Abstract
Here we report the activity of liposomes comprising egg phosphatidylcholine (PC) and stearylamine (SA) against Leishmania donovani parasites. Both promastigotes and intracellular amastigotes in vitro and in vivo were susceptible to SA-PC liposomes. A single dose of 55 mg of SA-PC liposomes/animal could significantly reduce the hepatic parasite burden by 85 and 68% against recent and established experimental visceral leishmaniasis, respectively, suggesting their strong therapeutic potential.
Of the various clinical forms of leishmaniasis, visceral leishmaniasis, or kala-azar, caused by Leishmania donovani, is the most severe and often is fatal if untreated. The pentavalent antimonial agents sodium stibogluconate and meglumine antimoniate, although moderately toxic, have remained the standard treatment for visceral leishmaniasis since the 1940s (5, 9). However, there are increasing reports of resistance to antimonial agents and high rates of relapses (17). In such cases, patients are treated with pentamidine or amphotericin B. Although powerful antileishmanial agents, these drugs remain a second line of defense because of their severe toxicities (3). However, the antimonial agents, pentamidine, and amphotericin B, when encapsulated in liposomes, are more effective for the treatment of leishmaniasis and are less toxic than the free drugs (4, 6, 15). Reduced toxicity and an improved therapeutic index with liposomal formulations, especially of amphotericin B, represent an alternative for the treatment of human visceral leishmaniasis (6, 20).
It was reported earlier that liposomes consisting of stearylamine (SA) and phosphatidylcholine (PC) are cytotoxic toward Trypanasoma cruzi (23), T. brucei gambiense (18), and Toxoplasma gondii (19). This remarkable effect of SA-PC liposomes, although not clearly understood, possibly occurs through interaction of the positively charged lipids with the negatively charged parasite membrane. Here we report the leishmaniacidal activity of SA-PC liposomes on L. donovani promastigotes and the antileishmanial effect of these liposomes (free of drug) on amastigotes in vitro and in vivo in murine models of visceral leishmaniasis.
Liposomes were prepared with egg lecithin (PC) (Centre for Biochemical Technology, Delhi, India) and SA (Fluka, Buchs, Switzerland) at a molar ratio of 7:2 as described earlier (1). Briefly, the thin dry film was dispersed in 20 mM phosphate-buffered saline (PBS) and vortexed, and the suspension was sonicated for 60 s in an ultrasonicator. The amount of liposomes, expressed as total lipid content, was the sum of the SA and PC contents. Liposomes containing only PC were prepared as described above with equivalent amounts of PC.
To study the effect of SA-PC liposomes on L. donovani promastigotes, freshly transformed promastigotes of L. donovani AG83 (106/450 μl of medium 199 containing 10% fetal bovine serum) were incubated with 50 μl of PBS or various concentrations of 22 mol% SA-PC liposomes at 37°C for 60 min, and their viability was determined microscopically by erythrocin B stain exclusion. More than 99% of the parasites were killed when incubated with 132 μg of SA-PC liposomes per ml, and very weak activity was detected at 6.6 μg of lipid per ml (Table 1). The number of viable promastigotes treated with similar amounts of PC liposomes (data not shown) as well as without liposomes appeared to be unchanged.
TABLE 1.
Effects of SA-PC liposome concentrations on L. donovani promastigotesa
| Treatment and final concn(μg/ml) |
Promastigote viability (%) |
|---|---|
| Control | 100 |
| SA-PC liposomes | |
| 6.6 | 94.0 |
| 13.2 | 53.5 |
| 33.0 | 27.3 |
| 66.0 | 7.9 |
| 132.0 | 0.5 |
Promastigotes (106/450 μl) were incubated with various concentrations of 22 mol% SA-PC liposomes for 1 h at 37°C.
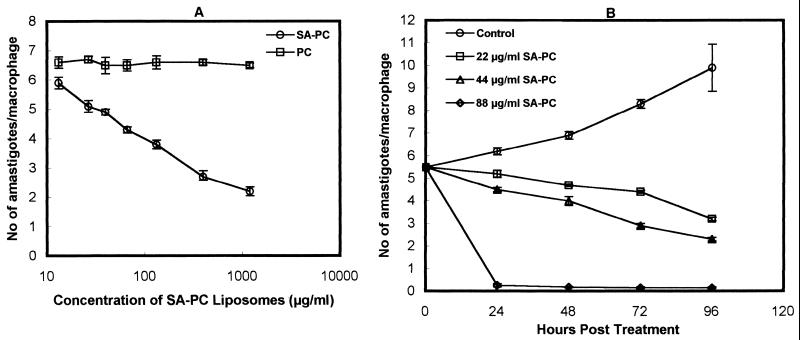
In vitro parasite killing by SA-PC liposomes was investigated with murine peritoneal macrophages (106 cells) infected with L. donovani promastigotes at a ratio of 1:10 at 37°C. Following infection for 3 h, the macrophages were treated with graded dosages of PC and 22 mol% SA-PC liposomes for 3 h. While 66% of the intracellular parasites were killed by 1,188 μg of SA-PC liposomes per ml, in comparison to untreated infected macrophages, no killing due to PC liposomes at similar concentrations was seen (Fig. 1A). The kinetics of antileishmanial activity of 22, 44, and 88 μg of SA-PC liposomes per ml (doses close to the 50% effective dose and lower) on infected macrophages revealed significant suppression of the parasites with increasing concentrations of lipid, with 88 μg of SA-PC liposomes per ml causing 95% killing of intracellular amastigotes in 24 h (Fig. 1B). Similar concentrations of PC liposomes had no significant effect on parasite multiplication.
FIG. 1.
(A) Effect of SA-PC liposomes on the survival of L. donovani amastigotes in mouse peritoneal macrophages. Cells were infected with L. donovani promastigotes and incubated with various concentrations (13.2 to 1,188 μg/ml) of SA-PC liposomes or PC liposomes prepared with equivalent concentrations of PC. Infected control macrophages contained 6.5 ± 0.26 amastigotes/macrophage. (B) Microbicidal effect of SA-PC liposomes on L. donovani-infected macrophages as a function of time. Macrophages were infected with L. donovani promastigotes and incubated with 22, 44, and 88 μg of SA-PC liposomes per ml for 0 to 96 h. Control infected cells were treated only with media. The results, expressed as mean ± standard deviation (n = 3), are those of one experiment representative of three performed.
To examine the therapeutic efficacy of SA-PC liposomes, female BALB/c mice (4 to 6 weeks old) were each infected intravenously (i.v.) with 2.5 × 107 promastigotes, freshly transformed in a biphasic medium consisting of medium 199 and blood agar. After 1 h of infection, the mice were randomly assigned to five groups; the first group was injected i.v. with PBS and used as a control group. The second group was treated i.v. with a single dose of 22 mg of 22 mol% SA-PC liposomes/animal (880 mg/kg of body weight). The third group was similarly treated with a single dose of 55 mg of SA-PC liposomes/animal (2,200 mg/kg of body weight). The fourth and the fifth groups were injected with single doses of 20 and 50 mg of only PC liposomes/animal (800 and 2,000 mg/kg of body weight), respectively. Three mice from each group were sacrificed on days 30 and 60 postinfection, and the liver parasite burden was determined as Leishman-Donovan units (the number of amastigotes per 1,000 cell nuclei × organ weight [in milligrams]) (1). In another set of experiments, BALB/c mice were first infected for 8 weeks and then given single- and multiple-dose liposome therapy. For the single-dose therapy, the mice were injected with 55 mg of SA-PC liposomes/animal; in the multiple-dose experiment, the animals received four doses of 22 mg of SA-PC liposomes/animal/day on alternate days. In parallel, groups of mice received a single dose of 50 mg of PC liposomes or four doses of 20 mg of PC liposomes/animal/day or PBS as a control. Mice were sacrificed on days 1, 15, and 30 posttreatment, and the levels of amastigotes in the liver and the spleen were estimated.
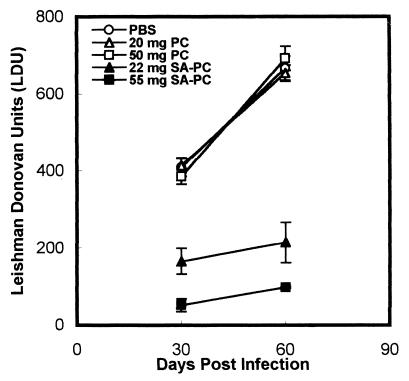
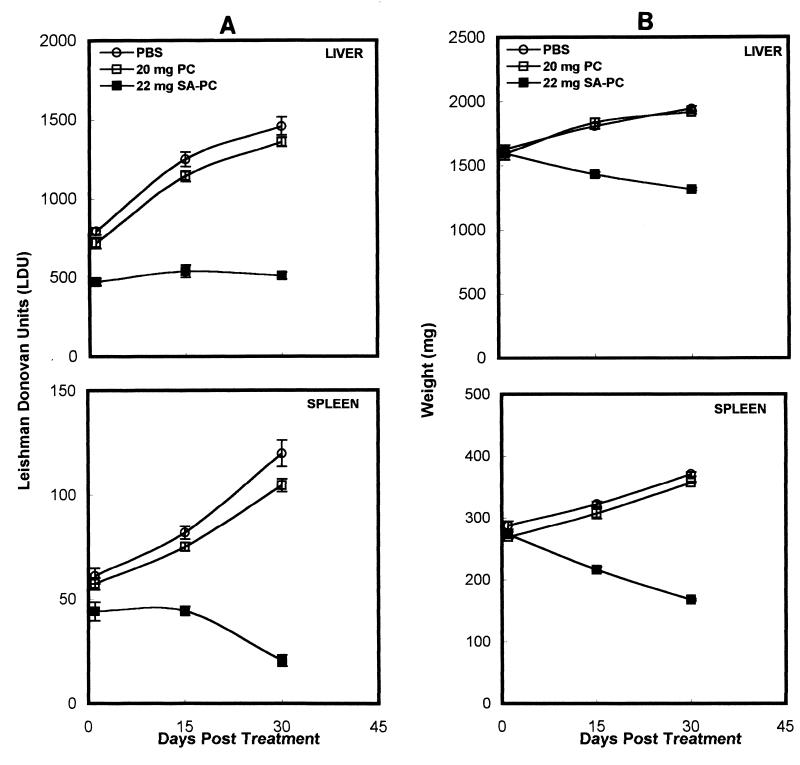
Single doses of both 22 and 55 mg of 22 mol% SA-PC liposomes induced a significant reduction in the liver parasite burden at days 30 and 60 postinfection, while PC liposomes (20 or 50 mg) alone had no effect (Fig. 2). BALB/c mice first infected for 8 weeks and then treated with a single dose of 55 mg of SA-PC liposomes showed significant reductions in the liver parasite burden—36% (P < 0.001), 53% (P < 0.001), and 68% (P value much less than 0.001)—and the splenic parasite burden—23% (P < 0.01), 47.7% (P < 0.001), and 79% (P value much less than 0.001)—at days 1, 15, and 30 posttreatment, respectively, compared to controls (Fig. 3). Correspondingly, on days 15 and 30 posttreatment, there were reductions in the weights of the livers—20.8% (P < 0.001) and 32.5% (P value much less than 0.001)—and spleens—33% (P < 0.001) and 55.5% (P value much less than 0.001)—in the SA-PC liposome-treated mice compared to controls (Fig. 3). A similar dose of PC liposome treatment had no significant effect (P > 0.05) on hepatic and splenic parasite burdens and hepatosplenomegaly in comparison to the results for controls. Treatment of BALB/c mice with four doses of 22 mg of SA-PC liposomes/animal on alternate days induced the suppression of hepatic and splenic parasites to levels not significantly different from those induced by a single dose of 55 mg of SA-PC liposomes/animal (data not shown).
FIG. 2.
Long-term activity of SA-PC liposomes against recent L. donovani infection. Mice were infected i.v. with freshly transformed promastigotes and treated i.v. 1 h later with 22 or 55 mg of SA-PC liposomes or with 20 or 50 mg of PC liposomes without SA. Control infected animals were injected with PBS. The liver parasite burden, expressed as Leishman-Donovan units, was estimated at days 30 and 60 postinfection. Data shown are the mean ± standard error from one experiment representative of two performed.
FIG. 3.
Antileishmanial activity of a single dose of SA-PC liposomes in established experimental visceral leishmaniasis. BALB/c mice infected for 8 weeks were treated i.v. with 55 mg of SA-PC liposomes or with 50 mg of PC liposomes. Control infected animals were injected with PBS. Mice were sacrificed 1, 15, and 30 days after completion of treatment. The liver and spleen parasite burdens, expressed as Leishman-Donovan units (A), and weights of the liver and the spleen (B) were measured. Data shown are the mean ± standard error from one experiment representative of two performed.
The toxicity of SA-PC liposomes for normal murine macrophages was investigated by measuring lactate dehydrogenase activity in the culture medium of liposome-treated macrophages (8). SA-PC liposomes at 1,188 and 396 μg of lipid per ml imparted 16.6 and 14.4% toxicity to normal macrophages, respectively, while 132 μg of SA-PC liposomes per ml showed less than 1% toxicity, as determined by the release of lactate dehydrogenase. Lower concentrations of SA-PC vesicles (66 to 13.2 μg/ml) had no toxic effect on in vitro-cultured macrophages.
Estimation of specific levels of enzymes, such as serum alkaline phosphatase and glutamine pyruvate transaminase (SGPT), related to normal liver function, at days 15 and 30 after injection of a single dose of 50 mg of PC liposomes or 55 mg of SA-PC liposomes revealed levels of alkaline phosphatase close to the normal levels. The level of SGPT increased with SA-PC treatment by day 15. This increase in SGPT, which was just above the normal range (5 to 35 U/ml), was reduced to the normal levels by day 30. Blood parameters, such as erythrocyte and leukocyte levels and hemoglobin content, and histological examinations of spleen and liver indicated the absence of toxicity with SA-PC liposomes under the given conditions (data not shown).
In this paper, we report on the remarkable in vitro and in vivo activities of positively charged liposomes against L. donovani parasites. Liposomes prepared with PC and SA had strong cytolytic activity toward L. donovani promastigotes and intracellular amastigotes, in comparison to the results obtained with similar treatment with PC liposomes and control treatment. The leishmaniacidal effect of these vesicles could be extended to in vivo infections with L. donovani, with a single dose of 55 mg of SA-PC lipid showing effective therapeutic activity for recent as well as established infections. The decline in the liver and splenic parasite burdens corresponded with a significant reduction in hepatomegaly and splenomegaly. This dose of SA-PC liposomes, however, was not toxic to the host.
The leishmaniacidal activity of SA-PC liposomes for intracellular amastigotes may be due to their preferential uptake by macrophages (12, 14) followed by their cytotoxic action on the parasites in the vacuoles. Leishmania parasites reside in the macrophages of the liver, spleen, and bone marrow, cells which are also responsible for the clearance of liposomes from the blood. The affinity of positively charged liposomes for the macrophages of the reticuloendothelial system is enhanced through interactions with serum (12), to which the liposomes are delivered, probably through the endocytic pathway. The adsorption or binding of liposomes to macrophages may be facilitated through nonspecific, electrostatic interactions of positively charged liposomes with the negatively charged cell membrane (10), followed by endocytosis (13, 22). Endosomes fuse with lysosomes, which then fuse with parasitophorous vacuoles, bringing liposomes into contact with parasites (2). While the mechanism of killing of parasites by SA-PC liposomes is not clear, it most probably occurs through direct interactions of the liposomes with the parasites, which could occur only if the SA-PC liposomes bypassed the fusion of the vesicles with the cells. Although cationic liposomes containing a neutral helper lipid, dioleoyl phosphatidylethanolamine, fuse with anionic endosomes (21), vesicles without dioleoyl phosphatidylethanolamine are pH resistant, and fusion with cells rarely occurs (22). Rather than fusing, it is possible that SA-PC liposomes persist for a long time and induce intracellular amastigote killing (Fig. 1 and 3). The interaction of liposomes with leishmanial parasites may occur through sialic acid (16) and lipophosphoglycan (7, 11), surface membrane components which contribute considerably to the negative charge.
In summary, the leishmaniacidal activity of SA-bearing positively charged liposomes and their low toxicity toward host cells warrant further investigation. Inclusion of antileishmanial drugs in these vesicles may target a disease synergistically, providing a new way to study liposome-mediated therapy of leishmaniasis.
Acknowledgments
Financial assistance from the Department of Science and Technology (DST), Government of India, is gratefully acknowledged. T.D. is a junior research fellow of DST, K.A. is a research associate of CSIR, and F.A. was a senior research fellow of CSIR.
REFERENCES
- 1.Afrin F, Ali N. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect Immun. 1997;65:2371–2377. doi: 10.1128/iai.65.6.2371-2377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alving C R. Delivery of liposome-encapsulated drugs to macrophages. Pharmacol Ther. 1983;22:407–424. doi: 10.1016/0163-7258(83)90010-4. [DOI] [PubMed] [Google Scholar]
- 3.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 4.Black C D V, Watson G J, Ward R J. The use of Pentostam liposomes in the chemotherapy of experimental leishmaniasis. Trans R Soc Trop Med Hyg. 1977;71:550–552. doi: 10.1016/0035-9203(77)90155-9. [DOI] [PubMed] [Google Scholar]
- 5.Bryceson A D M. Therapy in man. In: Killick P W, Kendrick R, editors. Leishmaniasis in biology and medicine. London, England: Academic Press Ltd.; 1987. pp. 848–907. [Google Scholar]
- 6.Davidson R N, Croft S L, Scott A, Miani M, Moody A H, Bryceson A D M. Liposomal amphotericin B in drug resistant visceral leishmaniasis. Lancet. 1991;337:1061–1062. doi: 10.1016/0140-6736(91)91708-3. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer D M. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol. 1977;41:341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- 8.Filion M C, Philips N C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim Biophys Acta. 1997;1329:345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 9.Herwaldt B L, Berman J D. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg. 1992;46:296–306. doi: 10.4269/ajtmh.1992.46.296. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Li S. Liposomal gene delivery: a complex package. Nat Biotechnol. 1997;15:620–621. doi: 10.1038/nbt0797-620. [DOI] [PubMed] [Google Scholar]
- 11.King D L, Chang Y D, Turco S J. Cell surface lipophosphoglycan of Leishmania donovani. Mol Biochem Parasitol. 1987;24:47–53. doi: 10.1016/0166-6851(87)90114-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Liu D. Serum independent liposome uptake by mouse liver. Biochim Biophys Acta. 1996;1278:5–11. doi: 10.1016/0005-2736(95)00196-4. [DOI] [PubMed] [Google Scholar]
- 13.Miller C R, Bondurant B, McLean S D, McGovern K A, O'Brien D F. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Fujiwara H, Hamaoka T, Mayumi T. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem Biophys Res Commun. 1997;240:793–797. doi: 10.1006/bbrc.1997.7749. [DOI] [PubMed] [Google Scholar]
- 15.New R R C, Chance M L, Thomas S C, Peters W. Anti-leishmanial activity of antimonials trapped in liposomes. Nature. 1978;272:55–56. doi: 10.1038/272055a0. [DOI] [PubMed] [Google Scholar]
- 16.Pimenta P F P, de Souza W. Leishmania mexicana amazonensis: surface charge of amastigote and promastigote forms. Exp Parasitol. 1983;56:194–206. doi: 10.1016/0014-4894(83)90063-2. [DOI] [PubMed] [Google Scholar]
- 17.Sundar S, Rosenkaimer F, Murray H W. Successful treatment of refractory visceral leishmaniasis in India using antimony plus interferon-γ. J Infect Dis. 1994;170:659–662. doi: 10.1093/infdis/170.3.659. [DOI] [PubMed] [Google Scholar]
- 18.Tachibana H, Yoshihara E, Kaneda Y, Nakae T. In vitro lysis of the bloodstream forms of Trypanosoma brucei gambiense by stearylamine-bearing liposomes. Antimicrob Agents Chemother. 1988;32:966–970. doi: 10.1128/aac.32.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachibana H, Yoshihara E, Kaneda Y, Nakae T. Protection of Toxoplasma gondii-infected mice by stearylamine-bearing liposomes. J Parasitol. 1990;76:352–355. [PubMed] [Google Scholar]
- 20.Torre-Cisneros J, Villanueva J L, Kindelan J M, Jurado R, Sanchez-Guijo P. Successful treatment of antimony-resistant visceral leishmaniasis with liposomal amphotericin B in patients infected with human immunodeficiency virus. Clin Infect Dis. 1993;17:625–627. doi: 10.1093/clinids/17.4.625. [DOI] [PubMed] [Google Scholar]
- 21.Vidal M, Hoekstra D. In vitro fusion of reticulocyte endocytic vesicles with liposomes. J Biol Chem. 1995;270:17823–17829. doi: 10.1074/jbc.270.30.17823. [DOI] [PubMed] [Google Scholar]
- 22.Woodle M C, Lasic D D. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 23.Yoshihara E, Tachibana H, Nakae T. Trypanocidal activity of stearylamine-bearing liposomes in vitro. Life Sci. 1987;40:2153–2159. doi: 10.1016/0024-3205(87)90005-1. [DOI] [PubMed] [Google Scholar]