequent employed by other vaccine's developers considering worldwide 15% of the candidates use only one dose and 62% two doses.10 However taken into account the course of the pandemic along the time with the emergency of new variants of concern, we consider that a three dose schedule would be a better approach to face this situation and potentially to achieve a longer duration of the immune response.
The safety and immunogenicity analyses indicated that three doses of Abdala at different strengths and with different immunization schedules in adults 19 to 80 years of age were safe and induced high immune responses, including neutralizing antibodies closely correlated with the anti-RBD IgG response.
The incidence of adverse reactions in the 25 μg and 50 μg groups were similar, indicating no dose-related safety. The adverse reactions reported were minimal, mostly mild and from the injection site, of short duration, resolved spontaneously. The most common symptom being injection-site pain, which is in accordance with previous findings for another COVID-19 vaccines.11, 12, 13
As anticipated, immune responses induced by the 0-28-56 days vaccination schedule were larger than those induced by the 0-14-28 days vaccination schedule, regardless of the dose. It was interesting to find that, with the long schedule, the response with the 25 μg group was closer to the 50 μg group in magnitude and quality of the generated antibodies, that explains why at the end of the schedule only differences between them were found in GMTs of anti-RBD IgG, but not for media of binding to ACE2 and neutralizing antibodies. However, quick antibody responses could be induced within a relatively short period of time by using a 0-14-28 days schedule. Although it can be argued that the longer vaccination schedule will induce not only a more robust antibody response but also potentially longer persistence of the response could be expected, the actual immune persistence of the two schedules needs to be verified in future studies. However, it was of interest to assess the results on day 56, after applying two doses of the long schedule versus the three doses of the short schedule, in line with the interest to use fewer doses, like other SARS-CoV-2 vaccines based mainly on different technological platforms. In that analysis, for both strengths of the vaccine, significantly higher GMT levels of anti-SARS-CoV-2 IgG antibodies were obtained after three doses of the schedule 0-14-28 days compared to only two doses of the schedule (0-28 days), which reinforced the original idea of the need for three doses of the candidate, under the experimental conditions assessed. Other studies also indicate that a relatively longer interval (21 or 28 days) between injections would be preferred, but the efficacy of the three and two dose schedules is unclear, and the optimal or minimum antibody titers that could protect people form COVID-19 are yet to be established.14 In phase 1-2 of Coronavac vaccine, quick antibodies response at 0-14 days were obtained but a more robust antibody response was obtained with the 0-28 schedule, although the persistence of the two schedules needs to be verified in future studies.11
Considering the capacity to generate a satisfactory immune response with both immunization schedules, including neutralization of the SARS-CoV-2 virus in cell cultures, which is one of the most important variables in relation to the functional capacity of antibodies induced by vaccination and taken into account as a determining practical premise, the exceptional pandemic conditions where it was necessary to immunize people in the shortest possible time, it was decided to continue to phase 2 trial with the three-dose short immunization schedule of 0-14-28 days. This schedule offered a favorable risk-benefit ratio, in terms of safety and immunogenicity. Although most of the COVID-19 vaccines already under emergency authorization are applied mainly in schedules of only two doses, and the three-dose schedule can be considered more complicated and time consuming for massive immunization programs, the short immunization schedule proposed in this trial (0-14-28 days) would allow in only one month to complete the vaccination regimen with good immunogenic profile and potentially a third dose instead of only two doses in this short period of time would be better. This proposal might be also suitable for emergency use, to achieve high vaccination coverage's in the exposed population of vital importance during the COVID-19 pandemic.
Phase 2 results were consistent with those obtained previously in phase 1 trial, where seroconversion percentages and GMTs of anti-RBD IgG, inhibition percentage of RBD-ACE2 binding neutralizing antibodies confirm the favorable immunogenic profile of the Abdala vaccine with better results and risk-benefit ratio for the 50 μg group. A better immunological performance of the vaccine was obtained in the age group age 19-54 years that the oldest individuals age 55-80 years regarding seroconversion rates and GMTs of anti-RBD IgG, but not in the percentages of individuals with Nab titers and GMTs. However, for each age stratum and overall for all study participants, the probability of success of each experimental group (RBD 25 μg and 50 μg) compared to the control (placebo) was estimated, and the hypothesis was fulfilled, since in all cases the probabilities were 1 or values very close to 1. This was demonstrated both overall and when stratified by age groups. GMTs of anti-RBD IgG and percentages of inhibition RBD-ACE2 binding also found differences in favor to the 50 μg group but not in the evaluation by day 56 of neutralizing antibodies. It is well-known that there is a large variation in the immune response to vaccination among different individuals, both in quantity and quality, and there is strong evidence that intrinsic factors, such as genetics, sex, age at vaccination, comorbidities, as well as vaccine-related factors (such as choice of vaccine products, adjuvants, and vaccination schedule) strongly influence vaccine responses.15 Age is considered an important factor influencing the immune response to vaccines, mainly in individuals in the extreme ages of life. This response is thought to be decreased in the earliest stages of life and also in the elderly, who also have a faster decrease in antibodies. This effect has been found for several vaccines where older people have lower antibody levels.16, 17, 18, 19, 20, 21, 22, 23 Taking this knowledge into account; it is not surprising to find lower seroconversion values in individuals aged 55-80 years, even though more than 80% of the subjects seroconverted. Other of COVID-19 vaccines have assess safety and immunogenicity in older individuals showing lower responses when compare with the youngest ages.24, 25, 26
It should be noted that since most vaccines induce very high antibody responses, small differences in antibody concentrations between groups of individuals may not be clinically significant in terms of protection or efficacy, and may be relevant only in individuals with poor responses, or may affect only the duration of protection, but this has to be proven in phase 3 efficacy trial and the evaluation of the immune response during longer follow-ups. In addition, the quality of the antibody response is an important factor since only a subset of the total detectable antibodies may have functional activity capable of neutralizing pathogens. In this sense, they have more value as potential correlates of efficacy than seroconversion values or geometric mean of titers, which assess the quantity of antibodies but not their functional activity.15 Moreover, the level of in vitro antibody response does not necessarily correlate with health outcomes, i.e., seroconversion does not mean complete protection against a disease, and non-seroconversion is not necessarily associated with susceptibility, not to mention that antibody levels decline over time, but seronegative individuals may still be protected through other immune mechanisms, as shown, for example, after hepatitis B vaccination.16
Neutralizing antibody titer is the most common correlate of protection against viral vaccines and it is highly correlated with protective effect and durability of protection. Results from previous studies on monoclonal antibodies and convalescent sera, as well as tests in animal models, have all confirmed the role of neutralizing antibodies in conferring protection against COVID-19.27,28 Neutralizing antibody responses to SARS-CoV-2 in hospitalized COVID-19 and convalescent patients are above 160 in more than 93% of convalescent sera.29,30 However, in the different clinical trials performed the GMT of the neutralizing antibodies varies for the different vaccines. In addition, comparisons between the different vaccines developed may not be reliable and indeed not be comparable, due to the lack of standards that could serve as reference points in the studies, and the different methods used to assess this response.31
Finally, when Pearson's linear correlation analyses were performed between the different immunogenicity variables, positive and highly significant correlations were found, as described in the corresponding results section, demonstrating the relationship that existed between these variables, indicating that the immune response for the vaccine is not only potent in quantity but also in the quality of the antibodies elicited.
This study has several limitations. First, immunogenicity was tested at day 14 and 28 after complete vaccination schedule, so the duration of the immune response cannot be assessed. Follow-up visits to evaluate long-term safety as well as the duration of the immune response at least 6 months after vaccination are underway. Second, we did not assess the T cell responses in this phase 1-2 trial. Third, the relevance of antibody response elicited by this vaccine to protection against COVID-19 disease has to be evaluated in phase 3 efficacy trials. Four, data of neutralizing antibody titers against emerging variants of SARS-CoV-2 require further studies currently ongoing.
The ethnic diversity of the Cuban population, the wide age range studied and its comorbidities, contributes to the generalisability (external validity, applicability) of the trial findings, although they are still limited the sample size.
In conclusion, the results of the phase 1-2 trial indicated that Abdala vaccine against SARS-CoV-2 was safe, well tolerated and induced humoral immune responses against SARS-CoV-2 among adults from 19 to 80 years of age. Our findings indicate that the a SARS-CoV-2 recombinant spike protein vaccine studied (Abdala) is a promising candidate that warrants testing in phase 3 studies, in a larger number of individuals older than 19 years of age and a three-dose schedule of 50 μg on days 0-14-28, evaluating vaccine efficacy in the prevention of symptomatic COVID-19 and progression to serious and critical forms of the disease.
Contributors
FHB concept and designed this study, was its main coordinator and chief investigator, and took part in the interpretation of the results and paper writing; MCRC, MPM, ZNR, LLL, ECA, JBA, JRN, SVP, MRD, ETF, JMFA, NAAM and LTL were clinical investigators of the trial, participated in volunteers recruitment, data acquisition and interpretation of the results; YMB, JQG, KaUP and KlUP took part in trial coordination and data monitoring; COCC, JLAH and MAV participated in the data management, statistical analyses and results interpretation; JLRR ensured masking of the investigational product and the cold chain throughout the process, as well as compliance with GCPs during vaccination; RRR contributed to the clinical laboratory determinations and the collection and masking of biological samples for immunogenicity analyses; GLP, GEGN, APD and ENR contributed to the immunogenicity analyses; MAA and MLF took part in the trial design and coordination; VLMG participated in the trial design, advice, analyses and interpretation of the results and paper writing. All authors had full access to and verify all the data in the study, and took the decision to submit the paper for publication. The authors FHB, MCRC, YMB, JQG, KaUP, KlUP, COCC, JLAH, MAV and VLMG, verified the study data.
Funding
Centre for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba.
Data sharing
The study protocol is provided in the appendix. Anonymised participant data will be made available when the trial is complete, upon requests directed to the corresponding author (hernandez.bernal@cigb.edu.cu). Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
Declaration of interests
Authors FHB, YMB, JQG, KaUP, KlUP, JLRR, MAV, MLF, GEGN, GLP, MAA, and VLMG, are employees of the Centre for Genetic Engineering and Biotechnology, Havana Network, where Abdala vaccine active ingredient is produced and the formulation was developed. The remaining authors have no conflict of interests. No honoraria, consulting fees or payments for seminar presentations, speeches or appearances have been received by any of the authors.
Acknowledgements
Special thanks to the volunteers in this trial, who maintained maximum adherence to the research protocol. Also, the authors wish to acknowledge the "Saturnino Lora" Hospital Direction and the Public Health Ministry of Cuba for its support. It was also commendable the work done by the independent data monitoring committee.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101383.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W., et al. A novel coronavirus from patients with pneumonia in China 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn DG, Shin HJ, Kim MH, et al. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan HA, Byrareddyb SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deming ME, Michael NL, Robb M, et al. Accelerating Development of SARS-CoV-2 Vaccines — The Role for Controlled Human Infection Models. N Engl J Med. 2020;383(10):e63. doi: 10.1056/NEJMp2020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. COVID-19 Risks and Vaccine Information for Older Adults. Available at: https://www.cdc.gov/aging/covid19/covid19-older-adults.html. Accessed 20 December 2021.
- 6.Naranjo CA, Shear NH, Busto U. In: Principles of medical pharmacology. Kalant H, Roschlau WHE, editors. Oxford University Press; New York: 1998. Adverse drug reactions; pp. 791–800. [Google Scholar]
- 7.CECMED. UMELISA SARS-COV-2 IGG (Cuban Sanitary Registry D2107-11). Available at: https://www.cecmed.cu/covid-19/aprobaciones/umelisa-sars-cov-2-igg. Accessed 27 December 2021.
- 8.Manenti A, Maggetti M, Casa E, et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J Med Virol. 2020;92(10):2096–2104. doi: 10.1002/jmv.25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharif N, Alzahrani KJ, Ahmed SN et al. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: a Systematic Review and Meta-Analysis. Front Immunol. 2021;12:714170. 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed]
- 10.World Health Organization (WHO). Draft landscape of COVID-19 candidate vaccines. WHO https://www.whoint/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (2021). Accessed 15 December 2021.
- 11.Yanjun Z, Gang Z, Hongxing P, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keech C, Albert G, Cho I, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W, Duan K, Zhang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: A randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084. doi: 10.1128/CMR.00084-18. -18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Sande MA, Waight P, Mendy M, et al. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis. 2006;193:1528–1535. doi: 10.1086/503433. [DOI] [PubMed] [Google Scholar]
- 17.Bayas JM, Vilella A, Bertran MJ, et al. Immunogenicity and reactogenicity of the adult tetanus diphtheria vaccine. How many doses are necessary? Epidemiol Infect. 2001;127(3):451–460. doi: 10.1017/s095026880100629x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Wielen M, Van Damme P, Chlibek R, et al. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine. 2006;24:5509–5515. doi: 10.1016/j.vaccine.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 19.D'Acremont V, Herzog C, Genton B. Immunogenicity and safety of a virosomal hepatitis A vaccine (Epaxal) in the elderly. J Travel Med. 2006;3:78–83. doi: 10.1111/j.1708-8305.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- 20.Wolters B, Junge U, Dziuba S, et al. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine. 2003;21:3623–3628. doi: 10.1016/s0264-410x(03)00399-2. [DOI] [PubMed] [Google Scholar]
- 21.Estevez ZC, Betancourt AA, Muzio-Gonzalez V, et al. Immunogenicity and safety assessment of the Cuban recombinant hepatitis B vaccine in healthy adults. Biologicals. 2007;35:115–122. doi: 10.1016/j.biologicals.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Tohme RA, Awosika-Olumo D, Nielsen C, et al. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine. 2011;29:9316–9320. doi: 10.1016/j.vaccine.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hainz U, Jenewein B, Asch E, et al. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23:3232–3235. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 24.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tebas P, Yang S, Boyer JD, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2020;31 doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu FC, Hou LH, Li JX, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;385:2272–2279. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Guo X, Xin Q, et al. Neutralizing Antibodies Responses to SARS-CoV-2 in COVID-19 Inpatients and Convalescent Patients. Clin Infect Dis. 2020;71:2688–2694. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee WT, Girardin RC, Dupuis AP, et al. Neutralizing Antibody Responses in COVID-19 Convalescent Sera. J Infect Dis. 2021;223:47–55. doi: 10.1093/infdis/jiaa673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Q, Mao Q, Zhang J, et al. COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.669339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.