Abstract
The pandemic of the coronavirus disease 2019 (COVID-19) has made biotextiles, including face masks and protective clothing, quite familiar in our daily lives. Biotextiles are one broad category of textile products that are beyond our imagination. Currently, biotextiles have been routinely utilized in various biomedical fields, like daily protection, wound healing, tissue regeneration, drug delivery, and sensing, to improve the health and medical conditions of individuals. However, these biotextiles are commonly manufactured with fibers with diameters on the micrometer scale (> 10 μm). Recently, nanofibrous materials have aroused extensive attention in the fields of fiber science and textile engineering because the fibers with nanoscale diameters exhibited obviously superior performances, such as size and surface/interface effects as well as optical, electrical, mechanical, and biological properties, compared to microfibers. A combination of innovative electrospinning techniques and traditional textile-forming strategies opens a new window for the generation of nanofibrous biotextiles to renew and update traditional microfibrous biotextiles. In the last two decades, the conventional electrospinning device has been widely modified to generate nanofiber yarns (NYs) with the fiber diameters less than 1000 nm. The electrospun NYs can be further employed as the primary processing unit for manufacturing a new generation of nano-textiles using various textile-forming strategies. In this review, starting from the basic information of conventional electrospinning techniques, we summarize the innovative electrospinning strategies for NY fabrication and critically discuss their advantages and limitations. This review further covers the progress in the construction of electrospun NY-based nanotextiles and their recent applications in biomedical fields, mainly including surgical sutures, various scaffolds and implants for tissue engineering, smart wearable bioelectronics, and their current and potential applications in the COVID-19 pandemic. At the end, this review highlights and identifies the future needs and opportunities of electrospun NYs and NY-based nanotextiles for clinical use.
Keywords: Electrospinning, Textile-forming technique, Nanoyarns, Tissue scaffolds, Wearable bioelectronics
Abbreviations: bFGF, basic fibroblast growth factor; CNT, carbon nanotube; COVID-19, coronavirus disease 2019; ECM, extracellular matrix; FDA, food and drug administration; GF, gauge factor; GO, graphene oxide; HAp, hydroxyapatite; HAVIC, human aortic valve interstitial cell; NGC, nerve guidance conduit; NHMR, neutral hollow metal rod; NMD, neutral metal disc; NY, nanofiber yarn; MeGel, methacrylated gelatin; MSC, mesenchymal stem cell; MSC-SC, MSC derived Schwann cell-like cell; MWCNT, multiwalled carbon nanotube; MY, microfiber yarn; PA6, polyamide 6; PA66, polyamide 66; PAN, polyacrylonitrile; PANi, polyaniline; PCL, polycaprolactone; PEO, polyethylene oxide; PGA, polyglycolide; PHBV, poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PLCL, poly(L-lactide-co-ε-caprolactone); PLGA, poly(lactic-co-glycolic acid); PLLA, poly(L-lactic acid); PMIA, poly(m-phenylene isophthalamide); PPDO, polydioxanone; PPy, polypyrrole; PSA, poly(sulfone amide); PU, polyurethane; PVA, poly(vinyl alcohol); PVAc, poly(vinyl acetate); PVDF, poly(vinylidene difluoride); PVDF-HFP, poly(vinylidene floride-co-hexafluoropropylene); PVDF-TrFE, poly(vinylidene fluoride trifluoroethylene); PVP, poly(vinyl pyrrolidone); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SC, Schwann cell; SF, silk fibroin; SWCNT, single-walled carbon nanotube; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor
Graphical abstract
1. Introduction
Biotextiles are one broad type of medical devices/materials with given textile structures and patterns and are used in specific biological environments, depending on their biocompatibility and biostability with cells and biological fluids. Biotextiles commonly include engineered textile scaffolds and implants [1], textile-based drug delivery carriers [2], hygiene textiles [3], and wearable bioelectronic devices [4]. For instance, the medical face masks are one typical biotextile, which are widely utilized to stop the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the COVID-19 pandemic. According to a case study, wearing a face mask could reduce 80% and 47% of the infection risk for healthcare workers and non-healthcare workers, respectively [5]. Another study reported that 17–45% and 24–65% of projected deaths could be prevented by appropriately wearing face masks in New York and Washington, respectively [6]. Actually, the use of biotextiles dates back to the ancient times, and they were first utilized to close or cover open wounds on human skin [7]. Nowadays, the widespread combination of biotechnology and textile engineering has brought various innovative biotextiles, which are becoming more important than anyone realizes [8,9].
The generation of biotextiles usually includes two main procedures [10,11]. Firstly, fiber-constructed yarns are produced from various natural or synthetic polymers using many different spinning methods. Melt spinning, dry spinning, and wet spinning are the three most notable spinning routes in fiber science and textile engineering [12]. The as-obtained yarns are then processed into diverse textile patterns with predetermined shapes, porosities, and mechanical properties using textile weaving, knitting, and braiding techniques, as well as other 3D textile-forming methods. Fibrous yarns are assuredly the primary processing unit for the fabrication of biotextiles, and the performances of the yarns are responsible for the performances of the final fabricated biotextiles. Currently, the commercially used yarns for the fabrication of biotextiles are constructed with microfibers with the diameters on the micrometer scale (typically larger than 10 μm). For implantable material applications, the fiber diameters are obviously larger than those of protein fibrils (diameters of several to several hundred nanometers) that exist in the native extracellular matrix (ECM) [13,14]. The unmatching fiber morphologies and sizes of as-fabricated biotextiles inevitably result in unsatisfactory cell interactions and therapeutic effects [15,16]. For wearable bioelectronic applications, the large fiber diameters of conventional microfibers negatively affect the device miniaturization, the weight, and the performance [17]. Therefore, reducing the fiber diameter of yarns for textile use is greatly required to improve the structures and properties of conventional microfibrous biotextiles [[18], [19], [20]].
Another key element for the fabrication of biotextiles, especially for implantable textiles, is the biomaterial selection. Previous studies demonstrated that the selected materials can notably influence the biological properties of biotextiles [21,22]. The commercialized textile products for implantable material applications are mainly fabricated from some non-degradable and bio-inert polymers, including cellulose, polyamide, polypropylene, polyester, poly(etheretherketone), poly(tetrafluoroethylene), carbon, etc. [23]. For instance, the commercial implantable textile products, such as ULTRAPRO, AQUACEL, INTERGARD, and TIGR Matrix, that are utilized to treat hernias, skin wounds, vascular injuries and disease, and pelvic organ prolapses, respectively, are either nonabsorbable or partially absorbable [12,24]. The clinical data suggests that these commercial implantable biotextiles have played an extremely important role in saving patients' lives and/or improving their quality of life, but there are still some drawbacks that need to be addressed, especially for implantable textiles [24]. For example, a strong foreign body response may take place after the non-degradable textile products are implanted, and the subsequent fibrosis completely engulfs the implanted textiles, leading to the defuctionalization of textile implants [25]. In addition, the long-term use of these non-degradable textile implants can release some debris from wear, which may be harmful for the surrounding cells and tissues, possibly causing cellular malformation, apoptosis, and even carcinogenesis [26,27]. Therefore, polymers with improved biocompatibility and controllable biodegradability are doubtlessly more appropriate for the fabrication of biotextile implants, which are expected to be gradually absorbed, accompanied by the generation and healing of diseased or damaged tissues and organs.
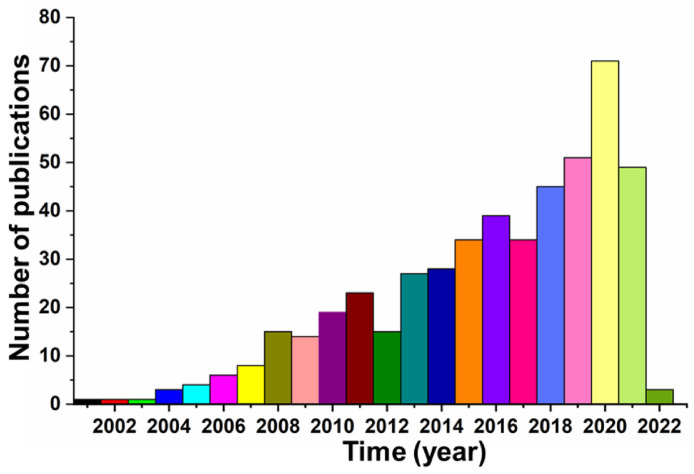
In the last two decades, electrospinning has been recognized as a feasible and versatile spinning method for generating fibers with diameters in the range of several to several hundred nanometers, which are at least ten times smaller than the fibers fabricated from traditional melt, dry, and wet spinning strategies [28]. The significantly decreased fiber diameter notably increased the specific surface area, and the ECM nanofibril-mimicking characteristics make electrospun nanofibers ideal materials for biomedical applications [29,30]. Importantly, a variety of biodegradable polymers with excellent biocompatibility and that originate from both natural and synthesized sources have been successfully processed into electrospun nanofibers, including silk fibroin (SF), collagen, gelatin, polydioxanone (PPDO), polycaprolactone (PCL), polyglycolide (PGA), poly(L-lactic acid) (PLLA), poly(lactic-co-glycolic acid) (PLGA), poly(L-lactide-co-ε-caprolactone) (PLCL), etc. [31,32]. In addition, various drugs and bioactive ingredients can be easily encapsulated into the nanofibers through the electrospinning technique, which can impart nanofibers with predetermined biological behaviors [33,34]. Moreover, a wide variety of post-treatment processes are perfectly suitable for the modification of electrospun nanofibers to further improve the properties and functions of as-prepared nanofibers [35,36]. The direct transformation of electrospun nanofibers into textile yarn-like structures, also named as nanofiber yarns (NYs), provides an innovative routine for renewing and updating the existing microfiber yarns (MYs) made from the traditional melt, dry, and wet spinning techniques [37]. Today, more and more studies are reporting modified electrospinning strategies for the fabrication of advanced NYs. 491 papers have been found using the terms “Electrospinning and Yarn” in the world-recognized database “Web of Science Core Collection”. The number of annual publications from 2000 to 2022 is shown in Fig. 1 . In the early 2000s, a few research groups started to modify the conventional electrospinning device to generate electrospun yarns. Since 2010, the number of publications related to electrospinning and yarns has been increasing rapidly every year. Moreover, some existing studies have already reported the innovative design and development of nanoarchitectured textiles using electrospun NYs for various biomedical applications in the most recent years.
Fig. 1.
Number of annual publications on Electrospun Yarns. The literary search is based on the terms “Electrospinning and Yarn” in the “Web of Science Core Collection” database from 2000 to 2022.
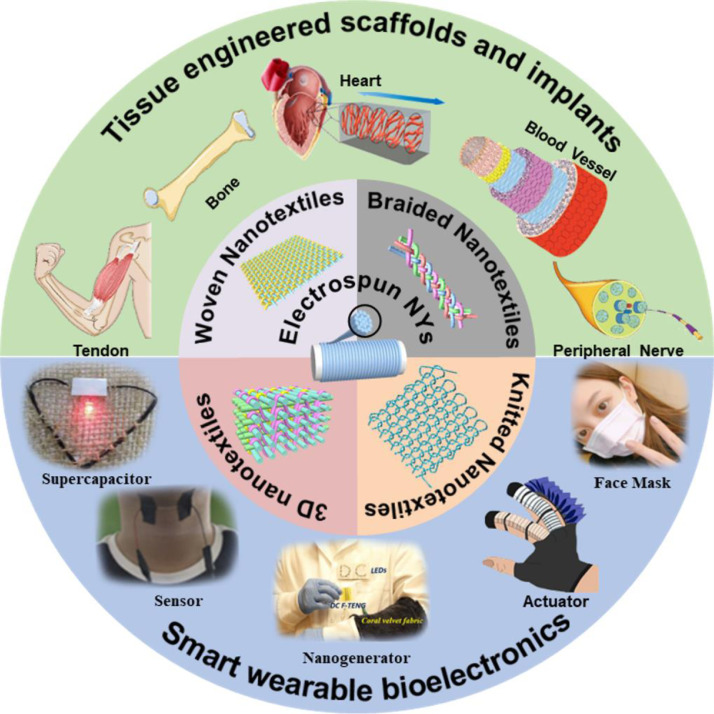
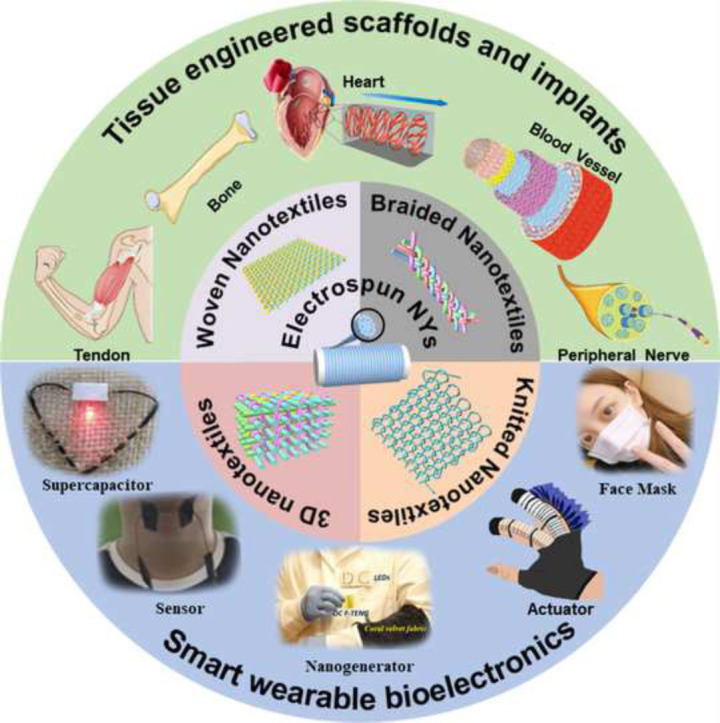
Although a lot of previous review papers introduced electrospinning strategies and their applications in biomedical engineering [[38], [39], [40], [41]], no review papers that systematically introduce the advances of electrospun nanofiber yarn-based textiles for biomedical applications were found. First, this review briefly introduces the basic principles of typical electrospinning techniques, including apparatuses and process parameters. Subsequently, this review highlights the recent progress in electrospun NY fabrication and summarizes the advantages and disadvantages of different strategies. Then, how the material composition and spinning process influence the morphologies, structures, and properties of electrospun NYs is discussed. This review also gives a comprehensive overview of the generation of nanotextiles that use electrospun NYs. Afterwards, this review presents some significant examples that highlight the advanced applications of electrospun NYs in biomedical fields. At the end of this review, we explore and identify the challenges, opportunities, and future needs of electrospun NYs and NY-based biotextiles for clinical use. Fig. 2 shows the whole schematic of the present review.
Fig. 2.
Fabrication of electrospun NY-based biotextiles and their applications in various biomedical fields. Some figure elements were rearranged and reprinted with permissions from Refs. [42], [43], [44], [45], [46], [47].
2. Principle and process of conventional electrospinning
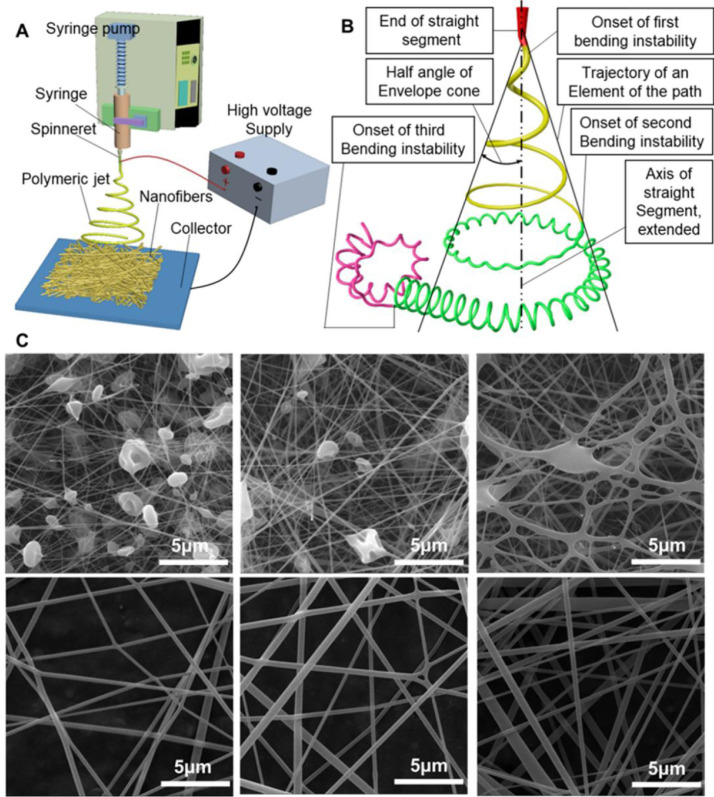
Electrospinning is a versatile, low cost strategy and is a simple process for producing polymeric nanofibers [48]. A conventional electrospinning device for lab research use is relatively simple, as shown in Fig. 3 A, mainly including one spinneret (usually in the form of a needle with a blunt tip), one syringe equipped with a syringe pump, one high voltage supply, and one conductive collector (usually in the form of an aluminum plate). Unlike the traditional melt spinning, dry spinning, or wet spinning, electrospinning employs a high-voltage electrostatic field to drive the whole spinning process. When an external electric field is applied to the spinneret, the electrostatic charges accumulate at the tip of a liquid droplet. Consequently, the electrostatic repulsion among the surface charges works against the surface tension and deforms and reshapes the droplet into a stretched cone (named a Taylor cone), and a jet is subsequently ejected from the Taylor cone when a threshold voltage is reached. This jet initially streams forward along a straight line and then experiences a complex whipping path due to bending instabilities, as shown in Fig. 3B [49]. The solvent volatilizes and the polymeric jet is elongated into a thinner diameter during the stretching and motion, and, finally, the nanofibers are generated and deposited on a preset collector. In general, a complicated electrohydrodynamic process occurs during electrospinning, which involves four distinct stages: liquid droplet charging and Taylor cone formation, jet initiation and straight segment formation, jet instability and whipping, and jet solidification and nanofiber deposition [50].
Fig. 3.
(A) Schematic of a basic electrospinning system. (B) Schematic of a pathway of an electrospun polymeric jet. (C) SEM images of electrospun nanofibers with different morphologies. The electrospun nanofibers were fabricated from different solution concentrations by dissolving cellulose acetate in a mixed solvent of acetone and N, N-Dimethylacetamide (2/1, v/v): 8% (w/w), 9% (w/w), 10% (w/w), 11% (w/w), 12% (w/w), 13% (w/w). (B) Redrawn based on Ref. [49]. (C) Reprinted with permission from Ref. [56].
Three typical instabilities occur with an electrically charged polymeric jet that affect the formation of nanofibers during the electrospinning process [[51], [52], [53]]. The Rayleigh instability is an axisymmetric varicose instability that tends to break the polymeric jet into small droplets. There is another axisymmetric instability, which happens under a much stronger electric field than the Rayleigh instability. The third type of instability is non-axisymmetric blending and whipping instability, which originates from the electrostatic repulsion among surface charges of polymeric jets as a result of a strong existing electrostatic field. To generate thinner nanofibers, it is significantly necessary to notably improve the whipping instability, which is responsible for bending and stretching of the jet during the jet movement [54,55]. Usually, the electrospinning strategy collects the nanofibers as meshes with dense packing densities and low thickness (usually < 1 mm). Three broad categories of factors affect the electrospinning process and the morphology and structure of collected polymeric nanofibers. Polymer solution variables are the first factor, which include molecular weight, concentration, viscosity, and solvent selection. The processing variables are another factor, referring to the applied voltage, solution feeding rate, spinning distance, spinneret, and collector selection. The third factor is environmental variables, including humidity, temperature, and atmospheric gas. An example of the influences of the polymer solution on the morphology of electrospun cellulose acetate nanofibers is shown in Fig. 3C [56]. Several recent reviews on how the process variables, environmental variables, and polymer solution variables influence the morphology and structure of electrospun nanofibers have been provided by Mailley et al. [57], Ibrahim and Klingner [58] and Haider et al. [59].
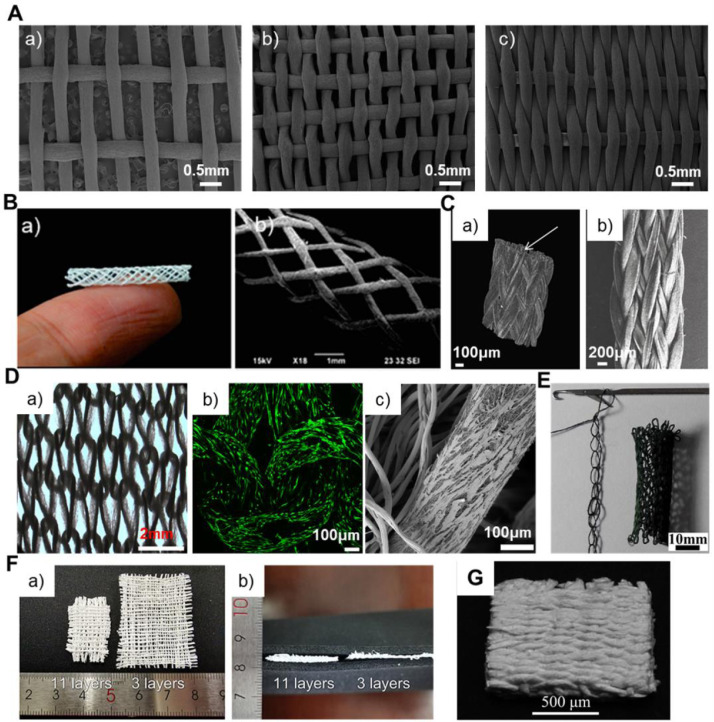
3. Electrospinning-based yarns
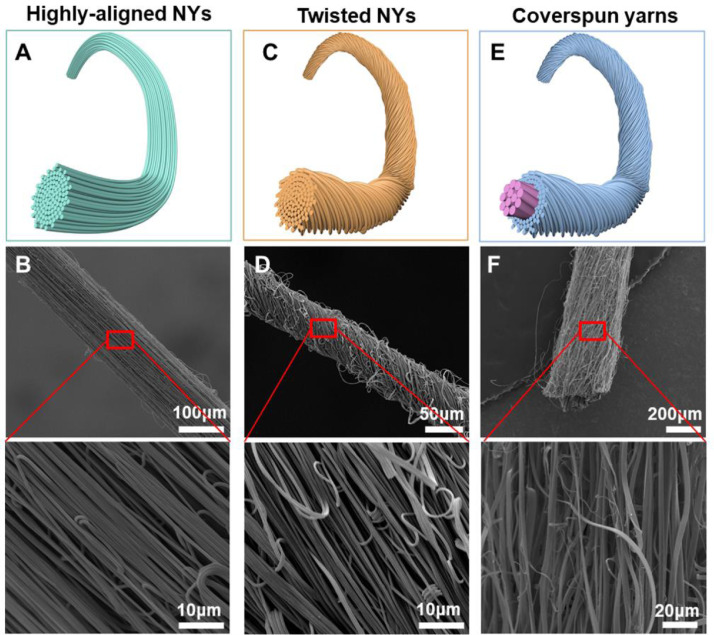
The idea of transforming electrospun nanofibers into yarn-like structures, i.e., NYs, was first reported by Anton Formhals in 1934 [60]. However, the electrospinning technique did not regain attention in the nanoscience community until the 1990s. In 2003, Ko et al. re-proposed the concept of employing modified electrospinning technology to generate NYs, but there were no detailed descriptions about their equipment [61]. Since then, modifying the conventional electrospinning devices to manufacture high quality NYs has become a long-term goal for fiber scientists and textile researchers. Over time, a series of traditional textile strategies have been introduced to modify the electrospinning technique for the production of NYs. At present, electrospinning-based yarns are mainly categorized into three different types, i.e., aligned NYs, twisted NYs, and coverspun yarns. Their schematics and morphologies are shown in Fig. 4 . The aligned NYs are constructed with numerous nanofibers that are uniaxially aligned along the yarn longitudinal direction, as shown in Fig. 4A and B [62]. The twisted NYs are also made of numerous nanofibers, but the nanofibers exhibit some twists as shown in Fig. 4C and D. The coverspun yarns are fabricated by coating numerous nanofibers on microfibers to generate a nanofiber-coated microfiber core-sheath structure, as shown in Fig. 4E and F. In addition, all the electrospinning-based yarns can also be categorized into discontinuous and continuous types in terms of the NY generating mechanisms and the continuity and length of the obtained yarn. For the discontinuous type, a limited yarn length (several millimeters to tens of centimeters) is generated at one time. As for the continuous type, at least several meters of yarns are manufactured at one time without the yarn breaking.
Fig. 4.
Different types of electrospun NYs. Schematic and SEM images of electrospun NYs with highly aligned fibrous structures (A, B) and with high twisting (C, D) and electrospun nanofiber-coated microfiber coverspun yarns with core-sheath structures (E, F). The blue dashed line indicates the inner microfibers of the coverspun yarns. (B) Reprinted with permission from Ref. [62].
3.1. Electrospun NY fabrication in a discontinuous manner
It is well-known that all the spinning methods include one necessary jet stretching process to refine and solidify fibers. The traditional melt spinning, dry spinning, and wet spinning utilize easily controlled compressed air, mechanical interaction, or a combination of both to realize the stretching of fibers Therefore, the stretching force is limited, and only microfibers with diameters larger than 10 μm can be generated [[63], [64], [65]]. In comparison, the dramatic stretching force caused by the complex three-dimensional whipping and bending instability during electrospinning is employed to stretch and solidify polymeric jets, and fibers with diameters of several to several hundred nanometers are formed [55]. However, the whipping and bending instability that are necessary for the nanofiber generation are also difficult to control, which creates significant hurdles for the continuous and controllable preparation of electrospun NYs. The reasonable control of the jet trajectory to arrange nanofibers into uniaxially aligned structures is one primary key process for the fabrication of high-quality electrospun NYs.
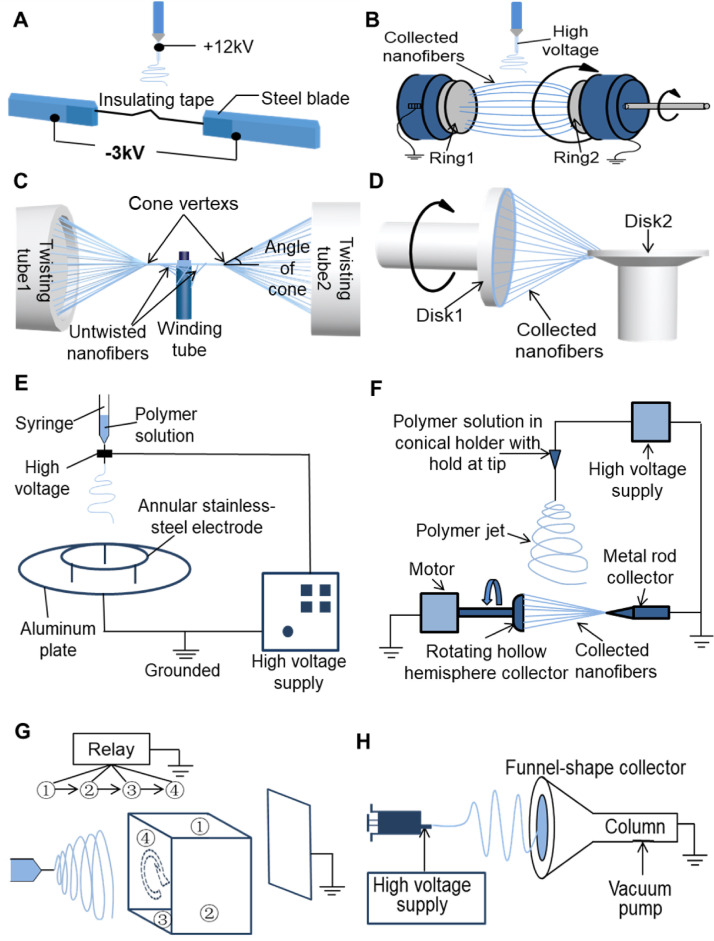
Currently, two oppositely-placed electrodes with a constant gap, constructed with two steel blades (Fig. 5 A) [66], two metal rings (Fig. 5B) [67,68], two tubes (Fig. 5C) [69], or two vertically-arranged metal disks (Fig. 5D) [70], were widely explored to balance the electrical field and achieve the alignment of electrospun nanofibers. The as-obtained nanofibers are further twisted into short electrospun NYs with a limited length in the range of several to tens of millimeters. The electrospun NYs generated by these methods commonly possess super-aligned fiber structures and controlled twisting degrees. Importantly, the fabrication devices are simple and versatile. Moreover, one conventional textile yarn supply device has been introduced to the vertically-arranged metal disk electrospinning system in Fig. 5D, which could generate nanofiber-coated microfiber coverspun yarns with controllable twists [71].
Fig. 5.
Schematic of some representative electrospinning devices for the fabrication of electrospun NYs in a discontinuous manner. (A) NY collector constructed with two steel blades. (B) NY collector constructed with two metal rings. (C) NY generator made from two twisting tubes and one winding tube. (D) Yarn generator made from two vertically arranged metal disks. (E) NY generator made by putting an annular stainless-steel electrode on an aluminum plate. (F) NY generator containing one hollow metal hemisphere and one metal rod with a sharp end. (G) Device with an auxiliary polyhedron electrode to facilitate NY formation. (H) Funnel NY-generating device. (A-H) were redrawn based on Refs. [[66], [67], [68],70,[72], [73], [74], [75], [76]].
In order to improve the yarn length, some relatively complicated devices have been designed and developed. For example, an annular stainless-steel collector on an aluminum plate was used to obtain polyacrylonitrile (PAN) nanofiber bundles in an annular shape (Fig. 5E), which were subsequently cut, pre-drafted, and twisted into short electrospun NYs with a length of 15 cm [72]. However, the short NYs obtained by this method presented an uneven and hairy appearance and a low breaking strength of roughly 5 cN/tex. In several other studies, a modified collector containing one hollow metal hemisphere and one metal rod with a sharp end (Fig. 5F) was designed to collect electrospun NYs with aligned structures and adjustable twists [73,74]. However, only 10 cm long NYs could be produced by this method. One study set an auxiliary polyhedron electrode between the spinning needle and collector, in Fig. 5G, and a rotating electric field was formed through a rapid change of the electric field of each plane in the polyhedron, which could directly twist the polyethylene oxide (PEO) nanofibers into NYs with diameters of 5 μm [75]. However, the spinning process was very unstable. Worse still, the diameters of as-obtained NYs were tiny (about 5 μm), resulting in poor mechanical properties. In another study, a funnel-shaped collecting device was developed for bundling and forming yarns, presented in Fig. 5H [76]. Specifically, the funnel-shaped collecting device could produce a high-speed airflow to bundle and twist nanofibers, but only false twisting processes were demonstrated to be produced by using this device. Moreover, the chaotically oriented fiber morphology was observed in the as-prepared NYs. In addition, some existing studies employed a high-speed rotating cylinder to collect aligned nanofiber mats and, subsequently, twist the nanofiber mats into short NYs [77,78]. As mentioned above, various modified electrospinning methods have been designed and implemented to manufacture discontinuous electrospun NYs, but it is not very realistic to further apply them into large-scale textile formation due to the limited yarn lengths. Therefore, the current trend is to develop devices that can fabricate electrospun NYs in a continuous manner.
3.2. Electrospun NY fabrication in a continuous manner
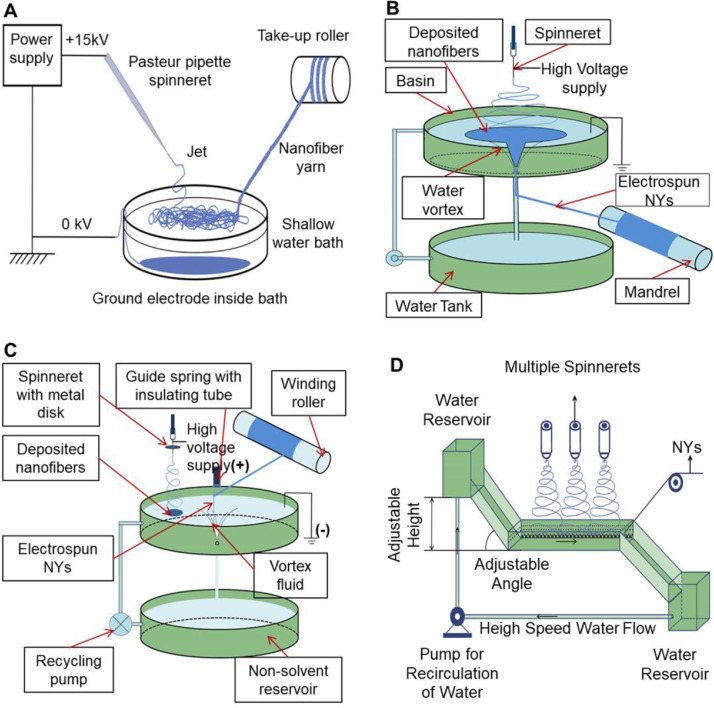
Several studies employed the mechanisms of wet spinning to modify the conventional electrospinning devices for the fabrication of NYs in a continuous manner. A similar electro-wet spinning method is presented to generate continuous NYs, as shown in Fig. 6 A [79,80]. A new collecting apparatus, consisting of a water coagulation bath, a nanofiber guiding bar, and a NY take-up cylinder, was utilized to take place of the metal plate collector used in conventional electrospinning. Polymeric nanofibers were directly deposited into a coagulation bath, and the assembled nanofibers were further stretched into yarn-like structures and pulled from the water to air with the aid of the guiding bar and take-up cylinder. Several synthetic polymers, including PCL, poly (vinylidene difluoride) (PVDF), poly(vinyl acetate) (PVAc), and PAN, have been electrospun into continuous NYs, demonstrating the feasibility of electro-wet spinning. By modifying the electro-wet spinning setup, a dynamic liquid support system was introduced to continuously collect the electrospun NYs, as shown in Fig. 6B [81]. The electrospun nanofibers were first deposited on the surface of the water in the upper basin. A small hole was created in the center of the upper basin to generate a water vortex when the water flowed out into the bottom water storage tank. The water vortex can draw the deposited nanofiber assembly into a NY. Poly(vinylidene floride-co-hexafluoropropylene) (PVDF-HFP) has been electrospun into NYs with highly aligned fibrous structures by using this dynamic electro-wet spinning method. To increase the controllability and stability of the NY fabrication process, Yousefzadeh et al. further modified the dynamic electro-wet spinning device [82]. As shown in Fig. 6C, an auxiliary disk electrode was applied onto the spinneret to concentrate the electrical field and limit the deposition area of nanofibers on the water's surface. A water vortex was created to assist in the NY formation. The NYs were guided through a spring tube and collected on a winding roller located above the basin hole. Importantly, this modified setup was demonstrated the ability to impart the NYs with a twist. Both untwisted and twisted PAN NYs were fabricated, and the twisted NYs presented obviously enhanced breaking strength compared to the untwisted NYs (13.9 ± 3.7 MPa vs 3.0 ± 0.3 MPa). Moreover, Fig. 6D shows an innovative nanofiber formation system constructed with multiple spinnerets [83], which significantly increased the production rate of PAN NYs and effectively improved the controllability and stability of the dynamic electro-wet spinning technique. Some existing studies also utilized the electro-wet spinning technique to fabricate electrospun PAN NYs [84,85]. Although some great efforts have been devoted to these electro-wet spinning methods, there are also some major limitations and drawbacks, including uncontrolled twisting parameters, high yarn breakage rate, and limited polymer source (only suitable for polymers insoluble in water).
Fig. 6.
Schematic of some reprehensive electro-wet spinning devices for the fabrication of electrospun NYs in a continuous manner. (A) Electro-wet spinning device for the generation of untwisted NYs. (B) Electro-wet spinning device for the generation of twisted NYs. (C) Electro-wet spinning device with an auxiliary disk electrode for the generation of twisted NYs with high controllability. (D) Electro-wet spinning device with multiple spinnerets for the large-scale production of untwisted NYs. (A-H) were redrawn based on Refs. [79], [80], [81], [82], [83].
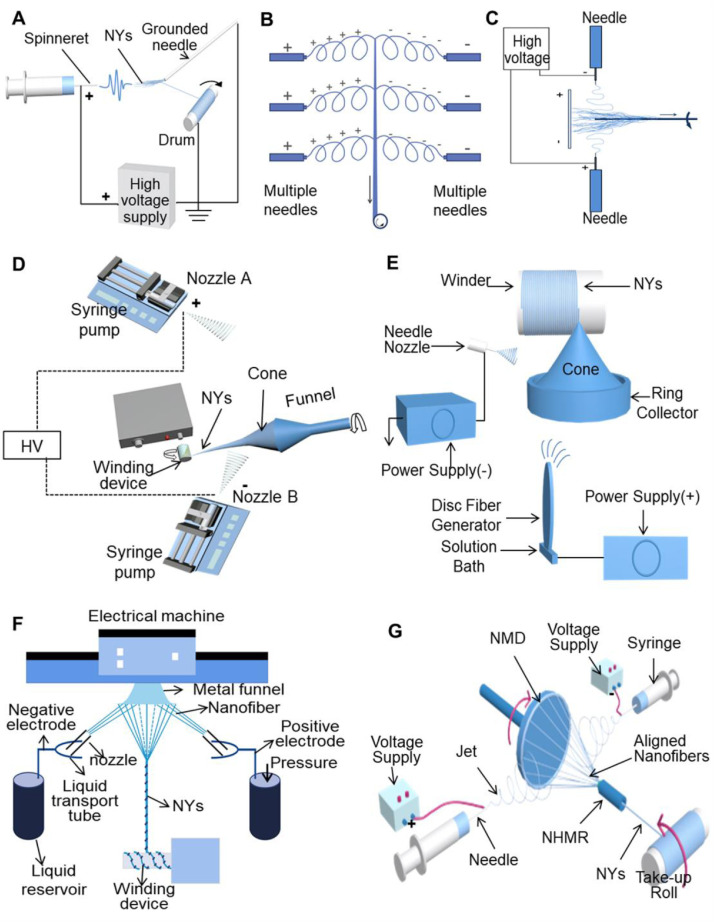
The mechanisms of traditional dry spinning were also employed to modify the conventional electrospinning device for the fabrication of continuous NYs. A self-bundling electrospinning method was reported to continuously generate NYs, as shown in Fig. 7 A [86,87]. A grounded needle tip was employed to induce the self-bundling behavior of nanofibers ejected from a high voltage charged needle tip, and the self-bundled NYs were further pulled back and wound onto a rotating drum. The conductivity of the spinning solution is the fatal factor for the self-bundling electrospinning. Organic salts should be added into the polymer solution to improve the conductivity. After adding benzyl triethylammonium chloride (BTEAC), four different types of synthetic polymers, including PAN, poly(L-lactic acid) (PLLA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly(m-phenylene isophthalamide) (PMIA), were electrospun into NYs, demonstrating the feasibility and versatility of this NY-forming technique. Although this self-bundling electrospinning technique presented simple and energy-saving features, the nanofibers’ self-bundling behavior was difficult to be precisely controlled during the NY-forming process, resulting in low quality NYs and a high yarn breakage rate. One study further designed a conjugated electrospinning apparatus to manufacture continuous poly(vinyl alcohol) (PVA) and poly(vinyl pyrrolidone) (PVP) NYs [88]. Positive and negative voltages were applied to two oppositely placed needles. The nanofibers were ejected from the two needles that carried opposite charges, which were attracted to each other and further bundled into a NYs. To increase the NY production rate of conjugated electrospinning, a multiple conjugate electrospinning system made of three sets of oppositely-placed needles was designed, as shown in Fig. 7B [89]. Continuous tricalcium phosphate encapsulated PLLA NYs were prepared by using this multiple conjugate electrospinning setup. The conjugated electrospinning device was also modified by placing a neutral collector (metal plate or cylinder) in the middle of two oppositely-placed needles, as shown in Fig. 7C [90,91]. By using a similar methods as used by Su et al., different polymers, including PAN, PLA [92], PLLA [93,94], and poly(acrylonitrile-co-methyl acrylate) [95], have been fabricated into electrospun NYs. One conventional textile yarn supply device was further introduced to the modified conjugate electrospinning system [96]. Two different nanofiber coverspun yarns, including nylon nanofiber-coated nylon microfiber yarns and PLA nanofiber-coated copper wire yarns, were manufactured. Some studies employed similar methods to develop some complicated yarn structures, such as hollow polyurethane (PU) NYs [97], hollow carbon nanotube (CNT)/polyamide 6 (PA6) NYs [98], PAN/CNT/Cotton coverspun yarns [99], poly(L-lactic acid) (PLLA)/ PA6 coverspun yarns [100] and multilayer PA6/PU/PA6 coverspun yarns [101].
Fig. 7.
Schematic of some representative conjugate electrospinning devices for the fabrication of electrospun NYs in a continuous manner. (A) Self-bundling electrospinning NY-forming device. (B) Multiple conjugate electrospinning NY-generating system. (C) Conjugated electrospinning NY-generating device modified by placing a neutral collector in the middle of two oppositely placed needles. (D) Conjugated electrospinning NY-generating setup modified with a funnel. (E) Hybrid needle-needleless electrospinning NY-fabricating system. (F) Modified multiple conjugate electrospinning NY-forming apparatus. (G) Conjugated electrospinning NY-generating device made by introducing an innovative collector constructed with NMD and NHMR. (A-G) were redrawn based on Refs. [62,86,89–91,102–105,109].
Fig. 7D shows a modified conjugate electrospinning device [102]. An intermediate funnel collector was employed to collect the oppositely charged nanofibers spun from two oppositely placed needles. A hollow nanofiber “cone” was formed on the edge of funnel, and PVDF-HFP NYs were produced by twisting and pulling away from the as-formed nanofiber cone. The highest number of twists of the NYs reached 7400 twists per meter. The same research group developed a hybrid needle-needleless electrospinning system, as shown in Fig. 7E, which extremely improved the productivity of PVDF-HFP NYs (∼240 m/h) [103]. The yarn and nanofiber diameters were in the ranges of 52 μm to 206 μm and 541 nm to 1.6 μm, respectively. Another research group developed double and multiple conjugate electrospinning devices to increase the production efficiency of nanofibers, thus resulting in the obvious improvement of yarn production efficiency (Fig. 7F) [104,105]. By using a similar device, as shown in Fig. 7D, various polymers containing PAN, poly(vinylidene fluoride trifluoroethylene) (PVDF-TrFE), PCL, and poly(sulfone amide) (PSA) have been fabricated into electrospun NYs [[106], [107], [108]].
It should be noticed that our previous study introduced an innovative collector into the conjugated electrospinning device (Fig. 7G). This method significantly improved the alignment and evenness of electrospun NYs. Importantly, continuous NYs with lengths in the range of tens to hundreds of meters could be produced. One collector was constructed with a neutral metal disc (NMD) placed oppositely from neutral hollow metal rod (NHMR), which were both in the middle of two oppositely placed needles. This homemade collector was demonstrated to effectively adjust the distribution of the external electric field and generate highly aligned nanofibers, and the obtained nanofibers were deposited in the gap between the edge of the NMD and the sharp end of NHMR. The collected nanofibers with super-aligned structures were then bundled and twisted into continuous NYs. Due to the highly aligned fibrous structure and excellent yarn evenness, the mechanical properties of the as-generated NYs were obviously enhanced. The PAN NYs showed high breaking stresses that ranged from 7.6 to 9.1 cN/tex, which were similar to the strength of textile-used cotton MYs [62,109,110]. In addition, one yarn supply and one tension device were further applied into this NY-forming system and could continuously provide conventional MYs. Several different types of nanofiber coverspun yarns, including PLGA nanofiber-coated PLA microfiber yarns, methacrylated gelatin (MeGel) nanofiber-coated PLA microfiber yarns, and MeGel/PLGA nanofiber-coated PLA microfiber yarns, were fabricated, which provided an effective finishing method for improving the properties of commonly-used textile MYs [111,112]. In general, various modified conjugate electrospinning methods are widely employed for the continuous generation of NYs, originated from a simple device, have widespread polymer spinnability, and have excellent handleability.
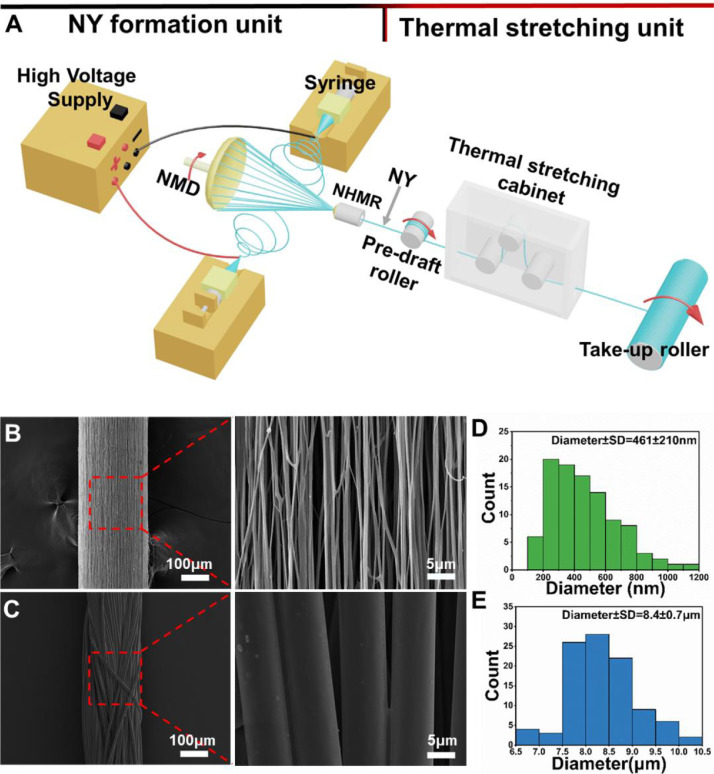
It is well-known that ideal electrospun NYs should not only present high nanofiber alignment and excellent yarn evenness but also possess appropriate mechanical properties that satisfy the fabrication requirements of various textile forming strategies. We summarized the index and reference of diameters and mechanical properties of electrospun NYs using different polymers and processing methods in Table 1 . It can be seen that both the twisting and hot stretching processes are effective ways to increase the mechanical properties of electrospun NYs. The twisting process utilizes physical force to improve the friction and adhesion among the nanofibers of electrospun NYs. In comparison, the hot stretching process could notably increase the fiber alignment and crystallinity of electrospun NYs, leading to improved mechanical performances. For example, one study investigated the effects of the hot stretching process on the mechanical properties of PAN NYs fabricated from self-bundling electrospinning [113]. They found that the mechanical properties of stretched NYs presented a remarkable improvement compared to unstretched NYs. For instance, the breaking strengths were 45 ± 2.5 MPa for unstretched NYs, 146 ± 9.6 MPa for 100% stretched NYs, and 372 ± 14.2 MPa for 300% stretched NYs. The tensile moduli were 0.8 ± 0.11 MPa for unstretched NYs, 5.2 ± 0.56 MPa for 100% stretched NYs, and 11.8 ± 0.46 MPa for 300% stretched NYs. Most Recently, another study introduced an integrated electrospinning device constructed with one nanoyarn-forming unit and one hot drawing unit to fabricate high performance PLLA NYs, as shown in Fig. 8 [132]. The hot drawing process was demonstrated to effectively increase the alignment and crystallinity of PLLA NYs, resulting in admirable mechanical properties that even surpassed the commercial PLLA MYs. The breaking load, breaking stress, and Young's modulus of 3-fold stretched PLLA NYs were 3.6 ± 0.1 N, 51.6 ± 0.8 MPa, and 1302.8±5.4 MPa, respectively. In comparison, the breaking load, breaking stress, and Young's modulus of commercial PLLA MYs were 2.7±0.1 N, 34.3±0.5 MPa, and 457.0±0.7 MPa, respectively [121]. A combination of twisting and hot stretching is obviously beneficial for synergistically improving the structural and mechanical properties of electrospun NYs.
Table 1.
Summarization of diameters and mechanical properties of some representative electrospun NYs using different polymers and processing methods.
| Materials | Yarn diameter | Fiber diameter | Young's modulus | Ultimate strength | Twisting | Hot stretching | Ref |
|---|---|---|---|---|---|---|---|
| PAN | /; / | ∼1300 nm; / |
0.8 GPa; >5.2-11.8 GPa |
45 MPa; 146-372 MPa |
×; × |
×; √ |
[113] |
| PAN | 1.16 tex; / |
411.8 nm; / |
1.66 GPa; 7.51 GPa |
58.08 MPa; 171.84 MPa |
√; √ |
×; √ |
[114] |
| PAN | 11.4-15.3 tex | 750-1000 nm | 0.55-0.64 N/tex | 0.45-0.54 N/tex | √ | × | [72] |
| PAN | 2.1 tex | 474 nm | 1440 MPa | 54.75 MPa | √ | × | [90] |
| PAN | 340.7 μm; / | /; / | 1.9 GPa; 4.5 GPa |
61.3 MPa; 116. 6 MPa |
√; √ |
×; √ |
[91] |
| PAN | 10-12 μm | / | 9.18 GPa | 100–180 MPa | √ | × | [69] |
| PAN | 39.9-71.3 tex | 220 ∼ 260 nm | / | 0.013-0.026 N/tex | √ | × | [115] |
| PAN | 1.51-1.78 tex | / | 1.68-1.88 N/tex | 0.076-0.091 N/tex | √ | × | [109] |
| PAN | 70-216 μm | 400-700 nm | / | 50.71 MPa | √ | × | [105] |
| PAN | 41.8-58.6 tex | / | / | 0.03-0.05 N/tex | √ | √ | [116] |
| PAN | 40-150 μm | 480-650 nm | 1.4-3.2 N/tex | 0.06-0.13 N/tex | √ | × | [110] |
| PAN | / | 1200-1650 nm | / | 3.80-4.25 MPa | √ | × | [106] |
| PCL | / | 330-440 nm | 12.44-68.14 MPa | 4.12-41.54 MPa | √ | × | [117] |
| PCL | / | 810-1320 nm | / | 1.56-2.03 MPa | √ | × | [106] |
| PLLA | 164 μm; / |
6000 nm; 2300-3200 nm |
0.037 N/tex; 0.012-0.34 N/tex |
0.0015 N/tex; 0.0039-0.01 N/tex |
√; √ |
×; √ |
[118] |
| PLLA | 209-435 μm | 461-763 nm | 0.2-0.6 N/tex | 0.04-0.08 N/tex | √ | √ | [119] |
| PLLA | 358-470 μm | 481-789 nm | 0.2-0.3 N/tex | 0.02-0.04 N/tex | √ | × | [94] |
| PLLA | 69.1 μm | 558.0 nm | 116.2 MPa | 23 MPa | × | × | [120] |
| PLLA | 241-494 μm | 449 -515 nm | 152.7-1191.5 MPa | 10.9-58.4 MPa | √ | √ | [121] |
| PLGA | / | 800 nm | 138.20 MPa | 59.48 MPa | √ | × | [117] |
| PLGA/PCL | / | 560 nm | 64.45 MPa | 5.40 MPa | √ | × | [117] |
| PLGA/PEO | 93 μm | 48 nm | / | 487.5 MPa | √ | × | [122] |
| PVDF-TrFE | 175-306 µm | 200-600 nm | 30.5 MPa | √ | × | [123] | |
| PVDF-TrFE | / | 790-970nm | / | 2.81-10.16 MPa | √ | × | [106] |
| PVDF-HFP | 30-450μm | 480-1500 nm | / | 60.4 MPa | √ | × | [102] |
| PVDF-HFP | 30-150 μm | 592 nm | / | 93.6 MPa | √ | × | [124] |
| PVDF-HFP | 46.2 μm | 631 nm | 334.0 MPa | 127.7 MPa | √ | √ | [125] |
| PVDF-HFP | 500 μm | / | / | 88.7 MPa | √ | × | [126] |
| PA66 | 84.7-175.3 μm | 90-220 nm | / | 86.75-118.56 MPa | √ | × | [127] |
| PA66 | 133-222 μm | 252-256 nm | 213-363 MPa | 64-88.4 MPa | √ | × | [128] |
| PA66 | 499-613 μm | 210-240 nm | 113-486 MPa | 13.7-23.9 MPa | × | × | [129] |
| PSA | 150-200 μm | 435-785 nm | / | 0.25-1.91 N/tex | √ | × | [130] |
| PPDO | 216 μm | 483 nm | 768 MPa | 190 MPa | √ | × | [131] |
Fig. 8.
(A) An innovative electrospinning system integrating one NY-forming unit and one thermal stretching unit. (B) SEM images of electrospun PLLA NYs. (C) SEM images of commercial PLLA MYs. (D) Fiber diameter distribution of the electrospun PLLA NYs in B. (E) Fiber diameter distribution of the commercial PLLA MYs in C. (A-E) Reprinted with permission from Ref. [132].
4. Electrospun NY-constructed biotextiles
In the textile industry, fiber-constructed yarns are the most widely used building blocks for the generation of various textiles. Microfibrous yarns have been extensively used in various textile processing technologies for thousands of years [1,9]. The application of electrospun NYs in the traditional textile processing techniques offers the potential to manufacture nanotextiles that exhibit superior characteristics and optical, electrical, mechanical, and biological properties due to size effects and surface/interface effects. The electrospun NY-constructed nanotextiles possess predetermined textile structures and patterns in one or multiple dimensions and open a new window to renew and update the existing microfibrous textiles. During traditional textile engineering, different textile-forming techniques, primarily including weaving, knitting, braiding, and other 3D textile-fabricating methods, can be easily adapted to fabricate nanotextiles and create different textile patterns, which notably affect the shape, structure, porosity, stability, and mechanical performances of as-generated biotextiles. Table 2 summarizes the features of each textile-forming strategy.
Table 2.
Summarization of various textile-forming methods.
| Textile Method | Schematic | Merits | Demerits |
|---|---|---|---|
| Weaving |  |
Controllable size, structure and porosity; Strong and anisotropic mechanical properties; Excellent durability |
Relatively inextensible in the warp and weft directions but opposite in the other bias directions |
| Braiding |  |
Excellent hierarchical organization; High strength and stiffness along the braiding direction; Excellent durability |
Lower porosity than woven and knitted patterns |
| Knitting |  |
Controllable size, structure and porosity; Easily stretchable in all the directions |
Lower Young's modulus than the other types of textiles |
| 3D textile-forming strategy |  |
3D structure; Controllable size and porosity; Enhanced mechanical properties along the thickness direction compared to other 2D textile patterns |
Complex fabrication system |
Weaving is a well-established textile creation method. In this process, one set of weft yarns are frequently interwoven into one set of warp yarns to generate different weaving patterns, commonly including plain, twill, and satin structures. Khil et al. first reported the use of electrospun NYs for a textile weaving strategy [79]. PCL NYs were fabricated using an electro wet-spinning device and further processed into a woven fabric with a plain pattern. Wu et al. developed a series of woven nanotextiles with different yarn weaving densities using high strength PLLA electrospun NYs, as shown in Fig. 9 A, and demonstrated the possibility of controlling the structure, pore size, and mechanical properties by using different NY weaving densities [121]. Importantly, they also found that the PLLA NY weaving density could dramatically affect the cell adhesion, growth, and proliferation. The PLLA NY-based woven nanotextiles are potential candidates for biomedical applications due to their controllable structures and properties.
Fig. 9.
(A) PLLA electrospun NY-constructed woven nanotextiles with different NY weaving densities. (B) PLLA electrospun NY-constructed braiding nanotextiles with a tube-like structure. (C) Solid braiding nanotextiles made from 24 strands of PPDO electrospun NYs. (D) Weft knitting nanotextiles constructed with a plied yarn. The plied yarn was composed with one PAN electrospun NY and one PLA MY. (E) PANI-coated PAN electrospun NY-constructed weft knitting nanotextiles with a tube-like structure. (F) 3D multilayered nanotextiles made from PLLA electrospun NYs. (G) 3D multilayered nanotextiles made from SF/PLLA electrospun NYs. (A) Reprinted with permission from Ref. [121]. (B) Reprinted with permission from Ref. [133]. (C) Reprinted with permission from Ref. [134]. (D) Reprinted with permission from Ref. [135]. (E) Reprinted with permission from Ref. [136]. (F) Reprinted with permission from Ref. [120]. (G) Reprinted with permission from Ref. [137].
Braiding is one of the most ancient textile strategies, originally developed by humans for generating ropes. In the braiding process, three or more yarns are interlaced into a diagonally overlapping structure to create different braided patterns. Joseph et al. braided PLLA electrospun NYs into a hollow structure for potential use as stents or vascular grafts, as shown in Fig. 9B [133]. Abhari et al. used PPDO electrospun NYs to fabricate a series of braided nanotextiles with different braiding densities [134]. A braided pattern fabricated with 24 strands of PPDO electrospun NYs is shown in Fig. 9C. Importantly, they demonstrated the feasibility of adjusting the structure, porosity, and mechanical properties of PPDO NY-based braiding patterns. Wu et al. also constructed a number of PLLA NY-based braiding nanotextiles and found that the NY-based braids significantly promoted the cell adhesion and proliferation compared to the braiding pattern fabricated using commercial PLLA MYs [121]. The braided nanotextiles made from electrospun NYs also showed extensive potential applications in biomedical textile products.
Knitting is another ancient textile method for creating clothes. Even now, people still have intensive interest in generating knitted clothes, hats, and gloves manually. During the knitting process, the yarns are set as a series of meandering loops and are interconnected into various patterns, which are commonly divided into two major categories, i.e., weft knitting and warp knitting. In general, the weft knitting patterns exhibit higher flexibility and stretchability than the warp knitting patterns. In one study, Wu et al. first generated a plied yarn constructed with one PAN electrospun NY and one commercial PLA MY and then knitted the plied yarn into a weft knitting pattern, as shown in Fig. 9D [135]. They further investigated the cellular behaviors of cells seeded on this weft knitting pattern and found that the cells preferred to adhere and proliferate on the PAN NYs compared to the PLA MYs. Moreover, the cells were found to elongate and align along the fiber orientation in the yarn loop, indicating the feasibility of controlling the cell growth by adjusting the knitting pattern. In another study, Wu et al. employed PANI-coated PAN NYs to successfully generate a tube-like weft knitting structure, as shown in Fig. 9E, demonstrating the potential of this knitted nanotextile for use in smart wearable textile applications [136]. Currently, no electrospun NYs have been reported to generate the warp knitting pattern because the flexibility, abrasive resistance, and tensile strength of the fabricated electrospun NYs are far behind the fabrication requirements of warp knitting techniques.
With the rapid development of textile techniques, some complex 3D textile-forming strategies have been explored and implemented. A 3D textile pattern can be achieved by using automated and programmable machines, which could extremely expand the applications of textile products in the biomedical fields. It is known that all the tissues and organs in the human body present 3D architectures and structures. The development of 3D electrospun NY-based textiles could maintain the unique features originating from electrospun nanofibers and, meanwhile, closely resembling the hierarchical and anisotropic characteristics of native tissues and organs. One study reported the fabrication of 3D textile patterns using PLLA electrospun NYs [120]. They utilized a noobing technique to process three orthogonal sets of NYs into a relatively complex 3D textile structure with multiple layers, as shown in Fig. 9F, and found that cells could easily penetrate all the layers of the textile scaffold and form a 3D cell-textile construction. Another study also developed a 3D multilayered textile pattern using electrospun SF/PLLA NYs, as shown in Fig. 9G [137]. Although electrospun NYs have been widely reported for generating different textile patterns, tremendous effort should still be made for the fabrication of more complex 3D textile patterns.
5. Tissue repair and regeneration applications
Tissue engineering is currently recognized as a promising treatment option for replacing the existing autografts, allografts, and xenografts in regenerative medicine. Biomaterial scaffolds, cells, and bioactive ingredients are three key elements in tissue engineering. Among them, biomaterial scaffolds are designed to resemble the components, structures, and various properties of native ECM, which promote the cell adhesion, growth, migration, proliferation, and differentiation. They also allow neo-tissue formation and regeneration with defined structures and functions [101]. Both electrospinning and textile forming strategies have attracted intense interest for fabricating fibrous biomaterial scaffolds and implants for diverse tissue engineering applications. Electrospun nanofibers possess structural similarities to the fibrils in native ECM. They also present a high surface area for increasing cell-material adhesion and interaction as well as adjustable chemical, physical, and biological properties for regulating cellular activities, remodeling ECM deposition, and facilitating tissue regeneration [39,138]. Unfortunately, most electrospun nanofibers are collected in the form of compact meshes, which exhibit unsatisfactory scaffold thickness and negatively affect the cellular infiltration and nutrition diffusion [135,139]. In comparison, textile-based scaffolds and grafts are designed and developed using textile technologies, which are more appropriate for resembling the hierarchical and anisotropic structures and strain-stiffening properties of native tissues. Therefore, constructing electrospun NYs and further processing them into diverse nanofibrous textile structures and patterns can combine and integrate the desirable characteristics from both electrospinning and textile fabrication techniques. These structures have been demonstrated to be great candidates for applications in surgical sutures and the repair and regeneration of various tissues, including peripheral nerves, tendons, bones, and cardiovascular tissues.
5.1. Surgical sutures
Surgical sutures are widely used and indispensable medical materials for closing wounded tissues and supporting their healing process after surgeries in clinics [140]. Among various commercial sutures, the absorbable synthesized sutures, including PLLA, PGA, PLGA, PLCL, and PPDO, have aroused more attention than the unabsorbable sutures, such as silk, nylon, and polypropylene, due to their higher biocompatibility, controllable degradation rates and mechanical properties, and minimal postoperative treatments. However, there are still some obvious limitations existing in these commercial absorbable sutures, which should be fully addressed. One key issue is mismatched fiber morphology and size. These sutures were all made of microfibers with diameters over 10 μm. Recently, to address this issue, several studies have been performed in innovative directions for employing electrospun NYs with great ECM fibril-mimicking characteristics as surgical sutures. From the perspective of suture composition, several absorbent synthesized polymers, such as PLGA [141], PLLA [142], PPDO [143], and PCL [144], were preliminarily investigated to electrospin them into NYs for surgical suture applications. It was found that the single electrospun NYs exhibited relatively low mechanical properties, which could not satisfy the requirements of practical applications [141]. One study utilized a multiple twisting technique to make 35 strands of NYs ply into one thread, which remarkably increased the mechanical properties of the NY-constructed thread [143]. They employed this nanofibrous thread as a suture for tendon repair. The results showed that the electrospun nanofibrous suture exhibited negligible immunogenicity, and significantly higher neovascularization was found in the nanofibrous suture than in the commercial microfibrous suture control. Several other studies reported the fabrication of electrospun nanofiber-coverspun yarns to enhance the mechanical properties of as-generated sutures [142,144,145].
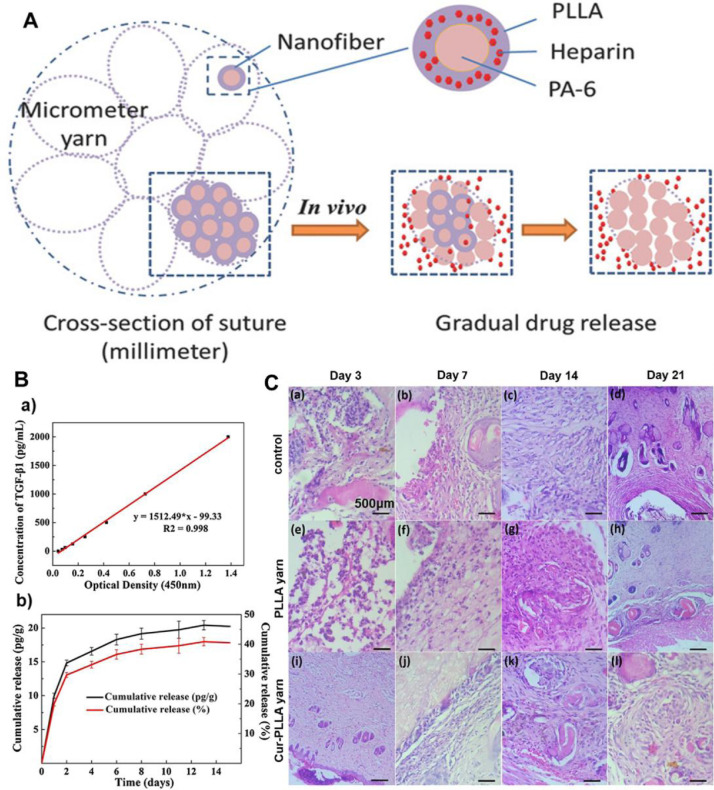
Another vital issue for the commercial sutures is a lack of necessary biological activity and function. The ideal suture should not only provide tissue securement but also promote wound healing. Although some effort was placed on exploring surface coating techniques to impart commercial sutures with drug-releasing functions, some key problems still remain. The application of electrospun NYs as surgical sutures can address these problems well. For example, a variety of bioactive materials, such as curcumin [142,144,145], vascular endothelial growth factor (VEGF) [142], aceclofenac [145], insulin [145], antimicrobial additives containing cefazolin [148], and silver nanoparticles [149], and biofunctional materials, i.e, carbon quantum dots [150], were incorporated into the nanofibers in NYs to exert predetermined biological functions during electrospinning. A schematic explanation about the release of heparin from electrospun NYs is shown in Fig. 10 A [142]. The growth factors and drugs exhibited sustained release behaviors when incorporated within electrospun NYs. For instance, Fig. 10B shows the release behavior of transforming growth factor-β1 (TGF-β1) from electrospun TGF-β1/PLGA nanofiber-coated PLGA microfibers coverspun yarns [146]. Moreover, another study demonstrated curcumin-loaded PLLA electrospun NYs as skin sutures [147], which exhibited a sustained release behavior with enhanced antibacterial and antiplatelet properties and improved cell migration and interaction in vitro as well as reduced inflammation and increased healing promotion performance in vivo, as shown in Fig. 10C.
Fig. 10.
Design and development of some representative drug-loaded electrospinning-based yarns for surgical suture application. (A) Schematic of the mechanisms of heparin release from electrospun heparin/PLLA nanofiber-coated PA6 microfibers coverspun yarns. (B) The cumulative release test of TGF-β1 from electrospun TGF-β1/PLGA nanofiber-coated PLGA microfibers coverspun yarns. (C) H&E staining of skin tissues sutured by using electrospun PLLA NYs and curcumin-loaded electrospun PLLA NYs for 21 days. (A) Reprinted with permission from Ref. [142]. (B) Reprinted with permission from Ref. [146]. (C) Reprinted with permission from Ref [147].
5.2. Peripheral nerves
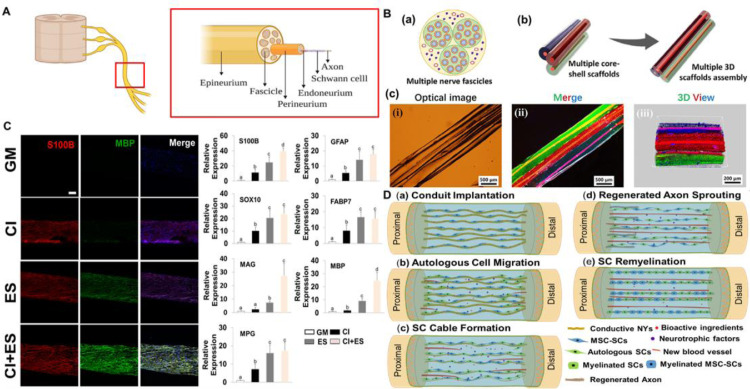
Native peripheral nerves exhibit a hierarchical cable-like structure, as shown in Fig. 11 A [153,154]. Motor or sensory axons are wrapped in a myelin sheath formed by Schwann cells (SCs) and surrounded by a layer of connective tissue (i.e., endoneurium). Multiple endoneurium-wrapped axons are bundled together with a layer of connective tissue (i.e., perineurium) to generate a series of fascicles, which are then grouped together with a layer of connective tissue (i.e., epineurium) into a nerve trunk. The peripheral nerve has a certain regeneration capacity. If a nerve injury gap (for humans) is less than 5 mm, an end-to-end coaptation is required to suture the two broken ends of the nerve trunk in a tension-free manner. For a larger defect gap, a nerve graft should be adopted to bridge the gap to assist in the nerve regeneration. A tube-like nerve graft, called a nerve guidance conduit (NGC), which can prevent the ingrowth of scar tissues and provide a relatively closed and concentrated environment for regeneration, is recognized as an effective structure for repairing the damaged nerves. Currently, most nerve grafts approved by the US Food and Drug Administration (FDA) are tube-like structures, such as Neurotube®, NeuroflexTM, and Neuragen® [155]. Unfortunately, the repair outcomes of these NGCs are unsatisfactory for large nerve defects, and they are not suggested to be utilized for nerve gaps over 30 mm in clinics [156,157]. The unsatisfactory efficacy is most probably due to the lack of ideal topographical and biological cues within the lumen of the NGCs.
Fig. 11.
Design and development of electrospun NYs and their potential applications as intraluminal fillings for peripheral nerve cell culture and tissue repair. (A) Schematic of peripheral nerve tissue. (B) Construction of three-column scaffolds made of conductive PCL/SF/CNT NYs and two layers of MeGel and alginate hydrogels. (a) The cross-sectional illustration of multiple nerve fascicles; (b) Schematic of the generation of three-column scaffolds. (c) Photograph (i), fluorescence image (ii), and 3D review image (iii) of as-prepared three-column scaffolds. The NYs were stained with a red color and the hydrogel shells were stained with red, green, and blue colors. (C)Phenotypic characterization of MSCs seeded on electrospun PPDO/CNT NYs and cultured under growth medium (GM), chemical induction (CI), electrical stimulation (ES), or a combination of CI and ES by using immunofluorescent staining and RT-PCR techniques. Bars that do not share letters are significantly different from each other, p < 0.05. (D) Illustration of the regenerative process using a multiple technique-integrated synergistic strategy containing conductive NYs, bioactive ingredients, and MSC-SCs to reconnect a large nerve gap. (A) Reprinted with permission from Ref. [151]. (B) Reprinted with permission from Ref. [152]. (C) Reprinted with permission from Ref. [131].
A variety of intraluminal filling materials, such as fibers, sponges, and hydrogels, are designed to improve the intraluminal microenvironment of NGCs, but a satisfactory regeneration treatment effect has still not been achieved. Most recently, the possibility of employing electrospun NYs as NGC fillers has been extensively explored. Compared to other filling materials, the electrospun NYs can better resemble the fascicle structures of native peripheral nerves, and the internal nanofibers of electrospun NYs can better replicate the size scale and longitudinal alignment of axons in fascicles [112]. Moreover, a bundle of electrospun NYs seems more like a natural nerve trunk. One study found that SCs cultured on the PLLA electrospun NYs presented a better spread morphology and proliferation rate than those cultured on electrospun PLLA nanofiber mats [158]. Biological cues have been combined with topographical guidance to improve the biological properties of electrospun NYs. Some bioactive polymers, including laminin [159] and MeGel [112], were utilized to construct electrospun NYs, which were found to significantly increase the biological activities of SCs. For example, MeGel was incorporated with PLGA to develop MeGel/PLGA nanofiber-coated PLLA microfiber yarns, which were demonstrated to notably promote cellular adhesion, migration, and proliferation as well as phenotypic maintenance [112]. In particular, a test was conducted to simulate in vivo cell migration after nerve injury, and it was found that the SCs migrated about 20 mm along the longitudinal axis of MeGel/PLGA/PLLA coverspun yarns after 14 days of culture.
Electrochemical cues have also been applied to integrate with the topographical cues provided by the electrospun NYs. For example, one study coated electrospun PCL NYs with polypyrrole (PPy) to form conductive NYs by in situ chemical polymerization, and it was demonstrated that the improved conductivity positively affected the growth and proliferation of SCs [160]. Another study fabricated conductive PCL/SF/CNTs NYs and further coated them with two layers of MeGel and alginate hydrogels to form three-column scaffolds, as shown in Fig. 11B [152]. The inner aligned NYs were demonstrated to guide the alignment and extension of neurites, and, meanwhile, the outer hydrogel shell was demonstrated to provide an epineurium-mimicking environment that protected the organization of nerve cells. In another study, CNTs were incorporated into PPDO nanofibers to generate conductive electrospun NYs, and it was demonstrated that increasing the CNT content could effectively promote the phenotypic maintenance of SCs [131]. This study also found that electrical stimulation notably increased the differentiation capacity of mesenchymal stem cells (MSCs) into SC-like cells on the CNT/PPDO electrospun NYs, as shown in Fig. 11C. Importantly, the synergistic effects of chemical induction and electrical stimulation remarkably promoted the maturation of SC-like cells and the secretion of multiple nerve growth factors. Moreover, Gopalakrishnan-Prema et al. fabricated several different types of NGCs by applying electrospun NYs with textile braiding techniques [161]. Electrospun PLLA NYs were braided with PPy-coated PLLA NYs, copper wires, or platinum wires to generate conductive NGCs. The braided platinum wires and electrospun PLLA NY NGCs exhibited improved bio-tolerability, enhanced neurite outgrowth, increased length of dorsal root ganglion compared to the braided NGCs made of other materials.
Unfortunately, all the existing studies have stayed at the in vitro level up until now. A tremendous effort should be made to speed up in vivo investigation and further clinical trials. Fig. 11D shows predicted regenerative mechanisms using multiple strategies to reconnect large nerve gaps. Conductive NYs loaded with cells, such as MSC-derived SC-like cells (MSC-SCs), and/or bioactive ingredients, such as various drugs, cytokines, and nucleic acids, can be employed as intraluminal filling materials and can exert synergistic effects. Conductive NYs can provide physical and electrochemical cues to guide the migration of autologous cells and directed regrowth of axons. They can also be utilized as carriers for loading bioactive ingredients and cells. Under the synergistic functions of multiple cues, ideal regenerative outcomes and functional recovery are expected.
5.3. Tendon
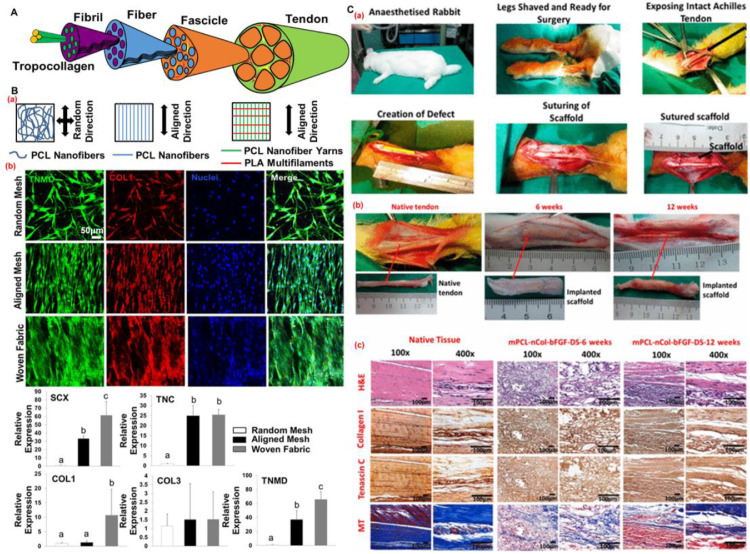
Tendon tissue is a dense connective tissue that presents a hierarchical fibrous organization along its longitudinal axis, as shown in Fig. 12 A. This consists mainly of tropocollagen molecules (∼1.5 nm in diameter), fibrils (50–500 nm in diameter), fibers (10–50 μm in diameters), and fascicles (50–400 μm in diameter) [162,163]. It has become clear that the replication of the hierarchical fibrous architecture of native tendon tissues is of significant importance for the design of scaffolds and grafts in tendon tissue engineering. More than 100 scientific papers have been published that report the exploration of electrospinning techniques to fabricate nanofibrous mats for tendon regeneration. Electrospun nanofibrous mats have been demonstrated to remarkably promote cellular adhesion, growth, proliferation, and migration as well as tenogenic differentiation. However, electrospun nanofibrous mats generally present low mechanical properties that cannot fulfill the rigorous mechanical requirements of native tendon tissues. Therefore, it is critical to develop some innovative strategies for improving the structures and mechanical properties of electrospun mats while maintaining their nanofiber characteristics. Several studies modified the typical electrospinning methods to fabricate mats made of aligned electrospun NYs and randomly distributed nanofibers, which exhibited relatively higher porosities and larger pore sizes than the typical random and aligned electrospun nanofiber mats, resulting in a higher cell proliferation rate [164,165]. Unfortunately, the mechanical properties of these mats were still low, and were even lower than typical electrospun mats.
Fig. 12.
Electrospun NYs and their biotextiles for promoting tenogenic differentiation and tendon regeneration. (A) Schematic of tendon tissue. (B) Comparative analysis of randomly electrospun PCL nanofiber mats, aligned nanofiber mats, and woven textile scaffolds made of electrospun PCL NYs and commercial PLA MYs. (a) Illustration of three different scaffolds; (b) Phenotypic characterization of MSCs seeded on the three different scaffolds by using immunofluorescent staining and RT-PCR techniques. Bars that do not share letters are significantly different from each other, p < 0.05. (C) In vivo analysis of tenocyte-loaded, braided mPCL-nCOL-bFGF textile scaffolds after in vitro dynamic stimulation for Achille's tendon reconstruction. (a) Photographs of the whole surgical procedure; (b) Photographs of harvested scaffold-tissue samples after 6 and 12 weeks of implantation; (c) Images of H&E staining, immunohistochemical staining, and Masson's trichrome (MT) staining of harvested scaffold-tissue samples after 6 and 12 weeks of implantation. (B) Reprinted with permission from Ref. [139]. (C) Reprinted with permission from Ref. [168].
Employing various textile forming techniques to process electrospun NYs into predetermined textile patterns provides a more appropriate strategy for addressing the drawbacks of electrospun nanofiber mats. Electrospun NYs are interwoven into woven nanotextiles to explore their potential in engineered tendon scaffold applications. The existing studies demonstrated that the woven nanotextiles exhibited controllable pore sizes, porosities, and mechanical and biological properties by adjusting the yarn weaving densities [139,166]. The woven nanotextiles were found to significantly enhance the tenogenic differentiation of stem cells, compared to the typical random and aligned electrospun nanofiber mats, by significantly increasing the gene expression levels of tenocyte-related markers, as shown in Fig. 12B. Biological cues have also been integrated with the topographical guidance provided by electrospun NYs to promote the cellular activities of tendon-associated cells. For example, thymosin beta-4 (Tβ4) was encapsulated into electrospun NYs, and they exhibited a sustained drug release profile for nearly one month and presented an additive effect on the promotion of the tenogenesis of stem cells [111]. Mechanical stimulation was also applied to accelerate the tenogenic differentiation of stem cells seeded on the electrospun NYs [167]. In addition, the mechanical stimulation has been integrated with the topographical cues of electrospun NY-based woven nanotextiles and a cellular coculture of human tenocytes and human umbilical vein endothelial cells to remarkably enhance the tenogenesis of stem cells [139].
The textile patterns fabricated by different textile-forming techniques dramatically affect the structures and mechanical properties of textile scaffolds, even if the same yarns are utilized. A multiple twist method has been utilized to ply 16 strands of single electrospun NYs into a thread with high mechanical performance that was more suitable for tendon regeneration applications than electrospun nanofiber mats [169]. A braiding technique was employed to generate braided nanotextiles from electrospun NYs, which exhibited notably enhanced tensile and suture-retention strengths and significantly promoted the tenogenic differentiation of stem cells compared to the aligned electrospun nanofiber mats [170]. In another study, basic fibroblast growth factor (bFGF) was loaded into electrospun PCL-micro/collagen-nano hybrid yarns (mPCL-nCOL-bFGF), and they were further fabricated into braided textile patterns for Achilles’ tendon reconstruction [168]. It was found that the tenocyte-seeded mPCL-nCol-bFGF scaffolds significantly enhanced the regeneration of tendon tissues in a rabbit Achilles tendon defect model after in vitro dynamic stimulation, as shown in Fig. 12C. A textile knitting technique was also utilized for generating NY-based tendon scaffolds. For instance, electrospun nanofiber coverspun yarns, employing microfibers as the core and electrospun nanofibers as the sheath, were first fabricated and then processed into knitted textile patterns [171]. An in vivo study using a rabbit patellar tendon defect model showed that the knitted textile scaffolds dramatically promoted the remodeling and regeneration process for neo-tissues.
5.4. Bone
Electrospun NYs and their nanotextiles also represent potential scaffolds for bone tissue engineering applications, originating from their ECM nanofibril-mimicking structures and controlled porosities. One study fabricated electrospun PLCL NYs, chopped them into short forms, and then encapsulated the short NYs into type I collagen hydrogels [172]. The NY-enhanced hydrogels significantly promoted the osteogenic differentiation of stem cells compared to hydrogels only. Importantly, the NY-enhanced hydrogel system was easily injected through a 16-gauge needle, which highlighted its potential as an injectable bone scaffold. In another study, electrospun SF/PLLA NYs were processed into textile patterns with 3D structures, which exhibited great mechanical performance, with a breaking strength of 180.4 MPa and a Young's modulus of 417.6 MPa [173]. A rabbit femoral condyle model experiment demonstrated that the 3D electrospun NY-based textile scaffolds significantly promoted the regeneration of new bone tissues.
Unlike other soft tissues, such as tendons and nerves, the ECM of bone tissue is composed of both organic (mainly type I collagen) and mineralized inorganic components. Along with type I collagen fibrils, the native bone ECM contains abundant hydroxyapatite (Ca10(PO4)6(OH)2, HAp) (an inorganic material, >70% of dry tissue weight), which remarkably influences the structure and properties of the bone ECM [174]. Therefore, various inorganic components were integrated into electrospun NY-based scaffolds to better resemble the complex organic-inorganic features and functions of natural bone ECM. For example, HAp particles were deposited on electrospun SF/PLCL NY-constructed scaffolds through a post-modification process, and they exhibited improved cellular adhesion, proliferation, and infiltration [175]. In another study, a post-soaking method was employed to deposit HA nanoparticles onto a 3D electrospun SF/PLLA NY-based textile scaffold, which was demonstrated to significantly improve cellular attachment and proliferation and to notably increase the osteogenic differentiation of stem cells [176]. It should be noticed that one study reported the development of electrospun PLLA NY-reinforced HAp/gelatin composite scaffolds and demonstrated that the scaffolds could notably promote the bone formation in a rabbit mandibular bone defect model [177] The regenerated bone tissues possessed a compressive strength that was almost similar to the native rabbit mandible. Moreover, a similar electrospun NY-enhanced scaffold was demonstrated to increase the formation of a mature lamellar bone in a rat femoral segmental defect model [178].
5.5. Cardiovascular system
Electrospun NYs and NY-constructed nanotextiles have also be explored for potential use in the repair and regeneration of the cardiovascular system, including vascular, cardiac, and heart valve leaflet tissues. The blood vessel is a long, tube-like structure that is mainly constructed of three complicated layers, i.e., intima, media, and adventitia [179]. Endothelial cells, smooth muscle cells, and fibroblasts are three main cell phenotypes in vascular tissues. Electrospun NYs have been demonstrated to improve the adhesion, elongation, proliferation [180,181], and angiogenic activity [182] of endothelial cells. The electrospun NYs could also support the differentiation of stem cells into smooth muscle cell-like phenotypes [135]. Several commercial FDA-approved textile vascular grafts, such as Gelsoft and Gelweave, have been utilized to replace diseased aortic vessels and have played key roles in saving patients’ lives [183,184]. In comparison to large aortic vessels, the repair of small blood vessels, with diameters less than 6 mm (for humans), remains a huge clinical challenge due to their tiny structures and complications after surgery. Most recently, one study developed an electrospun NY with a hollow structure for use as a vascular scaffold [185]. The wall thickness and outer diameter of the hollow NY scaffold were 156 ± 26.5 μm and 1.1 ± 0.15 mm, respectively. The hollow NY scaffold effectively supported the attachment and proliferation of endothelial cells in vitro, which is potentially valuable for the repair of small blood vessels.
The textile techniques also offer unique advantages for better mimicking the directional cellular alignment and anisotropic mechanical properties of cardiac tissues in the heart. In one study, conductive electrospun NYs were first fabricated and then interwoven into a woven pattern [42]. After that, one or multiple layers of woven fabric were integrated into a hydrogel system to engineer the anisotropy of 3D cardiac tissues. Researchers also seeded cardiomyocytes onto the woven nanotextiles and incorporated endothelial cells within the hydrogel to successfully achieve the endothelialization of engineered myocardium. In another study, a combined use of a woven nanotextile and a hydrogel was conducted to construct a nanotextile-enhanced hydrogel scaffold to engineer the anisotropic structure and properties of valvular tissues [186]. The composite scaffold exhibited mechanical properties similar to native valvular tissues and was found to support the growth and phenotypic maintenance of human aortic valve interstitial cells (HAVICs). Importantly, the composite scaffold could effectively inhibit the calcification of diseased HAVICs, which allows it to be potentially feasible as a living replacement for diseased valves.
6. Wearable textile devices and bioelectronics
Conductive fibrous yarns and textiles, which can be seamlessly integrated into everyday textile products, offer higher flexibility, stretchability, and breathability than solid and non-fibrous materials [4,187]. Among various fibrous assemblies, highly flexible and lightweight electrospun NYs exhibit high surface areas and enhanced conductivity, making them more appropriate candidates as the next generation of smart wearable devices and bioelectronics for monitoring, diagnosing, and managing medical conditions. The design and construction of conductive NYs with high flexibility and electrical performances are essential for nanofibrous textile bioelectronics. The material selection, referring to the matrix material and conductive materials, plays a key role in determining the physical properties of the fabricated devices. Three main categories of conductive materials, including conductive polymers, carbon nanomaterials, and metal nanomaterials, have been extensively investigated and utilized to fabricate conductive electrospun NYs. Table 3 summarizes the electrical performance and stretchability of some representative conductive or piezoelectric electrospun NYs. It was found that, compared to conductive polymers, the introduction of carbon nanomaterials, including multiwalled CNTs (MWCNTs), single-walled CNTs (SWCNTs), graphene, and MXene flakes, can significantly increase the conductivity of electrospun NYs. In addition, compared to the directed addition technique, the surface coating method is more beneficial for generating electrospun NYs with high conductivity. For instance, electrospun nylon NYs coated with MXene exhibited a high electrical conductivity (up to 1.2 × 105 S/m) [188]. Moreover, some piezoelectric polymers, including PVDF and PVDF-TrFE, were utilized to impart electrospun NYs with piezoelectric properties. For example, one study developed electrospun PVDF-TrFE NYs that exhibited a piezoelectric potential and a piezoelectric voltage constant of 500 mV and 0.412 mVm/N, respectively [189]. It should be also noticed that choosing a yarn matrix material with a high elasticity, such as PU, can impart the final fabricated conductive NYs with a high stretchability, which is especially meaningful for the parts of textiles undergoing frequent, large deformations during various daily movements. Tremendous effort should be made to further improve the conductivity or piezoelectricity of electrospun NYs while providing them with high stretchability, comfortability, structural stability, and reproducibility in the future. This section reviews the state-of-the-art applications of conductive or piezoelectric electrospun NYs in diverse wearable textile bioelectronics, including harvesters and storage systems, actuators, and sensors, as well as advanced face masks.
Table 3.
Summarization of electrical performances and stretchabilities of some representative conductive or piezoelectric electrospun NYs.
| Materials | Conductivity or Piezoelectric voltage constant | Stretchability | Ref |
|---|---|---|---|
| PANi/PVP | 4.1 × 10−2 S/m | / | [190] |
| PANi/PAN | 1.3 kΩ/m | ∼20% | [191] |
| PANi/PCL | 600 kΩ/m | / | [192] |
| Fe3O4/PANi/PAN | 0.091-0.629 S/m | / | [193] |
| PA6; MWCNT/PA6 |
1 × 10−13 S/m; 2.4 × 10−6 S/m |
/; / |
[98] |
| PA6; SWCNT/PA6 |
1 × 10−13 S/m; 3 × 10−5 S/m |
<61% | [194] |
| MWCNT-coated PA66 | 20 S/m | ∼125% | [195] |
| MWCNT-coated PAN | 0.28 S/m | >10% | [196] |
| Graphene/PAN | / | 119% | [197] |
| PAN-based carbon; Graphene/PAN-based carbon |
7700 S/m; 16500 S/m |
/; / |
[198] |
| Graphene/PAN-based carbon | 6644 S/m | 0.5% | [199] |
| PAN-based Carbon; PMMA-based Carbon; Copper nanoparticles/Carbon |
20634 S/m; 27181 S/m; 47213 S/m |
/; /; / |
[200] |
| MWCNT/SWCNT coated PU | 1300 S/m | 1200% | [201] |
| MXene flakes coated nylon; MXene flakes coated PU |
1.2 × 105 S/m; 7800 S/m |
43%; 263% |
[188] |
| Silver nanowire/PU | 40 kΩ/m | 500% | [202] |
| PPDO; CNT/PPDO |
1.73 × 10−8 S/m; 3.52 × 10−4 S/m |
64%; 53% |
[131] |
| PVDF | 0.4323 mVm/N | / | [203] |
| PVDF-TrFE | 0.412 mVm/N | / | [189] |
| PVDF-TrFE | / | 65% | [204] |
6.1. Harvesters and storage systems
Conductive electrospun NYs and nanotextiles have been explored as potential candidates for smart wearable bio-energy harvesting devices that can covert small-scale biological energy from human motion into renewable electrical power. Electrospun NYs with great piezoelectric properties have been utilized to harvest the energy from the cyclic compression forces of human physical movement [123]. Electrospun PVDF nanofibers were coated onto silver deposited nylon microfibers to obtain an electrospun nanofiber coverspun yarn-based nanogenerator [205]. The as-fabricated piezoelectric yarn nanogenerator exhibited a mean peak voltage, mean peak current, and power density of 0.52 V, 18.76 nA, and 5.54 µW/cm3, respectively, under a cyclic compression of 0.02 MPa at 1.85 Hz. More importantly, the yarn nanogenerator was demonstrated to possess excellent property retention, even after 50,000 cycles. In another study, PVDF and PAN hybrid nanofibers were also coated onto a silver wire to generate an electrospun NY-based nanogenerator that could generate high electrical outputs of 40.8 V and 0.705 μA/cm2 once applied with a cyclic compression of 5 N at 2.5 Hz [206]. Most recently, a triboelectric nanogenerator with a plain woven pattern was generated by interweaving electrospun PVDF-TrFE nanofiber-coated stainless-steel wire yarns with electrospun PA66 nanofiber-coated stainless-steel wire yarns [207]. The working mechanisms are shown in Fig. 13 A. An external material, such as a rubber film, cotton fabric, or polyester fabric, is put in contact with the as-generated nanogenerator by the action of an external applied force. The triboelectrification between the external material and nanogenerator will impart the PA66 and PVDF-TrFE nanofibers with opposite charges. An electrical potential difference will be generated between PA66 nanofibers and PVDF-TrFE nanofibers when the external material starts to pull away from nanogenerator. The electrons will flow from the inner electrode of the PVDF-TrFE nanofibers to the inner electrode of the PA66 nanofibers. An electrostatic equilibrium state, without a flow of electrons, will be reached when the maximum separation distance is reached between the external material and nanogenerator. After that, the external material starts to move towards the nanogenerator under the action of the external applied force, breaking the electrostatic equilibrium state and allowing the electrons to flow from the inner electrode of the PA66 nanofibers back to the inner electrode of the PVDF-TrFE nanofibers. Once the external material gets in contact with the nanogenerator again, a new cycle will start. Therefore, an alternating current can be periodically produced from the interaction of the external material and nanogenerator. This nanogenerator exhibited a maximum instantaneous power density of 93 mW/m2 and could light up 58 light-emitting diodes at the same time, indicating a big step for the use of biomechanical energy harvesting using electrospun NY-based nanogenerators.
Fig. 13.
Electropinning yarn-based wearable nanogenerator, supercapacitor, and electrochemical actuator. (A) Schematic illustration of the working principles of a NY-based triboelectric nanogenerator. (B) Fabrication and applications of a strong electrochemical actuator. (a) Schematic of the fabrication process; (b) SEM image of the CNT yarn; (c) SEM image of electrospun PVDF-HFP nanofiber-coated CNT coverspun yarn; (d) SEM image of a twisted coverspun yarn; (e) SEM image of a self-plied yarn using two twisted coverspun yarns; (g) Photograph of an obtained yarn muscle wrapped on a mandrel; (h) A dumbbell was lifted by the yarn muscle when a 4 V voltage was applied; (i) The grasping and releasing of a object with the weight of 56 mg by yarn-made grippers when a 4 V voltage was periodly applied. (A) Reprinted with permission from Ref. [207]. (B) Reprinted with permission from Ref. [208].
The design and construction of flexible, yarn-shaped supercapacitors are of remarkabe importance to realize the miniaturization and wearability of supercapacitors, which have been widely explored as innovative electrical storage devices for supplying electrical power for smart wearable electronics. Conductive electrospun NYs have been widely fabricated for use as the electrodes of yarn-shaped supercapacitors [43,[209], [210], [211]]. In one study, graphene oxide (GO) nanosheets and electrospun PAN-GO nanofibers were utilized to coat Ni-deposited cotton yarn, and the as-fabricated coverspun yarns were further deposited with PPy, as the capacitor electrodes, and then accompanied with PVA/H2SO4 electrolytes to obtain a yarn-like supercapacitors [209]. The specific capacitance and energy density of this yarn-like supercapacitor were found to be 28.34 mF/cm2 and 3.98 μWh/cm2, respectively. The yarn supercapacitor also exhibited a high capacitance retention and reached 90.2% after 1000 voltammetry cycles. The capacitance properties were relatively low. In another study, electrospun PAN nanofiber-coated carbon microfiber coverspun yarns were first developed, and a conductive polymer, PPy, was deposited onto the surface of coverspun yarns [43]. The PPy/PAN/Carbon yarns were then employed as electrodes to construct a yarn-shaped supercapacitor with the assembling of PVA/LiCl/H3PO4 gel electrolytes. The specific capacitance and energy density of this yarn-like supercapacitor were found to be 353 mF/cm2 and 247 µW/cm2, respectively. The yarn supercapacitor also exhibited a high capacitance retention and reached 82% after 2000 voltammetry cycles. Most interestingly, one study also reported the use of electrospun nanofiber-coated microfiber coverspun yarns with great photoactive characteristics to harvest solar energy with a 15.7% power conversion efficiency. The photoactive yarns were processed into a 30.5 mm × 30.5 mm woven pattern, which could generate a power density of 1.26 mW/cm2 under the exposure of one man-made sun (1000 W/m2) [212].
6.2. Actuators
The native muscle myofibril-like structure and high specific surface area of conductive electrospun NYs make them potentially fantastic candidates for bio-actuators and artificial muscle applications [[213], [214], [215]]. Conductive PANi was coated onto the surface of electrospun PU yarns to construct an electromechanical actuator, which exhibited an actuating strain of 1.65% and an actuating strength of 2.263 MPa [216]. In comparison, the PPy-coated electrospun SF yarns exhibited a relatively lower actuating strain of 0.9% and a relatively lower actuating strength of 70 kPa under cyclic electrical stimuli [217]. Most recently, one study developed a robust electrochemical actuator based on electrospun NYs, and the schematic illustration of fabrication process is shown in Fig. 13B [208]. A carbon nanotube yarn was coated with one layer of electrospun PVDF-HFP nanofibers, and this was further processed into twisted yarns. The two twisted yarns were processed into a complex coiled structure and cut in the middle to generate two coiled yarns, which were both soaked in an imidazolium ionic liquid (EMIBF4) solution to generate a yarn-based actuator. The actuator could generate a high actuating stress of 10.8 MPa, which was roughly 31 times that of the native skeletal muscles. Moreover, an artificial muscle made of three yarn actuators could lift a dumbbell of 10 g once a stimulated voltage of 4 V was applied.
6.3. Sensors
Conductive electrospun NYs are assuredly ideal materials for constructing wearable strain and pressure sensors for monitoring diverse human activities due to their high electric properties and great flexibility. Many efforts have been devoted to construct conductive NYs and their fabrics for wearable strain sensors [188,218]. One study developed a strain sensor by putting electrospun PU nanofibers on CNT-loaded PET microfibers and found that the gauge factors (GFs) in the strain ranges of 0%–15%, 15%–29%, and 29%–44% were 46.4, 353, and 980, respectively. The electrospun NY-based strain sensor could effectively monitor various real-time human activities, as shown in Fig. 14 A [47]. Another study first obtained a PAN electrospun NY, twisted it into a helical structure, and further processed it into a helical carbon NY using a carbonization process to generate a strain sensor. This sensor showed a potential in detecting the small strains and exhibited a GF of 37.3 to the tiny strain of 0.1%, which was demonstrated for monitoring different subtle human activities [219].
Fig. 14.
Sensory property analysis of some representative electrospun yarn-based wearable sensors. (A) Detection of various human motions using an electrospun PU/CNT/PET coverspun yarn sensor, including (a) finger bending, (b) wrist bending, (c) writing, (d) knee bending, (e) speaking, and (f) Drinking. (B) Detection of different motions using a helical CNT/PU NY sensor, including (a) 500% stretching, (b) 180° bending, and (c) 720° torsion cycles. (d) Photos of LED light during the large-scale stretching process. (e) Recording of resistance change with the strain increasing to 100%. (f) Detection of resistance variation under a cyclic tensile loading with 200% strain. (A) Reprinted with permission from Ref. [47]. (B) Reprinted with permission from Ref. [220].
Besides various resistivity sensors, capacitance sensors based on electrospun NYs have also been widely explored [221–223]. For example, one study electrospun one layer of GO/PU nanofibers onto Ni-coated cotton yarns and further interlaced the GO/PU/Ni/cotton yarns into a capacitive sensor with a textile weaving structure [222]. The nanotextile senor presented a wide sensing range of 0–5 N, a low detection limit of 0.001 N, and a fast response time (< 50 ms). Importantly, it could detect various subtle tactile changes. In order to maintain the excellent strain sensing properties, some studies showed that the composite technique was of significant importance for improving the durability and stability of the electrospun NY-based sensor [201,220]. For example, one study fabricated helical CNT/PU NYs with ultra stretchability and stability, as shown in Fig. 14B [220]. The NY-based sensor presented an excellent recoverability within the tensile strain of 900%.
6.4. Advanced face masks in the COVID-19 pandemic
An unpredicted outbreak of COVID-19 caused by SARS-CoV-2 has become a global public healthcare crisis. Face masks are one piece of essential protective equipment used to effectively prevent the human-to-human transmission of SARS-CoV-2 by stopping the spread of virus-containing saliva and respiratory droplets. The commercialized face masks are commonly made of three layers, i.e., cover layer, filter layer, and sheath layer, as shown in Fig. 15 A [224]. The cover and sheath layers mainly provide support to maintain the whole structure of the face masks and contribute a limited filtration efficiency. As a comparison, the core part of face masks is the middle filter layer, which supplies the filtering function [225]. Currently, the meltblown nonwovens, made of fibers with diameters in the range of 1-10 micrometers, are the main materials for the fabrication of the filter layer. However, the large diameter of the filter fibers leads to insufficient filtration efficiency for particles and aerosols with sizes less than 0.3 μm. Therefore, an electrostatic treatment, such as corona charging and tribocharging, is an essential process for improving the filtration efficiency of meltblown nonwoven filters [226,227]. The electrostatically charged nonwovens can effectively capture the aerosols (<0.3 μm) through the electrical attraction. Unfortunately, the charge dissipation during long-term storage or use will significantly reduce the filtration efficiency of the face masks. Moreover, one study shows that the filtration efficiency of nonwoven filters significantly dropped after ethanol sterilization treatment due to the large disappearance of the electrostatic charges [228]. All these reasons make the commercial face masks disposable. The recyclability of the discarded face masks has become a huge challenge all over the world.
Fig. 15.
(A) Structure and filtration mechanisms of commercial face masks. (a) Illustration of the structure of a face mask. (b) Photograph and (c) SEM image of a meltblown nonwoven filter layer. SEM analysis after filtration of (d) commercial polypropylene microfiber nonwoven, (e) cellulose diacetate nanofiber nonwoven, and (f) PAN nanofiber nonwoven. (g) Schematic of the filtering function of the three different layers in a face mask. (B) Structure and filtration mechanisms of a hypothetical face mask with reusable and self-powered characteristics using electrospun NY-based textiles as a filter layer. (A) Reprinted with permission from Refs. [224,225].
Electrospun NY-based textiles can serve as the filter layer to construct self-powered face masks that can potentially solve some fatal issues of single-use face masks [229,230]. Some polymers with tribo/piezoelectric characteristics, such as PVDF, PVDF-TrFE, and PVDF-HFP, can be fabricated into electrospinning-based NYs and further constructed into textile-shaped filter layers for face mask application [231,232]. The tribo/piezoelectric nanotextile filter layer can generate electrical charges during the breathing process, which can produce strong electrostatic attraction to effectively block the virus-containing aerosols [233,234]. The schematic and filtration mechanisms of the reusable tribo/piezoelectric nanotextile-based face masks is shown in Fig. 15B. Moreover, compared to the commercial meltblown microfibrous filter, the nanofibrous textile filter presented an obviously decreased fiber diameter and increased specific surface area, thus resulting in an improved filtration efficiency [235,236]. In addition, some antiviral components are more easily encapsulated into the NY-based textiles during electrospinning (performed at room temperature) than during the meltblown technique, which requires a high spinning temperature (usually > 100 °C) [236]. More extensive effort should be made to speed up the research and development of reusable and self-powered nanotextile-based face masks. It should be noticed that the same design philosophy also applies to the research and development of new types of protective clothing.
Recently, several groups have also incorporated bioactive components or biological sensors within face masks to effectively detect SARS-CoV-2. For example, Nguyen and colleagues integrated cell-free synthetic circuits, including CRISPR-based tools, into a face mask to detect SARS-CoV-2 [237]. They demonstrated that the wearable sensor-integrated face mask could successfully detect SARS-CoV-2 from exhaled aerosols at room temperature within 90 min. The worn face masks are also great resources for collecting and quantifying exhaled SARS-CoV-2 from hospitalized patients with COVID-19 [238,239]. Several groups have demonstrated that nanofibers, compared to traditional cottons or microfibers, can more effectively absorb SARS-CoV-2 and significantly increase the detection sensitivity [240,241]. Therefore, electrospun NYs and their nanotextiles can not only be used as physical barriers to passively filter out the viruses but also have great potential to serve as wearable devices to eliminate, detect, and quantify SARS-CoV-2.
7. Concluding remarks and future directions
For nearly two decades, some remarkable progress has been made in the design and implementation of innovative electrospinning devices for electrospun NY fabrication. Some tremendous endeavors have been devoted to better understand the mechanisms of nanofiber alignment and the formation mechanism of triangular spinning cones during the formation process of electrospun NYs. A series of continuous modifications based on electro-wet spinning and conjugate electrospinning have been explored to fabricate electrospun NYs in a continuous and controllable manner. On this basis, more complicated NY structures, including hollow NYs, double-layered coverspun NYs, and multilayered coverspun NYs, have been successfully produced. Numerous materials, including natural and synthesized polymers, and various functional additives, like conductive materials, nanomaterials, and bioactive ingredients, have been successfully utilized to construct electrospun NYs with predetermined properties and functions. As a result of the remarkable characteristics of electrospun NYs, many rapid advancements have been made in constructing electrospun NY-based biotextiles, and various polymeric electrospun NYs have been processed into various 2D woven textiles, knitted and braided patterns, and even 3D textile patterns. These nanotextiles have been demonstrated to possess the unique characteristics of electrospun nanofibers, including size and surface/interface effects and optical, electrical, mechanical, and biological properties, as well as predetermined textile structures.
The existing studies have clearly demonstrated the promising potential and possibility of using electrospun NYs and their nanotextiles to replace the currently existing MYs and MY-based textiles for various biomedical applications. However, a necessary step that should be moved forward with is to investigate the electrospun NYs and their nanotextiles in great depth and accelerate their clinical transformation. It remains very challenging to optimize and improve the compositions, morphologies, structures, and biological properties of both electrospun NYs and their nanotextiles. The low mechanics of electrospun NYs is one of the most severe hurdles holding back their application in the fabrication of biotextiles. Electrospun NYs generally present significantly weaker mechanical properties than the traditional MYs and are unable to meet the processing requirements of traditional textile braiding, weaving, and knitting techniques. Therefore, some more effort should be made on the improvement of the structures and mechanical properties of electrospun NYs in the future. For tissue engineered scaffold applications, more preclinical animal models should be utilized to evaluate the safety and effects of electrospun NY-based textile products. Meanwhile, for commercial fabrication and practical applications, future efforts should be devoted to the large-scale manufacturing of electrospun NYs with high reproducibility through simple manipulation procedures. Currently, a majority of electrospinning-based yarn formation devices were designed based on the needle-like spinneret, which presents a severely low nanofiber production scale. A needleless spinneret developed for conventional electrospinning with a high fiber production scale should be considered for the innovative design of an electrospun yarn-forming system. Moreover, all of the textile processing equipment is currently designed and implemented for traditional MYs, which is not necessarily applicable for innovative electrospun NYs due to the obviously different structures and properties of NYs and MYs. It is significantly important to explore the design and development of high value-added textile machines that are suitable for electrospun NYs. In summary, several vital issues regarding the optimization of the composition, morphology, structure, and biological properties; improvement of the production; and speeding up of clinical trials of electrospun NYs and NY-based textiles should be addressed to serve future commercialization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work has been supported by Shandong Science Foundation for Young Scholar (ZR2020QE090) and Start-up Grant of Qingdao University.
Reference
- 1.Jiang C., Wang K., Liu Y., Zhang C., Wang B. Application of textile technology in tissue engineering: A review. Acta Biomater. 2021;128:60–76. doi: 10.1016/j.actbio.2021.04.047. [DOI] [PubMed] [Google Scholar]
- 2.Rostamitabar M., Abdelgawad A.M., Jockenhoevel S., Ghazanfari S. Drug‐eluting medical textiles: From fiber production and textile fabrication to drug loading and delivery. Macromol. Biosci. 2021;21(7) doi: 10.1002/mabi.202100021. [DOI] [PubMed] [Google Scholar]
- 3.Mallakpour S., Azadi E., Hussain C.M. Recent breakthroughs of antibacterial and antiviral protective polymeric materials during COVID-19 pandemic and after pandemic: Coating, packaging, and textile applications. Curr. Opin. Colloid Interface Sci. 2021;55 doi: 10.1016/j.cocis.2021.101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J., Zambrano B.L., Woo J., Yoon K., Lee T. Recent advances in 1D stretchable electrodes and devices for textile and wearable electronics: materials, fabrications, and applications. Adv. Mater. 2020;32(5) doi: 10.1002/adma.201902532. [DOI] [PubMed] [Google Scholar]
- 5.Liang M., Gao L., Cheng C., Zhou Q., Uy J.P., Heiner K., Sun C. Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eikenberry S.E., Mancuso M., Iboi E., Phan T., Eikenberry K., Kuang Y., Kostelich E., Gumel A.B. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect. Dis. Model. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaishya R., Agarwal A.K., Tiwari M., Vaish A., Vijay V., Nigam Y. Medical textiles in orthopedics: An overview. J. Clin. Orthop. Trauma. 2018;9:S26–S33. doi: 10.1016/j.jcot.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saber D., Abd El-Aziz K. Advanced materials used in wearable health care devices and medical textiles in the battle against coronavirus (COVID-19): A review. J. Ind. Text. 2021 doi: 10.1177/15280837211041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Fan W., Sun Y., Chen W., Zhang Y. Application of antiviral materials in textiles: A review. Nanotechnol. Rev. 2021;10(1):1092–1115. doi: 10.1515/ntrev-2021-0072. [DOI] [Google Scholar]
- 10.Zille A., Almeida L., Amorim T., Carneiro N., Esteves M.F., Silva C.J., Souto A.P. Application of nanotechnology in antimicrobial finishing of biomedical textiles. Mater. Res. Express. 2014;1(3) doi: 10.1088/2053-1591/1/3/032003. [DOI] [Google Scholar]
- 11.Martel B., Campagne C., Massika N.B. When textiles help your recovery. Med. Sci. 2017;33(1):73–80. doi: 10.1051/medsci/20173301012. M S. [DOI] [PubMed] [Google Scholar]
- 12.Akbari M., Tamayol A., Bagherifard S., Serex L., Mostafalu P., Faramarzi N., Mohammadi M.H., Khademhosseini A. Textile technologies and tissue engineering: a path toward organ weaving. Adv. Healthc. Mater. 2016;5(7):751–766. doi: 10.1002/adhm.201500517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massella D., Argenziano M., Ferri A., Guan J., Giraud S., Cavalli R., Barresi A.A., Salaun F. Bio-functional textiles: Combining pharmaceutical nanocarriers with fibrous materials for innovative dermatological therapies. Pharmaceutics. 2019;11(8):403. doi: 10.3390/pharmaceutics11080403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang H., Luo J., Gulgunje P.V., Kumar S. Structural and functional fibers. Ann. Rev. Mater. Res. 2017;47:331–359. doi: 10.1146/annurev-matsci-120116-114326. [DOI] [Google Scholar]
- 15.Idumah C.I., Ezika A.C., Enwerem U.E. A review on biomolecular immobilization of polymeric textile biocomposites, bionanocomposites, and nano-biocomposites. J. Text. Inst. 2021 doi: 10.1080/00405000.2021.1957277. [DOI] [Google Scholar]
- 16.Mirjalili M., Zohoori S. Fabrication and characterization of zinc sulfide nanoparticles and nanocomposites prepared via a simple chemical precipitation method. J. Nanostruct. Chem. 2016;6(3):207–213. doi: 10.1007/s40097-016-0189-y. [DOI] [Google Scholar]
- 17.Dinis H., Mendes P. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. Biosens. Bioelectron. 2021;172 doi: 10.1016/j.bios.2020.112781. [DOI] [Google Scholar]
- 18.Tien N.D., Lyngstadaas S.P., Mano J.F., Blaker J.J., Haugen H.J. Recent developments in chitosan-based micro/nanofibers for sustainable food packaging, smart textiles, cosmeceuticals, and biomedical applications. Molecules. 2021;26(9):2683. doi: 10.3390/molecules26092683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aigner T.B., DeSimone E., Scheibel T. Biomedical applications of recombinant silk‐based materials. Adv. Mater. 2018;30(19) doi: 10.1002/adma.201704636. [DOI] [PubMed] [Google Scholar]
- 20.Akgol S., Ulucan-Karnak F., Kuru C.I., Kusat K. The usage of composite nanomaterials in biomedical engineering applications. Biotechnol. Bioeng. 2021;118(8):2906–2922. doi: 10.1002/bit.27843. [DOI] [PubMed] [Google Scholar]
- 21.Li G., Li Y., Chen G., He J., Han Y., Wang X., Kaplan D.L. Silk‐based biomaterials in biomedical textiles and fiber‐based implants. Adv. Healthc. Mater. 2015;4(8):1134–1151. doi: 10.1002/adhm.201500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo C., Li C., Mu X., Kaplan D.L. Engineering silk materials: From natural spinning to artificial processing. Appl. Phys. Rev. 2020;7(1) doi: 10.1063/1.5091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aibibu D., Hild M., Woeltje M., Cherif C. Textile cell-free scaffolds for in situ tissue engineering applications. J. Mater. Sci. Mater. Med. 2016;27(3):63. doi: 10.1007/s10856-015-5656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiao Y., Li C., Liu L., Wang F., Liu X., Mao J., Wang L. Construction and application of textile-based tissue engineering scaffolds: a review. Biomater. Sci. 2020;8(13):3574–3600. doi: 10.1039/D0BM00157K. [DOI] [PubMed] [Google Scholar]
- 25.Ma J., Xue Y., Liang X., Liao C., Tan Z., Tang B. Bi-directional regulatable mechanical properties of 3D braided polyetheretherketone (PEEK) Mater. Sci. Eng. C Mater. Biol. Appl. 2019;103 doi: 10.1016/j.msec.2019.109811. [DOI] [PubMed] [Google Scholar]
- 26.Tonndorf R., Aibibu D., Cherif C. Collagen multifilament spinning. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;106 doi: 10.1016/j.msec.2019.110105. [DOI] [PubMed] [Google Scholar]
- 27.Yao T., Choules B.D., Rust J.P., King M.W. The development of an in vitro test method for predicting the abrasion resistance of textile and metal components of endovascular stent grafts. J. Biomed. Mater. Res. Part B. 2014;102(3):488–499. doi: 10.1002/jbm.b.33026. [DOI] [PubMed] [Google Scholar]
- 28.Sun B., Long Y.Z., Zhang H.D., Li M.M., Duvail J.L., Jiang X.Y., Yin H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014;39(5):862–890. doi: 10.1016/j.progpolymsci.2013.06.002. [DOI] [Google Scholar]
- 29.Hong J., Yeo M., Yang G.H., Kim G. Cell-electrospinning and its application for tissue engineering. Int. J. Mol. Sci. 2019;20(24):6208. doi: 10.3390/ijms20246208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngadiman N.H.A., Noordin M.Y., Idris A., Kurniawan D. A review of evolution of electrospun tissue engineering scaffold: From two dimensions to three dimensions. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017;231(7):597–616. doi: 10.1177/0954411917699021. [DOI] [PubMed] [Google Scholar]
- 31.Schiffman J.D., Schauer C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008;48(2):317–352. doi: 10.1080/15583720802022182. [DOI] [Google Scholar]
- 32.Xu X., Ren S., Li L., Zhou Y., Peng W., Xu Y. Biodegradable engineered fiber scaffolds fabricated by electrospinning for periodontal tissue regeneration. J. Biomater. Appl. 2021;36(1):55–75. doi: 10.1177/0885328220952250. [DOI] [PubMed] [Google Scholar]
- 33.Luraghi A., Peri F., Moroni L. Electrospinning for drug delivery applications: A review. J. Control. Release. 2021;334:463–484. doi: 10.1016/j.jconrel.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Wen P., Wen Y., Zong M.H., Linhardt R.J., Wu H. Encapsulation of bioactive compound in electrospun fibers and its potential application. J. Agric. Food Chem. 2017;65(42):9161–9179. doi: 10.1021/acs.jafc.7b02956. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Li W., Li C., Zhou B., Zhou Y., Jiang L., Wen S., Zhou F. Fabrication of ultra-high working range strain sensor using carboxyl CNTs coated electrospun TPU assisted with dopamine. Appl. Surf. Sci. 2021;566 doi: 10.1016/j.apsusc.2021.150705. [DOI] [Google Scholar]
- 36.Zhou B., Liu Z., Li C., Liu M., Jiang L., Zhou Y., Zhou F.L., Chen S., Jerrams S., Yu J. A highly stretchable and sensitive strain sensor based on dopamine modified electrospun SEBS fibers and MWCNTs with carboxylation. Adv. Electron. Mater. 2021 doi: 10.1002/aelm.202100233. [DOI] [Google Scholar]
- 37.Wei L., Qin X. Nanofiber bundles and nanofiber yarn device and their mechanical properties: A review. Text. Res. J. 2016;86(17):1885–1898. doi: 10.1177/0040517515617422. [DOI] [Google Scholar]
- 38.Sinha M., Das B., Bharathi D., Prasad N., Kishore B., Raj P., Kumar K. Electrospun nanofibrous materials for biomedical textiles. Mater. Today Proc. 2020;21:1818–1826. doi: 10.1016/j.matpr.2020.01.236. [DOI] [Google Scholar]
- 39.Poshina D., Otsuka I. Electrospun Polysaccharidic Textiles for Biomedical Applications. Textiles. 2021;1(2):152–169. doi: 10.3390/textiles1020007. [DOI] [Google Scholar]
- 40.Rasouli R., Barhoum A., Bechelany M., Dufresne A. Nanofibers for biomedical and healthcare applications. Macromol. Biosci. 2019;19(2) doi: 10.1002/mabi.201800256. [DOI] [PubMed] [Google Scholar]
- 41.Barhoum A., Pal K., Rahier H., Uludag H., Kim I.S., Bechelany M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today. 2019;17:1–35. doi: 10.1016/j.apmt.2019.06.015. [DOI] [Google Scholar]
- 42.Wu Y., Wang L., Guo B., Ma P.X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano. 2017;11(6):5646–5659. doi: 10.1021/acsnano.7b01062. [DOI] [PubMed] [Google Scholar]
- 43.Yang J., Mao Z., Zheng R., Liu H., Shi L. Solution-blown aligned nanofiber yarn and its application in yarn-shaped supercapacitor. Materials. 2020;13(17):3778. doi: 10.3390/ma13173778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loey M., Manogaran G., Taha M.H.N., Khalifa N.E.M. A hybrid deep transfer learning model with machine learning methods for face mask detection in the era of the COVID-19 pandemic. Measurement. 2021;167 doi: 10.1016/j.measurement.2020.108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen P.H., Zhang W. Design and computational modeling of fabric soft pneumatic actuators for wearable assistive devices. Sci. Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-65003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C., Guo H., Chen L., Wang Y.C., Pu X., Yu W., Wang F., Du Z., Wang Z.L. Direct current fabric triboelectric nanogenerator for biomotion energy harvesting. ACS Nano. 2020;14(4):4585–4594. doi: 10.1021/acsnano.0c00138. [DOI] [PubMed] [Google Scholar]
- 47.Pan J., Hao B., Song W., Chen S., Li D., Luo L., Xia Z., Cheng D., Xu A., Cai G. Highly conductive and stretchable carbon nanotube/thermoplastic polyurethane composite for wearable heater. Compos. Part B Eng. 2020;183 doi: 10.1016/j.compositesb.2019.107683. [DOI] [Google Scholar]
- 48.Xue J., Wu T., Dai Y., Xia Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019;119(8):5298–5415. doi: 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reneker D.H., Yarin A.L. Electrospinning jets and polymer nanofibers. Polymer. 2008;49(10):2387–2425. doi: 10.1016/j.polymer.2008.02.002. [DOI] [Google Scholar]
- 50.Rahmati M., Mills D.K., Urbanska A.M., Saeb M.R., Venugopal J.R., Ramakrishna S., Mozafari M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021;117 doi: 10.1016/j.pmatsci.2020.100721. [DOI] [Google Scholar]
- 51.Reneker D.H., Yarin A.L., Fong H., Koombhongse S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000;87(9):4531–4547. doi: 10.1063/1.373532. [DOI] [Google Scholar]
- 52.Shin Y., Hohman M., Brenner M., Rutledge G. Experimental characterization of electrospinning: The electrically forced jet and instabilities. Polymer. 2001;42(25):09955–09967. doi: 10.1016/S0032-3861(01)00540-7. [DOI] [Google Scholar]
- 53.Yarin A.L., Koombhongse S., Reneker D.H. Bending instability in electrospinning of nanofibers. J. Appl. Phys. 2001;89(5):3018–3026. doi: 10.1063/1.1333035. [DOI] [Google Scholar]
- 54.Hohman M.M., Shin M., Rutledge G., Brenner M.P. Electrospinning and electrically forced jets. II. Applications. Phys. Fluids. 2001;13(8):2221–2236. doi: 10.1063/1.1384013. [DOI] [Google Scholar]
- 55.Liu F., Guo R., Shen M., Wang S., Shi X. Effect of processing variables on the morphology of electrospun poly[(lactic acid)‐co‐(glycolic acid)] nanofibers. Macromol. Mater. Eng. 2009;294(10):666–672. doi: 10.1002/mame.200900110. [DOI] [Google Scholar]
- 56.Wu S., Qin X., Li M. The structure and properties of cellulose acetate materials: A comparative study on electrospun membranes and casted films. J. Ind. Text. 2014;44(1):85–98. doi: 10.1177/1528083713477443. [DOI] [Google Scholar]
- 57.Mailley D., Hebraud A., Schlatter G. A review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021;306(7) doi: 10.1002/mame.202100115. [DOI] [Google Scholar]
- 58.Ibrahim H.M., Klingner A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020;90 doi: 10.1016/j.polymertesting.2020.106647. [DOI] [Google Scholar]
- 59.Haider A., Haider S., Kang I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018;11(8):1165–1188. doi: 10.1016/j.arabjc.2015.11.015. [DOI] [Google Scholar]
- 60.Anton F. Process and apparatus for preparing artificial threads. Google Patents. 1934:US1975504. [Google Scholar]
- 61.Ko F., Gogotsi Y., Ali A., Naguib N., Ye H., Yang G., Li C., Willis P. Electrospinning of continuous carbon nanotube‐filled nanofiber yarns. Adv. Mater. 2003;15(14):1161–1165. doi: 10.1002/adma.200304955. [DOI] [Google Scholar]
- 62.Wu S.H., Qin X.H. Effects of the stabilization temperature on the structure and properties of polyacrylonitrile-based stabilized electrospun nanofiber microyarns. J. Therm. Anal. Calorim. 2014;116(1):303–308. doi: 10.1007/s10973-013-3530-4. [DOI] [Google Scholar]
- 63.Puppi D., Chiellini F. Wet‐spinning of biomedical polymers: From single‐fibre production to additive manufacturing of three‐dimensional scaffolds. Polym. Int. 2017;66(12):1690–1696. doi: 10.1002/pi.5332. [DOI] [Google Scholar]
- 64.Imura Y., Hogan R., Jaffe M. Advances in Filament Yarn Spinning of Textiles and Polymers. Woodhead Publishing; 2014. Dry spinning of synthetic polymer fibers; pp. 187–202. [DOI] [Google Scholar]
- 65.Ohzawa Y., Nagano Y., Matsuo T. Studies on dry spinning. I. Fundamental equations. J. Appl. Polym. Sci. 1969;13(2):257–283. doi: 10.1002/app.1969.070130201. [DOI] [Google Scholar]
- 66.Teo W., Ramakrishna S. Electrospun fibre bundle made of aligned nanofibres over two fixed points. Nanotechnology. 2005;16(9):1878. doi: 10.1088/0957-4484/16/9/077. [DOI] [Google Scholar]
- 67.Dalton P.D., Klee D., Möller M. Electrospinning with dual collection rings. Polymer. 2005;46(3):611–614. doi: 10.1016/j.polymer.2004.11.075. [DOI] [Google Scholar]
- 68.Bazbouz M., Stylios G. Proceedings of the Green Chemistry and Engineering International Conference on Process Intensification and Nanotechnology. Heriot-Watt Research Portal; 2008. A spinning concept for ultrafine composite nanofibre yarns; pp. 145–160. [Google Scholar]
- 69.Yan H., Liu L., Zhang Z. Continually fabricating staple yarns with aligned electrospun polyacrylonitrile nanofibers. Mater. Lett. 2011;65(15–16):2419–2421. doi: 10.1016/j.matlet.2011.04.091. [DOI] [Google Scholar]
- 70.Bazbouz M.B., Stylios G.K. Novel mechanism for spinning continuous twisted composite nanofiber yarns. Eur. Polym. J. 2008;44(1):1–12. doi: 10.1016/j.eurpolymj.2007.10.006. [DOI] [Google Scholar]
- 71.Bazbouz M.B., Stylios G.K. A new mechanism for the electrospinning of nanoyarns. J. Appl. Polym. Sci. 2012;124(1):195–201. doi: 10.1002/app.31930. [DOI] [Google Scholar]
- 72.Liu C.K., Sun R.J., Lai K., Sun C.Q., Wang Y.W. Preparation of short submicron-fiber yarn by an annular collector through electrospinning. Mater. Lett. 2008;62(29):4467–4469. doi: 10.1016/j.matlet.2008.07.058. [DOI] [Google Scholar]
- 73.Lotus A., Bender E., Evans E., Ramsier R., Reneker D.H., Chase G.G. Electrical, structural, and chemical properties of semiconducting metal oxide nanofiber yarns. J. Appl. Phys. 2008;103(2) doi: 10.1063/1.2831362. [DOI] [Google Scholar]
- 74.Lotus A., Bhargava S., Bender E., Evans E., Ramsier R., Reneker D.H., Chase G.G. Electrospinning route for the fabrication of p-n junction using nanofiber yarns. J. Appl. Phys. 2009;106(1) doi: 10.1063/1.3157206. [DOI] [Google Scholar]
- 75.Gu B.K., Shin M.K., Sohn K.W., Kim S.I., Kim S.J., Kim S.K., Lee H., Park J.S. Direct fabrication of twisted nanofibers by electrospinning. Appl. Phys. Lett. 2007;90(26) doi: 10.1063/1.2753109. [DOI] [Google Scholar]
- 76.Li N., Hui Q., Xue H., Xiong J. Electrospun Polyacrylonitrile nanofiber yarn prepared by funnel-shape collector. Mater. Lett. 2012;79:245–247. doi: 10.1016/j.matlet.2012.04.005. [DOI] [Google Scholar]
- 77.Guan X., Xia H., Ni Q.Q. Shape memory polyurethane‐based electrospun yarns for thermo‐responsive actuation. J. Appl. Polym. Sci. 2021;138(24):50565. doi: 10.1002/app.50565. [DOI] [Google Scholar]
- 78.Nakashima R., Watanabe K., Lee Y., Kim B.S., Kim I.S. Mechanical properties of poly (vinylidene fluoride) nanofiber filaments prepared by electrospinning and twisting. Adv. Polym. Technol. 2013;32(S1):E44–E52. doi: 10.1002/adv.20268. [DOI] [Google Scholar]
- 79.Khil M.S., Bhattarai S.R., Kim H.Y., Kim S.Z., Lee K.H. Novel fabricated matrix via electrospinning for tissue engineering. J. Biomed. Mater. Res. Part B. 2005;72(1):117–124. doi: 10.1002/jbm.b.30122. [DOI] [PubMed] [Google Scholar]
- 80.Smit E., Bűttner U., Sanderson R.D. Continuous yarns from electrospun fibers. Polymer. 2005;46(8):2419–2423. doi: 10.1016/j.polymer.2005.02.002. [DOI] [Google Scholar]
- 81.Teo W.E., Gopal R., Ramaseshan R., Fujihara K., Ramakrishna S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer. 2007;48(12):3400–3405. doi: 10.1016/j.polymer.2007.04.044. [DOI] [Google Scholar]
- 82.Yousefzadeh M., Latifi M., Teo W.E., Amani-Tehran M., Ramakrishna S. Producing continuous twisted yarn from well‐aligned nanofibers by water vortex. Polym. Eng. Sci. 2011;51(2):323–329. doi: 10.1002/pen.21800. [DOI] [Google Scholar]
- 83.Liu J., Chen G., Gao H., Zhang L., Ma S., Liang J., Fong H. Structure and thermo-chemical properties of continuous bundles of aligned and stretched electrospun polyacrylonitrile precursor nanofibers collected in a flowing water bath. Carbon. 2012;50(3):1262–1270. doi: 10.1016/j.carbon.2011.10.046. [DOI] [Google Scholar]
- 84.Bin Y., Hao Y., Meifang Z., Hongzhi W. Continuous high-aligned polyacrylonitrile electrospun nanofibers yarns via circular deposition on water bath. J. Nanosci. Nanotechnol. 2016;16(6):5633–5638. doi: 10.1166/jnn.2016.11721. [DOI] [PubMed] [Google Scholar]
- 85.Yan T., Tian L., Pan Z. Structures and mechanical properties of plied and twisted polyacrylonitrile nanofiber yarns fabricated by a multi-needle electrospinning device. Fiber. Polym. 2016;17(10):1627–1633. doi: 10.1007/s12221-016-6553-1. [DOI] [Google Scholar]
- 86.Wang X., Zhang K., Zhu M., Yu H., Zhou Z., Chen Y., Hsiao B.S. Continuous polymer nanofiber yarns prepared by self-bundling electrospinning method. Polymer. 2008;49(11):2755–2761. doi: 10.1016/j.polymer.2008.04.015. [DOI] [Google Scholar]
- 87.Zhang K., Wang X., Yang Y., Wang L., Zhu M., Hsiao B.S., Chu B. Aligned and molecularly oriented semihollow ultrafine polymer fiber yarns by a facile method. J. Polym. Sci. Part B Polym. Phys. 2010;48(10):1118–1125. doi: 10.1002/polb.22003. [DOI] [Google Scholar]
- 88.Pan H., Li L., Hu L., Cui X. Continuous aligned polymer fibers produced by a modified electrospinning method. Polymer. 2006;47(14):4901–4904. doi: 10.1016/j.polymer.2006.05.012. [DOI] [Google Scholar]
- 89.Li X., Yao C., Sun F., Song T., Li Y., Pu Y. Conjugate electrospinning of continuous nanofiber yarn of poly (L‐lactide)/nanotricalcium phosphate nanocomposite. J. Appl. Polym. Sci. 2008;107(6):3756–3764. doi: 10.1002/app.27524. [DOI] [Google Scholar]
- 90.Dabirian F., Hosseini S. Novel method for nanofibre yarn production using two differently charged nozzles. Fibres Text. East. Eur. 2009;17(3):45–47. [Google Scholar]
- 91.Dabirian F., Ravandi S.H., Sanatgar R.H., Hinestroza J. Manufacturing of twisted continuous PAN nanofiber yarn by electrospinning process. Fiber. Polym. 2011;12(5):610–615. doi: 10.1007/s12221-011-0610-6. [DOI] [Google Scholar]
- 92.Su C.I., Liu Y.S., Hsu C.H., Lee J.Y., Lu C.H. Optimum parameters of the continuous process of electrospun nanofibrous yarn. Fiber. Polym. 2015;16(4):826–833. doi: 10.1007/s12221-015-0826-y. [DOI] [Google Scholar]
- 93.Maleki H., Gharehaghaji A.A., Criscenti G., Moroni L., Dijkstra P.J. The influence of process parameters on the properties of electrospun PLLA yarns studied by the response surface methodology. J. Appl. Polym. Sci. 2015;132(5) doi: 10.1002/app.41388. [DOI] [Google Scholar]
- 94.Maleki H., Gharehaghaji A., Dijkstra P. Electrospinning of continuous poly (L-lactide) yarns: Effect of twist on the morphology, thermal properties and mechanical behavior. J. Mech. Behav. Biomed. Mater. 2017;71:231–237. doi: 10.1016/j.jmbbm.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 95.Hosseini S.A., Pan N., Ko F. Dynamic mechanical relaxations of electrospun poly (acrylonitrile-co-methyl acrylate) nanofibrous yarn. Text. Res. J. 2017;87(18):2193–2203. doi: 10.1177/0040517516665265. [DOI] [Google Scholar]
- 96.Dabirian F., Ravandi S.A.H., Hinestroza J.P., Abuzade R.A. Conformal coating of yarns and wires with electrospun nanofibers. Polym. Eng. Sci. 2012;52(8):1724–1732. doi: 10.1002/pen.23109. [DOI] [Google Scholar]
- 97.Najafi S., Gharehaghaji A., Etrati S. Fabrication and characterization of elastic hollow nanofibrous PU yarn. Mater. Des. 2016;99:328–334. doi: 10.1016/j.matdes.2016.02.111. [DOI] [Google Scholar]
- 98.Ghane N., Mazinani S., Gharehaghaji A.A. Fabrication and characterization of hollow nanofibrous PA6 yarn reinforced with CNTs. J. Polym. Res. 2018;25(3):1–12. doi: 10.1007/s10965-018-1477-7. [DOI] [Google Scholar]
- 99.Ke G., Jin X., Cai G., Li W., Xu A. A novel composite cotton yarn with phase change and electrical conductivity functions. J. Ind. Text. 2021 doi: 10.1177/15280837211003166. [DOI] [Google Scholar]
- 100.Moghbelnejad Z., Gharehaghaji A.A., Yousefzadeh M., Hajiani F. Investigation of wicking phenomenon and tensile properties in three‐layer composite nanofibrous PA/PLLA yarn. Polym. Eng. Sci. 2021;61(2):576–585. doi: 10.1002/pen.25601. [DOI] [Google Scholar]
- 101.Sohanaki P., Ahamadloo E., Gharehaghaji A.A., Malek R. Fabrication and characterization of three-layer nanofibrous yarn (PA6/PU/PA6) Polym. Bull. 2021:1–20. doi: 10.1007/s00289-021-03835-2. [DOI] [Google Scholar]
- 102.Ali U., Zhou Y., Wang X., Lin T. Direct electrospinning of highly twisted, continuous nanofiber yarns. J. Text. Inst. 2012;103(1):80–88. doi: 10.1080/00405000.2011.552254. [DOI] [Google Scholar]
- 103.Shuakat M.N., Lin T. Highly-twisted, continuous nanofibre yarns prepared by a hybrid needle-needleless electrospinning technique. RSC Adv. 2015;5(43):33930–33937. doi: 10.1039/C5RA03906A. [DOI] [Google Scholar]
- 104.He J., Zhou Y., Qi K., Wang L., Li P., Cui S. Continuous twisted nanofiber yarns fabricated by double conjugate electrospinning. Fiber. Polym. 2013;14(11):1857–1863. doi: 10.1007/s12221-013-1857-x. [DOI] [Google Scholar]
- 105.He J., Qi K., Zhou Y., Cui S. Multiple conjugate electrospinning method for the preparation of continuous polyacrylonitrile nanofiber yarn. J. Appl. Polym. Sci. 2014;131(8) doi: 10.1002/app.40137. [DOI] [Google Scholar]
- 106.Levitt A.S., Knittel C.E., Vallett R., Koerner M., Dion G., Schauer C.L. Investigation of nanoyarn preparation by modified electrospinning setup. J. Appl. Polym. Sci. 2017;134(19) doi: 10.1002/app.44813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin S., Xin B., Zheng Y., Liu S. Effect of electric field on the directly electrospun nanofiber yarns: Simulation and experimental study. Fiber. Polym. 2018;19(1):116–124. doi: 10.1007/s12221-018-7734-2. [DOI] [Google Scholar]
- 108.Buzol Mülayim B., Göktepe F. Analysis of polyacrylonitrile nanofiber yarn formation in electrospinning by using a conical collector and two oppositely charged nozzles. J. Text. Inst. 2021;112(3):494–504. doi: 10.1080/00405000.2020.1768772. [DOI] [Google Scholar]
- 109.Wu S.H., Qin X.H. Uniaxially aligned polyacrylonitrile nanofiber yarns prepared by a novel modified electrospinning method. Mater. Lett. 2013;106:204–207. doi: 10.1016/j.matlet.2013.05.010. [DOI] [Google Scholar]
- 110.Wu S., Zhang Y., Liu P., Qin X. Polyacrylonitrile nanofiber yarns and fabrics produced using a novel electrospinning method combined with traditional textile techniques. Text. Res. J. 2016;86(16):1716–1727. doi: 10.1177/0040517515603808. [DOI] [Google Scholar]
- 111.Wu S., Zhou R., Zhou F., Streubel P.N., Chen S., Duan B. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/microfiber hybrid yarns for tendon tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;106 doi: 10.1016/j.msec.2019.110268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu S., Ni S., Jiang X., Kuss M.A., Wang H.J., Duan B. Guiding mesenchymal stem cells into myelinating schwann cell-like phenotypes by using electrospun core–sheath nanoyarns. ACS Biomater. Sci. Eng. 2019;5(10):5284–5294. doi: 10.1021/acsbiomaterials.9b00748. [DOI] [PubMed] [Google Scholar]
- 113.Wang X., Zhang K., Zhu M., Hsiao B.S., Chu B. Enhanced mechanical performance of self‐bundled electrospun fiber yarns via post‐treatments. Macromol. Rapid Commun. 2008;29(10):826–831. doi: 10.1002/marc.200700873. [DOI] [Google Scholar]
- 114.Dabirian F., Hosseini Y., Ravandi S.H. Manipulation of the electric field of electrospinning system to produce polyacrylonitrile nanofiber yarn. J. Text. Inst. 2007;98(3):237–241. doi: 10.1080/00405000701463979. [DOI] [Google Scholar]
- 115.Su C.I., Lai T.C., Lu C.H., Liu Y.S., Wu S.P. Yarn formation of nanofibers prepared using electrospinning. Fiber. Polym. 2013;14(4):542–549. doi: 10.1007/s12221-013-0542-4. [DOI] [Google Scholar]
- 116.Su C.I., Liu Y.S., Lu C.H., Lin C.W., Wu S.P. Polyacrylonitrile nanocomposite with carbon nanostructures: a review. Polym. Plast. Technol. Eng. 2015;54(11):1106–1112. doi: 10.1080/03602559.2014.986803. [DOI] [Google Scholar]
- 117.Bosworth L.A. Travelling along the clinical roadmap: developing electrospun scaffolds for tendon repair. Conf. Pap. Sci. 2014;304974 doi: 10.1155/2014/304974. Hindawi. [DOI] [Google Scholar]
- 118.Afifi A.M., Nakano S., Yamane H., Kimura Y. Electrospinning of continuous aligning yarns with a ‘funnel’target. Macromol. Mater. Eng. 2010;295(7):660–665. doi: 10.1002/mame.200900406. [DOI] [Google Scholar]
- 119.Maleki H., Barani H. Morphological and mechanical properties of drawn poly(l‐lactide) electrospun twisted yarns. Polym. Eng. Sci. 2018;58(7):1091–1096. doi: 10.1002/pen.24671. [DOI] [Google Scholar]
- 120.Li D., Tao L., Shen Y., Sun B., Xie X., Ke Q., Mo X., Deng B. Fabrication of multilayered nanofiber scaffolds with a highly aligned nanofiber yarn for anisotropic tissue regeneration. ACS Omega. 2020;5(38):24340–24350. doi: 10.1021/acsomega.0c02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu S., Liu J., Cai J., Zhao J., Duan B., Chen S. Combining electrospinning with hot drawing process to fabricate high performance poly (L-lactic acid) nanofiber yarns for advanced nanostructured bio-textiles. Biofabrication. 2021;13(4) doi: 10.1088/1758-5090/ac2209. [DOI] [PubMed] [Google Scholar]
- 122.Bae S., DiBalsi M.J., Meilinger N., Zhang C., Beal E., Korneva G., Brown R.O., Kornev K.G., Lee J.S. Heparin-eluting electrospun nanofiber yarns for antithrombotic vascular sutures. ACS Appl. Mater. Interfaces. 2018;10(10):8426–8435. doi: 10.1021/acsami.7b14888. [DOI] [PubMed] [Google Scholar]
- 123.Baniasadi M., Huang J., Xu Z., Moreno S., Yang X., Chang J., Quevedo-Lopez M.A., Naraghi M., Minary-Jolandan M. High-performance coils and yarns of polymeric piezoelectric nanofibers. ACS Appl. Mater. Interfaces. 2015;7(9):5358–5366. doi: 10.1021/am508812a. [DOI] [PubMed] [Google Scholar]
- 124.Shuakat M.N., Lin T. Direct electrospinning of nanofibre yarns using a rotating ring collector. J. Text. Inst. 2016;107(6):791–799. doi: 10.1080/00405000.2015.1061785. [DOI] [Google Scholar]
- 125.Ali U., Niu H., Abbas A., Shao H., Lin T. Online stretching of directly electrospun nanofiber yarns. RSC Adv. 2016;6(36):30564–30569. doi: 10.1039/C6RA01856D. [DOI] [Google Scholar]
- 126.Li Y., Guo F., Hao Y., Gupta S.K., Hu J., Wang Y., Wang N., Zhao Y., Guo M. Helical nanofiber yarn enabling highly stretchable engineered microtissue. Proc. Natl. Acad. Sci. 2019;116(19):9245–9250. doi: 10.1073/pnas.1821617116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hajiani F., Jeddi A.A., Gharehaghaji A. An investigation on the effects of twist on geometry of the electrospinning triangle and polyamide 66 nanofiber yarn strength. Fiber. Polym. 2012;13(2):244–252. doi: 10.1007/s12221-012-0244-3. [DOI] [Google Scholar]
- 128.Asghari Mooneghi S., Gharehaghaji A.A., Hosseini-Toudeshky H., Torkaman G. Tensile fatigue behavior of polyamide 66 nanofiber yarns. Polym. Eng. Sci. 2015;55(8):1805–1811. doi: 10.1002/pen.24019. [DOI] [Google Scholar]
- 129.Sensini A., Santare M.H., Eichenlaub E., Bloom E., Gotti C., Zucchelli A., Cristofolini L. Tuning the structure of nylon 6, 6 electrospun bundles to mimic the mechanical performance of tendon fascicles. Front. Bioeng. Biotechnol. 2021;9:230. doi: 10.3389/fbioe.2021.626433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jin S., Xin B., Zheng Y. Preparation and characterization of polysulfone amide nanoyarns by the dynamic rotating electrospinning method. Text. Res. J. 2019;89(1):52–62. doi: 10.1177/0040517517736474. [DOI] [Google Scholar]
- 131.Wu S., Qi Y., Shi W., Kuss M., Chen S., Duan B. Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction. Acta Biomater. 2022;139:91–104. doi: 10.1016/j.actbio.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu J., Zhai H., Sun Y., Wu S., Chen S. Developing high strength poly (L-lactic acid) nanofiber yarns for biomedical textile materials: A comparative study of novel nanofiber yarns and traditional microfiber yarns. Mater. Lett. 2021;300 doi: 10.1016/j.matlet.2021.130229. [DOI] [Google Scholar]
- 133.Joseph J., Nair S.V., Menon D. Integrating substrateless electrospinning with textile technology for creating biodegradable three-dimensional structures. Nano Lett. 2015;15(8):5420–5426. doi: 10.1021/acs.nanolett.5b01815. [DOI] [PubMed] [Google Scholar]
- 134.Abhari R., Mouthuy P.A., Vernet A., Schneider J., Brown C., Carr A. Using an industrial braiding machine to upscale the production and modulate the design of electrospun medical yarns. Polym. Test. 2018;69:188–198. doi: 10.1016/j.polymertesting.2018.05.014. [DOI] [Google Scholar]
- 135.Wu S., Duan B., Liu P., Zhang C., Qin X., Butcher J.T. Fabrication of aligned nanofiber polymer yarn networks for anisotropic soft tissue scaffolds. ACS Appl. Mater. Interfaces. 2016;8(26):16950–16960. doi: 10.1021/acsami.6b05199. [DOI] [PubMed] [Google Scholar]
- 136.Wu S., Liu P., Zhang Y., Zhang H., Qin X. Flexible and conductive nanofiber-structured single yarn sensor for smart wearable devices. Sens. Actuator B Chem. 2017;252:697–705. doi: 10.1016/j.snb.2017.06.062. [DOI] [Google Scholar]
- 137.Shao W., He J., Han Q., Sang F., Wang Q., Chen L., Cui S., Ding B. A biomimetic multilayer nanofiber fabric fabricated by electrospinning and textile technology from polylactic acid and Tussah silk fibroin as a scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;67:599–610. doi: 10.1016/j.msec.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 138.Nagarajan S., Belaïd H., Pochat-Bohatier C., Teyssier C., Iatsunskyi I., Coy E., Balme S., Cornu D., Miele P., Kalkura N.S. Design of boron nitride/gelatin electrospun nanofibers for bone tissue engineering. ACS Appl. Mater. Interfaces. 2017;9(39):33695–33706. doi: 10.1021/acsami.7b13199. [DOI] [PubMed] [Google Scholar]
- 139.Wu S., Wang Y., Streubel P.N., Duan B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater. 2017;62:102–115. doi: 10.1016/j.actbio.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Setiawati A., Jang D., Cho D., Cho S., Jeong H., Park S., Gwak J., Ryu S.R., Jung W.H., Ju B.G. An accelerated wound‐healing surgical suture engineered with an extracellular matrix. Adv. Healthc. Mater. 2021;10(6) doi: 10.1002/adhm.202001686. [DOI] [PubMed] [Google Scholar]
- 141.Haghighat F., Ravandi S.A.H. Mechanical properties and in vitro degradation of PLGA suture manufactured via electrospinning. Fiber. Polym. 2014;15(1):71–77. doi: 10.1007/s12221-014-0071-9. [DOI] [Google Scholar]
- 142.Ye Y.J., Zhou Y.Q., Jing Z.Y., Liu Y.Y., Yin D.C. Electrospun heparin‐loaded core–shell nanofiber sutures for Achilles tendon regeneration in vivo. Macromol. Biosci. 2018;18(7) doi: 10.1002/mabi.201800041. [DOI] [PubMed] [Google Scholar]
- 143.Rashid M., Dudhia J., Dakin S.G., Snelling S., Lach A., De Godoy R., Mouthuy P.-A., Smith R., Morrey M., Carr A.J. Histological evaluation of cellular response to a multifilament electrospun suture for tendon repair. PloS One. 2020;15(6) doi: 10.1371/journal.pone.0234982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharifisamani E., Mousazadegan F., Bagherzadeh R., Latifi M. PEG‐PLA‐PCL based electrospun yarns with curcumin control release property as suture. Polym. Eng. Sci. 2020;60(7):1520–1529. doi: 10.1002/pen.25398. [DOI] [Google Scholar]
- 145.Padmakumar S., Joseph J., Neppalli M.H., Mathew S.E., Nair S.V., Shankarappa S.A., Menon D. Electrospun polymeric core–sheath yarns as drug eluting surgical sutures. ACS Appl. Mater. Interfaces. 2016;8(11):6925–6934. doi: 10.1021/acsami.6b00874. [DOI] [PubMed] [Google Scholar]
- 146.Gu Z., Yin H., Wang J., Ma L., Morsi Y., Mo X. Fabrication and characterization of TGF-β1-loaded electrospun poly (lactic-co-glycolic acid) core-sheath sutures. Colloid Surf. B Biointerfaces. 2018;161:331–338. doi: 10.1016/j.colsurfb.2017.10.066. [DOI] [PubMed] [Google Scholar]
- 147.Richard A.S., Verma R.S. Bioactive nano yarns as surgical sutures for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;128 doi: 10.1016/j.msec.2021.112334. [DOI] [PubMed] [Google Scholar]
- 148.Maleki H., Gharehaghaji A., Toliyat T., Dijkstra P. Drug release behavior of electrospun twisted yarns as implantable medical devices. Biofabrication. 2016;8(3) doi: 10.1088/1758-5090/8/3/035019. [DOI] [PubMed] [Google Scholar]
- 149.Barani H. Antibacterial continuous nanofibrous hybrid yarn through in situ synthesis of silver nanoparticles: preparation and characterization. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;43:50–57. doi: 10.1016/j.msec.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 150.Cotrim M., Oréfice R. Biocompatible and fluorescent polycaprolactone/silk electrospun nanofiber yarns loaded with carbon quantum dots for biotextiles. Polym. Adv. Technol. 2021;32(1):87–96. doi: 10.1002/pat.5063. [DOI] [Google Scholar]
- 151.Xue W., Shi W., Kong Y., Kuss M., Duan B. Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact. Mater. 2021;6(11):4141–4160. doi: 10.1016/j.bioactmat.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang L., Wu Y., Hu T., Ma P.X., Guo B. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019;96:175–187. doi: 10.1016/j.actbio.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 153.Luca A.C.D., Raffoul W., Giacalone F., Bertolini M., Summa P.G.D. Tissue-engineered constructs for peripheral nerve repair: current research concepts and future perspectives. Plast. Aesthet. Res. 2015;2:213–219. doi: 10.4103/2347-9264.160889. [DOI] [Google Scholar]
- 154.Sarker M., Naghieh S., McInnes A.D., Schreyer D.J., Chen X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018;171:125–150. doi: 10.1016/j.pneurobio.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 155.Panagopoulos G.N., Megaloikonomos P.D., Mavrogenis A.F. The present and future for peripheral nerve regeneration. Orthopedics. 2017;40(1):e141–e156. doi: 10.3928/01477447-20161019-01. [DOI] [PubMed] [Google Scholar]
- 156.Lackington W.A., Ryan A.J., O’Brien F.J. Advances in nerve guidance conduit-based therapeutics for peripheral nerve repair. ACS Biomater. Sci. Eng. 2017;3(7):1221–1235. doi: 10.1021/acsbiomaterials.6b00500. [DOI] [PubMed] [Google Scholar]
- 157.Wu S., Kuss M., Qi D., Hong J., Wang H.J., Zhang W., Chen S., Ni S., Duan B. Development of cryogel-based guidance conduit for peripheral nerve regeneration. ACS Appl. Bio Mater. 2019;2(11):4864–4871. doi: 10.1021/acsabm.9b00626. [DOI] [PubMed] [Google Scholar]
- 158.Li D., Pan X., Sun B., Wu T., Chen W., Huang C., Ke Q., Ei-Hamshary H.A., Al-Deyab S.S., Mo X. Nerve conduits constructed by electrospun P (LLA-CL) nanofibers and PLLA nanofiber yarns. J. Mat. Chem. B. 2015;3(45):8823–8831. doi: 10.1039/C5TB01402F. [DOI] [PubMed] [Google Scholar]
- 159.Wu T., Li D., Wang Y., Sun B., Li D., Morsi Y., El-Hamshary H., Al-Deyab S.S., Mo X. Laminin-coated nerve guidance conduits based on poly (l-lactide-co-glycolide) fibers and yarns for promoting Schwann cells’ proliferation and migration. J. Mat. Chem. B. 2017;5(17):3186–3194. doi: 10.1039/C6TB03330J. [DOI] [PubMed] [Google Scholar]
- 160.Pan X., Sun B., Mo X. Electrospun polypyrrole-coated polycaprolactone nanoyarn nerve guidance conduits for nerve tissue engineering. Front. Mater. Sci. 2018;12(4):438–446. doi: 10.1007/s11706-018-0445-9. [DOI] [Google Scholar]
- 161.Gopalakrishnan-Prema V., Mohanan A., Shivaram S.B., Madhusudanan P., Raju G., Menon D., Shankarappa S.A. Electrical stimulation of co-woven nerve conduit for peripheral neurite differentiation. Biomed. Mater. 2020;15(6) doi: 10.1088/1748-605X/abaf06. [DOI] [PubMed] [Google Scholar]
- 162.Rinoldi C., Kijeńska-Gawrońska E., Khademhosseini A., Tamayol A., Swieszkowski W. Fibrous systems as potential solutions for tendon and ligament repair, healing, and regeneration. Adv. Healthc. Mater. 2021;10(7) doi: 10.1002/adhm.202001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang D., Zhang X., Huang S., Liu Y., Fu B.S.C., Mak K.K.L., Blocki A.M., Yung P.S.H., Tuan R.S. Engineering multi-tissue units for regenerative medicine: bone-tendon-muscle units of the rotator cuff. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120789. [DOI] [PubMed] [Google Scholar]
- 164.Yang C., Deng G., Chen W., Ye X., Mo X. A novel electrospun-aligned nanoyarn-reinforced nanofibrous scaffold for tendon tissue engineering. Colloid Surf. B Biointerfaces. 2014;122:270–276. doi: 10.1016/j.colsurfb.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 165.Xu Y., Wu J., Wang H., Li H., Di N., Song L., Li S., Li D., Xiang Y., Liu W. Fabrication of electrospun poly (L-lactide-co-ɛ-caprolactone)/collagen nanoyarn network as a novel, three-dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Eng. Part C Methods. 2013;19(12):925–936. doi: 10.1089/ten.tec.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Laranjeira M., Domingues R.M., Costa-Almeida R., Reis R.L., Gomes M.E. 3D mimicry of native‐tissue‐fiber architecture guides tendon‐derived cells and adipose stem cells into artificial tendon constructs. Small. 2017;13(31) doi: 10.1002/smll.201700689. [DOI] [PubMed] [Google Scholar]
- 167.Bosworth L., Rathbone S., Bradley R., Cartmell S. Dynamic loading of electrospun yarns guides mesenchymal stem cells towards a tendon lineage. J. Mech. Behav. Biomed. Mater. 2014;39:175–183. doi: 10.1016/j.jmbbm.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jayasree A., Kottappally Thankappan S., Ramachandran R., Sundaram M.N., Chen C.H., Mony U., Chen J.P., Jayakumar R., Biomater ACS. Bioengineered braided micro–Nano (multiscale) fibrous scaffolds for tendon reconstruction. Sci. Eng. 2019;5(3):1476–1486. doi: 10.1021/acsbiomaterials.8b01328. [DOI] [PubMed] [Google Scholar]
- 169.Mouthuy P.A., Zargar N., Hakimi O., Lostis E., Carr A. Fabrication of continuous electrospun filaments with potential for use as medical fibres. Biofabrication. 2015;7(2) doi: 10.1088/1758-5090/7/2/025006. [DOI] [PubMed] [Google Scholar]
- 170.Rothrauff B.B., Lauro B.B., Yang G., Debski R.E., Musahl V., Tuan R.S. Braided and stacked electrospun nanofibrous scaffolds for tendon and ligament tissue engineering. Tissue Eng. Part A. 2017;23(9–10):378–389. doi: 10.1089/ten.tea.2016.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cai J., Xie X., Li D., Wang L., Jiang J., Mo X., Zhao J. A novel knitted scaffold made of microfiber/nanofiber core–sheath yarns for tendon tissue engineering. Biomater. Sci. 2020;8(16):4413–4425. doi: 10.1039/D0BM00816H. [DOI] [PubMed] [Google Scholar]
- 172.Liu W., Zhan J., Su Y., Wu T., Ramakrishna S., Liao S., Mo X. Injectable hydrogel incorporating with nanoyarn for bone regeneration. J. Biomater. Sci. Polym. Ed. 2014;25(2):168–180. doi: 10.1080/09205063.2013.848326. [DOI] [PubMed] [Google Scholar]
- 173.Shao W., He J., Han Q., Sang F., Wang Q., Chen L., Cui S., Ding B. A biomimetic multilayer nanofiber fabric fabricated by electrospinning and textile technology from polylactic acid and Tussah silk fibroin as a scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;67:599–610. doi: 10.1016/j.msec.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y.S., Xia Y. Multiple facets for extracellular matrix mimicking in regenerative medicine. Nanomedicine. 2015;10(5):689–692. doi: 10.2217/nnm.15.10. [DOI] [PubMed] [Google Scholar]
- 175.Sun B., Li J., Liu W., Aqeel B.M., El-Hamshary H., Al-Deyab S.S., Mo X. Fabrication and characterization of mineralized P (LLA-CL)/SF three-dimensional nanoyarn scaffolds. Iran. Polym. J. 2015;24(1):29–40. doi: 10.1007/s13726-014-0297-9. [DOI] [Google Scholar]
- 176.Gao Y., Shao W., Qian W., He J., Zhou Y., Qi K., Wang L., Cui S., Wang R. Biomineralized poly (l-lactic-co-glycolic acid)-tussah silk fibroin nanofiber fabric with hierarchical architecture as a scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;84:195–207. doi: 10.1016/j.msec.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 177.Manju V., Anitha A., Menon D., Iyer S., Nair S.V., Nair M.B. Nanofibrous yarn reinforced HA-gelatin composite scaffolds promote bone formation in critical sized alveolar defects in rabbit model. Biomed. Mater. 2018;13(6) doi: 10.1088/1748-605X/aadf99. [DOI] [PubMed] [Google Scholar]
- 178.Anitha A., Joseph J., Menon D., Nair S.V., Nair M.B. Electrospun yarn reinforced nanoHA composite matrix as a potential bone substitute for enhanced regeneration of segmental defects. Tissue Eng. Part A. 2017;23(7–8):345–358. doi: 10.1089/ten.tea.2016.0337. [DOI] [PubMed] [Google Scholar]
- 179.Song H.H.G., Rumma R.T., Ozaki C.K., Edelman E.R., Chen C.S. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22(3):340–354. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kruse M., Greuel M., Kreimendahl F., Schneiders T., Bauer B., Gries T., Jockenhoevel S. Electro-spun PLA-PEG-yarns for tissue engineering applications. Biomed. Eng. Biomed. Tech. 2018;63(3):231–243. doi: 10.1515/bmt-2017-0232. [DOI] [PubMed] [Google Scholar]
- 181.Babu R., Reshmi C.R., Joseph J., Sathy B.N., Nair S.V., Varma P.K., Menon D. Design, development, and evaluation of an interwoven electrospun nanotextile vascular patch. Macromol. Mater. Eng. 2021;306(11) doi: 10.1002/mame.202100359. [DOI] [Google Scholar]
- 182.Rahimtoroghi E., Kasra M. Mechanical and cellular characterization of electrospun poly (l-lactic acid)/gelatin yarns with potential as angiogenesis scaffolds. Iran. Polym. J. 2021;30(6):623–632. doi: 10.1007/s13726-021-00916-x. [DOI] [Google Scholar]
- 183.Goeaubrissonniere O., Mercier F., Nicolas M.H., Bacourt F., Coggia M., Lebrault C., Pechere J.C. Treatment of vascular graft infection by in situ replacement with a rifampin-bonded gelatin-sealed Dacron graft. J. Vasc. Surg. 1994;19(4):739–744. doi: 10.1016/S0741-5214(94)70050-8. [DOI] [PubMed] [Google Scholar]
- 184.Amirghofran A.A., Karimi A., Emaminia A., Sharifkazemi M.B., Salaminia S. Brucellosis relapse causing prosthetic valve endocarditis and aortic root infective pseudoaneurysm. Ann. Thorac. Surg. 2011;92(4):E77–E79. doi: 10.1016/j.athoracsur.2011.03.144. [DOI] [PubMed] [Google Scholar]
- 185.Liu F., Liao X., Liu C., Li M., Chen Y., Shao W., Weng K., Li F., Ou K., He J. Poly (l-lactide-co-caprolactone)/tussah silk fibroin nanofiber vascular scaffolds with small diameter fabricated by core-spun electrospinning technology. J. Mater. Sci. 2020;55(16):7106–7119. doi: 10.1007/s10853-020-04510-z. [DOI] [Google Scholar]
- 186.Wu S., Duan B., Qin X., Butcher J.T. Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering. Acta Biomater. 2017;51:89–100. doi: 10.1016/j.actbio.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 187.Heo J.S., Eom J., Kim Y.H., Park S.K. Recent progress of textile‐based wearable electronics: a comprehensive review of materials, devices, and applications. Small. 2018;14(3) doi: 10.1002/smll.201703034. [DOI] [PubMed] [Google Scholar]
- 188.Levitt A., Seyedin S., Zhang J., Wang X., Razal J.M., Dion G., Gogotsi Y. Bath electrospinning of continuous and scalable multifunctional MXene‐infiltrated nanoyarns. Small. 2020;16(26) doi: 10.1002/smll.202002158. [DOI] [PubMed] [Google Scholar]
- 189.Park S., Kwon Y., Sung M., Lee B.S., Bae J., Yu W.R. Poling-free spinning process of manufacturing piezoelectric yarns for textile applications. Mater. Des. 2019;179 doi: 10.1016/j.matdes.2019.107889. [DOI] [Google Scholar]
- 190.Perdigao P., Faustino B.M.M., Faria J., Canejo J.P., Borges J.P., Ferreira I., Baptista A.C. Conductive electrospun polyaniline/polyvinylpyrrolidone nanofibers: Electrical and morphological characterization of new yarns for electronic textiles. Fibers. 2020;8(4):24. doi: 10.3390/fib8040024. [DOI] [Google Scholar]
- 191.Weerasinghe P., Wanasekara N.D., Dissanayake D., Banadara H.R.T., Tissera N., Wijesena R., de Silva K., Karalasingam A. Proceedings of the IEEE 14th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS) IEEE; 2019. All organic, conductive nanofibrous twisted yarns; pp. 308–311. [DOI] [Google Scholar]
- 192.Weerasinghe V.T., Dissanayake D.G.K., Perera W.P.T.D., Tissera N.D., Wijesena R.N., Wanasekara N.D. All-organic, conductive and biodegradable yarns from core–shell nanofibers through electrospinning. RSC Adv. 2020;10(54):32875–32884. doi: 10.1039/D0RA05430E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fan L., Ma Q., Tian J., Li D., Xi X., Dong X., Yu W., Wang J., Liu G. Novel nanofiber yarns synchronously endued with tri-functional performance of superparamagnetism, electrical conductivity and enhanced fluorescence prepared by conjugate electrospinning. RSC Adv. 2017;7(77):48702–48711. doi: 10.1039/C7RA09598H. [DOI] [Google Scholar]
- 194.Li J., Tian L., Pan N., Pan Z.J. Mechanical and electrical properties of the PA6/SWNTs nanofiber yarn by electrospinning. Polym. Eng. Sci. 2014;54(7):1618–1624. doi: 10.1002/pen.23705. [DOI] [Google Scholar]
- 195.Guan X., Zheng G., Dai K., Liu C., Yan X., Shen C., Guo Z. Carbon nanotubes-adsorbed electrospun PA66 nanofiber bundles with improved conductivity and robust flexibility. ACS Appl. Mater. Interfaces. 2016;8(22):14150–14159. doi: 10.1021/acsami.6b02888. [DOI] [PubMed] [Google Scholar]
- 196.Li Y., Góra A., Anariba F., Baji A. Enhanced tensile strength and electrical conductivity of electrospun polyacrylonitrile Yarns via post‐treatment. Polym. Compos. 2019;40(5):1702–1707. doi: 10.1002/pc.24920. [DOI] [Google Scholar]
- 197.Mehrpouya F., Foroughi J., Naficy S., Razal J.M., Naebe M. Nanostructured electrospun hybrid graphene/polyacrylonitrile yarns. Nanomaterials. 2017;7(10):293. doi: 10.3390/nano7100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Matsumoto H., Imaizumi S., Konosu Y., Ashizawa M., Minagawa M., Tanioka A., Lu W., Tour J.M. Electrospun composite nanofiber yarns containing oriented graphene nanoribbons. ACS Appl. Mater. Interfaces. 2013;5(13):6225–6231. doi: 10.1021/am401161b. [DOI] [PubMed] [Google Scholar]
- 199.Yan T., Pan Z. High conductivity electrospun carbon/graphene composite nanofiber yarns. Polym. Eng. Sci. 2018;58(6):903–912. doi: 10.1002/pen.24643. [DOI] [Google Scholar]
- 200.Zhou Y., He J., Wang H., Qi K., Ding B., Cui S. Carbon nanofiber yarns fabricated from co-electrospun nanofibers. Mater. Des. 2016;95:591–598. doi: 10.1016/j.matdes.2016.01.132. [DOI] [Google Scholar]
- 201.Li Y., Zhou B., Zheng G., Liu X., Li T., Yan C., Cheng C., Dai K., Liu C., Shen C. Continuously prepared highly conductive and stretchable SWNT/MWNT synergistically composited electrospun thermoplastic polyurethane yarns for wearable sensing. J. Mater. Chem. C. 2018;6(9):2258–2269. doi: 10.1039/C7TC04959E. [DOI] [Google Scholar]
- 202.Chen S., Zhang J., Zhang Q., Cai G., Xu A., Yan S. Highly stretchable and durable electrospinning polyurethane nanofiber composite yarn for electronic devices. Fiber. Polym. 2021 doi: 10.1007/s12221-021-2351-5. [DOI] [Google Scholar]
- 203.Peng H.K., Wu M.M., Wang Y.T., Li T.T., Sun F., Lou C.W., Lin J.H. Enhancing piezoelectricity of poly (vinylidene fluoride) nano‐wrapped yarns with an innovative yarn electrospinning technique. Polym. Int. 2021;70(6):851–859. doi: 10.1002/pi.6177. [DOI] [Google Scholar]
- 204.Hsu Y.H., Liu P.C., Lin T.T., Huang S.W., Lai Y.C. Development of an elastic piezoelectric yarn for the application of a muscle patch sensor. ACS Omega. 2020;5(45):29427–29438. doi: 10.1021/acsomega.0c03309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gao H., Minh P.T., Wang H., Minko S., Locklin J., Nguyen T., Sharma S. High-performance flexible yarn for wearable piezoelectric nanogenerators. Smart Mater. Struct. 2018;27(9) doi: 10.1088/1361-665X/aad718. [DOI] [Google Scholar]
- 206.Ma L., Zhou M., Wu R., Patil A., Gong H., Zhu S., Wang T., Zhang Y., Shen S., Dong K. Continuous and scalable manufacture of hybridized nano-micro triboelectric yarns for energy harvesting and signal sensing. ACS Nano. 2020;14(4):4716–4726. doi: 10.1021/acsnano.0c00524. [DOI] [PubMed] [Google Scholar]
- 207.Guan X., Xu B., Wu M., Jing T., Yang Y., Gao Y. Breathable, washable and wearable woven-structured triboelectric nanogenerators utilizing electrospun nanofibers for biomechanical energy harvesting and self-powered sensing. Nano Energy. 2021;80 doi: 10.1016/j.nanoen.2020.105549. [DOI] [Google Scholar]
- 208.Ren M., Qiao J., Wang Y., Wu K., Dong L., Shen X., Zhang H., Yang W., Wu Y., Yong Z. Strong and robust electrochemical artificial muscles by ionic‐liquid‐in‐nanofiber‐sheathed carbon nanotube yarns. Small. 2021;17(5) doi: 10.1002/smll.202006181. [DOI] [PubMed] [Google Scholar]
- 209.Zhao X., Li W., Li F., Hou Y., Lu T., Pan Y., Li J., Xu Y., He J. Wearable yarn supercapacitors coated with twisted PPy@ GO nanosheets and PPy@ PAN-GO nanofibres. J. Mater. Sci. 2021;56(32):18147–18161. doi: 10.1007/s10853-021-06500-1. [DOI] [Google Scholar]
- 210.Sun X., He J., Qiang R., Nan N., You X., Zhou Y., Shao W., Liu F., Liu R. Electrospun conductive nanofiber yarn for a wearable yarn supercapacitor with high volumetric energy density. Materials. 2019;12(2):273. doi: 10.3390/ma12020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen L., Li D., Chen L., Si P., Feng J., Zhang L., Li Y., Lou J., Ci L. Core-shell structured carbon nanofibers yarn@ polypyrrole@ graphene for high performance all-solid-state fiber supercapacitors. Carbon. 2018;138:264–270. doi: 10.1016/j.carbon.2018.06.022. [DOI] [Google Scholar]
- 212.Li Q., Balilonda A., Ali A., Jose R., Zabihi F., Yang S., Ramakrishna S., Zhu M. Flexible solar yarns with 15.7% power conversion efficiency, based on electrospun perovskite composite nanofibers. Sol. RRL. 2020;4(9) doi: 10.1002/solr.202000269. [DOI] [Google Scholar]
- 213.Gotti C., Sensini A., Fornaia G., Gualandi C., Zucchelli A., Focarete M.L. Biomimetic hierarchically arranged nanofibrous structures resembling the architecture and the passive mechanical properties of skeletal muscles: a step forward toward artificial muscle. Front. Bioeng. Biotechnol. 2020;8:767. doi: 10.3389/fbioe.2020.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ebadi S.V., Fashandi H., Semnani D., Rezaei B., Fakhrali A. Electroactive actuator based on polyurethane nanofibers coated with polypyrrole through electrochemical polymerization: a competent method for developing artificial muscles. Smart Mater. Struct. 2020;29(4) doi: 10.1088/1361-665X/ab73e5. [DOI] [Google Scholar]
- 215.Taghipoor F., Semnani D., Naghashzargar E., Rezaei B. Electrochemical properties of bi-component bundle of coaxial polyacrylonitrile/ polyaniline nanofibers containing TiO2 nanoparticles. J. Compos Mater. 2017;51(24):3355–3363. doi: 10.1177/0021998316687028. [DOI] [Google Scholar]
- 216.Gu B.K., Ismail Y.A., Spinks G.M., Kim S.I., So I., Kim S.J. A linear actuation of polymeric nanofibrous bundle for artificial muscles. Chem. Mat. 2009;21(3):511–515. doi: 10.1021/cm802377d. [DOI] [Google Scholar]
- 217.Severt S., Maxwell S., Bontrager J., Leger J., Murphy A. Mimicking muscle fiber structure and function through electromechanical actuation of electrospun silk fiber bundles. J. Mat. Chem. B. 2017;5(40):8105–8114. doi: 10.1039/C7TB01904A. [DOI] [PubMed] [Google Scholar]
- 218.Yan T., Wang Z., Pan Z.J. A highly sensitive strain sensor based on a carbonized polyacrylonitrile nanofiber woven fabric. J. Mater. Sci. 2018;53(16):11917–11931. doi: 10.1007/s10853-018-2432-z. [DOI] [Google Scholar]
- 219.Yan T., Zhou H., Niu H., Shao H., Wang H., Pan Z., Lin T. Highly sensitive detection of subtle movement using a flexible strain sensor from helically wrapped carbon yarns. J. Mater. Chem. C. 2019;7(32):10049–10058. doi: 10.1039/C9TC03065D. [DOI] [Google Scholar]
- 220.Gao Y., Guo F., Cao P., Liu J., Li D., Wu J., Wang N., Su Y., Zhao Y. Winding-locked carbon nanotubes/polymer nanofibers helical yarn for ultrastretchable conductor and strain sensor. ACS Nano. 2020;14(3):3442–3450. doi: 10.1021/acsnano.9b09533. [DOI] [PubMed] [Google Scholar]
- 221.Qi K., Wang H., You X., Tao X., Li M., Zhou Y., Zhang Y., He J., Shao W., Cui S. Core-sheath nanofiber yarn for textile pressure sensor with high pressure sensitivity and spatial tactile acuity. J. Colloid Interface Sci. 2020;561:93–103. doi: 10.1016/j.jcis.2019.11.059. [DOI] [PubMed] [Google Scholar]
- 222.You X., He J., Nan N., Sun X., Qi K., Zhou Y., Shao W., Liu F., Cui S. Stretchable capacitive fabric electronic skin woven by electrospun nanofiber coated yarns for detecting tactile and multimodal mechanical stimuli. J. Mater. Chem. C. 2018;6(47):12981–12991. doi: 10.1039/C8TC03631D. [DOI] [Google Scholar]
- 223.Choi S., Moon S.H., Kim T.K., Kim H.S. Fabrication of capacitive yarn torsion sensors based on an electrospinning coating method. Polym. Int. 2019;68(11):1921–1927. doi: 10.1002/pi.5902. [DOI] [Google Scholar]
- 224.Zhang Z., Ji D., He H., Ramakrishna S. Electrospun ultrafine fibers for advanced face masks. Mater. Sci. Eng. R Rep. 2021;143 doi: 10.1016/j.mser.2020.100594. S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tan N.P.B., Paclijan S.S., Ali H.N.M., Hallazgo C.M.J.S., Lopez C.J.F., Ebora Y.C., Appl ACS. Solution blow spinning (SBS) nanofibers for composite air filter masks. Nano Mater. 2019;2(4):2475–2483. doi: 10.1021/acsanm.9b00207. [DOI] [Google Scholar]
- 226.Wang N., Cai M., Yang X., Yang Y. Electret nanofibrous membrane with enhanced filtration performance and wearing comfortability for face mask. J. Colloid Interface Sci. 2018;530:695–703. doi: 10.1016/j.jcis.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 227.Javidpour L., Božič A., Naji A., Podgornik R. Electrostatic interactions between the SARS-CoV-2 virus and a charged electret fibre. Soft Matter. 2021;17(16):4296–4303. doi: 10.1039/D1SM00232E. [DOI] [PubMed] [Google Scholar]
- 228.Ullah S., Ullah A., Lee J., Jeong Y., Hashmi M., Zhu C., Joo K.I., Cha H.J., Kim I.S., Appl ACS. Reusability comparison of melt-blown vs nanofiber face mask filters for use in the coronavirus pandemic. Nano Mater. 2020;3(7):7231–7241. doi: 10.1021/acsanm.0c01562. [DOI] [PubMed] [Google Scholar]
- 229.Cheng Y., Wang C., Zhong J., Lin S., Xiao Y., Zhong Q., Jiang H., Wu N., Li W., Chen S. Electrospun polyetherimide electret nonwoven for bi-functional smart face mask. Nano Energy. 2017;34:562–569. doi: 10.1016/j.nanoen.2017.03.011. [DOI] [Google Scholar]
- 230.Shen H., Zhou Z., Wang H., Zhang M., Han M., Durkin D.P., Shuai D., Shen Y. Development of electrospun nanofibrous filters for controlling coronavirus aerosols. Environ. Sci. Technol. Lett. 2021;8(7):545–550. doi: 10.1021/acs.estlett.1c00337. [DOI] [PubMed] [Google Scholar]
- 231.Sanyal A., Sinha-Ray S. Ultrafine PVDF nanofibers for filtration of air-borne particulate matters: A comprehensive review. Polymers. 2021;13(11):1864. doi: 10.3390/polym13111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.He R., Li J., Chen M., Zhang S., Cheng Y., Ning X., Wang N. Tailoring moisture electroactive Ag/Zn@ cotton coupled with electrospun PVDF/PS nanofibers for antimicrobial face masks. J. Hazard. Mater. 2022;428 doi: 10.1016/j.jhazmat.2022.128239. [DOI] [PubMed] [Google Scholar]
- 233.Mariello M., Qualtieri A., Mele G., De Vittorio M. Metal-free multilayer hybrid PENG based on soft electrospun/-sprayed membranes with cardanol additive for harvesting energy from surgical face masks. ACS Appl. Mater. Interfaces. 2021;13(17):20606–20621. doi: 10.1021/acsami.1c01740. [DOI] [PubMed] [Google Scholar]
- 234.Liu G., Nie J., Han C., Jiang T., Yang Z., Pang Y., Xu L., Guo T., Bu T., Zhang C. Self-powered electrostatic adsorption face mask based on a triboelectric nanogenerator. ACS Appl. Mater. Interfaces. 2018;10(8):7126–7133. doi: 10.1021/acsami.7b18732. [DOI] [PubMed] [Google Scholar]
- 235.Canalli Bortolassi A.C., Guerra V.G., Aguiar M.L., Soussan L., Cornu D., Miele P., Bechelany M. Composites based on nanoparticle and pan electrospun nanofiber membranes for air filtration and bacterial removal. Nanomaterials. 2019;9(12):1740. doi: 10.3390/nano9121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bortolassi A.C.C., Nagarajan S., de Araújo Lima B., Guerra V.G., Aguiar M.L., Huon V., Soussan L., Cornu D., Miele P., Bechelany M. Efficient nanoparticles removal and bactericidal action of electrospun nanofibers membranes for air filtration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:718–729. doi: 10.1016/j.msec.2019.04.094. [DOI] [PubMed] [Google Scholar]
- 237.Nguyen P.Q., Soenksen L.R., Donghia N.M., Angenent-Mari N.M., de Puig H., Huang A., Lee R., Slomovic S., Galbersanini T., Lansberry G. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 2021;39(11):1366–1374. doi: 10.1038/s41587-021-00950-3. [DOI] [PubMed] [Google Scholar]
- 238.Wang X., Grobe N., Haq Z., Thwin O., Fuentes L.R., Maddux D., Kotanko P. Testing of worn face masks for timely diagnosis of SARS-CoV-2 in hemodialysis patients. J. Am. Soc. Nephrol. 2021;32(11):2728–2730. doi: 10.1681/ASN.2021060812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Williams C.M., Pan D., Decker J., Wisniewska A., Fletcher E., Sze S., Assadi S., Haigh R., Abdulwhhab M., Bird P. Exhaled SARS-CoV-2 quantified by face-mask sampling in hospitalised patients with COVID-19. J. Infect. 2021;82(6):253–259. doi: 10.1016/j.jinf.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McCarthy A., Saldana L., Ackerman D.N., Su Y., John J.V., Chen S., Weihs S., Reid S.P., Santarpia J.L., Carlson M.A. Ultra-absorptive nanofiber swabs for improved collection and test sensitivity of SARS-CoV-2 and other biological specimens. Nano Lett. 2021;21(3):1508–1516. doi: 10.1021/acs.nanolett.0c04956. [DOI] [PubMed] [Google Scholar]
- 241.Ding Z., Wang H., Feng Z., Sun M. Synthesis of dual-phase Ti3O5/Ti4O7 nanofibers for efficient adsorption of SARS-CoV-2. Mater. Lett. 2021;300 doi: 10.1016/j.matlet.2021.130167. [DOI] [PMC free article] [PubMed] [Google Scholar]