Abstract
Purpose
To describe point-of-care lung ultrasound (POC-LUS) artifact findings in children admitted to the pediatric intensive care unit (PICU) for acute respiratory failure (ARF).
Methods
This is a secondary analysis of a prospective observational study completed in a 21-bed PICU. Children > 37 weeks gestational age and ≤ 18 years were enrolled from December 2018 to February 2020. POC-LUS was completed and interpreted by separate physicians blinded to all clinical information. POC-LUS was evaluated for the presence of lung sliding, pleural line characteristics, ultrasound artifacts, and the ultrasound diagnosis.
Results
Eighty-seven subjects were included. A-lines were the most frequent artifact, occurring in 58% of lung zones (163/281) in those with bronchiolitis, 39% of lung zones (64/164) in those with pneumonia, and 81% of lung zones (48/59) in those with status asthmaticus. Sub-pleural consolidation was second most common, occurring in 28% (80/281), 30% (50/164), and 12% (7/59) of those with bronchiolitis, pneumonia, and status asthmaticus, respectively. The pattern a priori defined as bronchiolitis, pneumonia, and status asthmaticus was demonstrated in 31% (15/48), 10% (3/29), and 40% (4/10) of subjects with bronchiolitis, pneumonia, and status asthmaticus, respectively.
Conclusion
We found significant heterogeneity and overlap of POC-LUS artifacts across the most common etiologies of ARF in children admitted to the PICU. We have described the POC-LUS artifact findings in pediatric ARF to support clinicians using POC-LUS and to guide future pediatric POC-LUS studies. Determining the optimal role of POC-LUS as an adjunct in the care of pediatric patients requires further study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40477-022-00675-2.
Keywords: Acute respiratory failure, Critical care, Diagnostic imaging, Lung ultrasound, Pediatrics, Point-of-care ultrasound
Introduction
Acute respiratory failure (ARF) accounts for more than a quarter of all pediatric intensive care unit (PICU) admissions, making it one of the most common indications for admission [1]. Despite high disease burden, pediatric ARF’s epidemiology remains poorly described due to its diagnostic challenges [2]. Initial management focuses primarily on supportive care and treatment of the underlying disease process [2]. However, given frequent diagnostic uncertainty, many are initially treated with a multi-therapy approach until the etiology underlying the ARF becomes better defined, after which therapeutic de-escalation occurs. Accurately identifying the etiology leading to ARF early in the hospital course could improve the care these children receive by helping direct timely and necessary therapies while allowing cessation of unnecessary treatments. Point-of-care lung ultrasound (POC-LUS) has emerged as an invaluable tool in assessing adults with ARF, demonstrating high sensitivity and specificity in determining the cause of ARF on presentation [3, 4]. Therefore, it is prudent to establish if POC-LUS can be equally effective in determining the cause of ARF in infants and children.
While there exists a growing body of evidence supporting the use of POC-LUS in the diagnosis and care of pediatric patients in the emergency department (ED) and inpatient ward, relevance of this data to children with ARF in the PICU remains unclear. There is increasing use of point-of-care ultrasound (POCUS) in the PICU and many high quality reviews describing its use in pediatric ARF [5–7]. In addition to its diagnostic capabilities, benefits of POC-LUS include a rapid time to image acquisition, short exam time, real-time interpretation and integration into patient care, promotion of time spent at the bedside, low cost, and repeatability [7–10]. However, standardized clinical research in pediatric POC-LUS in the PICU is still lacking [10]; published pediatric POCUS guidelines recommend use of POC-LUS in critically ill pediatric patients based on low-quality evidence (level B and lower), and primarily on neonatal and adult intensive care unit (ICU) studies [8]. Given the increasing interest in diagnostic POCUS in pediatric ARF [5], our objective is to describe POC-LUS artifact findings in a previously published cohort of children admitted to the PICU with ARF to help guide clinicians and inform future POC-LUS research studies [11].
Methods
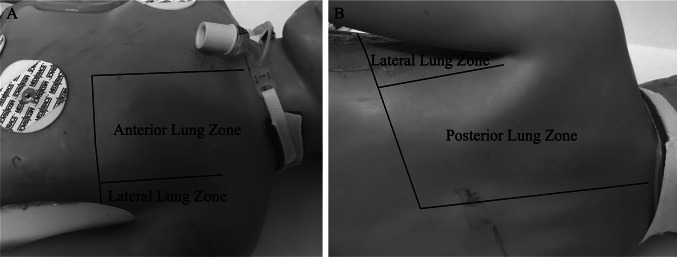
The Institutional Review Board at the University of Wisconsin-Madison approved this secondary analysis of a prospective observational study (completed December 2018 to February 2020) evaluating the sensitivity and specificity of POC-LUS in determining the etiology of ARF on PICU admission (IRB 2018-0711). The full study protocol has been previously published [11]. In summary, following informed consent, a POC-LUS was performed by one of three PICU physicians with 1–3 years clinical POCUS experience, who were not providing clinical care to the patient, and who were blinded to historical and clinical subject information (except as apparent at the bedside), scanning 3-zones (anterior, lateral, posterior) per hemi-thorax (Fig. 1) for the most abnormal sonographic finding(s) within each zone. A video clip of the most abnormal finding was saved to Q-path (Telexy Healthcare; British Columbia, Canada) and interpreted offline by a second physician, an intensivist with > 7 years clinical POCUS experience, who was blinded to all subject information. POC-LUS was examined for the presence of lung sliding, pleural line characteristics, ultrasound artifacts (examples in Fig. 2), and ultrasound diagnosis, and findings were recorded in Q-path. A second expert (an ultrasound-fellowship trained ED physician with > 7 years clinical POCUS experience) additionally reviewed the ultrasound diagnoses but due to loss of data during a Q-path system upgrade (documentation of the image location/lung zone), was not able to assess artifacts based on anatomic location. Thus, this report contains data only from the single intensivist reviewer. Ultrasound exams were completed within 14 h of PICU admission, as close to admission as feasible. Patients were diagnosed with ARF if the treating physician determined a clinical need for PICU admission and use of noninvasive or invasive respiratory support [2, 11].
Fig. 1.
Lung ultrasound scanning locations. Anterior zone located between the anterior axillary line and sternum, lateral zone between the anterior and posterior axillary lines, and posterior zone between the posterior axillary line and spine
Fig. 2.
Ultrasound artifact examples. Video examples of ultrasound artifacts in the online supplement
A literature review was conducted prior to the start of the study to define the ultrasound patterns of the most likely causes of ARF to be evaluated during the study. At the time of inception, there were no published POC-LUS studies conducted within the PICU. We thus incorporated literature evaluating subjects in the pediatric ED [12–15], pediatric ward [16–20], and adult ICUs [3, 4, 21] to define the patterns of the a priori determined most likely causes of pediatric ARF: status asthmaticus [4, 12], bronchiolitis/viral pneumonitis [15–17, 19], bacterial pneumonia [3, 14, 18, 20], acute respiratory distress syndrome (ARDS) [3, 4], and pulmonary edema [3, 4]. We also included definitions for pneumothorax [22, 23] and pleural effusion [24], since their findings are distinct and had been well described in the literature. As ARDS is a clinical syndrome rather than a discrete etiologic cause of respiratory failure with diagnostic criteria that overlap other causes of respiratory failure, we analyzed data for this manuscript based on the underlying etiologic cause of the ARF [11]. Studies were collated to define ultrasound patterns correlating with these likely etiologies (Supplementary Table 1), as previously published [11].
We compared the ultrasound diagnosis to the diagnostic reference standard (termed final diagnosis), an independent standardized review of the medical record (EMR) following hospital discharge by a physician not involved in the subject’s care and blinded to POC-LUS findings and diagnosis [11]. The final diagnoses were likewise developed using an evidence-based review of the literature incorporating the EMR documented history, clinical findings, laboratory tests, radiographic data, hospital management, and course to determine the etiology of the ARF that led to the hospitalization [11]. Disease defining characteristics are presented in Supplementary Table 2. Study comparisons are presented using descriptive statistics (numbers and percentages for categorical variables and medians with interquartile ranges for continuous variables). Categorical variables are compared between groups using the Pearson’s chi-squared or Fisher’s exact tests and continuous variables compared using the Kruskal–Wallis test. All use a two-tailed p value ≤ 0.05 as demonstrating statistical significance. Data were analyzed using R (version 3.6.3).
Results
Eighty-eight subjects were enrolled in the parent study, 87 of whom are included in this analysis. One subject was excluded because of an inability of two reviewers to congruently determine a single final diagnosis. There were 48 subjects (55%) with a final diagnosis of bronchiolitis/viral pneumonitis, 29 (33%) with pneumonia, and 10 (11%) with status asthmaticus. The cohort was 63% male (55/87), had a median age of 22 months (IQR 7.5–67); 66% had no medical history (57/87) while 34% had bronchopulmonary dysplasia or asthma (30/87); and 44% were receiving HFNC at the time POC-LUS was completed (38/87) with 42% on noninvasive positive pressure ventilation (37/87), 6% on invasive ventilation (5/87), and 8% receiving continuous nebulized therapy without other support (7/87) (Table 1). A detailed comparison of ultrasound artifact findings by disease process, hemi-thorax side, and lung zone location is shown in Supplementary Table 3.
Table 1.
Demographics of study population
| Bronchiolitis, n = 48 | Pneumonia, n = 29 | Status Asthmaticus, n = 10 | p-value | |
|---|---|---|---|---|
| Age in months (median, IQR) | 14.5 (4.8–41.3) | 25.0 (14.0–86.0) | 65.0 (37.3–76.8) | < 0.01 |
| Male sex (n, %) | 32 (67%) | 17 (59%) | 6 (60%) | 0.77 |
| Medical History (n, %)b | ||||
| None | 34 (71%) | 21 (72%) | 2 (20%) | < 0.01 |
| Asthma | 10 (21%) | 2 (7%) | 8 (80%) | < 0.01 |
| Bronchopulmonary dysplasiac | 5 (10%) | 4 (14%) | 0 (0%) | 0.10 |
| Chronic aspiration | 3 (6%) | 2 (7%) | 0 (0%) | 1.00 |
| Baseline pulmonary medications | 15 (45%) | 11 (38%) | 8 (80%) | 0.02 |
| Home respiratory support device | 7 (15%) | 4 (14%) | 0 (0%) | 0.59 |
| Respiratory Support (n, %)a | < 0.01 | |||
| HFNC | 22 (46%) | 15 (52%) | 1 (10%) | |
| CPAP | 7 (15%) | 2 (7%) | 0 (0%) | |
| BiPAP | 16 (33%) | 10 (34%) | 2 (20%) | |
| Mechanical ventilation | 3 (6%) | 2 (7%) | 0 (0%) | |
| Continuous nebulized therapy | 0 (0%) | 0 (0%) | 7 (70%) | |
| Respiratory Therapies (n, %)a | ||||
| B-agonist | 21 (44%) | 12 (41%) | 10 (100%) | < 0.01 |
| Corticosteroid | 9 (19%) | 3 (10%) | 10 (100%) | < 0.01 |
| Hypertonic saline | 3 (6%) | 2 (7%) | 0 (0%) | 1.00 |
| Ipratropium | 6 (13%) | 2 (7%) | 9 (90%) | < 0.01 |
| Magnesium | 2 (4%) | 1 (3%) | 2 (20%) | 0.14 |
| Racemic epinephrine | 0 (0%) | 3 (10%) | 0 (0%) | 0.07 |
| Antibiotics | 21 (44%) | 27 (93%) | 4 (40%) | < 0.01 |
| Respiratory Viral Panel (n, %)a | ||||
| Positived | 35 (97%) | 15 (79%) | 2 (66%) | 0.05 |
| Negatived | 1 (3%) | 4 (21%) | 1 (33%) | |
| Not obtainede | 12 (25%) | 10 (34%) | 7 (70%) | 0.03 |
| Length of stay (median, IQR) | ||||
| PICU (h) | 44 (31–63) | 64 (25–120) | 27 (23–46) | 0.08 |
| Hospital (h) | 94 (61–133) | 116 (64–211) | 46 (41–62) | < 0.01 |
BiPAP bilevel positive airway pressure, CPAP continuous positive airway pressure, HFNC high flow nasal cannula, IQR interquartile range, PICU pediatric intensive care unit
aReceiving when POC-LUS performed; bsum > 100% given patients with multiple underlying medical conditions; cbronchopulmonary dysplasia, chronic lung disease, or chronic respiratory failure diagnosis; dpercent of obtained tests; epercent of total patients
Ultrasound characteristics in bronchiolitis
An evaluation of sliding was not documented in 2.4% of imaged zones (7/288), was absent in 0.3% (1/281), and was present in 99.6% of interpreted zones (280/281). Evaluation of the pleural line was not documented in 7.3% of imaged zones (21/288), was thick and irregular in 21% (56/267), and thin and smooth in 79% of interpreted zones (211/267). Evaluation of ultrasound artifacts was not documented in 2.4% of imaged zones (7/288). A-lines were the most common artifact occurring in 58% of interpreted zones (163/281), sub-pleural consolidation was second most common occurring in 28% of lung zones (80/281), and multiple B-lines third most common occurring in 14.5% of lung zones (41/281). Lobar consolidation was found in 5.3% of lung zones (15/281).
Ultrasound characteristics in pneumonia
An evaluation of sliding was not documented in 2.9% of imaged zones (5/174), was absent in 0.6% (1/169), and present in 99.4% of interpreted zones (168/169). Evaluation of the pleural line was not documented in 16.7% of imaged zones (29/174), was thick and irregular in 23.4% (34/145), and thin and smooth in 76.6% of interpreted zones (111/145). Evaluation of ultrasound artifacts was not documented in 5.7% of imaged zones (10/174). A-lines were the most common artifact occurring in 39% of interpreted zones (64/164), sub-pleural consolidation was second most common occurring in 30.5% of lung zones (50/164), and multiple B-lines third most common occurring in 23.2% of lung zones (38/164). Lobar consolidation was found in only 14% of lung zones (23/164).
Ultrasound characteristics in status asthmaticus
An evaluation of sliding was made for all imaged lung zones, was absent in 1.7% (1/60), and present in 98.3% of interpreted zones (59/60). Evaluation of the pleural line was not documented in 6.7% of imaged zones (4/60), was thick and irregular in 5.3% (3/56), and thin and smooth in 94.6% of interpreted zones (53/56). Evaluation of ultrasound artifacts was not documented in 1.7% of imaged zones (1/60). A-lines were the most common artifact occurring in 81.4% of interpreted zones (48/59), sub-pleural consolidation was second most common occurring in 11.9% of lung zones (7/59), and multiple B-lines third most common occurring in 8.5% of lung zones (5/59). Lobar consolidation was not seen in subjects with status asthmaticus.
Overall
Diffuse A-lines were found in 17% of subjects (15/87), B-lines at any location in 47% (41/87), sub-pleural consolidation at any location in 63% (54/87), and lobar consolidation at any location in 33% of subjects (29/87) (Table 2). Diffuse A-lines demonstrating the pattern defined as status asthmaticus was demonstrated in 40% of subjects with a final diagnosis of status asthmaticus (4/10). Multiple B-lines (10%; 1/10), multiple B-lines with sub-pleural consolidations (20%; 2/10), and sub-pleural consolidations (30%; 3/10) were the other patterns identified. The pattern defining bronchiolitis consisting of either bilateral sub-pleural consolidation without other findings or bilateral sub-pleural consolidation with multiple B-lines was seen in 13% (6/48) and 13% (6/48) of subjects with a final diagnosis bronchiolitis, respectively. These patterns were also seen in 10% (3/29) and 7% (2/29) of subjects with pneumonia and 0% (0/10) and 10% (1/10) with status asthmaticus, respectively.
Table 2.
Comparison of the presence of specific ultrasound artifacts in each subject for each diagnosis
| Final diagnosis | Bronchiolitis, n = 48 | Pneumonia, n = 29 | Status asthmaticus, n = 10 | p-value |
|---|---|---|---|---|
| A-lines | 9 (19%) | 2 (7%) | 4 (40%) | 0.05 |
| B-linesa | 24 (50%) | 15 (52%) | 2 (20%) | 0.20 |
| Subpleural Consolidation | 29 (60%) | 19 (66%) | 7 (70%) | 0.85 |
| Lobar Consolidation | 13 (27%) | 16 (55%) | 0 (0%) | < 0.01 |
aMultiple or confluent B-lines
Diffuse A-lines were identified in 19% (9/48) and lobar consolidation identified in 27% (13/48) of subjects with a final diagnosis of bronchiolitis. A unilateral or bilateral lobar consolidation without other abnormalities was seen in only 10% (3/29) of subjects with a final diagnosis of pneumonia. Lobar consolidation with sub-pleural consolidation (21%; 6/29), with multiple B-lines (7%; 2/29), or with sub-pleural consolidation and multiple B-lines (17%; 5/29), were also common. There were 44% (13/29) of subjects diagnosed with pneumonia who did not have a lobar consolidation, including 7% (2/29) who only had identified A-lines. A comparison of individual ultrasound artifacts present in each disease process is shown in Table 2 and a comparison of ultrasound artifact patterns for each diagnosis is shown in Table 3.
Table 3.
Comparison of most frequent ultrasound artifact patterns for each diagnosis
| Final diagnosis | ||||
|---|---|---|---|---|
| Ultrasound pattern | Bronchiolitis, n = 48 | Pneumonia, n = 29 | Status Asthmaticus, n = 10 | p-value |
| Diffuse A-lines | 9 (19%) | 2 (7%) | 4 (40%) | 0.05 |
| Multiple B-lines | 0.86 | |||
| Unilateral B-lines | 5 (10%) | 2 (7%) | 0 (0%) | |
| Bilateral B-lines | 1 (2%) | 0 (0%) | 0 (0%) | |
| SPC only | 0.06 | |||
| Unilateral SPC | 4 (8%) | 0 (0%) | 3 (30%) | |
| Bilateral SPC | 6 (13%) | 3 (10%) | 0 (0%) | |
| SPC + Multiple B-lines | 0.75 | |||
| Unilateral SPC | 4 (8%) | 3 (10%) | 2 (20%) | |
| Bilateral SPC | 6 (13%) | 2 (7%) | 1 (10%) | |
| LC only | 0.61 | |||
| Unilateral LC | 2 (4%) | 2 (7%) | 0 (0%) | |
| Bilateral LC | 0 (0%) | 1 (3%) | 0 (0%) | |
| LC + Any other pattern | 0.01 | |||
| Unilateral LC | 11 (23%) | 9 (31%) | 0 (0%) | |
| Bilateral LC | 0 (0%) | 4 (14%) | 0 (0%) | |
| Any other pattern | 0 (0%) | 1 (3%) | 0 (0%) | 0.45 |
SPC subpleural consolidation, LC lobar consolidation
Discussion
Many studies have described POC-LUS artifact findings across a range of disorders that can cause children to develop respiratory distress and ARF [9, 12, 13, 15–17, 19, 22, 25–32]. These reports, however, have been limited in the number of children enrolled with ARF, which may affect the sensitivity and specificity of the POC-LUS exam [11]. It has been consistently described that children with more ultrasound abnormalities have prolonged clinical courses and higher resource utilization [12, 13, 16, 26, 29, 31]. Our study is the first to characterize diagnostic POC-LUS artifact findings in children admitted to the PICU for undifferentiated ARF, demonstrating significant heterogeneity with a range of sonographic findings across the most common causes of pediatric ARF: bronchiolitis/viral pneumonitis, pneumonia, and status asthmaticus. We found diffuse A-lines in 17% of all subjects, B-lines at any location in 47%, sub-pleural consolidation at any location in 63%, and lobar consolidation at any location in 33% of subjects.
Reports evaluating homogenous patient populations with known or suspected disease processes have found a high discriminatory value of POC-LUS. Meta-analyses evaluating the ability of POC-LUS to diagnose pneumonia find both sensitivity and specificity > 90% [25, 32]. Meta-analyses have not been conducted for other pediatric disease processes but individual studies likewise show high sensitivity and specificity identifying bronchiolitis [17, 19, 29, 32, 33]. Asthma exacerbation is the exception and to date, only a single study has specifically evaluated children with suspected asthma exacerbation and found abnormalities in 45% (27/60) [12], these included B-lines in 38% (23/60), large consolidations (≥ 1 cm) in 20% (12/60), pleural line abnormalities in 12% (7/60), and small consolidations (< 1 cm in size) in 10% (6/60). The authors hypothesized their findings might be attributable to the high incidence of asthma exacerbations triggered by viral respiratory tract infections. We likewise demonstrated a high incidence of POC-LUS abnormalities in children diagnosed with status asthmaticus finding 60% with any POC-LUS artifact abnormality, including 30% with unilateral sub-pleural consolidations and 30% with sub-pleural consolidations and multiple B-lines.
Few studies have evaluated children in undifferentiated respiratory distress or failure in which the suspected clinical diagnosis is unknown to the sonographer [9, 13, 27, 28]. Varshney et al. evaluated 94 children ≤ 2 years with signs of respiratory tract infection and wheezing in the ED and found sonographic abnormalities in 42% (39/94) [13]. Among those with bronchiolitis 46% demonstrated any abnormality (33/72), in those with asthma 0% demonstrated abnormalities (0/14), in those with pneumonia 100% demonstrated abnormalities (4/4), and in those with asthma and concomitant pneumonia 50% demonstrated ultrasound abnormalities (2/4) with a trend toward a greater proportion of abnormalities in those requiring hospitalization (67%, 4/6) compared with those discharged home (40%, 35/88). Importantly, and similar to our study, a wide range of findings was documented—among those with bronchiolitis, B-lines were present in 36% (26/72), small consolidations (< 1 cm size) in 25% (18/72), and large consolidation (≥ 1 cm) in 3% (2/72). Likewise, in those with pneumonia, B-lines were present in 100% (4/4), small consolidations in 50% (2/4) and large consolidations in 25% (1/4). In those with asthma and concomitant pneumonia, B-lines, small consolidations and large consolidations were present in 25% each (1/4 for each finding). Similarly, Buonsenso et al. evaluated 186 children with suspected acute lower respiratory tract infection 1 month to 17 years and found significant artifact overlap [28]. Consolidation was found in 100% of subjects with bacterial infection (67/67) of which 22% were < 1.5 cm (15/67), 55% were 1.5–4 cm (37/67), and 22% were > 4 cm in size (15/67); consolidation was also found in 85% with viral infection (65/76; 63% (41/65) < 1.5 cm, 35% (23/65) 1.5–4 cm, and 2% (1/65) > 4 cm) and in 88% with atypical infection (38/42; 50% (19/38) < 1.5 cm, 45% (17/38) 1.5–4 cm, and 5% (2/38) > 4 cm). Multiple consolidations, bilateral consolidations, and air bronchograms were found in all disease processes. While there was a trend toward larger consolidations in bacterial infections and smaller consolidations in viral infections, the calculated positive predictive value (PPV) of consolidation > 1.5 cm in predicting bacterial infection was 54% (52/95; 83% for consolidation > 4 cm (15/18)) and the PPV of consolidation < 1.5 cm in size predicting viral infection was 55% (41/75). The other two published studies evaluating children in undifferentiated respiratory distress or failure did not provide the frequency of disease specific artifact abnormalities [9, 27].
Our results and those of others indicate the need for more extensive research to determine the role of POC-LUS as an adjunct imaging modality in the care of children with respiratory distress and ARF. The unique physiology of pediatric patients, especially infants and young children, as well as an overlap of ultrasound findings, likely decreases the sensitivity and specificity of individual ultrasound artifacts in undifferentiated respiratory diseases, contrasting with the well-described and consistent findings in older children and adults [34]; it has even been shown that a majority of healthy infants demonstrate abnormal POC-LUS during the first 6 months of life [30, 35]. Atelectasis presenting as a POC-LUS consolidation, in particular, may be especially difficult to differentiate from pneumonia and is common in both asthma exacerbations and bronchiolitis [17, 25]. We found lobar consolidation in 33% of subjects [29/87; including 27% of subjects with bronchiolitis (13/38) and 55% with pneumonia (16/29)] and sub-pleural consolidation in 39% [34/87; including 42% in subjects with bronchiolitis (20/48), 28% with pneumonia (8/29), and 60% with status asthmaticus (6/10)]. The small size of pediatric airways coupled with a high propensity for further narrowing by secretions, edema, and bronchoconstriction results in a high resistance to airflow [2]. Pediatric airways also lack rigid cartilaginous support and collateral ventilation, making them more susceptible to dynamic compression, obstruction, and collapse [2].
Bronchiolitis is especially common in infants and young children and can affect proximal airways, distal parenchyma, or both [36]. As a result, the clinical signs, symptoms, and presentation are widely variable and overlap both with status asthmaticus and pneumonia, contributing to its clinical, and likely sonographic, diagnostic challenge. Similar to other studies, we found a small number of normal ultrasound patterns (diffuse A-lines) in children with bronchiolitis [13, 15, 16, 29, 31] and lobar consolidations [13, 16, 19, 29, 31]. These characteristics of pediatric physiology and pathophysiology may explain the modest study results of researchers who have used POC-LUS to evaluate children with undifferentiated respiratory distress or failure [9, 11, 13, 27, 28]. Of these, Ozkaya et al. demonstrated the highest sensitivity and specificity in determining the etiology of undifferentiated respiratory distress or failure but their study protocol also allowed the study physician clinical and historical information if unable to arrive at a diagnosis following blinded POC-LUS [9]. This is in comparison to those evaluating a known or suspected single disease entity and supports the idea that the current sonographic criteria that have been well defined in adult POC-LUS may not extrapolate to the pediatric population, and in particular, to the critically ill pediatric population [11, 34]. It may turn out that pediatric POC-LUS is best used to help clinicians differentiate underlying lung pathophysiology or disease severity to guide treatment [16, 26, 29, 37, 38], rather than to help determine a specific clinical diagnosis. As examples, using POC-LUS pre- and post-albuterol may aid in determining albuterol responsiveness; using POC-LUS pre- and post-ventilator adjustment to determine lung recruitability, and using POC-LUS to guide fluid administration/removal.
The major strength of our study is its blinded methodology, thus limiting confirmation bias of ultrasound findings but necessarily decreasing direct clinical applicability. While POC-LUS is not highly sensitive or specific when interpreted alone [9, 11, 13, 27, 28], we expect that diagnostic accuracy will be improved when interpreted along with clinical knowledge and integration of the patient history and physical examination. However, we are not aware of a study that has evaluated this question. We studied the most common causes of pediatric ARF but were not able to capture cases of pleural effusion, pneumothorax, or pulmonary edema; these etiologies are rare causes of ARF that are more commonly seen in more complicated cases, in patients with underlying systemic illness, or later in the disease course [our median time to POC-LUS 7.1 h (IQR, 3.5–10.4 h)] [11, 37–39]. Important limitations of this study that may have decreased the ability of POC-LUS to differentiate individual etiologies of ARF include: (1) examining only the most abnormal artifact within each zone and not incorporating dynamic ultrasound artifacts into our diagnostic schema, including not differentiating consolidations (sub-pleural or lobar) based on lesion size or evaluating for the presence/absence of mobile or static air bronchograms, both of which may decrease sensitivity and specificity of ultrasound [30, 37]; (2) exams were completed and interpreted by different providers, both without clinical knowledge of the subject, which may decrease the likelihood of identifying/recognizing important sonographic lesions; and (3) only a single interpretation of ultrasound artifacts was completed by a POC-LUS expert physician, which makes assessment of inter-rater reliability impossible. We also did not include any cases of SARS-CoV2 as the study was completed before the start of the COVID-19 pandemic, so the applicability of the findings to current PICU practice is unclear. The next step in systematically evaluating use of POC-LUS in the PICU will be to evaluate a non-blinded study protocol to determine which ultrasound findings change clinical management and how POC-LUS findings guide therapeutic decision making. Determining the utility of POC-LUS in objectively evaluating reversibility of bronchoconstriction, the effects of treatment on lung aeration, changes in ultrasound artifacts with therapy, and the effects of therapy on intra-alveolar edema could prove to be future patient-centered POC-LUS applications.
Conclusion
Use of diagnostic POC-LUS is common in pediatric ARF despite lack of robust supporting evidence and is mainly based on adult literature. We have found significant overlap in POC-LUS artifacts across common etiologies of pediatric ARF, including very commonly occurring B-lines and sub-pleural consolidations in patients with bronchiolitis, pneumonia, and status asthmaticus using a blinded study protocol. We herein describe POC-LUS findings across these diagnoses to support pediatric clinicians use of POC-LUS in patient care and to help guide future lung ultrasound research studies. There are also pathophysiologic considerations for why pediatric and adult POC-LUS may differ and we therefore call for additional research, including with the use of non-blinded study protocols, into the use of pediatric POC-LUS in ARF.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
RLD, PDK, and AMA contributed to the conception and design of the study, data collection, analysis and interpretation, manuscript preparation and gave final approval of the version to be published. EAC and JS contributed to data collection, manuscript preparation and gave final approval of the version to be published. MRL contributed to data analysis and interpretation, manuscript preparation and gave final approval of the version to be published. RLD is the guarantor of the paper, had full access to all study data, and takes responsibility for the integrity of the data and accuracy of analysis, from inception to published article.
Funding
This project was supported by the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS), Grant UL1TR002373. The content of the work and manuscript are solely the responsibility of the authors and do not represent the views of the NIH. The funding source had no involvement in the study design; collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to submit the article for publication. The authors have declared no other sources of funding related to this work.
Declarations
Conflict of interest
Dr. Al-Subu has a consulting agreement with Edwards Lifesciences LLC. The remaining authors have declared no conflicts of interest related to this work.
Study location
This work was completed at the University of Wisconsin-Madison, American Family Children’s Hospital Pediatric Intensive Care Unit.
Ethical approval
The Institutional Review Board at the University of Wisconsin-Madison approved this study (IRB 2018-0711). All parents provided written informed consent and patients provided assent when appropriate.
Prior data presentation
Study data was presented in poster format at the Wisconsin Pediatric Critical Care 2021 Regional Meeting (April 20, 2021; Virtual conference). Study registered on ClinicalTrials.gov (Identifier NCT03744169).
Consent to publish
The authors declare that the submitted work has not been published previously and that the work is not under consideration for publication elsewhere.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010;11:549–555. doi: 10.1097/PCC.0b013e3181ce7427. [DOI] [PubMed] [Google Scholar]
- 2.Schneider J, Sweberg T. Acute respiratory failure. Crit Care Clin. 2013;29:167–183. doi: 10.1016/j.ccc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Inglis AJ, Nalos M, Sue K-H, et al. Bedside lung ultrasound, mobile radiography and physical examination: a comparative analysis of diagnostic tools in the critically ill. Crit Care Resusc. 2016;18:124. [PubMed] [Google Scholar]
- 4.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlon TW, Kantor DB, Su ER, et al. Diagnostic bedside ultrasound program development in pediatric critical care medicine: results of a National Survey. Pediatr Crit Care Med. 2018;19:e561–e568. doi: 10.1097/PCC.0000000000001692. [DOI] [PubMed] [Google Scholar]
- 6.Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199:701–714. doi: 10.1164/rccm.201802-0236CI. [DOI] [PubMed] [Google Scholar]
- 7.Conlon TW, Nishisaki A, Singh Y, et al. Moving beyond the stethoscope: diagnostic point-of-care ultrasound in pediatric practice. Pediatrics. 2019 doi: 10.1542/peds.2019-1402. [DOI] [PubMed] [Google Scholar]
- 8.Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) Crit Care. 2020;24:65. doi: 10.1186/s13054-020-2787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Özkaya AK, Başkan Vuralkan F, Ardıç Ş. Point-of-care lung ultrasound in children with non-cardiac respiratory distress or tachypnea. Am J Emerg Med. 2019;37:2102–2106. doi: 10.1016/j.ajem.2019.05.063. [DOI] [PubMed] [Google Scholar]
- 10.Watkins LA, Dial SP, Koenig SJ, et al. The utility of point-of-care ultrasound in the pediatric intensive care unit. J Intensive Care Med. 2021 doi: 10.1177/08850666211047824. [DOI] [PubMed] [Google Scholar]
- 11.DeSanti RL, Al-Subu AM, Cowan EA, et al. Point-of-care lung ultrasound to diagnose the etiology of acute respiratory failure at admission to the PICU. Pediatr Crit Care Med. 2021;22:722–732. doi: 10.1097/PCC.0000000000002716. [DOI] [PubMed] [Google Scholar]
- 12.Dankoff S, Li P, Shapiro AJ, et al. (2017) Point of care lung ultrasound of children with acute asthma exacerbations in the pediatric ED. Am J Emerg Med 35:615–622. 10.1016/j.ajem.2016.12.057 [DOI] [PubMed]
- 13.Varshney T, Mok E, Shapiro AJ, et al. Point-of-care lung ultrasound in young children with respiratory tract infections and wheeze. Emerg Med J. 2016;33:603–610. doi: 10.1136/emermed-2015-205302. [DOI] [PubMed] [Google Scholar]
- 14.Milliner BHA, Tsung JW. Lung consolidation locations for optimal lung ultrasound scanning in diagnosing pediatric pneumonia. J ultrasound Med. 2017;36:2325–2328. doi: 10.1002/jum.14272. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JS, Hughes N, Tat S, et al. The utility of bedside lung ultrasound findings in bronchiolitis. Pediatr Emerg Care. 2017;33:97–100. doi: 10.1097/PEC.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 16.Basile V, Di Mauro A, Scalini E, et al. Lung ultrasound: a useful tool in diagnosis and management of bronchiolitis. BMC Pediatr. 2015;15:63. doi: 10.1186/s12887-015-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biagi C, Pierantoni L, Baldazzi M, et al. Lung ultrasound for the diagnosis of pneumonia in children with acute bronchiolitis. BMC Pulm Med. 2018;18:191. doi: 10.1186/s12890-018-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caiulo VA, Gargani L, Caiulo S, et al. Lung ultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatr Pulmonol. 2013;48:280–287. doi: 10.1002/ppul.22585. [DOI] [PubMed] [Google Scholar]
- 19.Caiulo VA, Gargani L, Caiulo S, et al. Lung ultrasound in bronchiolitis: comparison with chest X-ray. Eur J Pediatr. 2011;170:1427–1433. doi: 10.1007/s00431-011-1461-2. [DOI] [PubMed] [Google Scholar]
- 20.Urbankowska E, Krenke K, Drobczyński Ł, et al. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir Med. 2015;109:1207–1212. doi: 10.1016/j.rmed.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Ng C, Tsung JW. Point-of-care ultrasound for assisting in needle aspiration of spontaneous pneumothorax in the pediatric ED: a case series. Am J Emerg Med. 2014;32:488.e3–8. doi: 10.1016/j.ajem.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Raimondi F, Rodriguez Fanjul J, Aversa S, et al. Lung ultrasound for diagnosing pneumothorax in the critically ill neonate. J Pediatr. 2016;175:74–78.e1. doi: 10.1016/j.jpeds.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Kurian J, Levin TL, Han BK, et al. Comparison of ultrasound and CT in the evaluation of pneumonia complicated by parapneumonic effusion in children. AJR Am J Roentgenol. 2009;193:1648–1654. doi: 10.2214/AJR.09.2791. [DOI] [PubMed] [Google Scholar]
- 25.Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714–722. doi: 10.1542/peds.2014-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Mauro A, Cappiello AR, Ammirabile A, et al. Lung ultrasound and clinical progression of acute bronchiolitis: a prospective observational single-center study. Medicina (Kaunas) 2020 doi: 10.3390/medicina56060314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripathi S, Ganatra H, Martinez E, et al. Accuracy and reliability of bedside thoracic ultrasound in detecting pulmonary pathology in a heterogeneous pediatric intensive care unit population. J Clin Ultrasound. 2019;47:63–70. doi: 10.1002/jcu.22657. [DOI] [PubMed] [Google Scholar]
- 28.Buonsenso D, Musolino A, Ferro V, et al. Role of lung ultrasound for the etiological diagnosis of acute lower respiratory tract infection (ALRTI) in children: a prospective study. J Ultrasound. 2021 doi: 10.1007/s40477-021-00600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Supino MC, Buonsenso D, Scateni S, et al. Point-of-care lung ultrasound in infants with bronchiolitis in the pediatric emergency department: a prospective study. Eur J Pediatr. 2019;178:623–632. doi: 10.1007/s00431-019-03335-6. [DOI] [PubMed] [Google Scholar]
- 30.Buonsenso D, De Rose C, Ferro V, et al. Lung ultrasound to detect cardiopulmonary interactions in acutely ill children. Pediatr Pulmonol. 2022;57:483–497. doi: 10.1002/ppul.25755. [DOI] [PubMed] [Google Scholar]
- 31.Bueno-Campaña M, Sainz T, Alba M, et al. Lung ultrasound for prediction of respiratory support in infants with acute bronchiolitis: a cohort study. Pediatr Pulmonol. 2019;54:873–880. doi: 10.1002/ppul.24287. [DOI] [PubMed] [Google Scholar]
- 32.Heuvelings CC, Bélard S, Familusi MA, et al. Chest ultrasound for the diagnosis of paediatric pulmonary diseases: a systematic review and meta-analysis of diagnostic test accuracy. Br Med Bull. 2019;129:35–51. doi: 10.1093/bmb/ldy041. [DOI] [PubMed] [Google Scholar]
- 33.Di Mauro A, Ammirabile A, Quercia M, et al. Acute bronchiolitis: is there a role for lung ultrasound? Diagnostics (Basel, Switzerland) 2019 doi: 10.3390/diagnostics9040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buonsenso D, Musolino AM, Gatto A, et al. Lung ultrasound in infants with bronchiolitis. BMC Pulm Med. 2019;19:159. doi: 10.1186/s12890-019-0925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buonsenso D, Soldati G, Curatola A, et al. lung ultrasound pattern in healthy infants during the first 6 months of life. J Ultrasound Med Off J Am Inst Ultrasound Med. 2020;39:2379–2388. doi: 10.1002/jum.15347. [DOI] [PubMed] [Google Scholar]
- 36.Swaminathan AC, Carney JM, Tailor TD, Palmer SM. Overview and challenges of bronchiolar disorders. Ann Am Thorac Soc. 2020;17:253–263. doi: 10.1513/AnnalsATS.201907-569CME. [DOI] [PubMed] [Google Scholar]
- 37.Musolino AM, Tomà P, Supino MC, et al. Lung ultrasound features of children with complicated and noncomplicated community acquired pneumonia: a prospective study. Pediatr Pulmonol. 2019;54:1479–1486. doi: 10.1002/ppul.24426. [DOI] [PubMed] [Google Scholar]
- 38.Buonsenso D, Tomà P, Scateni S, et al. Lung ultrasound findings in pediatric community-acquired pneumonia requiring surgical procedures: a two-center prospective study. Pediatr Radiol. 2020;50:1560–1569. doi: 10.1007/s00247-020-04750-w. [DOI] [PubMed] [Google Scholar]
- 39.Randolph AG, Meert KL, O’Neil ME, et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334–1340. doi: 10.1164/rccm.200210-1175OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.