Abstract
Since octopuses are similar in appearance and can be processed into various forms, seafood fraud has been reported. In this study, we developed the PCR assay to simultaneously detect three octopuses (big blue octopus, giant Pacific octopus, and common octopus). Specific primer sets were designed based on COI gene. We observed that the specific PCR amplicon sizes were 84 bp for big blue octopus, 117 bp for giant Pacific octopus, and 166 bp for common octopus, respectively. This assay was then used to test for specificity and did not show cross-reactivity with 15 cephalopods families. The limit of detection of the multiplex PCR assay was 0.1 pg. Subsequently, 30 commercial food products were then monitored to evaluate the applicability of this assay. All products were specifically amplified, and three octopus species of interest were distinguished. Therefore, this assay can be used as an octopus authentication tool in the seafood industry.
Keywords: Big blue octopus, Giant Pacific octopus, Common octopus, Multiplex PCR, Species identification
Introduction
Seafood fraud is a global problem that is increasing yearly. Therefore, many related studies have been conducted on this issue in several countries (Donlan and Luque, 2019). Although seafood fraud has many forms, including species substitution, mislabeling of the species and their origin, in addition to adulteration, substituting high-value fish species with cheaper ones is one of the most common fraudulent activities in the fish and seafood sector (Hassoun et al., 2020). Thus, seafood authentication is important. Also, accurate and reliable methods for identifying seafood species are required.
Cephalopods consist of 28 families and fewer than 1000 species, many of which are commercially important marine resources (Chapela et al., 2003; Velasco et al., 2020). The major cephalopod-consuming countries are Japan, Korea, Argentina, Taiwan, China, Spain, Morocco, Mauritania, Greece, and Italy (Vaz-Pires et al., 2004). Octopus is a generic term for species belonging to the Octopods and is a valuable resource due to its high content of calcium, vitamins, and low calories (Espiñeira and Vieites, 2012). Among octopuses, the big blue octopus (Octopus cyanea), giant Pacific octopus (Enteroctopus dofleini), and common octopus (Octopus vulgaris) are the most common species in the Eastern Pacific, which makes them important as a fishery resource (Chande et al., 2021; Chapela et al., 2003; Kang et al., 2009; Lee et al., 2014; McKeown et al., 2018; Toussaint et al., 2012; Velasco et al., 2021). The identification methods for cephalopod species by morphological characteristics were based on teeth shape, sucker rings, number of sucker rows, and length and shape of fins and tails (Gebhardt and Knebelsberger, 2015; Sin et al., 2009). However, these species have similar morphological features, which makes them difficult to distinguish visually. Furthermore, identifying cephalopods through morphological characteristics can be inaccurate because sex, age, growth, sexual maturity, and environmental factors influence their features (Chapela et al., 2003).
As the processed seafood market gradually expands, several commercial octopus products have been identified worldwide, such as the frozen, cooked, and canned types. However, with processed foods, it is hard to identify through morphology because their morphological characteristics disappear during processing, which can lead to seafood fraud. In fact, species substitution of cephalopods is frequent, and some related cases have been reported (Espiñeira and Vieites, 2012; Santaclara et al., 2007). Therefore, to accurately identify octopus species and ensure correct labeling of commercial food products, specific, sensitive, and reliable detection methods are required.
To authenticate species, morphological, protein, and DNA-based assays have been used. Since protein is unstable during processing, such as when subjected to heat and dry treatments, protein-based assays are unsuitable for processed foods. In contrast, because DNA is more stable under heat and pressure, DNA-based assays can be applied to processed foods, and have therefore been widely used as analytical methods for reliable species identification (Wilwet et al., 2021). Among DNA-based methods, FINS (Forensically Informative Nucleotide Sequencing), PCR–RFLP (Restriction Fragment Length Polymorphism), DNA barcoding, and real-time PCR have been reported for cephalopod species identification (Espiñeira et al., 2010; Espiñeira and Vieites, 2012; Chapela et al., 2006; Shi and Huang, 2020; Sin et al., 2009; Ye et al., 2016). However, all these methods require technical complexity and are also time-consuming. Alternatively, studies have reported that the multiplex PCR method can detect several species simultaneously in a single reaction. It is also simple, fast, and low cost (Hou et al., 2015; Kim et al., 2018, 2021; Kim and Kim, 2017; Safdar and Junejo, 2015). Therefore, in this study, we developed an efficient and reliable multiplex PCR assay to identify three octopuses, using species-specific primer sets. Then, to confirm its applicability, this method was applied to commercial food products.
Methods/Experimental procedures
Samples
The National Institute of Food and Drug Safety Evaluation provided reference samples of big blue octopus (Octopus cyanea), giant Pacific octopus (Enteroctopus dofleini), and common octopus (Octopus vulgaris). Additionally, 14 non-target species, including the long arm octopus (Octopus minor), webfoot octopus (Octopus ocellatus), golden cuttlefish (Sepia esculenta), Japanese flying squid (Todarodes pacificus), Argentine shortfin squid (Illex argentinus), jumbo flying squid (Dosidicus gigas), diamondback squid (Thysanoteuthis rhombus), swordtip squid (Loligo edulis), spear squid (Loligo bleekeri), Japanese squid (Loliolus japonica), beka squid (Loliolus beka), and bigfin reef squid (Sepioteuthis lessoniana) were purchased online and from supermarkets in Korea. Afterward, all commercial products labeled as octopuses were collected from the market for monitoring. The commercial products used in this study were processed using various methods, and their origins were Korea, Philippine, Vietnam, Mauritania, Indonesia, and China. Finally, both raw and commercial samples were washed, cut, and stored at − 20 °C until experiment.
DNA extraction
Genomic DNA was extracted from 15 samples and 30 commercial products, using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), following the protocol described by the manufacturer. The extracted genomic DNA was used as template DNA. The purity and concentration of extracted DNA were then evaluated using a Maestro Nano spectrophotometer (Maestrogen, Las Vegas, NV, USA). Only DNA samples with a 260/280 nm ratio between 1.8 and 2.0 were used as the template for PCR.
Primer design
To design species-specific primers for each species, sequences of the mitochondrial cytochrome c oxidase subunit I (COI) gene of the 15 cephalopod species, including big blue octopus, giant Pacific octopus, and common octopus were downloaded from the GenBank database, following alignment using the Clustal Omega alignment system (http://www.ebi.ac.uk/Tools/msa/clustalo/). Primers were then designed using the Primer Designer Program version 3.0 (Scientific and Educational Software, Durham, NC, USA), after which primer synthesis was performed from Bionics (Seoul, Korea). Each primer sequence is shown in Table 1.
Table 1.
Sequence of primers used in this study
| Target species | Primer name | Sequence (5’ → 3’) | Target gene | Amplicon size (bp) | Accession No | Reference |
|---|---|---|---|---|---|---|
| Octopus cyanea | O.CYA F | CCG CAG TCG AAA GAG GTG TT | COIa | 84 | NC_039847.1 | This study |
| O.CYA R | CAA CAG ATG GTC CTA TAT GGG C | |||||
| Enteroctopus dofleini | E.DOF F | CAA TAC TAT CTA TTG GCC TTC | COI | 117 | MW092825.1 | This study |
| E.DOF R | GGA TAG CAA TAA TTA TAG TAG CA | |||||
| Octopus vulgaris | O.VUL F | GCA GGT ATT TCA TCA ATC CTT G | COI | 166 | NC_006353.1 | This study |
| O.VUL R | TAG TAA TTG CTC CAG CGA GT |
aCOI, cytochrome c oxidase subunit I
Simplex PCR
At the preliminary stage of this study, the specificity and sensitivity of developed primers were estimated. The specificity of species-specific primers was assessed with DNA extracted from the 15 cephalopod samples. Template DNA used was diluted to a concentration of 10 ng. Afterward, the sensitivity was evaluated using DNA that was serially diluted from 10 ng to 0.01 pg as a template.
Simplex PCR reactions were conducted in a 25 μL reaction mixture containing 10 × Buffer (Bioneer, Daejeon, Korea), 800 μM dNTPs (Bioneer), 0.5 unit Hot Start Taq DNA polymerase (Bioneer), 0.4 μM of each primer, and 10 ng template DNA. PCR conditions were 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s, with the final extension at 72 °C for 5 min, using a thermal cycler (Astec, Tokyo, Japan). Electrophoresis on 2% agarose gels (Sea-Kem, Rockland, ME) stained with ethidium bromide, using 0.5 × Tris–acetate EDTA (TAE) buffer for 15 min at 150 V was then used to analyze amplified PCR products.
Multiplex PCR
For multiplex PCR, various primer concentrations were tested and adjusted to optimize the PCR reaction. The specificity of the multiplex PCR was then checked using 10 ng DNA from the 15 cephalopods. The sensitivity was measured using a tenfold serially diluted template DNA from 10 ng to 0.01 pg.
In the case of multiplex PCR, since the three target species were amplified by the species-specific primers in the same mixture, a double concentration of Taq polymerase was used compared to simplex PCR. Multiplex PCR was conducted in a 25 μL reaction mixture containing 10 × Buffer (Bioneer, Daejeon, Korea), 800 μM dNTPs (Bioneer), 1 unit Hot Start Taq DNA polymerase (Bioneer), 0.2 μM big blue octopus primers, 1.2 μM giant Pacific octopus primers, 0.2 μM common octopus primers, and 10 ng of template DNA. Multiplex PCR conditions were identical to those used for simplex PCR. All PCR products were subsequently confirmed on a 3% agarose gel (Sea-Kem Rockland, ME) stained with ethidium bromide using 0.5 × Tris–acetate EDTA (TAE) buffer for 30 min at 150 V.
Application and intra-validation of the multiplex PCR assay to commercial octopus products
30 commercial food products labeled octopus were used for octopus species identification, using the developed multiplex PCR assay. For the experimental method applied to octopus processed food, DNA extraction was performed in the same way as the raw sample, and PCR is also the same as the multiplex PCR conditions. The 10 ng DNA extracted from commercial octopus products was used as a template. This method was validated with other PCR instruments of the same type.
Results and discussion
Specificity and sensitivity of the designed primers
We designed three species-specific primers that targeted the mitochondrial cytochrome oxidase subunit I (COI) gene. Since this gene was conserved within species and varied between species, they were commonly used for interspecies and intraspecies variability studies (Wang et al., 2019). Additionally, for the multiplex PCR assay, each primer was designed to differ by at least 30 bp to visually distinguish PCR amplicons on the agarose gel (big blue octopus, 84 bp; giant Pacific octopus, 117 bp; common octopus, 166 bp). Also, to increase the applicability of this assay to processed foods, short-sized (< 200 bp) primers were designed because DNA would have broken under high temperature and pressure during processing (Kim et al., 2019; Qin et al., 2016).
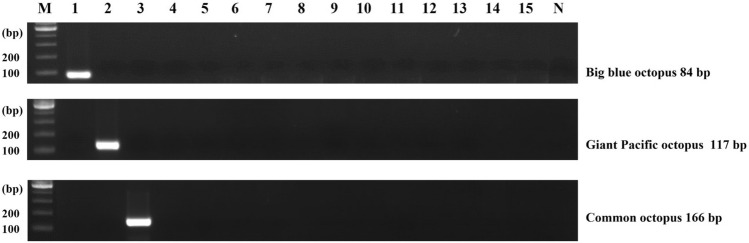
The specificity of species-specific primers was conducted using 15 cephalopod species. Octopus, squid, long arm octopus, and webfoot octopus all have common suckers on their arms. Therefore, it was difficult to identify them by shape if only the arms were sold separately. In this experiment, similar species were used as non-target species. As observed, nonspecific bands did not appear against the 15 cephalopods (Fig. 1). For the target octopus species, the sensitivity of each primer was further performed using serially diluted DNA in the range of 10 ng–0.01 pg. The sensitivity was 1 pg for the giant Pacific octopus and 0.1 pg for the big blue and common octopuses, respectively (Fig. 2). Thus, the limit of detection (LOD) from our study was 0.1 pg–1 pg, which was more sensitive than the 0.5 ng value obtained for the common octopus in a previous study (Velasco et al., 2021). These results therefore indicate that the primers designed in this study had a high specificity and sensitivity.
Fig. 1.
Specificity of simplex PCR assay. Lane M: 100 bp DNA ladder, lane 1: big blue octopus, lane 2: giant Pacific octopus, lane 3: common octopus, lane 4: long arm octopus, lane 5: webfoot octopus, lane 6: golden cuttlefish, lane 7: Japanese flying squid, lane 8: Argentine shortfin squid, lane 9: jumbo flying squid, lane 10: diamondback squid, lane 11: swordtip squid, lane 12: spear squid, lane 13: Japanese squid, lane 14: beka squid, lane 15: bigfin reef squid, lane N: non-template
Fig. 2.
Sensitivity of simplex PCR assay. Lane M: 100 bp DNA ladder, lanes 1–7: 10 ng to 0.01 pg of DNA from target species, and lane N: non-template
Specificity and sensitivity of the multiplex PCR assay
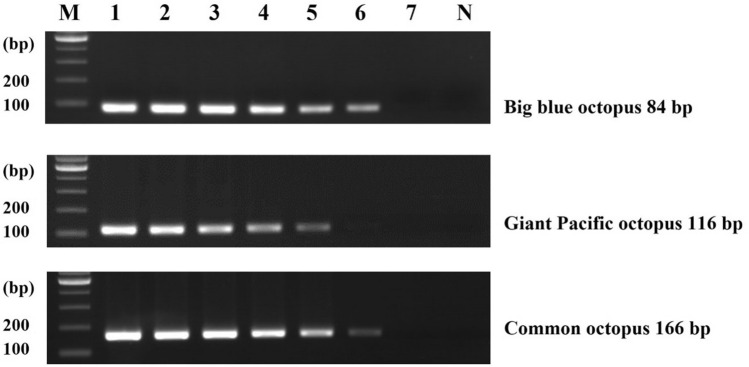
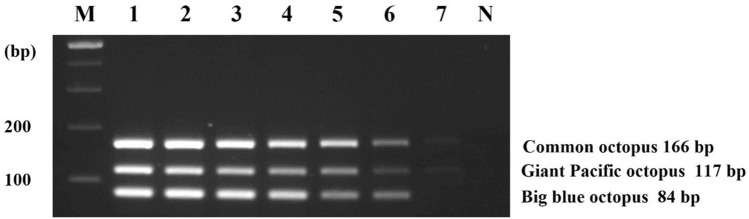
To develop the multiplex PCR assay, the concentration of three primers were optimized, considering cross-reactivities of their primer sets and their sensitivities. Previous studies have reported that the concentration of primers should be optimized to increase sensitivity and minimize non-specific interactions (Lee et al., 2022; Suh et al., 2020; Zha et al., 2010). We tested several conditions to determine the optimal concentration of each primer set. After testing different primer concentrations (data not shown), the following condition was determined to be the most suitable: 0.2 μM/1.2 μM/0.2 μM for big blue octopus primers, giant Pacific octopus primers, and common octopus, respectively. As shown in Fig. 3, the target species were successfully amplified to the expected sizes and no cross-reaction was detected. The sensitivity of this assay was then tested using tenfold serially diluted DNA (from 10 ng to 0.01 pg). The LOD of the multiplex PCR assay was measured as 0.1 pg for giant Pacific octopus, big blue octopus, and common octopus. (Fig. 4). Based on the sensitivity, the multiplex PCR assay was improved over the simplex PCR by adjusting the primer concentrations. Compared to the LOD of simultaneous detection of squid species, that of this assay (0.1 pg) was sensitive and useful as a tool for detecting small quantities of target DNA (Kim et al., 2015). Moreover, although similar previous studies showed that the sensitivity was 1 pg for the common cuttlefish and 0.25 ng for squid species, which was less sensitive than our method, it was possible to apply these methods to processed food products (Kim et al., 2015; Velasco et al., 2020). Therefore, this result indicated the developed multiplex PCR assay as suitable for identifying target species in both raw and processed samples.
Fig. 3.
Specificity of multiplex PCR assay. Lane M: 100 bp DNA ladder, lane P: positive control (10 ng of DNA from target species), lane 1: big blue octopus, lane 2: giant Pacific octopus, lane 3: common octopus, lane 4: long arm octopus, lane 5: webfoot octopus, lane 6: golden cuttlefish, lane 7: Japanese flying squid, lane 8: Argentine shortfin squid, lane 9: jumbo flying squid, lane 10: diamondback squid, lane 11: swordtip squid, lane 12: spear squid, lane 13: Japanese squid, lane 14: beka squid, lane 15: bigfin reef squid, lane N: non-template
Fig. 4.
Sensitivity of multiplex PCR assay. Lane M: 100 bp DNA ladder, lanes 1–7: 10 ng to 0.01 pg of DNA from target species, and lane N: non-template
Furthermore, although several methods for detecting octopuses have been reported, these detection methods used simplex PCR assay using species-specific primers or group-specific primers (Chung et al., 2018; Espiñeira and Vieites, 2012; Velasco et al., 2021), which were time-consuming or created difficulties during species identification. Espiñeira and Vieites (2012) reported the identification of octopus species using real-time PCR, which requires expensive equipment and skilled experimenters compared to multiplex PCR. Velasco et al. (2021) developed the recombinase polymerase amplification and lateral flow assay to discriminate common octopuses, but showed a lower sensitivity (0.5 ng) than that of this assay (0.1 pg). Chung et al (2018) analyzed SYBR-based real-time PCR using cephalopod group-specific primers, but there is a limitation in specificity as it was amplified from a cephalopod other than the target cephalopod. Therefore, since the multiplex PCR assay for octopus species had not been reported, the multiplex PCR assay reported in this study was a unique, effective, and sufficient detection method to study the three octopuses (big blue octopus, giant Pacific octopus, and common octopus).
Application and intra-validation of the multiplex PCR assay using commercial octopus products
The applicability of the multiplex PCR assay was demonstrated, using 30 commercial products that were labeled as octopuses. All commercial products were of various types, including parboiled, sushi, nuggets, stir-fried octopus, salted octopus, dumpling, nugget, salad, dried octopus, fish cake, takoyaki, porridge, roasted octopus, fried octopus, and boiled octopus (Table 2). The 10 ng DNA extracted from these commercial products was used as a template. From the application test, all products were identical, compared to the product’s sample label (Table 2). Subsequently, the test was validated using three PCR instruments in the laboratory. Experimental results were the same.
Table 2.
Application and validation of the multiplex PCR assay to commercial products
| No | Product type | Origin | Multiplex PCR results | ||
|---|---|---|---|---|---|
| Big blue octopus | Giant Pacific octopus | Common octopus | |||
| 1 | Parboiled | Korea | + + + | ||
| 2 | Parboiled | Korea | + + + | ||
| 3 | Parboiled | Korea | + + + | ||
| 4 | Parboiled | Korea | + + + | ||
| 5 | Parboiled | China | + + + | ||
| 6 | Sushi | Philippine | + + + | ||
| 7 | Sushi | Vietnam | + + + | ||
| 8 | Salted | Korea | + + + | ||
| 9 | Stir-fried | Mauritania | + + + | ||
| 10 | Salted | Korea | + + + | ||
| 11 | Fish cake | NL | + + + | ||
| 12 | Dumpling | NL | + + + | ||
| 13 | Dumpling | Philippine | + + + | ||
| 14 | Stir-fried | Philippine | + + + | ||
| 15 | Stir-fried | Mauritania | + + + | ||
| 16 | Nugget | Philippine | + + + | ||
| 17 | Salad | Korea | + + + | ||
| 18 | Boiled | Mauritania | + + + | ||
| 19 | Dumpling | China | + + + | ||
| 20 | Dried | Korea | + + + | ||
| 21 | Parboiled | Korea | + + + | ||
| 22 | Sushi | NL | + + + | ||
| 23 | Fish cake | China | + + + | ||
| 24 | Takoyaki | China | + + + | ||
| 25 | Porridge | Indonesia | + + + | ||
| 26 | Porridge | China | + + + | ||
| 27 | Dried | Korea | + + + | ||
| 28 | Roasted | Korea | + + + | ||
| 29 | Fried | Korea | + + + | ||
| 30 | Boiled | Korea | + + + | ||
The test was independently three times using three different instruments
NL, no label
+ means a positive result
In recent years, the proportion of the seafood processed food industry has increased more than that of raw materials (So and Kim, 2013). However, in the case of processed octopus foods (ex, fish cake, sausage, and porridge), there is a limit to visually check whether the target species is contained or not because the original properties was damaged after cutting, heating, sterilization or canning. Moreover, most octopus commercial food products were labeled “octopus” on their products rather than the species name. Additionally, many commercial octopus products, such as octopus arms and porridges were identified. It was difficult to distinguish the species with the naked eye, which is proposed to result in an intentional or nonintentional species substitution and mislabeling. In fact, due to the limitation of this visual classification, cases of adulteration, in which the squid arm was converted to the octopus arm and the octopus arm to sword squid’s arm have been reported (Kim et al., 2015). Also, based on results from the application test, domestic octopus species identified were the common octopus and giant Pacific octopus, besides imported octopus species, including the common octopus and big blue octopus. This result indicates that the relatively inexpensive big blue octopus was used only as imported commercial products. PCR amplicons were also detected in the products No. 19 and No. 23 which contain low contents of octopus (2% and 4.04% octopus, respectively), showing that our assay can be applied to products with low octopus concentrations.
With processed foods, the DNA analysis-based PCR method has advantages because protein denaturation occurs during processing, such as high-pressure sterilization. Additionally, since heat or pressure during the manufacturing process damages DNA, the size of the amplification product should be designed as small as 300 bp or less (Yao et al., 2021). However, among the DNA-based PCR methods, multiplex PCR analysis of octopus has not been previously reported. The multiplex PCR method can simultaneously detect multiple species in a short time at low cost, so this analysis can be efficiently applied to processed octopus foods. Therefore, in this study, we designed a method that can be applied to processed foods with the developed multiplex PCR. Through the application test, it was confirmed that our assay can be applied to processed foods and that the sensitivity of the primer was excellent. Furthermore, for a more efficient detection method, this assay can be combined with on-site detection using direct DNA extraction from processed food products.
Funding
This research was supported by the Ministry of Food and Drug Safety in Korea, grant number 17162MFDS065.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Chande MA, Mgaya YD, Benno LB, Limbu SM. The influence of environmental variables on the abundance and temporal distribution of Octopus cyanea around Mafia Island, Tanzania. Fisheries Research. 2021;241:105991. doi: 10.1016/j.fishres.2021.105991. [DOI] [Google Scholar]
- Chapela MJ, Sotelo CG, Perez-Martin RI. Molecular identification of cephalopod species by FINS and PCR-RFLP of a cytochrome b gene fragment. European Food Research and Technology. 2003;217:524–529. doi: 10.1007/s00217-003-0788-y. [DOI] [Google Scholar]
- Chapela MJ, Sotelo CG, Calo-Mata P, Pérez-Martín RI, Rehbein H, Hold GL, Quinteiro J, Rey-Méndez M, Rosa C, Santos AT. Identification of cephalopod species (Ommastrephidae and Loliginidae) in seafood products by forensically informative nucleotide sequencing (FINS) Journal of Food Science. 2006;67(5):1672–1676. doi: 10.1111/j.1365-2621.2002.tb08703.x. [DOI] [Google Scholar]
- Chung IY, Seo YB, Yang JY, Kwon S, Kim GD. Development and validation of quick and accurate Cephalopods grouping system in fishery products by real-time quantitative PCR based on mitochondrial DNA. Journal of Food Hygiene and Safety. 2018;33(4):280–288. doi: 10.13103/JFHS.2018.33.4.280. [DOI] [Google Scholar]
- Donlan CJ, Luque GM. Exploring the causes of seafood fraud: A meta-analysis on mislabeling and price. Marine Policy. 2019;100:258–264. doi: 10.1016/j.marpol.2018.11.022. [DOI] [Google Scholar]
- Espiñeira M, Vieites JM. Rapid method for controlling the correct labeling of products containing common octopus (Octopus vulgaris) and main substitute species (Eledone cirrhosa and Dosidicus gigas) by fast real-time PCR. Food Chemistry. 2012;135:2439–2444. doi: 10.1016/j.foodchem.2012.07.056. [DOI] [PubMed] [Google Scholar]
- Espiñeira M, Vieites JM, Santaclara FJ. Species authentication of octopus, cuttlefish, bobtail and bottle squids (families Octopodidae, Sepiidae and Sepiolidae) by FINS methodology in seafoods. Food Chemistry. 2010;121(2):527–532. doi: 10.1016/j.foodchem.2009.12.042. [DOI] [Google Scholar]
- Gebhardt K, Knebelsberger T. Identification of cephalopod species from the North and Baltic Seas using morphology, COI and 18S rDNA sequences. Helgoland Marine Research. 2015;69:259–271. doi: 10.1007/s10152-015-0434-7. [DOI] [Google Scholar]
- Hou B, Meng X, Zhang L, Guo J, Li S, Jin H. Development of a sensitive and specific multiplex PCR method for the simultaneous detection of chicken, duck and goose DNA in meat products. Meat Science. 2015;101:90–94. doi: 10.1016/j.meatsci.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Kang H, Kim Y, Kim S, Lee D, Choi Y, Chang D, Gwak SW. Maturity and spawning period of the common octopus, octopus vulgaris in the south sea of Korea. The Korean Journal of Malacology. 2009;25(2):127–133. [Google Scholar]
- Kim MJ, Kim HY. Species identification of commercial jerky products in food and feed using direct pentaplex PCR assay. Food Control. 2017;78:1–6. doi: 10.1016/j.foodcont.2017.02.027. [DOI] [Google Scholar]
- Kim H, Seo YB, Choi SS, Kim JH, Shin J, Yang JY, Kim GD. Development and validation of multiplex polymerase chain reaction to determine squid species based on 16s rRNA gene. Journal of Food Hygiene and Safety. 2015;30(1):43–50. doi: 10.13103/JFHS.2015.30.1.43. [DOI] [Google Scholar]
- Kim MJ, Yoo I, Yang SM, Suh SM, Kim HY. Development and validation of a multiplex PCR assay for simultaneous detection of chicken, turkey and duck in processed meat products. International Journal of Food Science and Technology. 2018;53:2673–2679. doi: 10.1111/ijfs.13876. [DOI] [Google Scholar]
- Kim MJ, Kim SY, Jung SK, Kim MY, Kim HY. Development and validation of ultrafast PCR assays to detect six species of edible insects. Food Control. 2019;103:21–26. doi: 10.1016/j.foodcont.2019.03.039. [DOI] [Google Scholar]
- Kim NYS, Park EJ, Lee SH, Mun KH, Yang JY, Kim JB. Development and validation of multiplex PCR assay for differentiating tunas and billfishes. Korean Journal of Food Science and Technology. 2021;30:497–503. doi: 10.1007/s10068-021-00893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SI, Yang JH, Lee HW, Kim JB, Cha HK. Maturity and spawning of the giant Pacific octopus, Octopus dofleini in the coast of Gangwondo, East Sea. Journal of the Korean society of Fisheries Technology. 2014;50(2):154–161. doi: 10.3796/KSFT.2014.50.2.154. [DOI] [Google Scholar]
- Lee GY, Suh SM, Lee YM, Kim HY. Multiplex PCR assay for simultaneous identification of five types of Tuna (Katsuwonus pelamis, Thunnus alalonga, T. albacares, T. obesus and T. thynnus) Foods. 2022;11(3):280. doi: 10.3390/foods11030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown NJ, Taylor AL, Shaw PW. Isolation and characterisation of polymorphic microsatellite loci for studies of the big blue octopus, Octopus cyanea. Marine Biodiversity. 2018;48:2233–2235. doi: 10.1007/s12526-017-0717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Hong Y, Kim HY. Multiplex-PCR assay for simultaneous identification of lamb, beef and duck in raw and heat-treated meat mixtures. Journal of Food Safety. 2016;36(3):367–374. doi: 10.1111/jfs.12252. [DOI] [Google Scholar]
- Safdar M, Junejo Y. A multiplex-conventional PCR assay for bovine, ovine, caprine and fish species identification in feedstuffs: Highly sensitive and specific. Food Control. 2015;50:190–194. doi: 10.1016/j.foodcont.2014.08.048. [DOI] [Google Scholar]
- Santaclara FJ, Espiñeira M, Vieites JM. Genetic Identification of Squids (Families Ommastrephidae and Loliginidae) by PCR–RFLP and FINS Methodologies. Journal of Agricultural and Food Chemistry. 2007;55:9913–9920. doi: 10.1021/jf0707177. [DOI] [PubMed] [Google Scholar]
- Shi R, Huang M, Wang J, He C, Ying X, Xiong X, Xiong X. Molecular identification of dried squid products sold in China using DNA barcoding and SYBR green real time PCR. Food Additives and Contaminants: Part A. 2020;37(7):1061–1074. doi: 10.1080/19440049.2020.1746411. [DOI] [PubMed] [Google Scholar]
- Sin YW, Yau C, Chu KH. Morphological and genetic differentiation of two loliginid squids, Uroteuthis (Photololigo) chinensis and Uroteuthis (Photololigo) edulis (Cephalopoda: Loliginidae), in Asia. Journal of Experimental Marine Biology and Ecology. 2009;369:22–30. doi: 10.1016/j.jembe.2008.10.029. [DOI] [Google Scholar]
- So WH, Kim HK. Effects of the enterprise image in Korean processed marine product industry on consumers’ product evaluation and purchase intention. The Journal of Fisheries Business Administration. 2013;44(1):1–14. doi: 10.12939/FBA.2013.44.1.001. [DOI] [Google Scholar]
- Suh SM, Kim MJ, Kim HI, Kim HJ, Kim HY. A multiplex PCR assay combined with capillary electrophoresis for the simultaneous detection of tropomyosin allergens from oyster, mussel, abalone, and clam mollusk species. Food Chemistry. 2020;317:126451. doi: 10.1016/j.foodchem.2020.126451. [DOI] [PubMed] [Google Scholar]
- Toussaint RK, Scheel D, Sage GK, Talbot SL. Nuclear and mitochondrial markers reveal evidence for genetically segregated cryptic speciation in giant Pacific octopuses from Prince William Sound, Alaska. Conservation Genetics. 2012;13:1483–1497. doi: 10.1007/s10592-012-0392-4. [DOI] [Google Scholar]
- Vaz-Pires P, Seixas P, Barbosa A. Aquaculture potential of the common octopus (Octopus vulgaris Cuvier, 1797): a review. Aquaculture. 2004;238:221–238. doi: 10.1016/j.aquaculture.2004.05.018. [DOI] [Google Scholar]
- Velasco A, Ramilo-Fernández G, Sotelo CG. A Real-time PCR method for the authentication of common cuttlefish (Sepia officinalis) in food products. Foods. 2020;9(3):286. doi: 10.3390/foods9030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco A, Ramilo-Fernández G, Denis F, Oliveira L, Shum P, Silva H, Sotelo CG. A new rapid method for the authentication of common octopus (Octopus vulgaris) in Seafood products using recombinase polymerase amplification (RPA) and lateral flow assay (LFA) Foods. 2021;10(8):1825. doi: 10.3390/foods10081825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hang X, Geng R. Molecular detection of adulteration in commercial buffalo meat products by multiplex PCR assay. Food Science and Technology. 2019;39(2):344–348. doi: 10.1590/fst.28717. [DOI] [Google Scholar]
- Wilwet L, Shakila RJ, Sivaraman B, Nayak BB, Kumar HS, Jaiswar AK, Jeyasekaran G. Rapid detection of fraudulence in seven commercial shrimp products by species-specific PCR assays. Food Control. 2021;124:107871. doi: 10.1016/j.foodcont.2021.107871. [DOI] [Google Scholar]
- Yao L, Qu M, Jiang Y, Guo Y, Li N, Li F, Tan Z, Wang L. The development of genus-specific and species-specific real-time PCR assays for the authentication of Patagonian toothfish and Antarctic toothfish in commercial seafood products. Journal of the Science of Food and Agriculture. 2021;104:1674–1683. doi: 10.1002/jsfa.11507. [DOI] [PubMed] [Google Scholar]
- Ye J, Feng J, Liu S, Zhang Y, Jiang X, Dai Z. Identification of four squid species by quantitative real-time polymerase chain reaction. Molecular and Cellular Probes. 2016;30(1):22–29. doi: 10.1016/j.mcp.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Zha D, Xing X, Yang F. A multiplex PCR assay for fraud identification of deer products. Food Control. 2010;21:1402–1407. doi: 10.1016/j.foodcont.2010.04.013. [DOI] [Google Scholar]