Abstract
BACKGROUND
Few contemporary data exist evaluating care patterns and outcomes in heart failure (HF) across the spectrum of kidney function.
OBJECTIVES
This study sought to characterize differences in quality of care and outcomes in patients hospitalized for HF by degree of kidney dysfunction.
METHODS
Guideline-directed medical therapies were evaluated among patients hospitalized with HF at 418 sites in the GWTG-HF (Get With The Guidelines–Heart Failure) registry from 2014 to 2019 by discharge CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration)-derived estimated glomerular filtration rate (eGFR). We additionally evaluated the risk-adjusted association of admission eGFR with in-hospital mortality.
RESULTS
Among 365,494 hospitalizations (age 72 ± 15 years, left ventricular ejection fraction [EF]: 43 ± 17%), median discharge eGFR was 51 ml/min/1.73 m2 (interquartile range: 34 to 72 ml/min/1.73 m2), 234,332 (64%) had eGFR <60 ml/min/1.73 m2, and 18,869 (5%) were on dialysis. eGFR distribution remained stable from 2014 to 2019. Among 157,439 patients with HF with reduced EF (≤40%), discharge guideline-directed medical therapies, including beta-blockers, were lowest in discharge eGFR <30 mL/min/1.73 m2 or dialysis (p < 0.001). “Triple therapy” with angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor–neprilysin inhibitor + beta-blocker + mineralocorticoid receptor antagonist was used in 38%, 33%, 25%, 15%, 5%, and 3% for eGFR ≥90, 60 to 89, 45 to 59, 30 to 44, <30 ml/min/1.73 m2, and dialysis, respectively; p < 0.001. Mortality was higher in a graded fashion at lower admission eGFR groups (1.1%, 1.5%, 2.0%, 3.0%, 5.0%, and 4.2%, respectively; p < 0.001). Steep covariate-adjusted associations between admission eGFR and mortality were observed across EF subgroups, but was slightly stronger for HF with reduced EF compared with HF with mid-range or preserved EF (pinteraction = 0.045).
CONCLUSIONS
Despite facing elevated risks of mortality, patients with comorbid HF with reduced EF and kidney disease are not optimally treated with evidence-based medical therapies, even at levels of eGFR where such therapies would not be contraindicated by kidney dysfunction. Further efforts are required to mitigate risk in comorbid HF and kidney disease.
Keywords: glomerular filtration rate, heart failure, kidney disease, outcomes, therapy
Despite contemporary advances in care, hospitalization for heart failure (HF) remains a frequent, costly, and highly morbid event, carrying a subsequent 5-year mortality of >75% across the syndrome’s spectrum of ejection fraction (EF) (1–3). Although several medical therapies have been demonstrated to reduce worsening HF events and mortality among individuals with heart failure with reduced ejection fraction (HFrEF), recent data suggest that implementation and titration of such therapies are suboptimal (4,5).
Kidney dysfunction frequently coexists with chronic HF, and the presence of both is associated with worse clinical outcomes than either condition alone (6–9). Whereas several contemporary classes of therapies for HFrEF (angiotensin receptor blockers [ARBs], angiotensin-converting enzyme [ACE] inhibitors, and mineralocorticoid receptor antagonists [MRAs]) have demonstrated clinical benefits among select individuals with chronic kidney disease (CKD) (10–12), historical data have suggested these therapies are infrequently used in this high-risk cohort (13–15) and contemporary data are lacking. As such, we evaluated clinical profiles, discharge medical therapies, and in-hospital mortality among patients hospitalized for HF across the spectrum of kidney function in the GWTG-HF (Get With The Guidelines–Heart Failure) registry.
METHODS
GWTG-HF STUDY DESIGN.
The GWTG-HF program objectives, design, and protocols have been previously described (16). Briefly, GWTG-HF was initiated by the American Heart Association in 2005 as a hospital-based quality improvement program that prospectively collects data regarding patients with a primary discharge diagnosis of HF from participating centers within the United States. Study personnel utilize standardized report forms to obtain information regarding demographics, comorbidities, hospital characteristics, vital signs and laboratory data, in-hospital treatments, left ventricular EF, in-hospital outcomes, discharge medications, and patient disposition at discharge. A point-of-service, web-based tool (IQVIA Platform, IQVIA Inc.) through the American Heart Association serves as a platform for collation of these data and is compliant with the Joint Commission and Centers for Medicare and Medicaid Services standards. Deidentified data are aggregated by the Duke Clinical Research Institute (Durham, North Carolina) and are independently monitored for quality assurance. A waiver for patient informed consent is granted under the Common Rule as the GWTG-HF registry’s primary purpose is for quality improvement. The protocol for GWTG-HF has been approved by or received waivers from the Institutional Review Boards at each participating site, and deidentified analyses were approved by the Institutional Review Board of Duke Clinical Research Institute.
STUDY POPULATION.
This analysis included hospitalizations of adult patients between January 1, 2014, and September 30, 2019, across 529 GWTG-HF sites. Patients excluded from the primary analysis were those who: 1) were under the age of 18 years; 2) left against medical advice, were transferred to an acute care facility, or discharged to hospice care; or 3) were missing estimated glomerular filtration rate (eGFR) data during their entire hospital stay. The following EF criteria were used to categorize patients: HFrEF (≤40%), heart failure with mid-range ejection fraction (HFmrEF) (41% to 49%), and heart failure with preserved ejection fraction (HFpEF) (≥50%). The CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation was used to calculate eGFR using demographics and serum creatinine (17). eGFR was calculated using laboratory data at discharge; if data at discharge were missing, then values closest to the time of discharge were used. The analysis considered each hospitalization as a unique episode of care and individual patients may have contributed to more than 1 hospitalization.
QUALITY MEASURES ACHIEVED AT DISCHARGE AMONG PATIENTS WITH HFrEF.
As part of the GWTG-HF registry, several quality measures are recorded at the time of discharge. For patients with HFrEF (EF ≤40%), the primary metrics of interest for this analysis were discharge use of ACE inhibitor, ARB, or angiotensin receptor–neprilysin inhibitor (ARNI), beta-blocker, and/or MRA, and prescription of all 3 classes of drugs (“triple therapy”: ACE inhibitor, ARB, or ARNI, along with beta-blocker and MRA). Rates of use of hydralazine/nitrate were also reported. An additional quality metric of interest for this analysis was post-discharge appointment made.
STATISTICAL ANALYSIS.
Patients were categorized into 6 prespecified eGFR groups according to the KDIGO (Kidney Disease: Improving Global Outcomes) classification (≥90 ml/min/1.73 m2, 60 to <90 ml/min/1.73 m2, 45 to <60 ml/min/1.73 m2, 30 to <45 ml/min/1.73 m2, <30 ml/min/1.73 m2, and receipt of dialysis). To assess variation in clinical profiles and in-hospital outcomes across the 6 discharge eGFR groups, Pearson chi-square tests were used to compare differences in categorical variables and Wilcoxon rank-sum tests were used to compare continuous variables. We evaluated the distribution of discharge eGFR by patient-reported history of CKD. The proportions of patients within each discharge eGFR category by calendar year were reported as absolute number and percentage. We further evaluated proportions of eGFR categories over time among each EF subtype (HFrEF, HFmrEF, HFpEF). Characteristics of patients with available and missing discharge eGFR were compared using standardized differences (>10% difference considered clinically relevant).
Among Black participants in GWTG-HF with available eGFR measurements and who were not on dialysis, we re-estimated the CKD-EPI–based eGFR with and without the race coefficient. We then evaluated the proportion of Black participants who would be reclassified to worse CKD stages if the race coefficient were removed.
For in-hospital mortality assessment, admission eGFR (or closest value) based on KDIGO categories was examined instead of discharge measurements to limit immortal person-time bias. Using multivariable logistic regression analyses, associations between admission eGFR and in-hospital mortality were adjusted for prespecified patient- and hospital-level clinical and sociodemographic covariates: age, sex, race, admission systolic blood pressure, admission heart rate, body mass index, diabetes, atrial fibrillation/flutter, prior myocardial infarction, prior stroke or transient ischemic attack, chronic obstructive pulmonary disease, hospital region, hospital teaching status, and hospital area income (based on zip code tabulation area). Associations were summarized as adjusted odds ratio (95% confidence intervals) with eGFR ≥90 ml/min/1.73 m2 as the reference group. We determined whether admission eGFR differentially predicted in-hospital mortality by EF subtype (HFrEF, HFmrEF, HFpEF) using interaction terms.
Among the hospitalized HFrEF cohort, we evaluated achievement of prespecified quality measures by discharge eGFR category using Pearson chi-square tests overall and stratified by sex and race/ethnicity. To understand whether variation in discharge medical therapies for HFrEF is driven by reduced kidney function as opposed to confounding factors such as hyperkalemia or hypotension, we performed a sensitivity analysis in which we evaluated rates of medical therapies within each eGFR category among participants with discharge systolic blood pressure >95 mm Hg and serum potassium <5.0 mEq/l. Analyses were performed using SAS version 9.4 (SAS Institute Inc.). Two-tailed testing was performed and p < 0.05 was considered statistically significant.
RESULTS
STUDY COHORT SELECTION.
Of 586,580 hospitalizations at any of the 529 GWTG-HF sites between January 1, 2014, and September 30, 2019, 221,086 were excluded (28,429 patients left against medical advice or were discharged to an acute care facility or hospice, 192,657 were missing eGFR data) (Figure 1). Thus, the primary analytic cohort consisted of 365,494 hospitalizations from 418 sites. There was minimal variation in demographic and clinical characteristics of patients who did and did not have available discharge eGFR; teaching hospitals and hospitals located in the Northeast and West regions of the United States were more likely to report eGFR data (Supplemental Table 1).
FIGURE 1. STROBE Flow Diagram for Study Patient Inclusion.
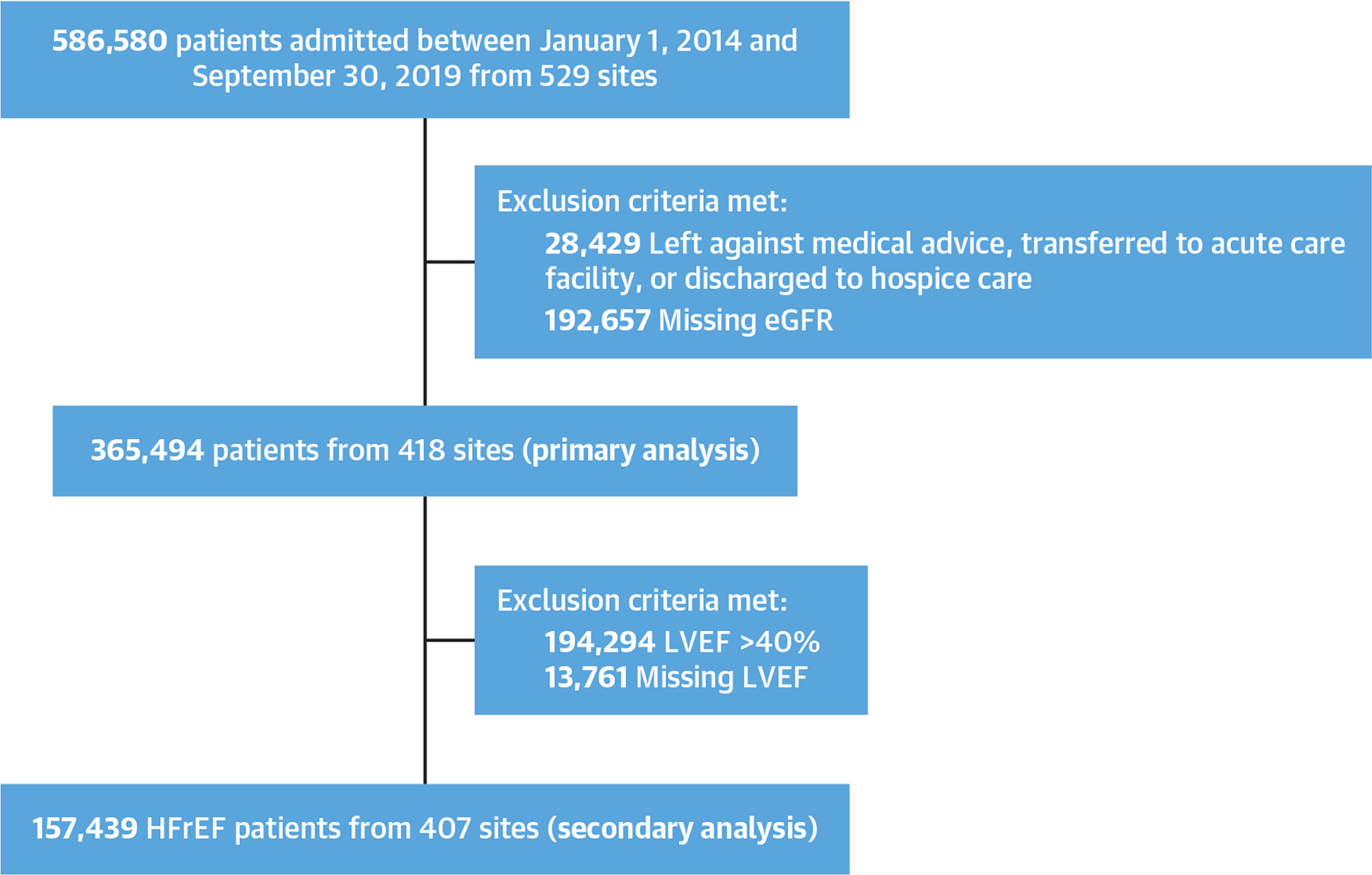
Flow diagram showing the inclusion process. eGFR = estimated glomerular filtration rate; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; STROBE = Strengthening the Reporting of Observational Studies in Epidemiology.
CLINICAL PROFILES BY DISCHARGE eGFR CATEGORIES.
Among 365,494 patients (mean age 72 ± 15 years, LVEF 43 ± 17%), median discharge eGFR was 51 (interquartile range: 34, 72) ml/min/1.73 m2 and 234,332 (64%) had eGFR <60 ml/min/1.73 m2. Overall, 18,869 patients (5%) were on dialysis and 35,759 (10%) had eGFR ≥90 ml/min/1.73 m2. Over time, the proportion of patients within each discharge eGFR category remained stable (Figure 2). These findings were consistent on stratification by EF subtype (HFrEF, HFmrEF, HFpEF) (Supplemental Tables 2, 3, and 4). Notably, there was wide variation in discharge eGFR among patients without a reported history of CKD (Supplemental Figure 1). Of patients without a reported history of CKD (n = 273,511), 148,505 (54%) had discharge eGFR <60 ml/min/1.73 m2.
FIGURE 2. Trends in Discharge eGFR in Hospitalized HF From 2014 to 2019.
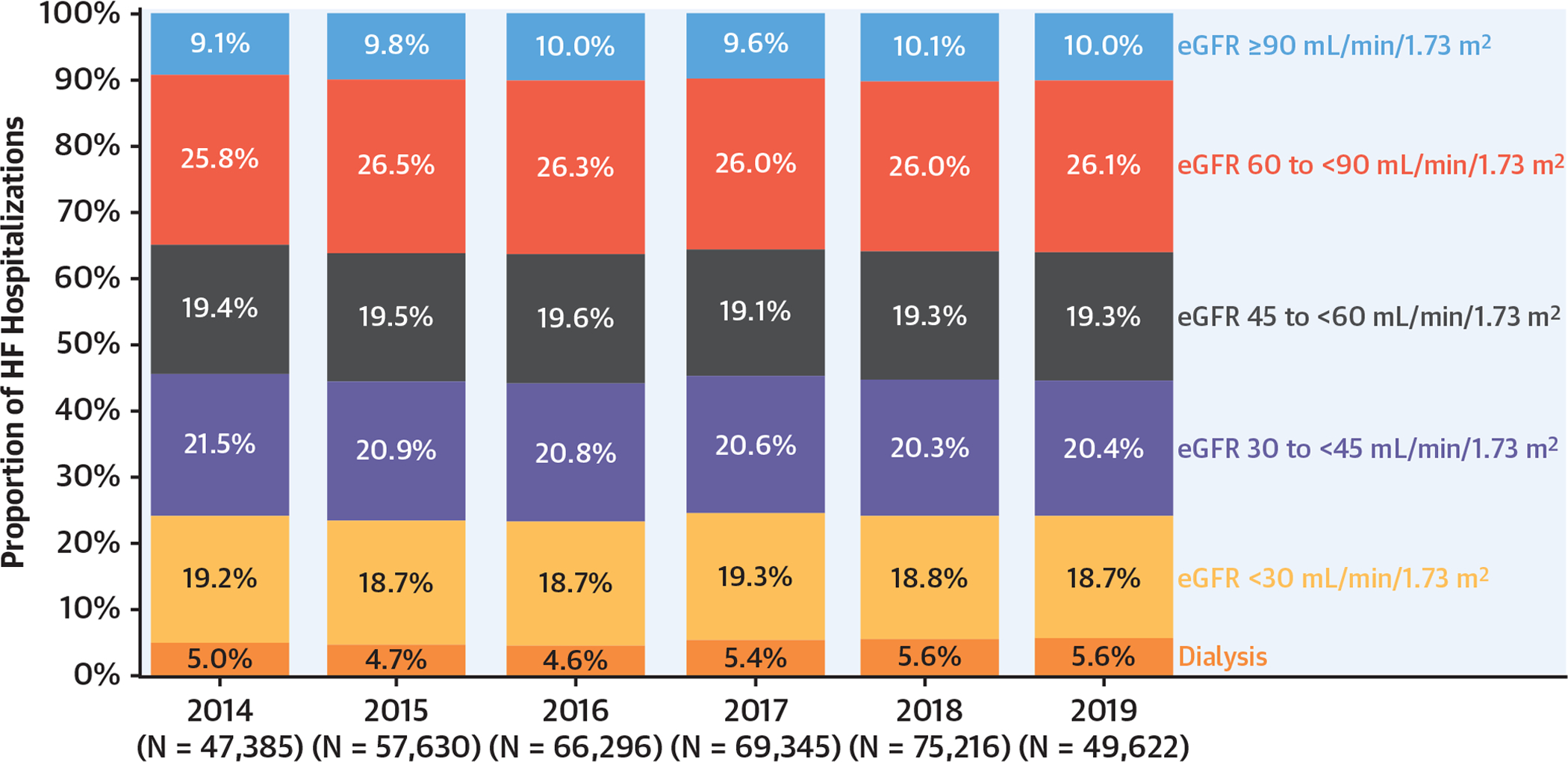
Trends in kidney function were determined by estimated glomerular filtration (eGFR) on discharge from hospital. HF = heart failure.
Prevalence of dialysis was similar (5%) in men and women, but it varied by race/ethnicity, was lowest among White patients (4%), and highest among Asian patients (11%) (Figure 3). Among patients not on dialysis, those with lower eGFR were older and more likely to be women and of White race, whereas patients on dialysis at discharge were younger (Table 1). Similarly, prevalence of atrial fibrillation/flutter was higher in lower eGFR categories except in the dialysis group, in which atrial fibrillation/flutter was less frequent. Across the entire spectrum of kidney function, lower eGFR was associated with higher EF and higher prevalence of hypertension, coronary artery disease, diabetes, peripheral vascular disease, and stroke/transient ischemic attack. Serum potassium concentrations were available at or near discharge in 198,544 participants (54.3%). Rates of potassium ≥5.0 mEq at discharge increased with lower kidney function ranging from 2.8% among participants with eGFR ≥90 ml/min/1.73 m2 to 19.4% among participants on dialysis (p < 0.001). Similarly, rates of potassium ≥5.5 mEq at discharge increased from 0.5% among participants with eGFR ≥90 ml/min/1.73 m2 to 6.8% among participants on dialysis (p < 0.001) (Supplemental Figure 2).
FIGURE 3. Distribution of Discharge eGFR by Sex and Race/Ethnicity.
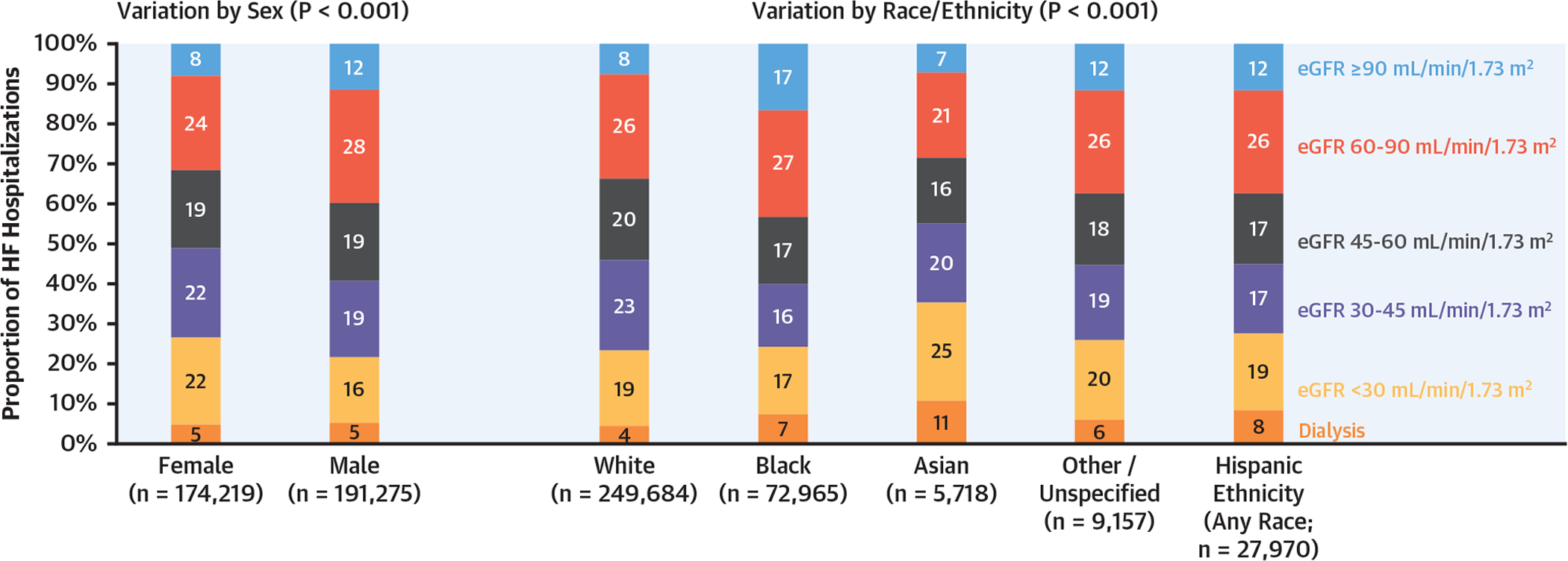
Shown is proportion of HF hospitalizations within each discharge eGFR category by sex and race/ethnicity. Abbreviations as in Figures 1 and 2.
TABLE 1.
Characteristics of Patients Hospitalized for HF by Discharge eGFR Category
| eGFR Category, ml/min/1.73 m2 | ||||||
|---|---|---|---|---|---|---|
| ≥90 (n = 35,759) |
60 to <90 (n = 95,403) |
45 to <60 (n = 70,699) |
30 to <45 (n = 75,704) |
<30 (n = 69,060) |
Dialysis (n = 18,869) |
|
| Demographics | ||||||
| Age, y | 58 ± 14 | 70 ± 15 | 74 ± 13 | 77 ± 13 | 76 ± 13 | 66 ± 14 |
| Female | 13,698 (38) | 41,277 (43) | 33,816 (48) | 39,054 (52) | 37,813 (55) | 8,561 (45) |
| Race | ||||||
| Black | 12,234 (34) | 19,372 (20) | 12,226 (17) | 11,504 (15) | 12,214 (18) | 5,415 (29) |
| White | 18,835 (53) | 65,182 (68) | 51,045 (72) | 56,439 (75) | 48,193 (70) | 9,990 (53) |
| Hispanic | 3,221 (9) | 7,259 (8) | 4,876 (7) | 4,881 (6) | 5,443 (8) | 2,290 (12) |
| Asian | 408 (1) | 1,221 (1) | 929 (1) | 1,143 (2) | 1,401 (2) | 616 (3) |
| Other | 1,061 (3) | 2,369 (2) | 1,623 (2) | 1,737 (2) | 1,809 (3) | 558 (3) |
| Medical history | ||||||
| AF/AFL | 8,826 (25) | 37,808 (40) | 32,220 (46) | 36,269 (48) | 28,433 (41) | 5,383 (29) |
| COPD/asthma | 13,832 (39) | 34,720 (36) | 25,612 (36) | 27,194 (36) | 23,032 (33) | 6,896 (37) |
| CVA/TIA | 3,756 (11) | 14,086 (15) | 12,066 (17) | 13,944 (18) | 12,946 (19) | 3,730 (20) |
| Peripheral vascular disease | 2,411 (7) | 8,850 (9) | 8,046 (11) | 10,438 (14) | 10,588 (15) | 3,448 (18) |
| Previous MI | 5,589 (16) | 17,502 (18) | 14,608 (21) | 16,857 (22) | 15,352 (22) | 4,393 (23) |
| Hypertension | 27,074 (76) | 78,147 (82) | 60,180 (85) | 65,903 (87) | 61,326 (89) | 17,097 (91) |
| Diabetes mellitus | 14,271 (40) | 36,749 (39) | 30,606 (43) | 37,134 (49) | 39,546 (57) | 12,002 (64) |
| Smoking history | 12,990 (36) | 19,803 (21) | 10,034 (14) | 7,976 (11) | 7,010 (10) | 3,469 (18) |
| LVEF, % | 39 ± 18 | 42 ± 18 | 43 ± 17 | 44 ± 17 | 46 ± 16 | 46 ± 16 |
| HFrEF ≤40% | 18,963 (55) | 44,737 (49) | 30,790 (45) | 30,865 (42) | 25,561 (39) | 6,523 (37) |
| HFmrEF 41% to 49% | 2,813 (8) | 8,346 (9) | 6,769 (10) | 7,467 (10) | 7,058 (11) | 2,139 (12) |
| HFpEF ≥50% | 12,756 (37) | 38,841 (42) | 30,594 (45) | 34,581 (47) | 33,749 (51) | 9,181 (51) |
| Any diuretic use | 18,903 (53) | 53,690 (56) | 40,213 (57) | 41,797 (55) | 33,834 (49) | 5,088 (27) |
| Loop diuretic | 18,154 (51) | 51,828 (54) | 38,853 (55) | 40,305 (53) | 32,607 (47) | 4,904 (26) |
| Thiazide diuretic | 885 (2) | 2,435 (3) | 2,049 (3) | 2,626 (3) | 2,434 (4) | 308 (2) |
| Diuretic type not specified | 441 (1) | 1,044 (1) | 759 (1) | 846 (1) | 725 (1) | 110 (1) |
| Discharge vital signs and laboratory measurements | ||||||
| Body mass index, kg/m2 | 32.9 ± 11.3 | 30.8 ± 10.0 | 30.6 ± 9.2 | 30.5 ± 8.9 | 30.4 ± 8.6 | 29.0 ± 8.3 |
| Heart rate, beats/min | 82 ± 15 | 79 ± 15.0 | 77 ± 15 | 76 ± 15 | 75 ± 15 | 78 ± 14 |
| Systolic blood pressure, mm Hg | 121 ± 20 | 123 ± 20 | 124 ± 20 | 125 ± 21 | 129 ± 22 | 132 ± 24 |
| Potassium, mEq/l* | 4.0 (3.7, 4.3) | 4.0 (3.7, 4.3) | 4.0 (3.7, 4.3) | 4.0 (3.7, 4.4) | 4.2 (3.8, 4.6) | 4.4 (4.0, 4.8) |
| Hospital characteristics | ||||||
| Teaching hospital | 29,405 (82) | 75,953 (80) | 55,967 (79) | 59,492 (79) | 54,704 (79) | 14,694 (78) |
| Region | ||||||
| West | 6,471 (18) | 17,127 (18) | 11,708 (17) | 11,413 (15) | 10,261 (15) | 3,043 (16) |
| South | 12,088 (34) | 29,219 (31) | 21,296 (30) | 22,011 (29) | 20,299 (29) | 6,062 (32) |
| Midwest | 7,326 (20) | 19,647 (21) | 15,034 (21) | 16,750 (22) | 14,478 (21) | 4,357 (23) |
| Northeast | 9,874 (28) | 29,410 (31) | 22,661 (32) | 25,530 (34) | 24,022 (35) | 5,407 (29) |
| Outcomes | ||||||
| In-hospital death | 367 (1) | 1,288 (1) | 1,263 (2) | 2,093 (3) | 3,922 (6) | 783 (4) |
| Length of stay, days | 4 (3, 6) | 4 (3, 6) | 4 (3, 6) | 4 (3, 6) | 4 (3, 7) | 4 (2, 7) |
Values are mean ± SD, n (%), or median (interquartile range). All P-value for comparisons across eGFR categories were ≤0.01.
Among 198,544 participants (54.3%) who had available data on potassium levels at discharge.
AF = atrial fibrillation; AFL = atrial flutter; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; eGFR = estimated glomerular filtration rate; HF = heart failure; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; MI = myocardial infarction; TIA = transient ischemic attack.
REMOVAL OF RACE FROM ESTIMATES OF KIDNEY FUNCTION.
Among the 67,550 Black participants in GWTG-HF with available eGFR measurements who were not on dialysis, CKD-EPI–based eGFR was re-estimated without the race coefficient (Figure 4). Overall, among 31,606 Black participants who had eGFR originally estimated as ≥60 ml/min/1.73 m2 when the race coefficient was included, 7,353 (23%) would be reclassified as eGFR 30 to 59 ml/min/1.73 m2 if the race coefficient were removed. Similarly, among 23,730 Black participants who had eGFR 30 to 59 ml/min/1.73 m2 when the race coefficient was included, 3,504 (15%) would be reclassified as eGFR below 30 ml/min/1.73 m2 if the race coefficient were removed (Supplemental Table 5).
FIGURE 4. eGFR Distribution With and Without Race Coefficient Among Black Participants.

Vertical lines are included at eGFR 30 and 60 ml/min/1.73 m2. For display purposes, eGFR above 150 mL/min/1.73 m2 are aggregated. CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; GWTG-HF = Get With The Guidelines–Heart Failure; other abbreviations as in Figures 1 and 2.
IN-HOSPITAL MORTALITY ACROSS ADMISSION eGFR CATEGORIES.
Median admission eGFR was 51 (IQR: 34, 72) ml/min/1.73 m2 and 234,445 (64%) had admission eGFR <60 ml/min/1.73 m2. There was a graded significant association between admission eGFR and in-hospital mortality: 1.1%, 1.5%, 2.0%, 3.0%, 5.0%, and 4.2% mortality rates for eGFR ≥90, 60 to 89, 45 to 59, 30 to 44, <30 ml/min/1.73 m2 and dialysis, respectively; p < 0.001. Similar patterns were observed in EF-based subgroups with the steepest gradient observed among patients with HFrEF (Figure 5A). After accounting for key patient- and hospital-level clinical and sociodemographic covariates, lower admission eGFR categories were independently associated with in-hospital mortality across EF subgroups (Figure 5B). The association between admission eGFR and in-hospital mortality was slightly stronger in the HFrEF compared with the HFmrEF and HFpEF subgroups (pinteraction = 0.045).
FIGURE 5. Associations of Admission eGFR and In-Hospital Mortality by LVEF Category.

(A) In-hospital mortality rates are displayed by admission eGFR and left ventricular ejection fraction (LVEF). (B) Risk-adjusted associations between admission eGFR and in-hospital mortality, adjusted for age, sex, race, admission systolic blood pressure, admission heart rate, body mass index, diabetes, atrial fibrillation/flutter, prior myocardial infarction, prior stroke or transient ischemic attack, chronic obstructive pulmonary disease, hospital region, hospital teaching status, and hospital area income (based on zip code tabulation area) are shown. Interaction analyses identified slightly stronger association among patients with HFrEF. Adj = adjusted; CI = confidence interval; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; OR = odds ratio; other abbreviations as in Figure 1.
QUALITY METRICS ACHIEVED BY DISCHARGE eGFR CATEGORY.
Of 365,494 patients included in the primary analytic cohort, the secondary analytic cohort to assess achievement of quality measures among patients with HFrEF excluded 194,294 individuals with EF >40% and 13,761 with missing EF data, leaving 157,439 participants from 407 sites for this analysis (Figure 1).
There was a graded, significant decrease in rates of beta-blocker prescription at discharge by eGFR category (90% of patients with eGFR ≥90 ml/min/1.73 m2 vs 79% of patients on dialysis; p < 0.001) (Central Illustration). A similar, but more marked, pattern of decrease in rates of MRA prescription by eGFR category was also noted (45% of patients with eGFR ≥90 ml/min/1.73 m2 vs 5% of patients on dialysis; p < 0.001). Whereas a similar pattern was noted with ACE inhibitor/ARB prescription across eGFR categories, rates were lowest among those with eGFR <30 ml/min/1.73 m2 (24%) and relatively higher among patients on dialysis (38%). Although ARNI prescription rates were low in the entire cohort (5.5%), there was a significant decrease in use across lower eGFR categories.
CENTRAL ILLUSTRATION. Prescription of Evidence-Based HFrEF Medical Therapies at Discharge by eGFR.
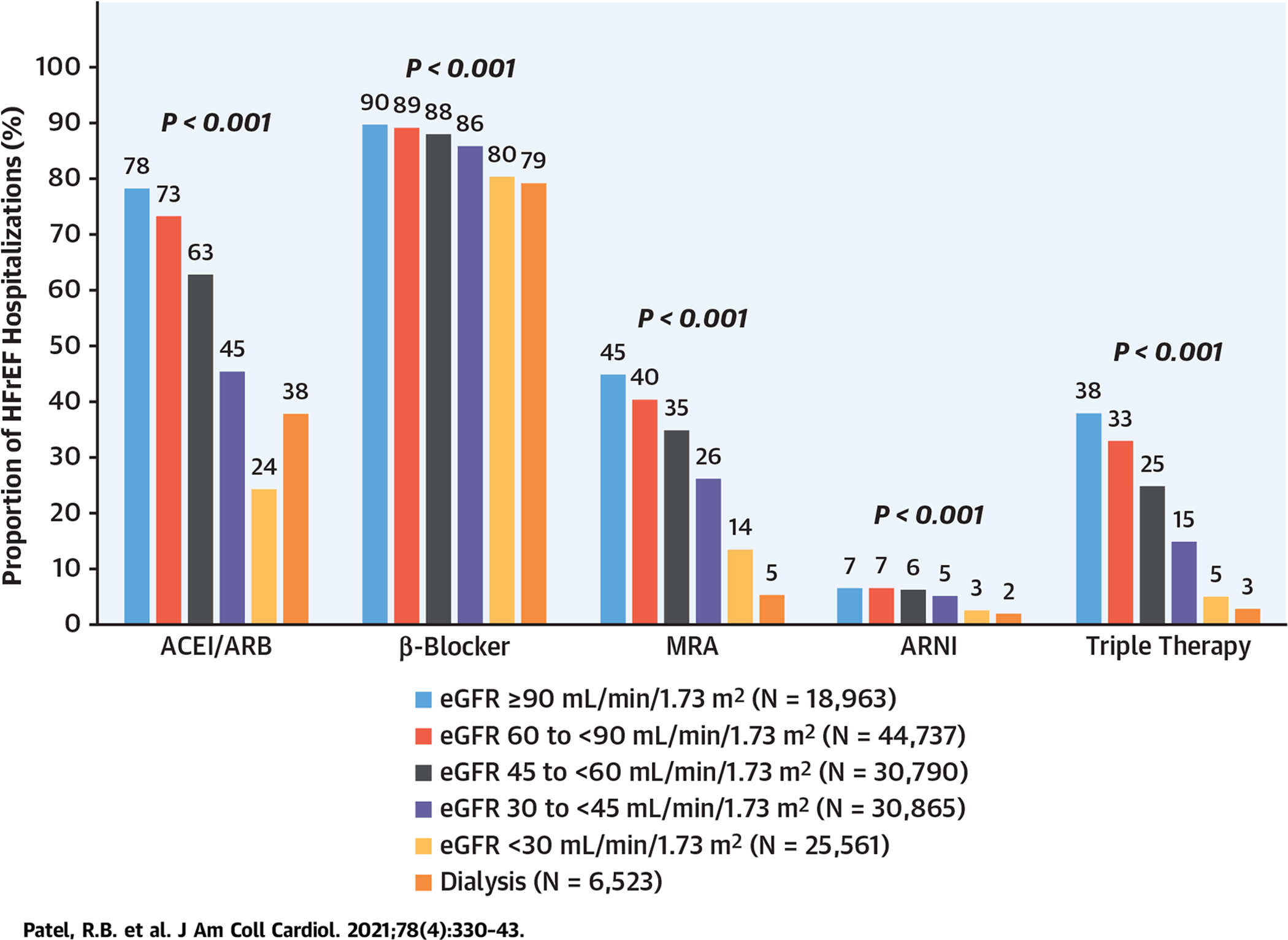
“Triple therapy” denotes prescription of 3 classes of drugs (angiotensin converting enzyme inhibitor [ACEI]/angiotensin receptor blocker [ARB]/angiotensin receptor–neprilysin inhibitor [ARNI], beta-blocker, and mineralocorticoid receptor antagonist [MRA]). eGFR = estimated glomerular filtration rate; HFrEF = heart failure with reduced ejection fraction.
In the overall cohort, 36,022 (23%) were prescribed an ACE inhibitor or ARB or ARNI along with both a beta-blocker and an MRA (ie, triple therapy). There was marked decrease in prescription rates of triple therapy with lower eGFR categories: 38%, 33%, 25%, 15%, 5%, and 3% for eGFR ≥90, 60 to 89, 45 to 59, 30 to 44, <30 ml/min/1.73 m2 and dialysis, respectively; p < 0.001. Rates of prescribing patterns by discharge eGFR categories were consistent on stratification by race/ethnicity and sex (Supplemental Tables 6, 7, 8, and 9). The rate of hydralazine/nitrate use was low in the overall cohort (0.5%). There was higher hydralazine/nitrate use with lower eGFR category, but absolute rates remained low: 0.4%, 0.3%, 0.4%, 0.5%, 0.8%, and 0.6% for eGFR ≥90, 60 to 89, 45 to 59, 30 to 44, <30 ml/min/1.73 m2 and dialysis, respectively; p < 0.001. In sensitivity analysis of participants with discharge systolic blood pressure >95 mm Hg and potassium <5.0 mEq/l, patterns of beta-blocker, ACE inhibitor/ARB, ARNI, MRA, and triple therapy (ACE inhibitor/ARB/ARNI + beta-blocker + MRA) use were consistent with less frequent use at lower discharge eGFR (Supplemental Table 10).
Lower discharge eGFR was also associated with a lower likelihood of having a post-discharge appointment made (70%, 67%, 65%, 60%, 54%, and 51% for eGFR ≥90, 60 to 89, 45 to 59, 30 to 44, <30 ml/min/1.73 m2 and dialysis, respectively; p < 0.001).
DISCUSSION
In this contemporary U.S.-based registry evaluating over 350,000 hospitalizations for HF across over 400 U.S. sites, we identify a number of key findings: 1) approximately 60% to 65% of patients have discharge eGFR below 60 ml/min/1.73 m2 and 5% are on dialysis; this high burden of kidney disease was similarly observed by sex and racial/ethnic groups; 2) patterns of kidney function at the time of discharge have remained stable from 2014 to 2019; 3) lower admission kidney function was significantly associated with higher in-hospital mortality in a graded fashion, particularly for HFrEF; and 4) evidence-based medical therapies, including ones without eGFR restrictions, are not optimally used in patients with comorbid HFrEF and CKD even at levels of eGFR where such therapies would not be contraindicated by kidney dysfunction, and could, in fact, attenuate the decline in kidney function.
Kidney disease may arise among patients with HF secondary to HF disease progression and subsequent overlapping pathophysiology (ie, cardiorenal syndrome) or as a result of shared cardiometabolic risk factors that drive both disease states in parallel (18). Regardless of mechanism, the prevalence of kidney disease is high among hospitalized adults with HF and its presence is associated with worse outcomes. In previous U.S. cohorts of hospitalized HF, the prevalence of kidney disease as defined by eGFR <60 ml/min/1.73 m2 consistently ranged between 64% and 68% (14,15,19–21). In these older cohorts, kidney dysfunction was also associated with higher in-hospital mortality (13–15). Previous data have demonstrated lower prescription rates of ACE inhibitors/ARBs and beta-blockers among patients with HFrEF with kidney disease at discharge after HF hospitalization (13–15). However, these prior studies reflect smaller samples, on less contemporary medical therapies, and used the less accurate MDRD (Modification of Diet in Renal Disease) equation for eGFR estimation (22). Our current investigation furthers the understanding of the burden and implications of kidney disease in hospitalized HF through race- and sex-stratified evaluation of a large, contemporary registry that includes data on more modern HFrEF medical therapies and leverages a more accurate estimation of eGFR for kidney disease classification. The stable prevalence of kidney disease in hospitalized HF offers insight into critical unmet needs regarding HF care. Across a 6-year time span from 2014 to 2019, the proportion of patients within each category of kidney dysfunction remained stable, and >60% of those hospitalized had eGFR <60 ml/min/1.73 m2 irrespective of left ventricular EF. These data extend data from another registry-based analysis that recently showed stably high rates of kidney disease (60% to 70%) among patients admitted for decompensated HFrEF or HFpEF from 2005 to 2014 (23).
Importantly, the CKD-EPI equation (used in the current study) and other contemporary eGFR calculators incorporate a race coefficient in GFR estimation. However, recent work has called the inclusion of race into question, suggesting that this may be 1 factor in contributing to bias and inequities in kidney health (24–27). We estimate 15% of Black participants who had eGFR 30 to 59 ml/min/1.73 m2 with traditional CKD-EPI estimation may be reclassified to an eGFR <30 ml/min/1.73 m2 if the race coefficient was removed. In our study of a higher-risk, hospitalized cohort, this relative shift was more pronounced than that observed in projections from nationally representative samples of the U.S. general population (28). Our projections suggest that removal of the race coefficient may result in substantial shifts in eGFR estimates among Black patients with HF, which could worsen existing inequities in prescription of key HF medications if clinicians are hesitant to prescribe them to patients with kidney disease. The clinical and therapeutic implications of this potential change in GFR estimation should therefore be carefully evaluated and perhaps coupled with clinician education to avoid this unintended consequence.
Previously, few therapeutic options were available to delay progression of kidney disease with HF and many early foundational therapies were thought to even accelerate kidney progression. However, more recently, sacubitril/valsartan was shown to slow eGFR decline in chronic HFrEF (29,30) and HFpEF (31) compared with renin-angiotensin system inhibitors. Whereas event rates of definitive kidney endpoints were low, for HFpEF, sacubitril/valsartan was shown to reduce the rates of progression to end-stage kidney disease by one-half, a prespecified secondary outcome. Furthermore, 2 sodium glucose co-transporter 2 inhibitors—dapagliflozin and empagliflozin—have demonstrated concordant benefits in attenuating eGFR decline during maintenance therapy (after an early, transient, and reversible eGFR dip) and reduced composite kidney outcomes in chronic HFrEF (32,33). Additionally, dapagliflozin and canagliflozin have been shown to reduce hospitalization for HF among patients with prevalent CKD (34,35). Finally, finerenone, a nonsteroidal, selective MRA that may have lower risk of hyperkalemia than spironolactone, reduced progression of kidney disease among those with CKD (12), and its effect on cardiovascular and kidney outcomes in HFpEF and HFmrEF is currently under investigation (FINEARTS-HF [Study to Evaluate the Efficacy and Safety of Finerenone on Morbidity and Mortality in Participants With Heart Failure and Left Ventricular Ejection Fraction Greater or Equal to 40%]; NCT04435626). These data support early integration and prioritization of therapies that have concordant benefits on heart and kidney health.
Consistent with previous data from ambulatory care cohorts (5,36,37), use of evidence-based medical therapies at hospital discharge was suboptimal among patients with HFrEF across the kidney function spectrum. Indeed, even among patients with HFrEF and eGFR ≥90 ml/min/1.73 m2, use of triple therapy was <40%. Use of evidence-based medical therapies was markedly lower among those with reduced kidney function. These findings are particularly notable given the high-risk nature of kidney disease for HFrEF, as we noted a stronger association of admission eGFR with in-hospital mortality in patients with HFrEF compared with those with HFmrEF and HFpEF. Whereas patients with advanced CKD were largely excluded from pivotal randomized clinical trials, available trial data suggest comparable efficacy of ACE inhibitors (38–40), ARBs (6), MRA (41), and ARNI (29) among patients with moderate CKD. The low utilization of MRAs among patients with reduced eGFR may be partially explained by contraindications to their use in current societal guidelines among patients with eGFR <30 ml/min/1.73 m2 or serum creatinine >2.5 mg/dl in men or serum creatinine >2 mg/dl in women (42). Nonetheless, we noted a stepwise reduction in MRA use with lower eGFR categories starting at eGFR levels <90 ml/min/1.73 m2, which are well above the threshold to reserve such therapies.
Although ACE inhibitors, ARBs, and ARNI therapy do not have strict contraindications by eGFR, use of such medications were significantly less common across lower eGFR categories until the dialysis stage, in which a slightly higher prescription rate was noted. The reasons for this are unknown but may be due to clinical concern for side effects (ie, hypotension, hyperkalemia, worsening kidney function), need for more frequent monitoring, or perception of greater non-HF competing risks or limited life expectancy. This clinical complexity may be particularly evident among those patients approaching dialysis initiation (43). The fluctuating and somewhat uncertain trajectory of kidney function over the course of HF hospitalization may also provide challenges to prescribing MRAs and ACE inhibitors/ARBs/ARNI therapies.
A potential barrier to the use of certain HF medical therapies among the CKD cohort is the clinical concern for hyperkalemia. The advent of novel potassium binders such as patiromer and sodium zirconium cyclosilicate may facilitate new or ongoing use of these therapies even in light of worsening CKD; their effects on clinical outcomes are currently being tested in dedicated clinical trials among at-risk patients (DIAMOND [Patiromer for the Management of Hyperkalemia in Subjects Receiving RAASi Medications for the Treatment of Heart Failure], NCT03888066; REALIZE-K [Study to Assess Efficacy and Safety of SZC for the Management of High Potassium in Patients With Symptomatic HFrEF Receiving Spironolactone], NCT04676646; OPRA-HF [Optimizing Aldosterone Receptor Antagonist Therapy by Sodium Zirconium Cyclosilicate in Heart Failure], NCT04789239). Furthermore, early introduction of therapies, such as sacubitril/valsartan and the sodium glucose co-transporter 2 inhibitors, may attenuate risks of hyperkalemia and enable new introduction or ongoing use of other elements of guideline-directed medical therapy (eg, MRA) (44–46). However, patients within lower eGFR categories were also less likely to receive prescriptions for beta-blockers, a therapy without contraindication based on kidney function or electrolytes. These findings may be explained by lower blood pressure or reduced cardiac output in these patients. Nonetheless, participants with lower eGFR had lower rates of use of all medical therapies for HFrEF even among patients with systolic blood pressure >95 mm Hg and serum potassium <5.0 mEq/l in our sensitivity analysis, suggesting that perceived risks with kidney dysfunction itself may drive underutilization of medical therapies. Overall, a risk-treatment paradox exists in the management of patients with HFrEF and comorbid CKD, such that patients with the highest mortality are treated with lesser disease-modifying medical therapies (47). As these observations may partially stem from quality-of-care gaps or medical contraindications, improving health outcomes in this high-risk population will require both broader implementation of available evidence-based strategies among eligible patients as well as ongoing identification of safe and effective novel treatment approaches in HF and reduced kidney function.
Despite being a higher risk cohort and having a greater need for more careful monitoring, patients with kidney dysfunction paradoxically were less likely to have timely post-hospital HF follow-up, which is guideline supported after hospitalization for HF. It is possible multimorbid patients face competing post-discharge priorities of multiple appointments or may have more limited access to ambulatory care. Kidney disease is also closely interlinked with race and social risk, which both powerfully influence quality of care, access, and follow-up. Targeted strategies are needed to improve transitional care for this at-risk cohort. These findings suggest that aggregate HF care may be less optimal among those with reduced kidney function across the spectrum of sex and race/ethnicity.
STUDY LIMITATIONS.
Main limitations of this analysis are the reliance on single time point measures of eGFR that may be dynamic during hospitalization for HF, which could influence medical therapies on discharge, and lack of data on post-discharge eGFR trajectory or clinical events (including progression to end-stage kidney disease). eGFR at discharge was preferred given completion of in-hospital decongestion and greater clinical stability at this time point. Additional analysis did confirm that most patients with prior histories of CKD had lower ranges of eGFR at hospital discharge. We did, however, note wide variation in discharge eGFR among patients without a reported history of CKD. These results may reflect both the variability of clinical definitions and documentation of CKD in practice. Participation in GWTG-HF is voluntary for patients and hospitals, so these findings may not be entirely generalizable to all treatment settings. Furthermore, eGFR was not available in all patients; however, minimal differences were observed in clinical profiles of patients with and without available eGFR to suggest systemic bias; instead, most variability was observed at the hospital-level, suggesting certain hospitals had incomplete reporting. Discharge potassium was missing in many but was still available in nearly 200,000 participants for detailed analysis. Dose of HFrEF medical therapies were not systematically captured in the GWTG-HF registry. Finally, given the deidentified nature of the registry, this analysis evaluated unique hospitalizations rather than unique patients, and some patients may have contributed to more than 1 hospitalization.
CONCLUSIONS
In a contemporary cohort of hospitalized HF, comorbid kidney disease appears to be highly prevalent, particularly for HFpEF, and has been stable over time. Despite higher in-hospital mortality with lower eGFR, rates of several evidence-based medications, including those without eGFR restrictions, are not optimally used in patients with comorbid HFrEF and CKD even at levels of eGFR where such therapies would not be contraindicated by kidney dysfunction and could, in fact, attenuate the decline in kidney function. Additionally, post-discharge appointment rates were significantly lower in worse kidney function categories. In HF, further efforts are required to implement strategies to delay onset and progression of CKD and mitigate risk in those with comorbid CKD.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Despite facing higher risks of mortality, patients with HFrEF and impaired kidney function are treated with lesser evidence-based medical therapies.
TRANSLATIONAL OUTLOOK:
More effective strategies are needed to implement available therapies that delay progression of CKD and reduce the risk of adverse events in patients who have concomitant HF and kidney dysfunction.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The GWTG-HF (Get With The Guidelines-Heart Failure) program is provided by the American Heart Association and sponsored, in part, by Novartis, Boehringer Ingelheim and Eli Lilly Diabetes Alliance, Novo Nordisk, Sanofi, AstraZeneca, and Bayer. This analysis, as a part of the TRANSLATE-HF research series, was supported by AstraZeneca. Dr Patel has received support from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (grant KL2TR001424). Dr Fonarow has received research funding from the NIH; and has served as a consultant for Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Medtronic, Merck, and Novartis. Dr Greene has received research support from Amgen, AstraZeneca, Bristol Myers Squibb, Merck, Novartis, and Pfizer; has served on advisory boards for Amgen, AstraZeneca, and Cytokinetics; and has served as a consultant for Amgen and Merck. Dr DeVore has received research funding through the Duke Clinical Research Institute from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, American Regent Inc., the National Heart, Lung, and Blood Institute (NHLBI), Novartis, and Patient-Centered Outcomes Research Institute; has served as a consultant for AstraZeneca; has received nonfinancial support from Amgen, Bayer, CareDx, InnaMed, LivaNova, Mardil Medical, Novartis, Procyrion, scPharmaceuticals, Story Health, and Zoll; and has received nonfinancial support from Abbott for educational activities outside the submitted work. Dr Butler has served as a consultant for Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, V-Wave Limited, and Vifor. Dr Joynt Maddox has received grants from NIH/NHLBI, NIH/National Institute on Aging, and Commonwealth Fund; has previously done U.S. Department of Health and Human Services contract work outside the submitted work; and has served on the Health Policy Advisory Committee for the Centene Corp. Dr Owens has served as a consultant for MyoKardia and Cytokinetics outside the submitted work. Dr Peterson has received grant funding from the NHLBI (grant R33HL143324-02); and has received personal fees from the American Heart Association outside the submitted work. Dr Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, NeuroTronik, Novartis, Respicardia, Sanofi Pasteur, and Theracos; and has served as a consultant for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GlaxoSmithKline, Ironwood, Merck, MyoKardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AOBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi Pasteur, DiNAQOR, Tremeau, CellProthera, and Moderna. Dr Vardeny has received funding from the NIH and the U.S. Food and Drug Administration; and has received personal fees from the American Heart Association. Dr Yancy’s spouse is employed by Abbott Labs Inc. Dr Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Relypsa, and Roche Diagnostics; has had speaker engagements with Novartis and Roche Diagnostics; and has participated on clinical endpoint committees for studies sponsored by Galmed and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ACE
angiotensin-converting enzyme
- ARB
angiotensin receptor blocker
- ARNI
angiotensin receptor–neprilysin inhibitor
- CKD
chronic kidney disease
- EF
ejection fraction
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- HFmrEF
heart failure with mid-range ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- IQR
interquartile range
- MRA
mineralocorticoid receptor antagonist
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol 2017;70:2476–86. [DOI] [PubMed] [Google Scholar]
- 2.Alter DA, Ko DT, Tu JV, et al. The average life-span of patients discharged from hospital with heart failure. J Gen Intern Med 2012;27:1171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail 2018;11:e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol 2019; 73:2365–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol 2018;72:351–66. [DOI] [PubMed] [Google Scholar]
- 6.Hillege HL, Nitsch D, Pfeffer MA, et al. , for the CHARM Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006;113:671–8. [DOI] [PubMed] [Google Scholar]
- 7.Bansal N, Katz R, Robinson-Cohen C, et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol 2017;2:314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damman K, Solomon SD, Pfeffer MA, et al. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. Eur J Heart Fail 2016; 18:1508–17. [DOI] [PubMed] [Google Scholar]
- 9.Beldhuis IE, Myhre PL, Claggett B, et al. Efficacy and safety of spironolactone in patients with HFpEF and chronic kidney disease. J Am Coll Cardiol HF 2019;7:25–32. [DOI] [PubMed] [Google Scholar]
- 10.Brenner BM, Cooper ME, de Zeeuw D, et al. , for the RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9. [DOI] [PubMed] [Google Scholar]
- 11.Lewis EJ, Hunsicker LG, Bain RP, et al. , for the Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329: 1456–62. [DOI] [PubMed] [Google Scholar]
- 12.Bakris GL, Agarwal R, Anker SD, et al. , for the FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–29. [DOI] [PubMed] [Google Scholar]
- 13.Ezekowitz J, McAlister FA, Humphries KH, et al. , for the APPROACH Investigators. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol 2004;44:1587–92. [DOI] [PubMed] [Google Scholar]
- 14.Heywood JT, Fonarow GC, Costanzo MR, et al. , for the ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13:422–30. [DOI] [PubMed] [Google Scholar]
- 15.Patel UD, Hernandez AF, Liang L, et al. Quality of care and outcomes among patients with heart failure and chronic kidney disease: a Get With the Guidelines–Heart Failure Program study. Am Heart J 2008;156:674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol 2006;5:179–86. [DOI] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH, Tighiouart H, et al. , for the CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation 2018;138:929–44. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin 2008;4:387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA 2003;289: 2517–24. [DOI] [PubMed] [Google Scholar]
- 21.Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med 2005; 165:2069–76. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita K, Mahmoodi BK, Woodward M, et al. , for the Chronic Kidney Disease Prognosis Consortium. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012;307:1941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey A, Vaduganathan M, Arora S, et al. Temporal trends in prevalence and prognostic implications of comorbidities among patients with acute decompensated heart failure: the ARIC Study Community Surveillance. Circulation 2020; 142:230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA 2019;322:113–4. [DOI] [PubMed] [Google Scholar]
- 25.Norris KC, Eneanya ND, Boulware LE. Removal of race from estimates of kidney function: first, do no harm. JAMA 2021;325:135–7. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Tighiouart H, Titan SM, Inker LA. Estimation of glomerular filtration rate with vs without including patient race. JAMA Intern Med 2020;180:793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diao JA, Wu GJ, Taylor HA, et al. Clinical implications of removing race from estimates of kidney function. JAMA 2021;325:184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bragg-Gresham J, Zhang X, Le D, et al. Prevalence of chronic kidney disease among black individuals in the US after removal of the Black race coefficient from a glomerular filtration rate estimating equation. JAMA Netw Open 2021;4: e2035636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. J Am Coll Cardiol HF 2018;6:489–98. [DOI] [PubMed] [Google Scholar]
- 30.McMurray JJ, Packer M, Desai AS, et al. , for the PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 31.Mc Causland FR, Lefkowitz MP, Claggett B, et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 2020;142:1236–45. [DOI] [PubMed] [Google Scholar]
- 32.Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation 2021;143:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zannad F, Ferreira JP, Pocock SJ, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation 2021; 143:310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. , for the DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383:1436–46. [DOI] [PubMed] [Google Scholar]
- 35.Perkovic V, Jardine MJ, Neal B, et al. , for the CREDENCDE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 36.Brunner-La Rocca HP, Linssen GC, Smeele FJ, et al. , for the CHECK-HF Investigators. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK-HF registry. J Am Coll Cardiol Heart Fail 2019;7:13–21. [DOI] [PubMed] [Google Scholar]
- 37.Komajda M, Anker SD, Cowie MR, et al. , for the QUALIFY Investigators. Physicians’ adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail 2016; 18:514–22. [DOI] [PubMed] [Google Scholar]
- 38.Bowling CB, Sanders PW, Allman RM, et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD Treatment trial. Int J Cardiol 2013;167:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed A, Love TE, Sui X, Rich MW. Effects of angiotensin-converting enzyme inhibitors in systolic heart failure patients with chronic kidney disease: a propensity score analysis. J Card Fail 2006;12:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swedberg K, Eneroth P, Kjekshus J, et al. , for the CONSENSUS Trial Study Group. Effects of enalapril and neuroendocrine activation on prognosis in severe congestive heart failure (follow-up of the CONSENSUS trial). Am J Cardiol 1990;66: 40D–4D; discussion 44D–5D. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira JP, Abreu P, McMurray JJV, et al. Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS-HF trial. Eur J Heart Fail 2019;21:345–51. [DOI] [PubMed] [Google Scholar]
- 42.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 43.Hein AM, Scialla JJ, Edmonston D, Cooper LB, DeVore AD, Mentz RJ. Medical management of heart failure with reduced ejection fraction in patients with advanced renal disease. J Am Coll Cardiol HF 2019;7:371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen L, Kristensen SL, Bengtsson O, et al. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA-HF. J Am Coll Cardiol HF 2021;9: 254–64. [DOI] [PubMed] [Google Scholar]
- 45.Desai AS, Vardeny O, Claggett B, et al. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol 2017;2: 79–85. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira JP, Zannad F, Pocock SJ, et al. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: EMPEROR-Reduced. J Am Coll Cardiol 2021;77:1397–407. [DOI] [PubMed] [Google Scholar]
- 47.Peterson PN, Rumsfeld JS, Liang L, et al. , for the American Heart Association Get With The Guidelines–Heart Failure Program. Treatment and risk in heart failure: gaps in evidence or quality? Circ Cardiovasc Qual Outcomes 2010;3: 309–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


