Abstract
Introduction and importance
Vertical transmission of the novel coronavirus, known as severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), has not yet been proven. However, several case reports and case series worldwide, including ours, support this certain type of transmission. Although COVID-19 has been mostly treated supportively, in some cases, including ours, medical treatment seems to be essential.
Case presentation
Herein, we present a case of a neonate born to an asymptomatic mother with no known history of COVID-19 during pregnancy who was diagnosed as an asymptomatic silent carrier following the confirmation of COVID-19 in her newborn. Although bacterial pneumonia, early-onset sepsis, and meconium aspiration syndrome were the possible differential diagnosis, positive COVID-19 real-time reverse transcriptase-polymerase chain reaction (RT-PCR) confirmed the diagnosis. Due to the neonate's critical lung involvement leading to a critical condition, remdesivir, intravenous immune globulin (IVIG) and corticosteroid were administered. The patient fully recovered and was discharged after around 20 days.
Clinical discussion
Although treatment in most cases of neonatal COVID-19 has been mainly supportive, in a few case reports remdesivir, corticosteroids and IVIG have been successfully used. Since a satisfying clinical improvement was not noticed following sepsis workup, all the three aforementioned medications were administered.
Conclusion
Immunomodulatory medications as well as antiviral therapy should be considered in severe neonatal COVID-19 cases, as were shown to be lifesaving in our patient. Interestingly, to date, this case seems to be the youngest survived patient who has received medicines other than supportive care.
Keywords: COVID-19, Neonate, IVIG, Remdesivir, Corticosteroid, Case report
Highlights
-
•
Vertical transmission of the virus should be considered in early onset neonatal COVID-19.
-
•
Critical COVID-19 necessitates medical treatment including IVIG, corticosteroids and/or antiviral therapy.
-
•
IVIG administration plays an important role and could be lifesaving in critical COVID-19 patients.
-
•
Magnesium sulfate 2% can be used as an adjuvant therapy to improve the patient's oxygenation.
1. Background
COVID-19 rapidly turned into a pandemic following the emergence of the first case in Wuhan, China in late November 2019 and is now the most serious health problem worldwide [1], [2], [3]. The disease involves children less than adults while neonates include a small proportion of the affected cases [4], [5], [6]. In Iran, 3,394,279 confirmed COVID-19 cases have been reported till July 3rd, 2021 leading to 86,041 deaths [7]. The vertical transmission of the virus has been increasing, although it has not yet been proven [4], [6], [8]. Our case along with some other reported cases from all around the world [9], [10], [11], [12], seem to further support this route of transmission. Children and specially neonates with COVID-19 are mostly asymptomatic or develop mild symptoms for which supportive care usually suffices [13], [14], [15]. To date, our case seems to be the youngest survived patient among the very few neonatal cases who have received antiviral therapy (remdesivir), IVIG and corticosteroids due to respiratory failure and the critically unstable status resulting from COVID-19 [6], [14], [16], [17], [18], [19].
2. Case presentation
Herein, a case of a neonate with COVID-19 born in HAKIM hospital, Neyshabur, Iran is reported in line with the SCARE 2020 criteria. [20]
On April 10th 2021 at 6:10 a.m. the obstetrician called the resuscitation team to attend the anticipated vaginal birth of a term (39w + 4d) baby boy. He was born to a 25-year-old mother (gravida 3, live 3) whose membranes had been ruptured 1 h prior to delivery. The mother had a history of hypothyroidism which had been under control as well as meconium-stained amniotic fluid. Fetal heart rate, amniotic fluid level and the non-stress test were all normal. The mother's vital signs were also in the normal range. It should be noted that COVID-19 screening is not routinely performed for all pregnant women in our medical center. Moreover, the mother did not report any signs or symptoms in favor of COVID-19 or any other infections.
The baby was born at 6.20 a.m. with good muscle tone and cried vigorously. Upon his birth our center's neonatologist was present in delivery room. His 1st minute Apgar score was 9. His birth weight, head circumference and length were within normal limits. However, 2 min after birth, respiratory distress initiated along with cyanosis and his 5th minute Apgar score reduced to 6. With respect to the oxygen saturation level (SpO2) of about 60%, nasal continuous positive airway pressure (NCPAP) was administered leading to a rise in SpO2 level. He was immediately transferred to the neonatal intensive care unit (NICU) where the SpO2 level dropped once again. On physical examination performed by the neonatologist, respiratory symptoms including tachypnea, mild intercostal and moderate subcostal retraction, nasal flaring and grunting were diagnosed. Pulses in all four extremities were full and symmetrical. His vital signs were as follows: temperature = 37.1 °C (Axillary), respiratory rate = 70 breaths/min, heart rate = 152 bpm, blood pressure = 69/42 mmHg and SpO2 = 60–65%. He had decreased sucking, but normal grasp and Moro reflexes. Despite the increase in the inspiratory fraction of oxygen (FIO2) to 0.6, he still suffered from a SpO2 of about 60–65%. Given the NCPAP failure, the mode was changed to noninvasive intermittent positive-pressure ventilation (NIPPV); yet the SpO2 did not increase. After endotracheal suctioning using a meconium aspirator (no secretions or meconium substance was noted), he was intubated. Despite symmetrical respiratory sounds, his SpO2 still remained at about 65% and did not rise, so the ventilator supporting set up was gradually increased.
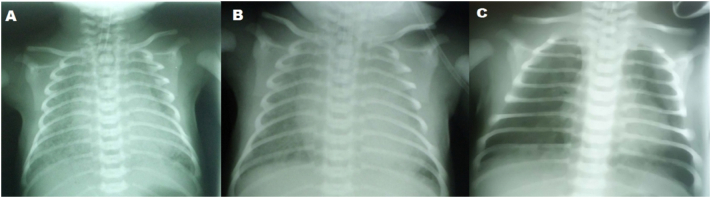
In the next step, sepsis workup was performed. Bedside chest radiograph (CXR) demonstrated diffuse patchy granular infiltrates compatible with meconium aspiration syndrome (MAS) (Fig. 1). The 1st venous blood gas (VBG) revealed respiratory acidosis (Fig. 2); therefore surfactant (Beraksurf ®) was administered (2.5 cc/kg) endotracheally through a feeding tube.
Fig. 1.
Chest Radiographies
Bilateral diffuse reticular and ground glass opacities in a newborn, without consolidation or pleural effusion (A). These abnormalities did not change significantly after twice intratracheal surfactant instillation (B). However, significant resolution of lesions was noted after remdesivir, corticosteroid and IVIG administration (C).
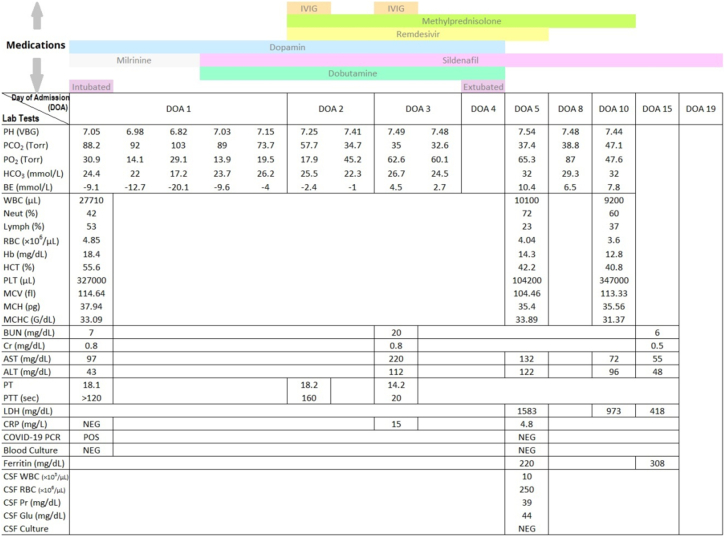
Fig. 2.
Laboratory tests and medications.
PH, potential of hydrogen; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; HCO3: bicarbonate; BE, base excess; VBG, venous blood gas; WBC, white blood cell; Neut, neutrophil; Lymph, lymphocyte; RBC, red blood cell; Hb, hemoglobin; HCT, hematocrit; PLT, platelet; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; BUN, blood urea nitrogen; Cr, creatinine; AST, aspartate transaminase; ALT, alanine transaminase; PT, prothrombin time; PTT, partial thromboplastin time; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCR, polymerase chain reaction; CSF, cerebrospinal fluid; Pr, protein; Glu, glucose; POS, positive; Neg, negative. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
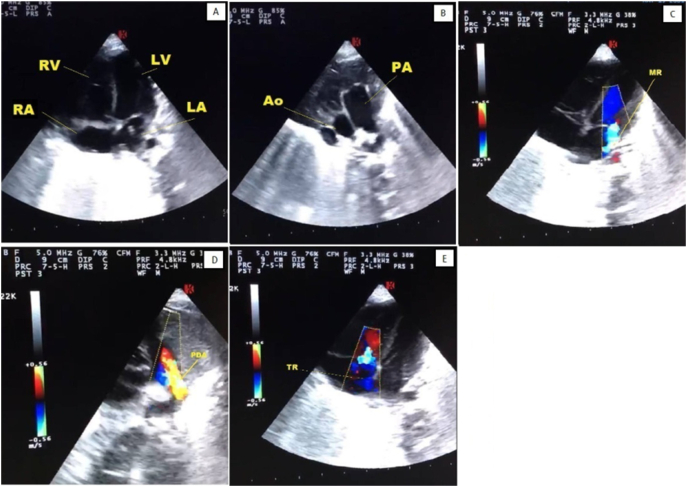
Despite the aforementioned interventions, SpO2 decreased even further. As pulmonary hypertension (pH) was also a probable diagnosis, milrinone (0.3 μg/kg/min) and dopamine (5μg/kg/min) which were later changed to sildenafil (1 mg/kg/dose four times a day) and dobutamine (5 μg/kg/min) upon cardiology consult were administered. Echocardiographic evaluation confirmed severe PH (Fig. 3). Captopril (0.01 mg/kg/dose twice a day) was also added to decrease the afterload. As the patient's condition did not improve following the 1st dose of surfactant administration and regarding severe lung involvement, COVID-19 real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on the endotracheal aspirate at the 4th hour of life.
Fig. 3.
Echocardiography
Transthoracic echocardiogram of the patient showing patent ductus arteriosus (D), mitral regurgitation (C), tricuspid regurgitation (E), dilated right atrium, right ventricle and main pulmonary artery (A,B) and pulmonary hypertension (PH).
The VBG taken 2 h after surfactant administration had worsened (Fig. 2). Hence, peak inspiratory pressure (PIP) was increased and the positive end-expiratory pressure (PEEP) was reduced. Regarding the CXR pattern taken 6 h after surfactant administration and the high ventilator supporting set up, the second dose of surfactant (1.25 cc/kg) was administered 10 h after the first one.
Gradually, SpO2 increased and the VBG improved (Fig. 2), and by the 24th hour of life although no significant improvement was noticed in his CXR, SpO2 reached 90% while the ventilator supporting set up was still quite high.
COVID-19 RT-PCR result was declared positive at 24 h of admission (day 2). Accordingly, it was decided to initiate remdesivir (5 mg/kg/dose on day one followed by 2.5 mg/kg/dose once daily on days 2–5), IVIG (1 g/kg/day, two consecutive days) and methylprednisolone (0.5 mg/kg/dose, twice a day) since the baby had diffuse lung involvement, was still intubated and had shown no improvement (day 2). Liver and kidney function tests were also performed and repeated every other day to monitor possible remdesivir induced toxicity (Fig. 2); ferritin level was also requested.
On day 4, inotropes were tapered and fentanyl dosage was reduced in preparation for the anticipated extubation. 48 h after the addition of remdesivir, IVIG and corticosteroid the need for ventilatory support decreased; finally he was successfully extubated and NIPPV was initiated due to his effective respiratory effort and persistent SpO2 of about 90–95%.
On Day 5 of his life (72 h following remdesivir, corticosteroid and IVIG administration) the inotropes were ceased and CXR was repeated (revealing significant improvement). COVID-19 PCR was also repeated and reported as negative.
After seven days of methylprednisolone administration, it was gradually tapered until discontinuation on day 14.
Due to the mild nonpersistent SpO2 drops, tachypnea of about 65–70 breaths/min and oxygen dependency, nebulized magnesium sulfate 2% (given by blender) was initiated on day 7 in order to decrease the airway hyperreactivity and inflammatory reactions. Magnesium sulfate 2% inhalation stopped the SpO2 drops in 48 h while tachypnea improved gradually; after about 13 days of administration, the patient was finally discharged with no need for further oxygen therapy. Regular follow up visits were planned for the patient. In his last follow up visit in our hospital's clinic about two months later he was quite well and no respiratory problem was noticed.
Upon a positive COVID-19 RT PCR in our patient, his mother was also tested and turned out to be positive the day after. As she did not develop any signs or symptoms compatible with COVID-19 in the next 20 days she was declared as a silent carrier.
3. Discussion
Our patient was born to an asymptomatic mother and became symptomatic at the second minute of life. He developed early respiratory distress, persistent hypoxemia and respiratory failure. These symptoms are compatible with severe MAS, pneumonia and sepsis while pneumonia or sepsis could each be the stimulus for the development of MAS [21]. Hence, sepsis workup was performed and antibiotics were initiated. Considering severe hypoxemia, persistent pulmonary hypertension resulting from MAS was highly suspected; so early treatment was initiated (nitric oxide is not available in our country).
As he was born during the peak incidence of COVID-19 in our district, when no improvement was achieved following surfactant administration (for suspected MAS-associated respiratory distress syndrome), COVID-19 was also suspected and was later confirmed. SARS-CoV-2 infection could also be a possible etiology for MAS.
The very early manifestation of the disease in our patient along with the positive COVID-19 PCR of both the neonate and his mother highly implies the vertical acquisition of the infection, as was also previously suggested by several other researchers [9], [17], [22], [23].
To the best of our knowledge, COVID-19 might undergo 4 phases in the body: viral entry and invasion, host immune response, the hyperinflammatory phase, and multiorgan dysfunction [24], [25]. According to the clinical symptoms and paraclinic findings, our patient seemed to be in the late phase 2 and early phase 3. Although treatment in most cases of neonatal COVID-19 has been mainly supportive, in a few case reports remdesivir which is approved in emergency situations for all ages [26], corticosteroids and IVIG have been successfully used [14], [16], [17], [18]. IVIG, a systemic immune modulator, has been successfully used in the early phase of acute respiratory distress leading to disease progression prevention and morbidity reduction [27].
Since our patient did not show any remarkable improvement in the first 48 h of antibiotics and surfactant administration and regarding the diagnosis of COVID-19, it seemed reasonable to initiate remdesivir, corticosteroid and IVIG. Eventually, 48 h after the addition of the mentioned medications, the need for ventilatory support decreased significantly and he was extubated successfully. Remarkable radiologic improvement developed in 4 days and was not typically compatible with bacterial pneumonia which is usually expected to persist for weeks [28]. Taken together, the patient's improvement seemed to be mainly related to the latter added medications.
Antibodies produced against viral agents are usually of the IgG class which is actively transported across the placenta. IgG activates the complement system through the classic pathway and amplifies the inflammatory response by increasing leukocyte chemotaxis and complement-mediated opsonization. At the time of birth IgG (from maternal origin) is the major antibody found in a newborn's circulation, whereas its concentration begins to decrease postnatally reaching a nadir at about 3–4 months of age. Sarah Gee et al. reported some immunity system alterations in neonates born to mothers with recent or ongoing SARS-CoV-2 infection, one of which is the reduced transfer of SARS-CoV-2-specific IgG to neonates due to impaired placental antibody transfer [29]. Taking this into account along with IVIG mechanisms of action [27], [30] and its effectiveness in viral clearance, radiologic remission and clinical improvement [31], [32], [33], it seems that the IVIG administration played an important role in viral clearance, radiologic remission and clinical improvement of our patient as well.
Furthermore, upon remdesivir initiation, kidney and liver function tests were monitored regularly. In this case aspartate transaminase (AST) and alanine transaminase (ALT) showed a slight increase which was less than the limit necessitating its discontinuation [34]. However, as they began to decrease in 2 days, the remdesivir course was completed successfully.
Magnesium sulfate 2%, a smooth muscle relaxant, exerts anti-cytokine effects, reduces airway hyperreactivity, and induces bronchodilation and vasodilation, hence improving the ventilation-perfusion mismatch. Therefore, it can be used as an adjuvant therapy to improve the patient's oxygenation (comparable to Nitric oxide) [35], [36]. Moreover, the systemic adverse reactions of magnesium sulfate 2% could be reduced through using its nebulized form instead of the systemic forms [35], [36]. In our patient magnesium sulfate 2% inhalation could have improved post-extubation transient hypoxemia. After about 20 days the patient was discharged with no need for oxygen therapy. In his follow up visits he was quite well and no respiratory problem was noticed.
4. Conclusion
This case is the youngest of the total six neonates who have been treated with remdesivir, IVIG and corticosteroids worldwide. Although further studies are recommended to better elucidate the appropriate treatment approach in neonates, the early administration of remdesivir along with corticosteroids and IVIG should be considered in severe neonatal COVID-19 cases. These medications in combination with supportive care could play a lifesaving role in such patients.
Abbreviations
- SARS-CoV-2
severe acute respiratory syndrome-coronavirus-2
- COVID-19
coronavirus disease 2019
- IVIG
intravenous immune globulin
- SpO2
oxygen saturation level
- NCPAP
nasal continuous positive airway pressure
- NICU
neonatal intensive care unit
- FIO2
inspiratory fraction of oxygen
- NIPPV
noninvasive intermittent positive-pressure ventilation
- CXR
chest X-ray; chest radiograph
- MAS
meconium aspiration syndrome
- VBG
venous blood gas
- PH
pulmonary hypertension
- RT-PCR
real-time reverse transcriptase-polymerase chain reaction
- PIP
peak inspiratory pressure
- PEEP
positive end-expiratory pressure
- AST
aspartate transaminase
- ALT
alanine transaminase
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
The authors gratefully acknowledge the financial support given by the Neyshabur University of Medical Sciences, Neyshabur, Iran (Grant Number: 9901222) (Ethics Code: IR.NUMS.REC.1400.014).
Ethical approval
Ethics Code: IR.NUMS.REC.1400.014.
Consent
Written informed consent was obtained from the parents for publication of this case report and accompanying images. A copy of the written consent in our native language is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
FK-M conceptualized and designed the study, drafted the initial manuscript, collected and designed data, carried out the initial analyses, reviewed and revised the manuscript. NP Pouralizadeh collected data, coordinated and supervised data collection. GP revised the manuscript structurally and scientifically. S S, MG and MP reported the radiograph, performed the echocardiography and helped in drafting the initial manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Registration of research studies
Not applicable.
Guarantor
The corresponding author is the guarantor of submission.
Declaration of competing interest
The authors have no conflict of interest to declare.
References
- 1.Gholipour S., Shamsizadeh Z., Moazeni M., Nikaeen M. Environmental aspects of the coronaviruses transmission: a narrative review. J. Isfahan Med. Sch. 2020:206–215. [Google Scholar]
- 2.Yousefi M., Oskoei V., Jafari A.J., Farzadkia M., Firooz M.H., Abdollahinejad B., Torkashvand J. Municipal solid waste management during COVID-19 pandemic: effects and repercussions. Environ. Sci. Pollut. Res. 2021;28:32200–32209. doi: 10.1007/s11356-021-14214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gholipour S., Nikaeen M., Manesh R.M., Aboutalebian S., Shamsizadeh Z., Nasri E., Mirhendi H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) contamination of high-touch surfaces in field settings. Biomed. Environ. Sci. 2020;33:925–929. doi: 10.3967/bes2020.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayak M., Panda S., Pradhan J.B., Mohakud N.K. Coronavirus disease 2019 in neonates-what is known and what needs to be known. Cureus. 2020;12 doi: 10.7759/cureus.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyedi S.J., Shojaeian R., Hiradfar M., Mohammadipour A., Alamdaran S.A. Coronavirus disease 2019 (COVID-19) outbreak in pediatrics and the role of pediatricians: a systematic review. Iran. J. Pediatr. 2020;30 [Google Scholar]
- 6.Kim D.-H. Clinical and Experimental Pediatrics. Vol. 64. 2021. Clinical implications of coronavirus disease 2019 in neonates; p. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO; 2021. World Health Organization. [Google Scholar]
- 8.Barrero-Castillero A., Beam K.S., Bernardini L.B., Ramos E.G.C., Davenport P.E., Duncan A.R., Fraiman Y.S., Frazer L.C., Healy H., Herzberg E.M. COVID-19: neonatal–perinatal perspectives. J. Perinatol. 2021;41:940–951. doi: 10.1038/s41372-020-00874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajith S., Reshmi V., Nambiar S., Naser A., Athulya B. Prevalence and risk factors of neonatal Covid-19 infection: a single-Centre observational study. J. Obstet. Gynaecol. India. 2021;71:235–238. doi: 10.1007/s13224-021-01436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni R., Rajput U., Dawre R., Valvi C., Nagpal R., Magdum N., Vankar H., Sonkawade N., Das A., Vartak S. Early-onset symptomatic neonatal COVID-19 infection with high probability of vertical transmission. Infection. 2021;49:339–343. doi: 10.1007/s15010-020-01493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trevisanuto D., Cavallin F., Cavicchiolo M.E., Borellini M., Calgaro S., Baraldi E. Coronavirus infection in neonates: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2021;106:330–335. doi: 10.1136/archdischild-2020-319837. [DOI] [PubMed] [Google Scholar]
- 14.Wardell H., Campbell J.I., VanderPluym C., Dixit A. Severe acute respiratory syndrome coronavirus 2 infection in febrile neonates. J. Pediatr. Infect. Dis. Soc. 2020;9:630–635. doi: 10.1093/jpids/piaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gharehbaghi G., Yousefzadegan S., Javid A., Riazi-Esfahani H., Mousavi A., Mahdavynia S., Zare Mahmoudabadi R., Radgoodarzi M., Vafaee-Shahi M. COVID-19 in children and neonates: a comprehensive review article. Iran. J. Pediatr. 2021;31 [Google Scholar]
- 16.Saikia B., Tang J., Robinson S., Nichani S., Lawman K.-B., Katre M., Bandi S. Neonates with SARS-CoV-2 infection and pulmonary disease safely treated with remdesivir. Pediatr. Infect. Dis. J. 2021;40:e194–e196. doi: 10.1097/INF.0000000000003081. [DOI] [PubMed] [Google Scholar]
- 17.Hopwood A.J., Jordan-Villegas A., Gutierrez L.D., Cowart M.C., Vega-Montalvo W., Cheung W.L., McMahan M.J., Gomez M.R., Laham F.R. Severe acute respiratory syndrome coronavirus-2 pneumonia in a newborn treated with remdesivir and coronavirus disease 2019 convalescent plasma. Journal of the Pediatric Infectious Diseases Society. 2021;10:691–694. doi: 10.1093/jpids/piaa165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frauenfelder C., Brierley J., Whittaker E., Perucca G., Bamford A. Infant with SARS-CoV-2 infection causing severe lung disease treated with remdesivir. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1701. [DOI] [PubMed] [Google Scholar]
- 19.Trieu C., Poole C., Cron R.Q., Hallman M., Rutledge C., Bliton K., Phillips A., Lawrence M., Boppana S.B., Pinninti S. Severe neonatal coronavirus disease 2019 presenting as acute respiratory distress syndrome. The Pediatric Infectious Disease Journal. 2020;39:e367–e369. doi: 10.1097/INF.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 20.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., Beamish A.J., Noureldin A., Rao A., Vasudevan B., The S.C.A.R.E. Guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84(2020):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Olicker A.L., Raffay T.M., Ryan R.M. Neonatal respiratory distress secondary to meconium aspiration syndrome. Children. 2021;8:246. doi: 10.3390/children8030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sisman J., Jaleel M.A., Moreno W., Rajaram V., Collins R.R., Savani R.C., Rakheja D., Evans A.S. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr. Infect. Dis. J. 2020;39:e265–e267. doi: 10.1097/INF.0000000000002815. [DOI] [PubMed] [Google Scholar]
- 24.Bohn M.K., Hall A., Sepiashvili L., Jung B., Steele S., Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology. 2020;35:288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar M., Al Khodor S. Pathophysiology and treatment strategies for COVID-19. J. Transl. Med. 2020;18:1–9. doi: 10.1186/s12967-020-02520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., Hall M.D. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS centralScience. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolahchi Z., Sohrabi H., Ekrami Nasab S., Jelodari Mamaghani H., Keyfari Alamdari M., Rezaei N. Potential therapeutic approach of intravenous immunoglobulin against COVID-19. allergyAsthma & Clinical Immunology. 2021;17:105. doi: 10.1186/s13223-021-00609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin R.J., Fanaroff A.A., Walsh M.C. Fanaroff and Martin's Neonatal Perinatal Medicine: Diseases of the Fetus and Infant. 2020. p. 1218. [Google Scholar]
- 29.Gee S., Chandiramani M., Seow J., Pollock E., Modestini C., Das A., Tree T., Doores K.J., Tribe R.M., Gibbons D.L. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 2021;22:1490–1502. doi: 10.1038/s41590-021-01049-2. [DOI] [PubMed] [Google Scholar]
- 30.Galeotti C., Kaveri S.V., Bayry J. Intravenous immunoglobulin immunotherapy for coronavirus disease-19 (COVID-19) Clin. Transl. Immunol. 2020;9 doi: 10.1002/cti2.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peshbahar S., Bendstrup E. Remarkable benefits of intravenous immunoglobulin (IVIG) in a patient with polymyositis-associated acute interstitial lung disease. Eur. Clin. Respir. J. 2020;7:1840706. doi: 10.1080/20018525.2020.1840706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gueta I., Shoenfeld Y., Orbach H. Intravenous immune globulins (IVIg) treatment for organizing pneumonia in a selective IgG immune deficiency state. Immunol. Res. 2014;60:165–169. doi: 10.1007/s12026-014-8571-7. [DOI] [PubMed] [Google Scholar]
- 33.Perricone C., Triggianese P., Bursi R., Cafaro G., Bartoloni E., Chimenti M.S., Gerli R., Perricone R. Intravenous immunoglobulins at the crossroad of autoimmunity and viral infections. Microorganisms. 2021;9:121. doi: 10.3390/microorganisms9010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panel C.-T.G. National Institutes of Health; 2022. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [PubMed] [Google Scholar]
- 35.Pourdowlat G., Mousavinasab S.R., Farzanegan B., Kashefizadeh A., Meybodi Z.A., Jafarzadeh M., Baniasadi S. Evaluation of the efficacy and safety of inhaled magnesium sulphate in combination with standard treatment in patients with moderate or severe COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:60. doi: 10.1186/s13063-021-05032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pooransari P., Pourdowlat G. Magnesium sulfate: APotential adjuvant treatment on COVID-19. Front. Emerg. Med. 2021;5 [Google Scholar]