Abstract
Objective
To determine the influence of immunoglobulins (Ig) level on the rate of infections in people with multiple sclerosis (pwMS) treated with ocrelizumab.
Methods
We enrolled 109 consecutive pwMS treated with ocrelizumab with a mean follow-up of 2.69±0.56 (1.36-4.27) years. We have retrospectively searched our electronic database and the following information was collected: age, sex, MS characteristics, number of ocrelizumab cycles, infections, duration of the infection, hospitalization due to infection, treatment of the infection, and COVID-19 characteristics. Ig levels were measured within 14 days before each ocrelizumab infusion.
Results
Number of pwMS with values of IgM and IgG below lower level of normal at baseline was 3 (2.8%) and 2 (2.8%), respectively; and before 6th cycle of ocrelizumab 5 (13.5%) and 5 (13.5%), respectively. Levels of IgM were steadily decreasing over time, while levels of IgG started to show statistically significant drop only after 5th cycle of ocrelizumab. 58.7% pwMS experienced infection during treatment, with a median number of infections per pwMS being 1, range 0-4. Female sex increased the risk of any infection (HR 2.561, 95%CI 1.382-4.774, p=0.003). Higher age and smaller drop in IgM before 3rd ocrelizumab cycle increased the risk for infection requiring hospitalization (HR 1.086, 95%CI 1.018-1.159, p=0.013 and HR 9.216, 95%CI 1.124-75.558, p=0.039, respectively). Longer disease duration increased the risk for COVID-19 (HR 1.075, 95%CI 1.002-1.154, p=0.045).
Conclusion
The present findings broaden limited real-world data on infection and COVID-19 risk in pwMS treated with ocrelizumab.
Keywords: Hypogammaglobulinemia, infections, COVID-19, multiple sclerosis, ocrelizumab
1. Introduction
Ocrelizumab is a recombinant humanized monoclonal antibody that targets CD20-expressing B cells, and is approved for the treatment of relapsing remitting (RRMS) and primary progressive multiple sclerosis (PPMS). Before the introduction of the ocrelizumab, a randomized controlled phase II study showed that rituximab, a chimeric monoclonal CD20 antibody, reduces inflammatory lesions on MRI, as well as the proportion of relapses. (Hauser et al., 2008) Results of this study led to wide off-label use of rituximab in the treatment of all MS phenotypes. It has later been identified from the results of real-world studies that rituximab use was associated with the highest rate of serious infections in people with multiple sclerosis (pwMS) treated with disease modifying therapies. (Luna et al., 2020) The presumed mechanism of increased risk of infections in pwMS on rituximab is hypogammaglobulinemia, one of the most reported laboratory values alterations associated with long-term rituximab treatment. (Chisari et al., 2022)
In both OPERA and ORATORIO clinical trials, which evaluated safety and efficacy of ocrelizumab in people with RRMS (pwRRMS) and people with PPMS (pwPPMS) respectively, infections were the most common adverse events, and pwPPMS in comparison with pwRRMS seem to have higher prevalence of infections. (Gabelić et al., 2021) Moreover, it was demonstrated that a decreased level of serum immunoglobulins, particularly IgG levels, is related to an increased risk of serious infections. (Derfuss et al., 2019, Baker et al., 2020)
The present study aims to determine the influence of immunoglobulins level on the rate of infections in pwMS treated with ocrelizumab.
The primary objective was to investigate the rates and relationship between hypogammaglobulinema and infections in pwMS treated with ocrelizumab.
The secondary objectives were to:
-
1
To investigate the predictors of infections in pwMS treated with ocrelizumab.
-
2
To investigate the rate of different types of infections in pwMS treated with ocrelizumab.
-
3
To investigate the rate and predictors of infections requiring hospitalization in pwMS treated with ocrelizumab.
-
4
To investigate the rate and predictors of COVID-19 in pwMS treated with ocrelizumab.
2. Methods
2.1. Study population
The use of ocrelizumab in pwMS was started in our Center in 2017, initially for pwPPMS, and several months later for pwRRMS. All consecutive patients were considered for inclusion in the study. Inclusion criteria were: 1) diagnosis of RRMS or PPMS, 2) at least 2 cycles of ocrelizumab (600 mg) administered. Exclusion criteria included those participants who stopped treatment after the first cycle due to any cause. If the participant stopped ocrelizumab after the second course, infections were considered up to the timepoint when other DMT was started. Ocrelizumab infusions were given as per summary of product characteristics (https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_hr.pdf, 2022).
The study protocol was approved by the ethical committees of the University Hospital Center Zagreb. The study followed the Declaration of Helsinki and the current European Regulation for Data Protection.
2.2. Medical visits
Patients visited the center twice for each ocrelizumab infusion (one laboratory visit (complete blood count, immunoglobulin (Ig) levels) and one infusion visit), and additional visits were scheduled in case of a relapse or adverse events. All examinations were performed by the same neurologists for each patient (TG, BB, IA and MH) and all participants were questioned whether they experienced any infection since the prior visit.. We have retrospectively searched our electronic database and the following information was collected: age, sex, MS phenotype (RRMS, PPMS), disease duration (years), expanded disability status scale (EDSS), disease activity in the year prior starting ocrelizumab (number of relapses, number of active lesions on the most recent MR), previous therapy, number of ocrelizumab cycles, infections (categorized as respiratory, urinary, skin, gastrointestinal and others), duration of the infection, hospitalization due to the infection, treatment of the infection, COVID-19 status (duration, hospitalization, vaccination). In December 2021, all participants were contacted by phone to check whether there was any missed infections or hospitalization in the electronic charts.
Ig levels were measured within 14 days before each ocrelizumab infusion. The cut-off value for IgG levels was ≥7 g/L. Corresponding levels for IgM were ≥0.4 g/L. Δ IgM and Δ IgG were calculated as IgM/IgG value prior 3rd cycle of ocrelizumab minus IgM/IgG value prior starting the therapy. 3rd cycle of ocrelizumab was chosen as the median cycle prior to infection.
2.3. Statistical analysis
Statistical analysis was performed with the IBM SPSS v25 software. The normality of the distribution was assessed with the Kolmogorov-Smirnov test. Differences between the quantitative variables were tested with the parametric independent sample t-test and non-parametric Mann-Whitney test. Survival analysis was performed in the form of Kaplan-Meier curves in order to estimate the cumulative risk of occurrence of specific event (any infection, infection requiring hospitalization and COVID-19), and in the form of the Cox regression model, which tested effect of age, sex, disease duration, EDSS, MS phenotype (RRMS or PPMS), Δ IgM and Δ IgG on the risk for specific outcomes (any infection, infection requiring hospitalization and COVID-19). P values less than 0.05 were considered as significant.
3. Results
We identified 109 consecutive pwMS who fulfilled inclusion criteria and were included in the final analysis with a cut-of date of December 20, 2021 with a mean follow-up of 2.69±0.56 (1.36-4.27) years. Demographic characteristics of the cohort are presented in Table 1 .
Table 1.
Demographic characteristics of the cohort (N=109).
| Age | 44.6±9.5 |
| Sex (females) | 75 (68.8%) |
| MS phenotype | |
| RRMS | 73 (67%) |
| PPMS | 36 (33%) |
| Disease duration (years) | 8.5±5.8 |
| EDSS | 3.5 (0-7.0) |
| Number of relapses in the previous year | 1 (0-3) |
| Number of active lesions in the most recent MRI (N=100) | 1 (0-20) |
| Previous DMTs | |
| Treatment naïve | 34 (31.2%) |
| 1 previous DMT | 58 (53.2%) |
| 2previous DMTs | 13 (11.9%) |
| ≥3previous DMTs | 4 (3.7%) |
| Previous DMTs* | |
| 1st line injectables | 51 (46.4%) |
| 1st line orals | 21 (19.1%) |
| Azathioprine | 1 (0.9%) |
| Fingolimod | 9 (8.2%) |
| Natalizumab | 1 (0.9%) |
| Alemtuzumab | 2 (1.8%) |
| Rituximab | 1 (0.9%) |
| Duration of previous therapies (months) | 64.3±47.9 |
| Number of ocrelizumab cycles | |
| 2 | 3 (2.8%) |
| 3 | 8 (7.3%) |
| 4 | 19 (17.4%) |
| 5 | 42 (38.5%) |
| 6 | 29 (26.6%) |
| 7 | 5 (4.6%) |
| 8 | 3 (2.8%) |
3.1. Primary objectives
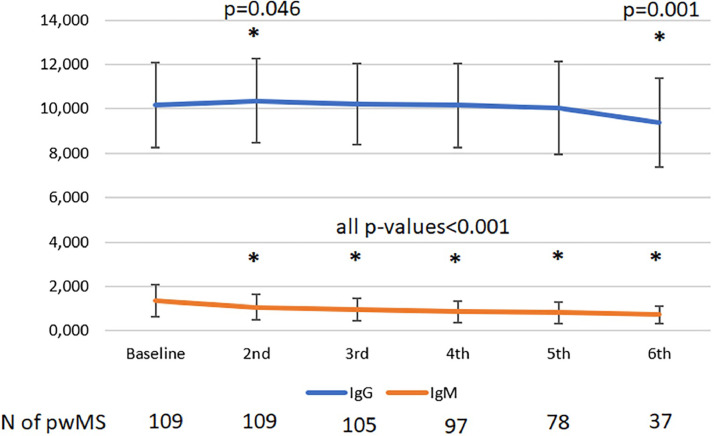
Rates of pwMS with levels of IgM and IgG below lower level of normal are presented in Fig. 1 . Number of pwMS with values of IgM and IgG below lower level of normal at baseline was 3 (2.8%) and 2 (2.8%), respectively; and before 6th cycle of ocrelizumab 5 (13.5%) and 5 (13.5%), respectively. Notwithstanding, levels of IgM are steadily decreasing over time, while levels of IgG started to show statistically significant drop only after 5th cycle of ocrelizumab (Fig. 2 ).
Fig. 1.
Proportion of pwMS with levels of IgM and IgG below lower level of normal.
Fig. 2.
Absolute levels of IgM and IgG before each new cycle of the ocrelizumab.
Rate of infections in the studied cohort is presented in the Table 2 and type of infections in supplementary Table 1. In Fig. 3 a, Kaplan-Meier survival curve is presented showing survival probability for the first infection. There was no difference in pwMS with any infection in Δ IgM and Δ IgG compared to pwMS without infection (-0.44±0.44 vs -0.36±0.23, p=0.252; and -0.08±0.23, p=0.160 vs 0.23±0.97, respectively).
Table 2.
Rate and characteristics of infections in the studied cohort (N=109).
| Any infection | |
|---|---|
| Number of pwMS having any infection | 64 (58.7%) |
| Median number of infections per pwMS | 1 (0-4) |
| Number of infections per pwMS (distribution) | |
| 0 | 45 (41.3%) |
| 1 | 37 (33.9%) |
| 2 | 19 (17.4%) |
| 3 | 7 (6.4%) |
| 4 | 1(0.9%) |
| Type of infection (number of events) | |
| Respiratory | 39 |
| Urinary | 40 |
| Skin | 14 |
| Gastrointestinal | 3 |
| Other | 4 |
| Duration of infections | 10 (2-42) |
| Number of episodes requiring specific treatment | 69 |
| Number of episodes requiring hospitalization | 9 |
| COVID-19 | |
| Number of pwMS with COVID-19 | 35 (32.1%) |
| Number of pwMS with COVID-19 requiring treatment (antibiotics, steroids, remdesivir, antibodies) | 14 (40%) |
| Number of pwMS with COVID-19 requiring hospitalization | 7 (20%) |
| Number of pwMS who received COVID-19 vaccine | 68 (62.4%) |
| 1 dose | 3 (2.8%) |
| 2 doses | 41 (37.6%) |
| 3 doses | 24 (22.0%) |
| Number of pwMS with COVID-19 after vaccination | 11 of 19 (57.9%) |
Fig. 3.
Kaplan-Meier survival curve showing survival probability for a) the first infection, b) infection requiring hospitalization and c) COVID-19.
3.2. Secondary objectives
Results of the univariable and multivariable Cox hazard model analysis investigating possible predictors of risk for any infection are presented in Table 3 . In a univariable model, female sex increased the risk of infection. Out of the total cohort nine (8.3%) pwMS had infection requiring hospitalization. There was no difference in PwMS with infection requiring hospitalization in Δ IgM and Δ IgG compared to pwMS without infection requiring hospitalization (-0.20±0.45 vs -0.42±0.36, p=0.074; and 0.50±2.30 vs 0.01±1.01, p=0.540, respectively). In Fig. 3b Kaplan-Meier survival curve is presented showing survival probability for the hospitalization. Higher age and smaller drop in IgM before 3rd ocrelizumab cycle increased the risk for infection requiring hospitalization.
Table 3.
Results of the univariable and multivariable Cox hazard models for predicting any infection, infection requiring hospitalization and COVID-19 in pwMS treated with ocrelizumab.
| Univariable COX hazard model | Multivariable Cox hazard model | |||||
|---|---|---|---|---|---|---|
| HR | 95% C.I. for HR | p value | HR | 95% C.I. for HR | p value | |
| Any infection | ||||||
| Age | 0.998 | 0.973-1.024 | 0.881 | |||
| Sex | 2.561 | 1.382-4.774 | 0.003 | |||
| Disease duration | 1.01 | 0.968-1.055 | 0.633 | |||
| EDSS | 0.962 | 0.822-1.127 | 0.635 | |||
| MS phenotype | 0.832 | 0.479-1.445 | 0.514 | |||
| Δ IgM | 0.841 | 0.387-1.829 | 0.663 | |||
| Δ IgG | 0.83 | 0.649-1.063 | 0.14 | |||
| Infection requiring hospitalization | ||||||
| Age | 1.099 | 1.030-1.173 | 0.004 | 1.086 | 1.018-1.159 | 0.013 |
| Sex | 0.641 | 0.169-2.431 | 0.513 | |||
| Disease duration | 0.216 | 0.963-1.180 | 0.216 | |||
| EDSS | 0.953 | 0.626-1.452 | 0.824 | |||
| MS phenotype | 1.913 | 0.478-7.655 | 0.359 | |||
| Δ IgM | 11.321 | 1.836-69.824 | 0.009 | 9.216 | 1.124-75.558 | 0.039 |
| Δ IgG | 1.452 | 0.896-2.355 | 0.13 | |||
| COVID-19* | ||||||
| Age | 1.005 | 0.965-1.047 | 0.802 | |||
| Sex | 1.33 | 0.583-3.033 | 0.498 | |||
| Disease duration | 1.09 | 1.025-1.158 | 0.006 | 1.075 | 1.002-1.154 | 0.045 |
| EDSS | 1.211 | 0.976-1.503 | 0.082 | |||
| MS phenotype | 1.071 | 0.454-2.527 | 0.875 | |||
| Δ IgM | 2.369 | 1.014-5.534 | 0.046 | 1.426 | 0.576-3.531 | 0.443 |
| Δ IgG | 0.938 | 0.687-1.280 | 0.686 | |||
adjusted to possible COVID-19 exposure (from March 2020) and number of COVID-19 vaccines received
Characteristics of COVID-19 in the studied cohort are presented in the Table 2. In Fig. 3c Kaplan-Meier survival curve is presented showing survival probability for the developing of COVID-19. There was no difference in PwMS with COVID-19 in Δ IgM and Δ IgG compared to pwMS without COVID-19 (-0.41±0.39 vs -0.40±0.36, p=0.944 and 0.19±1.39 vs -0.02±1.03, p=0.373, respectively).
Results of the univariable and multivariate Cox proportional hazard models, adjusted to possible COVID-19 exposure (from March 2020) and number of COVID-19 vaccines received, investigating predictors for COVID-19 are presented in Table 3. Longer disease duration increased the risk for COVID-19.
4. Discussion
In this observational, retrospective cohort study, 13.5% of pwMS developed low levels of IgM and/or IgG after 5th cycle of ocrelizumab. While levels of IgM were steadily decreasing over time, levels of IgG started to show statistically significant drop only after 5th cycle of ocrelizumab.
Hypogammaglobulinemia is the most frequently observed laboratory abnormality in pwMS treated with ocrelizumab. In a 7-year follow-up of pwMS enrolled in clinical trials or treated in real-world post marketing settings with ocrelizumab, there was a mean relative reduction of 55.8% in serum IgM levels in the OPERA population, characterized by a faster drop in the first year followed by a slower decline. Serum IgG levels decreased at an average rate of −0.33 g/L per year. (Hauser et al., 2021) Real-world data on hypogammaglobulinemia in pwMS on ocrelizumab are limited. In a cohort of pwMS treated with ocrelizumab in Melbourne, Australia, after a mean number of ocrelizumab doses of 4.6, 9.3% and 2.3% of pwMS had levels of IgM and IgG below lower limit of normal. (Seery et al., 2021) Data from the Danish MS registry showed that the rates of low levels of IgM and IgG among pwMS treated with CD20 depleting therapies (rituximab, ocrelizumab and ofatumumab) were 28% and 5%, respectively. (Oksbjerg et al., 2021) Overall, the largest body of evidence related to immunoglobulin levels exists for rituximab. When looking at the different classes of immunoglobulins, IgM was the most frequently affected immunoglobulin whose levels tend to stay low for longer periods than IgG after rituximab cessation and often remain low even after the level of B cells have returned to normal. (Kridin and Ahmed, 2020) As opposed to IgG levels, low IgM levels do not appear to be associated with serious consequences (Kridin and Ahmed, 2020). In several other studies with rituximab in pwMS and other conditions, low levels of IgM were present in >20% of participants during the course of treatment, and low IgG levels ranged from 3.0-4.2% pwMS. (Perriguey et al., 2021, Boleto et al., 2018, Isvy et al., 2012, Vollmer et al., 2020) Interestingly, none of the studies assessing the safety profile of cumulative doses of rituximab in rheumatoid arthritis demonstrated a higher risk of hypogammaglobulinemia with increasing numbers of rituximab cycles. (Boleto et al., 2018, Isvy et al., 2012) Only one study compared low levels of IgM and IgG between pwMS treated with ocrelizumab and rituximab. Levels of IgG dropped 0.16 g/L with each ocrelizumab infusion but remained stable with rituximab. In contrast, levels of IgM decreased to a similar extent with both drugs. (Evertsson et al., 2020)
Low levels of immunoglobulins, especially low levels of IgG have been associated with increased risk of infections in people treated with rituximab. (Perriguey et al., 2021, Marcinnò et al., 2018, Barmettler et al., 2018) Notwithstanding, in randomized clinical trials of rituximab in pwPPMS, serious infections occurred in 4.5% of rituximab-treated patients and in < 1.0% in the placebo, with no clear association to the number of infusions, which corroborates findings from large trials. (Chisari et al., 2022, Hawker et al., 2009)
In the current study, 58.7% pwMS treated with ocrelizumab experienced infection during treatment, with a median number of infections per pwMS being 1, range 0-4. In a 7-year follow-up of pwMS enrolled in clinical trials or treated in real-world post marketing settings with ocrelizumab, the overall rate of infections remained consistent with rates observed during the phase 3 clinical trials program, with a rate of serious infections fluctuating over time, but showed no meaningful year-on-year variation. (Hauser et al., 2021) Older age, higher serum IgA and IgG were associated with reduced odds of infection in pwMS treated with ocrelizumab. (Seery et al., 2021) Another study showed that pwMS with an infection requiring hospitalization were older, more commonly had comorbidities, had longer duration of treatment and higher EDSS scores. (Oksbjerg et al., 2021) Similarly, we have also shown that older age increases the risk for infections requiring hospitalization. In our cohort we didn't find association between IgG levels and infections, however, we found that smaller drop in IgM before 3rd ocrelizumab cycle increased the risk for infection requiring hospitalization. This observation has not so far been reported and warrants further investigation into possible mechanisms.
Several studies have indicated an association between B-cell depleting disease modifying therapy and higher probability of a more serious clinical course of COVID-19. (Sormani et al., 2021, Stastna et al., 2021) In this study we have investigated factors that increase the risk for acquiring COVID-19 in pwMS treated with ocrelizumab. In our cohort, longer disease duration increased the risk for COVID-19, however, there was no association between IgG and IgM levels and increased risk for COVID-19. There is an increasing amount of data suggesting an attenuated humoral response to SARS-COV-2 infection in pwMS using ocrelizumab. (Habek et al., 2021) As well, pwMS treated with ocrelizumab have an attenuated response to COVID-19 vaccines (Sormani et al., 2021). Therefore, extended interval dosing has been proposed as a risk mitigation strategy for COVID-19 infection as it seems that prolonging ocrelizumab dosing does not affect its efficacy (Rolfes et al., 2021, Barun et al., 2021). However, further studies are needed to assess whether this approach may improve the safety and vaccine readiness of pwMS during the COVID-19 pandemic.
The limitations of this study are retrospective design, the absence of a control group and moderate duration of observation time. However, the present findings broaden limited real-world data on infection and COVID-19 risk in pwMS treated with ocrelizumab.
Authors’ contributions
Study concept and design: Habek. Acquisition of data: Habek, Piskač, Gabelić, Barun, Adamec, Krbot Skorić. Analysis and interpretation of data: Habek, Piskač, Gabelić, Barun, Adamec, Krbot Skorić. Drafting of the manuscript: Habek. Critical revision of the manuscript for important intellectual content: Habek, Piskač, Gabelić, Barun, Adamec, Krbot Skorić. Administrative, technical, and material support: Habek, Piskač, Gabelić, Barun, Adamec, Krbot Skorić.
Financial & competing interest disclosure
MH: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
DP: Nothing to disclose.
BB: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals.
TG: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals.
IA: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
MKS: received consultation and/or speaker fees from: Sanofi Genzyme, Roche.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
No funding was received for this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.103798.
Appendix. Supplementary materials
References
- Baker D., Pryce G., James L.K., Marta M., Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: Benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020;44 doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- Barmettler S., Ong M.S., Farmer J.R., Choi H., Walter J. Association of Immunoglobulin Levels, Infectious Risk, and Mortality With Rituximab and Hypogammaglobulinemia. JAMA Netw Open. 2018;1(7) doi: 10.1001/jamanetworkopen.2018.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barun B., Gabelić T., Adamec I., Babić A., Lalić H., Batinić D., Krbot Skorić M., Habek M. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult Scler Relat Disord. 2021;48 doi: 10.1016/j.msard.2020.102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleto G., Avouac J., Wipff J., Forien M., Dougados M., Roux C., Kahan A., Dieude P., Allanore Y. Predictors of hypogammaglobulinemia during rituximab maintenance therapy in rheumatoid arthritis: A 12-year longitudinal multi-center study. Semin. Arthritis Rheum. 2018;48(2):149–154. doi: 10.1016/j.semarthrit.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Chisari C.G., Sgarlata E., Arena S., Toscano S., Luca M., Patti F. Rituximab for the treatment of multiple sclerosis: a review. J. Neurol. 2022;269:159–183. doi: 10.1007/s00415-020-10362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derfuss T., Weber M.S., Hughes R., Wang Q., Sauter A., Koendgen H., Hauser S.L., Bar-Or A., Hartung H.P. Serum immunoglobulin levels and risk of serious infections in the pivotal Phase III trials of ocrelizumab in multiple sclerosis and their open-label extensions. ECTRIMS Online Library. 2019;279399:65. 09/11/ [Google Scholar]
- Evertsson B., Hoyt T., Christensen A., Nimer F.A., Foley J., Piehl F. A comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs low dose of rituximab in multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6(4) doi: 10.1177/2055217320964505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelić T., Barun B., Adamec I., Krbot Skorić M., Habek M. Product review on MAbs (alemtuzumab and ocrelizumab) for the treatment of multiple sclerosis. Hum. Vaccin. Immunother. 2021;17(11):4345–4362. doi: 10.1080/21645515.2021.1969850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habek M., Jakob Brecl G., Bašić Kes V., Rogić D., Barun B., Gabelić T., Emeršič A., Horvat Ledinek A., Grbić N., Lapić I., Šegulja D., Đurić K., Adamec I., Krbot Skorić M. Humoral immune response in convalescent COVID-19 people with multiple sclerosis treated with high-efficacy disease-modifying therapies: A multicenter, case-control study. J. Neuroimmunol. 2021;359 doi: 10.1016/j.jneuroim.2021.577696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Kappos L., Montalban X., Craveiro L., Chognot C., Hughes R., Koendgen H., Pasquarelli N., Pradhan A., Prajapati K., Wolinsky J.S. Safety of Ocrelizumab in Patients With Relapsing and Primary Progressive Multiple Sclerosis. Neurology. 2021;97(16):e1546–e1559. doi: 10.1212/WNL.0000000000012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J., Bar-Or A., Panzara M., Sarkar N., Agarwal S., et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Hawker K., O’Connor P., Freedman M.S., Calabresi P.A., Antel J., Simon J., Hauser S., Waubant E., Vollmer T., Panitch H., Zhang J., Chin P., Smith C.H., OLYMPUS trial group Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009;66(4):460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- Isvy A., Meunier M., Gobeaux-Chenevier C., Maury E., Wipff J., Job-Deslandre C., Kahan A., Allanore Y. Safety of rituximab in rheumatoid arthritis: a long-term prospective single-center study of gammaglobulin concentrations and infections. Joint Bone Spine. 2012;79(4):365–369. doi: 10.1016/j.jbspin.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Kridin K., Ahmed A.R. Post-rituximab immunoglobulin M (IgM) hypogammaglobulinemia. Autoimmun. Rev. 2020;19(3) doi: 10.1016/j.autrev.2020.102466. [DOI] [PubMed] [Google Scholar]
- Luna G., Alping P., Burman J., Fink K., Fogdell-Hahn A., Gunnarsson M., Hillert J., Langer-Gould A., Lycke J., Nilsson P., Salzer J., Svenningsson A., Vrethem M., Olsson T., Piehl F., Frisell T. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2020;77:184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinnò A., Marnetto F., Valentino P., Martire S., Balbo A., Drago A., Leto M., Capobianco M., Panzica G., Bertolotto A. Rituximab-induced hypogammaglobulinemia in patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e498. doi: 10.1212/NXI.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksbjerg N.R., Nielsen S.D., Blinkenberg M., Magyari M., Sellebjerg F. Anti-CD20 antibody therapy and risk of infection in patients with demyelinating diseases. Mult Scler Relat Disord. 2021;52 doi: 10.1016/j.msard.2021.102988. [DOI] [PubMed] [Google Scholar]
- Perriguey M., Maarouf A., Stellmann J.P., Rico A., Boutiere C., Demortiere S., Durozard P., Pelletier J. Audoin B. Hypogammaglobulinemia and Infections in Patients With Multiple Sclerosis Treated With Rituximab. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1115. doi: 10.1212/NXI.0000000000001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_hr.pdf, accesed Jan 18, 2022.
- Rolfes L., Pawlitzki M., Pfeuffer S., Nelke C., Lux A., Pul R., Kleinschnitz C., Kleinschnitz K., Rogall R., Pape K., Bittner S., Zipp F., Warnke C., Goereci Y., Schroeter M., Ingwersen J., Aktas O., Klotz L., Ruck T., Wiendl H., Meuth S.G. Ocrelizumab Extended Interval Dosing in Multiple Sclerosis in Times of COVID-19. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1035. doi: 10.1212/NXI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery N., Sharmin S., Li V., Nguyen A.L., Meaton C., Atvars R., Taylor N., Tunnell K., Carey J., Marriott M.P., Buzzard K.A., Roos I., Dwyer C., Baker J., Taylor L., Spriggs K., Kilpatrick T.J., Kalincik T., Monif M. Predicting Infection Risk in Multiple Sclerosis Patients Treated with Ocrelizumab: A Retrospective Cohort Study. CNS Drugs. 2021;35(8):907–918. doi: 10.1007/s40263-021-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., Musc-19 Study Group Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021;89:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferrò M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A. CovaXiMS study group on behalf of the Italian Covid-19 Alliance in MS. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastna D., Menkyova I., Drahota J., Mazouchova A., Adamkova J., Ampapa R., Grunermelova M., Peterka M., Recmanova E., Rockova P., Rous M., Stetkarova I., Valis M., Vachova M., Woznicova I., Horakova D. Multiple sclerosis, neuromyelitis optica spectrum disorder and COVID-19: A pandemic year in Czechia. Mult Scler Relat Disord. 2021;54 doi: 10.1016/j.msard.2021.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer B.L., Wallach A.I., Corboy J.R., Dubovskaya K., Alvarez E., Kister I. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann Clin Transl Neurol. 2020;7(9):1477–1487. doi: 10.1002/acn3.51136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.