Abstract
Transcranial direct current stimulation (tDCS) is a promising tool for alleviating positive and negative symptoms of schizophrenia, but its role in functional outcome remains uncertain. This meta-analysis examined the effects of tDCS on general psychopathology symptoms (GPS) from the Positive and Negative Syndrome Scale (PANSS) because GPS are closely associated with daily functioning. Literature search using Medline and PsycINFO identified 8 RCTs with tDCS and PANSS. The GPS were significantly reduced after tDCS but there was no evidence for long-term treatment effects. Further research is needed to optimize the dosing of tDCS and to understand individual differences in treatment response.
Keywords: schizophrenia, general psychopathology, transcranial direct current stimulation, meta-analysis
1. Introduction
TDCS has emerged as a promising and safe brain stimulation tool for alleviating symptoms of schizophrenia. Recent meta-analyses indicate that tDCS improves positive and negative symptoms (Kim et al., 2019; Cheng et al., 2020) but it is unclear if there is an improvement in daily functioning. The general psychopathology symptoms (GPS) are measured separately from the positive and negative symptoms of PANSS, and are closely aligned with functional outcome. GPS consist of poor insight, anxiety, somatic concerns and motor retardation, and are likely to interfere with daily life and functional outcome, but they have been largely overlooked. We conducted a meta-analysis to investigate potential treatment effects of tDCS on the GPS. Furthermore, we sought to clarify the duration and variability of potential tDCS treatment effects across studies.
2. Methods
2.1. Literature search
A literature search based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2015) (see Figure 1) was conducted. Using the Medline and PsycINFO databases, we searched literatures published in English from 1950 to November 2020 utilizing the key words “transcranial direct current stimulation”, “tDCS”, “brain stimulation”, “schizophrenia”, “psychotic disorder”, “psychosis”, “general symptom”, “general psychopathology”, “positive and negative syndrome scale”, “PANSS”, “randomized controlled trial” and “RCT”. Inclusion criteria utilized the following criteria: randomized controlled trials (RCTs), tDCS applied to the cerebral cortex, and the collection of symptoms employing the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) and general psychopathology as outcome measures. Eight RCTs with active tDCS and sham conditions were identified.
Figure 1.
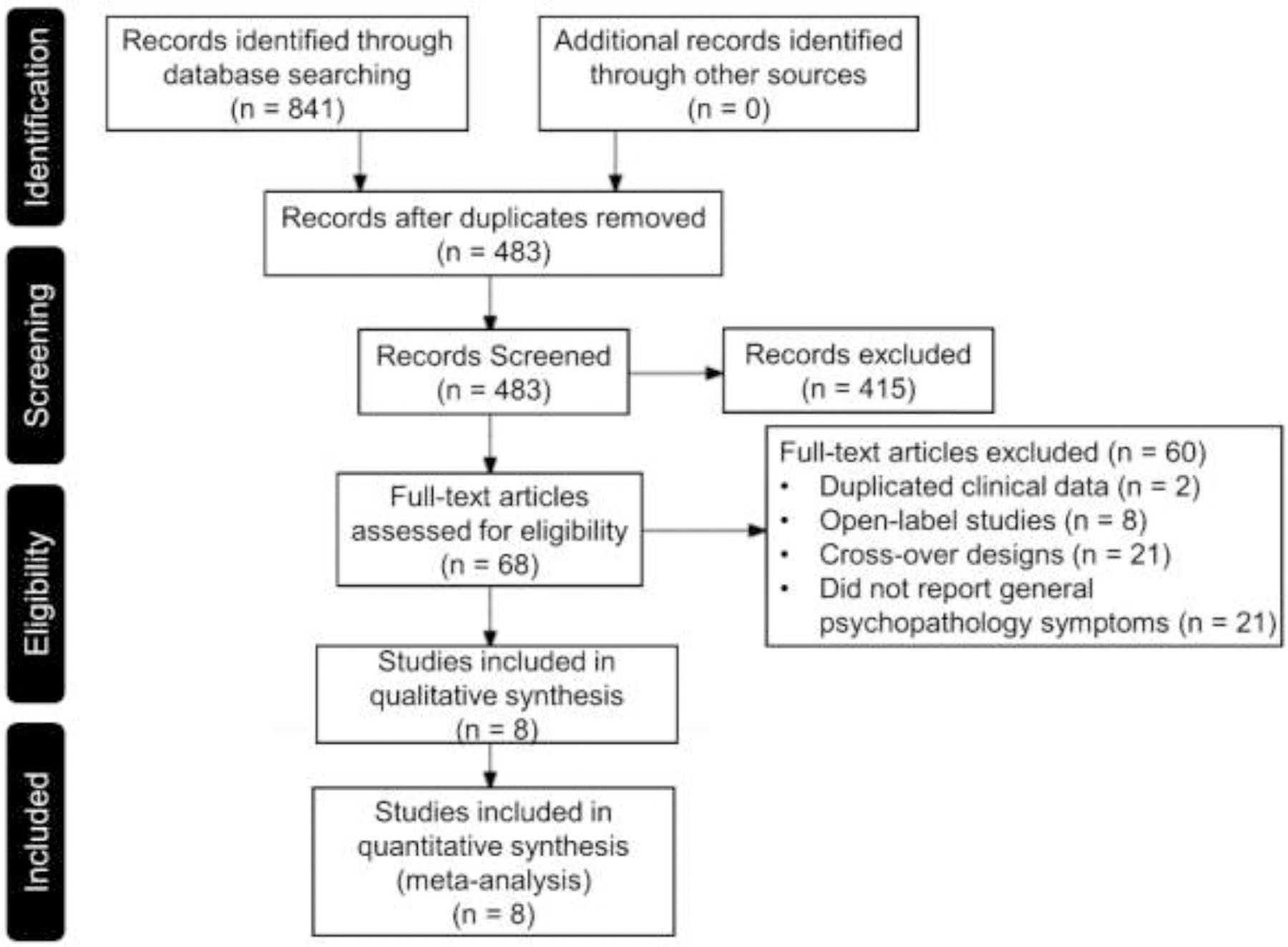
Literature search based on Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) guideline
2.2. Participants
There were 164 patients in the active condition and 165 patients in the sham condition. For studies that reported one-month or longer follow-up data, there were 108 and 110 patients in active and sham condition, respectively. Demographic and clinical characteristics (age, sex, daily dose of antipsychotics) and information about tDCS trials (e.g., parameters and sessions) were obtained (see Table 1). Notably, electrode placement was quite consistent across studies such that anodal stimulation was on left dorsolateral prefrontal cortex (DLPFC) while cathodal stimulation was on right DLPFC or left temporoparietal junction (TPJ).
Table 1.
Participant characteristics of the eight included studies
| Articles | N (sham, active) | Diagnosis | Mean Age | Sex (% female) | CPZ equivalent | Electrode Placement (Anode / Cathode) | Stimulation intensity(mA), area (cm2) | Sessions | Follow-up time point |
|---|---|---|---|---|---|---|---|---|---|
| Valiengo et al., 2020 | 100 (50, 50) | SZ with negative symptoms | 35.25 | 20.00% | 497.75 | L-DLPFC (F3) / L-TPJ (T3 and P3) | 2, 35 | 10 | 12 weeks |
| Chang et al., 2019 | 60 (30, 30) | SZ and SA | 44.28 | 55.00% | 493.60 | L-DLPFC / L-TPJ | 2, 35 | 10 | N/A |
| Lindenmayer et al., 2019 | 28 (13,15) | SZ with AVH (Drug resistant) | 40.20 | 14.29% | 891.81 | L-DLPFC / L-TPJ | 2, 35 | 8 | N/A |
| Gomes et al., 2018 | 24 (12,12) | SZ | 36.46 | 29.17% | N/A | L-DLPFC / R-DLPFC | 2,25 | 10 | 12 weeks |
| Jeon et al.. 2018 | 54 (28, 26) | SZ | 39.93 | 51.85% | 581.60 | L-DLPFC (F3) / R-DLPFC (F4) | 2,25 | 10 | 12 weeks |
| Mellin et al, 2018 | 14 (7,7) | SZ and SA | 34.22 | N/A | L-DLPFC (F3 and FP1) / L-TPJ (T3 and P3) | 2,25 | 10 | 4 weeks | |
| Fröhlich et al., 2016 | 26 (13,13) | SZ and SA with AVH | 41.69 | 15.38% | N/A | L-DLPFC (F3 and FP1) / L-TPJ (T3 and P3) | 2, 35 | 5 | 4 weeks |
| Mondino et al., 2016 | 23 (12,11) | SZ with AVH (Drug resistant) | 37.01 | 34.78% | 486.00 | L-DLPFC (F3 and FP1) / L-TPJ (T3 and P3) | 2, 35 | 10 | N/A |
Notes. SZ: patients with schizophrenia, SA: patients with schizoaffective disorder. AVH: auditory-verbal hallucination, L-DLPFC: left dorsolateral prefrontal cortex; R-DLPFC; right dorsolateral pref’ontal cortex L-TPJ; left temporoparietal junction; Sessions; the number of total sessions with transcranial direct current stimulation
2.3. Data analysis
Differences in pre- and post- treatment (mean and standard deviation values) of the PANSS were extracted from the studies. A random-effects model was used to minimize the type I error. The standardized mean differences (SMD) and variance-weighted variability ratios for each study were produced and analyzed. The variability ratio was used for comparing standard deviations between groups (active vs. sham) and determining which group had greater variability in PANSS scores. Increased variability might indicate a greater individual difference in response to treatment within the group (i.e., some people respond well to the treatment while others do not). For studies that reported one-month or longer follow-up data (5 studies), SMD of GPS score was evaluated. Results of effect sizes and variability ratios for each study are presented with their 95% confidence intervals (95% CI).
3. Results
3.1. The effect of tDCS on General psychopathology symptoms (GPS)
GPS scores from PANSS were significantly reduced after tDCS active condition compared to the sham condition (Cohen’s d = 0.31, 95% CI [0.05, 0.57]) (Figure 2-a), suggesting a significantly greater symptom reduction after the tDCS treatment in an active condition relative to a sham condition. Cohen’s d of .31 indicates a small effect size.
Figure 2.
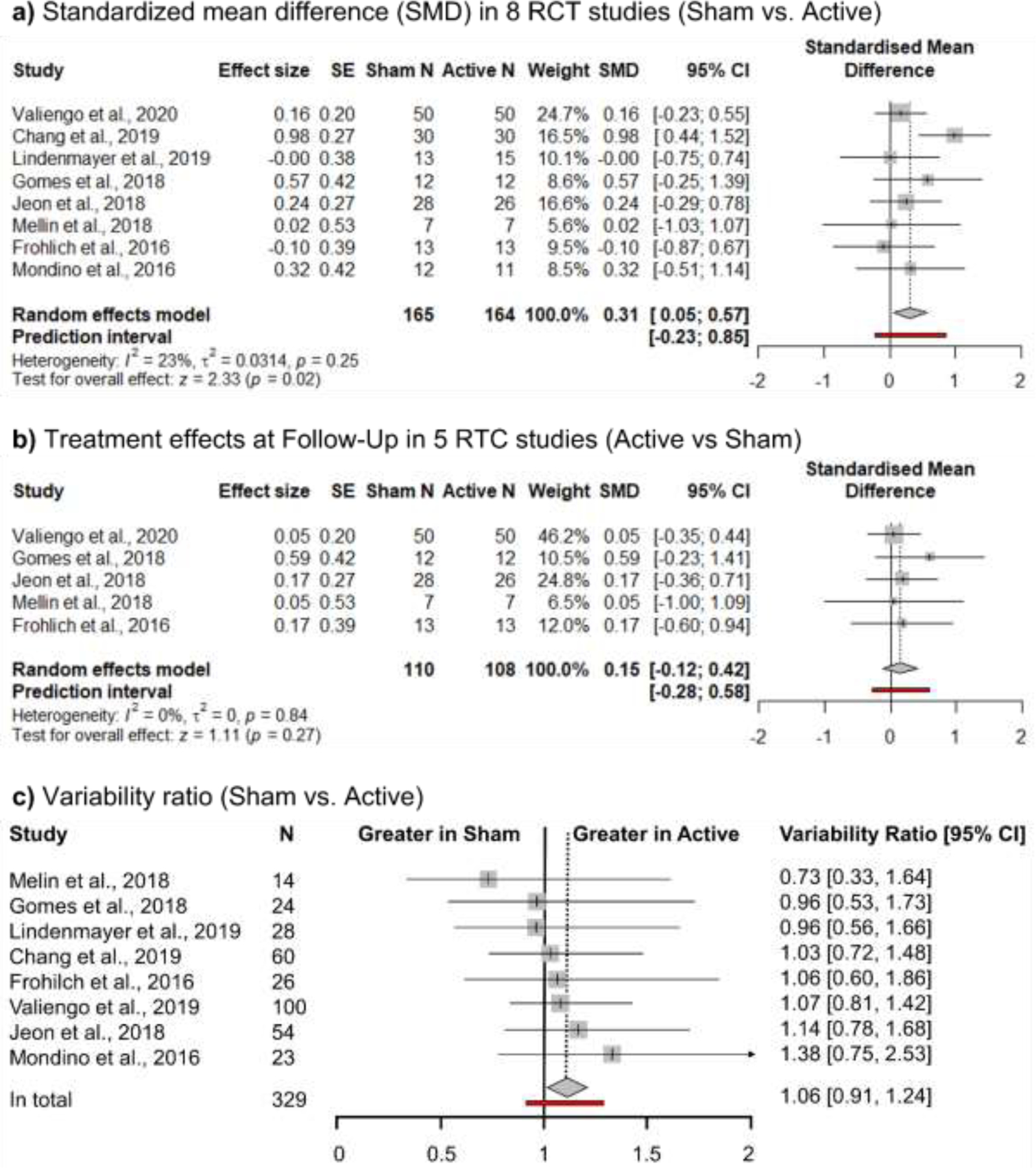
Forest Plots of the Results of the Meta-Analyses
3.2. The long-term treatment effect of tDCS on General psychopathology symptoms
We examined 5 studies that reported follow-up assessments. The effect size of long-term treatment effect at follow-up was very small and did not exceed the significance threshold (Cohen’s d = 0.15, 95% CI [−0.12, 0.42]) (Figure 2-b). This finding suggests that the treatment effect of tDCS is not durable. One month after the tDCS, there is no evidence of treatment effect.
3.3. Variability ratio of individual studies
The treatment group showed a 6% higher variability in general psychopathology scores than the control group (Variability ratio = 1.06, 95% CI: 0.91, 1.24) but it did not meet the significance threshold (Figure 2-c). However, interestingly, studies with relatively larger sample sizes (e.g., Jeon et al., 2018; Valiengo et al., 2019) showed higher variability in the active condition.
4. Discussion
The results of the meta-analysis suggest that tDCS improves the general psychopathology symptoms in the short-term, but there was no evidence for long-term treatment effects. Variability analysis suggests a higher variability in the treatment (active) condition than in the control (sham) condition but it is not possible to draw a firm conclusion due to the small sample size.
General psychopathology symptoms include a wide range of behaviors that contribute to functional outcome (e.g., poor insight, anxiety, somatic concerns and motor retardation). These behaviors are also associated with multiple neural mechanisms that only partially overlap. Therefore, the impact of tDCS may vary across these symptoms as well, and the symptom profile of each participant (i.e., individual differences) could influence the efficacy of the tDCS. Thus, baseline individual differences in symptoms could be a determining factor in the effectiveness of brain stimulation treatments.
There are caveats. It is possible that null results were under-reported. In other words, there is a risk of the “file drawer” problem (Rosenthal, 1979) but a bigger problem may be the neglect of the general psychopathology symptoms as treatment targets. Since only eight studies met our stringent criteria for inclusion in the meta-analysis, the small sample size is also a limitation.
Whilst the TDCS is a promising tool for targeting clinical symptoms of schizophrenia, its effects seem temporary. It is, however, important to remember that pharmacological treatments are not permanent either. Just as in pharmacotherapy, repeated stimulation is likely to be necessary. It is also important to identify the nature of individual differences in response to brain stimulation so that we can personalize treatments. Future research is needed to clarify optimal dosing, time course of effects, location of stimulation as well as individual differences in response to tDCS.
Highlights.
A meta-analysis was conducted to investigate potential treatment effects of tDCS on general psychopathology symptoms of schizophrenia
The tDCS active treatment significantly reduced general psychopathology symptoms
Long term treatment effect was not supported in one month or more follow-ups.
High variability observed in the treatment group was not statistically significant
Further research is needed to develop a more standardized protocol and individualized treatment.
Acknowledgements
We would like to thank members of the Park lab for their helpful comments and support.
Funding information
This work was supported by R01 MH110378 and Gertrude Conaway Vanderbilt Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None
CRediT authorship contribution statement
Hyeon-Seung Lee: Methodology, Formal analysis, Investigation, Data curation, Visualization, Writing - original draft, Writing - review & editing. Catherine Rast: Methodology, Data curation, Writing - original draft, Writing - review & editing. Sunil Shenoy: Methodology, Data curation, Writing - original draft, Writing - review & editing. Derek Dean: Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Geoffrey Woodman: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Resources, Supervision, Project administration, Funding acquisition. Sohee Park: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Resources, Supervision, Project administration, Funding acquisition.
References
- Chang CC, Kao YC, Chao CY, Chang HA, 2019. Enhancement of cognitive insight and higher-order neurocognitive function by fronto-temporal transcranial direct current stimulation (tDCS) in patients with schizophrenia. Schizophr Res 208, 430–438. [DOI] [PubMed] [Google Scholar]
- Cheng PWC, Louie LLC, Wong YL, Wong SMC, Leung WY, Nitsche MA, Chan WC, 2020. The effects of transcranial direct current stimulation (tDCS) on clinical symptoms in schizophrenia: A systematic review and meta-analysis. Asian J Psychiatr 53, 102392. [DOI] [PubMed] [Google Scholar]
- Fröhlich F, Burrello TN, Mellin JM, Cordle AL, Lustenberger CM, Gilmore JH, Jarskog LF, 2016. Exploratory study of once-daily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia. Eur Psychiatry 33 (1), 54–60. [DOI] [PubMed] [Google Scholar]
- Gomes JS, Trevizol AP, Ducos DV, Gadelha A, Ortiz BB, Fonseca AO, Akiba HT, Azevedo CC, Guimaraes LSP, Shiozawa P, Cordeiro Q, Lacerda A, Dias AM, 2018. Effects of transcranial direct current stimulation on working memory and negative symptoms in schizophrenia: a phase II randomized sham-controlled trial. Schizophr Res Cogn 12, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon DW, Jung DU, Kim SJ, Shim JC, Moon JJ, Seo YS, Jung SS, Seo BJ, Kim JE, Oh M, Kim YN, 2018. Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: a double-blind 12-week study. Schizophr Res 197, 378–385. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13 (2), 261–276. [DOI] [PubMed] [Google Scholar]
- Kim J, Iwata Y, Plitman E, Caravaggio F, Chung JK, Shah P, Blumberger DM, Pollock BG, Remington G, Graff-Guerrero A, Gerretsen P, 2019. A meta-analysis of transcranial direct current stimulation for schizophrenia: “Is more better?”. J Psychiatric Res 110, 117–126. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Kulsa MKC, Sultana T, Kaur A, Yang R, Ljuri I, Parker B, Khan A, 2019. Transcranial direct-current stimulation in ultra-treatment-resistant schizophrenia. Brain Stimul 12 (1), 54–61. [DOI] [PubMed] [Google Scholar]
- Mellin JM, Alagapan S, Lustenberger C, Lugo CE, Alexander ML, Gilmore JH, Jarskog LF, Fröhlich F, 2018. Randomized trial of transcranial alternating current stimulation for treatment of auditory hallucinations in schizophrenia. Eur Psychiatry 51, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J, 2016. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull 42 (2), 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R 1979. The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638–641. [Google Scholar]
- Valiengo LDCL, Goerigk S, Gordon PC, Padberg F, Serpa MH, Koebe S, Santos LA, Lovera RAN, Carvalho JB, Bilt M, Lacerda ALT, Elkis H, Gattaz WF, Brunoni AR, 2020. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psych 77 (2), 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]


