Abstract
Objective
To conduct a systematic review and meta-analysis to summarize evidence regarding differential changes in physical activity (PA) involvements and exercise habits in people with and without chronic diseases during the COVID-19 outbreak.
Data Sources
MEDLINE, Embase, SPORTDiscus, Cumulative Index to Nursing and Allied Health, PsycINFO, Cochrane Library, and Physiotherapy Evidence Database were searched from November 2019 to May 2021.
Study Selection
Two reviewers independently screened cross-sectional and longitudinal studies that investigated changes in PA-related outcomes in people with and without chronic diseases during the pandemic.
Data Extraction
PA-related outcomes and sedentary time were extracted from the included studies. Relevant risk of bias were assessed. Meta-analyses were conducted for each PA-related outcome, if applicable. Quality of evidence of each PA-related outcome was evaluated by Grading of Recommendations Assessment, Development, and Evaluation.
Data Synthesis
Of 1226 identified citations, 36 articles (28 with and 8 without chronic diseases) with 800,256 participants were included. Moderate evidence from wearable sensors supported a significant reduction in pooled estimates of step count (standardized mean differences [SMD]=−2.79, P<.01). Very limited to limited evidence substantiated significant decreases in self-reported PA-related outcomes and significant increases in sedentary behaviors among people with and without chronic diseases. Specifically, pooled estimates of metabolic equivalent-minute per week (SMD=−0.16, P=.02) and PA duration (SMD=−0.07, P<.01) were significantly decreased, while sedentary time (SMD=0.09, P=.04) showed significant increases in the general population (small to large effects). Very limited evidence suggested no significant PA changes among people in a country without lockdown.
Conclusions
During the pandemic, objective and self-reported assessments showed significant reductions in PA in people with and without chronic diseases globally. This mainly occurred in countries with lockdowns. Although many countries have adopted the “live with the coronavirus” policy, authorities should implement population-based strategies to revert the potential lockdown-related long-term deleterious effects on people's health.
Keywords: COVID-19, Exercise habit, Rehabilitation, SARS-CoV-2, Sedentary behavior
List of abbreviations: IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent task; MVPA, moderate to vigorous physical activity; PA, physical activity; SMD, standardized mean difference; T2D, type 2 diabetes; WHO, World Health Organization
The COVID-19 pandemic posed a menacing threat to global public health. After the first COVID-19 case reported in Wuhan, China, in December 2019,1 the disease has rapidly plagued the globe, inflicting unprecedented negative effects on the global socioeconomic and health care systems. As of September 2021, a total of 221 countries had been struck by COVID-19, resulting in more than 248 million infected cases and over 5 million deaths.2 Countries with lower national income and suboptimal medical services are more vulnerable to the negative consequences of the COVID-19 pandemic, including changes in health behaviors such as physical activity (PA) participation.3
Given the escalating number of confirmed COVID-19 cases and overburdened health care systems, the World Health Organization (WHO) declared the COVID-19 outbreak as a pandemic.2 Most governments implemented stringent measures, including travel ban, nationwide quarantine, social distancing, and lockdowns to suppress the outbreak.2 Approximately 4 billion people were confined to their homes, while more than 90 countries or regions had imposed lockdowns by April 2020.4
Prolonged lockdowns have a negative effect on people's physical, psychological, and social health.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Reduced PA or exercise participation, alongside increased sedentary behaviors, could compromise the physical and mental health of many individuals.40 , 41 The WHO recommends adults to perform 150-300 minutes of moderate intensity or 75-150 minutes of vigorous intensity aerobic PA every week.42 People with chronic diseases, who are recommended to do regular exercises to delay their disease progression,43 , 44 may be more susceptible to the adverse effect of reduced PA. Reduced PA in these patients not only may affect their disease progression but also increases their risk of developing additional inactivity-related diseases. Regular moderate to vigorous PA (MVPA) can boost immunity against community-acquired infectious diseases and increase potency of vaccination. Although an earlier systematic review has summarized the preliminary effects of the COVID-19 pandemic lockdown on PA changes of the public,45 it was limited by small representative samples and lack of assessments of evidence or meta-analyses regarding the effects of the pandemic on various PA-related outcomes among people with and without chronic diseases in countries with or without lockdowns. Because PA changes measured by wearable sensors may differ from those collected from self-reported PA questionnaires, comprehensive meta-analyses of various PA-related outcomes can better inform policy makers in developing tailored strategies to revert the adverse effects of physical inactivity in vulnerable subgroups during and after the pandemic. The current systematic review and meta-analysis addressed this gap to summarize the evidence regarding effects of the COVID-19 pandemic on PA-related outcomes in the people with and without chronic diseases who did not contract COVID-19.
Methods
The study protocol was registered on PROSPERO (CRD42021234936). The Preferred Reporting Items of Systematic Reviews and Meta-Analyses guidelines46 were adopted to report this review.
Search strategy
A systematic literature search was conducted on 7 databases (MEDLINE, EMBASE, SPORTDiscus, Cumulative Index to Nursing and Allied Health, PsycINFO, Cochrane Libraries, Physiotherapy Evidence Database) to identify articles published between November 1, 2019, and May 31, 2021, without any language restrictions. We searched these databases using a combination of 2 sets of keywords: [‘COVID’ OR ‘cov*’ OR ‘corona*’ OR ‘severe acute respiratory syndrome coronavirus 2’ OR ‘SARS*’] AND [‘physical activit*’ OR ‘activity level’ OR ‘exercise habit*’ OR ‘exercise routine*’ OR ‘lifestyle’] (appendix 1). Additional relevant articles were searched from the reference lists of the included studies. Forward citation tracking was conducted using Scopus. The corresponding authors of the included articles were contacted by emails to identify any additional relevant publications.
Selection criteria
Cross-sectional and longitudinal studies that investigated PA-related outcomes during the COVID-19 pandemic were included. Articles were excluded if the participants were actively or previously infected with COVID-19. Commentaries, letters to editors, reviews, conference proceedings, and qualitative studies were also excluded.
Study selection
All citations identified from database searches were exported to EndNote X9 (Clarivate).a After removing duplicates, 2 reviewers (K.Y.N., K.H.W.) independently screened the titles and abstracts following the selection criteria. They piloted on 100 abstracts to align discrepancy. They then independently screened the remaining references. Abstracts deemed relevant were included for full-text screening. The process was repeated for the full-text screening. Reviewers met to reach a consensus about the eligible articles. If disagreements persisted, a third reviewer (K.C.K.) arbitrated the disagreements. The interrater agreement was calculated using Cohen's κ.47
Data extraction
Two independent reviewers (K.Y.N., K.H.W.) used a standardized form to extract data related to authors, year of publication, study location, study design, data collection methods, response and attrition rate, participants’ demographics, definitions of PA and sedentary behaviors, changes in PA-related outcomes, and the corresponding statistics.
Risk of bias assessments
Two independent reviewers (K.Y.N., K.H.W.) used 2 separate tools to assess the quality of the included studies. Specifically, the methodological quality of cross-sectional studies was assessed by the 20-item Appraisal Tool for Cross-Sectional Studies.48 The tool only provides descriptive assessments without numeric scores. It is flexible for researchers to use it based on their priorities. Therefore, we rearranged the items into 6 domains: objectives and design, study participation, handling of nonrespondents, outcome measures, statistical analysis, and reporting.49 Similar to our previous reviews,49 , 50 each domain was ranked as low, moderate, or high based on the criteria listed in appendix 2. The Quality in Prognosis Studies tool was used to assess the methodological quality of longitudinal studies.51 , 52 The Quality in Prognosis Studies tool comprises 6 domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting.52 Each domain was rated as low, moderate, or high.52 From the quality of each domain, the overall methodological quality was graded as low, moderate, or high52 (see appendix 2).
Data synthesis
PA-related outcomes extracted from the included studies were categorized into 2 pairs of subgroups: (1) the people with and without chronic diseases and (2) countries with and without lockdown. If 2 or more included studies reported changes in a particular PA-related outcome during the pandemic in a given subgroup, the respective standardized mean differences (SMDs) were pooled for a meta-analysis using a random-effects model. All meta-analyses were performed using the Comprehensive Meta-analysis Version 3.3 software.b Statistical heterogeneity of the included studies was assessed by I² statistics and classified as low (I²<40%), moderate (I²=40%-59%), substantial (I²=60%-74%), and considerable (I²>75%) heterogeneity.53 The potential sources of heterogeneity of each meta-analysis were explained if substantial or considerable statistical heterogeneity was observed.53
Quality of evidence
The quality of evidence of each PA-related outcome was rated by the Grading of Recommendations Assessment, Development, and Evaluation.54 The Grading of Recommendations Assessment, Development, and Evaluation framework consists of 7 domains, 5 of which could downgrade the quality of evidence regarding the estimated effect size, while the other 2 domains could increase the confidence in the estimated effect size. The synthesized data was ranked as very limited, limited, moderate, or high quality of evidence regarding how the true effect lays close to the estimated effect (see appendix 2).55
Results
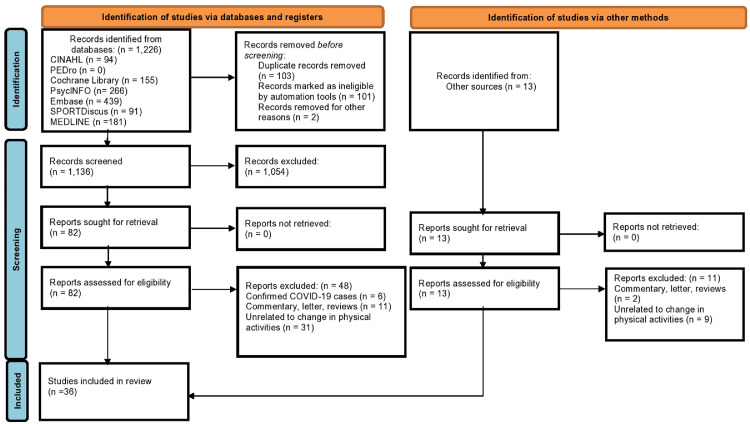
The literature search identified 1226 publications, while 13 records were identified through other sources (fig 1 ). After removing 103 duplicates, 1136 studies were eligible for the title and abstract screening. Of the 95 screened full-text articles, 36 articles were included.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 , 56 Fifty-nine full-text articles were excluded because they did not investigate PA changes (n=40); they included confirmed COVID-19 cases (n=6); or they were commentaries, letters or reviews (n=13). Our κ coefficients showed substantial (κ=0.75) and almost perfect (κ =0.93) agreements between the 2 reviewers (K.Y.N., K.H.W.) during the title/abstract screening and full-text screening, respectively.47
Fig 1.
Flowchart of the systematic review according to Preferred Reporting Items of Systematic Reviews and Meta-Analyses guidelines.
Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health; PEDro, Physiotherapy Evidence Database.
Characteristics of the included studies
The 36 included studies recruited 800,256 participants from Asia, Africa, Australia, Europe, and North and South America. Thirty-five studies were conducted in countries or regions with lockdowns,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 while a Swedish study was conducted without lockdown.56 The participants’ mean ages ranged from 7.3-74.0 years. Table 1 summarizes participants’ demographics in the included studies. Twenty-three studies adopted a cross-sectional design,6 , 7 , 9, 10, 11, 12 , 14 , 15 , 17 , 18 , 21, 22, 23, 24, 25 , 27 , 28 , 30, 31, 32 , 37 , 38 , 56 while 13 adopted a retrospective design.5 , 8 , 13 , 16 , 19 , 20 , 26 , 29 , 33, 34, 35, 36 , 39
Table 1.
Characteristics of the included studies
| Author | Country; Lockdown Policy | Study Sample Characteristics; % Male; Age (y) | Study Population | Definitions of PA or Exercises | PA-Related Variables | Outcome Measurement Tools (Validation) | Statistical Test |
|---|---|---|---|---|---|---|---|
| Cross-sectional studies | |||||||
| Ammar et al6 | Multiple countries; lockdown | N=1047; 46% men; Age: 18-35 y: 55.1% 36-55 y: 35.1% >55 y: 9.8% |
Healthy adults | PA as defined by IPAQ* | IPAQ score | IPAQ-Short Form (validated) | Paired t test |
| Arturo et al7 | Mexico; lockdown | N=37; 59% men; Age: 27.8 y±6.1 |
Healthy adults (Teachers) | PA as defined by IPAQ* | PA in MET-min/wk Change in PA levels in participants |
IPAQ (validated) | Student t test Descriptive statistics |
| Blom et al55 | Sweden; no lockdown | N=5599; 50% men; Age: 46.3 y±11.0 |
Healthy adults | Daily activity and exercise | Proportion of participants reported change in PA levels Proportion of participants reported change in exercise Proportion of participants reported change in sedentary time |
Customized questionnaire (unvalidated) |
Descriptive statistics |
| Chague et al9 | France; lockdown | N=124; 60.5% men; Age: 71.0 y±14.0 |
Patients with congestive heart failure | Not defined |
Proportion of participants reported change in PA levels Proportion of participants reported change in sedentary time |
Customized questionnaire (unvalidated) |
Descriptive statistics |
| Cooper et al10 | United States; lockdown | N=1607; 43% men; Age: 38.0 y±12.9 |
Healthy adults | Overall PA, walking for at least 30 min/d, PA as defined by IPAQ* | Proportion of participants reported change in PA levels Proportion of participants reported change in sedentary time |
IPAQ-Short Form (validated) | Descriptive statistics |
| Deng et al11 | Wuhan, China; lockdown | N=1607; 64.8% men; Age: <18 y: 1.2% 18-22 y: 97.9% >22 y: 0.9% |
Healthy students (university and college) | Regular exercise as defined as ≥3 times/wk and ≥60 min each time | Proportion of participants reported change in PA levels Proportion of participants reported change in exercise habit |
Customized questionnaire (unvalidated) |
Descriptive statistics |
| Di Renzo et al12 | Italy; lockdown | N=3533; 23.9% men; Age: 40.0 y±13.5 |
Healthy adults (internet users) | Sports training eg, walking; gym/run/swimming/soccer/volleyball/basketball/CrossFit/dance/yoga/aerobic fitness/martial arts/tennis/aerial gymnastics | Proportion of participants reported change in exercise habit | Customized questionnaire (unvalidated) |
Descriptive statistics |
| Đogaš et al14 | Croatia; lockdown | N=3027; 20.3% men; Median age: 40 y |
Healthy adults | Not defined | Duration of PA | Customized questionnaire (unvalidated) |
Paired t test |
| Duncan et al15 | United States; lockdown | N=3971; 30.8% men; Age: 50.4 y±16.0 |
Healthy adults (identical and same-sex fraternal twins) | Not defined | Proportion of participants reported change in PA levels |
Customized questionnaire (unvalidated) |
Descriptive statistics |
| Galle et al17 | Italy; lockdown | N=2125; 37.2% men; Age: 22.5 y±0.1 |
Undergraduate students | Not defined | Proportion of participants reported change in PA levels | Customized questionnaire (unvalidated) |
Descriptive statistics |
| Hu et al18 | China; lockdown | N=1033; 51.7% men; Age: 18-30 y: 61.7% 31-40 y: 27.2% >41 y: 11.1% |
Healthy adults | Exercise (such as running and dancing) |
Proportions of participants reported change in exercise habit Proportion of participants reported change in sedentary time |
IPAQ (validated) | Descriptive statistics |
| Lehtisalo et al21 | Finland; lockdown | N=613; 51.2% men; Age: 67.9 y±4.6 |
Older adults | Leisure time physical activity, housework or cleaning, gardening | Proportion of participants reported change in PA levels | Customized questionnaire (unvalidated) |
Descriptive statistics |
| Lesser et al22 | Canada; lockdown | N=1098; 19.6% men; Age: 42.0 y±15.0 |
Healthy adults | Walking/jogging/running/biking/cycling/weight training/online video/classes/yoga/home workout/hiking/home/yard work/other | Proportion of participants reported change in PA levels | Behavioral regulations in exercise (validated) Godin Leisure Questionnaire (validated) |
Descriptive statistics |
| Minsky et al23 | Israel; lockdown | N=279; 30.8% men; Age: 53.0 y±13.0 |
Adults with obesity | Not defined | Proportion of participants reported change in PA levels | Customized questionnaire (unvalidated) |
Descriptive statistics |
| Orlandi et al24 | Italy; lockdown | N=2218; 34.3% men; Age: 38.2 y±14.9 |
Healthy adults | PA as defined by IPAQ* | PA in MET-min/wk (converted from PA in MET-h/wk) | IPAQ (validated) | Paired t test |
| Paltrinieri et al25 | Italy; lockdown | N=2816; 23.2% men; Age: 18-44 y: 44.8% 45-64 y: 44.0% >65 y: 10.6% |
Healthy adults | Not defined | Proportion of participants reported change in PA levels | Customized questionnaire (unvalidated) |
Descriptive statistics |
| Persiani et al27 | Italy; lockdown | N=292; 49% men; Age: <35 y: 10.3% 35-50 y: 23% 50-70 y: 57.2% >70 y: 9.6% |
Patients with musculoskeletal pain | Not defined | Change in PA levels in participants Proportion of participants reported change in sedentary time |
Customized questionnaire (unvalidated) |
Descriptive statistics |
| Rodríguez-Nogueira et al28 | Spain; lockdown | N=472; 40% men; Age: 46.4 y±11.2 |
Healthy adults | Aerobics, strength exercise, and others | Change in PA levels in participants | Standardized Nordic Questionnaire (validated) | Descriptive statistics |
| Ruíz-Roso et al30 | Italy; lockdown | N=726; 39.8% men; Age: 10-15 y: 45.7% 16-19 y: 54.3% |
Adolescents | Active: PA ≥300 min/wk | Change in PA levels in participants | IPAQ (validated) | Descriptive statistics |
| Sonza et al31 | Brazil and Europe; lockdown | N=3194; 32.9% men; Age: 38.4 y±13.6 |
Healthy adults | Aerobics, resistance and strength exercise | Change in PA levels in participants Proportion of participants reported change in sedentary time |
Physical exercise level before and during social isolation questionnaire (validated) | Descriptive statistics |
| Stanton et al32 | Australia; lockdown | N=1491; 32.6% men; Age: 50.5 y±14.9 |
Healthy adults | PA as defined by Active Australia Survey† | Duration of PA Proportion of participants reported change in PA levels |
Active Australian Survey (validated) | Descriptive statistics Wilcoxon signed-rank test |
| Yamada et al37 | Japan; lockdown | N=1600; 50% men; Age: 74.0 y±5.6 |
Older adults | PA as defined by IPAQ* | Duration of PA | IPAQ-Short Form (validated) | Wilcoxon signed-rank test |
| Zhu et al38 | China; lockdown | N=889; 39% men; Age: 31.9 y±11.4 |
Healthy adults | Step counts | Step counts Duration of sedentary time |
Customized questionnaire (unvalidated) |
Paired t test |
| Retrospective studies | |||||||
| Al Fagih et al5 | Saudi Arabia; lockdown | N=82; 64.6% men; Median age: 65 y |
Patients with heart failure | Defined by algorithms of a cardiac implantable electronic device | Duration of PA | Cardiac implantable electronic devices | Wilcoxon signed-rank test |
| Barrea et al8 | Italy; lockdown | N=121; 33.5% men; Age: 44.9 y±13.3 |
Healthy adults | ≥30 min/d of aerobic exercise | Change in exercise habit in participants | Dichotomous questions (unvalidated) | χ2 test |
| Di Sebastiano et al13 | Canada; lockdown | N=2338; 9.8% men; Age: 18-65 y: 92% >65 y: 8% |
Healthy adults | MVPA: defined by a device specific definition, or ≥100 steps/min Light PA: <100 steps/min |
Duration of light PA Duration of MVPA Step counts |
Wearable devices or smartphone inbuilt accelerometer | 1-way repeated-measures ANOVA |
| Dunton et al16 | United States; lockdown | N=211; 47.4% men; Age: 8.7 y±2.6 |
Healthy children | 11 types of school-based PA enlisted in Active Where survey | Proportion of participants reported change in sedentary time | Active Where survey (validated) | Descriptive statistics |
| Jia et al19 | China; lockdown | N=10,082; 28.3% men; Age: 19.8 y±2.3 |
High school, college, and graduate students | PA as defined by IPAQ* | Duration of MVPA Duration of sedentary time |
IPAQ (validated) | Paired t test |
| Keel et al20 | United States; lockdown | N=90; 12.2% men; Age: 19.45 y±1.3 |
Undergraduate students | Not defined | Proportion of participants reported change in PA levels | Customized questionnaire (unvalidated) |
Descriptive statistics |
| Pépin et al26 | Multiple countries; lockdown | N=742,200; 62% men; Mean age: 43.4 y |
Healthy adults | Step counts | Step counts | Wearable pedometer | Wilcoxon signed-rank test |
| Rowlands et al29 | United Kingdom; lockdown | N=165; 55% men; Age: 64.2 y±8.3 |
Patients with T2D | Defined by accelerometer | Duration of MVPA Step counts (converted from acceleration in milligravitation units)‡ Frequency of continuous PA Duration of sedentary time |
Wearable accelerometer | Paired t test |
| Van Bakel et al33 | Netherlands; lockdown | N=1565; 73.1% men; Age: ≤65 y: 756 >65 y: 677 |
Patients with cardiovascular disease | MVPA determined by work, transportation, household, and leisure time | Duration of MVPA Duration of sedentary time |
Short Questionnaire to Assess Health-enhancing Physical Activity (validated) | Mann-Whitney U test |
| Vetrovsky et al34 | Czech Republic; lockdown | N=26; 69.2% men; Age: 58.8 y±9.8 |
Patients with heart failure | Step counts | Step counts | Wearable accelerometer | Paired t test |
| Wang et al35 | China; lockdown | N=3544; 65.4% men; Age: 51.6 y±8.9 |
Healthy adults | Step counts | Step counts | Smartphone inbuilt accelerometer | Generalized Estimating Equation |
| Weaver et al36 | United States; lockdown | n=362; 47.8% men; Age: 46.5 y±16.0 |
Healthy adults | PA as defined by IPAQ* | PA in MET-min/wk Duration of sedentary time |
IPAQ-Short Form (validated) | Paired t test |
| Zorcec et al39 | Macedonia; lockdown | N=72; 12.5% men; Age: 7.3 y±2.9 |
Children with chronic respiratory diseases | Not defined | Change in PA levels in participants | Customized questionnaire (unvalidated) |
Descriptive statistics |
Abbreviation: ANOVA, analysis of variance.
Definition of PA according to IPAQ: refer to appendix 3.
Definition of PA according to Active Australian Survey: refer to appendix 4.
Interpretation of acceleration in milligravitation units: refer to appendix 5.
Twenty-eight studies investigated changes in PA in people without chronic diseases.6, 7, 8 , 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 , 24, 25, 26 , 28 , 30, 31, 32 , 35, 36, 37, 38 , 56 Notably, 20 of them focused on adults,6, 7, 8 , 10 , 12, 13, 14, 15 , 18 , 22 , 24, 25, 26 , 28 , 31 , 32 , 35 , 36 , 38 , 56 2 on older people,21 , 37 4 on students,11 , 17 , 19 , 20 and 2 on children and adolescents.16 , 30 Eight studies investigated changes in PA among people with chronic diseases.5 , 9 , 23 , 27 , 29 , 33 , 34 , 39 Specifically, 4 focused on patients with cardiovascular diseases,5 , 9 , 33 , 34 1 on type 2 diabetes (T2D),29 1 on musculoskeletal pain,27 1 on adults with obesity,23 and 1 on children with chronic respiratory diseases.39
Risk of bias assessments
One study was rated as having low risk of bias,29 31 as moderate,6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 , 28 , 32, 33, 34, 35, 36, 37, 38, 39 , 56 and 4 as high.5 , 27 , 30 , 31 Of the 23 cross-sectional studies, the most common risks of bias were no sample size justification6 , 7 , 9, 10, 11, 12 , 14 , 15 , 18 , 21, 22, 23, 24, 25 , 27 , 30, 31, 32 , 37 , 38 , 56 and no description of nonresponders’ characteristics6 , 7 , 9, 10, 11, 12 , 14 , 15 , 17 , 18 , 21, 22, 23, 24, 25 , 27 , 28 , 30, 31, 32 , 37 , 38 , 56 (appendix 3). All the 13 retrospective studies did not provide information regarding the dropout participants nor accounted for potential confounders (appendix 4).5 , 8 , 13 , 16 , 19 , 20 , 26 , 29 , 33, 34, 35, 36 , 39
PA and sedentary time
The included studies used diverse definitions of PA (appendices 5-7, table 2 ). Five studies used accelerometers and/or pedometers to quantify PA levels.13 , 26 , 29 , 34 , 35 Eight studies used the International Physical Activity Questionnaire (IPAQ) to categorize PA into different levels.6 , 7 , 10 , 18 , 19 , 24 , 36 , 37 Fourteen studies had miscellaneous definitions of PA (eg, regular exercise for different durations or different leisure time PA),5 , 8 , 11, 12, 13 , 16 , 18 , 21 , 22 , 28 , 30 , 31 , 33 , 56 whereas 9 studies did not clearly define PA.9 , 14 , 15 , 17 , 20 , 23 , 25 , 27 , 39 For the 12 studies that investigated sedentary behaviors,9 , 10 , 16 , 18 , 19 , 27 , 29 , 31 , 33 , 36 , 38 , 56 1 used accelerometers to record sedentary time,29 4 used self-reported screen time,9 , 10 , 18 , 19 and 4 used self-reported sitting and/or couch time.10 , 16 , 27 , 36 However, 3 studies only mentioned sedentary behaviors without definitions.31 , 33 , 38
Table 2.
Summary of results on PA-related variables in people without chronic diseases
| Author | Study Sample Size | Study Population | Definition of PA-Related Outcomes | Statistical Test | Results | Level of Significance | Effect Size | Level of Evidence |
|---|---|---|---|---|---|---|---|---|
| Countries with lockdown measures | ||||||||
| Step counts (per d or per wk) (N=4) | ||||||||
| Di Sebastiano et al13 | N=2338 | Healthy adults | Step count/wk as measured by wearable devices or a smartphone inbuilt accelerometer | 1-way repeated-measures ANOVA | Decreased from 48,625±745 steps/wk to 43,395±705 steps/wk (by 10.8%) | P<.001 | Large | Moderate |
| Pépin et al26 | N=742,200 | Healthy adults | Step count/d as measured by a pedometer | Wilcoxon signed-rank test | Decreased from 5326±479 steps/d to 4752±925 steps/d (by 10.8%) | P<.001 | ||
| Wang et al35 | N=3544 | Healthy adults | Step count/d as measured by a smartphone in-built accelerometer | Generalized Estimating Equation | Decreased from 8097±4793 steps/d to 5440±4571 steps/d (by 32.8%) | Not reported | ||
| Zhu et al38 | N=889 | Healthy adults | Step count/day as measured by an unvalidated self-developed questionnaire | Paired t test | Decreased from 6247±4374 steps/d to 2714±3542 steps/d (by 56.6%) | P<.001 | ||
| Duration of light PA (N=1) | ||||||||
| Di Sebastiano et al13 | N=2338 | Healthy adults | Light PA as measured by an accelerometer: <100 steps/min | 1-way repeated-measures ANOVA | Decreased from 1000.5±17.0 min/wk to 874.1±15.6 min/wk (by 12.6%) | p<0.001 | Very limited | |
| Duration of MVPA (N=2) | ||||||||
| Di Sebastiano et al13 | N=2338 | Healthy adults | MVPA: defined by heart rate ≥60% heart rate maximum by a built-in monitor, or ≥100 steps/min measure by an accelerometer | 1-way repeated-measures ANOVA | Decreased from 194.2±5.2 min/wk to 176.7±5 min/wk (by 9.3%) | P<.001 | Very limited | |
| Jia et al19 | N=10,082 | High schools, colleges, and graduate schools students | PA as defined by IPAQ* | Paired t test | From 0.7±2.0 d/wk to 0.7±2.0 d/wk | P<.05 | ||
| Duration of PA (N=3) | ||||||||
| Đogaš et al14 | N=3027 | Healthy adults | Not defined | Paired t test | Decreased from 162.1 min/wk to 132.9 min/wk (by 18%) | P<.001 | Small | Very limited |
| Stanton et al32 | N=1491 | Healthy adults | PA as defined by Active Australian Survey† | Wilcoxon signed-rank test | Decreased to 312.5±363.5 min/wk | Not reported | ||
| Yamada et al37 | N=1600 | Healthy adults | PA as defined by IPAQ* | Wilcoxon signed-rank test | Decreased from 245 min/wk to 180 min/wk (by 27%) | P<.001 | ||
| Proportion of participants reporting changes in PA levels (N=8) | ||||||||
| Cooper et al10 | N=1607 | Healthy adults | PA as defined by IPAQ* | Descriptive statistics | Overall PA: n=413 reported decrease (25.7%) n=404 reported no change (25.1%) n=790 reported increase (49.1%) Low PA level: n=565 reported decrease (35.2%) n=574 reported no change (35.7%) n=468 reported increase (29.2%) Moderate PA level: n=519 reported decrease (32.3%) n=806 reported no change (50.2%) n=282 reported increase (17.6%) High PA level: n=566 reported decrease (35.2%) n=736 reported no change (45.8%) n=306 reported increase (19.0%) |
NA | Conflicting | |
| Duncan et al15 | N=3971 | Healthy adults (identical and same-sex fraternal twins) | Not defined | Descriptive statistics | n=1048 reported decrease (26.4%) n=1183 reported no change (29.8%) n=1740 reported increase (43.8%) |
NA | ||
| Galle et al17 | N=2125 | Undergraduate students | Not defined | Descriptive statistics | n=453 reported decrease (21.3%) n=640 reported no change (30.1%) n=1032 reported in increase (48.6%) |
NA | ||
| Keel et al20 | N=90 | Undergraduate students | Not defined | Descriptive statistics | n=54 reported decrease (61.5%) n=12 reported no change (13.6%) n=22 reported increase (24.9%) |
NA | ||
| Lehtisalo et al21 | N=2816 | Healthy adults | Leisure time physical activity, housework or cleaning, and gardening as measured by an unvalidated self-reported questionnaire | Descriptive statistics | n=91 reported decreased (34%) n=287 reported no change (50%) n=193 reported increased (16%) |
NA | ||
| Lesser et al22 | N=1098 | Healthy adults | Walking/jogging/running/biking/cycling/weight training/online video/classes/yoga/home workout/hiking/home/yard work/other as measured by the Behavioral Regulations in Exercise Questionnaire and Godin Leisure-Time Questionnaire | Descriptive statistics | n=372 reported decrease (33.9%) n=334 reported no change (30.4%) n=392 reported increase (35.7%) |
NA | ||
| Paltrinieri et al25 | N=2816 | Healthy adults | Not defined | Descriptive statistics | n=641 reported decrease (35.1%) n=972 reported no change (53.2%) n=97 reported increase (5.3%) n=116 were missing data (6.4%) |
NA | ||
| Stanton et al32 | N=1491 | Healthy adults | PA as defined by Active Australian Survey† | Descriptive statistics | n=308 reported decrease (20.7%) n=454 reported no change (30.5%) n=729 reported increase (48.9%) |
NA | ||
| Changes in number of participants involving in different PA categories (N=3) | ||||||||
| Rodríguez-Nogueira et al28 | N=472 | Healthy adults | Aerobics, strengthening exercise, others, and no exercise, as well as the frequency of doing exercise as measured by an unvalidated self-developed questionnaire Occasionally carry out PA: some d/mo Frequently carry out PA: 7 d/wk |
Descriptive statistics | Never carry out PA: increased from n=86 to n=113 (by 31%) Occasionally carry out PA: decreased from n=272 to n=213 (by 21.7%) Frequently carry out PA: increased from n=114 to n=146 (by 28.3%) |
NA | Very limited | |
| Ruíz-Roso et al30 | N=726 | Adolescents | Active: PA ≥300 min/wk as measured by IPAQ | Descriptive statistics | Active population: decreased from n=206 to n=135 (by 34.5%) Inactive population: increased from n=86 to n=157 (by 82.5%) |
NA | ||
| Sonza et al31 | N=3194 | Healthy adults | Aerobics, resistance, and strength exercise as measured by the Physical Exercise Level Before and During Social Isolation Questionnaire Level of activity reported by participants |
Descriptive statistics | “A bit active” population: increased from n=866 to n=1086 (from 27.1% to 34%) Active population: decreased from n=1412 to n=1044 (from 44.2% to 32.7%) Very active population: decreased from n=527 to n=265 (from 16.5% to 8.3%) |
NA | ||
| MET-min/wk (N=3) | ||||||||
| Arturo et al7 | N=37 | Healthy adults | PA as defined by IPAQ* | Student t test | Decreased from 1826 MET-min/wk to 552 MET-min/wk (by 69.8%) Low PA: increased 18.2% in MET-min/wk Moderate PA: increased 10.1% in MET-min/wk High PA: decreased 22.3% in MET-min/wk |
P=.005 | Small | Very limited |
| Orlandi et al24 | N=2218 | Healthy adults | PA as defined by IPAQ* | Paired t test | Decreased from 2269.2 MET-min/wk to 1728 MET-min/wk (by 23.8%) | P=.001 | ||
| Weaver et al36 | N=362 | Healthy adults | PA as defined by IPAQ* | Paired t test | Decreased from 2205±3342.7 MET-min/wk to 1616±2176.6 MET-min/wk (by 26.7%) | P<.001 | ||
| IPAQ scores (N=1) | ||||||||
| Ammar et al6 | N=1047 | Healthy adults | PA as defined by IPAQ* | Paired t test | Decreased from 5.04±2.51 to 3.83±2.84 (by 24%) | P<.001 | Very limited | |
| Proportion of participants reported changes in exercise duration (N=2) | ||||||||
| Deng et al11 | N=1607 | Healthy adults | Time spent on exercise during COVID-19 (including web-based physical education) as measured by an unvalidated self-developed questionnaire | Descriptive statistics | n=826 reported less time (51.4%) n=460 reported the same (28.6%) n=321 reported more (20.0%) |
NA | Limited | |
| Hu et al18 | N=1033 | Healthy adults | Exercise as defined by IPAQ (eg, dancing and running) at 4 mo before COVID-19 and 4 mo after COVID-19 as measured by an unvalidated self-developed questionnaire | Descriptive statistics | n=195 reported decrease (18.9%) n=654 reported no change (63.3%) n=184 reported increase (17.8%) |
NA | ||
| Proportion of participants reported doing regular exercise (N=2) | ||||||||
| Barrea et al8 | N=121 | Healthy adults | ≥30 min/d of aerobic exercise | χ2 test | Decreased from n=62 (51.2%) to n=39 (32.2%) | P=.004 | Conflicting | |
| Di Renzo et al12 | N=3533 | Healthy adults | At least once/wk of training (eg, walking/gym/run/swimming/soccer/volleyball/basketball/Cross Fit/dance/yoga/aerobic fitness/martial arts/tennis/aerial gymnastics) | Descriptive statistics | Reported training: increased from n=2173 to n=2198 (by 1.2%) Reported no training: decreased from n=1360 to n=1335 (by 1.8%) |
NA | ||
| Duration of sedentary time (N=3) | ||||||||
| Jia et al19 | N=10,082 | High school, college, and graduate school students | Screen time as measured by IPAQ | Paired t test | Increased from 4.9±1.9 h/d to 5.6±2.2 h/d (by 14.3%) | P<.001 | Small | Very limited |
| Weaver et al36 | N=362 | Healthy adults | Sitting time as measured by IPAQ-Short Form | Paired t test | Increased from 460.9±281.6 min/d to 494.5±211.5 min/d (by 7.4%) | P<.001 | ||
| Zhu et al38 | N=889 | Healthy adults | Sedentary time as measured by an unvalidated self-developed questionnaire | Paired t test | Increased from 5.3±2.7 h/d to 6.6±3.1 h/d (by 24.5%) | P<.001 | ||
| Proportion of participants reported changes in sedentary time (N=4) | ||||||||
| Cooper et al10 | N=1,607 | Healthy adults | Screen and sitting time as measured by IPAQ-Short Form | Descriptive statistics | Screen time: n=79 reported decrease (4.9%) n=662 reported no change (41.2%) n=866 reported increase (53.9%) Sitting time: n=91 reported decrease (5.7%) n=577 reported no change (35.9%) n=939 reported increase (58.5%) |
NA | Limited | |
| Dunton et al16 | N=211 | Healthy children | Sitting time as measured by the Active Where questionnaire | Descriptive statistics | n=8 reported decrease (4%) n=87 reported increase (41%) n=116 no results available (55%) |
NA | ||
| Hu et al18 | N=1033 | Healthy adults | Screen time as measured by IPAQ | Descriptive statistics | n=80 reported decrease (7.8%) n=247 reported no change (23.9%) n=706 reported increased (68.3%) |
NA | ||
| Sonza et al31 | N=3194 | Healthy adults | Sedentary behavior as measured by the Physical Exercise Level Before and During Social Isolation Questionnaire | Descriptive statistics | Sedentary population increased from n=390 (12.2%) to n=799 (25.0%) | NA | ||
| Countries without lockdown measures | ||||||||
| Proportion of participants reported changes in PA (N=1) | ||||||||
| Blom et al55 | N=5599 | Healthy adults | Daily activity as measured by an unvalidated self-developed questionnaire | Descriptive statistics | n=1456 reported decrease (26.0%) n=3527 reported no change (63.0%) n=616 reported increase (11.0%) |
NA | Very limited | |
| Proportion of participants reported changes in exercise duration (N=1) | ||||||||
| Blom et al55 | n=5599 | Healthy adults | Time spent on exercise during COVID-19 as measured by an unvalidated self-developed questionnaire | Descriptive statistics | n=1567 reported decrease (28%) n=3303 reported no change (59%) n=728 reported increase (13%) |
NA | Very limited | |
| Proportion of participants reported changes in sedentary time (N=1) | ||||||||
| Blom et al55 | N=5599 | Healthy adults | Sitting time as measured by an unvalidated self-developed questionnaire | Descriptive statistics |
n=448 reported decrease (8%) n=3695 reported no change (66%) n=1456 reported increase (26%) |
NA | Very limited | |
Abbreviations: ANOVA, analysis of variables; NA. not applicable.
Definition of PA according to IPAQ: refer to appendix 3.
Definition of PA according to Active Australian Survey: refer to appendix 4.
Thirteen PA-related variables were reported in the included studies. Of them, 12 were reported in studies involving people without chronic diseases (appendix 8), while 8 were reported in studies involving people with chronic diseases (appendix 9). Those studies conducted in regions involving lockdowns reported 12 PA-related variables (see appendices 8 and 9), whereas the Swedish study (without lockdown) reported 3 PA-related variables in people without chronic diseases (see appendix 8).
Quality of evidence regarding changes in PA during the pandemic in people without chronic diseases
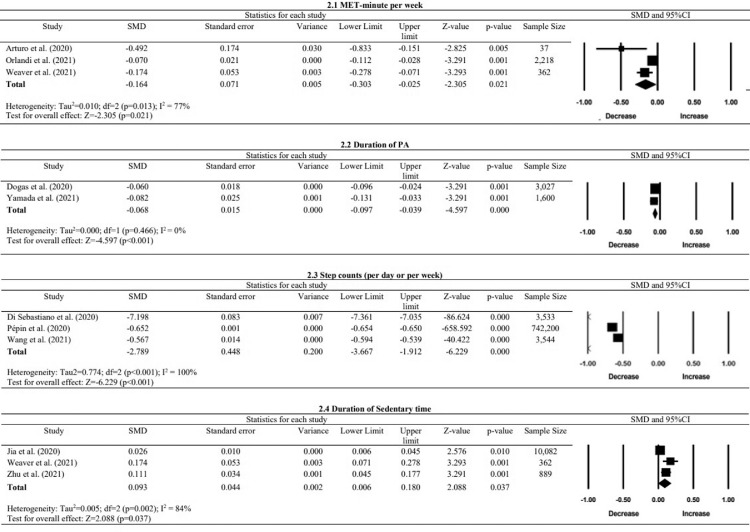
The included studies investigating changes in PA of people without chronic diseases were conducted in countries with5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 and without56 lockdowns. These changes in PA-related variables are summarized in fig 2 and table 2).
Fig 2.
Forest plots of PA in MET-min/wk, duration of PA, step counts, and duration of sedentary time of people without chronic diseases.
Step counts (per d/wk)
Moderate evidence from 4 studies consistently showed significant decreases in step counts after the outbreak as measured by accelerometers, pedometers, or a self-reported questionnaire.13 , 26 , 35 , 38 The meta-analysis showed a significant reduction in step counts with a large effect size (pooled SMD=−2.79, P<.01, I²=100%).
Durations of light PA and MVPA
Very limited evidence from 1 Canadian study revealed a significant reduction (12.6%) in the duration of light PA as measured by accelerometers during the pandemic.13 Likewise, very limited evidence from 2 studies suggested reduced durations of MVPA.13 , 19 Specifically, 1 study used accelerometers to detect significant decreases in the duration of MVPA (9.3%) after the outbreak.13 Another study used IPAQ to reveal a significant reduction in time spent on MVPA after lockdown.19
Duration of PA
Very limited evidence substantiated decreases in self-reported weekly PA duration.14 , 32 , 37 However, because 1 included study did not present the relevant statistical data,32 it was excluded from the meta-analysis. The meta-analysis showed a significant reduction in PA duration per week with a small effect size (pooled SMD=−0.07, P<.01, I²=0%).14 , 37
Proportion of participants reporting changes in PA levels
There was inconsistent evidence regarding the proportion of people reporting changes in PA levels during the outbreak. Five studies used customized questionnaires to evaluate changes in PA during lockdowns, although PA was not defined.15 , 17 , 20 , 21 , 25 They found that 20.7%-61.5% of participants reported decreases, 13.6%-53.2% reported no change, and 5.3%-48.6% reported increases in PA levels.15 , 17 , 20 , 21 , 25 Similarly, 3 included studies used validated questionnaires to evaluate changes in PA levels during lockdowns.10 , 22 , 32 They showed that 20.7%-33.9% of participants reported decreases, 25.1%-30.5% reported no change, and 35.7%-49.1% reported increases in PA levels.10 , 22 , 32 A Swedish study (without lockdown) used a customized questionnaire to reveal that 63.0% of participants reported no change in PA levels, and only 26.0% reported decreases in PA levels during the outbreak.56
Changes in number of participants involving in different PA categories
There was very limited evidence supporting the adoption of an inactive lifestyle during lockdowns.28 , 30 , 31 Although 3 studies revealed that more people were classified into the low PA category after the outbreak,28 , 30 , 31 1 of them reported approximately 30% increase in the number of people being categorized into “never performed PA” or “frequently performed PA” during the COVID-19 outbreak.28
Proportion of participants reported changes in their exercise duration
Limited evidence from 2 included studies supported that people living in countries with lockdowns reported either no change or decreases in their exercise duration, while only 17.8%-20.0% of people reported increases in their exercise duration.11 , 18 Conversely, up to 26% of participants reported increases in exercise in a country without lockdown.56
Proportion of participants reported doing regular exercise
There was conflicting evidence regarding the proportion of people participating in regular exercises.8 , 12 One included study reported no significant changes in the proportion of participants involved in regular exercise training after the outbreak,12 while another study revealed a 19% drop in the number of participants who exercised regularly.8 However, both studies used unvalidated questionnaires.
Metabolic equivalent-minute per week
Very limited evidence supported reduced estimated metabolic equivalent task (MET)-minute per week as measured by IPAQ.7 , 24 , 36 The PA reduction ranged from 23.8%-69.8%.7 , 24 , 36 The meta-analysis from 3 studies showed a significant reduction in MET-minute per week with a small effect size (pooled SMD=−0.16, P=.02, I²=77%).7 , 24 , 36
IPAQ scores
Very limited evidence from 1 study reported a 24.0% decrease in IPAQ scores, although it was described in MET-minutes.6
Sedentary time
Very limited evidence suggested approximately 7.4%-24.5% increases in sedentary time (defined by screen time, sitting, and sedentary activities) in the general public as measured by IPAQ19 , 36 or a self-developed questionnaire.38 Our meta-analysis showed a significant increase in sedentary time with a small effect size (pooled SMD=0.09, P=.04, I²=84%).19 , 36 , 38
Proportions of participants reported changes in sedentary time
There was limited evidence that a relatively larger proportion of participants reported increases in sedentary time in countries imposing lockdowns.10 , 16 , 18 , 31 Approximately 41.0%-68.3% of participants reported increases in their sedentary time.10 , 16 , 18 One study also found that the proportion of participants being classified as the “sedentary” category increased from 12.2% to 25.0%.31 All these studies adopted validated questionnaires to quantify sedentary time.10 , 16 , 18 , 31 Conversely, very limited evidence suggested that Swedish participants (without lockdown) were less likely to adopt a sedentary lifestyle.56 Only 26.0% of Swedish participants reported increased sitting time, while 66.0% reported no change.56
People with chronic diseases
Step counts (per d/wk)
Very limited evidence suggested significant decreases in step counts by 7.6% in patients with T2D.29 Similarly, there was very limited evidence that patients with cardiovascular diseases had approximately 16.4% reduction in step counts.34
Duration of MVPA
Very limited evidence supported no significant change in the duration of MVPA in patients with T2D as quantified by accelerometers.29 Conversely, very limited evidence substantiated 25.0% increases in MVPA among patients with cardiovascular diseases as measured by a validated questionnaire.33
Duration of PA
There was very limited evidence that patients with heart failure displayed an average of 0.8 hours reduction in daily duration of PA as measured by cardiac implantable electronic devices.5
Proportion of participants reported change in PA levels
Very limited evidence suggested significant decreases in PA levels among approximately 41% of patients with congestive heart failure 9 or adults with obesity.23
Changes in number of participants involving in different PA categories
Very limited evidence showed an increased number of participants classified as no activity in patients with musculoskeletal pain27 or low PA categories in children with chronic respiratory diseases.39 Specifically, 1 study showed a 82.5% increase in the number of participants being classified as “no PA,”27 while another study revealed a 237.5% increase in the number of participants being classified as having less than 1-2 hours of PA per day.39
Frequency of continuous PA
Very limited evidence supported an increase in the frequency of continuous PA in patients with T2D as recorded by an accelerometer.29 Rowlands et al revealed that the average frequency of 30- and 60-minute continuous PA in patients with T2D significantly increased from 0.65 d/wk to 1.0 d/wk and from 0.24 d/wk to 0.44 d/wk, respectively.29
Sedentary time
Very limited evidence supported a 3.0% increase in sedentary time in patients with T2D as measured by accelerometers29 and a 14.4% increase in patients with cardiovascular disease documented by a validated questionnaire.33
Proportion of participants reporting increases in sedentary time
Very limited evidence suggested that 46% of participants with congestive heart failure9 and 35% of participants with musculoskeletal pain27 reported increases in sedentary time.
Discussion
This systematic review and meta-analysis summarized evidence regarding changes in PA among people with and without chronic diseases during the COVID-19 pandemic. Twelve and 8 PA-related variables were used to evaluate changes in PA levels among people with and without chronic diseases, respectively. Moderate evidence from objective measurements (eg, accelerometers) substantiated decreases in step counts in people without chronic diseases during the pandemic. Very limited to limited evidence supported decreases in PA levels and exercise behaviors but increases in sedentary time in both groups, although 2 studies reported increases in continuous PA and MVPA among patients with T2D and cardiovascular diseases, respectively. People living in countries with lockdowns showed significant reductions in PA and increases in sedentary behaviors. The lockdown policy and closure of public facilities refrained people from performing PA.28 This is attested by the fact that most respondents in a country without lockdown reported no change in their PA and sedentary behaviors.56
Effects of physical inactivity on global health
It is well-known that physical inactivity adversely affect physical and mental health, as well as quality of life.57 Insufficient PA heightens the risk of developing noncommunicable diseases (eg, 24%, 16%, and 42% increase in the risk of having coronary heart disease, cardiovascular accident, and T2D, respectively).56 Because 23.3% and 27.5% of the global population had insufficient PA in 2010 and 2016, respectively,57 the WHO implemented an action plan between 2018 and 2030 to counteract physical inactivity58 and to reduce the global physical inactivity by 10% in 2025 and 15% in 2030.59 However, the COVID-19 pandemic could adversely affect the attainment of the original WHO 2025 global PA target.
Our meta-analysis and a prior systematic review45 highlight the negative effect of the COVID-19 pandemic on PA of people with and without chronic diseases globally. Home confinement policies and public facilities closure have heightened the prevalence of global physical inactivity. Lockdown-related physical inactivity may increase the incidence of noncommunicable diseases and related health care burdens.60 It is well known that regular MVPA boosts immunity against infectious diseases. An average energy expenditure of 500-1000 MET-minutes per week is associated with a lower risk of SARS-CoV-2 infection.61 PA can also improve an individual's depression and mood by the augmented release of endorphins.62 Thereby, health authorities should implement new strategies to promote active lifestyle (especially MVPA) during and after lockdowns. Because some people may fear going out even after lockdowns are lifted, governments should run proper campaigns and/or use mobile applications to promote indoor PA and exercises58 among people who hesitate to exercise outdoor during or after lockdowns.
Effect of physical inactivity on people with chronic diseases
Most of the included studies reported decreases in PA among people with chronic diseases during the pandemic. Physical inactivity may have greater detrimental effects on people with chronic diseases. Increased physical inactivity in patients with chronic heart conditions could heighten their morbidity63 and mortality rates.64 A 30-minute reduction of daily PA in any given month during 4 years in patients after implanting cardioverter defibrillators was associated with 48% increased hazard for death compared with active patients in the same month.65 Similarly, physical inactivity and suspended face-to-face physiotherapy treatments during lockdowns led to increased symptoms in patients with musculoskeletal pain compared with the prepandemic period.27 Increased frequency and intensity of pain in patients with osteoarthritis66 or chronic low back pain67 in turn may result in the adoption of a sedentary lifestyle. If this vicious cycle persists, these patients may experience other pain-related comorbidities (eg, depression). Imperatively, decreased PA levels8 and the suspension of routine medical care in children with chronic respiratory diseases may lead to weight gain and mental health issues during the pandemic.39
While reduced PA during the COVID-19 pandemic is prevalent, some people with chronic diseases reported increased PA during lockdowns. One included study reported no significant change in the duration of MVPA and even increases in the frequency of 30- and 60-minute exercise sessions among people with T2D.29 These findings might be attributed to the success of the British government in promoting exercise for health maintenance and permitting outdoor exercises during lockdowns. Similarly, increased population interest regarding the effects of PA and screen time on health might inspire some patients with chronic heart diseases to increase their MVPA during the pandemic.68 Physical activities may increase the levels of adiponectin that can dampen the proinflammatory pathway of T2D69 and reduce plaque formation in patients with heart diseases.69 These results underscore the importance of proper public health policies and/or strategies to minimize the negative effect of lockdowns on PA.
Changes in exercise formats
During lockdowns, exercise formats have shifted from outdoors to indoors70 and from on-field team sports to home-based individual exercises (eg, yoga).28 Additionally, tele-exercise has gained popularity. Some governments produced online exercise videos by physiotherapists to promote home-based training to the general public.71 Similarly, nongovernment organizations (eg, National Centre of Health, Physical Activity, and Disability) launched different campaigns (eg, #MoveInMay, online toolkits, workout videos) via social media to engage and educate people to perform PA.72 While some private companies (eg, ParticipACTION) used websites and/or mobile applications to provide users with exercise demonstration videos and guidelines, interactive team challenges, and rewarding schemes to counteract the lockdown-related physical inactivity,13 other companies embedded a body positional tracking system in a mirror to provide individualized home exercise training.73 Although telerehabilitation/telemedicine may facilitate home-based disease management, older people or underprivileged individuals may have difficulty in using telehealth.27 , 74 Future studies should investigate the optimal strategies for delivering telerehabilitation/tele-exercise to older people or people in low-income countries.
Validity and reliability of outcome measures
Wearable devices (eg, smart watches) allow objective PA measurements. All included studies13 , 26 , 29 , 34 , 35 using wearable devices consistently showed reduced PA in people with and without chronic diseases during the pandemic, except for 1 study investigating patients with diabetes.29 However, PA levels quantified by wearable devices rely on participants’ compliance.13 , 75 Studies that used wearable devices to collect PA data might underestimate the negative effects of the pandemic on PA because people using wearable sensors might be more health conscious and intend to monitor their PA levels to stay active.34
Most included studies used self-reported questionnaires to estimate PA during the pandemic, which was common in epidemiologic studies to investigate the prevalence of diseases or behaviors.76 However, self-reported PA levels may be subjected to recall bias. Therefore, PA levels estimated by online questionnaires might not be related to those measured by accelerometers.77 Further, while 30 included articles used different self-reported questionnaires to estimate PA,6, 7, 8, 9, 10, 11, 12 , 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 , 27 , 28 , 30, 31, 32, 33 , 36, 37, 38, 39 , 56 only 15 studies used self-reported PA questionnaires with reported reliability and validity.6 , 7 , 10 , 16 , 18 , 19 , 22 , 24 , 28 , 30, 31, 32, 33 , 36 , 37 The estimated effects of the COVID-19 pandemic on PA might have been more accurate had validated questionnaires been used. Therefore, an international consortium should be formed to determine a core set of PA questionnaires (eg, global physical activity questionnaire)58 to allow comparisons of PA levels across studies in the future.
Study designs
Given the sudden onset of the pandemic, most of the included studies adopted a cross-sectional design. Twenty-three included studies evaluated the current PA levels. The remaining studies used questionnaires (n=7) or wearable or implanted devices to retrospectively evaluate the changes in PA levels before and after the outbreak. Cross-sectional studies are needed during a pandemic to cost-effectively garner relevant information from large samples78 to inform policy making and help plan further prospective studies.79 However, retrospective studies using wearable or implanted devices to quantify changes in PA-related variables following the pandemic are also important because they help reveal causation.78
Study limitations
The current review had several limitations. First, the searched keywords might not be comprehensive enough to capture all relevant articles although most key articles have been included. Second, because diverse PA-related variables were used in the included studies, some variables were only used in 1 included study, which prevented the conduction of meta-analysis. Third, the included studies covered a range of chronic diseases, which prevented the conduction of meta-analysis of PA for each disease.
For limitations of the included studies, only 1 article reported PA-related variables in a country without lockdown, which limited its generalizability. Further, people's PA levels may change over time. Their PA levels showed the greatest decline at the beginning of lockdowns, but PA levels increased toward the end of lockdowns.13 , 26 Because some studies did not report the time of data collection, people's PA levels at a given time point might not illustrate changes in PA levels throughout the pandemic. Moreover, many included studies recruited participants by convenience sampling or recruiting from a single center, which might affect the generalizability of results. Finally, although 3 PA-related variables were pooled for meta-analyses, they showed substantial heterogeneity (I²>60%). The high heterogeneity might be attributed to differences in the sampling methods, delivery mode of questionnaires, durations spent on completing questionnaires, and socioeconomic status.
Conclusions
Moderate evidence from objective PA measurements demonstrated significant decreases in PA during the COVID-19 pandemic, while very limited to limited evidence from self-reported questionnaires revealed reduced PA and increased sedentary behaviors among people with and without chronic diseases during the COVID-19 pandemic globally. Tele-exercise may have the potential to help promote and/or maintain PA levels and exercise habits in people with and without chronic diseases. Future epidemiologic studies are warranted to determine the long-term effects of COVID-19 on the changes of PA and/or exercise habits and the associated health outcomes in people with and without chronic diseases worldwide. An international consortium should be established to determine the core set of PA measurements to allow comparisons of results across studies in the future. It would be prudent to include wearable devices or smartphones to reliably and objectively monitor PA. Collectively, the current review has laid the foundation for relevant stakeholders to develop and implement effective strategies to minimize the negative effects of similar outbreaks on PA and health of people with and without chronic diseases in the future.
Suppliers
a. EndNote X9; Clarivate.
b. Comprehensive Meta-analysis Version 3.3 software; Biostat.
Acknowledgement
We thank Mr Arturo Irisarri for screening full-text articles in Spanish in our study.
Footnotes
Presented to the Hong Kong Physiotherapy Association, December 12, 2021, Hong Kong, China.
Supported by the Health and Medical Research Fund-Commissioned Research on COVID-19 (COVID190222).
Disclosures: none
Supplemental Digital Content 1. Search strategy
| Steps | Keywords |
|---|---|
| 1 | COVID* or Cov* or Corona* or Severe acute respiratory syndrome coronavirus 2 or SARS* |
| 2 | Physical activit* or activity level or Exercise habit* or Exercise routine* or lifestyle |
| 3 | 1 and 2 |
Supplemental Digital Content 2. Determination of overall risk of bias of the included studies and the level of evidence for a given outcome
| Risk of bias of a study | |
|---|---|
| High risk of bias | For a study graded as high in at least two domains |
| Moderate risk of bias | For a study graded as moderate in at least one domain, and rated as low in other domains |
| Low risk of bias | For a study graded as low in all six domains |
| Level of evidence of PA-related outcome measures | |
|---|---|
| Strong evidence | Pooled results from two or more studies, at least two of them have high quality; or consistent narrative findings in multiple studies with high-quality |
| Moderate evidence | Significant pooled results from multiple statistically heterogeneous studies, at least one of them has high quality; or consistent findings from multiple studies with at least one high quality study |
| Limited evidence | Findings from a high-quality study, or consistent findings from multiple moderate- or low-quality studies |
| Very limited evidence | Findings from one moderate- or low-quality study |
| Conflicting evidence | Inconsistent findings |
Appendix 3. Risk of bias of the included cross-sectional studies
| Appraisal tool for Cross-Sectional Studies (AXIS) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Objective & study design |
Study participation |
Handling of non-respondents |
Outcome measures |
Statistical analysis |
Reporting |
Overall risk | ||||||||||||||||||||
| Original item number | 1 | 2 | S | 3 | 4 | 5 | 6 | 20 | S | 7 | 13* | 14 | S | 8 | 9 | S | 10 | 11 | S | 12 | 15 | 16 | 17 | 18 | 19* | S | |
| Ammar et al. (2021)6 | Y | Y | L | N | N | Y | Y | Y | M | N | N | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Arturo et al. (2020)7 | Y | Y | L | N | Y | N | N | Y | M | N | N | N | H | Y | Y | L | Y | Y | L | N | Y | Y | Y | Y | ? | M | Moderate |
| Blom et al. (2021)55 | Y | Y | L | N | Y | N | Y | Y | M | N | Y | Y | M | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | Y | L | Moderate |
| Chague et al. (2020)9 | Y | Y | L | N | Y | Y | Y | Y | M | Y | N | Y | M | Y | Y | L | Y | Y | L | Y | Y | Y | Y | N | Y | M | Moderate |
| Cooper et al. (2021)10 | Y | Y | L | N | Y | N | Y | Y | M | Y | ? | Y | M | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Deng et al. (2020)11 | Y | Y | L | N | Y | Y | Y | Y | M | Y | ? | N | H | Y | N | M | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Di Renzo et al. (2020)12 | Y | Y | L | N | Y | Y | Y | Y | M | N | ? | N | H | Y | Y | L | Y | N | M | Y | Y | Y | Y | Y | N | M | Moderate |
| Đogaš et al. (2020)14 | Y | Y | L | N | Y | Y | Y | Y | M | N | ? | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Duncan et al. (2020)15 | Y | Y | L | N | Y | Y | Y | Y | M | N | N | N | H | Y | N | M | N | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Galle et al. (2020)17 | Y | Y | L | Y | Y | Y | Y | Y | L | N | Y | N | H | Y | Y | L | N | Y | M | Y | Y | Y | Y | Y | N | M | Moderate |
| Hu et al. (2020)18 | Y | Y | L | N | Y | Y | Y | Y | M | Y | N | N | H | Y | Y | L | N | Y | M | Y | Y | Y | Y | Y | N | M | Moderate |
| Lehtisalo et al. (2021)21 | Y | Y | L | N | Y | Y | Y | Y | M | N | N | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Lesser et al. (2020)22 | Y | Y | L | N | Y | Y | Y | Y | M | N | ? | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Minsky et al. (2021)23 | Y | Y | L | N | Y | Y | Y | Y | M | N | Y | N | H | Y | N | M | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Orlandi et al. (2021)24 | Y | Y | L | N | Y | Y | Y | Y | M | N | ? | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Paltrinieri et al. (2021)25 | Y | Y | L | N | Y | Y | Y | Y | M | N | N | N | H | Y | N | M | N | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Persiani et al. (2021)27 | Y | Y | L | N | N | Y | N | Y | H | N | ? | N | H | Y | N | M | Y | Y | L | Y | Y | Y | Y | Y | N | M | High |
| Rodriguez-Nogueira et al. (2021)28 | Y | Y | L | Y | Y | Y | Y | Y | L | N | N | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Ruíz-Roso et al. (2020)30 | Y | Y | L | N | N | N | Y | Y | H | N | N | N | H | Y | N | M | Y | Y | L | Y | Y | Y | Y | Y | N | M | High |
| Sonza et al. (2021)31 | Y | Y | L | N | N | Y | Y | Y | H | N | N | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | High |
| Stanton et al. (2020)32 | Y | Y | L | N | Y | Y | Y | Y | M | N | N | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Yamada et al. (2020)37 | Y | Y | L | N | Y | Y | Y | Y | M | N | N | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| Zhu et al. (2021)38 | Y | Y | L | N | Y | Y | Y | Y | M | N | N | N | H | Y | Y | L | Y | Y | L | Y | Y | Y | Y | Y | N | M | Moderate |
| % of studies that reported “yes”/no bias | 100 | 100 | 9 | 83 | 78 | 87 | 100 | 83 | 13 | 13 | 100 | 74 | 83 | 96 | 96 | 100 | 100 | 100 | 96 | 15 | |||||||
H = High; M = Moderate; L = Low; ? = Uncertain; N = No; Y = Yes; S = Subscore
For item number 13 and 19 in the AXIS, a point is rewarded when the content is “N”.
Appendix 4. Risk of bias of the included retrospective studies
| Quality of Prognosis Studies Risk of Bias Assessment Instruments for Prognostic Factor Studies (QUIPS) | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Subject participation |
Study attrition |
Prognostic factor measurement |
Outcome measurement |
Study confounding |
Statistical analysis and reporting |
Overall risk | |||||||||||||||||||||||||||||||
| Original item number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | S | 1 | 2 | 3 | 4 | 5 | S | 1 | 2 | 3 | 4 | 5 | S | 1 | 2 | 3 | S | 1 | 2 | 3 | 4 | 5 | 6 | 7 | S | 1 | 2 | 3 | 4 | S | |
| Al Fagih et al. (2020)5 | P | N | Y | Y | P | U | P | H | N | N/A | N/A | N/A | N/A | H | P | P | Y | N | N/A | H | P | U | Y | H | N | N | N | N/A | N/A | N | N | H | Y | Y | Y | U | M | High |
| Barrea et al. (2020)8 | P | N | N | Y | P | Y | Y | M | N | N/A | N/A | N/A | N/A | H | P | N | Y | Y | N/A | M | Y | N | Y | M | N | N | N | N/A | N/A | N | N | H | Y | Y | Y | Y | L | Moderate |
| Di Sebastiano et al. (2020)13 | Y | N | Y | Y | P | Y | Y | M | Y | N | N/A | N/A | N/A | M | Y | Y | Y | Y | N/A | L | Y | P | P | M | N | N | N | N/A | N/A | N | N | H | Y | Y | Y | Y | L | Moderate |
| Dunton et al. (2020)16 | Y | P | Y | Y | P | U | Y | M | Y | P | Y | N | N/A | M | P | N | Y | N/A | N/A | H | Y | N | Y | M | Y | N | N | N/A | N/A | N | N | H | Y | Y | Y | Y | L | Moderate |
| Jia et al. (2020)19 | Y | Y | Y | Y | P | Y | Y | L | N/A | N/A | N/A | N/A | N/A | H | P | Y | Y | Y | N/A | M | Y | Y | Y | L | Y | Y | Y | Y | N/A | Y | N | M | Y | Y | Y | Y | L | Moderate |
| Keel et al. (2020)20 | Y | P | Y | P | N | U | Y | M | N/A | N/A | N/A | N/A | N/A | H | Y | Y | Y | U | N/A | M | Y | N | Y | M | P | N | P | Y | N/A | P | N | H | Y | Y | Y | U | L | Moderate |
| Pepin et al. (2020)26 | Y | N | Y | P | N | U | Y | M | N/A | N/A | N/A | N/A | N/A | H | Y | Y | Y | U | N/A | M | Y | Y | Y | L | P | N | P | U | N/A | P | N | H | P | Y | Y | U | M | Moderate |
| Rowlands et al. (2021)29 | Y | Y | Y | Y | Y | Y | Y | L | Y | N/A | N/A | N/A | N/A | H | Y | Y | Y | Y | N/A | L | Y | Y | Y | L | Y | Y | P | Y | N/A | Y | N | M | Y | Y | Y | U | L | Low |
| Van Bakel et al. (2021)33 | Y | P | Y | Y | P | Y | Y | M | P | N/A | N/A | N/A | N/A | H | Y | Y | Y | Y | N/A | L | Y | P | Y | M | Y | N | P | Y | N/A | Y | N | M | Y | Y | Y | U | L | Moderate |
| Vetrovsky et al. (2020)34 | Y | P | Y | N | N | U | Y | M | N/A | N/A | N/A | N/A | N/A | H | Y | Y | Y | Y | N | M | Y | Y | Y | L | P | Y | P | Y | N/A | P | N | M | Y | Y | Y | Y | L | Moderate |
| Wang et al. (2020)35 | Y | Y | Y | Y | P | U | Y | M | N | N/A | N/A | N/A | N/A | H | Y | Y | Y | U | N/A | M | Y | Y | Y | L | Y | Y | P | Y | N/A | Y | N | M | Y | Y | Y | Y | L | Moderate |
| Weaver et al. (2021)36 | Y | N | Y | Y | P | Y | Y | M | P | N/A | N/A | N/A | N/A | H | Y | Y | Y | Y | N/A | L | Y | Y | Y | L | P | P | P | Y | N/A | P | N | M | Y | Y | Y | Y | L | Moderate |
| Zorcec et al. (2020)39 | Y | N | Y | P | N | U | Y | M | N | N/A | N/A | N/A | N/A | H | Y | Y | Y | Y | N/A | L | Y | N | Y | M | Y | N | Y | Y | N/A | Y | N | M | Y | Y | Y | U | L | Moderate |
Y = Yes; N = No; P = Partial; U = Unclear; N/A = Not Applicable; S = Subscore
Appendix 5. Definitions of physical activity according to International Physical Activity Questionnaire (IPAQ) questionnaires79
| Definition of PA according to IPAQ79 | ||
|---|---|---|
| Category 1 | Low intensity | This is the lowest level of physical activity. Individuals who do not meet the criteria for the categories 2 or 3 are considered low intensity/inactive. |
| Category 2 | Moderate intensity | Any one of the following 3 criteria: • 3 or more days of vigorous activity for at least 20 minutes per day OR • 5 or more days of moderate-intensity activity or walking for at least 30 minutes per day; OR • 5 or more days of any combination of walking, moderate-intensity or vigorous intensity activities achieving a minimum of at least 600 MET-min/week. |
| Category 3 | High intensity | Any one of the following 2 criteria: • Vigorous-intensity activity on at least 3 days and accumulating at least 1,500 MET-min/week OR • 7 or more days of any combination of walking, moderate-intensity or vigorous intensity activities achieving a minimum of at least 3,000 MET-min/week |
MET-min = Metabolic equivalent of task-minutes
Appendix 6. Definitions of physical activity according to the Active Australian Survey
| Physical activity | Definition |
|---|---|
| Walking | Continuous in 10-minute intervals of walking |
| Gardening/yardwork | No description available |
| Other moderate activities | Activities that make you “breathe somewhat harder than normal and slightly increase heart rate” |
| Other vigorous activities | Activities that make you “breathe much harder than normal and have a greater effect on heart rate” |
Duration of total activity time = time spent on walking + time spent on moderate activities + (2 x time spent of vigorous activities)
Appendix 7. Interpretation of acceleration in milli-gravitation units
| Interpretation of acceleration in milli-gravitation units (mg)80 | |
|---|---|
| Time spent: | |
| 100-200 mg | Slow walking |
| 200-350 mg | Brisk walking |
| 350-500 mg | Fast walk / jog |
| 500-1000mg | Slow run |
| 1000-1500mg | Medium run |
| 1500-2000mg | Fast run |
| >2000mg | Sprint |
Conversion from milli-gravitation unit to number of steps based on suggestion of 1.7 mg being equivalent to ∼800 steps/day as suggested by Rowlands et al.29
Appendix 8. Quality of evidence of PA-related outcome variables in people without chronic diseases
| GRADE factors |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants | Number of studies | Number of cohorts | Univariate | Phase | Study limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Moderate/large effect size | Dose effect | Overall quality | |||
| Potential outcomes identified | + | 0 | - | ||||||||||||
| Lockdown | |||||||||||||||
| MET-minute per week | 2,617 | 3 | 3 | 3 | 1 | X | ✓ | ✓ | ✓ | X | X | X | + | ||
| Duration of PA | 6,118 | 3 | 3 | 3 | 1 | X | ✓ | ✓ | X | ✓ | X | X | + | ||
| Duration of light PA | 2,338 | 1 | 1 | 1 | 1 | X | N/A | X | X | ✓ | ✓ | ✓ | + | ||
| Duration of MVPA | 12,420 | 2 | 2 | 1 | 1 | 1 | X | X | X | X | ✓ | X | X | + | |
| Proportion of participants reported changes in PA levels | 16,014 | 8 | 8 | N/A | 1 | X | X | X | X | ✓ | N/A | N/A | - | ||
| Changes in number of participants involving in different PA categories | 4,392 | 3 | 3 | N/A | 1 | X | X | X | X | ✓ | N/A | N/A | + | ||
| IPAQ scores | 1,047 | 1 | 1 | 1 | 1 | X | N/A | ✓ | X | ✓ | X | X | + | ||
| Step counts (per day or per week) | 748,971 | 4 | 4 | 4 | 1 | X | ✓ | ✓ | X | ✓ | ✓ | ✓ | +++ | ||
| Proportion of participants reported changes in exercise duration | 2,640 | 2 | 2 | N/A | 1 | ✓ | ✓ | X | X | ✓ | N/A | N/A | ++ | ||
| Proportion of participants reported doing regular exercise | 3,654 | 2 | 2 | 1 | 1 | 1 | X | X | X | X | ✓ | N/A | N/A | +/- | |
| Duration of sedentary time | 11,333 | 3 | 3 | 3 | 1 | X | ✓ | ✓ | X | ✓ | ✓ | X | + | ||
| Proportion of participants reported changes in sedentary time | 6,045 | 4 | 4 | N/A | 1 | ✓ | ✓ | ✓ | X | ✓ | X | X | ++ | ||
| No lockdown | |||||||||||||||
| Proportion of participants reported changes in PA | 5,599 | 1 | 1 | N/A | 1 | ✓ | N/A | ✓ | X | X | X | X | + | ||
| Proportion of participants reported changes in exercise duration | 5,599 | 1 | 1 | N/A | 1 | ✓ | N/A | ✓ | X | X | X | X | + | ||
| Proportion of participants reported changes in sedentary time | 5,599 | 1 | 1 | N/A | 1 | ✓ | N/A | ✓ | X | X | X | X | + | ||
IPAQ = International Physical Activity Questionnaire; MET-min = metabolic equivalent-minute; MVPA = moderate-to-vigorous physical activity; PA = physical activities
Phase, phase of investigation. For univariate analysis: +, number of significant effects with a positive value; 0, number of non-significant effects; -, number of significant effects with a negative value. For GRADE factors: ✓, no serious limitations; ✕, serious limitations (or not present for moderate/large effect size, dose effect); ?, unable to rate item based on available information; N/A, not applicable. For overall quality of evidence: -, inconsistent; +, very low; ++, low; +++, moderate; ++++, high
PA = physical activities; MVPA = moderate-to-vigorous physical activity
Phase, phase of investigation. For univariate analysis: +, number of significant effects with a positive value; 0, number of non-significant effects; -, number of significant effects with a negative value. For GRADE factors: ✓, no serious limitations; ✕, serious limitations (or not present for moderate/large effect size, dose effect); ?, unable to rate item based on available information; N/A, not applicable. For overall quality of evidence: +, very low; ++, low; +++, moderate; ++++, high
Appendix 9. Quality of evidence of PA-related outcome variables for people with chronic diseases
| GRADE factors |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential outcomes identified | Number of participants | Number of studies | Number of cohorts | Univariate | Phase | Study limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Moderate/large effect size | Dose effect | Overall quality | |||
| + | 0 | - | ||||||||||||||
| Duration of PA | ||||||||||||||||
| Patients with heart failure | 82 | 1 | 1 | 1 | 1 | X | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Duration of MVPA | ||||||||||||||||
| Patients with type 2 diabetes mellitus | 165 | 1 | 1 | 1 | 1 | ✓ | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Patients with cardiovascular disease | 1,565 | 1 | 1 | 1 | 1 | ✓ | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Proportion of participants reported changes in PA levels | ||||||||||||||||
| Patients with congestive heart failure | 124 | 1 | 1 | N/A | 1 | X | N/A | ✓ | X | X | N/A | N/A | + | |||
| Adults with obesity | 279 | 1 | 1 | N/A | 1 | ✓ | N/A | X | X | ✓ | N/A | N/A | + | |||
| Changes in number of participants involving in different PA categories | ||||||||||||||||
| Patients with musculoskeletal pain | 292 | 1 | 1 | N/A | 1 | X | N/A | X | X | ✓ | N/A | N/A | + | |||
| Children with chronic respiratory diseases | 72 | 1 | 1 | N/A | 1 | ✓ | N/A | X | X | ✓ | N/A | N/A | + | |||
| Step counts (per day or per week) | ||||||||||||||||
| Patients with type 2 diabetes mellitus | 165 | 1 | 1 | 1 | 1 | ✓ | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Patients with heart failure | 26 | 1 | 1 | 1 | 1 | X | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Frequency of continuous PA | ||||||||||||||||
| Patients with type 2 diabetes mellitus | 165 | 1 | 1 | 1 | 1 | ✓ | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Duration of sedentary time | ||||||||||||||||
| Patients with type 2 diabetes mellitus | 165 | 1 | 1 | 1 | 1 | ✓ | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Patients with cardiovascular disease | 1,565 | 1 | 1 | 1 | 1 | ✓ | N/A | ✓ | X | ✓ | ✓ | N/A | + | |||
| Proportion of participants reported changes in sedentary time | ||||||||||||||||
| Patients with congestive heart failure | 124 | 1 | 1 | N/A | 1 | ✓ | N/A | ✓ | X | X | N/A | N/A | + | |||
| Patients with musculoskeletal pain | 292 | 1 | 1 | N/A | 1 | X | N/A | X | X | ✓ | N/A | N/A | + | |||
References
- 1.Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19) Postgrad Med J. 2020;96:753–758. doi: 10.1136/postgradmedj-2020-138234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Weekly epidemiological update on COVID-19 - 7 September 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—7-september-2021. Accessed September 15, 2021.
- 3.Bashir MF, Ma B, Shahzad L. A brief review of socio-economic and environmental impact of COVID-19. Air Qual Atoms Health. 2020;13:1403–1409. doi: 10.1007/s11869-020-00894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alasdair S. Coronavirus: half of humanity now on lockdown as 90 countries call for confinement. Available at:https://www.euronews.com/2020/04/02/coronavirus-in-europe-spain-s-death-toll-hits-10-000-after-record-950-new-deaths-in-24-hou. December 7, 2021.
- 5.Al Fagih A, Al Onazi M, Al Basiri S, et al. Remotely monitored inactivity due to COVID-19 lockdowns potential hazard or heart failure patients. Saudi Med J. 2020;41:1211–1216. doi: 10.15537/smj.2020.11.25449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammar A, Trabelsi K, Brach M, et al. Effects of home confinement on mental health and lifestyle behaviours during the COVID-19 outbreak: insights from the ECLB-COVID19 multicentre study. Biol Sport. 2021;38:9–21. doi: 10.5114/biolsport.2020.96857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arturo Hall-López J. Physical activity levels in physical education teachers before and during school suspension brought by the COVID-19 quarantine. FU Phys Ed Sport. 2020;18:475–481. [Google Scholar]
- 8.Barrea L, Pugliese G, Framondi L, et al. Does SARS-CoV-2 threaten our dreams? Effect of quarantine on sleep quality and body mass index. J Trans Med. 2020;18:318. doi: 10.1186/s12967-020-02465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chague F, Boulin M, Eicher JC, et al. Impact of lockdown on patients with congestive heart failure during the coronavirus disease 2019 pandemic. ESC Heart Fail. 2020;7:4420–4423. doi: 10.1002/ehf2.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper JA, vanDellen M, Bhutani S. Self-weighing practices and associated health behaviors during COVID-19. Am J Health Behav. 2021;45:17–30. doi: 10.5993/AJHB.45.1.2. [DOI] [PubMed] [Google Scholar]
- 11.Deng CH, Wang JQ, Zhu LM, et al. Association of web-based physical education with mental health of college students in Wuhan during the COVID-19 outbreak: cross-sectional survey study. J Med Internet Res. 2020;22:e21301. doi: 10.2196/21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Renzo L, Gualtieri P, Pivari F, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18:229. doi: 10.1186/s12967-020-02399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Sebastiano KM, Chulak-Bozzer T, Vanderloo LM, Faulkner G. Don't walk so close to me: physical distancing and adult physical activity in Canada. Front Psychol. 2020;11:1895. doi: 10.3389/fpsyg.2020.01895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Đogaš Z, Lušić Kalcina L, Pavlinac Dodig I, et al. The effect of COVID-19 lockdown on lifestyle and mood in Croatian general population: a cross-sectional study. Croat Med J. 2020;61:309–318. doi: 10.3325/cmj.2020.61.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan GE, Avery AR, Seto E, Tsang S. Perceived change in physical activity levels and mental health during COVID-19: findings among adult twin pairs. PloS One. 2020;15 doi: 10.1371/journal.pone.0237695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunton GF, Do B, Wang SD. Early effects of the COVID-19 pandemic on physical activity and sedentary behavior in children living in the U.S. BMC Public Health. 2020;20:1351. doi: 10.1186/s12889-020-09429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallè F, Sabella EA, Da Molin G, et al. Understanding knowledge and behaviors related to COVID-19 epidemic in Italian undergraduate students: the EPICO Study. Int J Environ Res Public Health. 2020;17:3481. doi: 10.3390/ijerph17103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z, Lin X, Chiwanda Kaminga A, Xu H. Impact of the COVID-19 epidemic on lifestyle behaviors and their association with subjective well-being among the general population in mainland China: cross-sectional study. J Med Internet Res. 2020;22:e21176. doi: 10.2196/21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia P, Zhang L, Yu W, et al. Impact of COVID-19 lockdown on activity patterns and weight status among youths in China: the COVID-19 Impact on Lifestyle Change Survey (COINLICS) Int J Obes (Lond) 2020;45:695–699. doi: 10.1038/s41366-020-00710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keel PK, Gomez MM, Harris L, Kennedy GA, Ribeiro J, Joiner TE. Gaining “the quarantine 15:” perceived versus observed weight changes in college students in the wake of COVID-19. Int J Eat Disord. 2020;53:1801–1808. doi: 10.1002/eat.23375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehtisalo J, Palmer K, Mangialasche F, Solomon A, Kivipelto M, Ngandu T. Changes in lifestyle, behaviors, and risk factors for cognitive impairment in older persons during the first wave of the coronavirus disease 2019 pandemic in Finland: results from the FINGER Study. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.624125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesser IA, Nienhuis CP. The impact of COVID-19 on physical activity behavior and well-being of Canadians. Int J Environ Res Public Health. 2020;17:3899. doi: 10.3390/ijerph17113899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minsky NC, Pachter D, Zacay G, et al. Managing obesity in lockdown: survey of health behaviors and telemedicine. Nutrients. 2021;13:1359. doi: 10.3390/nu13041359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlandi M, Rosselli M, Pellegrino A, et al. Gender differences in the impact on physical activity and lifestyle in Italy during the lockdown, due to the pandemic. Nutr Metab Cardiovasc Dis. 2021;31:2173–2180. doi: 10.1016/j.numecd.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Paltrinieri S, Bressi B, Costi S, et al. Beyond lockdown: the potential side effects of the SARS-CoV-2 pandemic on public health. Nutrients. 2021;13:1600. doi: 10.3390/nu13051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pépin JL, Bruno RM, Yang RY, et al. Wearable activity trackers for monitoring adherence to home confinement during the COVID-19 pandemic worldwide: data aggregation and analysis. J Med Internet Res. 2020;22:e19787. doi: 10.2196/19787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persiani P, De Meo D, Giannini E, et al. The aftermath of COVID-19 lockdown on daily life activities in orthopaedic patients. J Pain Res. 2021;14:575–583. doi: 10.2147/JPR.S285814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Nogueira Ó, Leirós-Rodríguez R, Benítez-Andrades JA, Álvarez-álvarez MJ, Marqués-Sánchez P, Pinto-Carral A. Musculoskeletal pain and teleworking in times of the COVID-19: analysis of the impact on the workers at two Spanish universities. Int J Environ Res Public Health. 2021;18:1–12. doi: 10.3390/ijerph18010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowlands AV, Henson JJ, Coull NA, et al. The impact of COVID-19 restrictions on accelerometer-assessed physical activity and sleep in individuals with type 2 diabetes. Diabet Med. 2021;38:e14549. doi: 10.1111/dme.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruíz-Roso MB, de Carvalho Padilha P, Matilla-Escalante DC, et al. Changes of physical activity and ultra-processed food consumption in adolescents from different countries during COVID-19 pandemic: an observational study. Nutrients. 2020;12:1–13. doi: 10.3390/nu12082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonza A, da Cunha de Sá-Caputo D, Sartorio A, et al. COVID-19 Lockdown and the behavior change on physical exercise, pain and psychological well-being: an international multicentric study. Int J Environ Res Public Health. 2021;18:3810. doi: 10.3390/ijerph18073810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanton R, To QG, Khalesi S, et al. Depression, anxiety and stress during COVID-19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environ Res Public Health. 2020;17:1–13. doi: 10.3390/ijerph17114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Bakel BMA, Bakker EA, de Vries F, Thijssen DHJ, Eijsvogels TMH. Impact of COVID-19 lockdown on physical activity and sedentary behaviour in Dutch cardiovascular disease patients. Neth Heart J. 2021;29:273–279. doi: 10.1007/s12471-021-01550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vetrovsky T, Frybova T, Gant I, et al. The detrimental effect of COVID-19 nationwide quarantine on accelerometer-assessed physical activity of heart failure patients. ESC Heart Fail. 2020;7:2093–2097. doi: 10.1002/ehf2.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhang Y, Bennell K, et al. Physical distancing measures and walking activity in middle-aged and older residents in Changsha, China, during the COVID-19 epidemic period: longitudinal observational study. J Med Internet Res. 2020;22:e21632. doi: 10.2196/21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver RH, Jackson A, Lanigan J, et al. Health behaviors at the onset of the COVID-19 pandemic. Am J Health Behav. 2021;45:44–61. doi: 10.5993/AJHB.45.1.4. [DOI] [PubMed] [Google Scholar]
- 37.Yamada M, Kimura Y, Ishiyama D, et al. Effect of the COVID-19 epidemic on physical activity in community-dwelling older adults in Japan: a cross-sectional online survey. J Nutr Health Aging. 2020;24:948–950. doi: 10.1007/s12603-020-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Q, Li M, Ji Y, et al. "Stay-at-home" lifestyle effect on weight gain during the COVID-19 outbreak confinement in China. Int J Environ Res Public Health. 2021;18:1813. doi: 10.3390/ijerph18041813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zorcec T, Jakovska T, Micevska V, Boskovska K, Cholakovska VC. Pandemic with COVID-19 and families with children with chronic respiratory diseases. Pril (Makedonska Akad Nauk Umet Odd Med Nauki) 2020;41:95–101. doi: 10.2478/prilozi-2020-0038. [DOI] [PubMed] [Google Scholar]
- 40.Biddle S. Physical activity and mental health: evidence is growing. World Psychiatry. 2016;15:176–177. doi: 10.1002/wps.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Lack of physical activity. Available at: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/physical-activity.htm. Accessed May 1, 2021.
- 42.Bull FC, Al-Ansari SS, Biddle S, et al. World Health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dempsey PC, Friedenreich CM, Leitzmann MF, et al. Global public health guidelines on physical activity and sedentary behavior for people living with chronic conditions: a call to action. J Phys Act Health. 2021;18:76–85. doi: 10.1123/jpah.2020-0525. [DOI] [PubMed] [Google Scholar]
- 44.Rausch Osthoff AK, Niedermann K, Braun J, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77:1251–1260. doi: 10.1136/annrheumdis-2018-213585. [DOI] [PubMed] [Google Scholar]
- 45.Stockwell S, Trott M, Tully M, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med. 2021;7 doi: 10.1136/bmjsem-2020-000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 48.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong CK, Mak RY, Kwok TS, et al. Prevalence, incidence, and factors associated with non-specific chronic low back pain in community-dwelling older adults aged 60 years and older: a systematic review and meta-analysis. J Pain. 2022;23:509–534. doi: 10.1016/j.jpain.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Wong AYL, Chan LLY, Lo CWT, et al. Prevalence/incidence of low back pain and associated risk factors among nursing and medical students: a systematic review and meta-analysis. PM R. 2021;13:1266–1280. doi: 10.1002/pmrj.12560. [DOI] [PubMed] [Google Scholar]
- 51.Cochrane Prognosis Methods Group. Review tools. Available at: https://methods.cochrane.org/prognosis/tools. Accessed December 7, 2021.
- 52.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 53.Higgins JPT, Thomas J, Chandler J, editors. Cochrane handbook for systematic reviews of interventions version 6.2. 2021. Available at: https://training.cochrane.org/handbook. Accessed December 20, 2021. [Google Scholar]
- 54.Huguet A, Hayden JA, Stinson J, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev. 2013;2:71. doi: 10.1186/2046-4053-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blom V, Lönn A, Ekblom B, et al. Lifestyle habits and mental health in light of the two COVID-19 pandemic waves in Sweden, 2020. Int J Environ Res Public Health. 2021;18:3313. doi: 10.3390/ijerph18063313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1· 9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 58.Amini H, Habibi S, Islamoglu AH, Isanejad E, Uz C, Daniyari H. COVID-19 pandemic-induced physical inactivity: the necessity of updating the Global Action Plan on Physical Activity 2018-2030. Environ Health Prev Med. 2021;26:32. doi: 10.1186/s12199-021-00955-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization . World Health Organization; Geneva: 2018. Global action plan on physical activity 2018-2030: more active people for a healthier world. [Google Scholar]
- 60.Lippi G, Henry BM, Sanchis-Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19) Eur J Prev Cardiol. 2020;27:906–908. doi: 10.1177/2047487320916823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SW, Lee J, Moon SY, et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med. 2021 Jul 22 doi: 10.1136/bjsports-2021-104203. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Dinas PC, Koutedakis Y, Flouris AD. Effects of exercise and physical activity on depression. Ir J Med Sci. 2011;180:319–325. doi: 10.1007/s11845-010-0633-9. [DOI] [PubMed] [Google Scholar]
- 63.Chelu MG, Gunderson BD, Koehler J, Ziegler PD, Sears SF. Patient activity decreases and mortality increases after the onset of persistent atrial fibrillation in patients with implantable cardioverter-defibrillators. JACC Clin Electrophysiol. 2016;2:518–523. doi: 10.1016/j.jacep.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure-time physical activity with cardiovascular mortality: a systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prev Cardiol. 2018;25:1864–1872. doi: 10.1177/2047487318795194. [DOI] [PubMed] [Google Scholar]
- 65.Kramer DB, Mitchell SL, Monteiro J, et al. Patient activity and survival following implantable cardioverter-defibrillator implantation: the ALTITUDE activity study. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, Chang RW, Ehrlich-Jones L, et al. Sedentary behavior and physical function: objective evidence from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2015;67:366–373. doi: 10.1002/acr.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park SM, Kim HJ, Jeong H, et al. Longer sitting time and low physical activity are closely associated with chronic low back pain in population over 50 years of age: a cross-sectional study using the sixth Korea National Health and Nutrition Examination Survey. Spine J. 2018;18:2051–2058. doi: 10.1016/j.spinee.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Ding D, Del Pozo Cruz B, Green MA, Bauman AE. Is the COVID-19 lockdown nudging people to be more active: a big data analysis. Br J Sports Med. 2020;54:1183–1184. doi: 10.1136/bjsports-2020-102575. [DOI] [PubMed] [Google Scholar]
- 69.Becic T, Studenik C, Hoffmann G. Exercise increases adiponectin and reduces leptin levels in prediabetic and diabetic individuals: systematic review and meta-analysis of randomized controlled trials. Med Sci (Basel) 2018;6:97. doi: 10.3390/medsci6040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garmin. Are we still moving? Available at:https://www.garmin.com/en-US/blog/general/the-effect-of-the-global-pandemic-on-active-lifestyles. Accessed December 7, 2021.
- 71.Elderly Health Service Depaetment of Health. Physical activity. Available at: https://www.elderly.gov.hk/english/fightvirus/physical_activity.html. Accessed December 20, 2021.
- 72.National Center of Health Physical Activity and Disability. NCHPAD COVID response. 2020. Available at: https://www.nchpad.org/fppics/NCHPAD%20COVID%20Response.pdf. Accessed December 20, 2021.
- 73.Hong Kong Trade Development Council Research. Navigating COVID-19: a smart fitness app. Available at:https://hkmb.hktdc.com/en/NTc3NDE1Njc4/hktdc-research/Navigating-Covid-19-A-Smart-Fitness-App. Accessed May 1, 2021.
- 74.Angotti M, Mallow GM, Wong A, Haldeman S, An HS, Samartzis D. COVID-19 and its impact on back pain. Global Spine J. 2022;12:5–7. doi: 10.1177/21925682211041618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duncan MJ, Wunderlich K, Zhao Y, Faulkner G. Walk this way: validity evidence of iphone health application step count in laboratory and free-living conditions. J Sports Sci. 2018;36:1695–1704. doi: 10.1080/02640414.2017.1409855. [DOI] [PubMed] [Google Scholar]
- 76.Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. doi: 10.2147/JMDH.S104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 78.Yee J, Niemeier D. Advantages and disadvantages: longitudinal vs. repeated cross-section surveys. Proj Battelle. 1996;94:16–22. [Google Scholar]
- 79.Caruana EJ, Roman M, Hernández-Sánchez J, Solli P. Longitudinal studies. J Thoracic Dis. 2015;7:E537–E540. doi: 10.3978/j.issn.2072-1439.2015.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rowlands AV, Fairclough SJ, Yates T, Edwardson CL, Davies M, Munir F, et al. Activity intensity, volume, and norms: utility and interpretation of accelerometer metrics. Med Sci Sports Exerc. 2019;51:2410–2422. doi: 10.1249/MSS.0000000000002047. [DOI] [PubMed] [Google Scholar]