Abstract
Background
Both midline catheters (MCs) and peripherally inserted central catheters (PICCs) can cause venous thromboembolism (VTE), but the prevalence associated with each is controversial.
Objective
To compare the risk of VTE between MCs and PICCs with a systematic review and meta‐analysis.
Methods
The Web of Science Core Collection, PubMed, Scopus, Embase, the Cochrane Library and ProQuest were searched from inception to January 2020. All studies comparing the risk of VTE between MCs and PICCs were included. Selected studies were assessed for methodological quality using the Downs and Black checklist. Two authors independently assessed the literature and extracted the data. Any different opinion was resolved through third‐party consensus. Meta‐analyses were conducted to generate estimates of VTE risk in patients with MCs versus PICCs, and publication bias was evaluated with RevMan 5.3.
Results
A total of 86 studies were identified. Twelve studies were recruited, involving 40,871 patients. The prevalence of VTE with MCs and PICCs was 3.97% (310/7806) and 2.29% (758/33065), respectively. Meta‐analysis showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.53, 95% CI: 1.33–1.76, p < .00001). Subgroup analyses by age showed that the prevalence of VTE with MCs was higher than that with PICCs in the adult group (RR=1.75, 95% CI: 1.38–2.22, p < .00001), and higher than that with PICCs in the other subgroups (RR=1.42, 95% CI: 1.19–1.69, p = .0001). Subgroup analyses by nation showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.50, 95% CI: 1.30–1.73, p < .00001) in US subgroup and higher than that with PICCs (RR=2.87, 95% CI: 1.24–6.65, p = .01) in the other nations. The sensitivity analysis shows that the results from this meta‐analysis are robust and all studies have no significant publication bias.
Conclusions
This study provides the first systematic assessment of the risk of VTE between MCs and PICCs. MCs are associated with a higher risk of VTE than PICCs in all patients and adults. The findings of this study have several important implications for future practice. However, the risk of VTE between MCs and PICCs in children is unclear.
Keywords: complication, intravenous therapy, midline catheter, peripherally inserted central catheter, systematic review, venous thromboembolism
Essentials.
The prevalence of venous thromboembolism (VTE) associated with midline catheters (MCs) and peripherally inserted central catheters (PICCs) is controversial.
The risk of VTE was compared between MCs and PICCs with a systematic review and meta‐analysis.
This study provides the first systematic assessment of the risk of VTE between MCs and PICCs.
MCs are associated with a higher risk of VTE than PICCs in all patients and adults.
1. INTRODUCTION
Venous thromboembolism (VTE) is a common complication in intravenous therapy. VTE is a potentially life‐threatening condition that includes deep vein thrombosis (DVT) and pulmonary embolism (PE). The predominant symptoms of DVT include pain, tenderness and swelling of the involved limb, while those of PE include dyspnoea, tachypnoea and pleuritic chest pain (Clay et al.,2018). VTE is a cause of significant economic burden in Europe (Cohen et al.,2007). Some studies have indicated the costs of VTE in the European Union were 1,800 euro after 3 months and 3,200 euro after 1 year, and in the United States, the costs of the initial VTE were approximately $3,000–9,500, $5,000 over 3 months, $10,000 after 6 months and $33,000 after 1 year, which represent a considerable impact on healthcare systems (Ruppert et al.,2011). VTE is related to vascular access devices (VADs). The prevalence of VTE caused by various VADs is different.
A midline catheter (MC) is approximately a 3‐ to 8‐inches long, thin, soft tube that is inserted in the antecubital area, and the tip of this catheter is at or below the axillary vein. The prevalence of VTE with MCs has been reported in many studies to be 0%–11.88% (Chopra et al.,2019; Lisova et al.,2018). A peripherally inserted central catheter (PICC) is a form of intravenous access that is inserted in a peripheral vein such as a vein in the arm or saphenous vein, and the tip reaches the superior vena or right atrium and thus becomes a central catheter. PICCs are utilized to obtain central venous access. For example, it may be used for long chemotherapy regimens, extended antibiotic therapy, total parenteral nutrition, or for the administration of substances that should not be peripherally administered. In some studies, the prevalence of VTE in PICCs was 0.5%‐13% (Al‐Asadi et al.,2019; Dai et al.,2019; Dhir et al.,2019; Jacques et al.,2018; Jumani et al.,2013; Kang, et al., 2017; Kang, et al., 2017; Noonan et al.,2018; Taxbro et al.,2019).
Many studies have shown that the prevalence of VTE in MCs and PICCs is controversial. In MCs and PICCs, which VADs leads to higher VTE prevalence? To provide evidence for the selection of appropriate VADs for intravenous therapy, the risk of VTE was compared between MCs and PICCs with a systematic review and meta‐analysis.
2. METHODS
2.1. Literature search
We followed the preferred reporting item for systematic reviews and meta‐analyses (PRISMA) guidelines in conducting this systematic review and meta‐analysis (Moher et al.,2009). We performed a serial literature search for English and non‐English papers during January 2020. The Web of Science Core Collection (Science Citation Index Expanded: 1900‐present; Social Sciences Citation Index: 1900‐present; Arts & Humanities Citation Index: 1975‐present; Conference Proceedings Citation Index‐ Science: 1996‐present; Conference Proceedings Citation Index‐ Social Science & Humanities: 1996‐present; Emerging Sources Citation Index: 2015‐present), PubMed (inception‐present), Scopus (inception‐present), Embase (inception‐present), the Cochrane Library (inception‐present) and ProQuest (inception‐present) were searched. We used Boolean logic with search terms including “midline catheter*,” “midline venous catheter*,” “midline peripheral catheter*,” “medium‐term intravenous access*,” “peripherally inserted central catheter*,” “PICC line,” “PICC,” “percutaneous indwelling central catheter*,” “peripherally inserted central venous catheter*,” “phlebothrombo*,” “venous thrombus*,” “venous thrombo*,” “deep vein thrombo*” and “pulmonary embolism*.” To search for all terms that begin with a word, enter the word followed by an asterisk. Box 1 provides a detailed search strategy for the Web of Science Core Collection.
BOX 1. Search strategy in the Web of Science Core Collection.
#1 TOPIC: (midline catheter*) OR TOPIC: (midline venous catheter*) OR TOPIC: (midline peripheral catheter*) OR TOPIC: (medium‐term intravenous access*)
#2 TOPIC: (peripherally inserted central catheter*) OR TOPIC: (peripherally inserted central venous catheter*) OR TOPIC: (percutaneous indwelling central catheter*) OR TOPIC: (PICC line) OR TOPIC: (PICC)
#3 TOPIC: (phlebothrombos*) OR TOPIC: (thrombus*) OR TOPIC: (venous thrombo*) OR TOPIC: (deep vein thrombo*) OR TOPIC: (pulmonary embolism*)
#4 #1 AND #2 AND #3
2.2. Study eligibility and selection criteria
Two authors independently determined study eligibility. Any differences in opinion about eligibility were resolved through another author as a third‐party consensus. Studies were included if they compared the complication of VTE between PICCs and MCs. Studies were excluded if they (1) were case reports, reviews, commentaries or studies that did not report the prevalence of VTE; (2) were non‐human studies; (3) were secondary research; (4) included several participants <10; (5) were a duplicate report; and (6) reported incomplete data, and the relevant data were not available.
2.3. Definition of variables and outcomes
PICCs was defined as catheters inserted in the basilic, cephalic or brachial veins of the upper extremities with tips that terminated in the superior vena or right atrium. MCs were defined as a typical 8‐ to 20‐cm long catheter and placed peripherally into the antecubital fossa or upper arm, with the tip located at or below the axillary vein. The primary outcome was the occurrence of VTE after MCs or PICCs placement. VTE included deep vein thrombosis (DVT) or pulmonary embolism (PT). DVT is a medical condition that occurs when a blood clot forms in a deep vein. These clots are developed in the deep vein of the arm (brachial, axillary, subclavian or internal jugular veins) and detected by compression ultrasonography, venography or CT scan. The occurrence of PE was based on reports of diagnosis in each study.
2.4. Data abstraction and validity assessment
Data were independently collected from all included studies on a template adopted from the Cochrane collaboration (Li et al., 2019). For all studies, we extracted author, publication year, study design, study location, study period, population, study indicator, number of MCs and PICCs, and number of VTE.
2.5. Quality of included papers assessment
The quality of the included studies was independently assessed by two authors. Because retrospective cohort studies and randomized controlled trials met the inclusion criteria, the quality of included papers was assessed according to the checklist for measuring study quality developed by Downs and Black (Downs & Black, 1998). This tool included 5 sections that included reporting (10 questions, total score of 11), external validity (3 questions, total score of 3), internal validity or bias (7 questions, total score of 7), internal validity or confounding (6 questions, total score of 6), power (1 question, total score of 5). Quality assessment of the studies was determined by the following cut‐off points: excellent (≥26), good (20–25), fair (15–19) and poor (≤14) (Ray‐Barruel et al., 2019). An overall quality score was assigned to individual studies.
2.6. Statistical analysis
The meta‐analyses were conducted using Review Manager software, version 5.3 (https://community.cochrane.org/help/tools‐and‐software/revman‐5). Dichotomous outcomes eligible in each study are reported as a risk ratio (RR) with an estimated 95% confidence interval (CI). Continuous outcomes are shown as the weighted mean difference (WMD) with the 95% CI, which were calculated from the mean, standard deviation (SD), P‐value and sample size in each study. Heterogeneity was assessed using Higgins I2, which evaluates the percentage of total variation across studies that were due to heterogeneity rather than by chance: 0%≤I2<25%, 25%≤I2<50%, 50%≤I2<75% and 75%≤I2 indicated no heterogeneity, low heterogeneity, moderate heterogeneity and severe heterogeneity, respectively. Thus, if I2>50%, which was considered to reflect substantial heterogeneity, a random effects model was used. If I2≤50%, which was considered to reflect no heterogeneity, a fixed effects model was employed. The chi‐square tests were also used to evaluate the heterogeneity: p <.1 indicates heterogeneity, while p >.1 indicates no heterogeneity. A p <.05 was considered statistically significant. The publication biases were judged by funnel plots.
2.7. IRB approval
This meta‐analysis study was approved by the institutional review board.
3. RESULTS
3.1. Eligible studies
A total of 86 studies were identified, and 12 duplicated articles were excluded. Fifty‐six studies, including case series (n = 6), reviews (n = 31), duplicate reports (n = 3) and other forms of publication (n = 16), were excluded after screening the title and abstract. After assessment of full‐text articles for eligibility, an additional six studies were excluded due to the following criteria: non‐thrombosis endpoint (n = 3), review (n = 1), commentary (n = 1) and no control group (n = 1). Finally, 10 comparative cohort studies and two RCTs were included in the present meta‐analysis. A total of 12 studies were selected for data extraction. The flow chart of the study selection is summarized in Figure 1.
FIGURE 1.
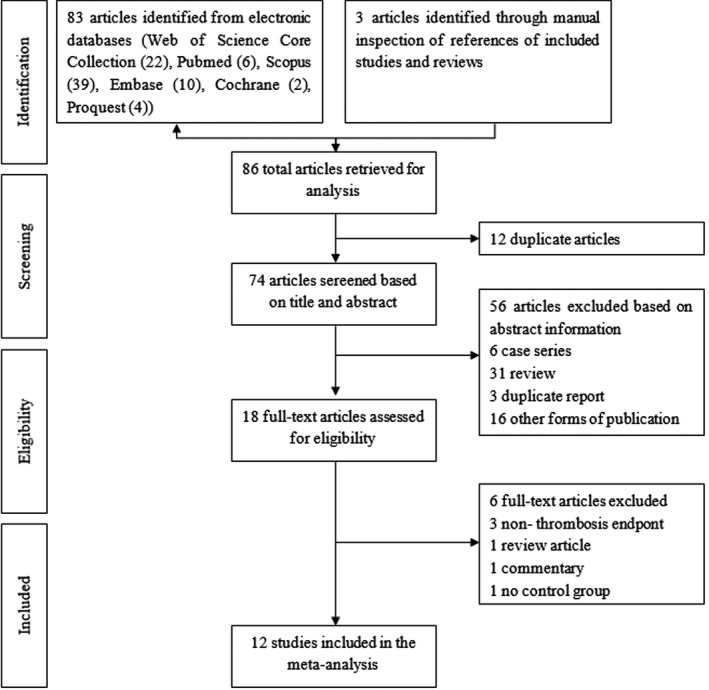
Summary of the literature identification and selection process
3.2. Study characteristics
A total of 12 studies were selected for inclusion in this meta‐analysis (Bahl et al.,2019; Benali et al.,2013; Caparas & Hu, 2014; Kaatz et al.,2019; Lisova et al.,2015; Moureau et al.,2002; Seo et al.,2020; Sharma et al.,2018; Sharp et al.,2014; Tso et al.,2017; Xu et al.,2016; Zohourian et al.,2019), with nine studies from the United States, accounting for 75.00%, and one study each from Australia (Sharp et al.,2014), Canada (Benali et al.,2013) and the Czech Republic (Lisova et al.,2015). Three studies were abstracts of meetings (Benali et al.,2013; Kaatz et al.,2019; Sharma et al.,2018). Overall, 40,871 participants were included; 7,806 (19.10%) were included in the MCs group; and 33,065 (80.90%) were included in the PICCs group. A summary of the included studies is presented in Table 1.
TABLE 1.
Characteristics of included studies with a comparison group
| Study | Study design | Study location | Study period | Population | Study indicators | MC group | PICC group | |||
|---|---|---|---|---|---|---|---|---|---|---|
| VTE | Total | VTE | Total | |||||||
| (Seo et al.,2020) | RC | New York, US | Nov. 2017 to Jul. 2018 | ≥18 years | Demographics, catheter‐related adverse events (local events, catheter dislodgment, infiltration, occlusion, VTE, extravasation, line‐associated infection), risk factors for complications, data on catheter gauge, the use of ultrasound during placement, the service placing the line, anticoagulation and/or antiplatelet therapy, in situ time of the midline, site of placement, total number of medications infused, medication type, duration of therapy, vesicant properties. | 2 | 82 | 0 | 50 | |
| (Kaatz et al.,2019) | RC | Michigan, US | NR | non‐surgical cancer patients | Demographics, device characteristics, catheter use, medical conditions, medications, 30 days after device placement rates of VTE | 3 | 160 | 83 | 2113 | |
| (Bahl et al.,2019) | RC | Michigan, US | Jul. 2016 to Aug. 2017 | ≥18 years | Demographics, date and time of line placement, type of line, diameter of line, number of line lumens, catheter‐to‐vein ratio, vein accessed, line laterality, number of attempts, indication for line, upper extremity venous duplex ultrasonography, VTE | 130 | 1094 | 102 | 1483 | |
| (Zohourian et al.,2019) | RC | Florida, US | NR | NR | Age, lumen size, major surgery, previous VTE, body mass index, catheter infection, thrombophilia, critically illness, inferior vena cava filters, pacemaker, implantable cardioverter defibrillator, anticoagulation, catheter insertion site | 6 | 123 | 91 | 1560 | |
| (Sharma et al.,2018) | RC | Missouri, US | NR | NR | DVT, location of DVT, type of catheter | 3 | 122 | 50 | 1365 | |
| (Tso et al.,2017) | RC | San Francisco, US | Feb. 25, 2008, to Oct. 31, 2014 | adult and paediatric | Age, sex, diagnosis, presence of aura, total cumulative dose (dihydroergotamine) or maximum dose reached (lidocaine), type of IV line used, number of days with a PICC or MC, DVT, PE | 8 | 110 | 13 | 205 | |
| (Xu et al.,2016) | RC | Pennsylvania, US | Jan‐May 2015 | 19–98 years | Demographics, comorbidity score, length of stay, insertion location, line duration, complications | 2 | 200 | 2 | 206 | |
| (Lisova et al.,2015) | RC | Prague, Czech Republic | During 2013 | 23–90 years | Complications (infection, VTE, occlusion, displacement) | 12 | 162 | 6 | 167 | |
| (Caparas & Hu, 2014) | RCT | New York, US | NR | ≥ one dose and ≥6 days vancomycin | Demographics, administration of other antibiotics, average number of days on vancomycin, dwell time, complications | 0 | 30 | 0 | 28 | |
| (Sharp et al.,2014) | RC | Adelaide, Australia | 2004 to 2010 | 18–47 years | Demographics, comorbid conditions, inpatients/outpatients, severity of exacerbation, lung function, adverse events (catheter‐related bloodstream infection, DVT, occlusion, pain, infiltration, bleeding, phlebitis, catheter leakage, dislodgement), and whether the VAD was removed unexpectedly | 3 | 231 | 0 | 97 | |
| (Benali et al.,2013) | RCT | Montreal, Canada | NR | ≤18 years and weighed ≥3 kg | Demographics, dwell time, catheter complications (DVT, infection) | 5 | 69 | 0 | 84 | |
| (Moureau et al.,2002) | RC | California, US | Apr. 1999 to Sep. 2000 | 1–101 years | Age, sex, type of VAD, principal diagnosis, complications by event and device type, underlying causes and outcomes of DVT dysfunction | 136 | 5423 | 411 | 25707 | |
Abbreviations: NR, not reported; RC, retrospective cohort study; RCT, randomized controlled trial.
3.3. Study quality
The study quality for all 12 independent studies is shown in Table 2. Four studies were poor (Benali et al.,2013; Kaatz et al.,2019; Lisova et al.,2015; Sharma et al.,2018), three studies were fair (Moureau et al.,2002; Tso et al.,2017; Zohourian et al.,2019), and five studies were good (Bahl et al.,2019; Caparas & Hu, 2014; Seo et al.,2020; Sharp et al.,2014; Xu et al.,2016).
TABLE 2.
Quality of included studies
| Study | reporting | External validity | Internal validity ‐ bias | Internal validity ‐ confounding | Power | Total |
|---|---|---|---|---|---|---|
| (Seo et al.,2020) | 9 | 3 | 5 | 2 | 2 | Good (21) |
| (Kaatz et al.,2019) | 6 | 1 | 3 | 0 | 1 | Poor (11) |
| (Bahl et al.,2019) | 9 | 2 | 3 | 3 | 3 | Good (20) |
| (Zohourian et al.,2019) | 6 | 2 | 4 | 2 | 3 | Fair (17) |
| (Sharma et al.,2018) | 3 | 1 | 2 | 1 | 2 | Poor (9) |
| (Tso et al.,2017) | 7 | 2 | 3 | 4 | 2 | Fair (18) |
| (Xu et al.,2016) | 10 | 3 | 4 | 4 | 2 | Good (23) |
| (Lisova et al.,2015) | 5 | 1 | 3 | 2 | 2 | Poor (13) |
| (Caparas & Hu, 2014) | 9 | 3 | 4 | 5 | 2 | Good (23) |
| (Sharp et al.,2014) | 9 | 3 | 4 | 3 | 3 | Good (22) |
| (Benali et al.,2013) | 4 | 1 | 4 | 4 | 1 | Poor (14) |
| (Moureau et al.,2002) | 7 | 2 | 4 | 3 | 3 | Fair (19) |
3.4. Meta‑analysis results
A total of 12 studies were analysed that involved 40,871 patients. The prevalence of VTE with MCs and PICCs was 3.97% (310/7806) and 2.29% (758/33065), respectively. Heterogeneity among the studies was low (I2=21%, p =.24). Thus, a fixed effects model was used. The meta‐analysis showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.53, 95% CI: 1.33–1.76, p <.00001). If the four poor studies were removed (Benali et al.,2013; Kaatz et al.,2019; Lisova et al.,2015; Sharma et al.,2018), heterogeneity among the studies was low (I2=0%, p =.68). Thus, a fixed effects model was used. The meta‐analysis showed that the prevalence of VTE with MCs was higher than in PICCs (RR=1.57, 95% CI: 1.36–1.82, p <.00001) (Figure 2).
FIGURE 2.
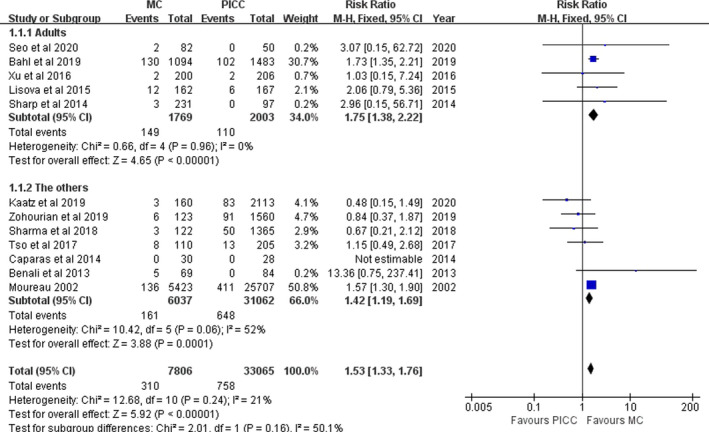
Pooled results for VTE between MCs and PICCs
Subgroup analyses were performed among adults and the others; the others subgroup included studies in children (Benali et al.,2013), children and adults (Moureau et al.,2002; Tso et al.,2017), and no reporting age of the participants (Caparas & Hu, 2014; Kaatz et al.,2019; Sharma et al.,2018; Tso et al.,2017; Zohourian et al.,2019). In the adult subgroup, heterogeneity among the studies was low (I2=0%, p =.96). The meta‐analysis showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.75, 95% CI: 1.38–2.22, p <.00001). In the others subgroup, heterogeneity among studies was moderate (I2=52%, p =.06). The meta‐analysis showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.42, 95% CI: 1.19–1.69, p =.0001) (Figure 2).
Subgroup analyses were performed among the United States and the other nations; the other nations’ subgroup included studies in Australia (Sharp et al.,2014), Canada (Benali et al.,2013) and the Czech Republic (Lisova et al.,2015). In the US subgroup, heterogeneity among the studies was low (I2=30%, p =.19). Meta‐analysis showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.50, 95% CI: 1.30–1.73, p <.00001). In the other nations’ subgroup, heterogeneity among the studies was low (I2=0%, p =.46). Meta‐analysis showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=2.87, 95% CI: 1.24–6.65, p =.01).
3.5. Sensitivity analyses
Sensitivity analyses were conducted. If Moureau et al (Moureau et al.,2002) were rejected, heterogeneity decreased significantly (I2=23%, p =.27) and the meta‐analysis showed that the prevalence of VTE was not significantly different between MCs and PICCs (RR=0.90, 95% CI: 0.58–1.42, p =.66) among the others subgroup. Heterogeneity and meta‐analysis results were not significantly different between the adult subgroup, US subgroup, the other nations’ subgroup and all studies. If Bahl et al. (2019) were rejected, heterogeneity (I2=0%, p =.90) and the meta‐analysis showed that the prevalence of VTE was not significantly different between MCs and PICCs (RR=1.98, 95% CI: 0.90–4.36, p =.09) among the adult subgroup. Heterogeneity and the meta‐analysis results were not significantly different in the others subgroup, US subgroup, the other nations’ subgroup and all studies.
There was no significant change in the heterogeneity, and meta‐analysis results after other studies were rejected one by one.
3.6. Publication bias analyses
Funnel plots of publication bias for studies examining VTE were assessed (Figure 3). The symmetry found in the funnel plots indicated no publication bias.
FIGURE 3.
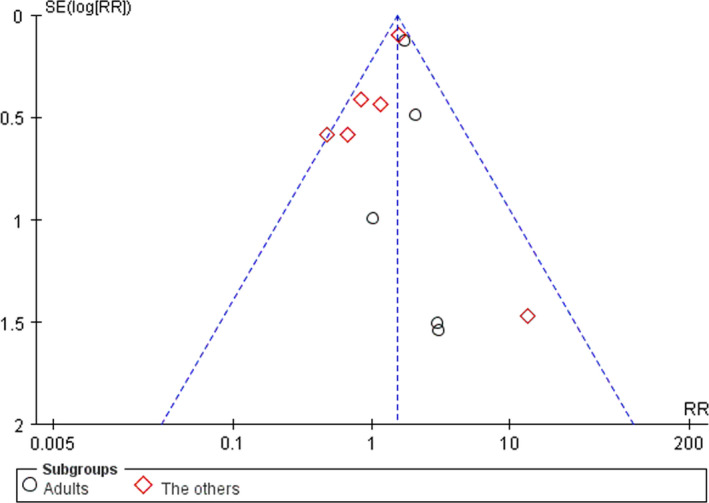
Funnel plots of publication bias for VTE
4. DISCUSSION
VTE is a serious complication in intravenous therapy. VTE can increase the length of hospitalizations, costs and mortality (Devani et al.,2017; Lyman et al.,2018; Mittal et al.,2018). The prevalence of VTE was different with different VADs. PICCs is long‐term use of central venous access. MCs is a new type of VAD, which has been widely used. Both MCs and PICCs can cause VTE in intravenous therapy. Many studies have shown that the risk of VTE in MCs and PICCs is different. In this study, the risk of VTE between MCs and PICCs was compared with a systematic review and meta‐analysis, to provide evidence for the selection of appropriate VADs for intravenous therapy. This is the first systematic assessment of the risk of VTE between MCs and PICCs.
In this study, we found that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.53, 95% CI: 1.33–1.76, p <.00001) in all patients. The meta‐analysis results have no significant changed after the four poor studies were removed. The sensitivity of overall studies is robust.
In subgroup analyses, we found that the prevalence of VTE with MCs was higher than that in MCs in adults (RR=1.75, 95% CI: 1.38–2.22, p <.00001). However, if Bahl et al. (2019) were rejected, the prevalence of VTE was not significantly different between MCs and PICCs among adult studies (RR=1.98, 95% CI: 0.90–4.36, p =.09). Therefore, a more contrastive study of VTE between MCs and PICCs in adults is still needed in the future. Only one study focused on the prevalence of VTE between PICCs and MCs in children, Benali et al. (2013) study indicated VTE has no significant difference between MCs and PICCs in children.
In the US subgroup, the meta‐analysis showed that the prevalence of VTE with MCs was higher than that with PICCs (RR=1.50, 95% CI: 1.30–1.73, p <.00001). The meta‐analysis results have no significant changed after other studies were rejected one by one. The sensitivity of overall studies is robust in the US subgroup. In the other nations’ subgroup, we have done nothing as three studies come from different countries.
4.1. Limitations
Some study limitations are listed. a. As nine studies were from the United States, and one study each was from Australia, Canada and the Czech Republic, and publication bias existed, it may be some research was not retrieved. b. Twelve studies were included, while three studies were abstracts presented at meetings. Some of the studies were of poor quality. c. Only published literature is included; unpublished results are not included. d. Most of the patients were adults, but few of them were children. This limitation means that study findings need to be interpreted cautiously.
5. CONCLUSIONS
The purpose of the current study was to determine the risk of VTE associated with MCs compared with PICCs with a systematic review and meta‐analysis. This study provides the first systematic assessment of the risk of VTE between MCs and PICCs. The findings indicate that the prevalence of VTE with MCs was higher than PICCs in all patients (RR=1.53, 95% CI: 1.33–1.76, p <.00001) and was higher than PICCs in adults (RR=1.75, 95% CI: 1.38–2.22, p <.00001). The findings of this study have several important implications for future practice. Further research is required to establish the risk of VTE between MCs and PICCs in children.
CONFLICT OF INTEREST
None of the authors have any financial and personal relationships with other people or organizations that could inappropriately influence their work.
AUTHOR CONTRIBUTIONS
H. Lu and Q. Yang: Design, acquisition, analysis, interpretation of data and drafting of the manuscript. L. Yang and K. Qu: Design, acquisition, analysis, interpretation of data and critical revision of the manuscript. B. Tian, Q. Xiao and X. Xin: Interpretation of data and critical revision of the manuscript. Y, Lv and X. Zheng: Design, analysis, interpretation of data and critical revision of the manuscript. H. Lu and Q. Yang had full access to all the study and take responsibility for the integrity of the data accuracy of the data analysis.
ETHICAL APPROVAL
This meta‐analysis study was approved by the institutional review board of the Department of Hepatobiliary and Pancreas Surgery, The First Affiliated Hospital, Xi'an Jiaotong University.
ACKNOWLEDGMENTS
This research was supported by grants from the fundamental research funds of the First Affiliated Hospital of Xi'an Jiao Tong University for funding this study (2017HL‐04).
Lu H, Yang Q, Yang L, et al. The risk of venous thromboembolism associated with midline catheters compared with peripherally inserted central catheters: A systematic review and meta‐analysis. Nurs Open. 2022;9:1873–1882. 10.1002/nop2.935
Huapeng Lu and Qinling Yang contributed equally to this study.
Funding information
The Fundamental Research Funds of the First Affiliated Hospital of Xi'an Jiao Tong University (2017Hl‐04) for funding this study
REFERENCES
- Al‐Asadi, O. , Almusarhed, M. , & Eldeeb, H. (2019). Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: Retrospective single Centre cohort study. Thrombosis Journal, 17, 2. 10.1186/s12959-019-0191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl, A. , Karabon, P. , & Chu, D. (2019). Comparison of venous thrombosis complications in midlines versus peripherally inserted central catheters: Are midlines the safer option?. Clinical and Applied Thrombosis/Hemostasis, 25, 1421729618. 10.1177/1076029619839150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benali, S. , Rypens, F. , Lacroix, J. , Dubois, J. , & Garel, L. (2013). Midline catheters versus peripherally inserted central catheters (PICC) in children: A randomized clinical trial. Pediatric Radiology, 43(S3), S541. [Google Scholar]
- Caparas, J. V. , & Hu, J. P. (2014). Safe administration of vancomycin through a novel midline catheter: A randomized, prospective clinical trial. The Journal of Vascular Access, 15(4), 251–256. 10.5301/jva.5000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, V. , Kaatz, S. , Swaminathan, L. , Boldenow, T. , Snyder, A. , Burris, R. , Bernstein, S. J. , & Flanders, S. (2019). Variation in use and outcomes related to midline catheters: Results from a multicentre pilot study. BMJ Quality & Safety, 28(9), 714–720. 10.1136/bmjqs-2018-008554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay, E. , Jamotte, A. , Verhamme, P. , Cohen, A. T. , Van Hout, B.A. , & Gumbs, P. (2018). Cost‐effectiveness of edoxaban compared to warfarin for the treatment and secondary prevention of venous thromboembolism in the UK. Journal of Market Access & Health Policy, 6(1), 1495974. 10.1080/20016689.2018.1495974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, A. T. , Agnelli, G. , Anderson, F. A. , Arcelus, J. I. , Bergqvist, D. , Brecht, J.G. & Spannagl, M. (2007). Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thrombosis and Haemostasis, 98(4), 756–764. [DOI] [PubMed] [Google Scholar]
- Dai, C. , Li, J. , Li, Q. M. , Guo, X. , Fan, Y‐y & Qin, H‐y , (2019). Effect of tunneled and nontunneled peripherally inserted central catheter placement: A randomized controlled trial. The Journal of Vascular Access, 21(4), 511–519. 10.1177/1129729819888120 [DOI] [PubMed] [Google Scholar]
- Devani, K. , Patil, N. , Simons‐Linares, C. R. , Patel, N. , Jaiswal, P. , Patel, P. , Patel, S. , Savani, C. , Sajnani, K. , Young, M. , & Reddy, C. (2017). Trends in hospitalization and mortality of venous thromboembolism in hospitalized patients with colon cancer and their outcomes: US perspective. Clinical Colorectal Cancer, 16(3), e199–e204. 10.1016/j.clcc.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Dhir, A. , DeMarsh, S. , Ramgopal, A. , Worley, S. , Auron, M. , Hupertz, V. , & Onimoe, G. (2019). Central venous line associated deep vein thrombosis in hospitalized children. Journal of Pediatric Hematology/oncology, 41(7), e432–e437. 10.1097/MPH.0000000000001512 [DOI] [PubMed] [Google Scholar]
- Downs, S. H. , & Black, N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiology and Community Health, 52(6), 377–384. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques, L. , Foeller, M. , Farez, R. , Kaljo, K. , Nugent, M. , Simpson, P. , & Klatt, T. (2018). Safety of peripherally inserted central catheters during pregnancy: A retrospective study. The Journal of Maternal‐Fetal & Neonatal Medicine, 31(9), 1166–1170. 10.1080/14767058.2017.1311314 [DOI] [PubMed] [Google Scholar]
- Jumani, K. , Advani, S. , Reich, N. G. , Gosey, L. , & Milstone, A. M. (2013). Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatrics, 167(5), 429–435. 10.1001/jamapediatrics.2013.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz, S. , Ratz, D. , McLaughlin, E. , Flandars, S. , Czilok, T. & Chopra, V. (2019). Risk of venous thromboembolism in hospitalized medical cancer patients with midline and peripherally inserted central catheters. Research and practice in thrombosis and haemostasis, 3, 706. 10.1002/rth2.12229 [DOI] [Google Scholar]
- Kang, J. , Chen, W. , Sun, W. , Ge, R. , Li, H. , Ma, E. , Su, Q. , Cheng, F. , Hong, J. , Zhang, Y. , Lei, C. , Wang, X. , Jin, A. , & Liu, W. (2017). Peripherally inserted central catheter‐related complications in cancer patients: A prospective study of over 50,000 catheter days. Journal of Vascular Access, 18(2), 153–157. 10.5301/jva.5000670 [DOI] [PubMed] [Google Scholar]
- Kang, J. R. , Long, L. H. , Yan, S. W. , Wei, W. W. , Jun, H. Z. & Chen, W. (2017). Peripherally inserted central catheter‐related vein thrombosis in patients with lung cancer. Clinical and Applied Thrombosis/Hemostasis, 23(2), 181–186. 10.1177/1076029615595880 [DOI] [PubMed] [Google Scholar]
- Li, T. , Higgins, J. P. , & Deeks, J. J. (2019). Cochrane Handbook for Systematic Reviews of Interventions Version. 6 2019‐12‐30, 2019, from https://training.cochrane.org/handbook/current
- Lisova, K. , Hromadkova, J. , Pavelková, K. , Zauška, V. , Havlin, J. , & Charvat, J. (2018). The incidence of symptomatic upper limb venous thrombosis associated with midline catheter: Prospective observation. The Journal of Vascular Access, 19(5), 492–495. 10.1177/1129729818761276 [DOI] [PubMed] [Google Scholar]
- Lisova, K. , Paulinova, V. , Zemanova, K. , & Hromadkova, J. (2015). Experiences of the first PICC team in the Czech Republic. British Journal of Nursing, 24(2), S4–S10. 10.12968/bjon.2015.24.Sup2.S4 [DOI] [PubMed] [Google Scholar]
- Lyman, G. H. , Culakova, E. , Poniewierski, M. S. , & Kuderer, N. M. (2018). Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thrombosis Research, 1641, S112–S118. 10.1016/j.thromres.2018.01.028 [DOI] [PubMed] [Google Scholar]
- Mittal, V. , Ahuja, S. , Vejella, S. S. , Stempel, J. M. , Palabindala, V. , Dourado, C.M. & Leighton, J.C. (2018). Trends and outcomes of venous thromboembolism in hospitalized patients with ovarian cancer results from nationwide inpatient sample database 2003 to 2011. International Journal of Gynecological Cancer, 28(8), 1478–1484. 10.1097/IGC.0000000000001335 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moureau, N. , Poole, S. , Murdock, M. A. , Gray, S. M. , & Semba, C. P. (2002). Central venous catheters in home infusion care: Outcomes analysis in 50,470 patients. Journal of Vascular and Interventional Radiology, 13(10), 1009–1016. 10.1016/s1051-0443(07)61865-x [DOI] [PubMed] [Google Scholar]
- Noonan, P. J. , Hanson, S. J. , Simpson, P. M. , Dasgupta, M. , & Petersen, T. L. (2018). Comparison of complication rates of central venous catheters versus peripherally inserted central venous catheters in pediatric patients. Pediatric Critical Care Medicine, 19(12), 1097–1105. 10.1097/PCC.0000000000001707 [DOI] [PubMed] [Google Scholar]
- Ray‐Barruel, G. , Xu, H. , Marsh, N. , Cooke, M. , & Rickard, C. M. (2019). Effectiveness of insertion and maintenance bundles in preventing peripheral intravenous catheter‐related complications and bloodstream infection in hospital patients: A systematic review. Infection, Disease & Health, 24(3), 152–168. 10.1016/j.idh.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Ruppert, A. , Steinle, T. , & Lees, M. (2011). Economic burden of venous thromboembolism: A systematic review. Journal of Medical Economics, 14(1), 65–74. 10.3111/13696998.2010.546465 [DOI] [PubMed] [Google Scholar]
- Seo, H. , Altshuler, D. , Dubrovskaya, Y. , Nunnally, M. E. , Nunn, C. , Ello, N. , Papadopoulos, J. , & Chen, X. J. (2020). The Safety of midline catheters for intravenous therapy at a large academic medical center. Annals of Pharmacotherapy, 54(3), 232–238. 10.1177/1060028019878794 [DOI] [PubMed] [Google Scholar]
- Sharma, V. , Bammes, N. O. , Paul, J. , & Howell, G. H. (2018). Incidence of upper extremity thrombosis after the implementation of midline catheters. American Journal of Respiratory and Critical Care Medicine, 197(MeetingAbstracts), A6817. [Google Scholar]
- Sharp, R. , Esterman, A. , McCutcheon, H. , Hearse, N. , & Cummings, M. (2014). The safety and efficacy of midlines compared to peripherally inserted central catheters for adult cystic fibrosis patients: A retrospective, observational study. International Journal of Nursing Studies, 51(5), 694–702. 10.1016/j.ijnurstu.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Taxbro, K. , Hammarskjöld, F. , Thelin, B. O. , Lewin, F. , Hagman, H. , Hanberger, H. , & Berg, S. (2019). Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: An open‐label, randomised, two‐centre trial. British Journal of Anaesthesia, 122(6), 734–741. 10.1016/j.bja.2019.01.038 [DOI] [PubMed] [Google Scholar]
- Tso, A. R. , Patniyot, I. R. , Gelfand, A. A. , & Goadsby, P. J. (2017). Increased rate of venous thrombosis may be associated with inpatient dihydroergotamine treatment. Neurology, 89(3), 279–283. 10.1212/WNL.0000000000004108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T. , Kingsley, L. , DiNucci, S. , Messer, G. , Jeong, J.‐H. , Morgan, B. , Shutt, K. , & Yassin, M. H. (2016). Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. American Journal of Infection Control, 44(12), 1458–1461. 10.1016/j.ajic.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Zohourian, H. , Schaubschlager, T. , Phan, L. , Polsinelli, E. , Hunter, K. , Timis, A. , Sanchez, D. , Maini, A. , Hardigan, P. , Carreon, A. , & Jani, V. (2019). Comparing incidence of thrombosis in PICC and midlines and evaluating the role of anticoagulation, site of insertion, and risk factors. Journal of the Association for Vascular Access, 24(1), 38–44. 10.1016/j.Java.2018.29.004 [DOI] [Google Scholar]


