Abstract
One of the major stored product pests, Indian meal moth causes the loss on the agriculture and food industries. This study was conducted to screen the insecticidal activity of ethanolic extracts and fractions partitioned by four different solvents [(1) n-hexane; (2) ether; (3) ethyl acetate; (4) water] from star anise (Illicium verum Hook. f.) against Plodia interpunctella larvae. Among all solvent-partitioned fractions, the strongest repellency was found for the n-hexane fraction of star anise extract. Solvent–solvent partitioning and chromatographic methods were further used to isolate and identify major anti-insect compounds from star anise extract. The results showed that trans-anethole (94.24%) was the major active compound showing an insect-repelling activity against P. interpunctella. Consequently, trans-anethole can be utilized as a main natural insect-repelling agent for controlling the P. interpunctella infestation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01053-8.
Keywords: Anti-insect, Insect-repellent, Indian meal moth, Trans-anethole, Solvent–solvent partitioning
Introduction
The Indian meal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae), is a major pest globally, which causes economic losses by damaging stored food products (Rees, 2004). Its larvae are attracted to grains, flour, stored cereal products, dried fruits and vegetables, processed foods, and meals (Prajapati et al., 2005). Infestations of P. interpunctella can generate direct or indirect economic costs through product losses, costs of pest control and quality loss, and consumer complaints (Phillips et al., 2000). Therefore, P. interpunctella should be controlled to preserve food during distribution and storage. Especially, an effective packaging design system using anti-insect materials can repel their presence from the food environment, assuring insect-free food with maintained food quality, cost, and consumer acceptance.
Controlling the P. interpunctella typically involves the use of synthetic insecticides (e.g. pyrethroids and organophosphates) and fumigants (e.g. methyl bromide or phosphine) (Kim et al., 2014). The pesticides most commonly used contain phosphine, organophosphates (DDVP, pirimiphos-methyl), and pyrethroids (lambda-cyhalothrin and deltamethrin). However, the use of pesticides has resulted in the resistance of P. interpunctella and their accumulation in food, with potentially fatal effects on humans (Arthur and Phillips, 2003; Attia, 1977; Phillips and Throne, 2009). There is thus a need to develop natural insect repellents for pest control.
In order to control pests in stored products, the use of plant materials as a form of extract, essential oil, and their component is an ancient practice used worldwide (Rajendran and Sriranjini, 2008; Tripathi and Dubey, 2004). These natural substances are classified in the Generally Recognized as Safe (GRAS) category established by the US Food and Drug Administration (Kumari et al., 2009). Essential oils (EOs), natural insect repellents, and bio-pesticides, among others, seem to be complementary or alternative options for reducing the harmful effects of synthetic pesticides and protecting stored foods (Tripathi and Dubey, 2004), like toxic, repellent, and antifeedant activities on insects infesting stored products (Isman, 2005; Regnault-Roger et al., 2012). Other researchers have suggested the applicability of EOs, such as cinnamon (Jo et al., 2013, 2015; Kim et al. 2020), clove (Kim et al., 2016, 2019), garlic (Lee et al., 2017), ginger (Lee et al., 2017), and black pepper (Lee et al., 2017) EOs as insect-repelling agents.
In this study, Illicium verum Hook. f., commonly known as star anise (SA), was selected as a source of the insect-repelling agent. It has been used as a spice in the food industry, and EO from SA (SAEO) as a flavoring in confectionery, pharmaceutical preparations, and health supplements (Ding et al., 2017). While the physiological mechanisms supporting the action of this SAEO are unexplored, it is proposed to have insect-repelling activity by exerting neurotoxic effects on insects (Lee et al., 2020). SA has also been shown to exert antimicrobial, antiviral, and antioxidant effects in various studies (De et al., 2002). However, EO has a low production yield of about only 2.0%, and it also needs some specific equipment (Balti et al., 2018). On the other hand, an ethanolic extract is more convenient to extract insect repellent agents, which can be a good natural insect repellent substitute for synthetic chemicals outstanding insect-repelling activity. However, there is a need for additional research as few studies on the isolation of major functional compounds of SA extract have been performed. Therefore, we aimed to (i) screen and identify anti-insect agents against P. interpunctella from SA extract and (ii) investigate a possible insect-repelling mechanism using an acetylcholinesterase (AChE) inhibition assay.
Materials and methods
Materials
SA (cultivated in Vietnam in 2020) was obtained from Gyeongdong Medicinal Herb Market (Seoul, Korea). It was washed with water (H2O) and air-dried at 22 ± 3˚C for 48 h. Then, the dried sample was finely pulverized with an electric grinder (DA280-S; Daesung Artron Co., Paju, Korea).
Reagents
For screening and isolation, n-hexane (Hex, purity ≥ 95%), diethyl ether (Et2O, purity ≥ 99%), ethyl acetate (EtOAc, purity ≥ 99%), ethyl alcohol (EtOH, purity ≥ 99.9%), methyl alcohol (MeOH, purity ≥ 99.9%), and acetic acid (purity ≥ 99.5%) were obtained from Daejung Chemicals & Metals Co., Ltd. (Siheung, Korea). Sulfuric acid (purity ≥ 95–98%), p-anisaldehyde (purity ≥ 98%), and trans-anethole (purity ≥ 95%) were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA). For AChE inhibition assay, acetylthiocholine iodide (ATChI) and 5,5’-dithio-bis acid (DTNB) were gained from Sigma-Aldrich Inc. (St. Louis, MO, USA).
Insects
Adults of P. interpunctella within 48 h of emergence were obtained and led to oviposition. The eggs were inoculated with artificial feed (a mixture of rice bran, yeast extract, glycerol, methyl p-hydroxybenzoate, and sorbic acid) and incubated in the dark at 30 °C with 60–70% relative humidity. Then, the collected third-instar larvae were used for experiments.
Extraction
The pulverized SA (100 g) was extracted in 500 mL of 80% (v/v) EtOH as an extraction solvent. The extraction was carried out using VC505 ultrasound (Sonics and Materials Inc., Danbury, CT, USA) with a probe (13-mm-diameter) for 2 h in a water bath (4 ± 1 °C). After extraction, the crude extracts were filtered through Whatman® No. 1 filter paper (Cytiva, Marlborough, MA, USA). Then, the solvent was removed from the extracts using a rotary evaporator (EYELA® N-1200B; Tokyo Rikakikai Co., Ltd., Tokyo, Japan) under a vacuum at 35 ± 5 °C. Concentrated crude EtOH extracts were kept at − 80 °C until further tests.
Fractionation
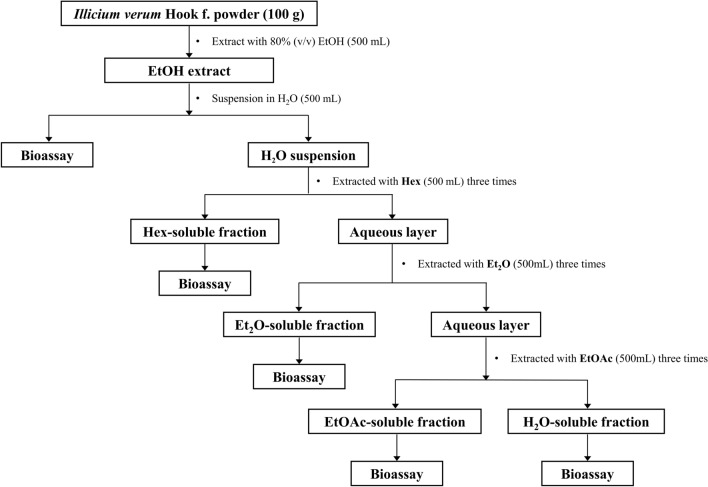
The EtOH extract was dispersed in H2O (500 mL) and further extracted with four solvents (Hex, Et2O, EtOAc, and H2O) in ascending order of polarity, as shown in the flowchart in Fig. 1. After solvent–solvent partitioning, each fraction was concentrated by using a vacuum rotary evaporator (EYELA® N-1200B) at 35 ± 5 °C, with the obtained samples being freeze-dried by a freeze-dryer (EYELA® FDU-2110; Tokyo Rikakikai Co., Ltd.). Through this process, four solvent-partitioned fractions [(1) Hex, (2) Et2O, (3) EtOAc, and (4) H2O fractions] from the extract of SA were obtained. All four fractions were stored at − 80 °C of ultra-low temperature freezer (CLN-52U; Nihon Freezer Co Ltd., Saitama, Japan) until further analyses.
Fig. 1.
Flowchart of stepwise solvent–solvent partitioning of star anise extracts and bioassay
Concentration gradient in insect repellent activity testing device
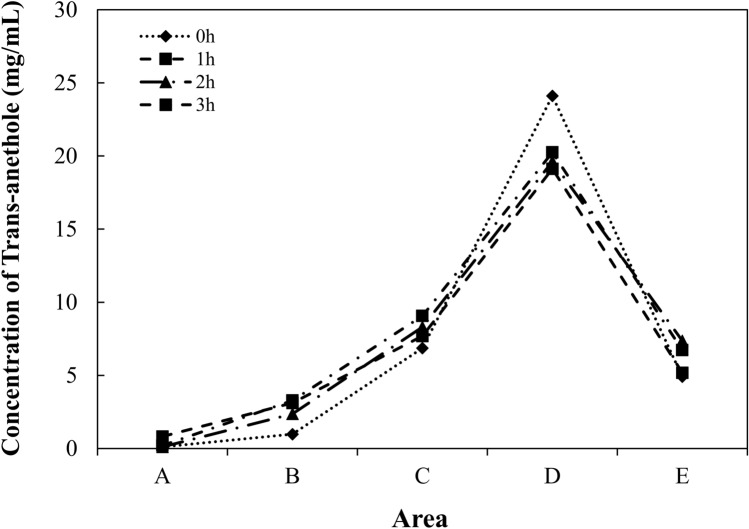
The concentration gradient of volatile compounds in the headspace of the device for testing insect-repelling activity was determined by a gas chromatography–flame ionization detector (GC-FID, Agilent 7890A; Agilent Technologies Inc., Santa Clara, CA, USA) equipped with HP-5 Capillary GC columns (30 m in length × 0.32 mm in inner diameter × 0.25 μm in film thickness; Agilent Technologies Inc., USA) according to method suggested by Park et al. (2018) and Lee et al. (2019) with slight modification. The prepared trans-anethole solution (0.5 mg/cm2) was spread onto filter paper (8.4 × 5 cm) and then put in the testing device. To identify the concentration gradient in the testing device, the concentration of trans-anethole in all areas (areas A–E) were measured. The release of trans-anethole into the headspace of the testing device was induced at 22 ± 3 °C for 1, 2 and 3 h. Then, trans-anethole was adsorbed for 15 min using a solid-phase microextraction (SPME) fiber coated with a 75-μm carboxen/polydimethylsiloxane. After that, the SPME fiber was manually injected into the injection port of the GC-FID and retained for 15 min. Both injector and detector temperatures were set to be 250 °C. The initial oven temperature was 80 °C, which was then increased to 250 °C at 5 °C/min. Nitrogen was used as a carrier gas with a split ratio of 2:1. The amount of trans-anethole adsorbed in the SPME fiber was calculated using Agilent software (Agilent OpenLAB CDC ChemStation C. 01.04, Agilent Technologies Inc.). The analysis was repeated three times.
Bioassays of fractions
The bioassays were performed based on our prior research (Lee et al., 2021) with some modifications using an insect-repelling activity measuring pipe device (Fig. S1). In this device, the perforated ends were covered with a calico cloth to form a concentration gradient of the test substance (insect-repelling agent) and hinder the insect from escaping. The control and testing filter papers were inserted into areas B and D, respectively. Therefore, area A + B, area C, and area D + E were regarded as control, neutral, and treated areas, respectively.
The Whatman® No. 1 filter paper was cut into 8.4 × 5 cm pieces and then soaked with 1 mL of solid contents at 0.05, 0.1, 0.2, 0.5, and 1 mg/cm2 for the anti-insect assay. The dilution solvent matched the one used to partition each fraction. Each pure solvent (1 mL) equivalent to the one used for the fractionation was used as a control. Then, filter papers were completely air-dried for 2 h to evaporate the solvents. The control and testing filter papers were inserted into areas B and D, respectively. Then, 10 third-instar larvae were positioned in the center of the device (area C), and feed was positioned in areas A and E to induce the movement of insects and to prevent cannibalism. The used testing device was then stored in a dark incubator at 30 °C. The larvae located in area A + B, area C, and area D + E (Ncontrol, Nneutral, and Ntreated) were counted at hourly intervals for 3 h. The percentage repellency (PR) for each fraction was determined using the following Eq. 1 contrived by Lee et al. (2021) for the first time:
| 1 |
where Ncontrol, Nneutral, and Ntreated are the numbers of larvae located in the control, neutral, and treated areas, respectively. The concentration gradient of the test material was formed for each area in the testing device, and the order of concentration was as follows: treated area > neutral area > control area. Accordingly, only the number of larvae positioned in the treated area, Ntreated could be a strong and positive index of the insect-repelling activity of the materials. On the other hand, the number of larvae in neutral and control areas, Nneutral and Ncontrol were regarded as the moderate and strong negative index, respectively. For such reasons, the following appropriate correction factor was assigned to each of the three variables in Eq. (1). The weighting factor, × 2, was assigned to Ntreated. Meanwhile, subtraction factors, × (–1) and × (–2), were given to Nneutral and Ncontrol, respectively.
Bioassay-guided isolation of Hex fraction
Among the obtained four fractions, the Hex fraction was selected as an optimal to have the strongest PR against the P. interpunctella larvae based on the “Bioassays of fractions” section. Therefore, the Hex fraction from SA extract was isolated into several subfractions using column chromatography (30 mm in diameter × 350 mm in height) on silica gel (silica gel 60; 0.063–0.200 mm, 70–230 mesh; Merck KGaA, Darmstadt, Germany) with Hex:EtOAc step-gradient elution, using consecutive ratios of 100:1, 50:1, 25:1, 15:1, 8:1, 4:1, 2:1, and 1:1 (v/v), followed by methanol. Subfractions (7 mL/tube) were obtained using a fraction collector (Spectra/Chrom® CF 2; Repligen Co., Waltham, MA, USA) and monitored by thin-layer chromatography (TLC) using anisaldehyde reagent (p-anisaldehyde:acetic acid:sulfuric acid = 1.0:48.5:0.5, v/v/v) after incubated at 100 °C for 5 min. Fluorescent materials were visualized at 254 and 366 nm wavelengths in a TLC darkroom (VL-4.LC; Vilber Lourmat, Collégien, France). As a result, 6 Hex subfractions (A1–6) were acquired from the Hex fraction. Each subfraction was concentrated under a vacuum evaporating condition (EYELA® N-1200B) at 35 ± 5 °C, and the resulting samples were lyophilized using a freeze-dryer (EYELA® FDU-2110). Then, lyophilized samples were stored in an ultra-low temperature freezer (CLN-52U; Nihon Freezer Co Ltd., Saitama, Japan) at –80 °C until analyses.
The bioassay of Hex subfractions was performed using the same methods and procedures as described in the “Bioassays of fractions” section. The testing filter paper was soaked with 1 mL of solid contents at 0.5 mg/cm2. In the case of the control, the filter paper was soaked with pure Hex solvent (1 mL). The values of Ncontrol, Nneutral, and Ntreated were counted at hourly intervals for 3 h. Then, each PR value was calculated using the above Eq. 1.
Gas chromatography–mass spectrometry (GC–MS) analysis of Hex fraction and subfractions
The compositions of the Hex fraction and final optimal subfractions were identified using gas chromatography-mass spectrometry (GC–MS, 7890A/5977A; Agilent Technologies, Inc.) equipped with HP‐5 capillary GC columns (30 m in length × 0.32 mm in inner diameter × 0.25 μm in film thickness; Agilent Technologies Inc., USA) with A2 and A3 fractions. An divinylbenzene/carboxen/polydimethylsiloxane coated SPME fiber with a needle size of 24 ga (Supelco Inc., Bellefonte, PA, USA) was used to adsorb the volatile headspace compounds for 30 min. The oven temperatures set for the analysis were as follows: initial temperature = 80 °C; final temperature = 250 °C; temperature increase rate = 3 °C/min; injector temperature = 250 °C; and detector temperature = 250 °C. The experimental conditions were as follows: carrier gas = helium (purity = 99.99%); flow rate = 1 mL/min; injection ratio = 2:1; and scan range = 10–800 m/z. GC/mass selective detector (MSD) ChemStation software (Agilent OpenLAB CDC ChemStation C. 01.04, Agilent Technologies Inc.) was used to analyze the resulting data.
Insect-repelling activity of trans-anethole
Trans-anethole was confirmed as a major component of Hex fraction and final optimal subfractions through the GC–MS identification (“GC–MS analysis of Hex fraction and subfractions” section). To confirm the insect-repelling activity of trans-anethole, a bioassay was carried out as described in the previous “Bioassays of fractions” section. Trans-anethole solution (1 mL) at different solid contents (0.05, 0.1, 0.2, 0.5, and 1 mg/cm2) and pure Hex were assigned to the experimental and negative controls, respectively. Each value (Ncontrol, Nneutral, and Ntreated) was counted at hourly intervals for 3 h. Then, each PR value was calculated using the above Eq. 1.
AChE-inhibiting activity of trans-anethole
AChE-inhibiting activity was measured using a modified version of a previously reported colorimetric method (Chang et al., 2017; Lee et al., 2017) with ATChI as the substrate. First, 30 larvae were soaked in phosphate buffer (1 mL) and homogenized using an SR30 homogenizer (M-TOPS Co., Ltd., Seoul, Korea). The extract was then centrifuged at 5000 rpm for 20 min using a Micro 17TR centrifuge (Hanil Science Industrial, Incheon, Korea), and the supernatant was used as a protein (enzyme source). The enzyme inhibitory activity was suppressed by adding 1 mL of trans-anethole (anti-insect agent) to the enzyme solution (79 mL) in 96-well plates (SPL Life Sciences, Bucheon, Korea) for 10 min. For the control, 1 mL phosphate buffer (0.1 M; pH 7.5) was used instead of the anti-insect agent. Subsequently, ATChI and DTNB were added. The pigment production of 5-thio-2-nitrobenzoate anions can be used to measure the hydrolysis of ATChI. This is formed by the reaction of DTNB and thiocholine released by hydrolysis of the enzyme. The AChE inhibitory activity was determined using a PowerWave XS microplate spectrophotometer (Bio-Tek Instruments Inc., Winooski, VT, USA), and AChE inhibition rates were obtained in triplicate using the following Eq. 2:
| 2 |
where Vmax is the maximum reaction velocity from monitoring hydrolysis of the substrate (ATChI) after treatment with anti-insect agents (chemical treatment group) or phosphate buffer in a control group.
Statistical analysis
Statistical analysis was carried out using SPSS software. Data for all fractions of extracts were compared using one-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison tests. The data are expressed as mean ± standard deviation (SD) with P < 0.05 set to indicate statistical significance.
Results and discussion
Extraction and fractionation
The yield of crude extracts of SA was 19.3% using 80% (v/v) ethanol as the extraction solvent, which was similar to the extraction yield (16.0%) of Hoque et al. (2011)’s study. Subsequently, the resulting yields of the partitioning by Hex, Et2O, EtOAc, and H2O were 37.0%, 7.5%, 4.6%, and 50.9%, respectively (Table S1).
Concentration gradient in insect repellent activity testing device
Figure 2 shows the trans-anethole concentration profile inside the headspace of the testing device for 0, 1, 2, and 3 h. As a result of identifying the concentration distribution of each area of trans-anethole in the testing device, the concentrations were in the following order, D > C > E > B > A, throughout the storage period. This meant that the ventilation parts prevented saturation of the test materials and induced the concentration gradient in the device. Meanwhile, the concentrations in each testing device area were in the following order: treated area (D + E) > neutral area (C) > control area (A + B). Because the trans-anethole volatiles attributed to the fumigant toxicity against P. interpunctella (Wang et al., 2021), the larvae gathered in control area, where the trans-anethole concentration was the lowest.
Fig. 2.
Concentration gradient of each area in pipe device (A–E) for measuring insect-repelling activity after the exposure time of 0, 1, 2, and 3 h; control areas of A (vent covered with calico cloth) and B (solvent-only attached area), C (neutral area), and treated areas of D (repellent-treated area) and E (vent covered with calico cloth)
Bioassays of fractions
Four solvent-partitioned fractions were acquired from the solvent–solvent partitioning process. Table 1 presents the PR values of the obtained fractions against P. interpunctella larvae. A PR value exceeding 50% was found for SAEO (0.5 and 1.0 mg/cm2) and the Hex fraction (0.5 and 1.0 mg/cm2) from SA extract. The insect-repelling activity in each fraction significantly increased (P ≤ 0.05) as the concentration increased. Particularly, the highest concentration of SAEO (1.0 mg/cm2) exhibited remarkable insect-repelling activity with a PR value of 94%. At the same concentration, the Hex fraction also showed the PR value of 91% after an exposure time of 3 h, significantly not differed (P > 0.05) from that of the SAEO. Thus, the Hex fraction was selected as the optimal partitioned fraction and further used in the other tests. On the other hand, the Et2O fraction, EtOAc fraction, and H2O fraction showed insect-attracting activity. The H2O fraction had the highest insect-attracting activity among all fractions. In addition, the attracting activity of each fraction also significantly increased (P ≤ 0.05) as the concentration increased. This might be because the water extract of SA contains large amounts of sugars, which could induce a behavioral attraction in larvae like a feeding stimulant (Dinesha et al., 2014; Lee et al., 2009). These results indicated that plant materials had not only insect-repelling components but also insect-attracting ones among their constituents. Therefore, fractionation and isolation processes were crucial to obtaining a single component presenting the strongest insect-repelling activity among the various constituents. Particularly noteworthy was the fact that even in the case of the Hex fraction, there was a rather insect-attracting activity at relatively low concentrations (0.05–0.2 mg/cm2). This might be because the relatively low, i.e., inappropriate concentration of using the insect repellent active substance may cause the insect-attracting activity. Therefore, it is essential to set an appropriate concentration of use to utilize natural substances as insect-repelling agents.
Table 1.
Percentage repellency (insect-repelling activity) against Plodia interpunctella larvae in star anise essential oil, ethanol extract, and four different solvents [(1) Hex, (2) Et2O, (3) EtOAc and (4) H2O] from star anise at different concentrations (0.05, 0.1, 0.2, 0.5, and 1 mg/cm2)
| Solvent | Concentration (mg/cm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 0.5 | 0.2 | |||||||
| Exposure time (h) | Exposure time (h) | Exposure time (h) | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| EO | 76 ± 13Aa | 85 ± 0Aa | 94 ± 8Aa | 70 ± 18Aa | 91 ± 13Aa | 91 ± 13Aa | −17 ± 22Ab | 17 ± 14Ab | 47 ± 11Ab |
| EtOH | −2 ± 39Ba | 28 ± 39Ba | 38 ± 34Ba | −28 ± 43Ba | −21 ± 40Bb | −10 ± 28Bb | −41 ± 36ABCa | −30 ± 29BCb | −32 ± 31BCb |
| Hex | 55 ± 35Aa | 76 ± 13Aa | 91 ± 13Aa | 39 ± 49Aa | 63 ± 30Aa | 64 ± 27Aa | −30 ± 30ABb | −14 ± 25Bb | 1 ± 46Bb |
| Et2O | −59 ± 8Ca | −36 ± 26Ca | −21 ± 30Ca | −72 ± 8Bab | −67 ± 20Cab | −59 ± 20Cb | −69 ± 10Cab | −62 ± 24Dab | −64 ± 32Cb |
| EtOAc | −80 ± 25Ca | −85 ± 23Db | −81 ± 28Db | −61 ± 13Ba | −46 ± 18BCa | −28 ± 50BCa | −59 ± 10BCa | −60 ± 15CDab | −64 ± 22Cab |
| H2O | −54 ± 41Ca | −64 ± 41CDab | −61 ± 47Dab | −56 ± 43Ba | −59 ± 45BCab | −60 ± 45Cab | −40 ± 17ABCa | −32 ± 23BCDa | −32 ± 20BCa |
| Solvent | Concentration (mg/cm2) | |||||
|---|---|---|---|---|---|---|
| 0.1 | 0.05 | |||||
| Exposure time (h) | Exposure time (h) | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| EO | −54 ± 9ABCc | −37 ± 31ABc | −22 ± 24Ac | −42 ± 13ABc | −37 ± 29Ac | −15 ± 40Ac |
| EtOH | −35 ± 11Aa | −18 ± 22Ab | −16 ± 23Ab | −33 ± 25Aa | −23 ± 24Ab | −20 ± 30Ab |
| Hex | −47 ± 43ABb | −58 ± 39Bc | −52 ± 38ABc | −76 ± 16Cb | −73 ± 17BCc | −78 ± 19Cc |
| Et2O | −78 ± 16Cb | −74 ± 30Bb | −74 ± 37Cb | −78 ± 4Cb | −83 ± 14Cb | −81 ± 14Cb |
| EtOAc | −65 ± 15ABCa | −67 ± 19Bab | −54 ± 22ABab | −58 ± 22BCa | −48 ± 28ABa | −35 ± 29Aa |
| H2O | −68 ± 17BCa | −67 ± 16Bab | −63 ± 10Cab | −78 ± 14Ca | −81 ± 21Cb | −84 ± 20Bb |
EO essential oil, EtOH ethyl alcohol, Hex n-hexane, Et2O diethyl ether, EtOAc ethyl acetate, H2O water. Data are expressed as mean ± standard deviation of five replicates. Different uppercase letters within the same column indicate a significant difference (P ≤ 0.05). Different lowercase letters within the same row indicate a significant difference (P ≤ 0.05)
Bioassay-guided isolation of Hex fraction
In the anti-insect assay for screening, the screening test confirmed that the Hex fraction had the strongest insect-repelling activity. Therefore, the Hex fraction was further isolated using column chromatography and TLC into 6 Hex subfractions (A1–6). The yields of A1–6 were 1274, 169, 164, 149, 141, and 229 g/100 kg of raw material, respectively (data not shown).
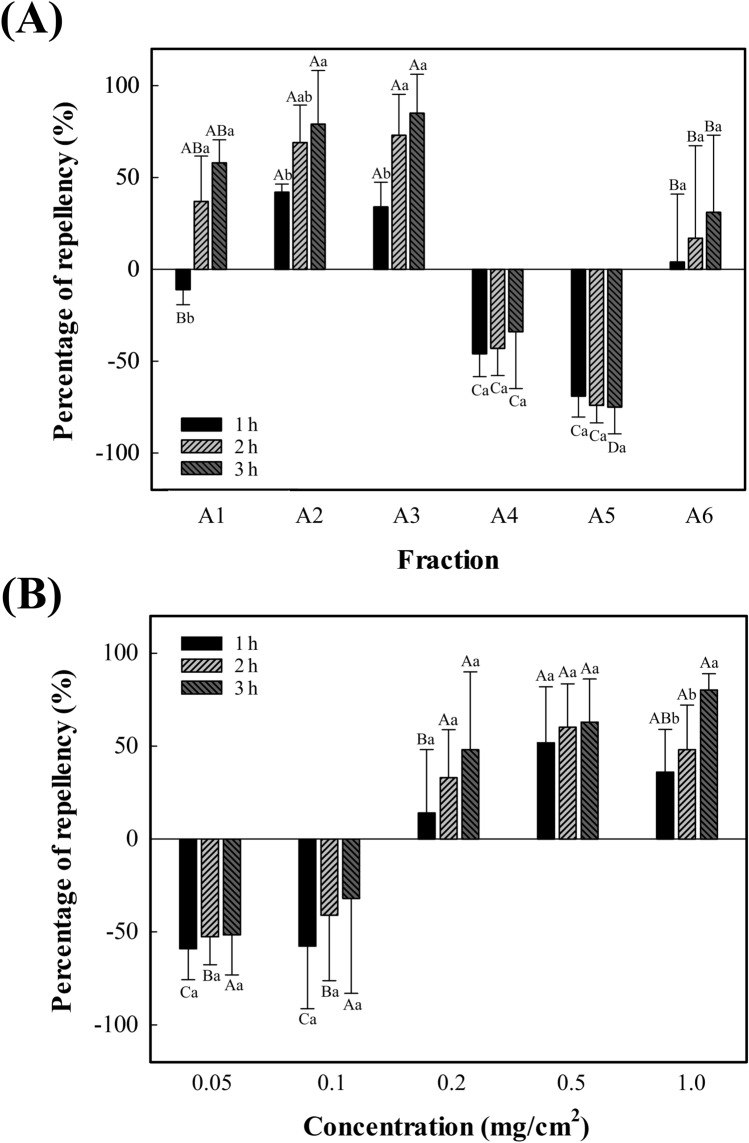
To isolate the Hex subfraction with the highest insect-repelling activity, the PR values of 6 Hex subfractions (A1–6) were measured. Regardless of the similar yields of A2–5, their PR values presented an opposite trend like A2–3 of positive PR values and A4–5 of negative PR values. In the isolation process, a specific solvent use can affect their yields of subfractions and their various constituents. This means that different constituents, having neutral, organic, basic, or polar properties, can be present in each subfraction (Bacon et al., 2017; Passari et al., 2019; Ribeiro de Souza et al., 2009). Particularly, A2 and A3 showed the highest PR values among subfractions having positive PR values (Fig. 3A). The PR values in A2 after exposure times of 1, 2, and 3 h were 42%, 69%, and 79%, respectively. Meanwhile, the PR values in A3 after 1, 2, and 3 h exposure times were 34%, 73%, and 85%, respectively. These two PR values were slightly higher than those in the Hex fraction at 0.5 mg/cm2. In actuality, the PR values in Hex fraction after exposure times of 1, 2, and 3 h were 39%, 63%, and 64%, respectively (Table 1), with no significant differences (P > 0.05) but lower standard deviations compared to those of A2 and A3. The removal of unnecessary components from the fractionation process might probably affect this, meaning that the content of a major single component with the strongest insect-repelling activity increased. These results presented that the isolation process was valid to increase the insect-repelling activity of the materials. Meanwhile, other Hex subfractions (A1 and A4-6) had very low PR values. From these results, we could predict that A2 and A3 contained the major single components having strong insect-repelling activity, and their mixture was selected as optimal for further identification.
Fig. 3.
Percentage repellency (%) against Plodia interpunctella larvae of (A) Hex subfractions (A1–6) from star anise extract at a concentration of 0.5 mg/cm2 and (B) trans-anethole at different concentrations (0.05, 0.1, 0.2, 0.5, and 1 mg/cm2), after the exposure time 1, 2, and 3 h. Each column represents the mean of triplicate experiments and error bars indicate the standard deviation. Different uppercase letters within the different fraction groups indicate a significant difference (P ≤ 0.05) and different lowercase letters within the different treatment times indicate a significant difference (P ≤ 0.05)
GC–MS analysis of Hex fraction and subfractions
Chemical constituents from the Hex fraction and subfraction (mixture of A1–3) were analyzed by GC–MS (Tables 2 and 3, respectively). In this analysis of the Hex fraction, 20 single components were identified (Table 2). The three major compounds of the Hex fraction were found to be trans-anethole (69.43%), p-anisaldehyde (5.73%), and phenol (2.18%). The trans-anethole was assumed to be the single key component showing strong insect-repelling activity in SA Hex fraction, inconstancy with the previous reports that trans-anethole was the main active compound in the extract or essential oil of SA (Patra et al., 2020; Singh et al., 2006). The differences in the composition of major compounds were potentially due to the differences in extraction methods and associated conditions including solvent type and extraction temperature (Sadeh et al., 2019).
Table 2.
Chemical composition of the Hex fraction identified by GC–MS analysis
| Number | Retention time (min) | Constituent | Formula | Composition (%) |
|---|---|---|---|---|
| 1 | 17.729 | Linalool | C10H18O | 0.18 |
| 2 | 21.544 | Terpinen − 4-ol | C10H18O | 0.1 |
| 3 | 22.149 | α-terpineol | C10H18O | 0.14 |
| 4 | 22.485 | Estragol | C10H12O | 0.29 |
| 5 | 25.443 | p-anisaldehyde | C8H8O2 | 5.73 |
| 6 | 27.208 | trans-anethole | C10H12O | 69.43 |
| 7 | 29.762 | Phenol | C6H6O | 2.18 |
| 8 | 30.653 | α-copaene | C15H24 | 0.25 |
| 9 | 30.905 | Linoleic acid | C18H32O2 | 2.57 |
| 10 | 32.569 | β-caryophyllene | C15H24 | 0.28 |
| 11 | 33.073 | trans-α-bergamotene | C15H24 | 0.35 |
| 12 | 34.014 | trans-β-farnesene | C15H24 | 0.13 |
| 13 | 35.98 | β-bisabolene | C15H24 | 0.16 |
| 14 | 38.333 | Methoxy-cinnaldehyde | C10H10O2 | 0.42 |
| 15 | 41.711 | T-muurolol | C15H26O | 0.21 |
| 16 | 42.434 | Chavicol | C9H10O | 1.79 |
| 17 | 51.912 | Palmitic acid | C16H32O2 | 1.35 |
| 18 | 57.441 | 9,12-octadecadienoic acid | C18H32O2 | 1.27 |
| 19 | 57.626 | Oleic acid | C18H34O2 | 1.28 |
| 20 | 62.433 | Bianisal, photoanethole | C16H16O2 | 1.1 |
| Total | 89.21 |
Table 3.
Chemical composition of the Hex subfraction identified by GC–MS analysis
| Number | Retention time (min) | Constituent | Formula | Composition (%) |
|---|---|---|---|---|
| 1 | 22.603 | Estragol | C10H12O | 0.5 |
| 2 | 25.56 | p-anisaldehyde | C8H8O2 | 0.27 |
| 3 | 27.493 | trans-anethole | C10H12O | 94.24 |
| 4 | 30.703 | α-copaene | C15H24 | 0.37 |
| 5 | 32.585 | β-caryophyllene | C15H24 | 0.23 |
| 6 | 33.089 | trans-α-bergamotene | C15H24 | 0.57 |
| 7 | 42.45 | Chavicol | C9H10O | 2.02 |
| Total | 98.2 |
In GC–MS analysis of the Hex subfraction, a total of seven single components were identified (Table 3). Among them, quantitatively, trans-anethole (94.24%) and chavicol (2.02%) were the two major compounds. Considering the results of the anti-insect assay of Hex subfractions, it was assumed that trans-anethole was the single key component showing the strongest insect-repelling activity. The chavicol was also reported to have insect-repelling or insecticidal activities against various insect pests (de Menezes et al., 2020). However, the chavicol was only present at a rate of 2.02%, meaning that chavicol would have only a slight effect on the insect-repelling activity of the Hex subfraction. Therefore, chavicol was not selected as the major single active compound having the strongest insect-repelling activity.
Insect-repelling activity of trans-anethole
Figure 3B presents the results of the bioassay on trans-anethole. The PR values over 0.5 mg/cm2 concentration showed strong insect repellent activity only after 1 h exposure time, similar to those of A2 and A3. Although the PR values for trans-anethole after an exposure time of 2 and 3 h looked somewhat lower than those of Hex subfractions A2 and A3 at the same concentration (0.5 mg/cm2), there were no significant differences among them (P > 0.05). This is because the trans-anethole, the single key component in SA having the most intensive insect-repelling activity, is the main constituent (approximately 94%) in Hex subfractions A2 and A3, as demonstrated in the previous “GC–MS analysis of Hex fraction and subfractions” section. Nevertheless, the slightly higher mean PR values of A2 and A3 subfractions might be based on the synergistic repellent effects coming from the mixture of various components. From the GC–MS analyses, trans-anethole and chavicol, having an insect-repelling activity, were found in Hex subfractions as two main constituents. Thus, a possible synergistic repellent effect could be expected through the interaction among the compounds present (Sarma et al., 2019). Another researcher also reported following similar results. For example, repelling activity against Aedes albopictus larvae was found to be stronger in Sophora alopecuroides extract than when applying its single dominant component (Shoukat et al., 2020). Accordingly, the use of subfractions (A2 and A3) showed better insect-repelling activity than the use of trans-anethole alone.
AChE-inhibiting activity of trans-anethole
Most insecticides present toxicity against pests based on the mechanism of AChE-inhibition. In insects, acetylcholine is present as a major neurotransmitter compound carrying electrical impulses to mediate muscle contractions. An AChE hydrolyzes the neurotransmitter to regulate nerve impulses at the postsynaptic membrane. Here, the AChE-inhibition assay was investigated against P. interpunctella to confirm the insect mortality of the volatile compounds, trans-anethole (Colovic et al., 2013; Lee et al., 2017; Rajashekar et al., 2014). The trans-anethole inhibited AChE by only 12.42 ± 7.59%. According to other researchers, trans-anethole had a low inhibitory activity of AChE (Yeom et al., 2012). On the other hand, other chemicals such as allyl mercaptan showed a high enzyme inhibition rate (> 50%) (Lee et al., 2017). Additionally, EOs and volatile compounds such as terpenes and sulfur-containing compounds are known to be active on various targets, including γ-aminobutyric acid (GABA)-gated chloride channels, octopamine receptors, tyramine receptors, nicotinic acetylcholine receptors, sodium channels, and possibly other targets associated with neurotoxicity (Tong and Coats, 2010). Therefore, anti-insect properties confirmed in this study could be due to other mechanisms rather than the AChE inhibition. These results indicate the value of further studies on the mechanism behind the anti-insect effects.
In this study, the Hex fraction from SA extract showed the strongest insect-repelling activity against P. interpunctella larvae compared to other different solvent fractions (Et2O, EtOAc, and H2O). Then, its fraction was further purified by a bioassay-guided isolation process to obtain a pure substance while removing unnecessary compounds in the Hex fraction. Among the various Hex subfractions, A2 and A3 exhibited the most intense insect-repelling activity against their target larvae, and the single key constituent in the two Hex fractions was found to be trans-anethole via GC–MS analysis. According to the bioassays of Hex subfractions (A2 and A3) and trans-anethole, there were no significant differences (P > 0.05) among their PR values, but the mean values of Hex subfractions were slightly higher than that of the single key constituent due to the synergy effect of major constituents, chavicol as well as the trans-anethole. Therefore, we show the usability of the Hex subfractions A2 and A3 as alternatives of trans-anethole, known as a famous insect-repellent single component. This study also supports the necessity of blending trans-anethole with other major components to obtain better insect-repelling activity, which is another issue for further studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was carried out with the support of R&D Program for Forest Science Technology (Project No. 2021375A00-2123-BD02) provided by Korea Forest Service (Korea Forestry Promotion Institute). This study was also supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (118048-03).
Declarations
Conflict of interest
The authors declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Inyoung Choi, Email: haunlzy@naver.com.
Seungyeon Kim, Email: ksyeon6855@naver.com.
Jung-Soo Lee, Email: food_passion@naver.com.
Yoonjee Chang, Email: ychang@kookmin.ac.kr.
Ja Hyun Na, Email: rhacoy@korea.ac.kr.
Jaejoon Han, Email: jjhan@korea.ac.kr.
References
- Arthur FH, Phillips TW. Stored-product insect pest management and control. pp. 341-348. In: Food Plant Sanitation. Hui YH, Bruinsma BL, Gorham JR, Nip WK, Tong PS, Ventresca P (ed). Marcel Dekker, New York, NY, USA (2003)
- Attia FI. Insecticide resistance in Plodia interpunctella (Hubner)(Lepidoptera: Pyralidae) in New South Wales. Australia. Australian Journal of Entomology. 1977;16:149–152. doi: 10.1111/j.1440-6055.1977.tb00076.x. [DOI] [Google Scholar]
- Bacon K, Boyer R, Denbow C, O'Keefe S, Neilson A, & Williams R. Antibacterial activity of jalapeño pepper (Capsicum annuum var. annuum) extract fractions against select foodborne pathogens. Food Science & Nutrition 5(3): 730–738 (2017) [DOI] [PMC free article] [PubMed]
- Balti MA, Hadrich B, Kriaa K, Kechaou N. Lab-scale extraction of essential oils from Tunisian lemongrass (Cymbopogon flexuosus) Chemical Engineering and Processing-Process Intensification. 2018;124:164–173. doi: 10.1016/j.cep.2017.12.012. [DOI] [Google Scholar]
- Chang Y, Lee SH, Na JH, Chang PS, Han J. Protection of grain products from Sitophilus oryzae (L.) contamination by anti-insect pest repellent sachet containing allyl mercaptan microcapsule. Journal of Food Science. 82: 2634-2642 (2017) [DOI] [PubMed]
- Colovic MB, Krsti DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Current Neuropharmacology. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De M, De AK, Sen P, Banerjee AB. Antimicrobial properties of star anise (Illicium verum Hook f) Phytotherapy Research. 2002;16:94–95. doi: 10.1002/ptr.989. [DOI] [PubMed] [Google Scholar]
- de Menezes CWG, Carvalho GA, Alves DS, de Carvalho AA, Aazza S, de Oliveira Ramos V Pinto JEBP, Bertolucci SKV. Biocontrol potential of methyl chavicol for managing Spodoptera frugiperda (Lepidoptera: Noctuidae), an important corn pest. Environmental Science and Pollution Research. 27: 5030-5041 (2020) [DOI] [PubMed]
- Dinesha R, Thammannagowda S, Prabhu M, Madhu C, Srinivas L. The antioxidant and DNA protectant activities of Star Anise (Illicium verum) aqueous extracts. Journal of Pharmacognosy and Phytochemistry. 2014;2:98–103. [Google Scholar]
- Ding X, Yang CW, Yang ZB. Effects of star anise (Illicium verum Hook.f.), essential oil, and leavings on growth performance, serum, and liver antioxidant status of broiler chickens. Journal of Applied Poultry Research. 26: 459-466 (2017)
- Hoque MS, Benjakul S, Prodpran T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocolloids. 2011;25:1085–1097. doi: 10.1016/j.foodhyd.2010.10.005. [DOI] [Google Scholar]
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of En Tomology. 2005;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Park KM, Min SC, Na JH, Park KH, Han J. Development of an anti-insect sachet using a polyvinyl alcohol−cinnamon oil polymer strip against Plodia interpunctella. Journal of Food Science. 2013;78:E1713–E1720. doi: 10.1111/1750-3841.12268. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Park KM, Na JH, Min SC, Park KH, Chang PS, Han J. Development of anti-insect food packaging film containing a polyvinyl alcohol and cinnamon oil emulsion at a pilot plant scale. Journal of Stored Products Research. 2015;61:114–118. doi: 10.1016/j.jspr.2015.01.005. [DOI] [Google Scholar]
- Kim H, Yu YS, Lee KY. Synergistic effects of heat and diatomaceous earth treatment for the control of Plodia interpunctella (Lepidoptera: Pyralidae) Entomological Research. 2014;44:130–136. doi: 10.1111/1748-5967.12058. [DOI] [Google Scholar]
- Kim J, Park NH, Na JH, Han J. Development of natural insect-repellent loaded halloysite nanotubes and their application to food packaging to prevent Plodia interpunctella infestation. Journal of Food Science. 2016;81:E1956–E1965. doi: 10.1111/1750-3841.13373. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon CS, Lee SE, Na JH, Han J. Development of insect-proof starch adhesive containing encapsulated cinnamon oil for paper box adhesion to inhibit Plodia interpunctella larvae infestation. Journal of Food Science. 2020;85:3363–3371. doi: 10.1111/1750-3841.15425. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon CS, Na JH, Han J. Prolonged insecticidal activity of clove oil-loaded halloysite nanotubes on Plodia interpunctella infestation and application in industrial-scale food packaging. Journal of Food Science. 2019;84:2520–2527. doi: 10.1111/1750-3841.14750. [DOI] [PubMed] [Google Scholar]
- Kumari R, Agrawal S, Singh S, Dubey NK. Supplemental ultraviolet-B induced changes in essential oil composition and total phenolics of Acorus calamus L. (sweet flag). Ecotoxicology and Environmental Safety. 72: 2013-2019 (2009) [DOI] [PubMed]
- Lee JE, Moon SR, Ahn HG, Cho SR, Yang JO, Yoon CM, Kim GH. Feeding behavior of Lycorma delicatula (Hemiptera: Fulgoridae) and response on feeding stimulants of some plants. Korean Journal of Applied Entomology. 2009;48:467–477. doi: 10.5656/KSAE.2009.48.4.467. [DOI] [Google Scholar]
- Lee JS, Chang Y, Park MA, Oh J, Han J. Insect-repellent activity of PET-based film with star anise essential oil and its pilot-scale production for food packaging. Food Packaging and Shelf Life. 25: 100539 (2020)
- Lee JS, Lee J, Choi I, Chang Y, Yoon CS, Han J. Isolation, screening, and identification of key components having intense insect repellent activity against Plodia interpunctella from 4 different medicinal plant materials. Journal of the Science of Food and Agriculture. 101: in press (2021) [DOI] [PubMed]
- Lee JS, Park MA, Yoon CS, Na JH, Han J. Characterization and preservation performance of multilayer film with insect repellent and antimicrobial activities for sliced wheat bread packaging. Journal of Food Science. 2019;84:3194–3203. doi: 10.1111/1750-3841.14823. [DOI] [PubMed] [Google Scholar]
- Lee SH, Chang Y, Na JH, Han J. Development of anti-insect multilayered films for brown rice packaging that prevent Plodia interpunctella infestation. Journal of Stored Products Research. 2017;72:153–160. doi: 10.1016/j.jspr.2017.05.001. [DOI] [Google Scholar]
- Passari LMZG, Ieda S Scarminio, Gustavo G Marcheafave, Roy E Bruns. Seasonal changes and solvent effects on fractionated functional food component yields from Mikania laevigata leaves. Food Chemistry 273: 151–158 (2019) [DOI] [PubMed]
- Park MA, Chang Y, Choi I, Bai J, Na JH, Han J. Development of a comprehensive biological hazard-proof packaging film with insect-repellent, antibacterial, and antifungal activities. Journal of Food Science. 2018;83:3035–3043. doi: 10.1111/1750-3841.14397. [DOI] [PubMed] [Google Scholar]
- Patra JK, Das G, Bose S, Banerjee S, Vishnuprasad CN, del Pilar Rodriguez-Torres M, Shin HS. Star anise (Illicium verum): Chemical compounds, antiviral properties, and clinical relevance. Phytotherapy Research. 2020;34:1248–1267. doi: 10.1002/ptr.6614. [DOI] [PubMed] [Google Scholar]
- Phillips TW, Berberet RC, Cuperus GW. Post-harvest integrated pest management. pp. 2690–2701. In: Encyclopedia of food science and technology. 2nd ed. Wiley. New York, NY, USA (2000)
- Phillips TW, Throne JE. Biorational approaches to managing stored-product insects. Annual Review of Entomology. 2009;55:375–397. doi: 10.1146/annurev.ento.54.110807.090451. [DOI] [PubMed] [Google Scholar]
- Prajapati V, Tripathi A, Aggarwal K, Khanuja S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresource Technology. 2005;96:1749–1757. doi: 10.1016/j.biortech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Rajashekar Y, Raghavendra A, Bakthavatsalam N. Acetylcholinesterase inhibition by biofumigant (Coumaran) from leaves of Lantana camara in stored grain and household insect pests. BioMed Research International. 2014;2014:1–6. doi: 10.1155/2014/187019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran S, Sriranjini V. Plant products as fumigants for stored-product insect control. Journal of Stored Products Research. 2008;44:126–135. doi: 10.1016/j.jspr.2007.08.003. [DOI] [Google Scholar]
- Rees D. Insects of stored products. pp. 2-3. CSIRO Publishing. Collingwood, VIC, Australia (2004)
- Regnault-Roger C, Vincent C, Arnason JT. Essential oils in insect control: low-risk products in a high-stakes world. Annual Review of Entomology. 2012;57:405–424. doi: 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- Ribeiro de Souza, EB, da Silva RR, Afonso S, Scarminio IS. Enhanced extraction yields and mobile phase separations by solvent mixtures for the analysis of metabolites in Annona muricata L. leaves. Journal of Separation Science. 32(23–24): 4176–4185 (2009) [DOI] [PubMed]
- Sadeh D, Nitzan N, Chaimovitsh D, Shachter A, Ghanim M, Dudai N. Interactive effects of genotype, seasonality and extraction method on chemical compositions and yield of essential oil from rosemary (Rosmarinus officinalis L.). Industrial Crops and Products. 138: 111419 (2019)
- Sarma R, Adhikari K, Mahanta S, Khanikor B. Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae) Scientific Reports. 2019;9:1–12. doi: 10.1038/s41598-019-45908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoukat RF, Shakeel M, Rizvi SAH, Zafar J, Zhang Y, Freed S, Xu X, Jin F. Larvicidal, ovicidal, synergistic, and repellent activities of Sophora alopecuroides and its dominant constituents against Aedes albopictus. Insects. 2020;11:246. doi: 10.3390/insects11040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Maurya S, DeLampasona M, Catalan C. Chemical constituents, antimicrobial investigations and antioxidative potential of volatile oil and acetone extract of star anise fruits. Journal of the Science of Food and Agriculture. 2006;86:111–121. doi: 10.1002/jsfa.2277. [DOI] [Google Scholar]
- Tong F, Coats JR. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pesticide Biochemistry and Physiology. 2010;98:317–324. doi: 10.1016/j.pestbp.2010.07.003. [DOI] [Google Scholar]
- Tripathi P, Dubey NK. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biology and Technology. 2004;32:235–245. doi: 10.1016/j.postharvbio.2003.11.005. [DOI] [Google Scholar]
- Wang Z, Xie Y, Sabier M, Zhang T, Deng J, Song X, Liao Z, Li Q, Yang S, Cao Y, Liu X, Zhou G. Trans-anethole is a potent toxic fumigant that partially inhibits rusty grain beetle (Cryptolestes ferrugineus) acetylcholinesterase activity. Industrial Crops and Products. 161: 113207 (2021)
- Yeom HJ, Kang JS, Kim GH, Park IK. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica) Journal of Agricultural and Food Chemistry. 2012;60:7194–7203. doi: 10.1021/jf302009w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.