Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has put pressure on healthcare services, forcing the reorganisation of traditional care pathways. We investigated how physicians taking care of severe asthma patients in Europe reorganised care, and how these changes affected patient satisfaction, asthma control and future care.
Methods
In this European-wide cross-sectional study, patient surveys were sent to patients with a physician-diagnosis of severe asthma, and physician surveys to severe asthma specialists between November 2020 and May 2021.
Results
1101 patients and 268 physicians from 16 European countries contributed to the study. Common physician-reported changes in severe asthma care included use of video/phone consultations (46%), reduced availability of physicians (43%) and change to home-administered biologics (38%). Change to phone/video consultations was reported in 45% of patients, of whom 79% were satisfied or very satisfied with this change. Of 709 patients on biologics, 24% experienced changes in biologic care, of whom 92% were changed to home-administered biologics and of these 62% were satisfied or very satisfied with this change. Only 2% reported worsening asthma symptoms associated with changes in biologic care. Many physicians expect continued implementation of video/phone consultations (41%) and home administration of biologics (52%).
Conclusions
Change to video/phone consultations and home administration of biologics was common in severe asthma care during the COVID-19 pandemic and was associated with high satisfaction levels in most but not all cases. Many physicians expect these changes to continue in future severe asthma care, though satisfaction levels may change after the pandemic.
Short abstract
Changes in severe asthma care caused by the #COVID19 pandemic include the transition to video/phone consultations and home administration of biologics. Patients are satisfied with the changes and show no evidence of poor asthma control. https://bit.ly/3MV6ywy
Introduction
Severe asthma, affecting around 3.7% of adults with asthma in Europe, is a heterogeneous chronic respiratory disease characterised by persistent symptoms, impaired lung function and frequent exacerbations most commonly triggered by viral infections, resulting in disease worsening and increased vulnerability [1, 2]. Treatment depends on complex regimes of high-dose maintenance medications, including biologics [3]. Traditional models of care for patients with severe asthma require frequent attendance to specialist centres and review by a multidisciplinary team to assess asthma control, monitor lung function and inflammation parameters, evaluate response and adherence to medication, check for adverse effects, and dispense or administer medication such as oral corticosteroids (OCS) and biologics [4, 5].
The coronavirus disease 2019 (COVID-19) pandemic has placed major challenges on healthcare services, forcing reorganisation of traditional care pathways and reducing the capacity for face-to-face consultations globally [6]. The crisis created considerable challenges to maintain access to and delivery of effective severe asthma care for many vulnerable patients. Several expert-opinion papers have provided recommendations for reorganisation of severe asthma care during the pandemic, though large-scale real-world data on how physicians managed in practice and the resultant impact on severe asthma patients are lacking [7–11].
The “Severe Heterogeneous Asthma Research collaboration, Patient-centred” (SHARP) is a Clinical Research Collaboration of the European Respiratory Society (ERS) that forms a network of severe asthma experts and patients from different European centres to promote patient-centred severe asthma research on a pan-European scale [12]. The aims of this European-wide survey-based study by SHARP are to investigate the effect of the pandemic on the organisation of severe asthma care: 1) from the physician perspective; 2) from the patient perspective, including the impact of changes in care and treatments on satisfaction with care and asthma control; and 3) to evaluate which aspects of reorganised care physicians expect to be continued in future care.
Methods
Design
This was a cross-sectional study in which a patient survey was sent to patients with severe asthma, and a physician survey was sent to severe asthma specialists. The survey was launched on 30 November 2020 and closed on 9 May 2021. Members of the European Lung Foundation's asthma Patient Advisory Group (PAG) and representatives of national respiratory patient organisations were actively involved in the conception and design of the study (details in supplementary file 1) [13].
Survey development and setting
The surveys were developed in an iterative manner by the authors, involving physicians (severe asthma experts), psychologists and patients. The patient surveys were translated by professional translators into the native languages of the 16 countries. The translations were reviewed by the SHARP National Leads. Physicians were asked to recruit severe asthma patients from their outpatient clinics for the patient survey and to complete the physician survey. Both online and paper versions of the patient survey were available, while only an online version was used for the physician survey. SurveyMonkey (SurveyMonkey, Momentive Inc, San Mateo, CA, USA) was used for the online survey. Paper versions of the patient survey were used if online versions were not available, and results from these paper version surveys were transferred into the SurveyMonkey system by the local research team. Data collection was anonymous.
Patient and physician selection
Patients were eligible for inclusion if they had physician-diagnosed severe asthma and had been followed up in a severe asthma clinic for at least 6 months from the beginning of the COVID-19 pandemic. Participating physicians included national leads from SHARP member countries and physicians in their Respiratory Societies, who were identified by the national leads to have significant experience treating severe asthma patients. All participating physicians were instructed not to exclude any severe asthma patient on their consultation hour when recruiting patients for the study.
Survey content
The patient survey consisted of multiple-choice questions including demographics, medication use, changes in care and (biologic) treatments, patient satisfaction with any changes in care or treatments, and patient perceptions of any change in asthma control induced by changes in care or treatments. Full patient and physician surveys are included in supplementary file 2 and 3, respectively. A scale ranging from 1 to 5 was used for answering questions about satisfaction, with a higher score meaning a higher level of satisfaction. “Satisfaction with care” was then calculated as a mean of the scores of seven questions (question 16A–G, in which 16C–G were reverse coded), “satisfaction with changes in care” as a mean of the scores of two questions (16H–I) and “satisfaction with changes to biologic treatments” consisted of the score of a single question (16J). A scale ranging from 1 to 5 was used for answering questions about patients’ perceived change in asthma control, with a higher score meaning a worsening in asthma. Change in asthma control due to “changes in care” was then calculated as a mean of the scores of three questions (question 17A–C), and change in asthma control due to “changes in biologic treatment” consisted of the score of a single question (17D). Questions 17A–D comprised statements indicating that asthma symptoms had got worse, with responses 1=strongly disagree, 2=disagree, 3=neither agree nor disagree, 4=agree, 5=strongly agree. The physician survey contained multiple-choice questions about the reorganisation of severe asthma care and treatments, the challenges they faced in reorganisation of care and physicians’ perspectives on which of these changes may be implemented in future care. The physician survey was conducted in English.
Ethics
Approval for the study was obtained from the medical ethical board of the Amsterdam University Medical Center (W20_463 # 20.512) and the ethical boards of every individual country where there was a requirement for ethics approval for survey-based studies. All patients and physicians provided digital or written informed consent for participation in this study.
Statistical analysis
Descriptive statistics and t-tests were used for comparisons between groups. p-values ≤0.05 were regarded as a statistically significant difference. Statistical analyses were performed using IBM SPSS v.25 software (IBM Corp., Armonk, NY, USA).
Results
Patient and physician participation
The physician survey was completed by 268 severe asthma specialists from 16 countries in Europe. Of 1119 returned patient surveys, 1101 were complete and included for analysis. Numbers of participating physicians and patients per country and baseline patient characteristics of included patients are shown in table 1.
TABLE 1.
Country breakdown of physician and patient respondents to questionnaires
| Country | Physicians n | Patients | |||
| n |
Female
n (%) |
Use of biologics
n (%) |
Daily OCS
n (%) |
||
| Belgium | 13 | 102 | 57 (56) | 86 (84) | 9 (9) |
| Estonia | 8 | 14 | 13 (93) | 6 (43) | 5 (36) |
| France | 28 | 15 | 10 (67) | 13 (87) | 5 (33) |
| Greece | 18 | 122 | 82 (67) | 74 (60) | 35 (29) |
| Hungary | 40 | 110 | 71 (65) | 71 (65) | 22 (20) |
| Italy | 31 | 52 | 38 (73) | 28 (54) | 13 (25) |
| Latvia | 4 | 54 | 33 (61) | 24 (44) | 19 (35) |
| Lithuania | 15 | 53 | 35 (66) | 41 (77) | 8 (15) |
| The Netherlands | 2 | 114 | 69 (61) | 79 (69) | 27 (24) |
| Romania | 31 | 12 | 5 (42) | 9 (75) | 3 (25) |
| Russian Federation | 13 | 55 | 34 (62) | 11 (20) | 9 (16) |
| Serbia | 15 | 74 | 50 (68) | 45 (60) | 30 (41) |
| Slovenia | 2 | 70 | 51 (73) | 64 (91) | 12 (17) |
| Sweden | 9 | 122 | 60 (49) | 67 (55) | 34 (28) |
| Switzerland | 19 | 57 | 25 (44) | 46 (81) | 19 (33) |
| UK | 20 | 75 | 43 (57) | 45 (60) | 31 (41) |
| Total | 268 | 1101 | 676 (61) | 709 (64) | 281 (26) |
Number of returned physician surveys per country, and number and characteristics of participating patients per country. OCS: oral corticosteroids.
Physician-reported changes in care during the COVID-19 pandemic
90% (242 of 268) of participating physicians reported at least one change in severe asthma care in their centre during the COVID-19 pandemic, and the nature of the changes are shown in table 2. Changes were either the result of “voluntary” physician-induced changes in reorganisations of severe asthma care or due to “involuntary” pandemic-induced changes, mainly concerning reduced staff or resource capacity.
TABLE 2.
Physician-reported changes in delivery of care
| Change in care | n (%) |
| Reorganisation of care by physicians (i.e. voluntary) | |
| Change to video/phone consultations | 122 (46) |
| Outpatient clinic continued with social distancing | 142 (53) |
| Urgent consultations only | 44 (16) |
| New patients postponed | 32 (12) |
| Switch to home-administered biologics | 102 (38) |
| Changes induced by the pandemic (i.e. involuntary) | |
| Reduced capacity outpatient clinic | 109 (41) |
| Reduced capacity lung function lab | 159 (59) |
| Fewer physicians available | 115 (43) |
| Fewer nurses available | 76 (28) |
Changes in severe asthma care during the coronavirus disease 2019 (COVID-19) pandemic as reported by the participating severe asthma specialists (n=268).
Patient-reported changes in care during the COVID-19 pandemic and impact on satisfaction with care and asthma control
Of 1101 included patients, 494 (45%) experienced a change in severe asthma care. Table 3 shows the nature of these changes in care and the associated levels of satisfaction with care as well as changes in care. Patients for whom care had changed were significantly less likely to be satisfied with care compared to patients who experienced no changes in care (p<0.001). In a further analysis of only those patients who were changed to video/phone consultations from face-to face the majority was satisfied, see figure 1.
TABLE 3.
Satisfaction scores with types of change in care and asthma control
| n (%) | Satisfaction with care | Satisfaction with changes in care | Effect on asthma control attributed to changes in care | |
| All patients (n=1101) | ||||
| No change | 607 (55) | 4.42±0.61# | ||
| Change | 494 (45) | 3.85±0.72# | 3.68±0.93 | 1.90±0.84 |
| Type of change reported (n=467) | ||||
| Phone/video consultations | 212 (45) | 3.96±0.67 | 3.81±0.87 | 1.80±0.78 |
| Monitored my asthma at home | 24 (5) | 3.55±0.76 | 3.65±0.86 | 2.24±0.70 |
| The location of my appointments was changed | 43 (9) | 3.90±0.68 | 3.78±0.91 | 1.86±0.87 |
| Attended alternative unit (e.g. ED) | 10 (2) | 3.66±0.92 | 3.55±1.28 | 2.50±1.25 |
| I chose to cancel appointments | 61 (13) | 3.60±0.74 | 3.30±1.00 | 2.07±0.96 |
| Cancelled or postponed by clinic | 117 (25) | 3.79±0.74 | 3.55±0.97 | 1.91±0.85 |
Data presented as mean±sd, unless otherwise stated. Patient-reported changes in severe asthma care during the coronavirus disease 2019 (COVID-19) pandemic and associated levels of satisfaction with care and changes in care, and patient-perceived effect on asthma control. Higher satisfaction scores indicate better satisfaction (range 1–5, 1=very low satisfaction and 5=very high satisfaction); higher asthma control scores indicate greater agreement with statements that changes in care induced worsening of asthma control (range 1–5, 1=strongly disagree and 5=strongly agree). ED: emergency department. #: t (1068)=15.82, p<0.001, d=0.96.
FIGURE 1.
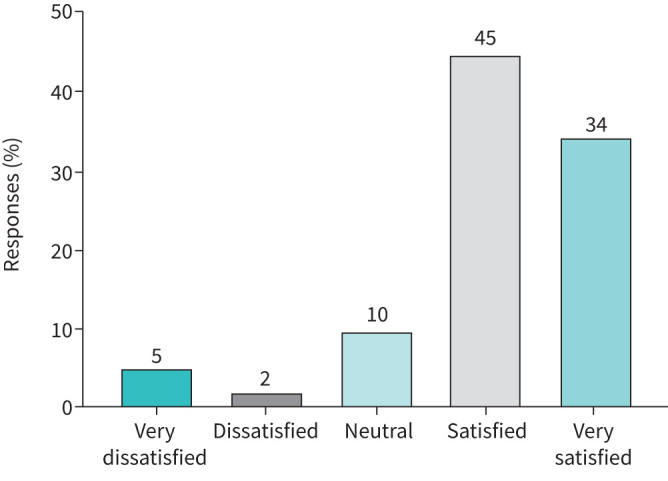
Satisfaction with change to video/phone consultations. A change to video/phone consultations was reported by 212 patients, of whom 207 indicated their satisfaction level with this change.
Table 3 also shows change in perceived asthma control. For those patients who reported a change, the mean score was 1.9 indicating that, on average, they disagreed with the three statements indicating poorer control. Reports of different types of change also showed mean levels indicating disagreement with the assertion that asthma symptoms had got worse.
Patient-reported changes in biologic care during the COVID-19 pandemic and impact on satisfaction with care and asthma control
Of 709 patients using asthma biologics at the start of the pandemic, 167 (24%) reported a change in their biologic treatment. The different types of changes in biologic care, and associated satisfaction ratings and impact on asthma control are presented in table 4. Patients on biologics reporting a change in provision of biologic care were significantly less satisfied with care than those who reported no change in provision of biologic care (p<0.001). In a further analysis of patients who experienced a change in biologic care during the pandemic, the large majority of patients reported a switch to home-administered biologics. Figure 2 shows that a small percentage of patients were not satisfied with this change. Only 3 out of 153 patients (2%) who switched to home administration of their biologic agreed or agreed strongly that their symptoms had worsened because of this change.
TABLE 4.
Satisfaction scores with types of change in biologic care and asthma control
| n (%) | Satisfaction with care | Satisfaction with changes in care | Effect on asthma control attributed to changes in biologic treatment | |
| All patients on biologics (n=709) | ||||
| No change | 542 (76) | 4.40±0.59# | ||
| Change | 167 (24) | 3.93±0.68# | 3.72±1.08 | 1.90±0.88 |
| Type of change reported (n=167) | ||||
| Switch to home administration | 153 (92) | 3.96±0.67 | 3.90±0.87 | 1.76±0.74 |
| Treatment less frequent | 4 (2) | 4.05±0.46 | 3.83±0.53 | 2.22±1.57 |
| Treatment postponed | 7 (4) | 3.63±0.84 | 3.92±1.02 | 2.05±0.83 |
| Treatment stopped | 3 (2) | 3.04±0.33 | 3.17±0.29 | 3.22±0.69 |
Data presented as mean±sd, unless otherwise stated. Patient-reported changes in biologic care during the coronavirus disease 2019 (COVID-19) pandemic and associated levels of satisfaction with care and changes in care, and patient-perceived effect on asthma control. Higher satisfaction scores indicate better satisfaction (range 1–5, 1=very low satisfaction and 5=very high satisfaction); higher asthma control scores indicate greater agreement with a statement that changes in biologic care induced worsening of asthma control (range 1–5, 1=strongly disagree and 5=strongly agree). Data of 709 patients on biologics; 26 did not complete the questions concerning satisfaction with care. #: t (674)=8.47, p<0.001, d=0.72.
FIGURE 2.
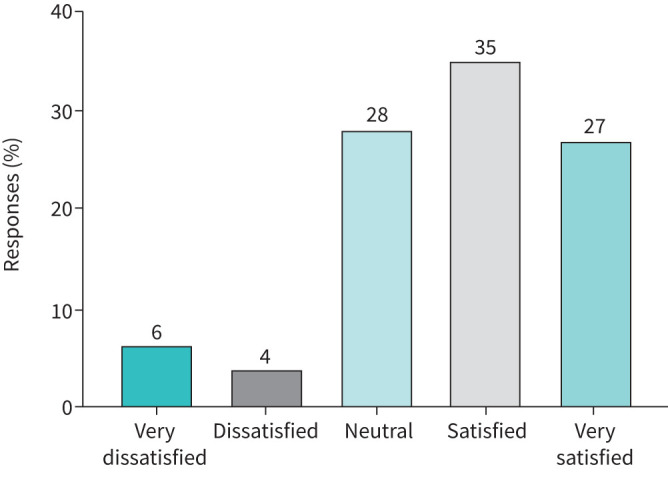
Satisfaction with change to home-administered biologics. Satisfaction with change to home-administered biologics in patients reporting this change in their biologic care (n=153).
Table 4 also shows the mean score of responses to a single statement indicating that change in biologic care produced a worsening of asthma control. On a scale ranging from 1 to 5 (in which 1=strongly disagree and 5=strongly agree), a mean score of 1.9 shows that on average patients who were on biologics disagreed with this statement. 92% of those patients reporting a change in biologic treatment reported that the change was due to home administration, and for these patients the mean was 1.76 indicating a slightly greater trend towards strong disagreement with the statement that asthma symptoms had worsened.
Physicians’ expected changes to future severe asthma care
The majority of participating physicians (78%) expect that certain aspects of reorganised care will be continued in the future. Figure 3 presents physicians’ beliefs about how severe asthma care will change as a result of the COVID-19 pandemic.
FIGURE 3.
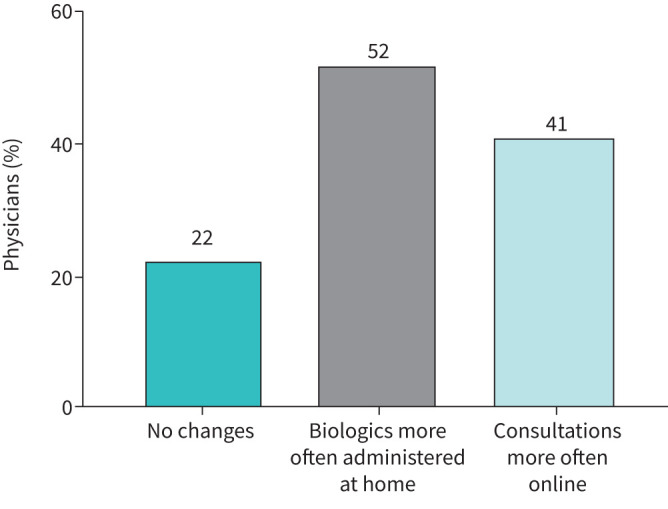
Physicians’ expected changes to future severe asthma care. Physicians’ beliefs about how asthma care will change following the pandemic (n=268).
Discussion
The results of this European-wide survey showed that both physicians and patients reported changes in severe asthma care during the COVID-19 pandemic. Physicians expected these changes to outlast the pandemic, and the majority of patients were satisfied by the changes that were made, the most common changes being the use of video/telephone consultations and home administration of biologics. There was no evidence that changes led to poorer perceived asthma control.
Although this study is the first that has investigated the effect of the pandemic on severe asthma care, our results can be compared to other disease areas. A global survey from the World Health Organisation showed that >50% of 163 participating countries reported disrupted outpatient services for non-communicable diseases with limited access, reduced staff capacity, alternate locations or different modes of care [6]. Consistent with the results of our study, replacement of face-to-face consultations into telemedicine deployments were reported in ∼60% of countries. Several other studies investigated patient satisfaction with video/phone consultations during the COVID-19 pandemic, both in allergy/immunology and other services (e.g. rheumatology, inflammatory bowel disease, oral/maxillofacial surgery, urology), and all confirmed high satisfaction levels in the majority of patients [14–20]. In addition, some other studies, mainly involving allergy/immunology clinics, reported increased prescriptions of home-administered biologics [21–23]. Apparently, even patients requiring complex care, including those with severe asthma, are willing to switch to a different type of care if circumstances demand it.
In our study changes in asthma care resulted from decisions made either by the hospital, the doctor or by the patients themselves, and changes took various forms. Some of the changes were due to reduced staffing, and low staffing will impact care irrespective of whether there is a pandemic. There was evidence of reduced satisfaction in care in those patients experiencing a change compared to those not experiencing a change, but it does not follow that change caused reduced satisfaction as other unknown factors also contribute to satisfaction levels. We found no evidence that any one type of change was associated with lower satisfaction than any other.
Slightly more than half of physicians in our study reported that the change to home administration of biologics would be more frequent in future care. In our study we found no evidence that home administration was associated with better or worse asthma control for the group as a whole. Although the majority were satisfied with that change, a small minority were not satisfied indicating the need to personalise this aspect of patient care post-pandemic.
Telemedicine in the field of asthma is not new, and several studies including meta-analyses suggested positive effects of telemedicine on asthma control and quality of life in asthma patients, though numerous human-related, technical and reimbursement barriers hampered widespread implementation [24–27]. The emergence of the COVID-19 pandemic seems to have accelerated the transition towards telemedicine modalities, although its precise role in future severe asthma care needs further exploration. In our study, satisfaction levels with video/phone consultations were high. 79% of patients were satisfied or very satisfied with this change, while only 7% of patients were not satisfied. Preferences in the mode of consultations may vary between patients or may vary over time in individual patients. In addition, previous reports suggested benefits to telemedicine modalities in asthma patients living in rural/remote areas, while other studies suggested decreased benefits in vulnerable patient populations, including those with lower socioeconomic status, with language barriers or poor internet access [28–30]. Better understanding of patient characteristics associated with dissatisfaction or poorer clinical outcome would allow for accurate patient selection and a personalised approach to telemedicine deployments in severe asthma patients. It is conceivable that a hybrid form of care delivery will emerge in future severe asthma care, in which virtual and face-to-face consultations are alternated, tailored to individual patient preferences and needs.
Limitations of this study include a possible underestimation of the proportion of patients with changes in care and the inability to calculate survey response rates, since numbers of provided surveys were incomplete. Further, we made no distinction between phone or video consultations, which are quite different modalities regarding logistics and patient–physician interaction, but a recent study in an allergy/immunology service evaluating patient satisfaction with in-person, video or phone consultations during the pandemic did not find a significant difference in satisfaction levels between these encounter modalities [19]. Lastly, we did not make comparisons between countries, because multiple factors could influence the results.
Conclusions and implications for clinical practice
Although severe asthma specialists across Europe reported numerous challenges in reorganisation of severe asthma care, this reorganisation was achieved with high levels of patient satisfaction and just limited effects on asthma control. Video/phone consultations and home-administered biologics were shown to work well for both physicians and most patients. For the small minority of patients who were dissatisfied, either face-to-face consultations are needed or assistance to improve their satisfaction with this mode of communication, consistent with previous research [29–31]. It remains to be seen whether the level of satisfaction with video/phone consultations will remain high after the pandemic. A personalised approach may be the way forward for a sustainable implementation of telemedicine modalities and home administration of injectable biologics in severe asthma care.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Guidance for Reporting Involvement of Patients and the Public (GRIPP)-2 form 00065-2022.SUPPLEMENT (196.9KB, pdf)
Patient survey 00065-2022.SUPPLEMENT2 (212.5KB, pdf)
Physician survey 00065-2022.SUPPLEMENT3 (170.6KB, pdf)
Acknowledgements
The SHARP CRC would like to acknowledge the support and expertise of the following individuals and groups without whom the study would not have been possible: Courtney Coleman (European Lung Foundation (ELF), Sheffield, UK), Emmanuelle Berret (European Respiratory Society, Lausanne, Switzerland), Elpiniki Papageorgiou-Georgatou (Medical Group of Athens, Athens, Greece), Petra Hirmann (Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands), Ekaterina Terekhova, Oksana Sebekina (both Russian Medical Academy of Continuous Professional Education of the Ministry of Healthcare of the Russian Federation, Moscow, Russia), Violeta Kolarov (Institute for Pulmonary Diseases of Vojvodina, Sremska Kamenica, Serbia), Agnes Csuth, Mohanad Abbas (both Allergy Center, Linköping, Sweden) and Jacqueline Otker (ELF Patient Advisory Group).
Provenance: Submitted article, peer reviewed.
Conflict of interest: A. Bourdin reports receiving grants or contracts outside the submitted work from AstraZeneca and Boeringher Ingelheim; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events as well as support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Chiesi and Amgen, outside the submitted work. Z. Csoma reports receiving honoraria for presentations from Astra/Zeneca, TEVA and Sanofi/Aventis (Hungary) outside the submitted work. E. Heffler reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Sanofi-Genzyme, Regeneron, Novartis, GSK, Circassia; Stallergenes-Greer and Nestlè Purina outside the submitted work. G.W. Canonica reports receiving consulting fees from AstraZeneca GSK, Novartis, Sanofi and Stallergenes Greer; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GSK, Novartis, Sanofi, Stallergenes Greer, Menarini, Chiesi and Mylan; participation on data safety monitoring or advisory boards for AstraZeneca, GSK, Novartis, Sanofi, Stallergenes Greer and Chiesi; all disclosures made outside the submitted work. G-J. Braunstahl reports grants or contracts from GSK, AstraZeneca and ALK Abello; consulting fees from GSK, Sanofi, ALK Abello, AstraZeneca and Novartis; payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from GSK, Sanofi, ALK Abello, AstraZeneca and Novartis; and is on the scientific board of the Dutch Lung Foundation, task force Asthma NVALT and editorial board NTVAAKI; all disclosures made outside the submitted work. S. van der Sar reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca and GSK; and support received from ALK for attending meetings and/or travel; all disclosures made outside the submitted work. N. Nenasheva reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca plc., Sanofi S.A., Teva Pharmaceuticals, Novartis International AG and Chiesi Farmaceutici S.p.A., outside the submitted work. A. Bossios reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AZ, GSK and Teva; support for attending meetings and/or travel received from Novartis; and participation on a data safety monitoring or advisory boards for AZ, GSK, Novartis, Teva and Sanofi; and is a member of the steering Committee of SHARP, Secretary of Assembly 5 (Airway diseases, asthma, COPD and chronic cough) of the European Respiratory Society and vice-chair of Nordic Severe Asthma Network; all disclosures made outside the submitted work. D. Aronsson reports receiving grants or contracts from ALK-Abello outside the submitted work. A. Egesten reports receiving honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, outside the submitted work. L. Ahlbeck reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca; and participation on data safety monitoring or advisory boards for AstraZeneca, Sanofi and GSK; all disclosures made outside the submitted work. S. Škrgat reports receiving honoraria for lectures and educational events supported by GSK, AstraZeneca, Sanofi, Chiesi, Pliva Teva and Medis; and participation on advisory boards of GSK, AstraZeneca, Chiesi and Sanofi; all disclosures made outside the submitted work. N. Edelbaher reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, Astra Zeneca, Chiesi, Pliva Teva, Krka, Novartis, Boehringer Ingelheim and Sanofi; and participation on advisory boards of GSK, Astra Zeneca, Chiesi, Novartis and Boehringer Ingelheim; all disclosures made outside the submitted work. J. Rüdiger reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from the Education of Swiss Emergency physicians; and participation on a data safety monitoring board or board for Boehringer Ingelheim; and is a member of the Board of the Swiss Society of Pneumology and President of the Thorax section of the Swiss Ultrasound Society; all disclosures made outside the submitted work. N. Pavlov reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, Novartis and OM Pharma; support for attending meetings and/or travel received from Boehringer Ingelheim; participation on data safety monitoring or advisory boards for AstraZeneca, GSK, Novartis and Sanofi; all disclosures made outside the submitted work. P. Gianella reports participation on an advisory board for Novartis about Xolair for nasal polyps, outside the submitted work. F. Charbonnier reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Sanofi, Novartis, Mundipharma, AstraZeneca and GSK; participation on data safety monitoring or advisory boards for Sanofi, Novartis, Mundipharma, AstraZeneca and GSK; all disclosures made outside the submitted work. R. Chaudhuri reports receiving grants or contracts from AstraZeneca; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, AstraZeneca, Teva, Chiesi, Sanofi and Novartis; support for attending meetings and/or travel received from Chiesi, Napp, Sanofi, Boehringer, GSK and AZ; and participation on data safety monitoring or advisory boards for GSK, AstraZeneca, Teva, Chiesi and Novartis; all disclosures made outside the submitted work. S.J. Smith is supported by the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement number 831434 for Taxonomy, Targets, Treatment, and Remission; the JU receives support from the European Union's Horizon 2020 research and innovation programme, and the European Federation of Pharmaceutical Industries and Associates; all disclosures made outside the submitted work. S. Doe reports receiving support for attending meetings and/or travel from GSK and Sanofi, outside the submitted work. L. Heaney reports grants or contracts from Medimmune, Novartis UK, Roche/Genentech Inc., GlaxoSmithKline, Amgen, Genentech/Hoffman la Roche, AstraZeneca, Medimmune, Aerocrine and Vitalograph; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for Novartis, Hoffman la Roche/Genentech Inc., Sanofi, GlaxoSmithKline, AstraZeneca, Teva and Circassia; support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Napp Pharmaceuticals; participation on data safety monitoring or advisory board for Novartis, Hoffman la Roche/Genentech Inc., Sanofi, Evelo Biosciences, GlaxoSmithKline, AstraZeneca, Teva, Theravance and Circassia; all disclosures made outside the submitted work. M. Masoli reports receiving grants or contracts from ERS SHARP to support the study “The burden of severe asthma on HRQoL across Europe”, outside the submitted work. P. Howarth is an employee of GSK. R. Djukanovic reports support for the present manuscript received from ERS, TEVA, GSK, Novartis, Sanofi and Chiesi; consulting fees from Synairgen for which the author is a co-founder and consultant and owns shares, outside the submitted work; and participation on a data safety monitoring or advisory board for Kymab (Cambridge), outside the submitted work. E. Bel reports support for the present manuscript from the ERS; grants or contracts received from GlaxoSmithKline and Teva, outside the submitted work; consulting fees received from AstraZeneca, GlaxoSmithKline, Sanofi, Sterna and Chiesi, outside the submitted work; honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Teva; participation on a data safety monitoring or advisory board for AstraZeneca, outside the submitted work; and leadership or fiduciary roles, unpaid, for SHARP-CRC and RAPSODI (Dutch registry), outside the submitted work. M. Hyland reports receiving grants or contracts from TEVA; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for GSK; all disclosures made outside the submitted work. The remaining authors have nothing to disclose.
Support statement: The SHARP CRC has been supported by financial and other contributions from the following consortium partners: European Respiratory Society, GlaxoSmithKline Research and Development Limited, Chiesi Farmaceutici SPA, Novartis Pharma AG, Sanofi-Genzyme Corporation and Teva Branded Pharmaceutical Products R&D, Inc. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med 2017; 377: 965–976. doi: 10.1056/NEJMra1608969 [DOI] [PubMed] [Google Scholar]
- 2.Hekking PPW, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 3.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2020; 55: 1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma (GINA) . Difficult-to-treat & severe asthma in adolescents and adult patients – Diagnosis and Management. V2.0. 2019. http://ginasthma.org/
- 5.Gibeon D, Heaney LG, Brightling CE, et al. Dedicated severe asthma services improve health-care use and quality of life. Chest 2015; 148: 870–876. doi: 10.1378/chest.14-3056 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . The impact of the covid-19 pandemic on noncommunicable disease resources and services: results of a rapid assessment. 2020. https://www.who.int/publications/i/item/9789240010291 Date last accessed: 10 November 2021.
- 7.Pfaar O, Klimek L, Jutel M, et al. COVID-19 pandemic: practical considerations on the organization of an allergy clinic—an EAACI/ARIA Position Paper. Allergy 2021; 76: 648–676. doi: 10.1111/all.14453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Licskai C, Yang C, Ducharme F, et al. Key highlights from the Canadian Thoracic Society Position Statement on the optimization of asthma management during the coronavirus disease 2019 pandemic. Chest 2020; 158: 1335–1337. doi: 10.1016/j.chest.2020.05.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaker MS, Oppenheimer J, Grayson M, et al. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract 2020; 8: 1477–1488.e5. doi: 10.1016/j.jaip.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persaud YK, Portnoy JM. Ten rules for implementation of a telemedicine program to care for patients with asthma. J Allergy Clin Immunol Pract 2021; 9: 13–21. doi: 10.1016/j.jaip.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaney T, Salman D, Samee T, et al. Assessment and management of adults with asthma during the covid-19 pandemic. BMJ 2020; 369: 1–5. [DOI] [PubMed] [Google Scholar]
- 12.Djukanovic R, Adcock IM, Anderson G, et al. The severe heterogeneous asthma research collaboration, patient-centred (SHARP) ERS clinical research collaboration: a new dawn in asthma research. Eur Respir J 2018; 52: 1–7. doi: 10.1183/13993003.01671-2018 [DOI] [PubMed] [Google Scholar]
- 13.Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017; 358: j3453. doi: 10.1136/bmj.j3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams L, Lester S, Hoon E, et al. Patient satisfaction and acceptability with telehealth at specialist medical outpatient clinics during the COVID-19 pandemic in Australia. Intern Med J 2021; 51: 1028–1037. doi: 10.1111/imj.15205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horgan TJ, Alsabbagh AY, Mcgoldrick DM, et al. Oral and maxillofacial surgery patient satisfaction with telephone consultations during the COVID-19 pandemic. Br J Oral Maxillofac Surg 2021; 59: 335–340. doi: 10.1016/j.bjoms.2020.08.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargsyan N, Karunaratne D, Masani A, et al. ENT telephone clinics during the coronavirus pandemic: an analysis of 400 telephone consultations at a district general hospital. Ear Nose Throat J 2021; in press [ 10.1177/01455613211028091]doi: 10.1177/01455613211028091 [DOI] [PubMed] [Google Scholar]
- 17.Efthymiadis A, Hart EJ, Guy AM, et al. Are telephone consultations the future of the NHS? The outcomes and experiences of an NHS urological service in moving to telemedicine. Future Healthc J 2021; 8: e15–e20. doi: 10.7861/fhj.2020-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier K, Kuruvilla M, Shih J. Patient satisfaction and utilization of telemedicine services in allergy: an institutional survey. J Allergy Clin Immunol Pract 2020; 9: 484–486. doi: 10.1016/j.jaip.2020.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mustafa SS, Vadamalai K, Ramsey A. Patient satisfaction with in-person, video, and telephone allergy/immunology evaluations during the COVID-19 pandemic. J Allergy Clin Immunol Pract 2021; 9: 1858–1863. doi: 10.1016/j.jaip.2021.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morais-Almeida M, Sousa C, Barbosa MT, et al. Telehealth: the future is now in allergy practice. J Allergy Clin Immunol Pract 2020; 8: 2836–2837. doi: 10.1016/j.jaip.2020.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Codispoti CD, Bandi S, Moy JN, et al. Running a virtual allergy division and training program in the time of COVID-19 pandemic. J Allergy Clin Immunol 2020; 145: 1357–1359. doi: 10.1016/j.jaci.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishna MT, Beck S, Gribbin N, et al. The impact of COVID-19 pandemic on adult and pediatric allergy & immunology services in the UK National Health Service. J Allergy Clin Immunol Pract 2021; 9: 709–722.e2. doi: 10.1016/j.jaip.2020.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malipiero G, Paoletti G, Puggioni F, et al. An academic allergy unit during COVID-19 pandemic in Italy. J Allergy Clin Immunol 2020; 146: 227. doi: 10.1016/j.jaci.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snoswell CL, Rahja M, Lalor AF. A systematic review and meta-analysis of change in health-related quality of life for interactive telehealth interventions for patients with asthma. Value Health 2021; 24: 291–302. doi: 10.1016/j.jval.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 25.Chongmelaxme B, Lee S, Dhippayom T, et al. The effects of telemedicine on asthma control and patients’ quality of life in adults: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2019; 7: 199–216.e11. doi: 10.1016/j.jaip.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 26.Bousquet J, Chavannes NH, Guldemond N, et al. Realising the potential of mHealth to improve asthma and allergy care: how to shape the future. Eur Respir J 2017; 49: 1700447. doi: 10.1183/13993003.00447-2017 [DOI] [PubMed] [Google Scholar]
- 27.Wu AC, Rehman N, Portnoy J. The good, the bad, and the unknown of telemedicine in asthma and allergy practice. J Allergy Clin Immunol Pract 2019; 7: 2580–2582. doi: 10.1016/j.jaip.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 28.Brown W, Odenthal D. The uses of telemedicine to improve asthma control. J Allergy Clin Immunol Pract 2015; 3: 300–301. doi: 10.1016/j.jaip.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 29.Tsao LR, Villanueva SA, Pines DA, et al. Impact of rapid transition to telemedicine-based delivery on allergy/immunology care during COVID-19. J Allergy Clin Immunol Pract 2021; 9: 2672–2679. doi: 10.1016/j.jaip.2021.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronenfeld JP, Penedo FJ. Novel coronavirus (COVID-19): telemedicine and remote care delivery in a time of medical crisis, implementation, and challenges. Transl Behav Med 2021; 11: 659–663. doi: 10.1093/tbm/ibaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner RM, Glied SA. Covid-induced changes in health care delivery — can they last? N Engl J Med 2021; 385: 868–870. doi: 10.1056/NEJMp2110679 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Guidance for Reporting Involvement of Patients and the Public (GRIPP)-2 form 00065-2022.SUPPLEMENT (196.9KB, pdf)
Patient survey 00065-2022.SUPPLEMENT2 (212.5KB, pdf)
Physician survey 00065-2022.SUPPLEMENT3 (170.6KB, pdf)


