Abstract
Background
Spinal muscular atrophy (SMA) is a progressive neuromuscular disorder that has a substantial impact on health-related quality of life for patients with SMA and their caregivers. Utility values (‘utilities’) are used in health economic analyses to incorporate individual or societal perspectives regarding the desirability of health outcomes such as a certain health state or change in health states over time.
Objectives
The primary objective of this systematic literature review (SLR) was to identify published utilities associated with patients with SMA and their caregivers and to determine the extent to which Health Technology Assessment (HTA) requirements of methods used to generate utilities are met. A secondary objective was to broaden the scope to identify utilities associated with other (non-SMA) neuromuscular disorders.
Methods
A comprehensive search to capture published utilities associated with patients with SMA and their caregivers was performed in 2019 and updated in 2021 using several electronic databases in addition to supplementary sources. As we anticipated that few published utilities associated with SMA would be identified, the search also captured utilities for other (non-SMA) neuromuscular disorders that may serve as useful surrogate values for studies of SMA and other rare diseases. Electronic searches were performed in Embase, MEDLINE, Evidence-Based Medicine Reviews, and EconLit via the Ovid platform and were supplemented by searches of the grey literature (reference lists, conference proceedings, global HTA body websites, and other relevant sources). Study eligibility criteria were based on the population, interventions, comparators, and outcomes (PICO) framework. The quality of the full-text publications was assessed using a checklist based on UK National Institute for Health and Care Excellence technical support documents.
Results
In total, 14 publications that reported SMA-related patient or caregiver utilities or disutilities met the eligibility criteria to be included in the SLR; the included studies demonstrate the substantial health-related quality-of-life burden of SMA on both patients with SMA and their caregivers. A variety of preference-based measures were used to derive utilities for patients with SMA and their caregivers. Different methods for collecting utility data included patient and proxy assessment of health states using questionnaires, vignette methodologies, structured forms of expert elicitation, and mapped data from results of clinical trials. A range of utilities was reported from both patient- and proxy-reported data, which reflects the degree of disability associated with early- and later-onset SMA. Methods for deriving utilities were assessed with respect to three reference cases from HTA bodies in the UK, the USA, and Canada. None of the 14 publications met the requirements of all three HTA bodies because of differing tariff requirements between countries; one study met the requirements of HTA bodies in Canada and the UK. Also, six studies did not report the method of valuation, which precluded analysis with respect to the HTA reference cases.
Conclusions
This SLR provides a comprehensive repository of the currently available utilities relevant to patients with SMA and their caregivers. This SLR provides recommendations for establishing consensus on the approach to generating utility values for the SMA patient population and their caregivers for health economic decisions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-021-01115-5.
Key Points for Decision Makers
| Most studies identified in the systematic literature review failed to meet the requirements of health technology assessment bodies in the UK, USA, and Canada because they used country-specific tariffs or did not report valuation methods. |
| This review highlights the need for age-appropriate and validated preference-based measures for paediatric patients and utility data collection of caregivers of patients with spinal muscular atrophy (SMA). |
| Consensus for future utility estimations in SMA should include health state descriptions that reflect the improvement in motor function yielded by treatment with disease-modifying therapies. |
Introduction
Spinal muscular atrophy (SMA) is a rare hereditary neuromuscular disease with an estimated incidence of 1 in 10,000 live births [1, 2]. There is currently no cure for SMA, and—in the absence of medical intervention—SMA is a principal genetic cause of infant mortality [3]. SMA results from homozygous deletions (~ 96%) or deletions and mutations (~ 4%) in the survival of motor neuron 1 (SMN1) gene [4]. A related gene, SMN2, produces insufficient levels of stable SMN protein to compensate for the SMN1 deficiency, and the number of SMN2 copies that an individual carries is generally inversely proportional to the severity of SMA [5, 6]. However, discordance between SMN2 copy number and SMA phenotype can occur as a result of genetic and epigenetic modifiers [7]. Insufficient levels of SMN protein causes motor neuron degeneration, which in turn leads to progressive muscle degeneration and symmetrical muscle weakness [2]. The traditional classification of SMA includes five types (Types 0–4) based on patient age at onset of disease symptoms and the highest motor milestone achieved [8–11]. Type 0 SMA causes foetal or neonatal death, whereas Type 4 SMA—the least severe form—typically manifests during adulthood, and individuals are ambulant [11, 12]. Without disease-modifying therapies (DMTs), infants with Type 1 SMA (also known as Werdnig–Hoffmann disease) cannot sit, and life expectancy may not exceed 2 years [9–12]. Type 2 SMA generally manifests between 7 and 18 months of age, and individuals can sit but never walk [11, 12], whereas patients with Type 3 SMA (also known as Kugelberg–Welander disease) who can walk progressively lose this ability [11, 12]. Standard of care (SOC) management for SMA cannot modify the pathology underlying the disease and is considered as palliative or supportive. SOC for SMA incorporates multidisciplinary input from a team including neurologists, respiratory specialists, gastroenterologists, geneticists, palliative care physicians, orthopaedic surgeons, and physical therapists [13, 14].
Between 2016 and 2021, three DMTs that use different routes of administration to increase SMN levels were approved by the US FDA and the European Medicines Agency. Nusinersen (SPINRAZA®; Biogen Inc., Cambridge, MA, USA), an antisense oligonucleotide that modifies SMN2 pre-messenger RNA splicing to increase functional SMN production, was approved in the USA and Europe in 2016 [15] and 2017 [16], respectively. Nusinersen is intrathecally administered to adult and paediatric patients with SMA [15, 16]. Following four loading doses of nusinersen, maintenance doses are required three times per year [15, 16]. Onasemnogene abeparvovec-xioi (ZOLGENSMA®; AveXis Inc., Bannockburn, IL, USA) is a single-dose intravenously administered adeno-associated virus vector-based gene-transfer therapy that facilitates the transfer of a copy of the SMN1 gene [17]. Onasemnogene abeparvovec was approved in the USA in 2019 for the treatment of children aged < 2 years with bi-allelic mutations in SMN1 [18] and in Europe in 2020 for the treatment of children (≤ 21 kg body weight) with an inherited mutation in SMN1 and up to three copies of the SMN2 gene [19]. Risdiplam (EVRYSDI™; Genentech Inc., South San Francisco, CA, USA) is a daily orally administered SMN2 splicing modifier that is distributed centrally and peripherally and increases SMN production [20]. Risdiplam was approved in the USA in 2020 for the treatment of SMA in patients aged ≥ 2 months [21] and in Europe on 30 March 2021 for patients aged ≥ 2 months with a clinical diagnosis of Type 1, 2, or 3 SMA or with one to four SMN2 copies [22].
The advent of DMTs for SMA has offered new management options and hope for patients with SMA. However, a lack of validated biomarkers has led to some ethical, medical, and financial concerns for the SMA community regarding how to interpret variability in treatment responses [23]. Whether the cost of a new medical intervention is justified by the expected health benefits is typically appraised by health technology assessment (HTA) bodies using decision-analytic models [24]. Quality-adjusted life-years (QALYs)—a combined measure of survival and health-related quality of life (HRQoL)—are the benefit outcome in the incremental cost-effectiveness ratio used by HTA bodies to make resource-allocation decisions [24]. QALYs are calculated using health state utility values (HSUVs or ‘utilities’), which incorporate individual or societal perspectives regarding the desirability of health outcomes such as a certain health state or change in health states over time [25]. HSUVs are indexed measures anchored between zero and one that reflect ‘death’ and ‘perfect health’, respectively; a negative value is considered ‘worse than death’ [26]. Disutility refers to the decrement in valued quality of life (utility) because of a particular symptom or complication [27].
HSUVs may be derived using direct and indirect approaches. Direct approaches include methods such as standard gamble (SG) or time trade-off (TTO) [28]. HSUVs may be estimated indirectly using generic preference-based measures (PBMs) that typically consist of a standardised HRQoL questionnaire from which health state descriptions are indirectly mapped to a utility scale by applying societal preferences (tariffs) to health states [29]. Generic PBMs are commonly used in clinical trials [30] and include instruments such as the EQ-5D [31] (including the EQ-5D-3L, EQ-5D-5L, and EQ-5D-Y versions) [32], and the Health Utilities Index Mark 2 and 3 (HUI2 and 3) [33]. As HSUV estimates affect the accuracy and quality of cost-utility models, HTA bodies may recommend a particular approach to HSUV derivation [28]. For example, the UK National Institute for Health and Care Excellence (NICE) recommends that the EQ-5D is used to derive HSUVs for adults [34]. As the EQ-5D-Y does not currently have a validated UK value set, NICE suggests that, for paediatric populations, alternative standardised and validated PBMs designed specifically for use in children should be considered [34]. A value set for the EQ-5D-Y has recently been developed but is currently only available for research purposes [35].
In the absence of robust EQ-5D (or other preference-based instruments) data collected directly from patients, HTA bodies may accept the mapping of or elicitation from disease-specific/generic HRQoL data to a generic PBM. For example, an algorithm developed by Khan et al. [36] has been used to map EQ-5D utility scores from Pediatric Quality of Life Inventory (PedsQL) generic core scales. This alternative approach to generating HSUVs is pertinent to a disease such as SMA that mostly affects paediatric patients. In cases where an established PBM scale is not available, direct preference elicitation can be used, for example, discrete choice experiments (DCEs) and other vignette approaches, including case history reviews by clinical experts [30].
To date, few HRQoL tools have been developed specifically for SMA to estimate utility or disutility data for economic evaluations. According to a previous study [37], in the limited number of SMA clinical trials in which utility data were collected using standardised measures, data collection methods varied from patient surveys to vignette methodologies, structured expert elicitation, and DCEs—a quantitative method in which competing scenarios are presented to determine trade-offs between medical treatment attributes [38].
The primary objective of this systematic literature review (SLR) was to identify published HSUVs associated with patients with SMA and their caregivers, with a secondary objective to broaden the scope to identify HSUVs associated with other (non-SMA) neuromuscular disorders. We identify available HSUVs for patients with SMA and their caregivers and determine the extent to which HTA body requirements of methods used to generate HSUVs are currently met. In addition, we emphasise the importance of developing a consensus approach in HSUVs for the SMA community, so that the needs of patients with SMA and their caregivers may be consistently assessed.
Methods
Search Strategy and Selection Criteria
An SLR was conducted to identify available HSUVs associated with SMA. The 2020 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed to isolate and screen scientific literature and extract data [39]. The following electronic databases were searched on 29 August 2019 via the Ovid platform: MEDLINE® (including epub ahead of print, in-process and other non-indexed citations, and daily update), Embase, and Evidence-Based Medicine Reviews. Additional searches of congress proceedings, reference lists of included publications, HTA bodies, and additional sources and websites such as Spinal Muscular Atrophy UK were conducted to identify relevant evidence (Table 1 in the electronic supplementary material [ESM]). The search was updated on 8 March 2021 to ensure that any recently published studies were captured. The full search strategies (up to 8 March 2021), including free text words, subject index headings (e.g. medical subject headings [MeSH]), the relationship between search terms (e.g. Boolean), and database start dates are provided in Table 2 in the ESM. The SLR search parameters were designed to capture HSUVs for relevant SMA health states derived using generic preference-based instruments, direct methods, mapping algorithms, vignette studies, patient/caregiver utilities and disutilities (Table 3 in the ESM). Eligibility criteria for the SLR were defined in terms of population, interventions, comparators, and outcomes (PICO) framework and study design [40], and there were no restrictions regarding geography, study country, or date of publication (Table 3 in the ESM). Studies reporting HSUVs for SMA-related health states were of primary interest for the review; however, given the anticipated paucity of evidence for SMA, we also identified HSUVs associated with other (non-SMA) neuromuscular conditions, such as myodystrophy, muscular dystrophy, and amyotrophic lateral sclerosis. Non-SMA HSUVs are not analysed further in this SLR but are presented in Table 4 in the ESM as they may serve as useful surrogate values for rare diseases for which utility values are lacking.
Data Extraction
Relevant data were extracted into summary tables by a first reviewer. A second reviewer checked the data extraction, and any inconsistencies were referred to a third reviewer and resolved through discussion.
Assessment of Bias and Quality of Evidence
The quality of eligible HSUV studies was assessed as recommended by NICE technical support documents 8–10 [41–43] and enabled justification of the use/non-use of different utility values or mapping algorithms in an economic model. In particular, the following issues were addressed: (1) whether response rates, loss to follow-up, or missing data level were likely to threaten the validity of the utility estimate; (2) whether the selection criteria yielded a population similar to that being modelled; (3) whether the utility incorporated a decrement for quality-of-life (QoL) loss from adverse events; and (4) whether the utility met the NICE reference case [34] (i.e. health states should be described by the patient and valued according to UK societal preferences).
Comparison of Available HSUVs with HTA Body Reference Cases
The final publications considered for inclusion in the SLR were compared with three HTA body reference cases to determine which publications, if any, met the requirements for HTA body submissions with respect to HSUVs. The HTA body reference cases included reviews published by NICE in 2013 [34], the Canadian Agency for Drugs and Technologies in Health (CADTH) in 2017 [44], and the US Institute for Clinical and Economic Review (US ICER Group) in 2018 [45]. Table 1 summarises the requirements of each reference case with respect to measurement and valuation of health effects. All three HTA bodies recommend that HRQoL should be measured in patient populations, but only NICE specifies which generic PBM should be used. NICE and the US ICER Group require UK and US tariffs, respectively, but CADTH accepts Canadian (or similar) societal preferences. Of the three HTA bodies, only NICE provides guidance for the measurement of utilities in paediatric populations. None of the HTA bodies provide recommendations for the measurement of utilities in caregivers of patients. Where no specific recommendations are provided, it is likely that HTA body requirements will be met if a PBM is used and country-specific societal preferences are considered. Therefore, the information in Table 1 summarises the ‘gold standard’ requirements for the measurement and valuation of health effects for each HTA body, but we note that utilities derived using methods deviating from the recommendations would also be acceptable with adequate justification, e.g. proxy respondents on behalf of paediatric patients. Using the HTA body requirements, five questions were created to assess the extent to which the studies identified in this SLR met the requirements of each HTA reference case. The questions were as follows:
Table 1.
Summary of HTA body reference cases with regards to measurement and valuation of health effects
| Measurement and valuation of health effects | HTA body reference cases | ||
|---|---|---|---|
| NICE [34] | CADTH [44] | US ICER Group [45] | |
| Instrument with which change in HRQoL should be measured (adult patients) | EQ-5D-3L or EQ-5D-5La | Generic PBM | Generic PBM |
| Population in which change in HRQoL should be measured | Patients | Patients | Patients |
| Preferences (tariffs) with which health states should be valued | UK societal preferences | Canadian (or similar) societal preferences | US societal preferences |
| Preferred method for valuing health states | Choice-based method (SG/TTO) | Not specified | Not specified |
| Preferred instrument for estimating utilities in paediatric populations | Standardised and validated PBMs of HRQoL designed specifically for use in children | Not specified | Not specified |
| Preferred instrument for estimating utilities in caregivers | Not specified | Not specified | Not specified |
CADTH Canadian Agency for Drugs and Technologies in Health, HRQoL health-related quality of life, HTA health technology assessment, ICER Institute for Clinical and Economic Review, NICE National Institute for Health and Care Excellence, PBM preference-based measure, SG standard gamble, TTO time trade-off
aWhere data were gathered using the EQ‑5D‑5L descriptive system, utility values in reference case analyses should be calculated by mapping the EQ-5D-5L descriptive system data to the EQ-5D-3L value set using mapping function developed by van Hout et al. [64]. If analyses use data gathered using both EQ‑5D‑3L and EQ‑5D‑5L descriptive systems, the EQ-5D-3L value set should be used to derive all utility values, with EQ-5D-5L mapped to EQ-5D-3L where needed
A1. Was a generic preference-based instrument used to describe health states?
A2. Was the selected instrument age appropriate?
B. Did patients describe the health states?
C. Were appropriate societal preferences used to value health states?
D. Was the TTO/SG method used to value health states?
Results
Description of Identified Studies
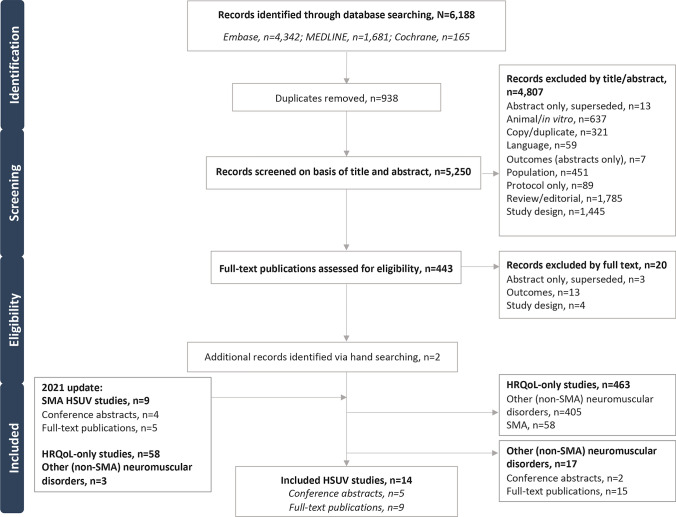
The original electronic database searches conducted in August 2019 identified a total of 6188 citations. Following removal of 938 duplicates, 5250 citations were screened on the basis of title and abstract. In total, 443 citations were deemed to be potentially relevant and were obtained for full-text review, and a further 20 articles were excluded. Handsearching yielded two additional relevant SMA publications. In total, this search identified four full-text publications [46–49] and one conference abstract and associated poster [50] that reported HSUVs for the population of interest. The updated search conducted in March 2021 yielded an additional nine publications, including five full-text publications [51–55] and four abstracts [56–59]; three abstracts had an associated poster [56, 57, 59]. Across the original and updated SLR search, 17 studies considering other (non-SMA) neuromuscular disorders and 463 studies reporting HRQoL data only (i.e. studies did not report HSUV data but administered a disease-specific and/or generic HRQoL instrument) were tagged and excluded. The list of the 17 other (non-SMA) neuromuscular disorders is shown in Table 4 in the ESM.
The final list of 14 publications that met the eligibility criteria to be included in the SLR (Fig. 1) consisted of nine full-text publications [46–49, 51–55] and five abstracts [50, 56–59]; four of the abstracts had an associated poster [50, 56, 57, 59].
Fig. 1.
Study flow chart. HRQoL health-related quality of life, HSUV health state utility value, SMA spinal muscular atrophy
Reporting of HSUVs by Respondent Type and PBM Instruments Used in Identified Studies
For simplicity, we refer to HSUVs that were generated from self-reported health states by patients with SMA as ‘patient-derived HSUVs’, whereas SMA health states that were valued by proxies (caregivers/parents/clinical experts) are designated as ‘proxy-derived HSUVs’. We refer to ‘mixed patient-/proxy-derived HSUVs’ in cases where a single utility value was estimated from a combination of patient and proxy respondents. An ‘overall’ HSUV represents a single utility value derived from a cohort of patients with SMA, i.e. ‘overall Type 1 SMA’ or ‘overall Types 1–3 SMA’.
A variety of instruments were used to describe SMA health states, which in turn were used to derive patient and caregiver utility and disutility values across the 14 studies. Table 2 provides an overview of the characteristics of the PBM instruments used in the studies, including applicability to paediatric patient populations, and Table 3 lists the instrument(s) used in each study. Some studies used a combination of instruments; for example, Peña-Longobardo et al. [55] used the EQ-5D-3L for patients and the EQ-5D-5L for caregivers because the EQ-5D-5L is only validated for adult respondents. The instruments for proxy-derived patient HSUVs included the EQ-5D-Y (clinical experts as proxies for patients; n = 1) [46]; the EQ-5D-3L (parents/caregivers as proxies for patients; n = 4) [47, 49, 50, 59]; the EQ-5D-5L (caregivers as proxies for patients; n = 2) [57]; and the HUI3 (parent/caregivers as proxies for patients; n = 1) [51]. Patient-reported HSUVs were generated using the EQ-5D-Y (n = 1) [53], EQ-5D-3L (n = 1) [55], EQ-5D-5L (n = 4) [52, 54, 55, 57], HUI3 (n = 1) [58], and PedsQL mapped to the EQ-5D-Y (n = 1) [48]. Instruments used to derive caregiver utilities included the CarerQoL (n = 1) [53], the EQ-5D (n = 1) [47], the EQ-5D-3L (n = 1) [59], and the EQ-5D-5L (n = 2) [55, 57].
Table 2.
Characteristics of PBM instruments used in studies identified in this systematic literature review
| PBM instrument | CarerQoL | EQ-5D | EQ-5D-Y | HUI3 | PedsQL 4.0 Generic Core Scales | PedsQL-3.0 NMM |
|---|---|---|---|---|---|---|
| References | [74] | [31] | [32] | [33] | [90] | [91] |
| Type of measure (generic or designed for specific disorders) | Generic (caregiver burden) | Generic | Generic | Generic | Generic | Designed for neuromuscular disorders such as SMA |
| Applicable population(s) and reporting method(s) | Informal caregivers: self-report (cannot be applied to CUAs that evaluate patient interventions [77]) | Patients: self-report (adults and childrena aged ≥ 12 years) |
Patients: self-report for childrena aged ≥ 12 years Proxy version for children aged 4–7 years |
Patients: adults and children aged ≥ 13 years: self-assessment, self- or interviewer-administered version Proxy assessment, self- or interviewer-administered version recommended for children aged 5–8 years Self-assessment, interviewer-administered version recommended for children aged 8–12 years |
Child self-report: formats for those aged 5–7, 8–12, and 13–18 years Parent-proxy report: (formats: children aged 2–4 [toddler], 5–17 [young child], 8–12 [child], and 13–18 [adolescent] years) |
Parallel child self-report and parent-proxy report: children aged 5–18 years Parent-proxy report: children aged 2–4 years |
| Domains assessed | Seven dimensions of caregiver burden: fulfilment; relational dimension; mental health dimension; social dimension; financial dimension; perceived support; physical dimension |
Five dimensionsb (each with three levels): mobility; self-care; usual activities; pain/discomfort; anxiety/depression Levels for each dimension: no problems, some problems, extreme problems |
Five dimensions (each with three levels): mobility; looking after myself; doing usual activities; having pain or discomfort; feeling sad, worried, or unhappy | Eight attributes (each with five or six levels): vision; hearing; speech; ambulation; dexterity; emotion; cognition; pain | Four scales: physical functioning (eight items); emotional functioning (five items); social functioning (five items); school functioning (five items) | Three scales: about my/my child’s neuromuscular disease (17 items); communication (three items); about our family resources (five items) |
CarerQoL carer-related quality of life, CUA cost-utility analyses, HUI Health Utilities Index, NMM neuromuscular module, PBM preference-based measure, PedsQL Pediatric Quality of Life Inventory, SMA spinal muscular atrophy
aFor children aged ≥ 12 years, the youth version of the EQ-5D (EQ-5D-Y) should be considered depending on study design
bThe second part of the EQ-5D questionnaire consists of a visual analogue scale in which patients rate their perceived health from 0 (the worst imaginable health) to 100 (the best imaginable health)
Table 3.
Characteristics of studies identified in the systematic literature review
| Publication | Study design | Study location | Method of elicitation | Valuation methoda | Population for whom utility is being measured | Respondent | Sample size and patient demographics |
|---|---|---|---|---|---|---|---|
| Belter et al. [51] | Survey | Global | HUI3 | NR | Patients with SMA |
Patients with SMA (aged ≥ 18 years) Proxy (parent/caregiver) for pts aged < 18 years |
Responses represented n = 478 patients with SMA: Type 1 (n = 121), Type 2 (n = 225), Type 3 (n = 132) Female (n = 284), male (n = 194) Mean ± SD age 17.1 ± 16.8 years |
| Binz et al. [52] | Prospective longitudinal study | Germany | EQ-5D-5L | Value set for EQ-5D-5L based on preferences of German population [92] | Adult patients with SMA | Adult patients with SMA (aged ≥ 18 years) | n = 18 patients with SMA: Type 2 (n = 6), Type 3 (n = 11), Type 4 (n = 1) |
| Chambers et al. [53] | Cross-sectional study | Australia | EQ-5D-Y | EQ-5D-3L Australian value sets used as a proxy as EQ-5D-Y value set unavailable | Patients with Type 1, 2 or 3 SMA | Patients with SMA (aged 0–21 years) and caregiver pairs |
n = 40 patientt–caregiver pairs Children with SMA: Type 1 (n = 4), Type 2 (n = 26), Type 3 (n = 10). Female (n = 21), male (n = 19). Mean age 9.5 years (range 1–23) |
| Lloyd et al. [46] | Cross-sectional survey | UK | Clinical experts assessed Types 1 and 2 SMA case studies using EQ-5D-Y and PedsQL-NMM (baseline states only) | EQ-5D-Y data were scored using UK EQ-5D-3L tariff | Patients with SMA | Clinical experts in SMA | n = 5. No additional information on patient demographics |
| Lo et al. [57] | Survey | UK |
DCE Caregiver HRQoL and disutilities: Preference-based measure: EQ-5D-5L (caregiver HRQoL and disutilities) |
DCE choice data analysed by a conditional logit model EQ-5D-5L utility scores were calculated using EQ-5D-5L crosswalk index values [64] |
Patients with Type 2 SMA and non-ambulatory Type 3 SMA and their caregivers |
Adult patients with SMA and caregivers as proxy for paediatric patients with SMA Caregiver HRQoL: self-reporting by caregivers |
Patients (n = 84), caregivers (n = 83) Sex: Adult patient survey: female (n = 61), male (n = 23) Sex of patient of caregiver survey respondents: female (n = 8), male (n = 75) Mean ± SD age: Adult patient survey respondents: 33.9 ± 11.5 years Age of patient of caregiver survey respondents: 8.4 ± 3.4 years |
| Lo et al. [56] | Survey | UK | DCE | UK societal preferences (trade-off) | Patients with SMA | UK general public aged ≥ 18 years |
n = 506 members of UK general public Female (n = 258), male (n = 248) Mean ± SD age 49 ± 17 years (range 18–82) |
| López-Bastida et al. [47] | Cross-sectional, retrospective study | Spain |
EQ-5D-3L (parents/caregivers as proxies) EQ-5D-5L (caregivers self-report) |
NR | Patients with SMA and their caregivers | Caregivers completed EQ-5D-3L on behalf of patients and self-completed EQ-5D-5L |
n = 81 patient–caregiver pairs Children with SMA Type 1 (n = 8), Type 2 (n = 60), Type 3 (n = 13) Sex of patients: female (n = 47), male (n = 34) Mean ± SD patient age 7.22 ± 5.47 years |
| Love et al. [58] | Cross-sectional survey | Canada | HUI3b | NR | Patients with Types 1, 2, and 3 SMA aged 0–18 years | Patients with SMA (aged 13–18 years) and caregivers of patients with SMA (aged 0–18 years) | n = 14 patients, n = 46 parents. No additional information on patient demographics |
| Malone et al. [48] | NA | Global | PedsQL data from CHERISH mapped to EQ-5D-Y using a published algorithm [36] | NA | Patients with Type 1 SMA | CHERISH trial participants were aged 2–12 years | NR |
| McMillan et al. [54] | Survey | Canada | EQ-5D-5L | NR | Patients with SMA and their caregivers | Surveys were self-completed by adult patients with SMA or proxy completed by caregivers (children with SMA or adults with SMA needing assistance) |
n = 965 patient and n = 962 caregiver responses met eligibility criteria Patient survey respondents with SMA: Type 1 (n = 241), Type 2 (n = 399), Type 3 (n = 283) Patients of caregiver survey respondents: Type 1 (n = 285), Type 2 (n = 423), Type 3 (n = 241), unknown (n = 9) Median age: Patient survey respondents: 8.50 (IQR 2.75–23.50) years Age of patient of caregiver survey respondents: 6.25 (IQR 2.00–11.42) years Sex: Patient survey respondents: female (n = 387), male (n = 576); non-respondents (n ≤ 5) Patients of caregiver survey respondents: female (n = 551), male (n = 406); non-respondents (n = 5) |
| Peña-Longobardo et al. [55] | Cross-sectional study | France, Germany, UK |
EQ-5D-3L (patients) EQ-5D-5L (caregivers) |
UK tariff | Patients with Types 1, 2, and 3 SMA and their caregivers | Children/adolescents with SMA and their caregivers |
n = 86 patients with SMA–caregiver pairs Patients with SMA: Type 1 (n = 23), Type 2 (n = 45), Type 3 (n = 18) Sex of patients: female (n = 51), male (n = 35) Mean ± SD patient age: France: 6.19 ± 6.13 years; Germany: 9.52 ± 6.19 years; UK: 5.55 ± 4.79 years |
| Rowell et al. [59] | Survey | UK | EQ-5D-3L (crosswalk) | UK tariff | HRQoL of caregivers of patients with SMA; HRQoL of patients was not measured | HRQoL: caregivers of patients with SMA |
n = 80 caregivers No additional information on patient demographics relevant to caregiver respondents |
| Sampson and Garau [49] | Review reporting data from a cross-sectional study [68] | France, Germany, Spain, UK | EQ-5D-3L | NR | Caregivers of patients with SMA | Caregivers of patients with SMA | NR |
| Thompson et al. [50] | Cross-sectional study | France, Germany, Spain, UK |
Three options: 1. Parent proxy using EQ-5D-3L 2. Case vignette study of physician-rated EQ-5D-5L and PedsQL (motor function health) 3. CHERISH mapped to EQ-5D using a published algorithm (unspecified) |
NR | Infants and young children with SMA |
Three options: 1. Parents of patients with SMA 2. Physicians 3. Parent-proxy assessments of CHERISH trial participants |
NR |
DCE discrete choice experiment, HRQoL health-related quality of life, HUI health utilities index, IQR interquartile range, NA not applicable, NMM neuromuscular module, NR not reported, PedsQL Pediatric Quality of Life Inventory, SD standard deviation, SMA spinal muscular atrophy
aDetails of how country-specific tariffs were derived are presented in Table 1 (question D).
bHUI2 values also reported in publication; HUI3 utility values only were extracted in this systematic literature review
Countries/regions from which utility data were derived included (Table 3) Australia (n = 1) [53], Canada (n = 2) [54, 58], Germany (n = 1) [52], Spain (n = 1) [47], the UK (n = 4) [46, 56, 57, 59], and Europe, including studies covering France, Germany, Spain, and the UK (n = 2) [49, 50], and France, Germany, and the UK (n = 1) [55]. Two publications derived global utility data using a database (n = 1) [51] and a global clinical trial (CHERISH; NCT02292537) (n = 1) [48].
In the majority of studies included in the SLR, the SMA patient populations did not receive treatment with a DMT and were managed with SOC. Some studies used clinical trial populations of patients with SMA treated with nusinersen or onasemnogene abeparvovec. Clinical trial populations included patients treated with nusinersen (CHERISH [patients with SMA aged 2–12 years with onset of clinical symptoms after 6 months of age][60] and ENDEAR [NCT02193074; infants aged ≤ 210 days with SMA and two SMN2 copies] [61]) and patients with SMA treated with onasemnogene abeparvovec (AVXS-101-CL-101 [NCT02122952; patients aged ≤ 6 months with bi-allelic SMN1 mutations and two SMN2 copies] [17] and START [NCT03421977; follow-up study of AVXS-101-CL-101]) [62]. No intervention-specific utilities were reported. Health states that were used to estimate HSUVs were typically aligned with SMA disease severity or disease status. Lloyd et al. [46] developed case studies to match health states of patients with infantile-onset and later-onset SMA in Sweden who were treated with nusinersen or SOC [63].
The studies included the following patient populations (Table 3): patients with Types 1, 2, and 3 SMA (n = 9) [47, 49–51, 53–55, 58, 59]; Types 1 and 2 SMA (n = 1) [46]; Type 1 SMA (n = 1) [48]; Types 2 and 3 (non-ambulant) SMA (n = 1) [57]; and Types 2, 3, and 4 SMA (n = 1) [52]. One study did not report SMA type because the study consisted of a DCE in which a sample of the UK population was surveyed about SMA health outcomes and health burden [56].
Mapping Algorithms
Malone et al. [48] used the mapping algorithm by Khan et al. [36] to map PedsQL data to the EQ-5D-Y scale. The algorithm was derived from PedsQL data obtained from healthy school children aged 11–15 years [36]. Lo et al. [57] used a mapping algorithm by van Hout et al. [64] to generate HSUVs from EQ-5D-5L crosswalk index values—transformation of an EQ-5D-3L value set to EQ-5D-3L values [64].
Published HSUVs for Patients with SMA
Proxy-Derived Utilities for Patients with SMA
Proxy-derived patient HSUVs were determined based on motor function state (e.g. sitting, standing, and walking, with or without support, and loss of ambulation) (Table 4). TA588 is a report by the NICE appraisal committee that considered evidence of the clinical benefits of nusinersen for the treatment of SMA submitted by Biogen Idec. Table 4 reports HSUVs preferred by the NICE Evidence Review Group (ERG) and HSUVs presented from three NICE appraisal committee meetings (ACM1–3). Further details of TA588 and ACM1–3 are described in Sect. 3.6 in this SLR.
Table 4.
Summary of published proxy-derived mean HSUVs for patients with SMA by type (standard deviation) [standard error] [95% confidence interval]
| Health state | Proxy-derived HSUVs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | NICE TA588 ERG-preferred valuesa [65] | NICE TA588 ACM1 [65] | NICE TA588 ACM2 [65] | NICE TA588 ACM3 [65] | Lloyd et al. [46] | Lo et al. [56] | López-Bastida et al. [47] | Love et al. [58] | Malone et al. [48] | Sampson and Garau [49] | Thompson et al. [50] |
| Publication description | NICE report | NICE report | NICE report | NICE report | Full-text publication | Abstract and associated poster | Full-text publication | Abstract | Full-text publication | Full-text publication | Abstract and associated poster |
| PBM used | EQ-5D-Y vignette [46, 70] | PedsQL mapped to EQ-5D | EQ-5D-Y vignette [46, 70] | Clinical experts | Clinical experts assessed Types 1 and 2 SMA case studies using EQ-5D-Y and PedsQL-NMM (baseline states only) | DCE survey of UK general population | EQ-5D-3L (caregivers as proxies) | HUI3b | PedsQL data from CHERISH mapped to EQ-5D-Y using a published algorithm [36] | EQ-5D-3L |
Three options: 1. Parent proxy using EQ-5D-3L 2. Case vignette study of physician-rated EQ-5D-5L and PedsQL (motor function health) 3. CHERISH mapped to EQ-5D using a published algorithm (unspecified) |
| Overall Types 1–3 SMA | – | – | – | – | – | – | 0.158 (0.44) | 0.31 (0.27) | – | 0.22c | UKd 0.167 |
| Type 1 SMA (early onset) | |||||||||||
| Baseline/overall Type 1 SMA | – | – | – | – | − 0.12 (0.19) | – | – | 0.14 (0.19) | – | – | – |
| Worsening | – | – | – | – | − 0.24 (0.14) | – | – | – | – | – | – |
| Improvement | – | – | – | – | − 0.17 (0.17) | – | – | – | – | – | – |
| None | − 0.240 | 0.733 | − 0.240 | − 0.020 | – | – | – | – | – | – | – |
| Mild | − 0.120 | 0.752 | − 0.120 | 0.100 | – | – | – | – | – | – | – |
| Moderate | − 0.170 | 0.752 | − 0.170 | 0.200 | – | – | – | – | – | – | – |
| Permanent ventilation | – | – | – | – | – | – | – | – | 0.730 | – | – |
| Non-sitting | – | – | – | – | – | – | – | – | 0.756 | – | – |
| Sitting with support | – | – | − 0.040 | 0.400 | – | – | – | – | 0.764e.f | – | – |
| Sitting without support | − 0.040 | 0.780 | – | – | − 0.04 (0.09) | – | – | – | 0.764e.f | – | – |
| Standing with support | 0.040 | 0.807 | 0.040 | 0.650 | – | – | – | – | – | – | – |
| Standing without support | 0.710 | 0.878 | 0.710 | 0.850 | 0.71 (0.14) | – | – | – | – | – | – |
| Walking with support | 0.520 | 0.807 | 0.520 | 0.750 | 0.52 (0.22) | – | – | – | 0.878e,g | – | – |
| Walking without support | 0.710 | 0.878 | 0.710 | 0.850 | 0.71 (0.14) | – | – | – | 0.878e,g | – | – |
| Types 2/3 SMA (late onset) | |||||||||||
| Overall Type 2 SMA | – | – | – | – | – | – | − 0.012 (0.347) | 0.24 (0.12) | – | – | – |
| Overall Type 3 SMA | – | – | – | – | – | – | 0.62 (0.27) | – | – | – | |
| Baseline Type 2 SMA | – | – | – | – | 0.04 (0.10) | – | – | – | – | – | |
| Worsening | – | – | – | – | − 0.13 (0.06) | – | – | – | – | – | 0.730 [0.0132] |
| Stabilisation of baseline function | – | – | – | – | – | – | – | – | – | – | 0.756 [0.0188] |
| Mild improvement | – | – | – | – | 0.04 (0.11) | – | – | – | – | – | 0.716 [0.0174] |
| Moderate improvement | – | – | – | – | 0.10 (0.09) | – | – | – | – | – | 0.764 [0.0142] |
| Permanent ventilation, Type 2 SMA | – | – | – | – | – | – | – | – | – | – | – |
| Cannot sit | – | – | – | – | – | − 0.408 [CI − 0.440 to − 0.337] (cannot sit, disutility) | – | – | – | – | – |
| Sitting with support | – | – | – | – | – | − 0.068 [CI − 0.083 to − 0.053] (sit with assistance, disutility) | – | – | – | – | – |
| Sitting without support | 0.040 | 0.733 | 0.040 | 0.400 | – | − 0.222 [CI − 0.242 to − 0.201] (sit but cannot stand, disutility) | – | – | – | – | – |
| Sits and rolls | 0.040 | 0.752 | 0.040 | 0.450 | – | – | – | – | – | – | – |
| Sits and crawls | 0.100 | 0.780 | 0.100 | 0.500 | – | – | – | – | – | – | – |
| Standing with support | 0.390 | 0.807 | 0.390 | 0.700 | 0.39 (0.29) | –0.068 [CI − 0.083 to − 0.053] (stand with assistance, disutility) | – | – | – | – | 0.807 [0.0182] |
| Standing without support | 0.720 | 0.807 | 0.720 | 0.850 | 0.72 (0.12) | – | – | – | – | – | 0.805 [0.0256] |
| Walking with support | 0.390 | 0.807 | 0.390 | 0.700 | 0.39 (0.29) | − 0.068 [CI − 0.083 to − 0.053] (walk with assistance, disutility) | – | – | – | – | 0.807 [0.0182] |
| Walking without support | 0.720 | 0.878 | 0.720 | 0.850 | 0.72 (0.12) | – | – | – | – | – | 0.878 [0.0297] |
| Loss of ambulation/motor function (with/without assistance) | – | – | – | – | − 0.12 (0.16) | – | – | – | – | – | 0.774 [0.0303] |
| Disutilities (non-motor function specific) | |||||||||||
| Respiratory support (any) | – | – | – | – | − 0.33 (0.27) | – | – | – | – | – | – |
| Respiratory support (< 16 h/day) | – | – | – | – | – | − 0.159 [CI − 0.174 to − 0.143] | – | – | – | – | – |
| Respiratory support (> 16 h/day) | – | – | – | – | – | − 0.304 [CI − 0.328 to − 0.281] | – | – | – | – | – |
| Oral vs. intrathecal drug administration | – | – | – | – | – | − 0.071 [CI − 0.085 to − 0.057] | – | – | – | – | – |
| Treatment reactions, 12 h/4 mo | – | – | – | – | – | − 0.057 [CI − 0.071 to − 0.042] | – | – | – | – | – |
| Treatment reactions, 1–2 days/4 mo | – | – | – | – | – | − 0.060 [CI − 0.078 to − 0.042] | – | – | – | – | – |
| Treatment reactions, 3–4 days/4 mo | – | – | – | – | – | − 0.087 [CI − 0.103 to − 0.071] | – | – | – | – | – |
| Ophthalmological monitoring before/during treatment if symptoms present | – | – | – | – | – | − 0.024 [CI − 0.036 to − 0.012] | – | – | – | – | – |
| Ophthalmological monitoring before/during treatment 2 ×/year for 2 years | – | – | – | – | – | − 0.023 [CI − 0.037 to − 0.009] | – | – | – | – | – |
| SMA after scoliosis surgery | – | – | – | – | − 0.22 (0.22) | – | – | – | – | – | – |
| Gastric/NG tube | – | – | – | – | − 0.17 (0.17) | – | – | – | – | – | – |
| Contraception | – | – | – | – | – | − 0.012 [CI − 0.021 to − 0.002] | – | – | – | – | – |
ACM appraisal committee meeting, CI confidence interval, DCE discrete choice experiment, ERG evidence review group, HSUV health state utility value, HUI2/3 Health Utilities Index Mark 2/3, mo months, NG nasogastric, NICE National Institute for Health and Care Excellence, NMM neuromuscular module, PBM preference-based measure, PedsQL Pediatric Quality of Life Inventory, SD standard deviation, SMA spinal muscular atrophy, TA technology assessment
– Indicates not assessed in publication
aERG-preferred (dis)utility values are from Lloyd et al. [46, 70]
bHUI3 utility values only were extracted into current table; HUI2 values also reported in publication (not extracted)
cData referenced from López-Bastida et al. [68]; however, utility data for SMA are not reported in López-Bastida et al.; therefore, the review by Sampson and Garau is included as a primary source
dValue for UK; mean (SD) values for France, Germany and Spain also reported in publication
eOnly a single health state reported; no differentiation for with and without support
fHealth state functionally equivalent to Type 2 SMA
gHealth state functionally equivalent to Type 3 SMA
The HSUVs reported by Lloyd et al. [46] were estimated by clinical experts who reviewed case studies of health states used in economic models of nusinersen treatment for Types 1 and 2 SMA [63]. In some cases, treatment with nusinersen improved health states such that the initial classification of SMA type would no longer apply. For example, patients with type 1 SMA who achieved the ability to sit without support or walk/stand without support could be reclassified according to maximum motor milestone function as patients with Type 2 or 3 SMA, respectively, but are assigned based on original diagnosis [46].
Four studies [47, 49, 50, 58] reported a proxy-derived overall HSUV for Types 1, 2, and 3 SMA, which ranged from 0.158 [47] to 0.31 [58] (Table 4). The baseline/overall Type 1 SMA HSUV was estimated at − 0.12 [46] to 0.14 [58]. Within health states of Type 1 SMA, HSUVs ranged from − 0.240 (requires permanent ventilation) [65] to 0.71 (stands/walks without support; treated patient who could be reclassified as a patient with Type 3 SMA) [46], with substantial differences between some states (Table 4). Type 1 SMA health states describing no improvement or worsening from baseline had an HSUV below zero, reflecting a health state considered to be worse than death [46]. Similar to Type 1 SMA, the overall proxy-derived patient HSUV for Type 2 SMA was worse or better than death (− 0.012 vs. 0.24), depending on which study was examined [47, 58]. Type 2 SMA HSUVs ranged from − 0.13 (worsened) to 0.72 (stands/walks without support) [46]. The ‘baseline’ and ‘mild improvement’ states were valued equally (0.04), with a small increase in utility reported for the ‘moderate improvement’ health state (0.10). A greater increase in HSUVs for the standing/walking states was observed (0.39 [with support] and 0.72 [without support]). The ‘loss of ambulation’ state estimate was − 0.12 for Type 2 SMA (Table 4). Within motor function states of Type 1 SMA, one study [48] reported HSUVs that ranged from 0.730 (requires permanent ventilation) to 0.878 (stands/walks without support), with no substantial differences reported in relation to other estimates of motor function states such as non-sitting (0.756) and sitting without support (0.764) (Table 4).
Proxy-Derived Disutilities for Patients with SMA
Disutilities associated with specific interventions or treatment considerations such as respiratory support; oral versus intrathecal drug administration route; treatment reactions; ophthalmological monitoring; SMA health state after scoliosis surgery; use of gastric or nasogastric tube; and required use of contraception (Table 4) were assessed by a DCE survey of the UK population [56]. Two studies reported proxy-derived patient disutilities associated with SMA [46, 56]. Any type of respiratory support was associated with a disutility of − 0.33 [46]. This estimate was similar to respiratory support for > 16 h per day (− 3.04) as determined by a DCE [56]. SMA health state after scoliosis surgery and use of gastric/nasogastric tube had disutility estimates of − 0.22 and − 0.17, respectively [46]. The DCE also revealed that patients preferred daily oral administration of treatment to intrathecal injection of treatment every 4 months (− 0.071), and disutility estimates increased with longer treatment reactions over 4 months (− 0.057 vs. − 0.087 for 12 h and 3–4 days, respectively) [56]. Disutilities for ophthalmological monitoring were similar if monitoring occurred before and during treatment when symptoms were present (− 0.024) and before and during treatment twice a year for 2 years (− 0.023) [56]. Required use of contraception was associated with a disutility estimate of − 0.012 [56].
Patient-Derived Utilities for Patients with SMA
Patient-derived HSUVs were reported in three of the studies identified in the SLR [52, 55, 58] (Table 5). In contrast to proxy-derived estimates, the overall HSUV for Types 1, 2, and 3 SMA were considered better than death in the three studies in which these estimates were determined [52, 55, 58], with HSUVs ranging from 0.167 [55] to 0.46 [52]. A patient-reported baseline/overall HSUV for Type 1 SMA was considered better than death, with an estimate of 0.29 [58] (Table 5). Love et al. [58] estimated the patient-reported baseline/overall HSUV for Types 2 and 3 SMA health states as 0.23 and 0.41, respectively (Table 5), which aligns with the corresponding increase in disease severity between Types 2 and 3 SMA [11].
Table 5.
Summary of published patient-derived and mixed patient–proxy-derived mean HSUVs for patients by SMA type (standard deviation)
| Health state | Patient-derived HSUVs | Mixed patient–proxy-derived HSUVs | ||||
|---|---|---|---|---|---|---|
| Reference | Binz et al. [52] | Love et al.a [58] | Peña-Longobardo et al. [55] | Belter et al. [51] | Chambers et al. [53] | McMillan et al. [54] |
| Publication type | Full-text publication | Abstract | Full-text publication | Full-text publication | Full-text publication | Full-text publication |
| PBM used | EQ-5D-5L | HUI3 | EQ-5D-3L and EQ-5D-5L | HUI3 | EQ-5D-Y | EQ-5D-5L |
| Overall Types 1–3 SMA | 0.46 (0.37)b | 0.33 (0.28) | 0.167 (0.277)c | – | 0.115 (0.227) | 0.49 (0.26) |
| Baseline/overall Type 1 SMA | – | 0.29 (0.36) | – | – | 0.104 (0.278) | 0.32 |
| Worsening | – | – | – | – | – | – |
| Improvement | – | – | – | – | – | – |
| None | – | – | – | – | – | – |
| Mild | – | – | – | – | – | – |
| Moderate | – | – | – | – | – | – |
| Permanent ventilation | – | – | – | − 0.05 (0.10) | – | – |
| Non-sitting | – | – | – | 0.06 (0.10) | – | – |
| Sitting (with support) | – | – | – | 0.11 (0.21)d | – | – |
| Sitting without support | – | – | – | – | – | – |
| Standing (with support) | – | – | – | – | – | – |
| Standing without support | – | – | – | – | – | – |
| Walking (with support) | – | – | – | – | – | – |
| Walking without support | – | – | – | – | – | – |
| Overall Type 2 SMA | – | 0.23 (0.16) | – | – | 0.067 (0.158) | 0.46 |
| Overall Type 3 SMA | – | 0.41 (0.02) | – | – | 0.252 (0.332) | 0.65 |
| Baseline | – | – | – | – | – | – |
| Worsening | – | – | – | – | – | – |
| Stabilisation of baseline function | – | – | – | – | – | – |
| Mild improvement | – | – | – | – | – | – |
| Moderate improvement | – | – | – | – | – | – |
| Permanent ventilation, Type 2 SMA | – | – | – | 0.10 (0.11) | – | – |
| Non-sitting | – | – | – |
Type 2 SMA: 0.12 (0.12) Type 3 SMA: 0.14 (0.13) |
– | – |
| Sitting (with support) | – | – | – |
Type 2 SMA: 0.26 (0.16)d Type 3 SMA: 0.23 (0.11)d |
– | – |
| Sitting without support | – | – | – | – | – | |
| Standing (with support) | – | – | – | – | – | – |
| Standing without support | – | – | – | – | – | – |
| Walking (with support) | – | – | – |
Type 2 SMA: 0.44 (0.12) Type 3 SMA: 0.35 (0.21) |
– | – |
| Walking without support | – | – | – |
Type 2 SMA: 0.58 (0.15) Type 3 SMA: 0.64 (0.24) |
– | – |
| Loss of ambulation/motor function (with/without assistance) | – | – | – | – | – | – |
| Disutilities | NR | NR | NR | NR | NR | NR |
| Contraception | – | – | – | – | – | – |
HSUV health state utility value, HUI2/3 Health Utilities Index Mark 2/3, NR not reported, PBM preference-based measure, SMA spinal muscular atrophy
– Indicates not assessed in publication
aHUI3 utility values only were extracted into current table; HUI2 values also reported in publication (not extracted)
bStudy sample included patients with the following SMA types: Type 2 (n = 6); Type 3 (n = 11); and Type 4 (n = 1)
cValue for UK; mean (standard deviation) values for France and Germany also reported
dOnly a single health state reported; no differentiation for with and without support
Mixed Patient-/Proxy-Derived Utilities for Patients with SMA
Three studies identified in the SLR assessed patient HSUVs using mixed patient-/proxy-derived SMA health states [51, 53, 54] (Table 5). Similar to the patient baseline/overall HSUV for Types 1, 2, and 3 SMA that was determined using proxy and patient assessments of health states, the mixed patient-/proxy-derived HSUV was considered better than death as reported by two studies, although estimates ranged from 0.115 [53] to 0.49 [54] (Table 5). When mixed patient-/proxy-derived health states were used to generate the overall HSUV for Type 1 SMA, the estimate ranged from 0.104 [53] to 0.32 [54]. One study estimated mixed patient-/proxy-derived motor function health states for Type 1 SMA in which permanent ventilation was considered worse than death (− 0.05) [51]. The same study found an increase in the HSUV for sitting without support (0.11) as an SMA health state compared with sitting with support (0.11) [51]. Two studies [53, 54] estimated the overall HSUVs for Types 2 and 3 SMA using mixed patient-/proxy-derived health states (Table 5). Lower HSUV estimates were reported for Types 2 and 3 SMA (0.067 and 0.252, respectively) by Chambers et al. [53] compared with the corresponding estimates (0.46 and 0.65, respectively) from the study performed by McMillan et al. [54]. Another study reported that the mixed patient-/proxy-derived HSUV in patients with Type 2 SMA was considered better than death (0.10) [51]. Non-sitting was considered a minimally worse health state for patients with Type 2 compared with Type 3 SMA (0.12 and 0.14, respectively) [51]. According to one study, sitting with support for patients with Type 2 SMA was considered a slightly better health state (0.26) than was sitting without support for patients with Type 3 SMA (0.23) [51]. For patients with walking as a health state, the same study estimated that walking with support had a higher HSUV for patients with Type 2 SMA (0.44) than for patients with Type 3 SMA (0.35) [51]. In contrast, it was estimated that walking without support had a higher HSUV for patients with Type 3 SMA (0.64) than for patients with Type 2 SMA (0.58) [51].
Utilities for Caregivers of Patients with SMA
Five studies [47, 53, 55, 57, 59] presented HSUVs for caregivers of patients with SMA (Table 6). The overall HSUV for caregivers of patients with Types 1, 2, and 3 SMA derived using the EQ-5D varied across two studies from 0.484 [47] to 0.852 [55], whereas an overall caregiver HSUV of 0.708 was reported when values were derived from responses to the CarerQoL questionnaire [53].
Table 6.
Summary of published mean HSUVs for caregivers of patients with SMA (standard deviation)
| Health state | Chambers et al. [53] | Lo et al. [57] | López-Bastida et al. [47] | Peña-Longobardo et al. [55] | Rowell et al. [59] |
|---|---|---|---|---|---|
| Reference | |||||
| Publication type | Full-text publication | Abstract and associated poster | Full-text publication | Full-text publication | Abstract |
| PBM used |
CarerQoLa HRQoL score: 0=worse caregiving 1=best caregiving |
EQ-5D-5L | EQ-5D-5L | EQ-5D-5L | EQ-5D-3L (crosswalk) |
| Overall Types 1–3 SMA | 0.708 (0.148) | – | 0.484 (0.448) | 0.852 (0.155)b | – |
| Overall Type 1 SMA | 0.714 (0.091) | – | – | – | – |
| Overall Type 2 SMA | 0.703 (0.134) | – | 0.472 (0.475) | – | – |
| Overall Types 2/3 SMA | – | 0.940 (0.091) | – | – | – |
| Motor function: sits with support | – | 0.862 (0.127) | – | – | – |
| Motor function: sits independently for longer | – | 0.939 (0.092) | – | – | – |
| Motor function: stands with assistance | – | 0.964 (0.074) | – | – | – |
| Respiratory function: mechanical support <16 h | – | 0.915 (0.099) | – | – | – |
| Respiratory function: no mechanical support | – | 0.968 (0.075) | – | – | – |
| Overall Type 3 SMA | 0.715 (0.202) | – | – | – | – |
| Not specified | – | – | – | – | 0.739 (0.205) |
CarerQoL carer-related quality of life, HRQoL health-related quality of life, QALY quality-adjusted life-year, PBM preference-based measure, SMA spinal muscular atrophy
– Indicates not assessed in publication
aNote that CarerQoL utilities cannot be used to calculate QALYs, because the scale is anchored 0–100 (worst informal care situation to best informal care situation) rather than 0–1 (death to full health).
bValue for UK; mean (standard deviation) values for France and Germany are also reported
Previous HTA Submissions for the Treatment of SMA
Three HTA submissions that considered the cost effectiveness of DMTs (nusinersen or onasemnogene abeparvovec) for the treatment of SMA were identified. The recipient HTA bodies and the year of publication of review were as follows: CADTH, 2019 [66], NICE, 2019 [65], and the US ICER Group, 2019 [67].
The submission to CADTH aimed to present the cost effectiveness of nusinersen compared with SOC in Canada for patients with Types 1, 2, and 3 SMA [66]. Utility values for the model were derived from studies that the CADTH review group did not consider to be appropriate for the estimation of utilities. For Types 1 and 3 SMA, utilities were derived from a vignette study, in which five experts in SMA rated health state descriptions relating to the health states within the models (reference not provided in the CADTH report but is likely to be Lloyd et al. [46]). For Type 2 SMA, utility values were obtained from a mapping study that used both HRQoL values reported in the CHERISH trial and EQ-5D values (reference not provided in the CADTH report).
The original HTA submission to NICE by Biogen Idec aimed to present the cost effectiveness of nusinersen compared with SOC for the treatment of patients with SMA [65]. For patients with later-onset disease, utility values were derived from PedsQL data collected in CHERISH (ACM1 data in Table 4), which were then mapped to the EQ-5D using a published mapping algorithm [36]. The impact of SMA on caregivers was also captured by applying caregiver disutilities to each health state, based on the cross-sectional study of patients with SMA from López-Bastida et al. [68]; caregiver utility data were redacted in the NICE HTA submission [69]. The review of the HTA submission undertaken by the NICE ERG highlighted that the utility values employed by the manufacturer had poor face validity. The utility values used in the base-case analysis reflected the experience of patients with later-onset SMA and appeared to be higher than expected for patients with severe conditions such as SMA. Additionally, the difference between the more severe health states (‘no milestones achieved’) and the best health states (‘stands’/‘walks unaided’) were small. However, independent searches undertaken by the ERG did not identify any further published studies reporting EQ-5D utilities in patients with SMA. Of the available datasets, the NICE ERG expressed a preference for the (dis-)utilities reported in the vignette studies by Lloyd et al. [46, 70], which are listed as NICE ERG TA588-preferred values in Table 4. Analyses using the ERG’s preferred values from vignette studies (ACM2) and non-preference-based estimates generated by the manufacturers’ clinical advisers (ACM3) are shown in Table 4.
The submission to the US ICER Group aimed to present the cost effectiveness of nusinersen and onasemnogene abeparvovec, each compared with SOC, for the treatment of patients with SMA from a US healthcare perspective [67]. Robust utility data for the population of interest were lacking (with many identified studies lacking face validity), so HSUVs for various health states were derived from several sources that were judged to be relevant: 0.19 for the ‘permanent ventilation’ health state from Thompson et al. [50], 0.6 for the ‘sitting’ health state from Tappenden et al. [71], and general population HSUVs ranging from 0.922 to 0.736 (for age ranges 18–29 and ≥ 80 years, respectively) from Sullivan and Ghushchyan [72], which were used for the ‘walking’ health state.
Comparison of Utilities Identified in the SLR with HTA Body Reference Cases
The relevance of the identified studies in the SLR to the recommendations from CADTH [44], NICE [34], and the US ICER Group [45], referred to as HTA body reference cases in this SLR, for conducting health technology appraisals was determined (Table 7). Overall, four studies [46, 55, 57, 59] were considered as meeting the requirements of the NICE and CADTH reference cases; UK societal preferences were considered acceptable for CADTH for the study by Peña-Longobardo et al. [55]. The four studies failed to meet the requirements of the US ICER Group reference case because they used a UK (non-US) tariff. Two studies [57, 59] reported utilities that reflected only the health states of adult caregivers of patients with SMA. Two studies [46, 55] reported patient utilities; in one study [55], utilities were derived directly from patients. It should be noted that an age-appropriate instrument was not used to measure HRQoL (EQ-5D-3L and -5L versions). In the second study [46], the EQ-5D-Y was used to derive utilities from a panel of clinical experts, so additional justification may therefore be required to support the use of these utilities in economic analyses. Two studies did not meet the requirements of the three HTA body reference cases. In one publication [56], a DCE survey rather than a preference-based instrument was used to derive utilities. In one publication [48], a mapping algorithm was used to convert PedsQL data to utilities; this study is unlikely to be considered to meet the HTA body reference cases given the availability of preference-based utilities in SMA. One publication used an Australian tariff [53], and one publication used a German tariff [52]. These studies therefore did not meet the requirements of the NICE and US ICER Groups because they used non-UK and non-US tariffs, respectively. It is also unclear whether Australian and German societal preferences would be considered sufficiently similar to Canadian societal preferences to meet the CADTH reference case. Two studies [46, 57] were not relevant to the generation of patient utilities using societal preferences. The remainder of publications [47, 49–51, 54, 58] did not report the method of valuation (i.e. the tariff used), so it was not possible to determine whether the HTA body reference case requirements were met. Furthermore, four of these studies [47, 49, 50, 54] did not appear to consider the age appropriateness of the instrument(s) selected (Table 7).
Table 7.
Relevance of identified HSUVs to HTA body reference cases
| Study | Questions to assess relevance of identified HSUVs to HTA body reference cases | Is the study consistent with HTA bodya reference cases? | ||||||
|---|---|---|---|---|---|---|---|---|
| (A) Instrument selection | (B) Did patients describe the health states? | (C) Were appropriate societal preferences used to value health states? | (D) Was the TTO/SG method used to value health states? | HTA body | Consistent with reference case (Yes [with justification]/no/unclear) | If no/unclear, specify which question | ||
| (A1) Was a generic preference-based instrument used to describe health states? | (A2) Was the selected instrument age appropriate? | |||||||
| Belter et al. [51] | Yes: utilities were derived using the HUI3 | Yes: parents completed HUI3 on behalf of paediatric patients and adult patients self-completed the questionnaire | Partially: a proportion of questionnaires were completed by patients (adults), but the majority were completed by a parent/caregiver | Unclear | Unclear | CADTH | Unclear | C |
| US ICER Group | Unclear | C | ||||||
| NICE | Unclear | C/D | ||||||
| Binz et al. [52] | Yes: utilities were derived using the EQ-5D-5L | Yes: study considered adult patients (aged ≥18 years) only | Yes: utilities were derived directly from patients | Yes: German tariff | No: EQ-VTv2.0 was assumed from a published reference [92] | CADTH | Unclear | C |
| US ICER Group | No | C | ||||||
| NICE | No | C/D | ||||||
| Chambers et al. [53] | Yes: utilities were derived using the EQ-5D-Y | Yes: youth version appropriate for proxy respondents for a paediatric population | Partially: questionnaires were completed by patients or by caregivers on behalf of their child | Yes: Australian tariff | Yes: TTO | CADTH | Unclear | C |
| US ICER Group | No | C | ||||||
| NICE | No | C | ||||||
| Lloyd et al. [46] | Yes: utilities were derived using the EQ-5D-Y | Yes: youth version appropriate for proxy respondents for a paediatric population | No: utilities were derived from clinical experts on behalf of patients | Yes: UK tariff | NR | CADTH | Yes—with justification | – |
| US ICER Group | No | C | ||||||
| NICE | Yes—with justification | – | ||||||
| Lo et al. [56] | No: a DCE was used to estimate disutilities | NA | No: the sample included members of the UK general population | NA | NA | CADTH | No | A1/C |
| US ICER Group | No | A1/C | ||||||
| NICE | No | A1/C/D | ||||||
| Lo et al. [57] | Yes: utilities were derived using the EQ-5D-5L | Yes: appropriate for adult caregivers | No: utilities were derived for caregivers of patients with Type 2 SMA | Yes: UK tariff | Yes: values mapped to EQ-5D-3L TTO value | CADTH | Yes | – |
| US ICER Group | No | C | ||||||
| NICE | Yes | – | ||||||
| López-Bastida et al. [47] | Yes: utilities were derived using the EQ-5D (3L and 5L) | No: youth version more appropriate for paediatric population | No: utilities were derived from caregivers on behalf of patients | Unclear | Yes: TTO | CADTH | Unclear | A2/C |
| US ICER Group | Unclear | A2/C | ||||||
| NICE | Unclear | A2/C | ||||||
| Love et al. [58] | Yes: utilities were derived using the HUI3 | Yes: HUI3 completed by patients aged 13–18 years and by parents of younger children | Yes: utilities were derived from both patients and their parents | Unclear | Unclear | CADTH | Unclear | C |
| US ICER Group | Unclear | C | ||||||
| NICE | Unclear | C | ||||||
| Malone et al. [48] | No: PedsQL data were mapped to the EQ-5D-Y using a published algorithm [36] | Yes: PedsQL appropriate for paediatric population | Yes: pts completed the PedsQL, which was mapped to the EQ-5D | NA | NA | CADTH | No | A1/C |
| US ICER Group | No | A1/C | ||||||
| NICE | No | A1/C/D | ||||||
| McMillan et al. [54] | Yes: utilities were derived using the EQ-5D-5L | Partially: appropriate for self-completing adults; youth version available for children | Partially: surveys were self-completed by adult patients or proxy-completed by caregivers (children or adults needing assistance) | Unclear | Unclear | CADTH | Unclear | A2/C |
| US ICER Group | Unclear | A2/C | ||||||
| NICE | Unclear | A2/C/D | ||||||
| Peña-Longobardo et al. [55] | Yes: utilities were derived using the EQ-5D (3L and 5L) | No: youth version more appropriate for paediatric/adolescent population | Yes: utilities were derived directly from patients and caregivers | Yes: UK tariff was assumed from a published reference [93] | Yes: TTO | CADTH | Yes | – |
| US ICER Group | No | C | ||||||
| NICE | Yes | – | ||||||
| Rowell et al. [59] | Yes: utilities were derived using the EQ-5D-3L | Yes: appropriate for adult caregivers | No: utilities were derived for caregivers of patients with Type 2 SMA | Yes: UK tariff (assumed; refers to crosswalk value set) | Yes: TTO (assumed; refers to crosswalk value set) | CADTH | Yes | – |
| US ICER Group | No | C | ||||||
| NICE | Yes | – | ||||||
| Sampson and Garau. [49] | Yes: utilities were derived using the EQ-5D-3L | No: youth version more appropriate for paediatric population | No: utilities were derived from caregivers on behalf of patients | Unclear | Unclear | CADTH | Unclear | A2/C |
| US ICER Group | Unclear | A2/C | ||||||
| NICE | Unclear | A2/C/D | ||||||
| Thompson et al. [50] | Yes: utilities were derived using the EQ-5D-3L | No: youth version more appropriate for paediatric population | No: utilities were derived from parents on behalf of patients | Unclear | Unclear | CADTH | Unclear | A2/C |
| US ICER Group | Unclear | A2/C | ||||||
| NICE | Unclear | A2/C/D | ||||||
CADTH Canadian Agency for Drugs and Technologies in Health, DCE discrete choice experiment, EQ-VTv2.0 EQ-Valuation Technology Vision 2.0, HSUV health state utility value, HTA health technology assessment, HUI3 Health Utilities Index Mark 3, ICER Institute for Clinical and Economic Review, NA not applicable, NICE National Institute for Health and Care Excellence, NR not reported, PedsQL Pediatric Quality of Life Inventory, SG standard gamble, SMA spinal muscular atrophy, TTO time trade-off
aHTA body reference cases were NICE [34], CADTH [44], and the US ICER Group [45]
Quality Assessment
Quality assessment of the included studies highlighted several limitations associated with the HSUVs reported. For example, sample sizes were often unclear, and there was a consistent absence of details regarding the patient recruitment process, response rates to instruments, loss to follow-up, and missing data. These factors are likely to restrict the usefulness of the studies for informing economic evaluation. Further details of quality assessment of the studies are shown in Table 5 in the ESM.
Discussion
The aim of this SLR was to identify utilities associated with patients with SMA and their caregivers in the published literature. The studies identified in this SLR consistently demonstrated that SMA has a substantial HRQoL burden on both patients and caregivers. The impact of SMA on patients and their caregivers is an active research area, as indicated by the number of eligible studies for this SLR captured in 2019 compared with the number in the 2021 literature searches. In addition, we identified 17 studies of other (non-SMA) neuromuscular disorders that report utilities that may serve as useful surrogate values for studies of SMA and other rare diseases.
The estimation of reliable utilities is important as they are a key factor affecting incremental cost-effectiveness ratios in economic evaluations of rare disorders (along with discount rates, drug costs, and health state costs). Overall, we identified 14 publications reporting utilities/disutilities associated with patients with SMA and their caregivers. Nine of the identified studies were full-text publications [46–49, 51–55], and five were conference abstracts [50, 56–59], four of which had an associated poster available [50, 56, 57, 59]. Consistent with prior findings [50], we found that methods of measuring utilities generated different and sometimes ambiguous results. The 14 identified studies employed a variety of methods for collecting utility data for SMA, including patient surveys, vignette methodologies, DCE surveys, and structured forms of expert elicitation. Each method has known limitations, such as a lack of validated utility measures, unvalidated vignettes, poor mapping functions, and methods that relied on significant assumptions [37]. Utility estimates are also influenced by the type of instrument used to determine health states, whether health states are mapped or not, and whether direct methods such as SG/TTO or indirect methods are used.
Relevance of Published Utilities to HTA Body Reference Cases
The method of valuation of health states likely contributed to variation of estimated utilities for comparable health states, in addition to the extent to which studies met the recommendations of the three HTA body reference cases. Four studies [46, 55, 56, 59] used a UK societal preference to value health states, which aligns with NICE recommendations for HTA submissions [34]. Two of the remaining eight studies in which tariffs may have been applicable to generate patient utilities used non-UK tariffs [52, 53], and in six studies the valuation methods were unclear [47, 49–51, 54, 58]. Two studies [48, 59] were not relevant to the generation of patient utilities using societal preferences. Four studies [46, 55, 57, 59] were considered to meet the requirements of the NICE and CADTH reference cases (with justification/assumptions of acceptable alternative tariffs needed for some studies, such as acceptance of a UK instead of a Canadian tariff to meet CADTH requirements). The four studies did not meet the requirements of the US ICER Group because they used a non-US tariff. Six studies did not report the method of valuation, so it was not possible to determine whether HTA body requirements were met. Although no precise recommendation on an age-appropriate PBM is provided by CADTH and the US ICER Group, NICE recommends that a validated standardised age-appropriate PBM for paediatric patient populations is used. Despite variations in published utilities for patients with SMA, information from the identified studies in this SLR may be considered appropriate for informing economic evaluations, even though they are not fully aligned with the three specified HTA body reference cases.
Utilities of Caregivers of Patients with SMA
The substantial burden that SMA places on caregivers of patients with SMA [13, 14, 73] is often not included in HTA submissions. In this SLR, we identified five studies that reported utilities for caregivers of patients with SMA [47, 53, 55, 57, 59]. Variations in reported utilities may reflect the different instruments used in the studies to assess caregiver HRQoL, including the EQ-5D [31] and the CarerQoL [74]. Additional instruments have been developed to assess caregiver HRQoL, such as the Carer Experience Scale [75] and the ASCOT-Carer [76]. It should be noted that the CarerQoL cannot be applied to cost-utility analyses that evaluate patient interventions [77]. For such analyses, the instrument used to determine caregiver HRQoL should be the same as used for the patient [77].
Limitations of Individual Studies Identified in this SLR
Findings from this review must be interpreted in light of the caveats of the individual studies. Generally, the studies included in the SLR were of moderate quality. The validity of the data was jeopardised by small or unclear sample sizes and limited reporting of details regarding the patient recruitment process, response rates to instruments, loss to follow-up, and handling of missing data. Direct comparison of utilities across studies is difficult because of the significant heterogeneity between patient populations, for example, geographical location and SMA type characteristics. In addition, limited reporting of methodology in conference abstracts can reduce the reliability of the data. General inherent limitations of the SLR process, including the location and selection of studies and the influence of publication bias on reporting, must also be taken into consideration [78]. There is currently no value set available for the EQ-5D-Y, which may have quality implications for the studies that used the adult value set to derive utilities for children with SMA. HTA bodies may consider adult tariffs acceptable for application to the EQ-5D-Y because of the lack of a validated value set, but this goes against EuroQoL recommendations [35]. A value set for EQ-5D-Y, which is currently only available for research purposes [35], should be used in future studies that estimate utilities for paediatric patients with SMA.
Considerations of Patient and Proxy Assessments to Generate Utilities for Patients with SMA
An SLR published in 2019 revealed that the QoL of patients with SMA varied broadly across global studies, based on whether patient-self and caregiver-proxy assessments were used [79]. The 14 studies identified in this SLR further reveal the disparity of utility values when assessed by self-reported responses or responses by proxy to HRQoL questionnaires. When using indirect methods to determine utilities, unintentional bias from proxy assessments can lead to inaccurate health state descriptions that minimise or inflate health state descriptions. For example, parents and caregivers usually have knowledge of one child with SMA, which may skew their interpretation of a particular health state. In contrast, clinical experts may be familiar with several paediatric patients with SMA, which could confound their assessments of individual case studies. In studies that used mixed patient-/proxy-reported descriptions of health states, it is particularly challenging to determine the source of variation in utility values between studies. As Types 1 and 2 SMA in particular are diagnosed in childhood, it may be considered appropriate to obtain data from proxy respondents. Given the difficulties associated with obtaining utilities directly from children (most HRQoL instruments are not designed for use in this age group) [80, 81], the use of proxy respondents may be considered appropriate in this indication. Indeed, NICE specifies that where it is not possible to obtain measurements of HRQoL directly from patients, data should be obtained from the person who acts as their caregiver in preference to healthcare professionals [34].
One study identified in the SLR [48] used PedsQL data obtained directly from patients with Type 1 SMA, mapping PedsQL scores to the EQ-5D-Y using a published algorithm [36]. This method was also used in the ACM1 submission to NICE by the manufacturer of nusinersen [65]. According to recommendations from NICE [34], the mapping function chosen should be based on data sets containing both HRQoL measures and health-related benefits observed in relevant clinical trials (its statistical properties should be fully described and the choice justified), and how well the function fits the data should be adequately demonstrated. Furthermore, sensitivity analyses to explore variation in the use of the mapping algorithms on the outputs should also be presented [34]. The NICE ERG reported that responses from a healthy population would be very different from those from patients with SMA. For their mapping algorithm, Khan et al. [36] had few responses at the more severe end of the EQ-5D, and this may have affected the accuracy of the derived mapping functions. Therefore, the use of mapping may overestimate the utility values for those at the severe end, primarily because of the lack of data to accurately fit a regression model.
Consideration of Health State Descriptions and Instrument Choice that Reflect Emerging Phenotypes and Meaningful Change in Patients with SMA
It is challenging to define the health states and treatment efficacy parameters required for such analyses for rare genetic diseases, which often exhibit heterogeneity in disease progression and responses to treatment [82]. Treatment efficacy measures used in clinical trials may not be a reliable measure of treatment responses in the real-world setting. For instance, an intervention that prevents SMA disease progression may be as meaningful to one patient as a clinically defined improvement is to another patient [83]. Importantly, treatment with DMTs has resulted in the emergence of phenotypes of patients with SMA that do not align with the traditional classification system of the disease; for example, patients with Type 1 SMA who can sit and patients with Type 2 SMA who can walk independently [84]. As treatment with DMTs is most effective in patients with SMA who are presymptomatic [85], SMA classification is likely to focus more heavily on genotyping [86], particularly in relation to SMN2 copy number (which is inversely correlated with disease severity). Even though the correlation between SMN2 copy number and classical SMA phenotype is not absolute [7], treatment guidelines based on SMN2 copy number have been created for infants who are likely to develop SMA at a later age [87].
Reclassification of SMA according to motor function has been suggested to better reflect gains in mobility achieved by patients following treatment with DMTs. Consideration of new health state descriptions for studies that evaluate utilities in patients with SMA is warranted as existing motor function scales used may not adequately capture changes related to functional abilities [88], which may in turn affect HRQoL. For example, a change in the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders score cannot record the ability of a patient with SMA to perform fine movements of the fingers or to turn their head to the side, even though these abilities are valued by patients and their families. Fine movements of the fingers may later enable an older patient with SMA to control a motorised wheelchair independently.
Even though PBMs often included domains that focussed on gains in mobility, they may not be sensitive enough to detect other important disease-related changes for patients with SMA. Indeed, the HTA review of nusinersen by NICE found that SMA health states used to derive utilities may not reflect the benefits of gaining specific motor skills such as independence or the ability to perform self-care [65]. Furthermore, NICE reported that, although differences in utilities between SMA states were minimal, the ability to learn how to write or undertake formal education was not captured by the assessed health states [65]. Other PBMs would be of interest, such as the HUI and 36-item Short Form Survey (SF-36), which are more granular than the EQ-5D. Some HRQoL tools appear to be more appropriate for specific groups of patients with SMA (e.g. non-ambulant patients), with many developed specifically for adults. A recent review found that there are no specific scales for Type 1 SMA [88], probably because survival for these patients was limited [11] before the recent introduction of DMTs for SMA [85].
The Need for Consideration of SMA Utility Data in Future Clinical Trial Design
A combination of multiple tools, such as those that measure activities of daily living and caregiver burden, may enable patient and caregiver perspectives to be captured more effectively. The SMA community would benefit from reaching a consensus regarding PBMs and methods to generate utility values for patients and their caregivers that could be most efficiently integrated into clinical trial assessments or follow-up of patients in registries. A task force report from The Professional Society for Health Economics and Outcomes Research provides extensive recommendations for the consideration of utility assessments in early planning of clinical trial designs [28], including (1) the incorporation of health state assessments within the trial and follow-up periods to generate utilities that are important for economic evaluations, (2) choosing the most appropriate instrument and respondent type for the study population demographics (e.g. patient age for SMA), (3) standardisation of utility data collection across clinical trials of an intervention, and (4) considerations of how trial sample size will affect uncertainty of utility estimates [28]. Recommendations for study design and methodological approaches for economic evaluations in SMA have been recently published in an SLR [89].
Conclusions
This SLR provides a comprehensive repository of the currently available utilities relevant to patients with SMA and their caregivers. Overall, the included studies demonstrate the substantial HRQoL burden of SMA on both populations. This SLR also highlights a paucity of utility/disutility evidence for SMA, with available utility data also frequently failing to meet the stringent requirements of HTA body reference cases. The absence of robust utility data highlights the importance of global, regional, and/or local data collection platforms and disease registry networks and supports a recommendation for early planning in future clinical trial design to help generate utility data for economic evaluations in SMA. In the absence of higher-quality evidence, data from the 14 identified studies and the 17 studies in other (non-SMA) neuromuscular disorders may be considered appropriate for informing economic evaluations regarding rare neuromuscular disorders. In any case, the choice of utility inputs for economic evaluations should be fully justified, and estimates should be thoroughly tested through comprehensive sensitivity analysis (potentially using surrogate estimates from relevant diseases).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Rosalind Carney, DPhil, of MediTech Media Ltd for providing medical writing support, which was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Declarations
Funding
This study was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Disclosure statement
This article is published in a special edition journal supplement wholly funded by F. Hoffmann-La Roche Ltd.
Conflicts of Interest
During the preparation of this manuscript, C. Simone Sutherland and Noman Paracha were employed by F. Hoffmann-La Roche Ltd, and they own stocks in Roche. Pollyanna Hudson and Stephen Mitchell are employed by Mtech Access, which was commissioned by Roche and therefore received a consulting fee to conduct the SLR presented in the manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
CSS and NP conceptualised the study. All authors contributed to the study design. PH and SM conducted the literature search, data extraction, initial summary of results, and quality assessment. All authors contributed to data interpretation and development of the manuscript, including figures, tables, and electronic supplementary material. All authors approved the final version of the manuscript.
References
- 1.Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. 2017;12(1):124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996;3(2):97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- 3.Wirth B, Karakaya M, Kye MJ, Mendoza-Ferreira N. Twenty-five years of spinal muscular atrophy research: from phenotype to genotype to therapy, and what comes next. Annu Rev Genomics Hum Genet. 2020;21:231–261. doi: 10.1146/annurev-genom-102319-103602. [DOI] [PubMed] [Google Scholar]
- 4.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA) Hum Mutat. 2000;15(3):228–237. doi: 10.1002/(SICI)1098-1004(200003)15:3<228::AID-HUMU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Crawford TO, Paushkin SV, Kobayashi DT, Forrest SJ, Joyce CL, Finkel RS, et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE. 2012;7(4):e33572. doi: 10.1371/journal.pone.0033572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saffari A, Kolker S, Hoffmann GF, Weiler M, Ziegler A. Novel challenges in spinal muscular atrophy—How to screen and whom to treat? Ann Clin Transl Neurol. 2019;6(1):197–205. doi: 10.1002/acn3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubowitz V. Very severe spinal muscular atrophy (SMA type 0): an expanding clinical phenotype. Eur J Paediatr Neurol. 1999;3(2):49–51. doi: 10.1016/S1090-3798(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 9.Munsat TL, International SMA Collaboration International SMA consortium meeting. (26-28 June 1992, Bonn, Germany) Neuromuscul Disord. 1991;1(2):81. doi: 10.1016/0960-8966(91)90052-T. [DOI] [PubMed] [Google Scholar]
- 10.Munsat TL, Davies KE. International SMA consortium meeting: (26–28 June 1992, Bonn, Germany) Neuromuscul Disord. 1992;2(5–6):423–428. doi: 10.1016/s0960-8966(06)80015-5. [DOI] [PubMed] [Google Scholar]
- 11.Talbot K, Tizzano EF. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017;24(9):529–533. doi: 10.1038/gt.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis. 2011;6:71. doi: 10.1186/1750-1172-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103–115. doi: 10.1016/j.nmd.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197–207. doi: 10.1016/j.nmd.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration . SPINRAZA® (nusinersen) [package insert] Cambridge: Biogen Inc.; 2016. [Google Scholar]
- 16.European Medicines Agency. Spinraza. 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/spinraza. Accessed 06 Nov 2020.
- 17.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration . ZOLGENSMA® (onasemnogene abeparvovec-xioi) [package insert] Bannockburn: AveXis Inc.; 2019. [Google Scholar]
- 19.European Medicines Agency. ZOLGENSMA. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma. Accessed 06 Nov 2020.
- 20.Ratni H, Ebeling M, Baird J, Bendels S, Bylund J, Chen KS, et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA) J Med Chem. 2018;61(15):6501–6517. doi: 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- 21.Food and Drug Administration . EVRYSDI™ (risdiplam) [package insert] South San Francisco: Genentech Inc.; 2020. [Google Scholar]
- 22.European Medicines Agency. EVRYSDI™ (risdiplam). 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/evrysdi. Accessed 05 May 2021.
- 23.Messina S, Sframeli M. New treatments in spinal muscular atrophy: positive results and new challenges. J Clin Med. 2020 doi: 10.3390/jcm9072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health. 2000;21:587–611. doi: 10.1146/annurev.publhealth.21.1.587. [DOI] [PubMed] [Google Scholar]
- 25.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015. [Google Scholar]
- 26.Brazier J, Deverill M, Green C. A review of the use of health status measures in economic evaluation. J Health Serv Res Policy. 1999;4(3):174–184. doi: 10.1177/135581969900400310. [DOI] [PubMed] [Google Scholar]
- 27.York Health Economics Consortium. Disutility [online]. 2016.
- 28.Wolowacz SE, Briggs A, Belozeroff V, Clarke P, Doward L, Goeree R, et al. Estimating health-state utility for economic models in clinical studies: an ISPOR good research practices task force report. Value Health. 2016;19(6):704–719. doi: 10.1016/j.jval.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;339:b2688. doi: 10.1136/bmj.b2688. [DOI] [PubMed] [Google Scholar]
- 30.Matza LS, Stewart KD, Lloyd AJ, Rowen D, Brazier JE. Vignette-based utilities: usefulness, limitations, and methodological recommendations. Value Health. 2021;24(6):812–821. doi: 10.1016/j.jval.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 32.Wille N, Badia X, Bonsel G, Burstrom K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res. 2010;19(6):875–886. doi: 10.1007/s11136-010-9648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NICE. Guide to the methods of technology appraisal, April 2013 [online]. 2013.
- 35.EuroQol Research Foundation. EQ-5D-Y user guide, 2020.2020. https://euroqol.org/publications/user-guides. Accessed 02 Nov 2021.
- 36.Khan KA, Petrou S, Rivero-Arias O, Walters SJ, Boyle SE. Mapping EQ-5D utility scores from the PedsQL generic core scales. Pharmacoeconomics. 2014;32(7):693–706. doi: 10.1007/s40273-014-0153-y. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd A, Dean R, Jensen I, Maru B, Dabbous O. PRM209—How do we measure utilities outside of trials in rare diseases? Value Health. 2018;21:S392. doi: 10.1016/j.jval.2018.09.2327. [DOI] [Google Scholar]
- 38.Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al. Eliciting public preferences for healthcare: a systematic review of techniques. Health Technol Assess. 2001;5(5):1–186. doi: 10.3310/hta5050. [DOI] [PubMed] [Google Scholar]
- 39.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12–A13. doi: 10.7326/ACPJC-1995-123-3-A12. [DOI] [PubMed] [Google Scholar]
- 41.Brazier JE, Longworth L. NICE DSU Technical Support Document 8: an introduction to the measurement and valuation of health for NICE submissions. 2011. http://www.nicedsu.org.uk. Accessed 12 Nov 2020. [PubMed]
- 42.Papaioannou D, Brazier JE, Paisley S. NICE DCU Technical Suport Document 9: The identification, review and synthesis of health state utility values from the literature. 2011. http://www.nicedsu.org.uk. Accessed 12 Nov 2020. [PubMed]
- 43.Longworth L, Rowen D. NICE DSU Technical Support Document 10: the use of mapping methods to estimate health state utility values. 2011. http://www.nicedsu.org.uk. Accessed 12 Nov 2020. [PubMed]
- 44.CADTH. Guidelines for the economic evaluation of health technologies: Canada 4th Edition, March 2017 [online]. 2017.
- 45.ICER. ICER’s reference case for economic evaluations: principles and rationale, July 2018 [online]. 2018.
- 46.Lloyd AJ, Thompson R, Gallop K, Teynor M. Estimation of the quality of life benefits associated with treatment for spinal muscular atrophy. Clinicoecon Outcomes Res. 2019;11:615–622. doi: 10.2147/CEOR.S214084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.López-Bastida J, Peña-Longobardo LM, Aranda-Reneo I, Tizzano E, Sefton M, Oliva-Moreno J. Social/economic costs and health-related quality of life in patients with spinal muscular atrophy (SMA) in Spain. Orphanet J Rare Dis. 2017;12(1):141. doi: 10.1186/s13023-017-0695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malone DC, Dean R, Arjunji R, Jensen I, Cyr P, Miller B, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Mark Access Health Policy. 2019;7(1):1601484. doi: 10.1080/20016689.2019.1601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampson C, Garau M. How should we measure quality of life impact in rare disease? Recent learnings in spinal muscular atrophy. In: Office of Health Economics. Office of Health Economics. 2019. https://www.ohe.org/publications/how-should-we-measure-quality-life-impact-rare-disease-recent-learnings-spinal-muscular. Accessed 13 Nov 2019.
- 50.Thompson R, Vaidya S, Teynor M. The utility of different approachs to developing health utilities data in childhood rare diseases; a case study in spinal muscular atrophy (SMA) Value Health. 2017;20(9):A725–A726. doi: 10.1016/j.jval.2017.08.1962. [DOI] [Google Scholar]
- 51.Belter L, Cruz R, Jarecki J. Quality of life data for individuals affected by spinal muscular atrophy: a baseline dataset from the cure SMA community update survey. Orphanet J Rare Dis. 2020;15(1):217. doi: 10.1186/s13023-020-01498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Binz C, Schreiber-Katz O, Kumpe M, Ranxha G, Siegler H, Wieselmann G, et al. An observational cohort study on impact, dimensions and outcome of perceived fatigue in adult 5q-spinal muscular atrophy patients receiving nusinersen treatment. J Neurol. 2021;268(3):950–962. doi: 10.1007/s00415-020-10227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chambers GM, Settumba SN, Carey KA, Cairns A, Menezes MP, Ryan M, et al. Prenusinersen economic and health-related quality of life burden of spinal muscular atrophy. Neurology. 2020;95(1):e1–e10. doi: 10.1212/WNL.0000000000009715. [DOI] [PubMed] [Google Scholar]
- 54.McMillan HJ, Gerber B, Cowling T, Khuu W, Mayer M, Wu JW, et al. Burden of spinal muscular atrophy (SMA) on patients and caregivers in Canada. J Neuromuscul Dis. 2021 doi: 10.3233/JND-200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pena-Longobardo LM, Aranda-Reneo I, Oliva-Moreno J, Litzkendorf S, Durand-Zaleski I, Tizzano E, et al. The economic impact and health-related quality of life of spinal muscular atrophy: an analysis across Europe. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17165640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lo SH, Paracha N, Ali S, Lloyd A. PRO92 estimating disutilities in spinal muscular atrophy using a stated preference survey: a UK general public study. Value Health. 2020;23:S345. doi: 10.1016/j.jval.2020.04.1313. [DOI] [Google Scholar]
- 57.Lo SH, Paracha N, Gorni K, Lloyd A. PRO93 do caregivers and patients value the avoidance of lumbar punctures in spinal muscular atrophy? A stated preference survey. Value Health. 2020;23:S346. doi: 10.1016/j.jval.2020.04.1314. [DOI] [Google Scholar]
- 58.Love D, Hicks R, Wei Y, Aldana EZ, Almobarak S, Campbell C. P.218Utility based health related quality of life in children and adolescents with spinal muscular atrophy. Neuromuscul Disord. 2019;29:S130. doi: 10.1016/j.nmd.2019.06.332. [DOI] [Google Scholar]
- 59.Rowell J, Vincent SA, Saberian S, Scoto M, Muntoni F. PRO22 a real world study investigating the resource use and burden associated with spinal muscular atrophy (SMA) from the perspective of patients and carers in the UK. Value Health. 2020;23:S693. doi: 10.1016/j.jval.2020.08.1758. [DOI] [Google Scholar]
- 60.Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 61.Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 62.Mendell JR, Al-Zaidy SA, Lehman KJ, McColly M, Lowes LP, Alfano LN, et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78(7):834–841. doi: 10.1001/jamaneurol.2021.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuluaga-Sanchez S, Teynor M, Knight C, Thompson R, Lundqvist T, Ekelund M, et al. Cost effectiveness of nusinersen in the treatment of patients with infantile-onset and later-onset spinal muscular atrophy in Sweden. Pharmacoeconomics. 2019;37(6):845–865. doi: 10.1007/s40273-019-00769-6. [DOI] [PubMed] [Google Scholar]
- 64.van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 65.NICE. TA588 Nusinersen for treating spinal muscular atrophy, July 2019 [online]. 2019.
- 66.CADTH. Nusinersen (Spinraza) 2019 April 2019.
- 67.ICER. Spinraza® and Zolgensma® for spinal muscular atrophy: effectiveness and value 2019 April 3, 2019.
- 68.López-Bastida J, Oliva-Moreno J, Linertova R, Serrano-Aguilar P. Social/economic costs and health-related quality of life in patients with rare diseases in Europe. Eur J Health Econ. 2016;17(Suppl 1):1–5. doi: 10.1007/s10198-016-0780-7. [DOI] [PubMed] [Google Scholar]
- 69.NICE. Position statement on use of the EQ-5D-5L value set for England (updated October 2019) [online]. 2019.
- 70.Lloyd A, Gallop K, Thompson R, Vaidya S, Teynor M. Estimation of the health-related quality of life benefits of treatment for spinal muscular atropy (SMA) Value Health. 2017;20(9):A559. doi: 10.1016/j.jval.2017.08.911. [DOI] [Google Scholar]
- 71.Tappenden P, Hamilton J, Kaltenthaler E, Hock E, Rawdin A, Mukuria C, et al. Nusinersen for treating spinal muscular atrophy: a single technology appraisal. School of Health and Related Research (ScHARR); 2018. [Google Scholar]
- 72.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Mak. 2006;26(4):410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farrar MA, Carey KA, Paguinto SG, Chambers G, Kasparian NA. Financial, opportunity and psychosocial costs of spinal muscular atrophy: an exploratory qualitative analysis of Australian carer perspectives. BMJ Open. 2018;8(5):e020907. doi: 10.1136/bmjopen-2017-020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brouwer WB, van Exel NJ, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res. 2006;15(6):1005–1021. doi: 10.1007/s11136-005-5994-6. [DOI] [PubMed] [Google Scholar]
- 75.Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Mak. 2011;31(3):458–468. doi: 10.1177/0272989X10381280. [DOI] [PubMed] [Google Scholar]
- 76.Rand SE, Malley JN, Netten AP, Forder JE. Factor structure and construct validity of the adult social care outcomes toolkit for carers (ASCOT-Carer) Qual Life Res. 2015;24(11):2601–2614. doi: 10.1007/s11136-015-1011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoefman RJ, van Exel J, Brouwer WBF. Measuring care-related quality of life of caregivers for use in economic evaluations: CarerQol Tariffs for Australia, Germany, Sweden, UK, and US. Pharmacoeconomics. 2017;35(4):469–478. doi: 10.1007/s40273-016-0477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2(1):9–14. doi: 10.4103/2249-4863.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Landfeldt E, Edstrom J, Sejersen T, Tulinius M, Lochmuller H, Kirschner J. Quality of life of patients with spinal muscular atrophy: a systematic review. Eur J Paediatr Neurol. 2019;23(3):347–356. doi: 10.1016/j.ejpn.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Eiser C, Morse R. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess. 2001;5(4):1–157. doi: 10.3310/hta5040. [DOI] [PubMed] [Google Scholar]
- 81.Spieth LE, Harris CV. Assessment of health-related quality of life in children and adolescents: an integrative review. J Pediatr Psychol. 1996;21(2):175–193. doi: 10.1093/jpepsy/21.2.175. [DOI] [PubMed] [Google Scholar]
- 82.Facey K, Granados A, Guyatt G, Kent A, Shah N, van der Wilt GJ, et al. Generating health technology assessment evidence for rare diseases. Int J Technol Assess Health Care. 2014;30(4):416–422. doi: 10.1017/S0266462314000464. [DOI] [PubMed] [Google Scholar]
- 83.Stolte B, Bois JM, Bolz S, Kizina K, Totzeck A, Schlag M, et al. Minimal clinically important differences in functional motor scores in adults with spinal muscular atrophy. Eur J Neurol. 2020;27(12):2586–2594. doi: 10.1111/ene.14472. [DOI] [PubMed] [Google Scholar]
- 84.Sansone VA, Walter MC, Attarian S, Delstanche S, Mercuri E, Lochmuller H, et al. Measuring outcomes in adults with spinal muscular atrophy—challenges and future directions—meeting report. J Neuromuscul Dis. 2020;7(4):523–534. doi: 10.3233/JND-200534. [DOI] [PubMed] [Google Scholar]
- 85.Wirth B. Spinal muscular atrophy: in the challenge lies a solution. Trends Neurosci. 2021;44(4):306–322. doi: 10.1016/j.tins.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Waldrop MA, Kolb SJ. Current treatment options in neurology-SMA therapeutics. Curr Treat Options Neurol. 2019;21(6):25. doi: 10.1007/s11940-019-0568-z. [DOI] [PubMed] [Google Scholar]
- 87.Glascock J, Sampson J, Haidet-Phillips A, Connolly A, Darras B, Day J, et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5(2):145–158. doi: 10.3233/JND-180304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Messina S, Frongia AL, Antonaci L, Pera MC, Coratti G, Pane M, et al. A critical review of patient and parent caregiver oriented tools to assess health-related quality of life, activity of daily living and caregiver burden in spinal muscular atrophy. Neuromuscul Disord. 2019;29(12):940–950. doi: 10.1016/j.nmd.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Paracha N, Hudson P, Mitchell S, Sutherland CS. Systematic literature review to assess economic evaluations in spinal muscular atrophy (SMA) Pharmacoeconomics. 2021 doi: 10.1007/s40273-021-01095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Iannaccone ST, Hynan LS, Morton A, Buchanan R, Limbers CA, Varni JW, et al. The PedsQL in pediatric patients with spinal muscular atrophy: feasibility, reliability, and validity of the pediatric quality of life inventory generic core scales and neuromuscular module. Neuromuscul Disord. 2009;19(12):805–812. doi: 10.1016/j.nmd.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ludwig K, von der Schulenburg JMG, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(6):663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.