Abstract
Background
Sodium oxybate has been recognized as a gold standard for the treatment of disrupted nighttime sleep due to narcolepsy. Its short half-life and immediate-release formulation require patients to awaken 2.5–4 h after their bedtime dose to take a second dose. A novel extended-release, once-nightly sodium oxybate formulation (ON-SXB; FT218) is under US Food and Drug Administration review for the treatment of adults with narcolepsy.
Objective
A phase III trial of ON-SXB in individuals with narcolepsy type 1 (NT1) or 2 (NT2) [the REST-ON trial; NCT02720744] has been conducted and the primary results reported elsewhere. Secondary objectives from REST-ON were to assess the efficacy of ON-SXB on disrupted nighttime sleep; the results of this analysis are reported here.
Methods
In the double-blind, phase III REST-ON trial, patients aged ≥ 16 years were randomly assigned 1:1 to ON-SXB (1 week, 4.5 g; 2 weeks, 6 g; 5 weeks, 7.5 g; 5 weeks, 9 g) or placebo. Secondary endpoints included polysomnographic measures of sleep stage shifts and nocturnal arousals and patient-reported assessments of sleep quality and refreshing nature of sleep at 6, 7.5, and 9 g; post hoc analyses included changes in time spent in each sleep stage, delta power, and assessments in stimulant-use subgroups for prespecified endpoints.
Results
In total, 190 participants (n = 97, ON-SXB; n = 93, placebo) were included in the efficacy analyses. All three ON-SXB doses demonstrated a clinically meaningful, statistically significant decrease vs placebo in the number of transitions to wake/N1 from N1, N2, and rapid eye movement (REM) stages (all doses p < 0.001) and the number of nocturnal arousals (p < 0.05 ON-SXB 6 g; p < 0.001 7.5 and 9 g). Sleep quality and refreshing nature of sleep were significantly improved with all three ON-SXB doses vs placebo (p < 0.001). Post hoc analyses revealed a significant reduction in time spent in N1 (p < 0.05 ON-SXB 6 g; p < 0.001 7.5 and 9 g) and REM (all p < 0.001) and increased time spent in N3 with ON-SXB vs placebo (all p < 0.001), with a significant increase in delta power (p < 0.01 ON-SXB 6 g; p < 0.05 7.5 g; p < 0.001 9 g) and increased REM latency (ON-SXB 7.5 g vs placebo; p < 0.05). Significant improvements in disrupted nighttime sleep were observed regardless of concomitant stimulant use.
Conclusions
The clinically beneficial, single nighttime dose of ON-SXB significantly improved disrupted nighttime sleep in patients with narcolepsy.
Clinical Trial Registration
ClinicalTrials.gov NCT02720744.
Key Points
| One of the most frequently reported symptoms of narcolepsy is disturbed nocturnal sleep/disrupted nighttime sleep, characterized by a disruption in sleep architecture and sleep continuity. FT218 is an investigational, novel, once-nightly formulation of sodium oxybate. |
| In this randomized controlled clinical trial, both subjective and objective measures of disrupted nighttime sleep were significantly improved for the once-nightly formulation of sodium oxybate at 6, 7.5, and 9 g vs placebo. |
| The results from this trial support the use of a once-nightly formulation of sodium oxybate for the nocturnal symptoms of narcolepsy, as well as an improvement in patients’ perception of their sleep, including in those taking concomitant stimulants and/or wake-promoting agents. |
Introduction
Narcolepsy is a chronic sleep disorder affecting approximately 1 out of every 2000 individuals [1, 2]. Narcolepsy is characterized by a pentad of symptoms, including excessive daytime sleepiness (EDS), cataplexy, disrupted nighttime sleep (DNS; also known as disturbed nocturnal sleep), sleep paralysis (SP), and hypnagogic/hypnopompic hallucinations [1, 3]. Although DNS is universally seen as a narcolepsy disease-related symptom, a wide range of prevalence estimates is reported (~ 30–95%) [4]. Numerous factors such as the presence of rapid eye movement (REM) sleep behavior disorder, nightmares, periodic limb movements of sleep, obstructive sleep apnea, depression, anxiety, hallucinations, sleep paralysis, and obesity contribute to DNS [4–7]. Furthermore, varying definitions of DNS in narcolepsy are employed in both the literature and clinical practice, contributing to the wide range of DNS prevalence rates [4, 8]. A recently validated 15-item self-administered questionnaire, the Narcolepsy Severity Scale, includes an item to assess the presence and frequency of DNS [8]. In a study of patients with narcolepsy type 1 (NT1) that used the Narcolepsy Severity Scale, DNS was reported to be the third most frequent symptom (95.5% of untreated patients with three symptoms), exceeded only by EDS and cataplexy. Despite its high prevalence, it is unclear whether clinicians routinely assess or monitor this symptom. Additionally, sleep fragmentation is a frequent finding in polysomnographic (PSG) recordings in individuals with narcolepsy. Less time in slow-wave sleep (SWS), frequent awakenings/arousals, more N1 sleep, and more frequent awakenings from deeper stages of sleep are consistently found on PSG recordings in patients with narcolepsy [4].
Sodium oxybate (SXB), the sodium salt of ɣ-hydroxybutyrate, has been recognized as the gold standard for disrupted sleep due to narcolepsy [9]. However, the immediate-release formulations require the patients to awaken for the second dose 2.5–4 h after the first dose owing to the short half-life of ɣ-hydroxybutyrate (i.e., 30–60 min) [10, 11]. This required awakening disrupts sleep continuity. Results describing improvements in DNS with immediate-release SXB have been published; however, the data are presented as bifurcated segments of sleep [12]. This bifurcation and disruption of sleep highlights an unmet medical need in the treatment of nighttime symptoms in patients with narcolepsy.
FT218 is an investigational, extended-release, once-nightly formulation of SXB (ON-SXB). In a randomized, open-label, crossover pilot study evaluating the pharmacokinetic properties of ON-SXB, a single 6-g dose was shown to be bioequivalent in drug exposure (i.e., area under the curve) to two 3-g doses of immediate-release SXB administered 4 h apart [13]. In the 13-week, phase III, REST-ON clinical trial (NCT02720744) of patients with narcolepsy, all three evaluated doses of ON-SXB (6, 7.5, and 9 g) were effective on all three coprimary endpoints and demonstrated clinically meaningful and statistically significant improvements compared with placebo in EDS as assessed by the Maintenance of Wakefulness Test, overall condition as measured by Clinical Global Impression of Improvement, and frequency of cataplexy attacks, all with p < 0.001 [14]. Significant results were observed at week 3, the earliest formal assessment with the 6-g dose. In REST-ON, ON-SXB was generally well tolerated. Most adverse events were mild or moderate in severity and diminished over time. The most common adverse reactions with ON-SXB were consistent with the well-characterized safety profile of SXB and included vomiting, dizziness, and enuresis [10, 15].
Secondary endpoints of the REST-ON trial reported here included both objective and subjective assessments of DNS in patients with narcolepsy. These assessments include PSG measures of sleep instability (i.e., shifts to wake or N1 from N1, N2, N3, and REM) and nocturnal arousals (NAs), as well as patient-reported assessments of both sleep quality and refreshing nature of sleep.
Methods
Study Design
REST-ON was a multicenter, randomized, double-blind, placebo-controlled, phase III clinical trial (NCT02720744). The study design, which consisted of a 3-week screening period, a 13-week treatment period, and a 1-week follow-up period, was described previously [14]. The study was approved by an institutional review board or independent ethics committee for each study center. REST-ON was performed in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, International Council for Harmonisation guidelines, and any applicable regulatory requirements at the local and national level. Written informed consent was provided by all adult participants (≥ 18 years old). Young adults (16 and 17 years old) must have been capable of giving assent followed by consent from a legally authorized guardian.
Participants
Eligible participants were aged 16 years or older, with a diagnosis of NT1 or narcolepsy type 2 (NT2) as defined by the criteria listed in the International Classification of Sleep Disorders, Third Edition [16]. Participants who previously used SXB were excluded (except use of SXB ≤ 4.5 g for ≤ 2 weeks and ≥ 1 year before study entry), as were those who had a diagnosis of sleep apnea (apnea-hypopnea index ≥ 15) or any other sleep disorder known to cause EDS as determined by PSG findings and sleep history. Concomitant stimulant use was allowed (initiated ≥ 3 weeks before starting the screening process and the same stimulant regimen continued throughout the entire study period). Detailed participant inclusion/exclusion criteria can be found in the primary REST-ON publication [14].
Treatment
Stratified randomization was performed based on the presence of narcolepsy type (NT1 or NT2), with patients assigned in a 1:1 ratio to treatment with ON-SXB or placebo. Doses of study medication were 4.5 g for 1 week, 6 g for 2 weeks, 7.5 g for 5 weeks, and 9 g for 5 weeks taken once nightly at bedtime. The dosing paradigm was designed this way to demonstrate the efficacy and safety of 6, 7.5 and 9 g of ON-SXB. All study personnel were blinded to the study treatments, and a double-blind approach was used to ensure the integrity of the study blinding.
Assessments
Secondary efficacy endpoints were change from baseline in (1) frequency of sleep stage transitions, (2) NAs, and (3) patient-reported quality of sleep and refreshing nature of sleep. Overnight, in-clinic PSG was performed at baseline and at weeks 3, 8, and 13. Stage transitions were assessed as the number of shifts from N1 (light sleep), N2, N3 (deep sleep), and REM sleep to wake and from N2, N3, and REM sleep to N1. Nocturnal arousals were defined as the number of transient arousals on nocturnal PSG following the American Academy of Sleep Medicine Scoring Guidelines for PSG [17], i.e., an abrupt shift of electroencephalography frequency including alpha, theta, and/or frequencies greater than 16 Hz (but not spindles), greater than 3 s of changed frequency on electroencephalography, at least 10 s of stable sleep preceding the change, and in REM sleep, an increase in submental electromyography for at least 1 second. Quality of sleep and refreshing nature of sleep were assessed using a visual analog scale (VAS) and were recorded daily in an electronic sleep diary. The VAS ranged from 1 to 100, with 1 indicating “did not sleep”/“not refreshed” and 100 indicating “slept very well”/“refreshed.” Post hoc analyses included the amount of time spent in N1, N2, N3, and REM sleep; REM latency; shifts from REM to wake/N1; and delta power for non-REM sleep in the whole-night recording as an objective measure of DNS and the assessment of prespecified endpoints associated with DNS (i.e., shifts, arousals, sleep quality, and refreshing nature of sleep) in subgroups of participants who were vs who were not taking concomitant stimulants and/or wake-promoting medications.
Statistical Analyses
Efficacy analyses were based on the modified intent-to-treat population, defined as all patients randomized to treatment with at least one efficacy measurement after receiving either the 6-g dose of ON-SXB or placebo. Least-squares mean (LSM) differences vs placebo, associated 95% confidence intervals (CIs), and p-values were calculated. LSM change from baseline in the PSG and VAS measures were analyzed using a mixed-effects model for repeated measures that included treatment, time at which measurements were taken, treatment-by-time interaction, site (USA or non-USA), and baseline score as fixed effects, and subjects as random effects. For stage shifts, the p-value was estimated using a mixed-effects model for repeated measures with additional criteria including change from baseline in the number of wakefulness or N1 stages after sleep onset as the response variable, covariate of baseline number of wakefulness or N1 stages after sleep onset, and unstructured variance-covariance structure. Mean VAS responses were averaged over the 14 days preceding the test day. Post hoc analyses included changes from baseline in total time spent in N1, N2, N3, and REM sleep; REM latency; and delta power. All statistical tests were performed using a two-sided alpha test with a 5% overall significance level, unless otherwise noted. Secondary efficacy endpoints did not take multiplicity into account.
Results
Patient Disposition and Demographics
Of the 413 candidates who were screened, 222 (53.8%) were randomized 1:1 to ON-SXB or placebo (n = 111, each), and 212 of those randomized (95.5%) received one or more doses of the study drug. Baseline demographics and clinical characteristics of all randomized patients in the REST-ON trial were generally similar between treatment arms; full patient demographics and disposition information have been previously published [14]. The majority of patients were female (n = 144; 67.9%) and white (n = 160; 75.5%) with a mean age of 31.2 years (range, 16–72 years). Overall, 162 (76.4%) participants had NT1 (ON-SXB, n = 80 [74.8%]; placebo, n = 82 [78.1%]) and 50 (23.6%) had NT2 (ON-SXB, n = 27 [25.2%]; placebo, n = 23 [21.9%]). The modified intent-to-treat population consisted of 190 (85.6%) participants; 145 (65.3%) participants had NT1 (ON-SXB, n = 73 [65.8%]; placebo, n = 72 [64.9%]).
Only one participant had prior SXB exposure (≤ 4.5 g for ≤ 2 weeks and ≥ 1 year before study entry). In total, 63% of participants in the modified intent-to-treat population were receiving concomitant stimulants. The most common concomitant stimulants were modafinil (ON-SXB, 21.5%; placebo, 21.0%), armodafinil (ON-SXB, 12.1%; placebo, 6.7%), dextroamphetamine (ON-SXB, 9.3%; placebo, 7.6%), mixed amphetamine salts (ON-SXB, 10.3%; placebo, 5.7%), lisdexamfetamine (ON-SXB, 4.7%; placebo, 5.7%), and methylphenidate (ON-SXB, 10.3%; placebo, 6.7%).
Efficacy
Stage Shifts
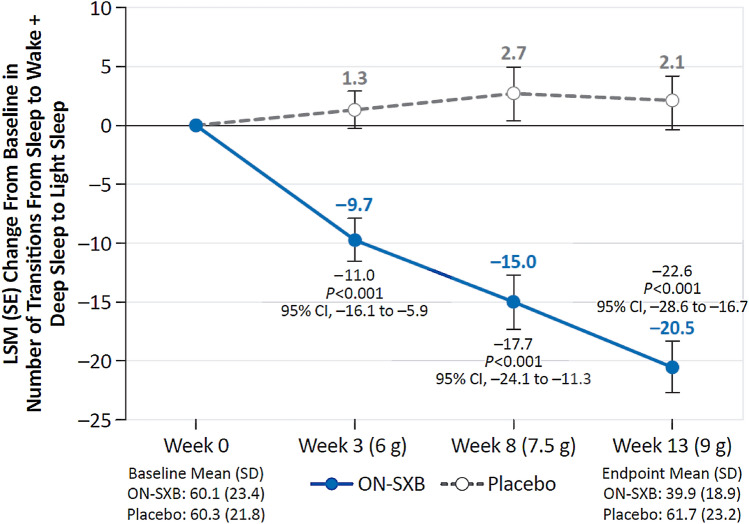
The number of stage shifts was similar between the ON-SXB and placebo arms at baseline (60.1 and 60.3, respectively). The total number of transitions from sleep to wake or N1 from N1, N2, N3, and REM was significantly reduced with ON-SXB vs placebo at week 3 for the 6-g dose (LSM change from baseline, − 9.7 vs 1.3; p < 0.001); at week 8 for the 7.5-g dose (− 15.0 vs 2.7, respectively; p < 0.001); and at week 13 for the 9-g dose (– 20.5 vs 2.1, respectively; p < 0.001) (all Fig. 1).
Fig. 1.
Change from baseline in sleep stage shifts (modified intent-to-treat population). A mixed-effects model for repeated measures was used for analyses of sleep stage shifts. Least-squares mean (LSM) change from baseline in sleep stage shifts for patients receiving once-nightly sodium oxybate (ON-SXB) or matching placebo. CI confidence interval, SD standard deviation, SE standard error
NAs
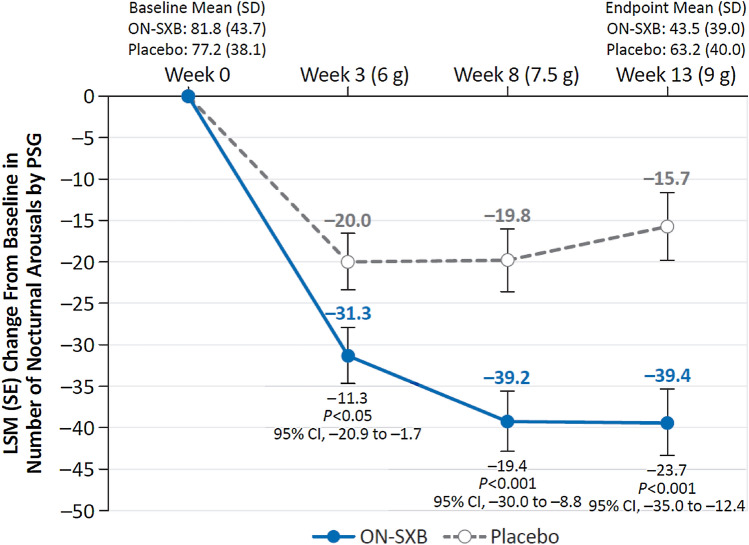
At baseline, the number of NAs was similar in the ON-SXB and placebo arms (81.8 and 77.2, respectively). The LSM change from baseline in the number of NAs was significantly reduced with ON-SXB vs placebo at week 3 for the 6-g dose (− 31.3 vs − 20.0, respectively; p < 0.05); at week 8 for the 7.5-g dose (− 39.26 vs − 19.8, respectively; p < 0.001); and at week 13 for the 9-g dose (− 39.4 vs − 15.7, respectively; p < 0.001) (all Fig. 2).
Fig. 2.
Change from baseline in nocturnal arousals (modified intent-to-treat population). A mixed-effects model for repeated measures was used for analyses of nocturnal arousals. Least-squares mean (LSM) change from baseline in nocturnal arousals for patients receiving once-nightly sodium oxybate (ON-SXB) or matching placebo. CI confidence interval, PSG polysomnography, SD standard deviation, SE standard error
Sleep Quality
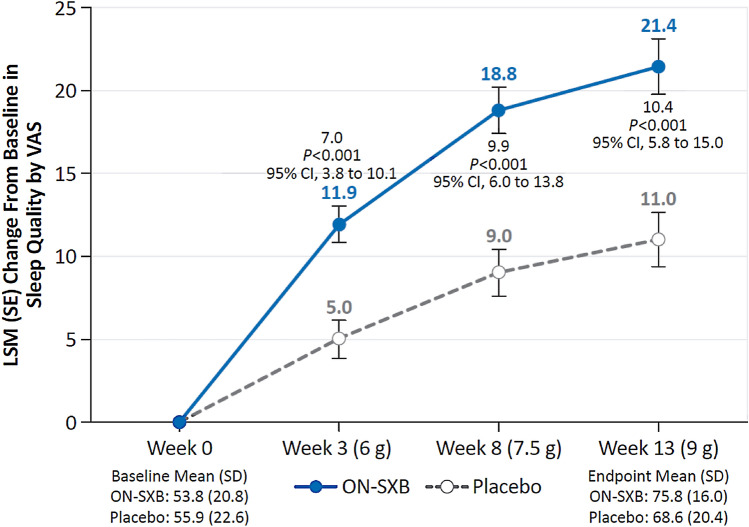
Patient-reported sleep quality was similar between the ON-SXB and placebo arms at baseline (mean VAS score, 53.8 and 55.9). Patient-reported sleep quality was significantly improved with ON-SXB vs placebo at week 3 for the 6-g dose (LSM change from baseline, 11.9 vs 5.0, respectively; p < 0.001); at week 8 for the 7.5-g dose (18.8 vs 9.0, respectively; p < 0.001); and at week 13 for the 9-g dose (21.4 vs 11.0, respectively; p < 0.001) (all Fig. 3).
Fig. 3.
Change from baseline in sleep quality (modified intent-to-treat population). A mixed-effects model for repeated measures was used for sleep quality analyses. Least-squares mean (LSM) change from baseline in patient-reported sleep quality for patients receiving once-nightly sodium oxybate (ON-SXB) or matching placebo. CI confidence interval, SD standard deviation, SE standard error, VAS visual analog scale
Refreshing Nature of Sleep
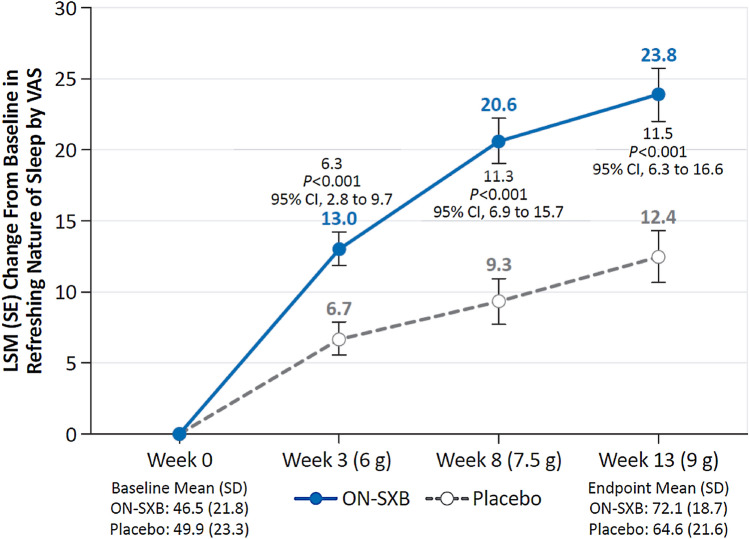
Refreshing nature of sleep reported by participants at baseline was similar between the ON-SXB and placebo arms (mean VAS score, 46.5 and 49.9, respectively). The LSM change from baseline in patient-reported refreshing nature of sleep was significantly increased with ON-SXB vs placebo at week 3 for the 6-g dose (13.0 vs 6.7, respectively; p < 0.001); at week 8 for the 7.5-g dose (20.6 vs 9.3, respectively; p < 0.001); and at week 13 for the 9-g dose (23.8 vs 12.4, respectively; p < 0.001) (all Fig. 4).
Fig. 4.
Change from baseline in refreshing nature of sleep (modified intent-to-treat population). A mixed-effects model for repeated measures was used for refreshing nature of sleep analyses. Least-squares mean (LSM) change from baseline in patient-reported refreshing nature of sleep for patients receiving once-nightly sodium oxybate (ON-SXB) or matching placebo. CI confidence interval, SD standard deviation, SE standard error, VAS visual analog scale
Post Hoc Analyses
A significantly reduced amount of time was spent in N1 with ON-SXB vs placebo at week 3 for the 6-g dose (p < 0.05); at week 8 for the 7.5-g dose (p < 0.001); and at week 13 for the 9-g dose (p < 0.001) (all Table 1). No significant change in time spent in N2 was observed at any dose of ON-SXB vs placebo. A significantly increased amount of time was spent in N3 with ON-SXB vs placebo at week 3 for the 6-g dose (p < 0.001); at week 8 for the 7.5-g dose (p < 0.001); and at week 13 for the 9-g dose (p < 0.001). A significant increase in delta power for non-REM sleep occurred with all doses of ON-SXB vs placebo (p < 0.01 ON-SXB 6 g [week 3]; p < 0.05 ON-SXB 7.5 g [week 8]; p < 0.001 ON-SXB 9 g [week 13]). The change from baseline in time in REM was significantly reduced with ON-SXB vs placebo at week 3 for the 6-g dose (p < 0.001); at week 8 for the 7.5-g dose (p < 0.001); and at week 13 for the 9-g dose (p < 0.001). Rapid eye movement latency increased with ON-SXB at all doses (range, 14.2–20.8 min) vs a decrease at all timepoints with placebo (range − 2.4 to − 3.4 min; p < 0.05 ON-SXB 7.5 g [week 8] vs placebo); while not statistically significant at week 3 (6-g dose) and week 13 (9-g dose), numerical increases in REM latency were observed.
Table 1.
Post hoc analysis: change from baseline in time spent in sleep stages, delta power, REM, and REM latency (modified intent-to-treat population)
| Parameter | Week 0 | Week 3 | Week 8 | Week 13 | ||||
|---|---|---|---|---|---|---|---|---|
| ON-SXB n = 97 |
Placebo n = 93 |
ON-SXB 6 g n = 97 |
Placebo n = 93 |
ON-SXB 7.5 g n = 97 |
Placebo n = 93 |
ON-SXB 9 g n = 97 |
Placebo n = 93 |
|
| N1 sleep | ||||||||
| Time spent in N1 sleep at baseline, minutes | ||||||||
| Mean (SD) | 40.2 (22.1) | 42.6 (21.2) | ||||||
| Change from baseline in N1, minutes | ||||||||
| LSM (SE) | − 6.2 (1.7) | − 0.3 (1.7) | − 10.3 (2.0) | 0.73 (1.9) | − 13.2 (1.8) | 0.15 (1.7) | ||
| LSMD (95% CI) | − 5.9 (− 10.7 to − 1.1)* | − 11.0 (− 16.5 to − 5.5)*** | − 13.4 (− 18.4 to − 8.4)*** | |||||
| N2 sleep | ||||||||
| Time spent in N2 sleep at baseline, minutes | ||||||||
| Mean (SD) | 215.9 (52.2) | 211.6 (48.3) | ||||||
| Change from baseline in N2, minutes | ||||||||
| LSM (SE) | – 8.5 (4.1) | − 1.9 (4.1) | 2.7 (4.4) | − 1.0 (4.3) | − 11.5 (5.0) | 2.0 (4.7) | ||
| LSMD (95% CI) | − 6.6 (− 18.1 to 4.9) | 3.6 (− 8.4 to 15.7) | − 13.5 (− 27.1 to 0.1) | |||||
| N3 sleep | ||||||||
| Time spent in N3 sleep at baseline, minutes | ||||||||
| Mean (SD) | 68.8 (31.5) | 68.8 (28.1) | ||||||
| Change from baseline in N3, minutes | ||||||||
| LSM (SE) | 27.6 (3.0) | 5.5 (3.0) | 30.4 (3.7) | 3.6 (3.6) | 39.5 (4.3) | 1.1 (4.1) | ||
| LSMD (95% CI) | 22.1 (13.6–30.5)*** | 26.8 (16.6–37.0)*** | 38.4 (26.7–50.1)*** | |||||
| Delta power in NREM | ||||||||
| Baseline delta power in NREM | ||||||||
| Mean (SD) | 460.8 (336.8) | 545.6 (656.5) | ||||||
| Change from baseline in delta power in NREM | ||||||||
| LSM (SE) | 239.4 (62.4) | − 6.5 (63.5) | 400.3 (95.3) | 90.2 (101.3) | 640.8 (110.8) | 67.2 (106.3) | ||
| LSMD (95% CI) | 245.8 (69.0–422.7)** | 310.0 (33.3–586.7)* | 573.6 (266.8–880.4)*** | |||||
| Total REM sleep | ||||||||
| Total REM sleep at baseline, minutes | ||||||||
| Mean (SD) | 76.7 (30.8) | 78.3 (27.7) | ||||||
| Change from baseline in total REM sleep, minutes | ||||||||
| LSM (SE) | − 16.1 (2.6) | 0.6 (2.6) | − 21.3 (2.7) | 5.9 (2.7) | − 22.8 (3.3) | 1.7 (3.1) | ||
| LSMD (95% CI) | − 16.7 (− 23.9 to − 9.5)*** | − 27.2 (− 34.8 to − 19.6)*** | − 24.5 (− 33.4 to − 15.6)*** | |||||
| REM latency | ||||||||
| Baseline REM latency, minutes | ||||||||
| Mean (SD) | 69.1 (68.0) | 56.0 (45.9) | ||||||
| Change from baseline in REM latency, minutes | ||||||||
| LSM (SE) | 14.2 (6.8) | − 3.4 (6.8) | 19.7 (7.6) | − 3.4 (7.5) | 20.8 (8.6) | − 2.4 (8.1) | ||
| LSMD (95% CI) | 17.6 (− 1.4 to 36.6) | 23.1 (2.0–44.2)* | 23.2 (− 0.21 to 46.6) | |||||
Mixed-effects model for repeated measures used to assess significance at each timepoint
CI confidence interval, LSM least-squares mean, LSMD least-squares mean difference, NREM non-rapid eye movement, ON-SXB once-nightly sodium oxybate, REM rapid eye movement, SD standard deviation, SE standard error
*p < 0.05; **p < 0.01; ***p < 0.001
The efficacy of ON-SXB vs placebo was investigated in the subgroups of participants who did or did not receive concurrent stimulant treatment during the trial (Table 2). The number of sleep shifts was significantly reduced with ON-SXB vs placebo at all three doses in both the stimulant use subgroup (6 g, p < 0.01; 7.5 g, p < 0.001; 9 g, p < 0.001) and the no stimulant use subgroup (all p < 0.001). A significant reduction from baseline in NAs with ON-SXB vs placebo was also observed with the 7.5-g and 9-g doses in the stimulant use subgroup (p < 0.01 and p < 0.001, respectively) and at all doses in the no stimulant use subgroup (6 g and 7.5 g, p < 0.05; 9 g, p ≤ 0.01). Significant improvements in patient-reported sleep quality with ON-SXB vs placebo were observed at all assessed doses in both the stimulant use (6 g and 7.5 g, p < 0.01; 9 g, p < 0.05) and no stimulant use (all p < 0.001) subgroups. Similarly, improvements in patient-reported refreshing nature of sleep with ON-SXB vs placebo were observed at all assessed doses in both the stimulant use (6 g and 9 g, p < 0.05; 7.5 g, p < 0.001) and no stimulant use subgroups (6 g and 7.5 g, p < 0.01; 9 g, p < 0.001).
Table 2.
Post hoc analysis: sleep stage shifts, NAs, sleep quality, and refreshing nature of sleep by stimulant use (modified intent-to-treat population)
| Baseline | Week 3 | Week 8 | Week 13 | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | ON-SXB | Placebo | ON-SXB 6 g | Placebo | ON-SXB 7.5 g | Placebo | ON-SXB 9 g | Placebo |
| Sleep stage shifts | ||||||||
| Stimulant use | n = 66 | n = 53 | ||||||
| Baseline, mean (SD) | 59.2 (24.5) | 64.0 (22.8) | ||||||
| LSM change from baseline (SE) | − 8.4 (2.2) | 0.6 (2.4) | − 12.8 (3.0) | 3.4 (3.1) | − 19.6 (2.6) | 1.5 (2.6) | ||
| LSMD (95% CI) | − 9.0 (− 15.5 to − 2.5)** | − 16.2 (− 24.8 to − 7.6)*** | − 21.1 (− 28.5 to − 13.8)*** | |||||
| No stimulant use | n = 31 | n = 40 | ||||||
| Baseline, mean (SD) | 62.1 (21.0) | 55.3 (19.5) | ||||||
| LSM change from baseline (SE) | – 13.4 (2.8) | – 3.5 (2.5) | – 20.2 (3.6) | 2.8 (3.2) | – 23.4 (4.1) | 4.1 (3.6) | ||
| LSMD (95% CI) | − 16.9 (– 24.5 to – 9.3)*** | – 23.0 (– 32.8 to – 13.3)*** | − 27.6 (– 38.4 to – 16.7)*** | |||||
| NAs | ||||||||
| Stimulant use | n = 66 | n = 53 | ||||||
| Baseline, mean (SD) | 78.5 (46.4) | 76.4 (30.6) | ||||||
| LSM change from baseline (SE) | – 26.6 (4.2) | – 20.2 (4.5) | – 35.8 (4.2) | – 17.1 (4.4) | – 36.0 (4.6) | – 14.7 (4.5) | ||
| LSMD (95% CI) | – 6.4 (– 18.7 to 5.9) | – 18.8 (– 30.8 to – 6.8)** | – 21.3 (– 34.1 to – 8.5)*** | |||||
| No stimulant use | n = 31 | n = 40 | ||||||
| Baseline, mean (SD) | 89.0 (36.8) | 78.2 (46.7) | ||||||
| LSM change from baseline (SE) | – 40.2 (6.2) | – 20.7 (5.5) | – 45.6 (7.7) | – 23.5 (6.8) | – 46.0 (8.2) | – 17.0 (7.2) | ||
| LSMD (95% CI) | – 19.5 (– 36.0 to – 3.0)* | – 22.1 (– 42.6 to – 1.6)* | – 29.0 (– 50.8 to – 7.1)** | |||||
| VAS sleep qualitya | ||||||||
| Stimulant use | n = 66 | n = 53 | ||||||
| Baseline, mean (SD) | 54.7 (19.1) | 55.5 (19.0) | ||||||
| LSM change from baseline (SE) | 10.7 (1.3) | 5.4 (1.4) | 18.3 (1.6) | 10.9 (1.7) | 19.5 (1.9) | 13.0 (2.0) | ||
| LSMD (95% CI) | 5.4 (1.6–9.1)** | 7.4 (2.9–12.0)** | 6.5 (1.1–11.9)* | |||||
| No stimulant use | n = 31 | n = 40 | ||||||
| Baseline, mean (SD) | 51.8 (24.3) | 56.5 (27.0) | ||||||
| LSM change from baseline (SE) | 14.7 (2.1) | 4.5 (1.8) | 19.4 (2.8) | 6.2 (2.5) | 24.9 (3.2) | 8.2 (2.8) | ||
| LSMD (95% CI) | 10.2 (4.6–15.9)*** | 13.2 (5.7–20.8)*** | 16.7 (8.1–25.3)*** | |||||
| VAS refreshing nature of sleepa | ||||||||
| Stimulant use | n = 66 | n = 53 | ||||||
| Baseline, mean (SD) | 49.0 (20.4) | 50.9 (21.8) | ||||||
| LSM change from baseline (SE) | 11.5 (1.4) | 7.0 (1.6) | 20.5 (1.7) | 11.7 (1.8) | 22.6 (2.0) | 15.1 (2.1) | ||
| LSMD (95% CI) | 4.5 (0.3–8.7)* | 8.8 (3.9–13.6)*** | 7.6 (1.8–13.3)* | |||||
| No stimulant use | n = 31 | n = 40 | ||||||
| Baseline, mean (SD) | 41.3 (24.1) | 48.6 (25.3) | ||||||
| LSM change from baseline (SE) | 16.1 (2.4) | 6.4 (2.0) | 20.6 (3.4) | 6.1 (2.9) | 25.7 (3.8) | 8.8 (3.2) | ||
| LSMD (95% CI) | 9.7 (3.5–15.8)** | 14.5 (5.6–23.4)** | 16.9 (7.0–26.8)*** | |||||
Mixed-effects model for repeated measures used to assess significance at each timepoint
CI confidence interval, LSM least-squares mean, LSMD least-squares mean difference, NA nocturnal arousal, ON-SXB once-nightly sodium oxybate, REM rapid eye movement, SD standard deviation, SE standard error, VAS visual analog scale, *p < 0.05; **p ≤ 0.01; ***p < 0.001
aVAS of 1–100, with 1 indicating “did not sleep”/“not refreshed” and 100 indicating “slept very well”/“refreshed”
Discussion
The data reported in this paper expand our understanding of the efficacy of ON-SXB for the treatment of the symptoms of narcolepsy. These results demonstrate that ON-SXB is associated with clinically significant improvements in sleep continuity, sleep architecture, and patient satisfaction with both the quality and refreshing nature of their sleep. Reductions in the number of transitions from sleep to wake and deeper stages of sleep to light sleep with ON-SXB treatment vs placebo were statistically significant, and NAs were also significantly reduced with ON-SXB treatment. Consistent with these improvements, a significant reduction in the length of time spent in N1, a significant increase in the time spent in N3, and increased delta power further support ON-SXB positively affecting sleep in patients with narcolepsy. In further alignment with the clinical efficacy demonstrated by the REST-ON primary efficacy results [14], significant improvements in patient-reported sleep quality and refreshing nature of sleep with ON-SXB treatment were reported. Overall, these significant findings provide additional support for the efficacy of ON-SXB in patients with narcolepsy. Moreover, they illustrate that for patients with narcolepsy, pharmacotherapy can improve nocturnal symptoms and patient perceptions of both sleep quality and feeling refreshed after sleep.
A previous assessment of twice-nightly SXB also showed increases in time spent in N3 and corresponding decreases in N1 and REM sleep, whereas N2 remains unaffected [12]; however, these data are presented separated into the first and the second half of the night owing to the need to awaken for the second middle-of-the-night dose required to cover a full night of sleep [10]. A required awakening for the second dose 2.5–4 h after the first bedtime dose by definition is a disruption of sleep. Although immediate-release SXB has been shown to increase the overall amount of time spent in SWS [12, 18, 19], the larger increase was observed in the second half of the night [12], which is in contrast to when SWS typically occurs following the homeostatic drive to sleep after a period of wakefulness [20].
Slow-wave sleep is considered the deepest and most restorative stage of sleep and is associated with sleep quality and maintenance of sleep [20–23]. A recently completed systematic review and meta-analysis showed that SWS is diminished in people with narcolepsy compared with controls (n = 65 studies) and similar in NT1 vs NT2 (n = 41 studies) [24]. During SWS, there is a reduction in sympathetic activity; a reduction in this sleep stage may adversely affect blood pressure and contribute to hypertension [25–27]. Whether the increase in SWS with ON-SXB confers additional clinical benefits merits further research.
A significant reduction in overall REM sleep was demonstrated with all three ON-SXB doses, which was previously demonstrated with the 9-g dose of twice-nightly SXB [12]. ON-SXB increased REM sleep latency at all doses compared with placebo, which was statistically significant at the 7.5-g dose. The clinical implications of SXB modifying REM sleep are not entirely known, although nocturnal REM suppression may theoretically decrease SP, hypnagogic/hypnopompic hallucinations, and vivid dreaming.
The magnitude of change in measures of DNS was greater for participants who were not taking concomitant stimulants, although no statistical comparisons were made between groups given that these are post hoc analyses. Significant improvements in sleep stage transitions, NAs, and patient-reported sleep quality and refreshing nature of sleep were observed with ON-SXB vs placebo regardless of concurrent stimulant use. Polypharmacy may be necessary for patients with narcolepsy, and many take stimulants for daytime sleepiness [28, 29]. Thus, these findings provide reassuring evidence that the efficacy of ON-SXB for the treatment of DNS in narcolepsy will likely be maintained whether or not the patients are using concurrent stimulants.
The analyses conducted in this trial have some limitations. Efficacy results presented are secondary endpoints, not powered for a multiplicity analysis, and some were conducted post hoc. However, improvements with ON-SXB treatment were consistent over time and increased in a dose-dependent manner, further substantiating the results and decreasing the likelihood of a type 1 error.
Conclusions
The efficacy of a single bedtime dose of SXB was demonstrated for the treatment of DNS in participants with narcolepsy. Clinically relevant improvements were observed in both objective measures (i.e., PSG-recorded decreases in stage shifts and NAs) and subjective findings with participants reporting both improved quality and refreshing nature of sleep. At all doses evaluated (6, 7.5, and 9 g), ON-SXB treatment demonstrated a significant consolidation of nocturnal sleep, a significant improvement in time spent in deep sleep, and a significant decrease in time spent in lighter sleep vs placebo.
Acknowledgments
The authors thank the REST-ON trial participants and their families. People who participate in clinical trials play a key role in advancing science and medicine and we would like to acknowledge those individuals who participated in this trial. A statistical review of the manuscript was provided by Robert Flikkema, PhD, with funding from Avadel Pharmaceuticals (Chesterfield, MO, USA). Medical writing support was provided by Judy Fallon, PharmD, and Jennifer Fetting, PhD, of The Curry Rockefeller Group, LLC (Tarrytown, NY, USA), and was funded by Avadel Pharmaceuticals (Chesterfield, MO, USA).
Declarations
Funding
This study was funded by Avadel Ireland. Avadel was involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Open access publication was funded by Avadel Ireland.
Conflicts of interest/Competing interests
TR is a consultant for Jazz Pharmaceuticals, Takeda Pharmaceutical Co., Orexo, Avadel Pharmaceuticals, Eisai, Merck & Co., and Idorsia. YD has served as a consultant or on advisory boards for Avadel Pharmaceuticals, Jazz Pharmaceuticals, UCB, Takeda Pharmaceutical Co., Theranexus, Harmony Biosciences, Bioprojet Pharma, and Idorsia. MJT has served as a consultant or on advisory boards for Axsome Therapeutics, Balance Therapeutics, Eisai, Avadel Pharmaceuticals, Harmony Biosciences, Jazz Pharmaceuticals, NLS Pharmaceuticals, Suven Life Sciences Ltd., and Takeda Pharmaceutical Co. CK is a consultant of Avadel Pharmaceuticals and XW Pharma. BCC is a member of the speakers bureaus and has received honoraria from Jazz Pharmaceuticals, Eisai, and Harmony Biosciences. He is an advisor and has received consulting fees and honoraria from Jazz Pharmaceuticals, Eisai, Harmony Biosciences, and Avadel Pharmaceuticals. He is a member of the speakers bureau for Merck & Co. Inc., Jazz Pharmaceuticals, Eisai, and Harmony Biosciences. RB is a shareholder in WaterMark Medical and Healthy Humming, LLC; serves on the board of directors for the National Sleep Foundation and WaterMark Medical; is a consultant for Jazz Pharmaceuticals, Takeda Pharmaceutical Co., Avadel Pharmaceuticals, and Oventus; has received industry-funded research grants from Avadel Pharmaceuticals, BrescoTec, Bayer, Idorsia, Suven Life Sciences Ltd, Jazz Pharmaceuticals, Balance, Vanda, Merck & Co., Eisai, Philips, FRESCA Medical, Takeda Pharmaceutical Co., LivaNova, Roche, and Sommetrics; and has served on speakers bureaus for Jazz Pharmaceuticals, Eisai, and Harmony Biosciences. RR received research grant funding by Avadel Pharmaceuticals to conduct the current study. He has also received research grant support from Jazz Pharmaceuticals, Eisai, Merck & Co., Apnimed, Inc., Idorsia, Biohaven, and Suven Life Sciences Ltd. He has participated in advisory boards for Jazz Pharmaceuticals, Eisai, and Harmony Biosciences. DS is an employee of Avadel Pharmaceuticals. JD is a consultant to, stockholder, and former employee of Avadel Pharmaceuticals.
Ethics approval
The REST-ON protocol was approved by the centers’ institutional review board or appropriate independent ethics committee.
Consent to participate
All participants provided written informed consent (and assent for participants aged 16 or 17 years of age) before participation.
Consent for publication
Not applicable.
Availability of data and material
The data underlying this article will be shared on reasonable request to the corresponding author.
Code availability
Not applicable.
Authors’ contributions
TR, YD, JD, and DS participated in study conception and design. TR, YD, CK, MJT, BCC, RB, RR, JD, and DS participated in the acquisition and/or analysis of data. TR, YD, CK, MJT, BCC, RB, RR, JD, and DS participated in drafting a significant portion of the manuscript or figures, approved the final version for submission, and agree to be accountable for the work presented.
Footnotes
At the time the study was conducted, Dr. Jordan Dubow's affiliation was Clinical Development and Medical Affairs, Avadel Pharmaceuticals, 16640 Chesterfield Grove Road, Suite 200, Chesterfield, MO 63005, USA
References
- 1.Kornum BR, Knudsen S, Ollila HM, et al. Narcolepsy. Nat Rev Dis Primers. 2017;3:16100. doi: 10.1038/nrdp.2016.100. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth JRWT, Koepsell TD, Ton TG, et al. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 4.Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9:955–965. doi: 10.5664/jcsm.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamelak M. Narcolepsy and depression and the neurobiology of gammahydroxybutyrate. Prog Neurobiol. 2009;89:193–219. doi: 10.1016/j.pneurobio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Alfano CA, Reynolds K, Scott N, et al. Polysomnographic sleep patterns of non-depressed, non-medicated children with generalized anxiety disorder. J Affect Disord. 2013;147:379–384. doi: 10.1016/j.jad.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargens TA, Kaleth AS, Edwards ES, et al. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep. 2013;5:27–35. doi: 10.2147/NSS.S34838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauvilliers Y, Barateau L, Lopez R, et al. Narcolepsy Severity Scale: a reliable tool assessing symptom severity and consequences. Sleep. 2020;43:zsaa009. doi: 10.1093/sleep/zsaa009. [DOI] [PubMed] [Google Scholar]
- 9.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jazz Pharmaceuticals . Xyrem (sodium oxybate oral solution, CIII) Full prescribing information. Palo Alto: Jazz Pharmaceuticals; 2020. [Google Scholar]
- 11.Barateau L, Lopez R, Dauvilliers Y. Treatment options for narcolepsy. CNS Drugs. 2016;30:369–379. doi: 10.1007/s40263-016-0337-4. [DOI] [PubMed] [Google Scholar]
- 12.Black J, Pardi D, Hornfeldt CS, et al. The nightly use of sodium oxybate is associated with a reduction in nocturnal sleep disruption: a double-blind, placebo-controlled study in patients with narcolepsy. J Clin Sleep Med. 2010;6:596–602. doi: 10.5664/jcsm.27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seiden D, Tyler C, Dubow J. Pharmacokinetics of FT218, a once-nightly sodium oxybate formulation in healthy adults. Clin Ther. 2021;43(672):e1–14. doi: 10.1016/j.clinthera.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Kushida CA, Shapiro CM, Roth T, et al. Once-nightly sodium oxybate (FT218) demonstrated improvement of symptoms in a phase 3 randomized clinical trial in patients with narcolepsy. Sleep. 2021 doi: 10.1093/sleep/zsab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U. S. Xyrem Multicenter Study Group A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42–49. doi: 10.1093/sleep/25.8.42. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine . International classification of sleep disorders. 3. Darien: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 17.Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4) J Clin Sleep Med. 2017;13:665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scrima L, Hartman PG, Johnson FH, Jr, et al. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study. Sleep. 1990;13:479–490. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- 19.Lapierre O, Montplaisir J, Lamarre M, et al. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: further considerations on REM sleep-triggering mechanisms. Sleep. 1990;13:24–30. doi: 10.1093/sleep/13.1.24. [DOI] [PubMed] [Google Scholar]
- 20.Akerstedt T, Hume K, Minors D, et al. Good sleep: its timing and physiological sleep characteristics. J Sleep Res. 1997;6:221–229. doi: 10.1111/j.1365-2869.1997.00221.x. [DOI] [PubMed] [Google Scholar]
- 21.Parekh A, Mullins AE, Kam K, et al. Slow-wave activity surrounding stage N2 K-complexes and daytime function measured by psychomotor vigilance test in obstructive sleep apnea. Sleep. 2019;42:zsy256. doi: 10.1093/sleep/zsy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5:S6–15. doi: 10.5664/jcsm.5.2S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dijk DJ, Groeger J, Deacon S, et al. Association between individual differences in slow wave sleep, slow wave activity and sleep continuity in young, middle-aged and older men and women. Eur Neuropsychopharmacol. 2006;16:S538. doi: 10.1016/S0924-977X(06)70749-0. [DOI] [Google Scholar]
- 24.Zhang Y, Ren R, Yang L, et al. Polysomnographic nighttime features of narcolepsy: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101488. doi: 10.1016/j.smrv.2021.101488. [DOI] [PubMed] [Google Scholar]
- 25.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease: a review of the recent literature. Curr Cardiol Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennum PJ, Plazzi G, Silvani A, et al. Cardiovascular disorders in narcolepsy: review of associations and determinants. Sleep Med Rev. 2021;58:101440. doi: 10.1016/j.smrv.2021.101440. [DOI] [PubMed] [Google Scholar]
- 27.Bosco A, Lopez R, Barateau L, et al. Effect of psychostimulants on blood pressure profile and endothelial function in narcolepsy. Neurology. 2018;90:e479–e491. doi: 10.1212/WNL.0000000000004911. [DOI] [PubMed] [Google Scholar]
- 28.Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34:9–27. doi: 10.1007/s40263-019-00689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maski K, Trotti LM, Kotagal S, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17:1881–1893. doi: 10.5664/jcsm.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]