Abstract
Purpose:
The current study aims to quantify the effect of brief behavioral treatment for insomnia (BBTI) studies through meta-analysis.
Method:
Searches were performed from inception to February 2020, reporting on the effects of BBTI using randomized controlled trials (RCT) (adults aged 32 to 84). The main outcome measures were sleep onset latency (SOL), wake after sleep onset (WASO), sleep efficiency (SE%), and total sleep time (TST).
Results:
BBTI showed improved SOL compared with control group in mean difference at early (−15.42 [95% CI: −33.05 to −12.01; I2 =49%]) and late follow-up (−10.52 [95% CI: −1.12 to 0.54; I2=93%]). This was statistically significant at early follow-up, but not at late follow-up. The improvement of WASO by BBTI over the control group was shown at early follow-up (−17.47 [95% CI: −2.67 to 0.45; I2=90%]), and was statistically significant. For WASO, a non-statistically significant improvement of BBTI over the control group was shown at late follow-up (−12.77 [95% CI: −22.47 to −3.08; I2=0%]). SE% was shown improved statistically significant by BBTI over control group at early (4.47 [95% CI: −0.35 to 9.29; I2=98%]) and at late follow-up (6.52 [95% CI: −4.00 to 17.05; I2=89%]). The TST was shown no improvement by BBTI at early follow-up in mean difference (−2.97 [95% CI −38.83 to 32.90; I2=96%]). At late follow-up, TST was shown improvement in BBTI with mean difference (14.52 [95% CI: −31.64 to 60.68; I2=94%]) compared with the control group.
Conclusion:
Current evidence suggests that BBTI can be considered preliminarily efficacious and can be used for samples of middle-aged and older adults.
Keywords: insomnia, behavioral treatment, brief treatment, BBTI, meta-analysis
An insomnia diagnosis consists of subjective complaints about difficulty initiating or maintaining sleep, early morning awakening, and/or experiencing chronically non-restorative sleep consistently over time, despite having adequate sleep opportunity (Buysse et al., 2017). Insomnia is the most prevalent sleep disorder (Morin & Benca, 2012), with symptoms occurring in 30 to 35% of the general population (Morin et al., 2015). Negative public health outcomes from insomnia, include: decline in cognitive functioning (Wardle-Pinkston et al., 2019), functional status (Spira et al., 2014), and immune system (Savard et al., 2003; Asif et al., 2017), as well as increased susceptibility to chronic disease and mortality risk (Fernandez-Mendoza, & Vgontzas, 2013; Javaheri & Redline, 2017; Li et al., 2014; Parthasarathy et al., 2015). Insomnia further negatively impacts our society by reducing work productivity and increased work absenteeism, and healthcare costs (Daley et al., 2009; Léger & Bayon, 2010).
Although insomnia can occur on a short term situational, recurrent, or persistent basis, those who are most negatively affected suffer prolonged insomnia (Morin & Benca, 2012). Two commonly recommended treatments for chronic insomnia are cognitive behavioral therapy (CBT) for insomnia and pharmacotherapy (Winkelman, 2015). While pharmacotherapy drugs such as benzodiazepine or Z-drugs (e.g., zopiclone, zolpidem, or zaleplon) are considered treatment options, limited evidence for long-term efficacy exists along with numerous side-effects and addiction risks (Atkin et al., 2018). Current evidence supports CBT for insomnia as the first-line treatment for insomnia due to its substantial clinical efficacy and good management stability (Edinger et al., 2021; Qaseem et al., 2016; Riemann et al., 2017).
CBT for insomnia includes a combination of stimulus control (establishing regular schedules and bedroom-sleep association, consolidating sleep to nighttime), arousal reduction (implementing calming pre-bedtime activities such as utilizing relaxation-imagery techniques), cognitive therapy, improving sleep hygiene practices, and sleep restriction (temporarily limiting hours staying in bed to increase sleep efficiency) (Morin et al., 2006). A number of recent systematic reviews and meta-analyses provide evidence on the efficacy of CBT for insomnia with clinically meaningful effect sizes (Thakral et al., 2020; Trauer et al., 2015; van Straten et al., 2018). However, challenges remain in implementing CBT for insomnia with time and labor-intensive sessions. In addition, there is a dire shortage of specialty-trained clinical psychologists, and varying patient’s willingness and availability to attend up to 8 treatment sessions (Morin, 2010; Troxel et al., 2012).
To overcome these limitations, Buysse and colleagues (Buysse et al., 2011; Germain et al., 2006) developed a brief, manualized behavioral treatment program entitled brief behavioral treatment for insomnia (BBTI). The primary components of BBTI consist of stimulus control and sleep restrictions, targeted to facilitate sleep onset, consolidate sleep, promote restorative sleep, and improve alertness during daytime (Troxel et al., 2012). Designed to be relevant in the general medical settings including primary care or community settings, BBTI has become more accessible and acceptable for patients with or without comorbidities and for those taking medications (Troxel et al., 2012). Moreover, the brief sessions can be administered over a short period of time, deliverable by a healthcare professional without needing intensive specialty training in behavioral sleep (Troxel et al., 2012). As a result, BBTI is increasingly being acknowledged as a practical and constructive method of treating insomnia that can be implemented across various population groups (Edinger et al., 2021).
To the best of our knowledge, there is no meta-analysis of the efficacy of BBTI in the literature. A recently published review provides a brief overview of BBTI with important insights on its effectiveness in tandem with limitations and future direction for research (Gunn et al., 2019). However, the previous review omits quantifying the effects, and appraisal of relevant empirical evidence. Therefore, we conducted the first comprehensive meta-analysis of studies examining the effects of BBTI across the general population. Rather than restricting our sample to certain types of comparison conditions or age groups, we decided to include all types of control groups (i.e. waitlist controls, passive controls, and attention controls). Hence, our review sought to conduct a systematic appraisal of all currently available RCT studies on BBTI on the general population suffering with insomnia, and evaluate the evidence for the effects of BBTI compared with a variety of control conditions.
METHODS
Data Source
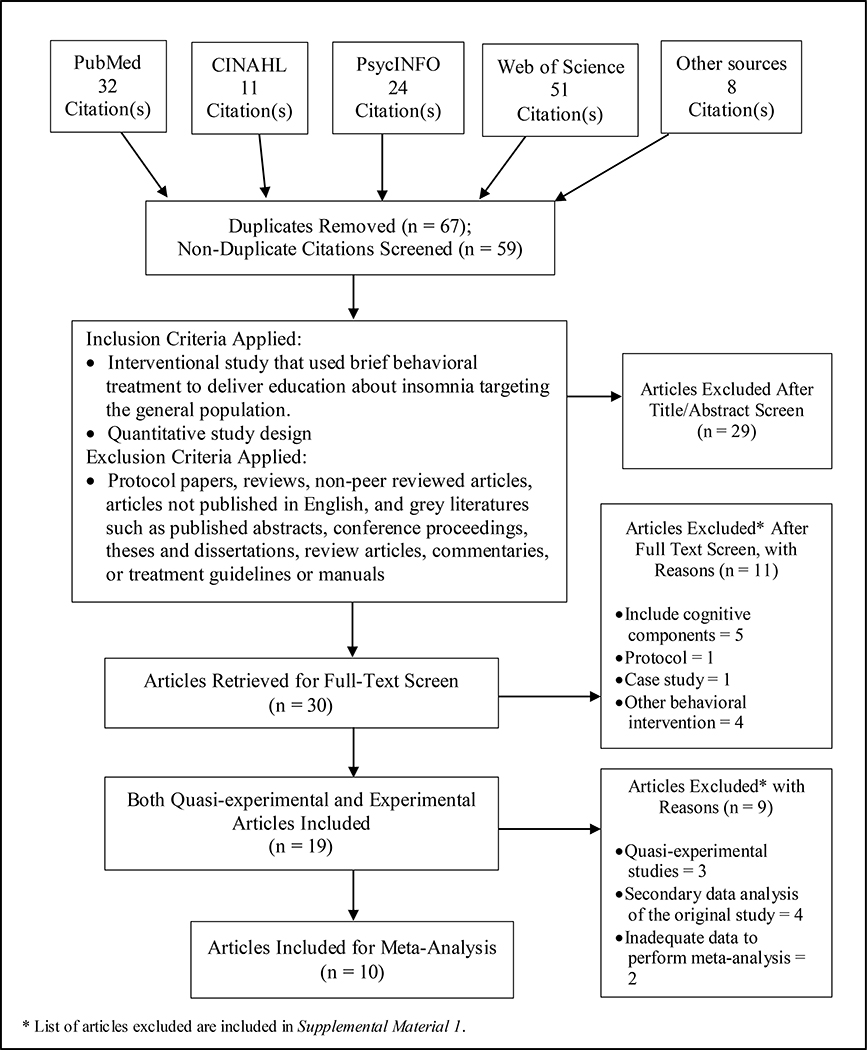
Employing Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Moher et al., 2015), a systematic, structured electronic search on academic databases including PubMed, CINAHL, PsycINFO, and Web of Science was performed. In addition, a backward search (snowballing) was conducted of reference lists of identified articles and review, as well as, forward search (citation tracking) until no additional relevant articles were found. The following search terms were used: (brief behavioral intervention OR brief behavioral treatment OR brief behavioural intervention OR brief behavioural treatment) AND insomnia. The search was conducted on February 15, 2020, and included articles published since inception to February 2020. Figure 1 shows the flow of documents through the identification, screening, eligibility, and inclusion stages of the review.
Figure 1.
Flow chart of the literature search process
Eligibility Criteria
Studies were eligible for inclusion if the intervention used BBTI treatment components to deliver education about insomnia targeting the general population. For the purpose of this review, BBTI has been defined as the multicomponent behavioral intervention that integrates principles of stimulus control (Bootzin et al., 1991) and/sleep restriction (Spielman et al., 1987), but does not include cognitive restructuring or relaxation components (e.g., imagery rehearsal therapy, mindfulness, yoga etc.) during the treatment as outlined by Troxel and colleagues (2012). There were no inclusion or exclusion criteria regarding sample characteristics to study samples that demonstrate a range of insomnia severity, from clinical samples to community samples of all ages with or without other co-morbid conditions suffering from symptoms of insomnia.
Peer reviewed publications of quantitative study designs including only RCTs were eligible for inclusion in the meta-analysis. In addition, all studies that met the inclusion criteria, yet were secondary data analysis of an included original study have been excluded to avoid duplicating outcomes. However, there was an exception where we used some data from the secondary data analysis (McCrae et al., 2020) that were not reported in the original study (McCrae et al., 2018) to perform our meta-analysis. We excluded studies that did not follow the BBTI previously defined. Protocol papers, case studies, reviews, non-peer reviewed articles, non-English publications, grey literature, published abstracts, conference proceedings, theses, dissertations, review articles, commentaries, and treatment guidelines or manuals were also excluded. We have furthermore included a list of pertinent reference of articles that were considered but excluded with reasons (Supplemental Material 1).
Statistical Analysis
A meta-analysis was performed on the mean differences and 95% confidence intervals (CI) calculated for each study to examine the effectiveness of the BBTI compared to control conditions. The main outcome measures of interest were sleep diary measures of sleep onset latency (SOL), wake after sleep onset (WASO), sleep efficiency (SE%), and total sleep time (TST). These were selected based on the most commonly and consistently reported sleep measures that were also relevant when interpreting findings similar to a previously published meta-analysis study on CBT for insomnia (Trauer et al., 2015). Secondary outcome measures included actigraphy and polysomnography (PSG) assessed SOL, WASO, SE%, and TST. We estimated the mean and variance from the median, range and size of a sample using Hozo et al.’s method (2005) for the studies reporting median and range instead of mean and standard deviation. A simplified mean estimation is given by
and the sample standard deviation is estimated by
Where a = the minimum value, m = the median, b = the maximum value.
Three time points were defined as follows for the purpose of the analysis: baseline, early follow-up (1 week to < 8 weeks after completion of the treatment), and late follow-up (8 to 24 weeks after completion of the treatment). All analyses used random-effects models. Heterogeneity in meta-analysis measures the variation in mean difference among studies. Heterogeneity was assessed using the statistic (25%=low, 50%=moderate, 75%=high; Higgins et al., 2003), where measures the proportion of observed variance that reflects the difference in effect size (Borenstein et al., 2011). The Knapp-Hartung method produced more robust pooled estimates of the variance. The effect sizes were calculated based on Cohen’s d and interpreted as: 0.2=small, 0.5=medium, 0.8=large (Cohen, 1992). All analyses were conducted using meta package in R, version 3.6.1 (R Foundation for Statistical Computing) according to the recent standard procedures (Balduzzi et al., 2019).
Quality Assessment
Quality of the studies were assessed for all included studies by two independent reviewers (GD, SD) using the RCT assessment quality domains tool from the Agency for Healthcare Research and Quality (AHRQ) evaluation tools (West et al., 2002). Detailed description of each domain, criteria and evaluation is provided in Table 2.
Table 2.
Methodological quality assessment for randomized controlled trials by domains
| First author, year | Domains |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study questiona | Study populationb | Randomizationc | Blindingd | Interventione | Outcome measuref | Statistical analysisg | Resultsh | Discussioni | Fundingj | |
|
| ||||||||||
| Buysse, 2011 | ● | ● | ● | ○ | ● | ● | ● | ● | ● | ● |
| Dean et al., 2020 | ● | ● | ● | ○ | ● | ● | ◐ | ● | ● | ● |
| Fuller et al., 2016 | ● | ● | ● | ◐ | ◐ | ● | ● | ● | ● | ● |
| Germain, 2006 | ● | ● | ● | ○ | ● | ● | ● | ● | ● | ● |
| Germain, 2014 | ● | ● | ● | ○ | ● | ● | ◐ | ● | ● | ● |
| Harris, 2019 | ● | ● | ● | ◐ | ● | ● | ◐ | ● | ● | ● |
| McCrae, 2018 * | ● | ● | ● | ◐ | ● | ● | ● | ● | ● | ● |
| McCrae, 2020 * | ● | ● | ● | ○ | ● | ● | ◐ | ● | ● | ● |
| Pallesen, 2003 | ● | ● | ● | ○ | ● | ● | ◐ | ● | ● | ● |
| Wang, 2016 | ● | ● | ● | ◐ | ● | ● | ◐ | ● | ● | ● |
| Watanabe, 2011 | ● | ● | ● | ◐ | ● | ● | ● | ● | ● | ● |
Note. ●=domain completely address; ◐=domain partially address; ○=domain not addressed.
Study question: Was it focused and appropriate?
Study population: Was it adequately described including identifying inclusion/exclusion criteria and sample size justification?
Randomization: Was it adequately used with similar baseline groups using a concealment method?
Blinding: Was it to treatment allocation by double-blinding appropriate personnel?
Intervention: Was it detailed and reproducible for all groups with treatment fidelity?
Outcome measure: Was primary and secondary outcome measure specified with standard, valid and reliable assessment methods?
Statistical analysis: Was an appropriate analytic technique used including power calculations, lose to follow up and treating missing data?
Result: were the outcome effect and measures of precision provided?
Discussion: Were conclusions supported by results with the identification of limitations?
Funding: Was funding or sponsorship supporting the study reported?
Overlapping studies
RESULTS
To determine the studies for the meta-analysis, after excluding the overlap of original studies and those providing insufficient data, 10 RCTs were used for the pooled estimates to perform meta-analysis.
Characteristics of Included Studies
A total of 496 participants with insomnia of various age groups (ranging from middle to older adults) were included in the meta-synthesis. Ages varied from 32 to 84 years old, but the majority (76%) of the participants were adults older than 50 years old (n=377). Sex was predominantly female (60%) and all studies were conducted in US (n=10). The studies’ population samples included: 5 studies of adults from the general population without significant psychiatric or physical health comorbidities (Buysse et al., 2011; Fuller et al., 2016; Germain et al., 2006; McCrae et al., 2018; Pallesen et al., 2003). Other studies focused on unique populations: one study of adult lung cancer survivors (Dean et al., 2020); one study of combat-exposed veterans (Germain et al., 2014); one study of adult patients with symptomatic heart failure (Harris et al., 2019); one study of adults with treatment resistant insomnia (Wang et al., 2016); and one study with adult psychiatric outpatients (Watanabe et al., 2011). The range of populations demonstrates the broad incidences of insomnia in a variety of groups.
Outcome Measures
Sleep diary data were used to measure subjective sleep outcomes (SOL, SE%, WASO, TST) in the majority of the studies (n=8). Actigraphy sleep parameter data was used in one study, and another study obtained sleep outcome data quantified from the Pittsburgh Sleep Quality Index (PSQI) data. The most prevalent self-reported sleep questionnaires used were PSQI (n=7) and Insomnia Severity Index (ISI, n=5). Table 1 provides the detailed description of the study characteristics.
Table 1.
Included randomized control trial studies of brief-behavioral treatment for insomnia (BBTI)
| First author, year | Sample size | Sample characteristics (age, sex) | Study design | Assessment times | Outcome measures | Statistical analysis | Main results | Drop-out rate (overall %) |
|---|---|---|---|---|---|---|---|---|
| Buysse, 2011 | 79 | Adults (Mean age 71.7 years, 70% female) | RCT | T1 – Week 0 T2 – Week 4 (post-Tx) T3 – 6 month (only for Tx group) |
SE%, SOL, TST, WASO, SQ (SD, actigraphy, and PSG), ESS, PSQI, SF-36, HAM-A, HAM-D, MMSE, PHQ |
Independent samples t-test, chi-square test, Fisher’s exact test, repeated-measures MANOVA (Cohen’s d effect sizes) | BBTI group: SD: ↓SOL, ↓WASO, ↑SE%, SQ > control at T2; Actigraphy: sig. ↓WASO, SOL, ↑SE > control, sig. ↓TST for Tx group at T2; PSG: no sig. group differences; Sig. change in depression, self-reported SQ and general health in Tx group at T2; No sig. change in anxiety or sleepiness for both groups at T2; 55% of Tx group no longer met insomnia criteria (40% response, and 25% remission), and 13% of control group no longer met insomnia criteria with 20% response and <5% remission at T3 |
3.8% and at T3, 68.4% |
| Dean et al., 2020 | 40 | Adult lung cancer survivors (53–82 years, 60% female) | RCT (pilot) | T1 – Week 0 T2 – Week 5 (post-Tx) |
SE%, SOL, SQ, TST, WASO, feeling rested (SD), ISI, DBAS, PSQI, FACT-L, HADS, POMS-SF, ESS |
ANCOVA, McNemar’s test | BBTI group: SD: ↑85% SE% (.02) at T2; ↑85% SE% (.04); feeling rested (.007), SQ (.029) > control at T2; Sig. ↓mean ISI (<.001) > control at T2; Sig. ↑mean FACT-L (.049) > control at T2; No sig. changes in PSQI, DBAS, ESS, POMS-F, HADS between groups Control group: SD: No sig. change in SE% at T2; ↓ (worsening) mean FACT-L at T2; |
25% |
| Fuller et al., 2016 | 46 | Adults (mean age 53.7 years, 72% female) | Cluster-RCT | T1 – Week 0 T2 – Week 4 (post-Tx) T3 – Week 12 |
SE%, SOL, TST, WASO (SD), ISI, DBAS, DASS | Descriptive statistic, independent samples t-test, Mann-Whitney U-test, Shapiro-Wilk test, dependent sample t-test, Wilcoxon signed-rank test, repeated-measure MANOVA, linear mixed-model analysis | BBTI group: Sig. ↓(improved) ISI > control (0.05) at T3; SD: Sig. ↑TST > control (0.04) at T3; Sig. ↓(improved) dysfunctional beliefs about sleep (DBAS) > control (0.003) at T3; Sig. ↓(improved) depression, anxiety, and stress (DASS) > than control (0.03) at T3 No sig. change in SD: WASO, SOL, and SE% in both groups |
21.7% |
| Germain, 2006 | 35 | Adults (60+ years, 71% female) | RCT | T1 – Week 0 T2 – Week 4 (post-Tx) |
SE%, SOL, TST, WASO (SD), PSQI, HAM-A, HAM-D |
Independent samples t-test (Cohen’s d effect sizes) |
BBTI group: SD: ↓SOL (<.05), WASO (<.05), ↑SE% (.05-.10) > control at T2; no sig. change in TST between groups; Sig. ↓(improved) PSQI (<.01), depression (<.05) > control at T2; No sig. change in anxiety level between groups at T2; 71% and 53% met criteria for response (reduction of 3 points or more on the PSQI or at least 10% ↑ in SE%), and remission (PSQI <5 or less or SE% >85%), respectively at T2. Control group: 39% and 17% met criteria for response and remission, respectively at T2 |
0% |
| Germain, 2014 | 40 | Combat-exposed veterans (Mean age 38.4 years, 15% female) | RCT | T1 – Week 0 T2 – Week 4 (post-Tx) T3 – Week 24 (6 mo.) |
ISI, PSQI, PSQI-A for PTSD, PCL-C, BDI, BAI | Independent samples t-test, chi-square test |
BBTI group: Sig. ↓(improved) ISI (.03), ↓(improved) PSQI (.02), ↓(improved) depression severity at T2 and maintained at T3; No sig. change in disruptive nocturnal behaviors or daytime symptoms of PTSD or anxiety at T2; Sig. ↓(improved) ISI (.03), ↓(improved) PSQI (.02) > control at T2; 77% and 53% for response and remission, respectively > control at post-Tx, but no sig. difference between groups Control group: No sig. change in disruptive nocturnal behaviors or daytime symptoms measures at T2 |
37.5% |
| Harris, 2019 | 23 | Symptomatic heart failure patients (Mean age 55.7 years, 69.6% female) | RCT (pilot) | T1 – Week 0 T2 – Week 4 (post-Tx) |
SE%, TST, SQ (SD), ISI, PSQI, HADS, KCCQ, SCHFI (self-care), WAIS-IV, TMT (executive function), VPA II and II, memory), 60ftWT (functional status) | Pearson correlation, ANOVA, chi-square test, independent samples t-test, repeated-measure MANOVA (Hedges’ g effect sizes) |
BBTI group: SD: Sig. ↑SE% (<.01); Sig. ↓(improved) ISI (<.01), PSQI (<.01); 58.3% met clinically meaningful improvements in insomnia (ISI total score reduction of ≥6 points), 25% no longer have insomnia symptoms at T2; Sig. ↓(improved) anxiety (<.01) and depression (<.001); No sig. change in heart-failure related quality of life, self-care, or cognitive outcomes Control group: No sig. change in sleep outcomes, anxiety, depression, heart-failure related quality of life, self-care, or cognitive outcomes |
8.7% |
| McCrae, 2018 | 62 | Adults (65+ years, 42% female) | RCT | T1 – Week 0 T2 – Week 5 (post-Tx) T3 – Week 12 (3mo.) |
SE%, SOL, SQ, TST, WASO, (SD and actigraphy), GDS, BDI-II, STAI-Y, neuropsychological battery |
Chi-square test, ANOVA, ANCOVA, Bonferroni and Greenhouse-Geisser correction (Hedges’ g and Cohen’s d effect sizes) |
BBTI group: SD: ↓SOL (.01), WASO (p<.01), ↑SE% (p<.01), SQ (p<.01) > control at T2; sig. ↓SOL, WASO, ↑SE%, SQ (all improved, .05) at T2 and T3; Control group: SD: no sig. change in SOL, WASO, SE%, TST, or SE% at T2 or T3; Actigraphy: ↓WASO (.01) for both groups at T2, but no sig. change at T3; no sig. change for SOL, SE%, or TST for both groups; ↓depressive symptoms for both groups at T2 and T3; No sig. changes in neuropsychological performance, mood or anxiety for both groups |
35.5% |
| McCrae, 2019 | SE, SOL, TST, WASO (SD), SDMT, letter series test (daily cognitive performance outcome measures) | Independent samples t-test, chi-square test, multilevel modeling | BBTI group: ↑TST associated with improved next day letter series performance at T2 (.01); ↑WASO associated with improved next day cognitive performance at T2 for both groups |
|||||
| Pallesen, 2003 | 55 | Adults (60–84 years, 83.6% female) | RCT | T1 – Week 0 T2 – Week 4 (post-Tx) T3 – 6 month |
SE%, SOL, TST, WASO, daytime sleepiness, days (%) of hypnotic use (SD), SII (insomnia severity), WHO (Ten) (life satisfaction) |
Repeated-measure ANOVA, chi-square test | No sig. diff. in treatment effects between two interventions Changes from pre- to posttreatment for all subjects: SD: Sig. ↓SOL (<.001), ↓WASO (<.02), ↓days (%) of hypnotic use (<.05), ↑TST (<.001), ↑SE% (<.001), ↑daytime alertness (<.001), ↑perceived life satisfaction (<.007) at T2; Clinical sig. improvements at T2: 12.5% of subjects with sleep onset insomnia; 20% of subjects with sleep maintenance insomnia; No clinical sig. to wait-list control group; No sig. change in any sleep parameters and perceived life satisfaction for control group at T2 |
14.5% |
| Wang, 2016 | 79 | Adults with treatment resistant insomnia (32–50 years, 54% female) | RCT | T1 – Week 0 T2 – Week 4 (post-Tx) |
SE%, SOL, SQ, TIB, TST, WASO (SD), DBAS, ESS, ISI, PSQI |
Mann-Whitney U test, chi-square test, repeated-measure ANOVA (Cohen’s d effect sizes) |
BBTI group: SD: Sig. ↓SOL, TIB, WASO, ↑SE% > than control; ↓sleep medication use > than control (<.05); Sig. improved PSQI, ESS, DBAS, ISI scores (all <.05) > than control; SD: Sig. change in SE%, SOL, TIB, WASO, SQ in both groups (<.05); Sig. change in PSQI, ESS, DBAS, ISI in both groups |
3.8% |
| Watanabe, 2011 | 37 | Adult psychiatric outpatients (Mean age 50.5 years, 62.6% female) | RCT | T1 – Week 0 T2 – Week 4 (post-Tx) T3 – Week 8 |
PSQI, ISI, GRID-HAM-D | Mixed linear model | BBTI group: Sig. ↓(improved) in insomnia severity (ISI, .01, .036) at T2 and T3, respectively, > control; Sig. ↓(improved) PSQI (.004, .003), ↑SE% (.003, .015) at T2 and T3, respectively, > control; Sig. difference in depression (.004, .013) at T2 and T3, respectively, > control; 40% at T2 and 50% at T3 no longer met insomnia criteria, 55% at T2 and 50% at T3 no longer met depression criteria Control group: 5.9% at T2 and 0% at T3 no longer met insomnia criteria, 5.9% at T2 and 5.9% at T3 no longer met depression criteria |
8.1% |
Abbreviations. ANCOVA=analysis of covariance; ANOVA=analysis of variance; BAI=Beck Anxiety Inventory; BDI=Beck Depression Inventory; BDI-II=Beck Depression Inventory-Second Edition; BBTI=brief behavioral treatment for insomnia; DASS=Depression, Anxiety, and Stress Scale; DBAS=Dysfunctional Beliefs and Attitudes about Sleep Scale; ESS=Epworth Sleepiness Scale; FACT-L=Functional Assessment of Cancer Therapy-Lung Scale; GDS=Geriatric Depression Scale; GRID-HAM-D=Hamilton Depression Rating Scale; HADS=Hospital Anxiety and Depression Scale; HAM-A=Hamilton Anxiety Rating Scale; HAM-D=Hamilton Depression Rating Scale; ISI=Insomnia Severity Index; KCCQ=Kansas City Cardiomyopathy Questionnaire; MMSE=Mini-Mental State Examination; PCL-C=Posttraumatic stress disorder checklist – civilian version; PHQ=Patient Health Questionnaire; POMS-SF=Profile of Mood State-Short Form; PSG=polysomnography; PSQI=Pittsburgh Sleep Quality Index; PSQI=Pittsburgh Sleep Quality Index-Addendum; MANOVA=multivariate analysis of variance; SCHFI=Self-Care of Heart Failure Index; SCI=Sleep Condition Indicator; SD=sleep diary; SDMT=Symbol Digit Modalities Test; SF-36=Medical Outcomes Study Short Form-36; SE%=sleep efficiency; SII=Sleep Impairment Index; SOL=sleep onset latency; SQ=sleep quality; STAI-Y=State Trait Anxiety Inventory-Form Y; TMT=Trail Making Test; Tx=treatment; TST=total sleep time; RCT=randomized control trial; VPA (I and II)=Verbal Paired Associates I and II; WAIS-IV=Wechsler Adult Intelligence Scale - Fourth Edition; WASO=wake after sleep onset; WHO (Ten)=Well-Being Index-10 item; 60ftWT=Sixty-Foot Walk Test; ↑=increase; ↓= decrease
Interventions
BBTI was delivered through two face-to-face individual sessions (n=5) with two phone call follow-ups. One study delivered BBTI via three individual sessions with no phone call follow-up (Fuller et al., 2016), two studies via four individual sessions with no follow-up (Pallesen et al., 2003; Watanabe et al., 2011), and one study had an in-person 3-month follow-up (McCrae et al., 2018). Across studies, interventionists ranged from registered nurses and nurse practitioners, pharmacists, social worker, clinical psychologists, to students in health science programs. Detailed descriptions of the characteristic of BBTI programs are summarized in Supplemental Material 2.
BBTI along with follow-up sessions included brief phone calls that emphasized reviewing treatment progress in addition to relapse prevention and adherence reinforcement, discussing any sleep challenges identified in weekly sleep diaries, and assessing the need to increase time allowed in bed if shown improvement. For control and comparative conditions, BBTIs were compared to waitlist (n=1), treatment as usual (n=2), information materials hand-outs (n=3), self-monitoring (n=2), healthy eating program (n=1), and sleep hygiene education (n=1).
Main Efficacy Meta-Analysis
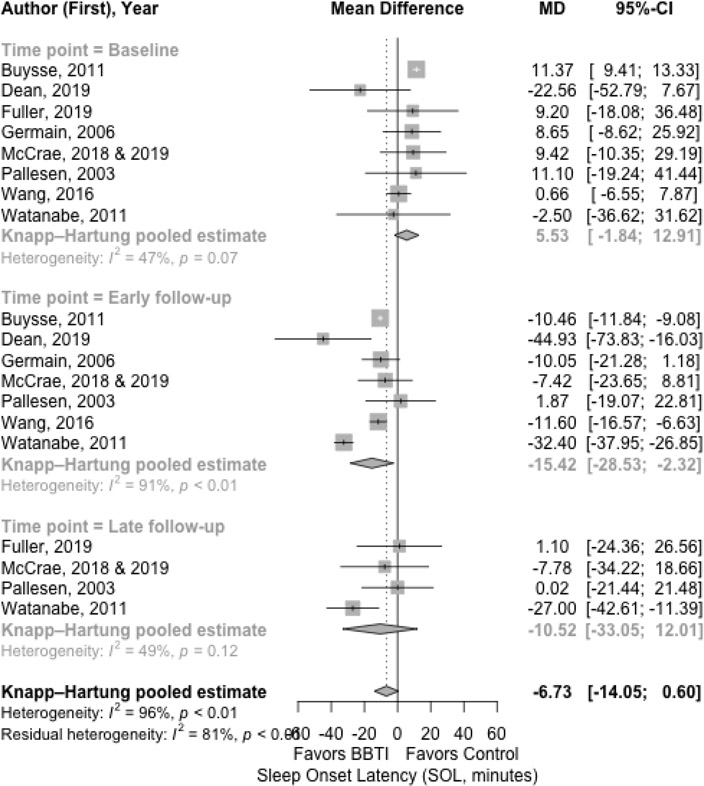
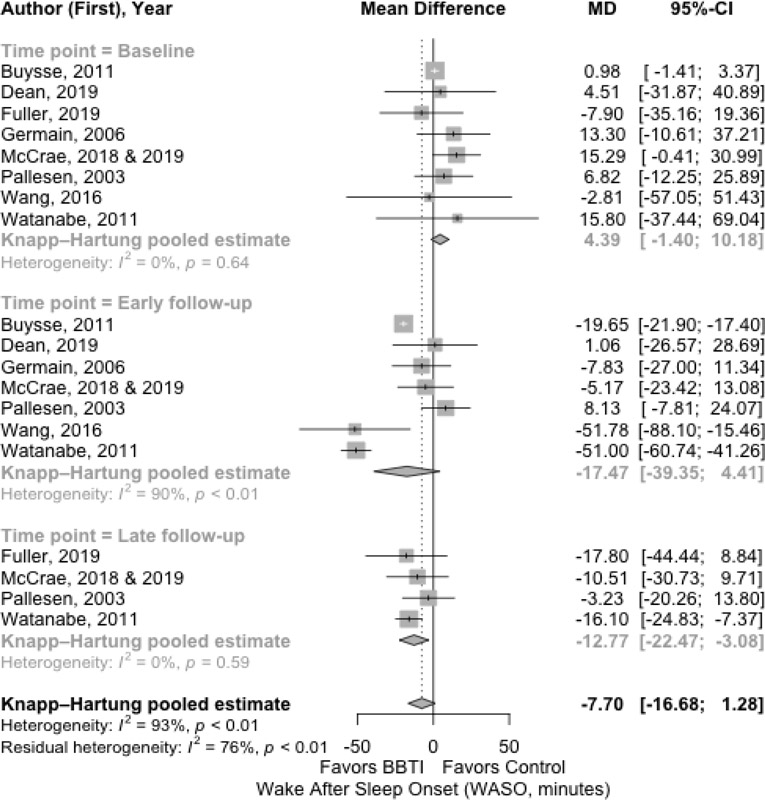
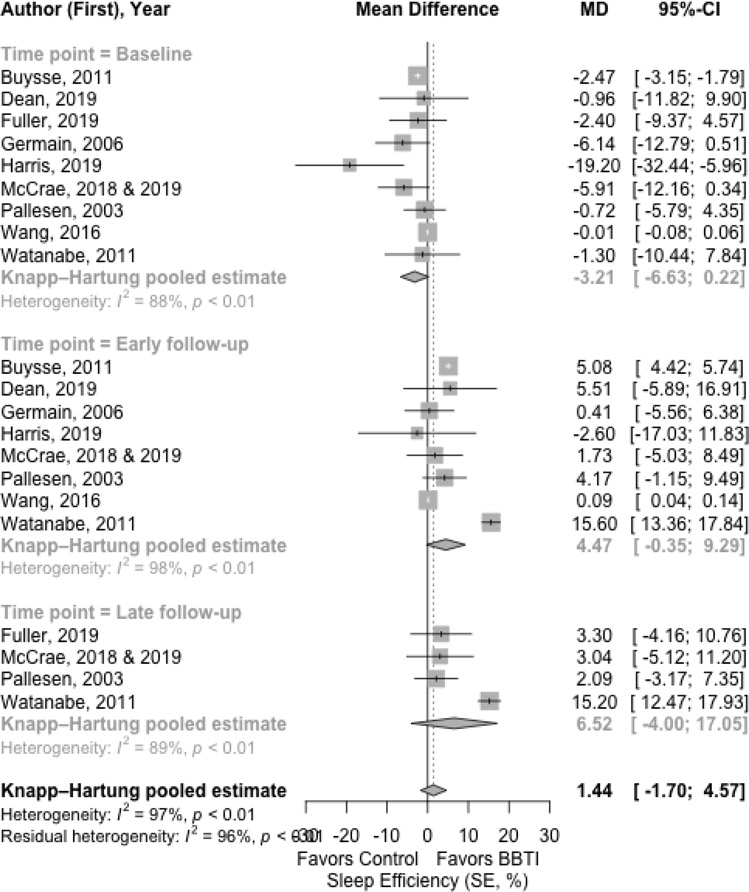
The results for the main outcomes of sleep diary measures with mean difference are presented in Figure 2, Figure 3, Figure 4, and Figure 5 for SOL, WASO, SE%, and TST, respectively. The results with effect size are shown in Supplemental Material 3.
Figure 2.
Meta-analysis of the effect of BBTI on SOL (sleep diary measured)
Figure 3.
Meta-analysis of the effect of BBTI on WASO (sleep diary measured)
Figure 4.
Meta-analysis of the effect of BBTI on SE% (sleep diary measured)
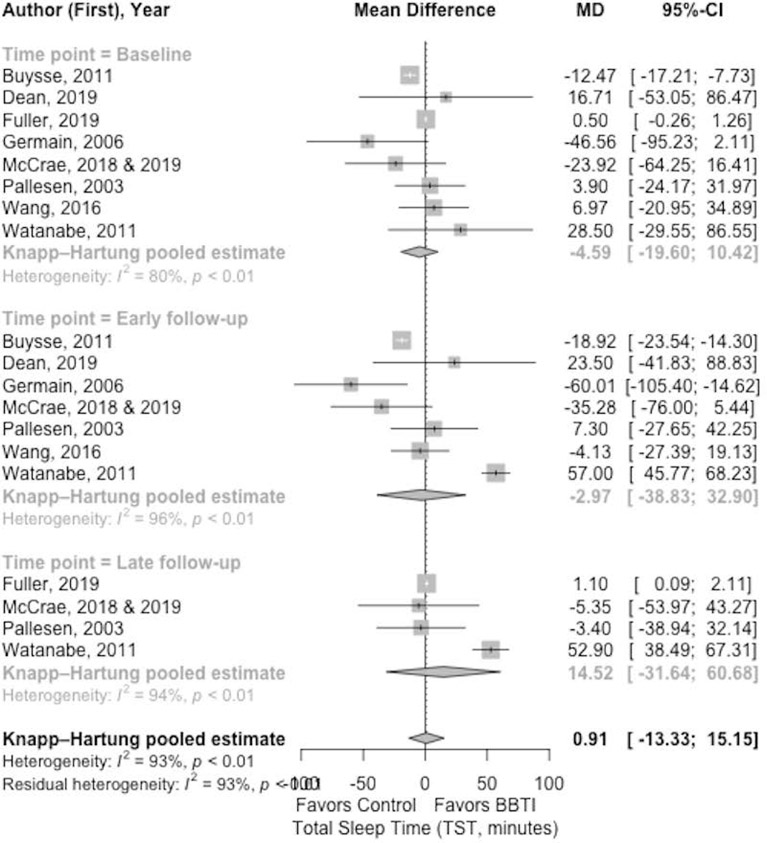
Figure 5.
Meta-analysis of the effect of BBTI on TST (sleep diary measured)
The SOL was statistically significantly improved after BBTI intervention compared to control with a large effect size at early follow-up (Figure 2). At baseline, the pooled estimate mean difference between BBTI group and control group was 5.53 minutes (95% CI: 12.91 to −1.84 minutes; =47%). The control group at baseline had better SOL than the BBTI group with a non-significant small effect size (d = 0.37 [95% CI: −0.39 to 1.13; =89%]). At early follow-up, there was statistically significant improvement in SOL (−15.42 minutes [95% CI: −2.32 to −28.54 minutes; =91%]) for BBTI intervention. The BBTI group had better SOL than the control group with a significant large effect size (d=−1.39 [95% CI: −2.75 to −0.03; =93%]). Additionally, the improvement was observed at late follow-up (−10.52 minutes [95% CI: 12.01 to −33.05 minutes; =49%]), but was not statistically significant. The BBTI group had better SOL than the control group with a non-significant small effect size (d=−0.29 [95% CI: −1.12 to 0.54; =93%]).
The WASO had improvement after BBTI interventions at early follow-up, with the pooled mean difference of −17.47 minutes (95% CI: 4.41 to −39.35 minutes; 90%). However, the statistical significance was not achieved for WASO during the late follow-up. The large effect size was statistically significant (d=−1.11 [95% CI: −2.67 to 0.45; =95%]) at early follow-up. Improvement was maintained but was not statistically significant at late follow-up, with the pooled mean difference of −12.77 minutes (95% CI: −3.08 to −22.47 minutes; =0%) (Figure 3), and the effect size was small and not statistically significant (d=−0.48 [95% CI: −0.99 to 0.12; =47%]).
The BBTI intervention had improvement in SE% both at early and late follow-up each with a large effect size (Figure 4). At baseline, the pooled estimate of mean difference of SE% between BBTI group and control group was −3.21% (95% CI: −6.63% to 0.22%; =88%) favoring control group. There was statistically significant improvement in both the early follow-up (4.47% [95% CI: −0.35% to 9.29%; =98%]) for BBTI intervention and at late follow-up (6.52% [95% CI: −4.00%, 17.05%; =89%]). The effect size comparing BBTI and control group was large (d =1.12 [95% CI: −0.30 to 2.54; =93%]) and was statistically significant at early follow-up, and likewise, was large (d=1.03 [95% CI: −1.55 to 3.61; =91%]) and statistically significant at late follow-up.
At the baseline, there was a statistically significant difference in TST for BBTI intervention (Figure 5) versus control (−4.59 minutes [95% CI: 10.42 to −19.60 minutes; =80%]) favoring control group. The effect size comparing BBTI intervention and control group was statistically significant (d=−0.14 [95% CI: −0.59 to 0.30; =74%]), favoring control group. At early follow-up, there was no improvement (−2.97 minutes [95% CI: 32.90 to −38.83 minutes; =96%]) for BBTI intervention when compared to control. BBTI intervention revealed statistically significant improvement at late follow-up (14.52 minutes [95% CI: 60.68 to −31.64 minutes, 60.68; =94%]). The BBTI intervention showed statistical significance improvement over the control group on TST with effect size at early follow-up (d=0.02 [95% CI −1.40 to 1.44; =93%]) and late follow-up (d=0.7 [95% CI: −1.07 to 2.47; =88%]). Overall, there was considerable heterogeneity in the analysis due to the limited number of studies considered.
Secondary End Points
In order to compare the effect of BBTI on sleep diary outcomes, with its effects on the same outcomes measured by different methods, actigraphy and polysomnography (PSG) measures were considered at the post-treatment time point. These results along with effect sizes of ISI and PSQI are summarized in Supplemental Material 4. For actigraphy, 2 studies (Buysse et al., 2011; McCrae et al., 2018) were available for meta-analysis, estimates for effect size were reversed to those seen in the sleep diary measures of the same estimates. In other words, although the effect size comparing BBTI and control group with sleep diary measures (i.e., SOL, WASO, SE%, and TST) were shown statistically significant, this was not seen in actigraphy measures evaluated through two existing studies. For PSG, only one study was analyzed (Buysse et al., 2011) and the effect size estimates for SOL was similar to the result in the sleep diary measure while the other three variables were completely opposite to the results in the sleep diary measures. For ISI, 5 studies were available for meta-analysis, the BBTI group was observed statistically significantly better than control group with a large effect size (d=−0.96 [95% CI: −2.24 to 0.32; =92%]). For PSQI, 7 studies were available for meta-analysis, the BBTI group was observed statistically significantly better than control group with a large effect size (d=−1.18 [95% CI: −2.35 to 0.01; =90%]).
Risk of Bias
Table 2 summarizes the methodological quality assessment for RCT by domains. All studies fully addressed the study question, study population, randomization, outcome measure, results, discussion, and funding. The most common deficit was the blinding which was often omitted. Only four studies discussed blinding of data collectors. Four of nine studies did not include a power analysis to determine the sample size.
DISCUSSION
To our knowledge, this is the first meta-analysis to examine the effects of BBTI. Due to the subjective assessment of the insomnia diagnosis, subject’s daily sleep diary measures SOL, WASO, SE%, and TST were used to calculate the mean differences. Overall, the present meta-analysis demonstrated that BBTI is an effective treatment for insomnia at early follow-up, as it produced meaningful improvements on several subjective sleep parameters. There were marked and statistically significant improvements in mean difference in SOL and WASO during the early follow-up (1 week to <8 weeks after the completion of BBTI). For SE%, statistically significant improvements were shown at both early and at late follow-up (8 to 24 weeks after completion of BBTI). For TST, statistically significant improvement was shown at only late follow-up.
The sleep diary results from the current review are lower, but somewhat comparable to a meta-analysis involving 20 studies of CBT for insomnia in adults with chronic insomnia that revealed improvements of 19 minutes in SOL, 26 minutes in WASO, 9.9 SE% post-treatment and 7.6 minutes of TST (Trauer et al., 2015). Our results included follow-up improvement of 15 (early) and 11 (late) minutes for SOL, 17 (early) and 13 (late) minutes for WASO, 4 (early) and 7 (late) for SE% and 3 (early) and 15 (late) minutes for TST. While Trauer et al. (2015) applied narrow inclusion criteria, which minimized heterogeneity of included studies, the current review used broad criterion for included studies which increased heterogeneity and results may have been negatively impacted.
Overall, BBTI was associated with improved insomnia symptoms of medium to large magnitude and can be considered preliminarily efficacious. Despite the encouraging results from this meta-analysis, it is not without its limitations. Hence, there are some considerations, and future directions for continued work in the field. BBTI may be more accessible and adoptable as an insomnia treatment option. First, although sleep diary measures were used to calculate the mean differences, there was an exception of one study where the sleep measures were abstracted from PSQI which is a past 30-day recall (Watanabe et al., 2011), and another only reporting SE% measure (Harris et al., 2019). Second, the current review is limited by the substantial methodological and statistical heterogeneity between studies. Fairly wide inclusion criteria were applied in the search due to the limited number of available studies. Included studies were also comprised of participants with varied range of ages, varying levels of insomnia symptom severity, comorbidity, and clinical vs. non-clinical samples. Although these methodological differences may have contributed to statistical heterogeneity, it is important to recognize that these could potentially represent the actual variety of samples and methods employed with BBTI treatment in various settings.
Thirdly, measures of remission for insomnia disorder were not included due to inconsistency of measures. Of the eleven studies included in this review, five included measures of remission. The PSQI was used in two (Buysse et al., 2011; Germain et al., 2006), the ISI was used in two (Germain et al., 2014; Watanabe et al., 2011), the PSQI and ISI were used in one (Harris et al., 2019) to define remission. Fourthly, for the similar reasons that effect sizes in pilot studies may be deemed unreliable, statistical outcomes from pilot studies may also have similar limitations. Thus, current findings need to be interpreted with caution and should be replicated with other larger adequately powered studies. More specifically, more RCTs are needed to examine the effectiveness of this treatment compared to treatment as usual, attention controls or other behavioral treatments in order to account for fluctuations in the SOL, WASO, SE%, and TST over follow up periods effecting assessment. Consistent and prolonged follow up periods are also needed to examine the long-term effects of BBTI as most of the reviewed studies followed their participants for ranges of 1 to 6 months. Lastly, our study did not control for publication bias. Although meta-analyses play an important role in evaluating the consistency of study findings and provide guidelines for future studies, it has the potential for publication bias and can be limited by the data available from published studies that likely report positive and statistically significant findings (Dwan et al., 2013).
The American Academy of Sleep Medicine clinical practice guidelines on behavioral and psychological treatments for chronic insomnia in adults strongly recommend utilizing CBT for insomnia relative to conditional recommendations suggested for use of single or multicomponent of BBTI (Edinger et al., 2021). A recently published noninferiority RCT study compared BBTI and CBT for insomnia at 1-week post treatment follow-up, and reported an inconclusive finding indicating BBTI as noninferior than CBT for insomnia (Bramoweth et al., 2020). Hence, the critical question that continues to remain unanswered is how effective BBTI is compared to the gold standard CBT for insomnia treatment given the same assessment periods. Included studies excluded all published research that included cognitive restructuring components, however, it could be interesting to evaluate studies if incorporating cognitive outcomes in BBTI verses those without, yields different results. It may also be important to determine moderators of treatment, such as age subgroups (i.e., young/middle-aged adults vs. older adults), as incorporating sleep hygiene practices and safety recommendations catered to directed age subgroups might yield better patient outcomes. Moreover, a recently published RCT compared a fully-automated, individually tailored BBTI and compared its short-term effect on insomnia and its severity with self-guided BBTI, self-monitoring with sleep diaries, or a waitlist control group (Okajima et al., 2020). Okajima and colleagues (2020) found that individually tailored BBTI showed significantly improved ISI scores than the waitlist group at 1-month follow-up, but this did not continue at 3-month follow-up. Digitalized/internet-delivered BBTI may serve as an important step towards broader dissemination and implementation, but it is important to first determine efficacy of different study designs including group-delivered BBTI and also include additional outcomes (i.e., daytime dysfunction) for future studies. Accordingly, the current meta-analysis has important implications for future research as the literature on BBTI continues to grow.
CONCLUSIONS
People with insomnia suffer consequences of poor sleep that influences their overall health and quality of life, as well as public well-being. BBTI offers several advantages as compared to CBTI by requiring fewer sessions that are briefer, and may be delivered by a wide range of healthcare providers (e.g., nurses, pharmacists, social workers) in non-specialized medical settings. As such, BBTI may be more easily implemented in general medical settings such as primary care or specialty care settings such as comprehensive cancer centers. The existing RCTs investigating the effect of BBTI, yielded statistically significant improvements in insomnia by subjective sleep measures. This suggests that BBTI is a generally acceptable treatment that shows favorable outcomes especially 1–2 months following the treatment. The research literature on BBTI is relatively small but growing, which highlights the importance of conducting high quality studies with large scale RCTs to examine efficacy.
Supplementary Material
Supplemental Material 1. List of articles that were considered but excluded with reasons
Supplemental Material 2. Characteristics of brief behavioral treatment for insomnia (BBTI) programs
Supplemental Material 3. Effect sizes
Supplemental Material 4. Secondary outcomes
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Nursing Research (NINR) of the National Institutes of Health (NIH) under award number 5R01NR018215 (Dean). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
DECLARATION OF INTEREST STATEMENT
None
REFERENCES
- Asif N, Iqbal R, & Nazir CF (2017). Human immune system during sleep. American Journal of Clinical and Experimental Immunology, 6(6), 92. [PMC free article] [PubMed] [Google Scholar]
- Atkin T, Comai S, & Gobbi G (2018). Drugs for insomnia beyond benzodiazepines: Pharmacology, clinical applications, and discovery. Pharmacological Reviews, 70(2), 197–245. 10.1124/pr.117.014381 [DOI] [PubMed] [Google Scholar]
- Balduzzi S, Rücker G, & Schwarzer G (2019). How to perform a meta-analysis with R: A practical tutorial. Evidence-Based Mental Health, 22(4), 153–160. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootzin RR, Epstein D, & Wood JM (1991). Stimulus control instructions. In Case studies in insomnia (pp. 19–28). Springer, Boston, MA. 10.1007/978-1-4757-9586-8_2 [DOI] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, & Rothstein HR (2011). Introduction to meta-analysis. John Wiley & Sons. [Google Scholar]
- Bramoweth AD, Lederer LG, Youk AO, Germain A, & Chinman MJ (2020). Brief behavioral treatment for insomnia vs. cognitive behavioral therapy for insomnia: Results of a randomized noninferiority clinical trial among veterans. Behavior Therapy, 51(4), 535–547. 10.1016/j.beth.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, Begley A, Houck PR, Mazumdar S, Reynolds CF, & Monk TH (2011). Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine, 171(10), 887–895. 10.1001/archinternmed.2010.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Rush AJ, & Reynolds CF (2017). Clinical management of insomnia disorder. JAMA, 318(20), 1973–1974. 10.1001/jama.2017.15683 [DOI] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J, & Baillargeon L (2009). Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Medicine, 10(4), 427–438. 10.1016/j.sleep.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Dean GE, Weiss C, Jungquist CR, Klimpt ML, Alameri R, Ziegler PA, Steinbrenner LM, Dexter EU, Dhillon SS, Lucke JF, & Dickerson SS (2020). Nurse-delivered brief behavioral treatment for insomnia in lung cancer survivors: A pilot RCT. Behavioral Sleep Medicine, 18(6), 774–786. 10.1080/15402002.2019.1685523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwan K, Gamble C, Williamson PR, Kirkham JJ, & Reporting Bias Group. (2013). Systematic review of the empirical evidence of study publication bias and outcome reporting bias—an updated review. PloS One, 8(7), e66844. 10.1371/journal.pone.0066844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, Sateia M, Troxel WM, Zhou ES, Kazmi U, Heald JL, & Martin JL (2021). Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 17(2), 255–262. 10.5664/jcsm.8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, & Vgontzas AN (2013). Insomnia and its impact on physical and mental health. Current Psychiatry Reports, 15(12), 418. 10.1007/s11920-013-0418-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JM, Wong KK, Hoyos C, Krass I, & Saini B (2016). Dispensing good sleep health behaviours not pills–a cluster‐randomized controlled trial to test the feasibility and efficacy of pharmacist‐provided brief behavioural treatment for insomnia. Journal of Sleep Research, 25(1), 104–115. 10.1111/jsr.12328 [DOI] [PubMed] [Google Scholar]
- Germain A, Moul DE, Franzen PL, Miewald JM, Reynolds CF, Monk TH, & Buysse DJ (2006). Effects of a brief behavioral treatment for late-life insomnia: Preliminary findings. Journal of Clinical Sleep Medicine, 2(04), 403–406. [PubMed] [Google Scholar]
- Germain A, Richardson R, Stocker R, Mammen O, Hall M, Bramoweth AD, Begley A, Rode N, Frank E, Haas G, & Buysse DJ (2014). Treatment for insomnia in combat-exposed OEF/OIF/OND military veterans: preliminary randomized controlled trial. Behaviour Research and Therapy, 61, 78–88. 10.1016/j.brat.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn HE, Tutek J, & Buysse DJ (2019). Brief behavioral treatment of insomnia. Sleep Medicine Clinics, 14(2), 235–243. 10.1016/j.jsmc.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Harris KM, Schiele SE, & Emery CF (2019). Pilot randomized trial of brief behavioral treatment for insomnia in patients with heart failure. Heart & Lung, 48(5), 557–560. 10.1016/j.hrtlng.2019.06.003 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, & Hozo I (2005). Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology, 5(1), 13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S, & Redline S (2017). Insomnia and risk of cardiovascular disease. Chest, 152(2), 435–444. 10.1016/j.chest.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger D, & Bayon V (2010). Societal costs of insomnia. Sleep Medicine Reviews, 14(6), 379–389. 10.1016/j.smrv.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, Ma J, & Gao X (2014). Association between insomnia symptoms and mortality: A prospective study of US men. Circulation, 129(7), 737–746. 10.1161/CIRCULATIONAHA.113.004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS, Curtis AF, Williams JM, Dautovich ND, McNamara JP, Stripling A, Dzierzewski JM, Chan WS, Berry RB, McCoy KJ, & Marsiske M (2018). Efficacy of brief behavioral treatment for insomnia in older adults: Examination of sleep, mood, and cognitive outcomes. Sleep Medicine, 51, 153–166. 10.1016/j.sleep.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS, Curtis AF, Williams JM, Dautovich ND, McNamara JP, Stripling A, Dzierzewski JM, Berry RB, McCoy KM, & Marsiske M (2020). Effects of brief behavioral treatment for insomnia on daily associations between self-reported sleep and objective cognitive performance in older adults. Behavioral Sleep Medicine, 18(5), 577–588. 10.1080/15402002.2019.1632201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, & Stewart LA (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Review, 4(1):1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM (2010). Chronic insomnia: Recent advances and innovations in treatment developments and dissemination. Canadian Psychology, 51(1), 31. 10.1037/a0018715 [DOI] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, & Lichstein KL (2006). Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep, 29(11), 1398–1414. 10.1093/sleep/29.11.1398 [DOI] [PubMed] [Google Scholar]
- Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, & Spiegelhalder K (2015). Insomnia disorder. Nature Reviews Disease Primers, 1(1), 1–18. 10.1038/nrdp.2015.26 [DOI] [PubMed] [Google Scholar]
- Morin CM, & Benca R (2012). Chronic insomnia. Lancet, 379(9821), 1129–1141. 10.1016/S0140-6736(11)60750-2 [DOI] [PubMed] [Google Scholar]
- Okajima I, Akitomi J, Kajiyama I, Ishii M, Murakami H, & Yamaguchi M (2020). Effects of a tailored brief behavioral therapy application on insomnia severity and social disabilities among workers with insomnia in Japan: A randomized clinical trial. JAMA Network Open, 3(4), e202775–e202775. 10.1001/jamanetworkopen.2020.2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen S, Nordhus IH, Kvale G, Nielsen GH, Havik OE, Johnsen BH, & Skjøtskift S (2003). Behavioral treatment of insomnia in older adults: An open clinical trial comparing two interventions. Behaviour Research and Therapy, 41(1), 31–48. 10.1016/S0005-7967(01)00122-X [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Vasquez MM, Halonen M, Bootzin R, Quan SF, Martinez FD, & Guerra S (2015). Persistent insomnia is associated with mortality risk. American Journal of Medicine, 128(3), 268–275. 10.1016/j.amjmed.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, & Denberg TD (2016). Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 165(2), 125–133. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, Espie CA, Garcia-Borreguero D, Gjerstad M, Gonçalves M, & Spiegelhalder K (2017). European guideline for the diagnosis and treatment of insomnia. Journal of Sleep Research, 26(6), 675–700. 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- Savard J, Laroche L, Simard S, Ivers H, & Morin CM (2003). Chronic insomnia and immune functioning. Psychosomatic Medicine, 65(2), 211–221. 10.1097/01.PSY.0000033126.22740.F3 [DOI] [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, & Thorpy MJ (1987). Treatment of chronic insomnia by restriction of time in bed. Sleep, 10(1), 45–56. 10.1093/sleep/10.1.45 [DOI] [PubMed] [Google Scholar]
- Spira AP, Kaufmann CN, Kasper JD, Ohayon MM, Rebok GW, Skidmore E, Parisi JM, & Reynolds IIICF (2014). Association between insomnia symptoms and functional status in US older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 69(7), S35–S41. 10.1093/geronb/gbu116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral M, Von Korff M, McCurry SM, Morin CM, & Vitiello MV (2020). Changes in dysfunctional beliefs about sleep after cognitive behavioral therapy for insomnia: A systematic literature review and meta-analysis. Sleep Medicine Reviews, 49, 101230. 10.1016/j.smrv.2019.101230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, & Cunnington D (2015). Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Annals of Internal Medicine, 163(3), 191–204. 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- Troxel WM, Germain A, & Buysse DJ (2012). Clinical management of insomnia with brief behavioral treatment (BBTI). Behavioral Sleep Medicine, 10(4), 266–279. 10.1080/15402002.2011.607200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, & Lancee J (2018). Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews, 38, 3–16. 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wu X, Zhong Z, & Li G (2016). Brief behavioral treatment for patients with treatment-resistant insomnia. Neuropsychiatric Disease and Treatment, 12, 1967. 10.2147/NDT.S110571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle-Pinkston S, Slavish DC, & Taylor DJ (2019). Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Medicine Reviews, 48, 101205. 10.1016/j.smrv.2019.07.008 [DOI] [PubMed] [Google Scholar]
- Watanabe N, Furukawa TA, Shimodera S, Morokuma I, Katsuki F, Fujita H, Sasaki M, Kawamura C, & Perlis ML (2011). Brief behavioral therapy for refractory insomnia in residual depression: An assessor-blind, randomized controlled trial. Journal of Clinical Psychiatry, 72(12), 1651–1658. 10.4088/JCP.10m06130gry [DOI] [PubMed] [Google Scholar]
- West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, & Lux L (2002). Systems to rate the strength of scientific evidence. AHRQ Evidence Report Summaries, AHRQ Publication No. 02-E015. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK11930/. [PMC free article] [PubMed] [Google Scholar]
- Winkelman JW (2015). Insomnia disorder. New England Journal of Medicine, 373(15), 1437–1444. 10.1056/NEJMcp1412740 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material 1. List of articles that were considered but excluded with reasons
Supplemental Material 2. Characteristics of brief behavioral treatment for insomnia (BBTI) programs
Supplemental Material 3. Effect sizes
Supplemental Material 4. Secondary outcomes