Abstract
Chestnut inner shell was fermented in solid state with Aspergillus sojae, and then extracted using ethanol (95%) to analyze its cosmeceutical activity and phenolic composition. The fermentation significantly increased the antioxidant activity, and in vitro cosmeceutical activities. The ethanol extract showed the higher activities than ethyl acetate and water extracts. DPPH radical scavenging activity of the alcoholic extract was 80.53%, and tyrosinase and elastase inhibition activities were 101.01%, and 76.73%, respectively, after 10 days of fermentation. Kojic acid, a secondary metabolite of A. sojae was produced by the fermentation as a major bioactive component. Gallic acid, ellagic acid, and coumaric acid appeared the major phenolic acids in the alcoholic extract from fermented chestnut inner shell. The alcoholic extract from chestnut inner shell fermented by A. sojae may be used as an effective and bioactive cosmeceutical.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01044-9.
Keywords: Chestnut inner shell, Aspergillus sojae, Fermentation, Antioxidant activity, Phenolic acid
Introduction
Chestnut (Castanea crenata S. et Z) trees are widely distributed in the Northern Hemisphere, and its fruit is consumed as food, mostly in East Asia including China, Korea, and Japan. Chestnut fruit is covered with multiple shells which are removed during peeling process. Those shells consist of hard outer shell and soft inner shell, and the inner shell contains a large quantity of natural polyphenols such as tannins and catechins (Vázquez et al., 2008). In general, natural polyphenols have antioxidant activity with health-promoting effect, and thus could be used as a nutraceutical to prevent various degenerative diseases such as cancer, diabetes, and metabolic syndromes. The polyphenols are also considered as effective ingredients in retarding skin aging as anti-melanogenic, and antioxidation activities (Roh et al., 2017).
The phenolic acids in chestnut inner shell were reported to inhibit or eliminate the formation of cell-damaging free radicals (Kwak et al., 2013). The antioxidant capacity may help to delay the aging process of skin which results from both intrinsic oxidative deterioration and extrinsic factors typically through UV irradiation. In the aging process, several enzymes including tyrosinase, elastase, hyaluronidase, and matrix metalloproteinase I involve. It is known that tyrosinase is the key enzyme in the formation of melanin (eumelanin and pheomelanin) through the oxidation of tyrosine, and dihydroxyphenylalanine. Elastase is a proteolytic enzyme that causes the degradation of skin tissues through the breakdown of elastin which bundles collagen and maintains the elasticity of skin. Therefore, the elastin degradation leads to the formation of wrinkles (Desmiaty et al., 2020). Various phenolic acids in plants such as cinnamic acids, gallic acids, and ferulic acids have the inhibitory activities against tyrosinase and elastase. It was also known that those phenolic acids may act in synergic manner on those inhibitory effects (Yu et al., 2019). The inhibitors against those enzymes in plant tissues may be considered as potent cosmeceuticals which are effective in retarding the skin aging.
Fermentation is one of the bioconversion processes used to enhance the bioactivity of natural products, either by enhancing the extractability of inherent active components or by producing the secondary metabolites. Solid-state fermentation has been used in food processing, which proceeds with limited amount of free water, and is suitable for fungal fermentation. It costs less and more convenient in downstream process for industrial utilization of natural products than liquid fermentation. Various fungi have been used in solid-state fermentation for foods, and thus are categorized as the food ingredients of generally recognized as safe (GRAS).
Aspergillus sojae is a fungal strain which is used for the preparation of soy sauces and pastes, which is classified as safe as a GRAS ingredient in foods (Kim et al., 2017). It is capable of hydrolyzing various food compounds through enzymatic actions from amylase, protease, and xylanase during the fermentation (Szendefy et al., 2006). In addition, kojic acid, one of the secondary metabolites of Aspergillus strains, is used as a key ingredient in functional cosmetics as antioxidant and tyrosinase inhibitor (Terabayashi et al., 2010). No study has been carried out on the fermentation of chestnut inner shell using Aspergillus strains for the utilization of chestnut by-product as a cosmeceutical.
In this study, chestnut inner shell was fermented using Aspergillus sojae, which had been chosen through a preliminary study among various GRAS Aspergillus strains, and then extracted using different solvents including ethanol, ethyl acetate, and water. The antioxidant and in vitro cosmeceutical activities, as well as phenolic composition in the extracts were analyzed.
Materials and methods
Materials
Chestnut inner shell was purchased from Jangmung Food Co. (Seoul, Korea). Aspergillus sojae KCCM 60,354 was purchased from the Korean Culture Center of Microorganisms (Seoul, Korea), and the spores were prepared by culturing the fungus on potato dextrose agar plates. The chemical and standard compounds were of analytical grade and were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Fermentation and extraction
The chestnut inner shell was mixed with water at a ratio of 1:1 (w/v), and the mixture was sterilized by autoclaving (121 °C for 20 min). The sterilized chestnut inner shell was mixed with fresh A. sojae spores (1% w/w) and the mixture was incubated at 25 °C for 14 days. The samples were occasionally collected during the fermentation and then extracted using 95% ethanol (1:10 w/v ratio). In a preliminary study, ethyl acetate and boiling water were also tested to compare with ethanol, but the extracts showed the lower antioxidant and cosmeceutical activities than the alcoholic extract. The dispersions were shaken at 100 rpm and 25 °C for 24 h for extraction. The dispersions were then filtered through Whatman No. 1 filter paper and centrifuged at 2,600 ×g for 15 min at ambient temperature, and then the supernatant was collected. These extraction processes were repeated twice, and the collected supernatants were evaporated to dryness. The dried extracts were then re-dissolved in 50% ethanol for the analysis in a ratio of 1:200 (w/v).
Total phenolic content
Total phenolic content (TPC) was evaluated using the method proposed by Ainsworth and Gillespie (2007). The dissolved extract sample was mixed with 10% (v/v) Folin–Ciocalteu reagent and 700 mM Na2CO3 in a volume ratio of 1:2:8. The reaction mixture was incubated at 25 °C for 2 h. The TPC was calculated by measuring the absorbance of the mixture at 765 nm using a standard curve with gallic acid.
Total flavonoid content
Total flavonoid content (TFC) was determined using the method introduced by Ritthibut et al. (2021). The extract sample (600 µL) was mixed with 2% aluminum chloride (600 µL). The reaction mixture was incubated at 25 °C for 30 min, and its absorbance was measured at 420 nm. The TFC was calculated using the regressive equation with quercetin as the standard.
DPPH radical scavenging activity
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was determined following the method of Ritthibut et al. (2021). A reaction mixture containing 50 µL of the extract sample, 450 µL of 100 mM Tris-HCl buffer (pH 7.4), and 1 mL of 0.1 mM DPPH solution in methanol was prepared. Then, the mixture was incubated at 25 °C for 30 min in the dark. The absorbance of the mixture was measured at 517 nm using a spectrophotometer.
Tyrosinase inhibition activity
Tyrosinase inhibition activity was evaluated using the method introduced by Ritthibut et al. (2021). A reaction mixture containing the sample dissolved in 5% DMSO, 50 mM potassium phosphate buffer (P-buffer) of pH 6.5, and tyrosinase (240 U/mL) in P-buffer in a ratio of 1:2:1 was prepared. The mixture was incubated at 25 °C for 10 min. Then, 0.85 mM l− 3,4-dihydroxyphenylalanine (l-DOPA; reaction mixture:l-DOPA = 4:1) was added to the mixture. This mixture was incubated at 25 °C for 5 min, and its absorbance was measured at 490 nm using a microplate spectrophotometer (Bio-Tek). The tyrosinase inhibition activity (%) was calculated using following formula. Tyrosinase inhibition activity (%) = [{(AE − AB) – (ASE − AS)}/ (AE − AB)] × 100; AE, AB, ASE, and AS mean absorbance of blank with sample, absorbance of blank without sample, absorbance of sample with enzyme, and absorbance of sample without enzyme, respectively.
Elastase inhibition activity
Elastase inhibition activity was determined using the method introduced by Ritthibut et al. (2021). A reaction mixture containing 30 µL of the sample, 100 µL of 2 mM Tris buffer pH 8.0, and 10 µL of elastase (1.1 U/mL) in Tris buffer was prepared. This mixture was incubated at 25 °C for 10 min. Then, 40 µL of 3 mg/mL N-Succ-Ala-Ala-Ala-p-nitroanilide was added to this mixture. This mixture was incubated at 25 °C for 20 min, and its absorbance was measured at 410 nm using a microplate spectrophotometer (Bio-Tek). The elastase inhibition activity (%) was calculated using following formula. Elastase inhibition activity (%) = [{(AE - AB) – (ASE – AS)}/ (AE - AB)] × 100; AE, AB, ASE, and AS mean absorbance of blank with sample, absorbance of blank without sample, absorbance of sample with enzyme, and absorbance of sample without enzyme, respectively.
Determination of activity by solvent
The sterilized chestnut inner shell was mixed with fresh A. sojae spores (1% w/w) and the mixture was incubated at 25 °C for 10 days. The chestnut inner shell fermented for 10 days were extracted using ethyl acetate, boiling water, and 95% ethanol (1:10 w/v ratio). The dispersions were shaken at 100 rpm and 25 °C for 24 h for extraction. The dispersions were then filtered through Whatman No. 1 filter paper and centrifuged at 2,600 ×g for 15 min at ambient temperature, and then the supernatant was collected. These extraction processes were repeated twice, and the collected supernatants were evaporated to dryness. DPPH radical scavenging activity, tyrosinase inhibition activity, and elastase inhibition activity were measured using the dried extract.
Determination of kojic acid
Kojic acid content was determined using a high-performance liquid chromatography (HPLC, DIONEX UltiMate 3000, Thermo Scientific, Sunnyvale, CA, USA) with a reverse-phase AcclaimTM 120 C18 column (5 μm, 4.6 × 250 mm) and a UV detector (270 nm). A gradient of 1% acetic acid (solvent A) and acetonitrile (solvent B) was used as the mobile phase. Gradient elution was performed from 0 to 10 min using 10–60% solvent B, 10 to 14 min using 60–90% B, 14 to 20 min using 90% B, and 20 to 26 min using 10% B. The flow rate was 1.0 mL/min, and the temperature was set at 30 °C. Kojic acid was used as the standard.
Phenolic acid composition
Phenolic acids present in the chestnut inner shell extracts were analyzed using a HPLC with a reverse-phase AcclaimTM 120 C18 column. The mobile phase contained 0.02% trifluoroacetic acid in methanol (solution A) and 0.02% trifluoroacetic acid in water (solution B) and was eluted in gradation. Gradient elution was performed from 0 to 30 min using 90–85% B, 30 to 45 min using 85–75% B, 45 to 60 min using 75–50% B, 60 to 65 min using 50–10% B, 65 to 75 min using 10% B, and 75 to 90 min using 90% B. The flow rate was set to 1.0 mL/min, and the column temperature was set at 40 °C. Different wavelengths were detected depending on the phenolic acids. Gallic acid and ellagic acid were detected at 280 nm. Coumaric acid, ferulic acid, and sinapic acid were detected at 320 nm.
Statistical analysis
All data were obtained in triplicates. Statistical analyses were evaluated using the SPSS software program (Statistics version 25, IBM Corp., Armonk, NY, USA) for one way analysis of variation test under Duncan’s multiple range test (p < 0.05).
Results and discussion
Total phenolic and flavonoid contents
The total phenolic and flavonoid contents (TPC and TFC, respectively) of the alcoholic extract from the fermented chestnut inner shells are shown in Fig. 1. The fermentation induced gradual increases in TPC and TFC. The increased in TPC appeared more significant than that in TFC. TPC showed the largest increase from 8 to 10 days of fermentation, and then showed a tendency to decrease slightly. TFC showed the greatest increase on the 8 and 14 days of fermentation but decreased more than TPC before or after the 8 days. The residual amount of flavonoids in the extracts was much less than that of phenolics, and its change during the fermentation was relatively minor. Phenolic components in chestnut inner shells are mainly tannins (Ham et al., 2015), which might be readily released through the hydrolytic action of the enzymes released from fungi (Kimura et al., 1999). From lentils too, residual phenolic compounds could be readily released by a fungal fermentation (Magro and de Castro, 2020). In addition, the release of phenolic and flavonoid compounds from chestnut inner shells might depend on the polarity of solvent used for extraction (Dai and Mumper, 2010). Ham et al. (2015) reported that alcohol was effective for the extraction of polyphenols from various plant tissues. However, phenolic compounds may be oxidized by polyphenol oxidase during fermentation (Haile and Kang, 2019). It is also possible that the phenolics are consumed as carbon source by the fungi for growth (Ademiluyi and Oboh, 2011). As shown in Fig. 1, the total phenolic and flavonoid contents were decreased as the fermentation proceeded over 8 days, indicating the possible consumption by the fungi.
Fig. 1.
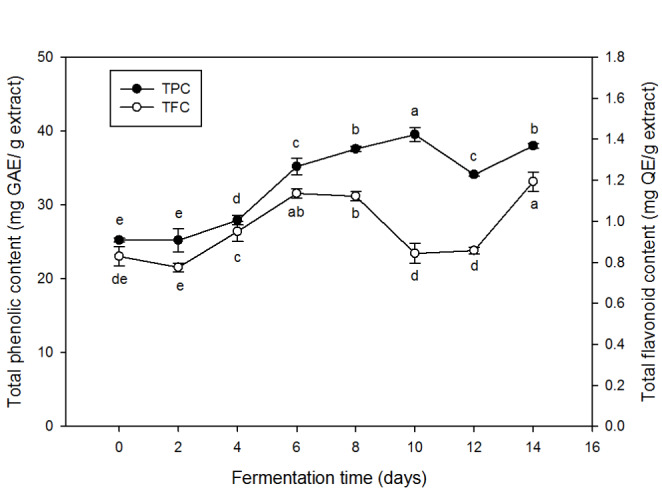
Total phenolic (TPC) and total flavonoid contents (TFC) in alcoholic extracts (95% ethanol) from chestnut inner shell fermented with A. sojae for 14 days. Values are averages of triplicates, and different alphabets indicate difference in statistical significance (p < 0.05)
DPPH radical scavenging activity
DPPH radical scavenging activity of the chestnut shell extracts at different periods of fermentation is shown in Fig. 2. The DPPH radical scavenging activity of the chestnut inner shell extracts appeared positively related to the total phenolic content (Figs. 1 and 2). As the TPC increased, the radical scavenging activity was increased by the fermentation. The activity reached to the maximum (84%) on the eighth day of fermentation but decreased afterward. The loss of phenolics in the late stage of fermentation might be responsible for the decrease in the activity. The positive relation between TPC and radical scavenging activity proved that the phenolic compounds were the key substances responsible for the antioxidant activity of the fermented chestnut inner shell extracts (Zhu et al., 2020). Reactive oxygen species (ROS) are the common initiator causing the cellular damages and skin aging. Various phenolic compounds in natural plants have antioxidant activity by scavenging the ROS (Adegbola et al., 2020). Numerous studies have reported that the plant extracts such as pomegranate peel, mango peel, and green tea exhibit radical scavenging activity, resulting in protective effects against cellular damage (Lee et al. 2020).
Fig. 2.
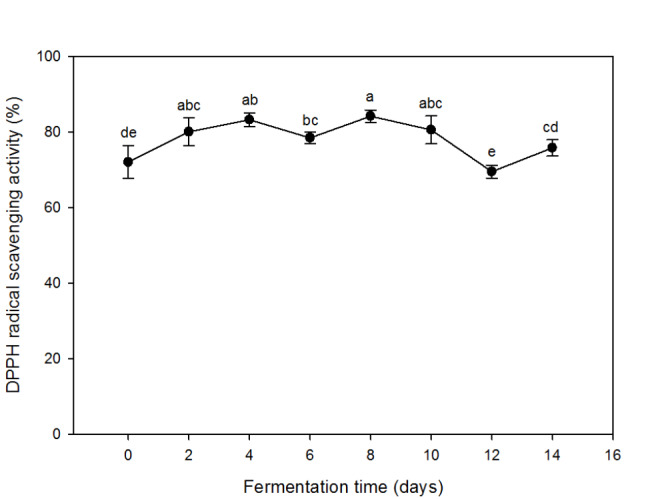
DPPH radical scavenging activity of alcoholic extracts (95% ethanol) from chestnut inner shell fermented with A. sojae for 14 days. Values are averages of triplicates, and different alphabets indicate difference in statistical significance (p < 0.05)
Overall data revealed that the solid state fermentation with A. sojae used in this study induced the release of phenolic components from chestnut inner shell, contributing the increase in radical scavenging activity. However, extensive fermentation over 8 days in this case might cause the loss of phenolics resulting the decrease in antioxidant activity.
Tyrosinase and elastase inhibition activities
Tyrosinase and elastase inhibition activities of the chestnut inner shell extracts at different periods of fermentation are shown in Fig. 3. Native chestnut inner shell itself was highly active in tyrosinase inhibition (53%). The fungal fermentation with A. sojae raised the activity, reaching to its maximum (over 100%) after 10 days of fermentation (Fig. 3), which was double of the activity of native chestnut inner shell extract. The change in tyrosinase inhibition activity during the fermentation appeared being related positively to the total phenolic content (Figs. 1 and 3). Among the phenolic compounds in natural plant tissues, gallic acid and ellagic acid are known to have the major tyrosinase inhibitors. In addition, isoeugenol, 4-hydroxybenxyl alcohol, pyrogallol, cinnamic acid, and hydroxystilbenes have been reported as tyrosinase inhibitors (Garcia-Jimenez et al., 2018). Not only those phenolic compounds, but some of the amino acids and their derivatives were reported to act as tyrosinase inhibitors (Panzella and Napolitano, 2019). It was observed that there was a positive correlation between antioxidant activity and tyrosinase inhibition activity (Figs. 2 and 3).
Fig. 3.
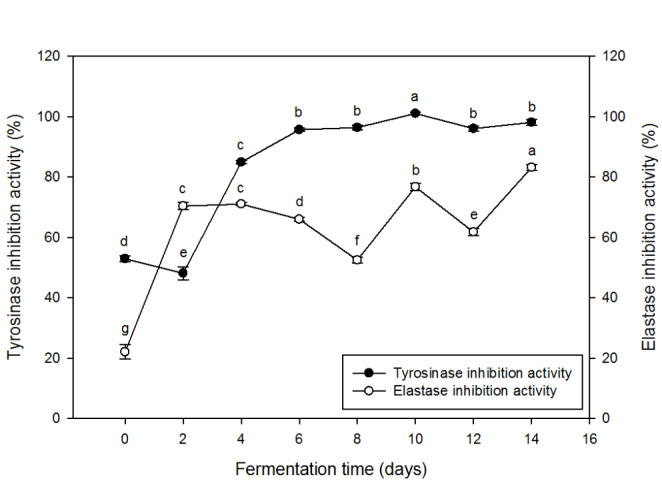
Tyrosinase inhibition and elastase inhibition activities of alcoholic extracts (95% ethanol) from chestnut inner shell fermented with A. sojae for 14 days. Values are averages of triplicates, and different alphabets indicate difference in statistical significance (p < 0.05)
The fermentation with A. sojae induced a positive result in elastase inhibition too (Fig. 3). Compared to the native sample (23% activity), the fermented chestnut inner shell showed the activity higher than 50%. It was also reported that the fungi of the genera Rhizopus and Aspergillus enhanced the elastase inhibitory activity of fermented rice (Abd Razak et al., 2019). It was noteworthy that the activity increase was substantial in the early stage of fermentation, and the increase afterward appeared relatively minor. This trend was somewhat different from that observed for tyrosinase inhibition activity. Gallic acid which is abundant in chestnut shells is one of the phenolic components having a strong inhibition activity against elastase. However, some of the non-phenolic components released from the chestnut inner shell through microbial fermentation might have the activity for elastase inhibition. Certain peptides found in plant tissues were reported potent in the inhibition of elastase activity (Ahmad et al., 2020). Additional study is needed to understand the key substances for elastase inhibition in the fermented chestnut shell. Therefore, it was confirmed that the alcoholic extract from the chestnut inner shell fermented for 10 days had a strong activity for the inhibition against tyrosinase and elastase.
Effect of solvents on activities
Ethyl acetate and boiling water were compared with ethanol as extraction solvents. As shown in Fig. 4, ethanol appeared more effective than ethyl acetate and water in extracting the antioxidants from the fermented chestnut inner shell (80.5%). The ethyl acetate extract showed a slight decrease in the antioxidant activity after the fermentation. After 10 days of the fungal fermentation, the ethanol extract showed the complete inhibition to tyrosinase, and near 80% of inhibition to elastase. Most phenolic components in plant tissues are readily extractable in alcohols. In general, it has been reported that antioxidants such as phenolic acid can be extracted from plant tissues using various solvents including alcohol, ethyl acetate and other organic solvents (Dzah et al., 2020). Among them, ethanol extract is the most effective for extracting antioxidants in plants. In a preliminary study, it was confirmed that total phenolic and flavonoid contents were higher in alcohol than those in ethyl acetate and water (supplementary Tables 1 and 2). Previous studies have also confirmed that ethanol extract from fermented rice bran is suitable for extracting bioactive substances (Ritthibut et al., 2021). It was noteworthy that the increases in enzyme inhibitory activity were much higher than the increases in radical scavenging activity (Fig. 4). The effectiveness of alcoholic extraction approved that the phenolics were the major bioactive compounds in the fermented chestnut inner shell.
Fig. 4.
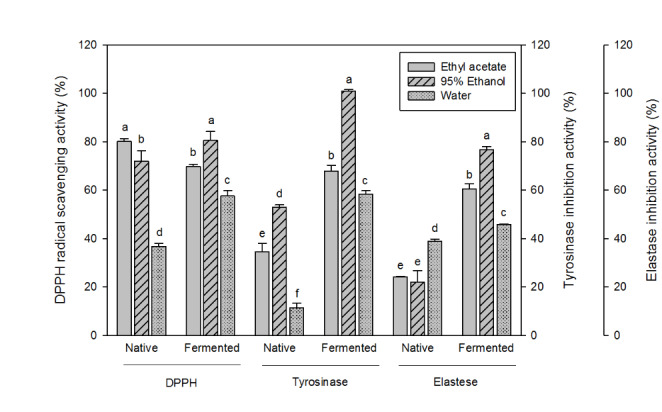
DPPH radical scavenging activity, tyrosinase inhibition activity, and elastase inhibition activity of alcoholic extracts from native and fermented (10 days) chestnut inner shells. Values are averages of triplicates, and different alphabets indicate difference in statistical significance (p < 0.05)
Phenolic acid and kojic acid contents
The amounts of phenolic and kojic acids in the alcoholic extract from fermented chestnut inner shell are shown in Table 1. Native chestnut inner shell was absent of kojic acid, but the fermented chestnut inner shell (10 days) contained approximately 50 mg/g in the extract, indicating that the kojic acid was produced as a fungal metabolite. The kojic acid in the fermented chestnut inner shell contributed the increase in tyrosinase inhibition activity. Several studies were carried out on the production of kojic acid by fermentation using various plant materials such as corn stalk, sugarcane molasses, and sugarcane bagasse with Aspergillus strains (Shehata and Sabry, 2020). A recent study reported that A. sojae was able to produce kojic acid from rice husk too (Chib et al., 2019).
Table 1.
Amounts of kojic acid and phenolic acids* in the ethanol extract (95%) from native and fermented (10 days) chestnut inner shells
| Sample | Kojic acid | Gallic acid | Ellagic acid | Coumaric acid | Ferulic acid | Sinapic acid |
|---|---|---|---|---|---|---|
| (mg/g extract) | (µg/g extract) | (µg/g extract) | (µg/g extract) | (µg/g extract) | (µg/g extract) | |
| Native | ND** | 1389.75 ± 25.96b | ND | ND | 71.03 ± 4.14a | 24.99 ± 4.01b |
| Fermented | 49.97 ± 0.12a | 5616.22 ± 23.99a | 330.17 ± 9.61a | 21.34 ± 0.05a | 71.08 ± 0.03a | 145.65 ± 0.08a |
*Values are averages of three replicates, and the different letters indicate difference in statistical significance (p < 0.05)
**ND: Not detected
The amount of phenolic acids (gallic acid, ellagic acid, coumaric acid, ferulic acid, and sinapic acid) in the alcohol extract from chestnut inner shell was also increased by the fungal fermentation (Table 1). Gallic acid and ellagic acid appeared to be the major phenolic acids in native chestnut inner shell, and their amounts in the extract were substantially increased by the fermentation. The ellagic and coumaric acids which were absent in the extract from native chestnut inner shell were produced by the fermentation. Those phenolic acids existed in native shell but became extractable by the fermentation. The amounts of ferulic and sinapic acids in native chestnut shell were relatively minor. It was noteworthy that the amount of ferulic acid was not changed by the fermentation whereas the level of sinapic acid was increased. In the chestnut inner shell, gallic acid, coumaric acid, ferulic acid, and sinapic acid, were supposed to have strong antioxidant and tyrosinase inhibition activities (Kwak et al., 2013). Ozer et al. (2018) reported that coumaric acid also exhibited antioxidant activity and tyrosinase inhibition activity. In Rubus rosifolius leaf extract, ellagic acid was reported as the key compound to inhibit the elastase activity (Desmiaty et al., 2020). Various plant tissues including fruits, leaves, barks, roots, and stems contain the substances exhibiting tyrosinase and elastase inhibition activities (Opperman et al., 2020). The bioactivity of the individual phenolic acids found in plant tissues may not be identical. Additional study is needed to analyze the activity of the residual phenolic acids in the fermented chestnut inner shells.
Fermentation using different microorganisms has been performed to increase the extractability and bioactivity of the phenolic compounds in plant tissues. Wu et al. (2018) reported an increase in the tyrosinase inhibition activity with the extract of Magnolia officinalis fermented with A. niger. Chang et al. (2007) reported that the isoflavones isolated from soybean germ acted as tyrosinase inhibitor after a fermentation with A. oryzae. Different plant tissues were fermented by Lactobacillus buchneri to raise the elastase inhibition activity (Kang et al., 2020). Furthermore, chestnut inner shell contains tannin, which is composed of gallic acid and flavanol, including catechin, epichatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, and thearubigins (Kim et al., 2004). Through the solid-state fermentation, the phenolic components were readily extracted from the chestnut shell. It was supposed that the antioxidant activity was mainly from the phenolic compounds, but some of non-phenolic compounds might involve in the inhibition of enzyme activity. Some organic acids and peptides originated from the fermented plant tissues were reported to have the inhibitory activity against tyrosinase (Parvez et al., 2007).
The phenolic acids in plant tissues normally exist in bound forms with other materials forming insoluble matrices (Aguilar-Hernández et al., 2017). Possibly, microbial fermentation allows those phenolic acids to release through the action of various enzymes. The release of phenolic acids from the shell was highly dependent on the specificity of enzymes produced by fungus (Table 1). Overall data confirmed that the total amount of phenolic acids in the alcoholic extract was greatly increased by the fungal fermentation with A. sojae (Fig. 4). It was also found that the alcohol was effective in extracting both kojic and phenolic acids from the fermented chestnut shell. As one of the fungal metabolites, typically when Aspergillus strains were used, kojic acid might function as one of the major cosmeceuticals.
Finally, chestnut inner shell could be used as a source of cosmeceutical compounds which may become extractible through fungal fermentation. A solid state fermentation using A. sojae significantly increased the extractable amounts of phenolic compounds including ellagic acid and gallic acid from chestnut inner shell. Not only the phenolic acids, kojic acid as a key substance inhibiting tyrosinase was produced as a fungal metabolite. Alcohols appeared proper solvents to extract the phenolic compounds from the fermented chestnut inner shell. The alcoholic extract had strong antioxidant activity, and inhibitory activity against tyrosinase and elastase. The fungal fermentation may be used to enhance the cosmeceutical activity of various plant tissues.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [grant number NRF-2021R1A6A3A01086642] and a Korea University grant.
Declarations
Conflict of interest
The authors disclose the following: Ritthibut, Oh, and Lim declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nuntinee Ritthibut, Email: ma_mueyly@naver.com.
Seung-Taik Lim, Email: limst@korea.ac.kr.
Su-Jin Oh, Email: skyohsujin@gmail.com.
References
- Abd Razak DL, Jamaluddin A, Abd Rashid NY, Ghani Abd, Manan MA. Assessment of fermented broken rice extracts for their potential as functional ingredients in cosmeceutical products. Annals of Agricultural Sciences. 2019;64(2):176–182. doi: 10.1016/j.aoas.2019.11.003. [DOI] [Google Scholar]
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nature protocols. 2007;2(4):875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Adegbola PI, Adetutu A, Olaniyi TD. Antioxidant activity of Amaranthus species from the Amaranthaceae family–A review. South African Journal of Botany. 2020;133:111–117. doi: 10.1016/j.sajb.2020.07.003. [DOI] [Google Scholar]
- Ademiluyi AO, Oboh G. Antioxidant properties of condiment produced from fermented bambara groundnut (Vigna subterranea L. Verdc) Journal of Food Biochemistry. 2011;35(4):1145–1160. doi: 10.1111/j.1745-4514.2010.00441.x. [DOI] [Google Scholar]
- Aguilar-Hernández I, Afseth NK, López-Luke T, Contreras-Torres FF, Wold JP. Ornelas-Soto, N. Surface enhanced Raman spectroscopy of phenolic antioxidants: A systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vibrational Spectroscopy. 2017;89:113–122. doi: 10.1016/j.vibspec.2017.02.002. [DOI] [Google Scholar]
- Ahmad S, Saleem M, Riaz N, Lee YS, Diri R, Noor A, Elsebai MF. The Natural Polypeptides as Significant Elastase Inhibitors. Frontiers in Pharmacology. 2020;11:688. doi: 10.3389/fphar.2020.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TS, Ding HY, Tai SSK, Wu CY. Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food chemistry. 2007;105(4):1430–1438. doi: 10.1016/j.foodchem.2007.05.019. [DOI] [Google Scholar]
- Chib S, Dogra A, Nandi U, Saran S. Consistent production of kojic acid from Aspergillus sojae SSC-3 isolated from rice husk. Molecular biology reports. 2019;46(6):5995–6002. doi: 10.1007/s11033-019-05035-8. [DOI] [PubMed] [Google Scholar]
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmiaty Y, Mulatsari E, Saputri FC, Hanafi M, Prastiwi R, Elya B. Inhibition of pancreatic elastase in silico and in vitro by Rubus rosifolius leaves extract and its constituents. Journal of Pharmacy And Bioallied Sciences. 2020;12(3):317. doi: 10.4103/jpbs.JPBS_271_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzah CS, Duan Y, Zhang H, Boateng NAS, Ma H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends in Food Science & Technology. 2020;99:375–388. doi: 10.1016/j.tifs.2020.03.003. [DOI] [Google Scholar]
- Garcia-Jimenez A, García-Molina F, Teruel-Puche JA, Saura-Sanmartin A, Garcia-Ruiz PA, Ortiz-Lopez A, Munoz-Munoz J. Catalysis and inhibition of tyrosinase in the presence of cinnamic acid and some of its derivatives. International journal of biological macromolecules. 2018;119:548–554. doi: 10.1016/j.ijbiomac.2018.07.173. [DOI] [PubMed] [Google Scholar]
- Haile M, Kang WH. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation. 2019;5(1):29. doi: 10.3390/fermentation5010029. [DOI] [Google Scholar]
- Ham JS, Kim HY, Lim ST. Antioxidant and deodorizing activities of phenolic components in chestnut inner shell extracts. Industrial Crops and Products. 2015;73:99–105. doi: 10.1016/j.indcrop.2015.04.017. [DOI] [Google Scholar]
- Kang YM, Hong CH, Kang SH, Seo DS, Kim SO, Lee HY, An HJ. Anti-Photoaging Effect of Plant Extract Fermented with Lactobacillus buchneri on CCD-986sk Fibroblasts and HaCaT Keratinocytes. Journal of Functional Biomaterials. 2020;11(1):3. doi: 10.3390/jfb11010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Uyama H, Kobayashi S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochemical and biophysical research communications. 2004;320(1):256–261. doi: 10.1016/j.bbrc.2004.05.163. [DOI] [PubMed] [Google Scholar]
- Kimura I, Yoshioka N, Tajima S. Purification and characterization of a β-glucosidase with β-xylosidase activity from Aspergillus sojae. Journal of bioscience and bioengineering. 1999;87(4):538–541. doi: 10.1016/S1389-1723(99)80107-8. [DOI] [PubMed] [Google Scholar]
- Kim KM, Lim J, Lee JJ, Hurh BS, Lee I. Characterization of Aspergillus sojae isolated from Meju, Korean traditional fermented soybean brick. Journal of microbiology and biotechnology. 2017;27(2):251–261. doi: 10.4014/jmb.1610.10013. [DOI] [PubMed] [Google Scholar]
- Kwak SY, Yang JK, Choi HR, Park KC, Kim YB, Lee YS. Synthesis and dual biological effects of hydroxycinnamoyl phenylalanyl/prolyl hydroxamic acid derivatives as tyrosinase inhibitor and antioxidant. Bioorganic & medicinal chemistry letters. 2013;23(4):1136–1142. doi: 10.1016/j.bmcl.2012.10.107. [DOI] [PubMed] [Google Scholar]
- Lee SB, Choi EH, Jeong KH, Kim KS, Shim SM, Kim GH. Effect of catechins and high-temperature-processed green tea extract on scavenging reactive oxygen species and preventing Aβ1–42 fibrils’ formation in brain microvascular endothelium. Nutritional neuroscience. 2020;23(5):363–373. doi: 10.1080/1028415X.2018.1507618. [DOI] [PubMed] [Google Scholar]
- Magro AEA, de Castro RJS. Effects of solid-state fermentation and extraction solvents on the antioxidant properties of lentils. Biocatalysis and Agricultural Biotechnology. 2020;28:101753. doi: 10.1016/j.bcab.2020.101753. [DOI] [Google Scholar]
- Opperman L, De Kock M, Klaasen J, Rahiman F. Tyrosinase and Melanogenesis Inhibition by Indigenous African Plants: A Review. Cosmetics. 2020;7(3):60. doi: 10.3390/cosmetics7030060. [DOI] [Google Scholar]
- Ozer MS, Kirkan B, Sarikurkcu C, Cengiz M, Ceylan O, Atılgan N, Tepe B. Onosma heterophyllum: Phenolic composition, enzyme inhibitory and antioxidant activities. Industrial Crops and Products. 2018;111:179–184. doi: 10.1016/j.indcrop.2017.10.026. [DOI] [Google Scholar]
- Panzella L, Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics. 2019;6(4):57. doi: 10.3390/cosmetics6040057. [DOI] [Google Scholar]
- Parvez S, Kang M, Chung HS, Bae H. Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2007;21(9):805–816. doi: 10.1002/ptr.2184. [DOI] [PubMed] [Google Scholar]
- Ritthibut N, Oh SJ, Lim ST. Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT. 2021;135:110273. doi: 10.1016/j.lwt.2020.110273. [DOI] [Google Scholar]
- Roh E, Kim JE, Kwon JY, Park JS, Bode AM, Dong Z, Lee KW. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Critical reviews in food science and nutrition. 2017;57(8):1631–1637. doi: 10.1080/10408398.2014.1003365. [DOI] [PubMed] [Google Scholar]
- Shehata RM, Sabry SM. Biotechnological Production of Kojic Acid Synthesized by Endophytic Fungi, Aspergillus oryzae Isolated from Euphorbia peplis. Egyptian Academic Journal of Biological Sciences, G. Microbiology. 2020;12(2):35–47. doi: 10.21608/eajbsg.2020.107645. [DOI] [Google Scholar]
- Szendefy J, Szakacs G, Christopher L. Potential of solid-state fermentation enzymes of Aspergillus oryzae in biobleaching of paper pulp. Enzyme and microbial technology. 2006;39(6):1354–1360. doi: 10.1016/j.enzmictec.2006.06.016. [DOI] [Google Scholar]
- Terabayashi Y, Sano M, Yamane N, Marui J, Tamano K, Sagara J, Higa Y. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genetics and Biology. 2010;47(12):953–961. doi: 10.1016/j.fgb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Vázquez G, Fontenla E, Santos J, Freire MS, González-Álvarez J, Antorrena G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Industrial crops and products. 2008;28(3):279–285. doi: 10.1016/j.indcrop.2008.03.003. [DOI] [Google Scholar]
- Wu, L., Chen, C., Cheng, C., Dai, H., Ai, Y., Lin, C., & Chung, Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Research International, 2018. (2018). 10.1155/2018/5201786 [DOI] [PMC free article] [PubMed]
- Yu Q, Fan L, Duan Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food chemistry. 2019;297:124910. doi: 10.1016/j.foodchem.2019.05.184. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhang J, Li C, Liu S, Wang L. Morinda citrifolia L. leaves extracts obtained by traditional and eco-friendly extraction solvents: Relation between phenolic compositions and biological properties by multivariate analysis. Industrial Crops and Products. 2020;153:112586. doi: 10.1016/j.indcrop.2020.112586. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


