Abstract
Purpose of Review
This review discusses the mechanisms, clinical implications, and treatments of left atrial (LA) myopathy in comorbid atrial fibrillation (AF) and heart failure (HF) across the spectrum of ejection fraction.
Recent Findings
AF and HF are highly comorbid conditions. Left atrial (LA) myopathy, characterized by impairments in LA structure, function, or electrical conduction, plays a fundamental role in the development of both AF and HF with preserved ejection fraction (AF-HFpEF) along with AF and HF with reduced ejection fraction (AF-HFrEF). While the nature of LA myopathy in AF-HFpEF is unique from that of AF-HFrEF, LA myopathy also leads to progression of both of these conditions.
Summary
There may be a vulnerable cohort of AF-HF patients who have a disproportionate degree of LA myopathy compared with left ventricular (LV) dysfunction. Further investigations are required to identify therapies to improve LA function in this cohort.
Keywords: Left atrium, Heart failure with preserved ejection fraction, Atrial fibrillation, Myopathy, Cardiac function, Disease mechanism
Introduction
Atrial fibrillation (AF) and heart failure (HF) are common cardiovascular diseases with increasing prevalence worldwide. Recent statistics indicate that the global burden of comorbid AF and HF (AF-HF) is approximately 30 million individuals, and this figure is predicted to rise as the prevalence of common predisposing risk factors for AF and HF increases [1–6]. It is currently estimated that between 25 and 60% of patients with HF have comorbid AF. Importantly, patients with comorbid AF and HF have a worse prognosis than patients with either condition in isolation [3–6].
AF commonly burdens HF syndromes across the spectrum of ejection fraction. In fact, over 50% of community-based individuals with HF will be burdened by AF during their lifetime [3, 5]. One common mechanism driving both AF and HF is abnormal left atrial (LA) function, or LA myopathy. Indeed, LA dysfunction at baseline is independently associated with incident AF [7]. Additionally, LA myopathy has been associated with future development of HF [8]. These findings suggest that LA myopathy may in fact drive both AF and HF to exist. Furthermore, LA myopathy may progress as a result of AF and HF, thus causing a sustained cycle of disease progression. As such, this review discusses the mechanisms, clinical implications, and treatments of LA myopathy in comorbid AF and HF (AF-HF).
Defining LA Myopathy
Given its significance in the pathophysiology of cardiovascular disease, representatives from the European Heart Rhythm Association (EHRA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Society of Electrophysiology and Cardiac Stimulation (SOLAECE) formed the EHRAS working group to create the following consensus definition of LA myopathy.
“Any complex of structural, architectural, contractile, or electrophysiological changes affecting the atria with the potential to produce clinically relevant manifestations [9]”
In addition, the EHRAS group developed four main classes of LA myopathy based on the nature of LA damage [9]:
Class I: cardiomyocyte-dependent
Class II: primarily fibroblast-dependent
Class III: mixed cardiomyocyte and fibroblast-dependent
Class IV: primarily non-collagen deposits
Both AF and HF cause LA myopathy via class II or class III mechanisms; however, the common comorbidities associated with AF and HF may lead to LA myopathy through any one of the mechanisms listed above. For example, obesity is associated with class III and class IV, diabetes mellitus and obstructive sleep apnea with class I and class III, and hypertension with classes I–III [9, 10].
The LA has 3 main mechanical functions: (1) a reservoir during ventricular systole; (2) a conduit during passive ventricular filling, and (3) a booster during active ventricular filling [11, 12]. In addition to the histologic/pathologic framework behind LA myopathy based upon the EHRAS definition, LA myopathy can also be defined clinically as an abnormality in any of the 3 aspects of LA function. Recent advances in imaging technology have enhanced the ability to diagnose LA myopathy via a variety of modalities (Table 1, Fig. 1) [27–35].
Table 1.
Methods to identify left atrial myopathy
| Parameter | How to determine LA myopathy | Advantages | Disadvantages |
|---|---|---|---|
| LA maximum volume [13] | -Increased LA volumes at the end of atrial diastole as assessed by biplane method of discs | -Cost-effective -Technology readily available -Associated with poor outcomes in AF-HF |
-Potential for foreshortening leading to improper calculation of LA volume -Increased LA volume may not occur until the late stages of LA myopathy |
| LA minimum volume [14] | -Increased LA volumes at the end of atrial systole as assessed by biplane method of discs | -Cost-effective -Technology readily available -Associated with poor outcomes in AF-HF -Greater association with LA myopathy than LA maximum volume |
-Potential for foreshortening leading to improper calculation of LA volume -Increased LA volume may not occur until the late stages of LA myopathy |
| LA emptying fraction [15, 16] | -Decreased LA emptying fraction, as calculated by the equation: ((LA maximum volume − LA minimum volume)/(LA maximum volume)) | -Cost-effective -Technology readily available -Associated with poor outcomes in AF-HF |
-Potential for foreshortening leading to improper calculation of LA volumes -Decreased LA emptying fraction may not occur until the late stages of LA myopathy |
| LA Functional Index [17] | -Decreased LA functional index as calculated by the equation: ((LA emptying fraction × outflow tract velocity time integral)/(LA max volume indexed to BMI)) |
-Cost-effective -Technology readily available -Can be calculated independent of rhythm -Provides a functional estimate of LA mechanics |
-Potential for foreshortening leading to improper calculation of LA volumes -Calculations may be impacted by patient age, heart rate, and LA size |
| Mitral inflow Doppler pattern [18] | -Decreased A-wave velocity | -Cost effective -Technology readily available |
-Cannot be measured in setting of AF -Low velocities may only be present in later stages of disease |
| LA tissue Doppler [19] |
-Decreased a′ tissue velocity (< 5 cm/s) | -Cost effective -Technology readily available |
-Potential for inaccuracy due to foreshortened views of the LA -Cannot be measured in the setting of AF -Low velocities may only be present in later stages of disease |
| LA strain [20] | -Decreased reservoir, conduit or booster strain | -Cost-effective -Technology readily available -Potential to post-process images if strain is not obtained on acquired images -Sensitive measurement of atrial dynamics that can detect changes to LA myocardium early in the disease course -Holds significant prognostic value in AF-HF |
-Potential for inaccuracy due to foreshortened views of the LA -Manufacturer-dependent algorithms may lead to discrepant measurements -Potential for user-related error when tracing the endocardial border |
| Cardiac MRI [21] | -Presence of macroscopic LA scar (late gadolinium enhancement) | -Sensitive and noninvasive method of evaluating LA fibrosis -Ability to provide additional information about myocardial structure |
-High cost -Limited availability -Long duration of exam -Dependent on patient participation -Requires specific imaging protocol that may be center-dependent |
| Four-dimensional flow MRI [22] | -Decreased velocity of flow as determined by post-processing calculations | -Ability to quantify flow through the LA -Sensitive to subtle decreases in flow velocity which infer LA myopathy |
-High cost -Limited availability of 4D flow MRI technology -Long duration of exam -Dependent on heart rate and patient participation -Does not provide information about LA myocardial tissue |
| Electroanatomic mapping [23] | -Areas of decreased voltage in the LA prior to ablation | -High sensitivity in determining areas of impaired LA conduction | -Very high cost -Invasive procedure -Does not provide information about LA mechanical function |
Abbreviations: LA left atrium, AF-HF comorbid atrial fibrillation and heart failure, BMI body mass index, AF atrial fibrillation, MRI magnetic resonance imaging
Fig. 1.
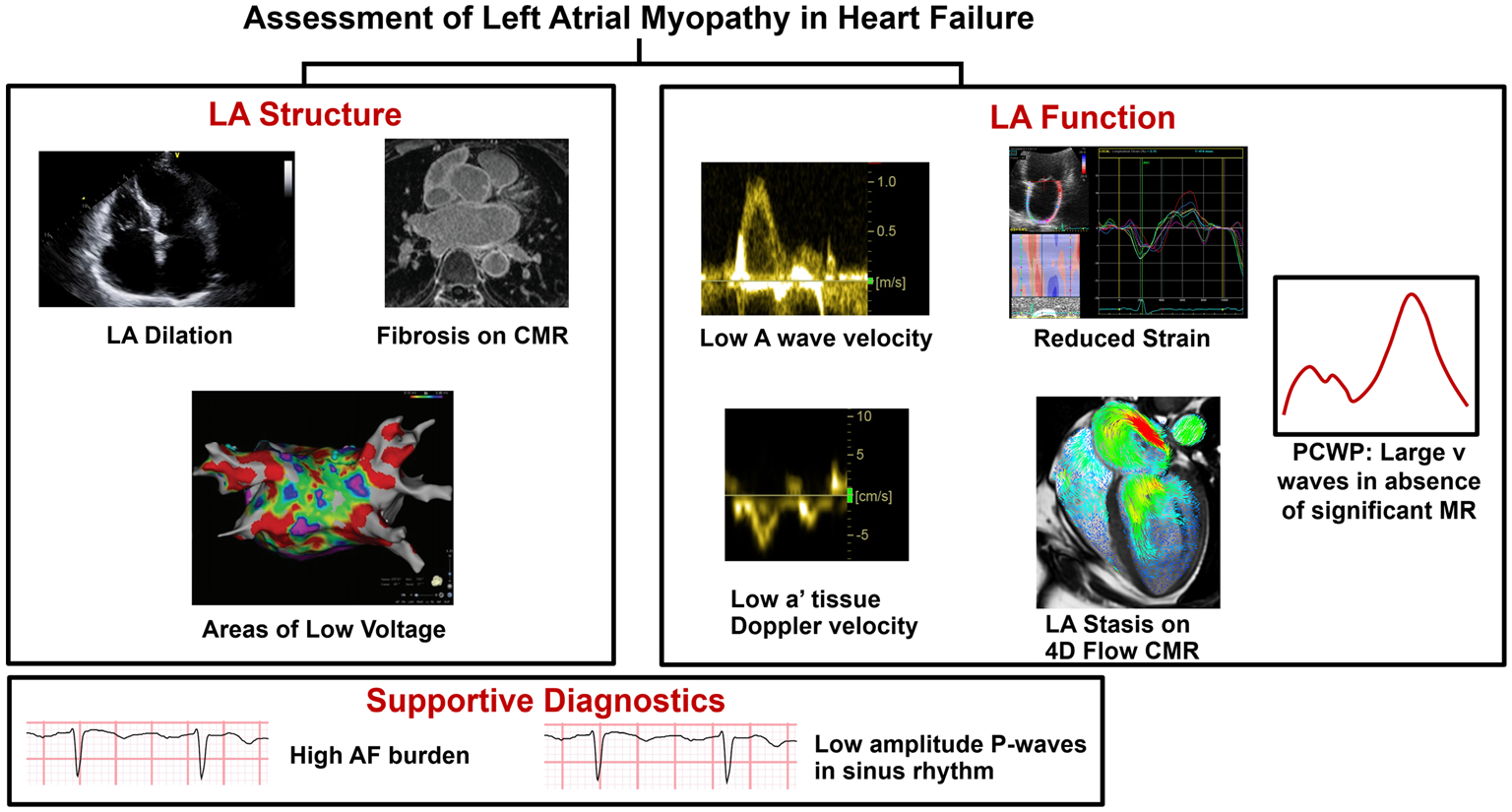
Comprehensive Assessment of LA myopathy in heart failure. LA myopathy is identified by alterations in LA structure or function. LA structural abnormalities may be determined by LA dilation, evidence of fibrosis, or areas of low voltage (red) on electroanatomic mapping. LA functional impairments can be diagnosed via low A wave velocity or low a′ tissue Doppler velocity, decreased strain, LA stasis on 4D flow cardiac MRI, or high v-waves on invasive hemodynamics. Portions of this figure were reproduced with permission from Zhao et al. [24], Rivner et al. [25], and Fidock et al. [26] (https://creativecommons.org/licenses/by/4.0/). Abbreviations: AF atrial fibrillation, CMR cardiac magnetic resonance, LA left atrium, MR mitral regurgitation, PCWP pulmonary capillary wedge pressure
Identification of LA Myopathy in Patients with Atrial Fibrillation and Heart Failure
While there is currently no laboratory biomarker that can provide unique insight into the presence of LA myopathy, there are several imaging modalities that may give valuable information regarding the health of the LA among patients with AF-HF (Table 1, Fig. 1). Systematic evaluation for LA myopathy through these mechanisms may be important to identify HF patients who are at particularly high risk for decompensation due to LA mechanical dysfunction. There is not a single gold-standard test to diagnose LA myopathy, and different diagnostic methods may be favored in specific clinical scenarios. If the diagnosis is unclear, a combination of multiple modalities likely has the highest sensitivity for identifying LA myopathy. Due to its cost-effectiveness and ability to detect subtle changes in myocardial dynamics, echocardiography (and specifically speckle-tracking) may have the broadest clinical applicability.
Electrocardiography and Continuous Rhythm Monitoring
In HF, burden of AF likely correlates with degree of LA myopathy. As such, those patients with persistent/permanent AF have high likelihood of LA mechanical failure that is manifested by high degree of atrial arrhythmia [36, 37]. Deep learning algorithms applied to electrocardiograms are able to identify patients at risk of developing AF [38]. Given the strong connection between AF and LA myopathy, these same algorithms may also be able to identify patients at risk for LA myopathy. Furthermore, among those patients in sinus rhythm but with a history of AF, low amplitude P-waves may signify the presence of LA myopathy due to the presence of LA scar/fibrosis [39, 40].
Echocardiography
Echocardiography is a primary imaging modality used to evaluate LA myopathy. Historically, increased LA volume was used to infer LA myopathy. However, this is a relatively crude measure of LA myopathy, and often presents later in the disease course. Similarly, both LA emptying fraction and LA function index indicate poor LA mechanical function, but may also present late in the LA myopathy disease process [15, 17]. Doppler imaging can provide valuable information about LA mechanics prior to changes in chamber size [29–31]. Specifically, decreased a′ tissue Doppler velocities during periods of sinus rhythm indicate reduced atrial booster function. Similarly, reduced A-wave velocities on mitral inflow Doppler are suggestive of poor atrial booster function [9].
Speckle-tracking echocardiography, or strain imaging, provides detailed information about myocardial dynamics by following the displacement of individual speckles across the cardiac cycle. As such, strain imaging of the LA can quantify each key role of the LA: reservoir, conduit, and booster functions. LA strain is sensitive to minor changes in myocardial dynamics and may detect subtle changes in LA myocardial function prior to macroscopic changes being observed [32, 33]. LA strain may diagnose atrial myopathy via reduced LA reservoir or contractile strain at rest, or suboptimal increase in LA reservoir strain after a volume challenge [41–44]. LA reservoir strain also has prognostic ability in AF-HF, as patients with low values demonstrate worse prognoses than patients with normal values [32, 33].
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance (CMR) has also been shown to detect LA myopathy. Four-dimensional flow CMR can assess the function of the LA via blood flow through the chambers of the heart. This allows for visualization of stasis in the LA, which may indicate LA myopathy. In addition to evaluating LA function, CMR also provides details about LA tissue, and can detect LA fibrosis via specific patterns of late gadolinium enhancement [34, 35]. The importance of LA fibrosis will be discussed in subsequent sections.
Electroanatomic Mapping
Invasive electroanatomic mapping (EAM) can allow for detailed visualization of the structure and corresponding electrical activity of atrial tissue. By integrating electrical activity obtained through a sensing catheter and LA structure, a 3D map of the LA is created which depicts areas of normal and impaired electrical conduction. Areas of preserved voltage indicate normal atrial tissue, while areas of low voltage indicate LA fibrosis or scar; these are two factors that can significantly impact LA mechanics and are associated with LA myopathy [45]. Among patients with AF-HF who are undergoing AF ablation, EAM may provide useful ancillary information at the time of the procedure regarding LA myopathy.
Invasive Hemodynamics
Invasive hemodynamic measurements may provide insight into LA reservoir function among patients with AF-HF. During LA filling through ventricular systole, decreased LA compliance (i.e., poor reservoir function) leads to a marked rise in LA pressure, manifesting as high v-waves. Thus, the presence of large v-waves on pulmonary capillary wedge pressure tracings in the absence of significant mitral regurgitation is concerning for LA myopathy.
LA Myopathy in AF-HFpEF
Clinical Importance
LA myopathy is common among patients with AF-HFpEF, and the degree of LA myopathy serves as both a diagnostic and prognostic marker in this patient population. Patients with AF-HFpEF have been shown to have high degrees of LA myopathy in multiple studies. In a meta-analysis of studies assessing LA strain parameters, LA reservoir, conduit, and booster strain were all significantly lower in AF-HFpEF patients than healthy controls [15]. Furthermore, a dose-dependent effect of time spent in AF (AF burden) on degree of LA myopathy in AF-HFpEF patients has been observed. In a study of 285 patients with HFpEF and 146 healthy controls, patients with HFpEF and high burdens of AF demonstrated lower LA reservoir strain compared to patients with HFpEF and lower burdens of AF and healthy controls [46••]. In addition, abnormal LA strain parameters predict progression of patients with HFpEF and paroxysmal AF to persistent or permanent AF [46••]. Similarly, among patients with AF, abnormal LA strain predicts development of HFpEF [46••].
LA myopathy is characterized by unique hemodynamic and biomarker profiles [43]. Patients with LA myopathy and AF-HFpEF develop LA volume overload, which leads to increased pulmonary vascular resistance, impaired oxygen consumption, and right ventricular dysfunction. These altered hemodynamics ultimately lead to worsening of symptoms from both AF and HFpEF [42, 47]. Additionally, worse baseline LA function in HFpEF is associated with elevation in biomarkers of neurohormonal activation and myocardial necrosis at baseline, and increased natriuretic peptide levels over time [48]. In aggregate, these findings suggest that LA myopathy may drive increased congestion in AF-HFpEF.
The degree of LA myopathy is directly related to rates of hospitalization and mortality in patients with AF-HFpEF [49]. In numerous studies of patients with AF-HFpEF, survival was lowest in patients with both the highest burden of AF and most significant LA myopathy [42, 46, 50]. Accordingly, it has been suggested that in patients with AF-HFpEF, the degree of LA myopathy may be more indicative of disease progression and symptomology than the amount of left ventricular dysfunction [43].
LA myopathy certainly plays a substantial role in the propagation of both AF and HFpEF in tandem. Similarly, AF and HFpEF lead to propagation of LA myopathy. In order to interrupt this cycle of disease progression between LA myopathy and AF-HFpEF, the mechanisms of LA myopathy in AF-HFpEF patients must be understood.
Mechanisms of LA Myopathy in AF-HFpEF
There are multiple mechanisms of LA myopathy in AF-HFpEF. Unique to patients with HFpEF, myocardial remodeling may occur in the LA independent of the left ventricle (LV) [3, 51, 52••]. In HFpEF, LA myopathy may occur out of proportion to LV myopathy, and is associated with a distinct proteomic signature, characterized by alterations in plasma proteins associated with cardiomyocyte stretch, extracellular matrix remodeling, and immune dysregulation [52••]. Additionally, the acute and chronic hemodynamic changes associated with AF and HFpEF lead to loss of coordinated atrial contraction, abnormal heart rates, impaired ventricular filling, and decreased cardiac output. As AF-HFpEF patients are dependent on the LA filling a noncompliant left ventricle, acute hemodynamic changes ensue. These altered hemodynamics lead to a variety of neurohormonal and inflammatory changes, all of which are associated with increased LA fibrosis [53, 54]. In patients with AF-HF, fibrosis preferentially impacts the atria due to the increased concentration of fibroblasts in the atria as compared to the ventricles [55].
LA fibrosis is an important mediator of LA myopathy in patients with AF-HFpEF via alterations of the LA electrophysiological substrate and mechanics [3, 51, 56, 57]. From an electrophysiological standpoint, LA fibrosis leads to abnormal ion channel expression in LA myocardial cells [58]. In canine models of HF, those with atrial fibrosis demonstrated reduced expression of IKur and IKs. This change in expression corresponds to a quicker action potential, and may serve as an electrical stimulus for the development or propagation of AF [59, 60]. In addition, increased degrees of fibrosis are associated with higher expression of the Na-Ca exchanger which may cause inappropriate delays after myocardial depolarization and further predispose to atrial arrhythmias [60]. Accordingly, LA fibrosis leads to an interruption of normal electrical conduction and creates a substrate for micro- and macro-reentrant rhythms within the atrium [61]. The atrial arrhythmias that are propagated from LA fibrosis in turn cause additional LA fibrosis and LA myopathy in AF-HFpEF patients.
In addition to electrophysiological alterations, LA fibrosis is associated with LA myopathy by leading to abnormal LA mechanics. Patients with confirmed LA fibrosis on CMR have demonstrated impaired LA strain on echocardiography [61]. Furthermore, the degree of LA fibrosis is oftentimes associated with the magnitude of mechanical abnormality.
There are multiple mechanisms of LA fibrosis in patients with AF-HFpEF, including activation of the renin-angiotensin-aldosterone system (RAAS), inappropriate autonomic response, increase in inflammation, oxidative stress, and impaired myocardial hemodynamics [62–64].
AF-HFpEF patients who have decreased cardiac output and increased LA wall stress oftentimes demonstrate inappropriate activation of the RAAS and sympathetic nervous systems [62–65]. By overactivation of the autonomic nervous system, patients with AF-HFpEF may develop a pro-arrhythmic state by non-equal shortening of refractory periods. This serves as an additional substrate for AF and propagation of atrial fibrosis and myopathy [65]. Furthermore, inappropriate RAAS activation in AF-HFpEF patients leads to an increased concentration of angiotensin II which is associated with stimulation of fibroblasts and subsequent LA fibrosis [66–68].
In addition, both AF and HFpEF are pro-inflammatory conditions. Patients with either HFpEF or AF have increased concentrations many markers of inflammation including TNFa and IL-6 which are associated with development of LA fibrosis via activation of metalloprotease enzymes [69–71]. NADPH oxidase, another marker of inflammation associated with increased production of reactive oxygen species and LA fibrosis, is also increased in patients with AF-HFpEF [69–71].
An additional mediator of cardiac inflammation in patients with AF-HFpEF is density and composition of epicardial adipose tissue. Increased concentrations of epicardial adipose tissue are associated with local inflammatory reactions and enhanced oxidative stress [57]. Taken together, the increased inflammatory milieu observed in patients with AF-HFpEF is associated with higher degrees of LA fibrosis and subsequent LA myopathy [57, 72, 73].
Finally, from a mechanical perspective, patients with AF-HFpEF have relatively high LA pressure, which further drives LA dysfunction. Whether LA pressures are increased due to LV diastolic dysfunction and subsequent elevation in filling pressures, intrinsic LA stiffness, or “atrial” mitral regurgitation, LA dysfunction ensues through an increase in LA wall stress in a non-uniform manner. The regional differences in wall stress within the LA create a nidus for further LA fibrosis [3].
Due to the significant LA myopathy in patients with AF-HFpEF, restoring sinus rhythm and appropriate LA mechanics proves difficult. A number of treatments for LA myopathy itself have been proposed for this population, which will be discussed in the next section.
Potential Treatments for LA Myopathy in AF-HFpEF
Once LA myopathy is identified among patients with AF-HFpEF, therapies should ideally be tailored for 1 of 2 major purposes: (1) to mitigate symptoms and clinical consequences of LA myopathy (i.e., LA decongestion), and (2) to reverse or halt the progression of LA dysfunction (Fig. 2).
Fig. 2.
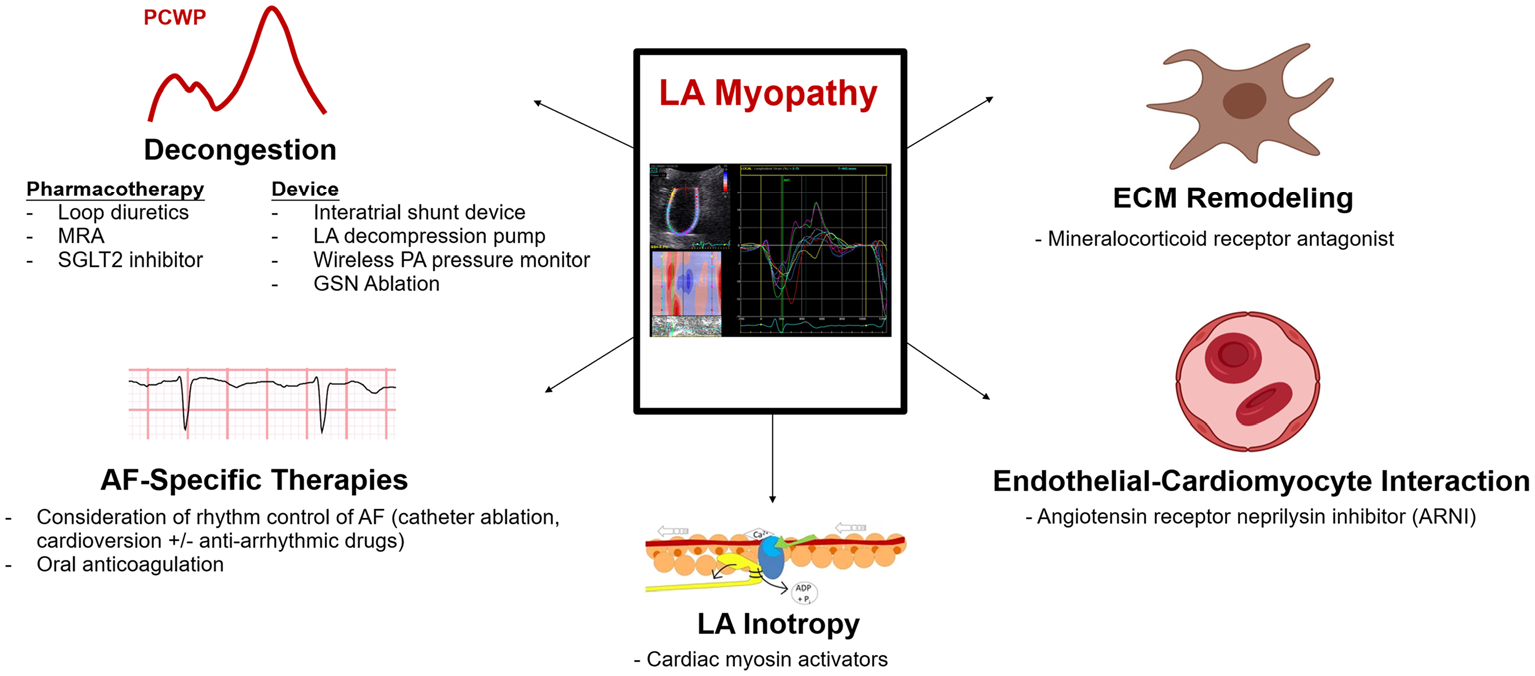
Potential therapies for left atrial myopathy in atrial fibrillation and heart failure with preserved ejection fraction. Potential therapeutics for LA myopathy in patients with AF-HFpEF include mechanical LA decongestion via device or pharmacotherapy, direct targeting the LA myopathic substrate, and rhythm control. While several therapies are under investigation to decongest the LA, further investigation is required to identify treatments that reverse the progression of LA myopathy. Portions of this figure were reproduced with permission from England et al. [74] (https://creativecommons.org/licenses/by/2.0/). Portions of this figure were created using BioRender.com. Abbreviations: AF atrial fibrillation, ECM extracellular matrix, GSN greater splanchnic nerve, LA left atrial, MRA mineralocorticoid receptor antagonist, PA pulmonary artery, PCWP pulmonary capillary wedge pressure, SGLT2 sodium glucose cotransporter-2
Decongestive Therapies
As a result of decreased chamber compliance and reduced LV filling, LA myopathy often leads to congestion via pulmonary venous hypertension, manifesting as dyspnea with low levels of exertion. Thus, therapies to decongest the LA may provide symptomatic benefit. While loop diuretics are a mainstay of therapy for this purpose, their role may be limited because oftentimes, LA pressure and volume overload may exist out of proportion to systemic venous congestion. As a result, loop diuretics may result in improvement in LA congestion, but at a cost of reduced venous preload, which may lead to symptoms of lightheadedness. Mineralocorticoid receptor antagonists (spironolactone) may also be considered for LA decongestion in combination with loop diuretics [75]. In addition, dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor with diuretic properties, reduced incidence of AF compared with placebo in the Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial [76]. Further investigation is required to understand if SGLT2 inhibitors directly benefit LA function. Nonetheless, among patients with con-comitant diabetes and AF-HFpEF, SGLT2 inhibitors should be considered to enhance decongestion. Finally, AF-HFpEF patients with LA myopathy may particularly benefit from continuous wireless pulmonary artery pressure monitoring to identify progressive pulmonary venous hypertension and increase decongestive therapies prior to further clinical decompensation.
Device therapies that improve LA pressure overload through LA unloading may provide sufficient LA decongestion without reducing systemic venous pressures. The ongoing REDUCE LAP-HF II trial (NCT03088033) is a sham-controlled randomized clinical trial that is evaluating the effect of an inter-atrial shunt device (IASD) on cardiovascular death and HF hospitalizations in HFpEF. Notably, the IASD reduced exercise pulmonary capillary wedge pressure in a mechanistic, sham-controlled, phase 2 trial of HFpEF patients [77]. Additionally, an LA to coronary sinus shunt device has demonstrated promise in improving functional status in an unblinded, single-arm experience [78]. Simulation models of an LA decompression pump in HFpEF have also suggested potential benefit [79]. Finally, among patients with both LA myopathy and systemic venous congestion, blood volume re-distribution away from the heart and to the splanchnic venous system offers promise [80]. The effect of endovascular ablation of the greater splanchnic nerve, the sympathetic regulator of splanchnic vascular capacitance, on pulmonary capillary wedge pressure in HFpEF is under investigation (NCT04592445). Taken together, patients with AF-HFpEF may be more likely to benefit from such LA unloading strategies due to the contribution of AF toward LA myopathy severity [6, 81]. The effect of LA unloading directly upon LA myopathy requires further understanding.
Direct Targeting of LA Myopathic Substrate
While therapies that directly target LA myopathy are currently lacking, there are several treatments that require further investigation. As LA fibrosis is a key link between LA myopathy, AF, and HFpEF, reduction of the factors that lead to LA fibrosis has been a popular area of investigation. Inflammation is a key mediator of LA fibrosis, and LA epicardial adipose tissue increases inflammation via a variety of mechanisms. In a study investigating the effect of weight loss on atrial adipose tissue density, researchers demonstrated that weight loss not only decreased LA adipose tissue quantity but was also associated with a reduced burden of AF and HFpEF symptoms [82]. While there are multiple mechanisms behind this finding, it is possible that decreasing LA adipose tissue also decreases the pro-inflammatory effects of adipose on the LA and improves LA myopathy [82]. Similarly, statins have been proposed as a treatment for LA myopathy in patients with AF-HFpEF due to their pleiotropic anti-inflammatory effects and reduction of LA adipose tissue mass [83–85]. Additionally, metformin is associated with a reduction in LA adipose tissue mass and AF burden in observational studies, suggesting a potential benefit at the level of the LA [85–87]. Unfortunately, spironolactone, a therapy with anti-fibrotic properties, did not reduce exercise capacity among patients with AF in the Improved Exercise Tolerance in heart Failure With Preserved Ejection Fraction by Spironolactone on Myocardial Fibrosis in Atrial Fibrillation (IMPRESS-AF) trial, suggesting it may not have a direct effect upon the LA [88].
Other neurohormonal therapies may directly improve LA function in AF-HFpEF. Given a particular suggestion of benefit of the angiotensin receptor-neprilysin inhibitor sacubitril-valsartan among individuals with AF in the Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor with Angiotensin Receptor Blocker Global Outcomes in HFpEF (PARAGON-HFpEF) trial, additional data are required to understand LA-specific effects of this therapy [89]. Additionally, beyond controlling heart rates and allowing for an increased LV diastolic filling time, beta blockers may also help improve LA myopathy by lengthening the relative refractory period of atrial myocytes [90]. However, while beta blockers are a mainstay of treatment for AF in isolation, their specific role in improving LA myopathy in AF-HFpEF requires further investigation.
Improvement in LA booster function through cardiac myosin activation is a potential target for LA myopathy in HFpEF that also warrants further investigation. Among HFrEF patients in sinus rhythm, danicamtiv, a novel cardiac myosin activator, increased LA function index and LA emptying fraction [91••]. Future studies evaluating the effect of such therapies on clinical outcomes in AF-HFpEF patients who can maintain sinus rhythm are warranted.
Rhythm Control
Restoration of sinus rhythm is an attractive treatment strategy that may confer beneficial effects upon LA mechanical function in AF-HFpEF. Unfortunately, maintaining sinus rhythm in patients with AF-HFpEF proves to be difficult. In a prospective cohort study of 157 patients with HFpEF and 100 patients without HF who underwent ablation for drug refractory AF, a significantly higher percentage of patients without HFpEF demonstrated freedom from AF 1 year following ablation [92]. Interestingly, 30% of patients with HFpEF in this study demonstrated at least 1 grade of improvement in diastolic dysfunction, but 8% of patients exhibited worsening diastolic dysfunction [92]. While some patients with AF-HFpEF improve with catheter ablation of AF, ablation may paradoxically worsen disease burden in patients with severe LA myopathy. The increased area of scar formed in the LA as a result of catheter ablation of AF can be associated with impaired LA mechanics, and increased LA stiffness, thus serving as a substrate for progression of both AF and HFpEF [81, 93–95]. Further investigation is necessary to understand ablation options that may result in restoration of sinus rhythm without worsening LA myopathy [81, 93, 94].
Accordingly, while a subset of patients with AF-HFpEF demonstrate symptomatic improvement with rhythm control, other patients have a less favorable response [43, 92, 96, 97]. A number of clinical trials, including Treatment of Atrial Fibrillation in Preserved Cardiac Function Heart Failure (NCT04160000) and Ablation Versus Medical Management of Atrial Fibrillation in HFpEF (NCT04282850), are currently ongoing to investigate the relative benefit of, and optimal modalities for, rhythm control in AF-HFpEF patients [43]. Further large-scale trials are necessary to determine specific patient characteristics that are associated with favorable responses to rhythm control in AF-HFpEF patients [6]. The degree of LA myopathy may be a significant factor in determining optimal candidates for rhythm control strategies.
LA Myopathy in AF-HFrEF
Clinical Importance
Despite being present in both AF-HFrEF and AF-HFpEF, the nature of LA myopathy in the 2 disease states is considerably unique [98, 99]. Generally, LA myopathy in AF-HFrEF manifests via eccentric LA remodeling and reduced LA ejection fraction rather than by LA stiffness and noncompliance [50]. As shown in a study of 189 patients with HF, those who had AF-HFrEF demonstrated greater eccentric remodeling, and lower LA ejection fraction than control patients [50].
In addition to being a powerful indicator of disease progression in patients with comorbid AF and HFrEF, LA myopathy plays a critical role in the development of comorbid AF-HFrEF. Among patients with AF and a moderately reduced ejection fraction, LA myopathy may precipitate HFrEF exacerbations [100]. Furthermore, the presence of AF may precipitate HFrEF exacerbations by decreasing ventricular preload. Without active atrial booster function, and with a shorter diastolic period for passive filling, the LV cannot completely fill. In an LV with decreased function at baseline, LA myopathy further lowers stroke volume and is associated with a subsequent decrease in cardiac output. While LA myopathy may be an underlying mediator that drives poor outcomes in AF-HFrEF, it appears that LA myopathy may play a less substantial primary role in this syndrome, as compared with AF-HFpEF.
Mechanisms of LA Myopathy in AF-HFrEF
In patients with AF-HFrEF, impaired antegrade flow from the LV is associated with both progressive LA hypervolemia and compensatory increases in afterload. This subsequently leads to elevated LA pressure which causes eccentric LA dilation [50]. In addition, the regional differences in LA wall stress caused by hypervolemia lead to focal areas of LA fibrosis. Together, this series of mechanical and structural changes to the LA is associated with significant LA myopathy that serves as a nidus for further AF and HFrEF [50, 64, 100].
Patients with AF-HFrEF and tachycardia-mediated cardiomyopathy are particularly likely to develop LA myopathy. Because of their persistent tachycardia, and even shorter refractory periods, AF-HFrEF patients demonstrate further decreased LA contractile function due to a relative decrease in calcium transport across myocardial membranes [100, 101]. This is associated with increased fibrosis due to remodeling of the extracellular matrix, and propagation of LA myopathy [102]. Additionally, functional mitral regurgitation secondary to LV dilation and/or AF itself (atrial mitral regurgitation) leads to LA pressure and volume overload, which further drives pathologic LA remodeling.
In addition to the mechanical changes detailed above, AF-HFrEF is associated with a variety of neurohormonal changes which precipitate LA myopathy. AF-HFrEF patients exhibit relative increases in norepinephrine and endothelin [100, 103, 104]. As a result, afterload is further increased which not only worsens symptoms from AF-HFrEF but also contributes to progression of LA myopathy by causing a higher burden of LA fibrosis [103, 104]. Patients with AF-HFrEF also often have RAAS overactivation. This is associated with inappropriately high concentrations of angiotensin II, which lead to an increased density of LA fibrosis and subsequent LA myopathy. Indeed, translational studies have suggested that interruption of the RAAS axis in patients with HFrEF may decrease the incidence of AF, with reductions in LA myopathy likely contributing to this phenomenon [100, 105]. Regardless of the mechanism, LA myopathy in patients with AF-HFrEF leads to further disease progression due to the associated decreases in LA booster function and LV preload. This subsequently is associated with a progressive increase in systemic vascular resistance and LA wall stress, decrease in cardiac output, and further neurohormonal changes. Again, these changes create a vicious cycle that, unless interrupted, is associated with significant morbidity and mortality.
Treatments for LA Myopathy in AF-HFrEF
There is a lack of data regarding the therapeutic role of improving LA myopathy in patients with AF-HFrEF. As opposed to HFpEF, which may be considered a syndrome of diastolic dysfunction and LA failure, HFrEF is defined by direct LV systolic dysfunction. As such, LA myopathy is typically a downstream (i.e., secondary) effect of LV pathology in HFrEF. However, many of the medical therapies that improve mortality in HFrEF may do so by not only improving LV function directly but also by improving LA myopathy.
As reported above, patients with AF-HFrEF have inappropriate activation of the sympathetic nervous system. This is associated both with worsening symptoms from AF-HFrEF, and progression of LA myopathy. Accordingly, the use of beta blockers in this population is an attractive treatment strategy to reduce the rate of LA remodeling. By decreasing adrenergic tone and subsequent oxygen consumption, beta blockers may help treat LA myopathy and improve overall LV function [90].
RAAS inhibition is another therapeutic target for LA myopathy in patients in AF-HFrEF. In the Valsartan Heart Failure Trial (Val-HeFT), patients with HFrEF who were started on valsartan had a lower rate of incident AF than patients in a control group. A mechanism of this finding is likely the interruption of LA fibrosis, and subsequent LA myopathy, conferred by RAAS inhibition among patients taking valsartan [105–107]. Although not specifically studied in an HFrEF population with existing AF, use of ACE or ARB medications in these patients may be associated with similarly beneficial effects on LA myopathy.
Similar to AF-HFpEF, cardiac myosin activation is a potential target for LA myopathy in HFrEF that warrants further investigation, given the improvements in LA contractile function noted with danicamtiv, a novel cardiac myosin activator, among HFrEF patients who were in sinus rhythm [91••].
Rhythm control with antiarrhythmic drugs, cardioversion, or catheter ablation may help treat LA myopathy in patients with AF-HFrEF, but can be associated with lower success rates than in patients without comorbid HFrEF [108]. Meta analyses have demonstrated ~ 60% freedom from AF 2 years after ablation among patients with AF-HFrEF when allowing for multiple procedures and re-initiation of antiarrhythmic drugs [108]. Among AF-HFrEF patients who do remain in sinus rhythm, an increase of LVEF by 8–21%, along with significant improvement in morbidity from HFrEF is observed [100, 109–111]. However, the creation of further scar in the LA via catheter ablation of patients with AF-HFrEF may be associated with more LA myopathy in certain patients, and make the LA substrate more prone to AF [81, 94]. In addition, when compared to healthy controls, there is also a higher rate of procedural complications among patients with AF-HFrEF who undergo catheter ablation [112]. Data suggest that in patients without HF, early attempts at restoration of sinus rhythm upon AF diagnosis result in reduced adverse clinical outcomes [113]. It remains unclear if such findings extend to those with HFrEF, and appropriate selection of ablation candidates is necessary in this population [81, 94].
Conclusion
In this review, we discussed the clinical importance, mechanisms, and treatments of LA myopathy in patients with AF and HF, across the spectrum of ejection fraction. In general, the presence of LA myopathy may precede development of AF and HF, and the effects of AF and HF on the LA lead to progression of LA myopathy. This creates a cycle by which LA myopathy begets AF and HF, while AF and HF simultaneously beget further LA myopathy.
While the nature of LA myopathy in AF-HFpEF is unique from that in AF-HFrEF, many of the fundamental mechanisms of its development overlap. The LA undergoes progressive remodeling through mechanical changes and overactivation of both RAAS and the autonomic nervous system. This remodeling ultimately leads to both electrical and mechanical failure of the LA which allows for rapid progression of both AF and HF in affected patients.
Given the more prominent pathologic role of LA dysfunction in driving poor outcomes among AF-HFpEF patients, specific treatment of LA myopathy may confer particularly beneficial effects in this cohort. Patients with AF-HFpEF may benefit from LA-specific treatment strategies in the future, which include LA unloading through device therapies. Further investigation is warranted to identify potential medical targets to directly improve LA function in AF-HFpEF. Conversely, the literature on therapies to treat AF-HFrEF is largely extrapolated from large trials of HFrEF patients, and specific trials to assess LA myopathy in this population are lacking. Regardless of ejection fraction, there may be a vulnerable cohort of AF-HF patients who have a disproportionate degree of LA myopathy compared with LV dysfunction. Further efforts are required to identify these patients and investigate therapies aimed to specifically improve LA function.
Funding
SJS is supported by research grants from the National Institutes of Health (R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423). RBP is supported by a research grant from the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001424).
Conflict of interest
SJS has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapies, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Eidos, Eisai, GSK, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Sanofi, Shifamed, Tenax, and United Therapeutics. The other authors declare no competing interests.
Footnotes
Human and Animal Rights This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8): 837–47. 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12): 1123–33. 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Patel RB, Vaduganathan M, Shah SJ, Butler J. Atrial fibrillation in heart failure with preserved ejection fraction: insights into mechanisms and therapeutics. Pharmacol Ther. 2017;176:32–9. 10.1016/j.pharmthera.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516–25. 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 5.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–92. 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RB, Passman RS, Shah SJ. Embarking upon atrial fibrillation management in heart failure with preserved ejection fraction: charting a course. J Cardiovasc Electrophysiol. 2020;31(9):2284–7. 10.1111/jce.14635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel RB, Delaney JA, Hu M, Patel H, Cheng J, Gottdiener J, et al. Characterization of cardiac mechanics and incident atrial fibrillation in participants of the Cardiovascular Health Study. JCI Insight. 2020;5(19). 10.1172/jci.insight.141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, et al. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging. 2014;7(6):570–9. 10.1016/j.jcmg.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18(10):1455–90. 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guichard JB, Nattel S. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol. 2017;70(6):756–65. 10.1016/j.jacc.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Blume GG, McLeod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, et al. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12(6):421–30. 10.1093/ejechocard/jeq175. [DOI] [PubMed] [Google Scholar]
- 12.Hoit BD. Assessment of left atrial function by echocardiography: novel insights. Curr Cardiol Rep. 2018;20(10):96. 10.1007/s11886-018-1044-1. [DOI] [PubMed] [Google Scholar]
- 13.Kanagala P, Arnold JR, Cheng ASH, Singh A, Khan JN, Gulsin GS, et al. Left atrial ejection fraction and outcomes in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging. 2020;36(1):101–10. 10.1007/s10554-019-01684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10(2):282–6. 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 15.Khan MS, Memon MM, Murad MH, Vaduganathan M, Greene SJ, Hall M, et al. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2020;22(3):472–85. 10.1002/ejhf.1643. [DOI] [PubMed] [Google Scholar]
- 16.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98(10):813–20. 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas L, Hoy M, Byth K, Schiller NB. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr. 2008;9(3):356–62. 10.1016/j.euje.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Mattioli AV, Castelli A, Andria A, Mattioli G. Clinical and echocardiographic features influencing recovery of atrial function after cardioversion of atrial fibrillation. Am J Cardiol. 1998;82(11): 1368–71. 10.1016/s0002-9149(98)00643-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Yip GW, Yu CM. Approaching regional left atrial function by tissue Doppler velocity and strain imaging. Europace. 2008;10 Suppl 3:iii62–9. 10.1093/europace/eun237. [DOI] [PubMed] [Google Scholar]
- 20.Donal E, Galli E, Schnell F. Left atrial strain: a must or a plus for routine clinical practice? Circ Cardiovasc Imaging. 2017;10(10). 10.1161/CIRCIMAGING.117.007023. [DOI] [PubMed] [Google Scholar]
- 21.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903. 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markl M, Carr M, Ng J, Lee DC, Jarvis K, Carr J, et al. Assessment of left and right atrial 3D hemodynamics in patients with atrial fibrillation: a 4D flow MRI study. Int J Cardiovasc Imaging. 2016;32(5):807–15. 10.1007/s10554-015-0830-8. [DOI] [PubMed] [Google Scholar]
- 23.Hansen BJ, Zhao J, Fedorov VV. Fibrosis and atrial fibrillation: computerized and optical mapping: a view into the human atria at submillimeter resolution. JACC Clin Electrophysiol. 2017;3(6): 531–46. 10.1016/j.jacep.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaho Y, Dagher L, Huang C, Miller P, Marrouche N. Cardiac MRI to manage atrial fibrillation. Arrhythmia Electrophysiol Rev. 2020;9(4). 10.15420/aer.2020.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivner H, Mitrani RD, Goldberger JJ. Atrial myopathy underlying atrial fibrillation. Arrhythmia Electrophysiol Rev. 2020;9(2):61–70. 10.15420/aer.2020.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidock B, Barker N, Balasubramanian N, Archer G, Fent G, Al-Mohammad A, et al. A systematic review of 4D-flow MRI derived mitral regurgitation quantification methods. Front Cardiovasc Med. 2019;6:103. 10.3389/fcvm.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caselli S, Canali E, Foschi ML, Santini D, Di Angelantonio E, Pandian NG, et al. Long-term prognostic significance of three-dimensional echocardiographic parameters of the left ventricle and left atrium. Eur J Echocardiogr. 2010;11(3):250–6. 10.1093/ejechocard/jep198. [DOI] [PubMed] [Google Scholar]
- 28.Suh IW, Song JM, Lee EY, Kang SH, Kim MJ, Kim JJ, et al. Left atrial volume measured by real-time 3-dimensional echocardiography predicts clinical outcomes in patients with severe left ventricular dysfunction and in sinus rhythm. J Am Soc Echocardiogr. 2008;21(5):439–45. 10.1016/j.echo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Hesse B, Schuele SU, Thamilasaran M, Thomas J, Rodriguez L. A rapid method to quantify left atrial contractile function: Doppler tissue imaging of the mitral annulus during atrial systole. Eur J Echocardiogr. 2004;5(1):86–92. 10.1016/s1525-2167(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 30.Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Changes in regional left atrial function with aging: evaluation by Doppler tissue imaging. Eur J Echocardiogr. 2003;4(2):92–100. 10.1053/euje.2002.0622. [DOI] [PubMed] [Google Scholar]
- 31.Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ, et al. Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study). Am J Cardiol. 2003;91(9): 1079–83. 10.1016/s0002-9149(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 32.Mirza M, Caracciolo G, Khan U, Mori N, Saha SK, Srivathsan K, et al. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two-dimensional speckle strain study. J Interv Card Electrophysiol. 2011;31(3):197–206. 10.1007/s10840-011-9560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai WC, Lee CH, Lin CC, Liu YW, Huang YY, Li WT, et al. Association of left atrial strain and strain rate assessed by speckle tracking echocardiography with paroxysmal atrial fibrillation. Echocardiography. 2009;26(10):1188–94. 10.1111/j.1540-8175.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 34.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311(5):498–506. 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 35.McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7(1):23–30. 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corradi D, Callegari S, Manotti L, Ferrara D, Goldoni M, Alinovi R, et al. Persistent lone atrial fibrillation: clinicopathologic study of 19 cases. Heart Rhythm. 2014;11(7):1250–8. 10.1016/j.hrthm.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, et al. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129(14):1472–82. 10.1161/CIRCULATIONAHA.113.004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394(10201):861–7. 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 39.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, et al. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132(4):278–91. 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT Jr, Nazarian S, et al. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45(9):2786–8. 10.1161/STROKEAHA.114.006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6(7):749–58. 10.1016/j.jcmg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9(3). 10.1161/CIRCIMAGING.115.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel RB, Shah SJ. Therapeutic targeting of left atrial myopathy in atrial fibrillation and heart failure with preserved ejection fraction. JAMA Cardiol. 2020;5(5):497–9. 10.1001/jamacardio.2020.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63(6):493–505. 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 45.Morton JB, Sanders P, Vohra JK, Sparks PB, Morgan JG, Spence SJ, et al. Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation. 2003;107(13):1775–82. 10.1161/01.CIR.0000058164.68127.F2. [DOI] [PubMed] [Google Scholar]
- 46.Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76(9):1051–64. 10.1016/j.jacc.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study characterizes the hemodynamic profile and outcomes among patients with HFpEF based on the degree of LA myopathy.
- 47.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3452–62. 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel RB, Alenezi F, Sun JL, Alhanti B, Vaduganathan M, Oh JK, et al. Biomarker profile of left atrial myopathy in heart failure with preserved ejection fraction: insights from the RELAX Trial. J Card Fail. 2020;26(3):270–5. 10.1016/j.cardfail.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail. 2014;7(6):1042–9. 10.1161/CIRCHEARTFAILURE.114.001276. [DOI] [PubMed] [Google Scholar]
- 50.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8(2):295–303. 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 51.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63(2):236–44. 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 52.Patel RB, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R, et al. Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants in the PROMIS-HFpEF study. Sci Rep. 2021;11(1):4885. 10.1038/s41598-021-84133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study identified and comprehensively characterized HFpEF patients with disproportionate degree of LA myopathy compared with LV myopathy.
- 53.Shannon RP. The relationship between altered load and impaired diastolic function in conscious dogs with pacing induced heart failure. Adv Exp Med Biol. 1993;346:337–45. 10.1007/978-1-4615-2946-0_33. [DOI] [PubMed] [Google Scholar]
- 54.Riegger AJ, Liebau G. The renin-angiotensin-aldosterone system, antidiuretic hormone and sympathetic nerve activity in an experimental model of congestive heart failure in the dog. Clin Sci (Lond). 1982;62(5):465–9. 10.1042/cs0620465. [DOI] [PubMed] [Google Scholar]
- 55.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117(13):1630–41. 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101(8):839–47. 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 57.Packer M, Lam CSP, Lund LH, Redfield MM. Interdependence of atrial fibrillation and heart failure with a preserved ejection fraction reflects a common underlying atrial and ventricular myopathy. Circulation. 2020;141(1):4–6. 10.1161/CIRCULATIONAHA.119.042996. [DOI] [PubMed] [Google Scholar]
- 58.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1(1):62–73. 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 59.Sridhar A, Nishijima Y, Terentyev D, Khan M, Terentyeva R, Hamlin RL, et al. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc Res. 2009;84(2):227–36. 10.1093/cvr/cvp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108(12):1461–8. 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 61.Naqvi TZ. The stiff left atrium is to atrial fibrillation as the stiff left ventricle is to diastolic heart failure. Circ Arrhythm Electrophysiol. 2016;9(3). 10.1161/CIRCEP.116.003952. [DOI] [PubMed] [Google Scholar]
- 62.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55(21):2299–307. 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 63.Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med. 2011;17(10):556–63. 10.1016/j.molmed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Hunter RJ, Liu Y, Lu Y, Wang W, Schilling RJ. Left atrial wall stress distribution and its relationship to electrophysiologic remodeling in persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5(2):351–60. 10.1161/CIRCEP.111.965541. [DOI] [PubMed] [Google Scholar]
- 65.Arora R Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: implications for therapies targeting the atrial autonomic nervous system. Circ Arrhythm Electrophysiol. 2012;5(4):850–9. 10.1161/CIRCEP.112.972273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104(21):2608–14. 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 67.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC Jr, Kasi VS, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165(3):1019–32. 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–9. 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 69.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52(4):306–13. 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 70.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51(1): 68–74. 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 71.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79(9):944–56. 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciuffo L, Nguyen H, Marques MD, Aronis KN, Sivasambu B, de Vasconcelos HD, et al. Periatrial fat quality predicts atrial fibrillation ablation outcome. Circ Cardiovasc Imaging. 2019;12(6): e008764. 10.1161/CIRCIMAGING.118.008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76(10):1197–211. 10.1016/j.jacc.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 74.England J, Loughna S. Heavy and light roles: myosin in the morphogenesis of the heart. Cell Mol Life Sci. 2013;70(7):1221–39. 10.1007/s00018-012-1131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pandey A, Garg S, Matulevicius SA, Shah AM, Garg J, Drazner MH, et al. Effect of mineralocorticoid receptor antagonists on cardiac structure and function in patients with diastolic dysfunction and heart failure with preserved ejection fraction: a meta-analysis and systematic review. J Am Heart Assoc. 2015;4(10): e002137. 10.1161/JAHA.115.002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141(15):1227–34. 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
- 77.Feldman T, Mauri L, Kahwash R, Litwin S, Ricciardi MJ, van der Harst P, et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364–75. 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 78.Simard T, Labinaz M, Zahr F, Nazer B, Gray W, Hermiller J, et al. TCT-87 Levoatrial to coronary sinus shunting as a novel strategy for symptomatic heart failure: first-in-human experience. J Am Coll Cardiol. 2019;74(13_Supplement):B87. 10.1016/j.jacc.2019.08.128. [DOI] [Google Scholar]
- 79.Burkhoff D, Maurer MS, Joseph SM, Rogers JG, Birati EY, Rame JE, et al. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail. 2015;3(4):275–82. 10.1016/j.jchf.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 80.Shah SJ, Zirakashvili T, Shaburishvili N, Shaishmelashvili G, Sivert H, Sivert K, et al. Endovascular ablation of the right greater splanchnic nerve for the treatment of heart failure with preserved ejection fraction-first in-human clinical trial. J Card Fail. 2020;26(12). 10.1016/j.cardfail.2020.11.023. [DOI] [Google Scholar]
- 81.Packer M Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J. 2019;40(23):1873–9. 10.1093/eurheartj/ehz284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakazato R, Rajani R, Cheng VY, Shmilovich H, Nakanishi R, Otaki Y, et al. Weight change modulates epicardial fat burden: a 4-year serial study with non-contrast computed tomography. Atherosclerosis. 2012;220(1):139–44. 10.1016/j.atherosclerosis.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang WT, Li HJ, Zhang H, Jiang S. The role of statin therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Br J Clin Pharmacol. 2012;74(5):744–56. 10.1111/j.1365-2125.2012.04258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Zhang Y, Gao M, Wang J, Wang Q, Wang X, et al. Statin therapy for the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Pharmacotherapy. 2011;31(11): 1051–62. 10.1592/phco.31.11.1051. [DOI] [PubMed] [Google Scholar]
- 85.Packer M Drugs that ameliorate epicardial adipose tissue inflammation may have discordant effects in heart failure with a preserved ejection fraction as compared with a reduced ejection fraction. J Card Fail. 2019;25(12):986–1003. 10.1016/j.cardfail.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. 10.1186/s12933-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen WJ, Greulich S, van der Meer RW, Rijzewijk LJ, Lamb HJ, de Roos A, et al. Activin A is associated with impaired myocardial glucose metabolism and left ventricular remodeling in patients with uncomplicated type 2 diabetes. Cardiovasc Diabetol. 2013;12:150. 10.1186/1475-2840-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shantsila E, Shahid F, Sun Y, Deeks J, Calvert M, Fisher JP, et al. Spironolactone in atrial fibrillation with preserved cardiac fraction: the IMPRESS-AF Trial. J Am Heart Assoc. 2020;9(18): e016239. 10.1161/JAHA.119.016239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–20. 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 90.Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, et al. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384(9961):2235–43. 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 91.Voors AA, Tamby JF, Cleland JG, Koren M, Forgosh LB, Gupta D, et al. Effects of danicamtiv, a novel cardiac myosin activator, in heart failure with reduced ejection fraction: experimental data and clinical results from a phase 2a trial. Eur J Heart Fail. 2020;22(9): 1649–58. 10.1002/ejhf.1933 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This trial evaluated the effect of a novel myosin activator on left atrial contractile function in patients with HFrEF.
- 92.Cha YM, Wokhlu A, Asirvatham SJ, Shen WK, Friedman PA, Munger TM, et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011;4(5):724–32. 10.1161/CIRCEP.110.960690. [DOI] [PubMed] [Google Scholar]
- 93.Park JW, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, et al. Atrial fibrillation catheter ablation increases the left atrial pressure. Circ Arrhythm Electrophysiol. 2019;12(4):e007073. 10.1161/CIRCEP.118.007073. [DOI] [PubMed] [Google Scholar]
- 94.Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011;8(9):1364–71. 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 95.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–67. 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Machino-Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62(20): 1857–65. 10.1016/j.jacc.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 97.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–70. 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123(18):2006–13; discussion 14. 10.1161/CIRCULATIONAHA.110.954388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Triposkiadis F, Harbas C, Kelepeshis G, Sitafidis G, Skoularigis J, Demopoulos V, et al. Left atrial remodeling in patients younger than 70 years with diastolic and systolic heart failure. J Am Soc Echocardiogr. 2007;20(2):177–85. 10.1016/j.echo.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 100.Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016;13(3):131–47. 10.1038/nrcardio.2015.191. [DOI] [PubMed] [Google Scholar]
- 101.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34(8):951–69. 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 102.Spinale FG, Tomita M, Zellner JL, Cook JC, Crawford FA, Zile MR. Collagen remodeling and changes in LV function during development and recovery from supraventricular tachycardia. Am J Phys. 1991;261(2 Pt 2):H308–18. 10.1152/ajpheart.1991.261.2.H308. [DOI] [PubMed] [Google Scholar]
- 103.Wasmund SL, Li JM, Page RL, Joglar JA, Kowal RC, Smith ML, et al. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation. 2003;107(15):2011–5. 10.1161/01.CIR.0000064900.76674.CC. [DOI] [PubMed] [Google Scholar]
- 104.Tuinenburg AE, Van Veldhuisen DJ, Boomsma F, Van Den Berg MP, De Kam PJ, Crijns HJ. Comparison of plasma neurohormones in congestive heart failure patients with atrial fibrillation versus patients with sinus rhythm. Am J Cardiol. 1998;81(10): 1207–10. 10.1016/s0002-9149(98)00092-7. [DOI] [PubMed] [Google Scholar]
- 105.Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54(2):456–61. 10.1016/s0008-6363(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 106.Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, et al. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J. 2005;149(3):548–57. 10.1016/j.ahj.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 107.Cohn JN, Tognoni G. Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–75. 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 108.Anselmino M, Matta M, D’Ascenzo F, Bunch TJ, Schilling RJ, Hunter RJ, et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7(6):1011–8. 10.1161/CIRCEP.114.001938. [DOI] [PubMed] [Google Scholar]
- 109.Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351(23):2373–83. 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 110.Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7(1):31–8. 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 111.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417–27. 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 112.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–413. 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 113.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–16. 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]


