Abstract
Background
With effective antiretroviral therapy, there is an emerging population of adults aged 50 years or older with human immunodeficiency virus (HIV). Frailty is an increasingly recognized clinical state of vulnerability associated with disability, hospitalization, and mortality. However, there is a paucity of large studies assessing its prevalence in people with HIV (PWH) aged 50 or older.
Methods
PubMed was systematically searched for studies published between January 2000 and August 2020 reporting the prevalence of frailty in PWH aged 50 or older. The pooled prevalence of frailty and prefrailty was synthesized using a random-effects meta-analysis.
Results
Of the 425 studies identified, 26 studies were included in the analysis, with a total of 6584 PWH aged 50 or older. The included studies were published between 2012 and 2020, and all studies used the Fried frailty phenotype to define frailty. The overall pooled prevalence of frailty and prefrailty was 10.9% (95% confidence interval [CI], 8.1%–14.2%) and 47.2% (95% CI, 40.1%–54.4%), respectively. A high degree of heterogeneity was observed (I2 = 93.2%). In the subgroup analysis, HIV-related variables and other demographic variables were examined, and heterogeneity disappeared only in the group of a longer duration since HIV diagnosis (I2 = 0%).
Conclusions
The pooled prevalence of frailty and prefrailty defined by the Fried frailty phenotype was assessed in PWH aged 50 or older. Findings from this study quantified the proportion of this specific population with this common geriatric syndrome. Future studies identifying effective strategies for frailty screening and intervention are required for this vulnerable population.
Keywords: frailty, HIV, meta-analysis, prefrailty, systematic review
The studies identified by our systematic review demonstrated a wide range of frailty prevalence among people with HIV aged 50 or older. The meta-analysis showed the pooled prevalence of frailty and prefrailty in this population was 10.9% and 47.2%, respectively.
The proportion of older people with human immunodeficiency virus (HIV) has substantially increased due to the use of antiretroviral therapy (ART) resulting in improved survival rates. Approximately 10%–30% of the 36 million plus people with HIV (PWH) worldwide are now aged 50 or over. This number is expected to triple over the next 3 decades [1, 2]. A previous meta-analysis revealed that after starting combination ART, the overall life expectancy of HIV-infected individuals in high-income countries was an additional 43.3 years at age 20 and 32.2 years at age 35 [3]. According to recent literature [4], the term “older patients” with HIV has been defined as adults aged 50 or older since PWH tend to suffer from geriatric conditions at a relatively younger age than the general population.
Frailty is a common geriatric syndrome and one of the most serious global public health challenges [5]. Frailty is often defined as a clinical state of vulnerability characterized by reduced physiological reserves and a loss of resistance to stressors caused by multisystem physiological dysregulation and accumulated age-related deficits [6]. The Fried [7] frailty phenotype, first validated in the Cardiovascular Health Study, is one of the most commonly used definitions of frailty in contemporary research [8]. It conceptualizes frailty as a distinct phenotype defined by the presence of 3 or more of the following 5 criteria: weight loss, exhaustion, weakness, slow gait speed, and decreased physical activity [9]. Prefrailty is defined as meeting 1 or 2 of these criteria. The Fried phenotype definition of frailty is frequently used in epidemiological studies because (1) there is an increasing consensus that frailty is a definable clinical state with multiple signs and symptoms, (2) clinical manifestations of frailty may be organized into a self-perpetuating cycle (“cycle of frailty”) of progressing clinical observations, (3) frailty is syndromal in presentation, and (4) it provides an a priori theoretical framework for mechanistic investigations underlying frailty [10]. In fact, a growing number of studies have shown that frailty is associated with adverse outcomes [11, 12]. For example, a study conducted by Rothman et al [12] showed that frailty defined by the frailty phenotype was independently associated with chronic disability, long-term nursing home stays, and death. In addition, a meta-analysis conducted by Vermeiren et al [13] revealed that frailty increases the risk of mortality, the loss of activities of daily living, hospitalization, physical limitation, falls, and fractures. Given these negative impacts on older adults, the proper screening and assessment of frailty and appropriate interventions are critical for optimizing the care of frail patients.
Growing evidence suggests that frailty is a significant risk factor for various negative health consequences in PWH. For example, Desquilbet et al [14] showed that frailty assessed with the Fried frailty phenotype before the initiation of HIV treatment was significantly associated with an increased risk of a composite outcome of acquired immunodeficiency syndrome and mortality. Another study, conducted by Piggott et al [15], showed that the combination of HIV infection and frailty assessed using the Fried criteria was associated with a more than 7-fold increased risk of death. In addition, frailty was also associated with an increased risk of falls, hospitalizations, polypharmacy, and multi-comorbidities among patients with HIV [16]. Moreover, previous studies have suggested an earlier onset of frailty in PWH. For example, a study conducted by Rajasuriar et al [17] revealed that multiple geriatric conditions, including frailty, occur more frequently among PWH aged even in their 30s and 40s.
Because PWH appear to suffer from frailty more frequently and at a younger age than the general population, frailty may be more prevalent in the newly emerging papulation of “older” PWH. Previous studies primarily focused on younger or middle-aged patients, given that the data on older adults are limited [18]. Only 1 systematic review assessed frailty prevalence, defined by the Fried phenotype criteria, in PWH [18]. In this study, a meta-analysis of data from 7 studies showed a pooled frailty prevalence of 8.6%. However, most samples included were young or middle-aged, with only 2 studies involving PWH aged 50 or older [19, 20]; therefore, the evidence of frailty status in older PWH remains scarce. Because frailty in older PWH is an emerging clinical challenge, it is essential to characterize the prevalence of frailty specific to this older population. Therefore, we conducted a systematic review and a meta-analysis to evaluate the prevalence of frailty and prefrailty in PWH aged 50 or older.
METHODS
Search Strategy and Study Selection
A systematic review of the literature was conducted in October 2020 based on a protocol developed a priori by following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statements [21]. The protocol was registered and is available at PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=209152). We searched PubMed without language restrictions. The PubMed explosion function was used. Cross-sectional studies that reported the prevalence of frailty or provided sufficient data to calculate the prevalence of frailty in patients with HIV were eligible. The search was conducted on August 11, 2020, for articles published in January 2000 or later. Medical Subject Headings (MeSH) and free-text terms used were as follows: “Frailty (MeSH)” OR “frail” AND “HIV (MeSH)” OR “Human immunodeficiency virus.” The reference lists of relevant articles were scrutinized. We contacted the corresponding authors of potentially eligible studies for the additional data required to conduct our meta-analysis. Studies were considered potentially eligible if they met the following inclusion criteria: sampled community-dwelling PWH aged 50 or older, defined frailty status using the Fried frailty phenotype, and provided sufficient data to demonstrate the prevalence of frailty and/or prefrailty. Although there is no gold standard definition or criteria for diagnosing frailty, the majority of studies evaluating frailty prevalence among PWH had used the Fried phenotype to identify frailty. Moreover, the prevalence of frailty can vary significantly if different frailty criteria are used, even with the same cohort [22]. Therefore, we only selected studies that had used the Fried frailty phenotype, to obtain consistent results and reduce heterogeneity. When a study included PWH younger than 50 years old and the data for PWH aged 50 or older was provided, the study was still considered eligible, and we only included data of PWH aged 50 or older. Randomized controlled trials, editorials, reviews, dissertations, and conference abstracts were excluded. If 2 or more studies used the same cohort, the one with the largest sample size was chosen. The studies identified during the systematic review were screened independently by 2 researchers (Y.Y. and G.K.), including their titles, abstracts, and full texts to ensure eligibility. Any discrepancies were solved by discussion.
Data Extraction
The information collected included the first author, cohort name if any, study location, sample size, the proportion of female participants, mean age, age range, frailty criteria, frailty assessment time, mean current CD4 cell count, mean nadir CD4 cell count, percentage of patients with undetectable viral load (VL), years since HIV diagnosis, HIV treatment duration, and the prevalence of frailty and prefrailty. Data were extracted directly from the studies included or were provided by the corresponding authors upon request. When the frailty prevalence was not shown, it was calculated using the crude numbers of participants.
Methodological Quality Assessment
Two investigators (Y.Y. and T.K.) appraised the methodological quality of the studies selected using the Joanna Briggs Institute Critical Appraisal Checklist for Analytical Cross-Sectional Studies [23], which consists of 8 items to be answered either “yes,” “no,” “unclear,” or “not applicable.” Of the 8 items, “strategies for confounders” was not answered because confounding factors do not affect the analysis of prevalence. We added the number of “yes” responses for each study reaching a score ranging from 0 to 7, with a higher score indicating better methodological quality. Scores of 4 or higher were considered to have adequate methodological quality.
Statistical Analysis
The pooled prevalence of frailty and prefrailty was calculated using a proportion meta-analysis. Heterogeneity across the studies was examined using a χ2 test, and the degree of heterogeneity was quantified using the I2 statistic. We selected a random-effects meta-analysis because a high degree of heterogeneity was expected. In addition, publication bias was assessed by visually inspecting funnel plots. All analyses were conducted using StatsDirect (version 3.3.4; StatsDirect, Cheshire, United Kingdom), and a 2-sided P value of less than .05 was considered statistically significant.
Several subgroup meta-analyses were conducted to explore potential factors affecting the pooled prevalence of frailty. Factors considered in the subgroup analysis were mean age (<55 vs >55), percentage of female gender (0%–36% vs 50%–100%), location (United States/Canada, Europe, and Asia/others), frailty assessment year (2013–2014 vs 2015–2018), mean current CD4 cell count (466–574 vs 592–673 cells/μL), mean nadir CD4 cell count (50–159 vs 161–208 cells/μL), percentage of patients with undetectable VL (69.5%–92.6% vs 93.3%–100%), mean duration since diagnosis of HIV (11.1–17.5 vs 19.1–23.2 years), and mean duration of treatment (2.2–10.0 vs 11.3–18.3 years).
RESULTS
Selection Processes
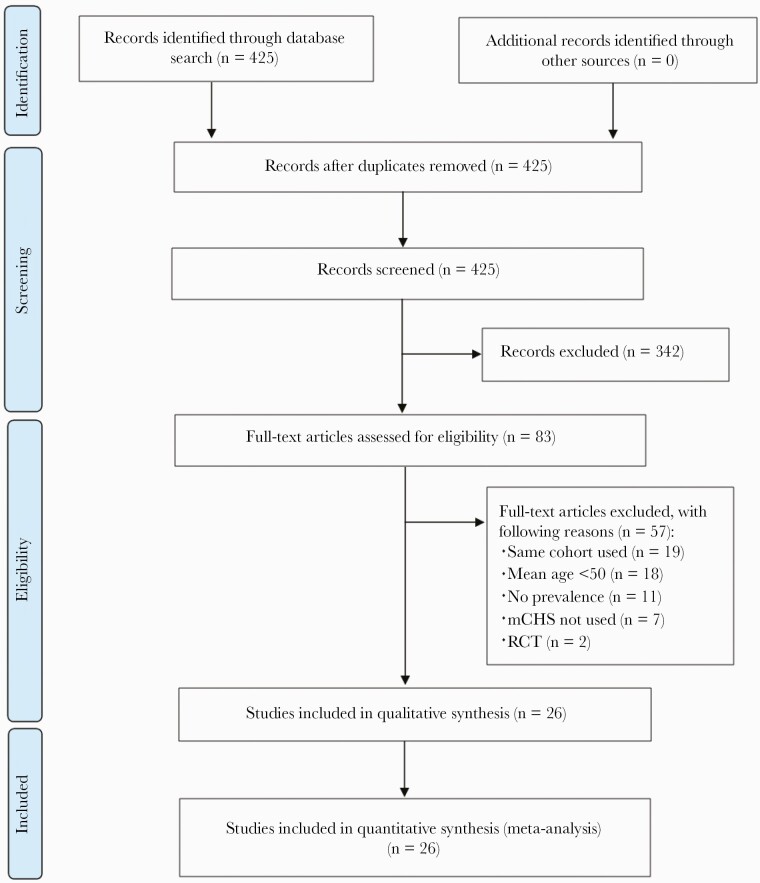
The PubMed search identified 425 articles, and no additional articles were found from other sources. All 425 articles were screened. The title and abstract screening excluded 342 articles, and the full texts of 83 articles were reviewed. Of these, 57 studies were removed for the following reasons: the same cohort used (n = 19), mean age <50 years old (n = 18), missing data (n = 11), the modified Cardiovascular Health Study (mCHS) had not been used for frailty diagnosis (n = 7), and randomized controlled trials (n = 2). Twenty-six studies were included for quantitative synthesis by meta-analysis. The flow chart of the literature search is summarized in Figure 1.
Figure 1.
Flow chart of systematic literature review. mCHS, modified Cardiovascular Health Study; RCT, randomized controlled trials.
Study Characteristics
Table 1 summarizes the 26 studies on the prevalence of frailty in PWH aged 50 or older [16, 19, 24–47]. The frailty assessments were conducted between 2003 [43] and 2018 [35, 40]. The prevalence of prefrailty was reported in 22 of the 26 studies. The size of the cohorts in each study ranged from 27 [35] to 1016 [32]. Two studies [28, 41] consisted only of male participants, and 2 other studies [31, 46] consisted of only female participants, whereas the rest used mixed-gender cohorts with the range of 6.4% [19] to 50% [43] female participants. The mean age of the cohorts ranged from 50.1 [38] to 61.3 years [47], although the exact mean age was not reported in 5 studies. Sixteen studies were conducted in the United States and Canada, 5 studies were conducted in the European Union, and 5 studies were conducted in Asia or other regions. Regarding HIV-related variables, the mean current CD4 cell count was reported in 18 of the 26 studies, ranging from 466 [37] to 673 cells/μL [36], and the mean nadir CD4 cell count was reported in 18 studies, ranging from 50 [33] to 208 cells/μL [40]. The percentage of patients with undetectable VL was reported in 19 studies, ranging from 69.5% [38] to 100% [19, 40]. The mean duration since diagnosis of HIV was reported in 14 studies, ranging from 11.1 [29] to 23.2 years [24]. The mean duration of treatment was reported in 7 studies, ranging from 2.2 [25] to 18.3 years [24].
Table 1.
Summary of Included Studies on the Prevalence of Frailty and Prefrailty in People With HIV Aged 50 or Older
| Author/Year /Study |
Location | Sample Size | Female (%) | Age (Range) | Frailty Criteria | Frailty Assessment Time | Current CD4 (Cells/μL) |
Nadir CD4 (Cells/μL) |
Undetectable VL (%) | Years since HIV Diagnosis (Years) | Treatment Duration (Years) | Frailty Prevalence | Prefrailty Prevalence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erlandson 2012 | US | 359 | 15 | 50.8 | mCHS | 2010 | 551 | 84 | 95 | 14.6 | - | 27/359 (7.5%) | 166/359 (46.2%) |
| Sandkovsky 2013 | US | 20 | 25 | 56.5 | mCHS | - | 658 | 161 | 95 | 14.2 | 11.3 | 4/20 (20%) | - |
| Althoff 2014 MACS | US | 898 | 0 | 51.3 | mCHS | 2009 | 543 | - | 74.2 | - | 10.0 | 257/898 (28.6%) | - |
| Önen 2014 SUN Study | US | 118 | - | (>50) | mCHS | 2011 | - | - | - | - | - | 8/118 (6.8%) | 52/118 (44.1%) |
| Greene 2015 SCOPE |
US | 155 | 6.4 | 57 | mCHS | - | 567 | 174 | 100 | 21 | - | 14/155 (9.0%) | 87/155 (56.1%) |
| Smit 2015 CARE | US | 50 | 50 | 56.8 | mCHS | 2003 | 554 | - | - | 20.1 | 9.8 | 8/50 (16.0%) | 22/50 (44%) |
| Kooij 2016 AGEhIV Cohort Study |
Netherlands | 521 | 11.3 | 52.8 | mCHS | 2011 | 563 | 180 | 93.3 | 12.0 | 9.8 | 55/521 (10.6%) | 264/521 (50.7%) |
| Rees 2016 | US | 55 | - | (>50) | mCHS | 2010 | - | - | - | - | - | 14/55 (25.5%) | - |
| Young 2016 | US | 61 | 100 | 57.8 | mCHS | 2005 | 595 | - | - | - | - | 7/61 (11.5%) | - |
| Brañas 2017 | Spain | 117 | 19.7 | 61.3 | mCHS | 2015 | 638 | 156 | 99.1 | 17.5 | - | 18/117 (15.4%) | 61/117 (52.1%) |
| Bregigeon 2017 | France | 175 | 30.9 | 55 | mCHS | 2013 | 592 | 148 | 84.3 | 23.2 | 18.3 | 14/175 (8.0%) | 111/175 (63.4%) |
| Ding 2017 | China | 345 | 22.3 | 52.7 | mCHS | 2015 | - | - | 92.6 | - | 2.2 | 21/345 (6.1%) | 97/345 (28.1%) |
| Paul 2018 | US | 122 | 20.5 | 57.5 | mCHS | - | 546 | 81 | 94 | 17.4 | - | 21/122 (17.2%) | 54/122 (44.3%) |
| Petit 2018 | France | 502 | 27.3 | 59.3 | mCHS | 2014 | - | - | 87.5 | - | - | 32/502 (6.4%) | 287/502 (57.2%) |
| Yeoh 2018 | Australia | 93 | 0 | 60 | mCHS | 2015 | 613 | 157 | 92.5 | 20.4 | - | 10/93 (10.8%) | 49/93 (52.7%) |
| Zamudio-Rodríguez 2018 | Mexico | 206 | 14.1 | 60.5 | mCHS | 2015 | - | 159 | 96.6 | 11.1 | - | 6/206 (2.9%) | 54/206 (26.2%) |
| Blanco 2019 | Spain | 131 | - | (>50) | mCHS | - | - | - | - | - | - | 8/131 (6.1%) | 57/131 (43.5%) |
| Fatukasi 2019 WIHS | US | 670 | 100 | (>50) | mCHS | 2016 | - | - | - | - | - | 35/670 (5.2%) | 88/670 (13.1%) |
| Kelly 2019 HAILO | US | 1016 | 19 | 51 | mCHS | 2014 | 621 | - | 91 | - | - | 62/1016 (6.1%) | 390/1016 (38.4%) |
| Morgello 2019 NNTC | US | 332 | 28 | 59.7 | mCHS | 2016 | 530 | 50 | 77 | - | - | 73/332 (22.0%) | 181/332 (54.5%) |
| Wulunggono 2019 | Indonesia | 27 | - | (>50) | mCHS | 2018 | - | - | - | - | - | 1/27 (3.7%) | 17/27 (63.0%) |
| Zeballos 2019 | Brazil | 201 | 36.3 | 55 | mCHS | 2017 | 673 | 194 | 88.6 | 17 | 16 | 39/201 (19.4%) | 99/201 (49.3%) |
| Chow 2020 HAHC-CVD | US | 73 | 11 | 51 | mCHS | 2012 or earlier | 466 | - | 84 | - | - | 5/73 (6.8%) | 26/73 (35.6%) |
| Rubtsova 2020 MDSA-HIAS |
US | 63 | 25.4 | 50.1 | mCHS | 2015 | 641 | 189 | 69.5 | 17.4 | - | 5/63 (7.9%) | 37/63 (58.7%) |
| Sun-Suslow 2020 | US | 178 | 12.4 | 54.3 | mCHS | 2017 | 607 | 185 | 94.4 | 19.1 | - | 15/178 (8.4%) | 81/178 (45.5%) |
| Walmsley 2020 | Canada | 96 | 19.8 | 56 | mCHS | 2018 | 574 | 208 | 100 | 21 | - | 10/96 (10.4%) | 79/96 (82.3%) |
Abbreviations: CD4, cluster of differentiation 4; HIV, human immunodeficiency virus; mCHS, modified Cardiovascular Health Study; US, United States; VL, viral load.
Methodological Quality Assessment
The 8-item Joanna Briggs Institute Critical Appraisal Checklist was used for all 26 studies, and the scores were all 7 of 7 (Supplementary Table S1). Therefore, all the studies included were considered of adequate methodological quality.
Meta-Analysis of the Prevalence of Frailty and Prefrailty
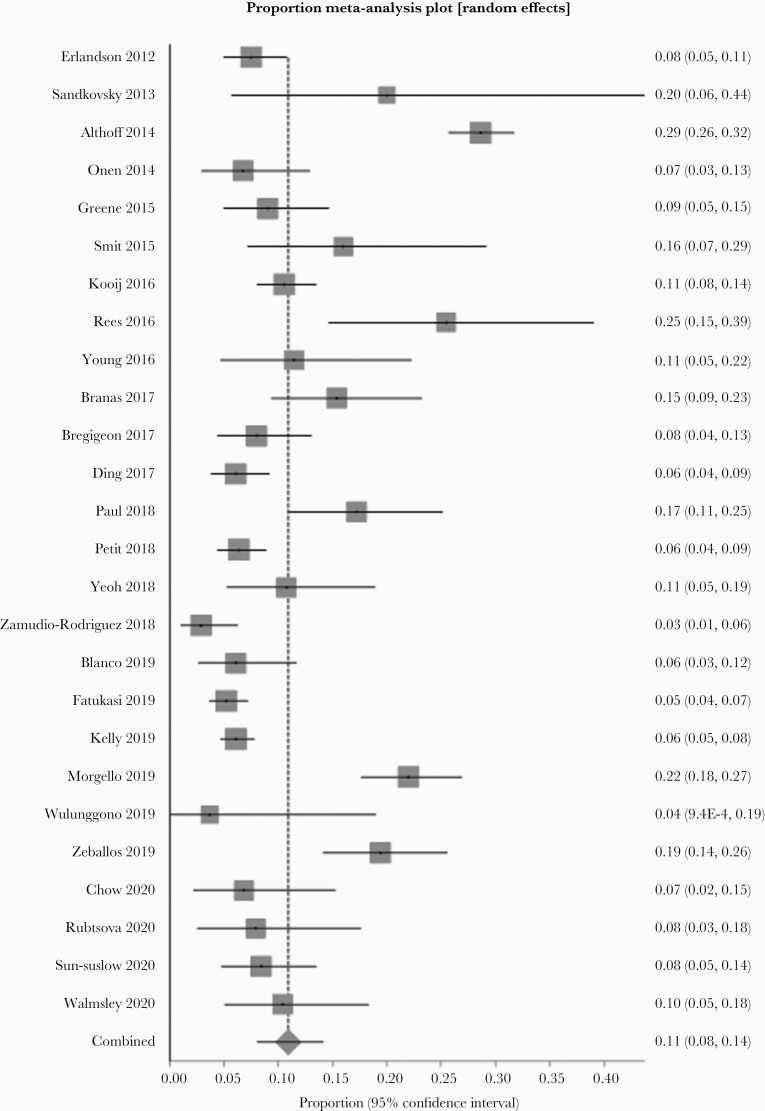
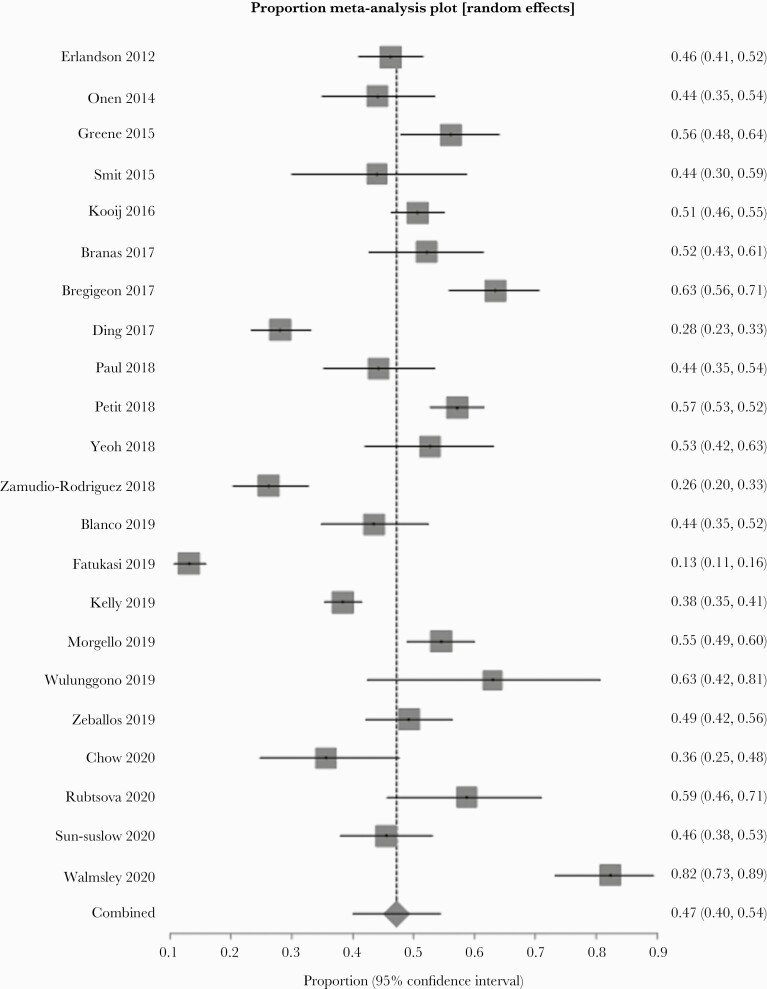
A random-effect meta-analysis showed that the pooled prevalence of frailty and prefrailty was 10.9% (n = 26, 95% confidence interval [CI ]= 8.1%–14.2%, P < .001) and 47.2% (n = 22, 95% CI = 40.1%–54.4%, P < .001), respectively. There was a high degree of heterogeneity (frailty: I2 = 93%, P < .001; prefrailty: I2 = 96%, P < .001). The forest plots for the prevalence of frailty and prefrailty are shown in Figures 2 and 3, respectively. No significant asymmetry was observed in the funnel plots.
Figure 2.
Forest plot of the prevalence of frailty.
Figure 3.
Forest plot of the prevalence of prefrailty.
Subgroup Meta-Analysis
Table 2 presents the findings of the subgroup analysis. Again, there was a high degree of heterogeneity persisting across all subgroups (I2 = 74%–97%, P < .02), except for the group with the longest duration (19.1–23.2 years) since HIV diagnosis (I2 = 0%, P = .66).
Table 2.
Subgroup Analyses of Cross-Sectional Associations Between HIV Status and Frailty
| Variables | Number of Cohorts | Pooled Prevalence (95% CI) | P for Heterogeneity | I2 |
|---|---|---|---|---|
| Total | 26 | 10.9 (8.1–14.2) | <.001 | 93% |
| Subgroup | ||||
| Mean/Median Age | ||||
| <55 | 11 | 10.2 (5.7–15.8) | <.001 | 96% |
| >55 | 10 | 12.2 (7.8–17.5) | <.001 | 88% |
| Female Sex (%) | ||||
| 0–36 | 19 | 11.2 (7.8–15.2) | <.001 | 94% |
| 50–100 | 3 | 10.0 (3.9–18.6) | <.01 | 79% |
| Location | ||||
| US/Canada | 16 | 12.5 (8.1–17.6) | <.001 | 95% |
| Europe | 5 | 9.0 (6.4–12.0) | .02 | 68% |
| Asia/others | 5 | 8.5 (3.4–15.4) | <.001 | 89% |
| Frailty Assessment Year | ||||
| 2003–2014 | 11 | 11.5 (6.7–17.4) | <.001 | 96% |
| 2015–2018 | 11 | 10.0 (6.3–14.6) | <.001 | 90% |
| Current CD4 (Cells/μL) | ||||
| 466–574 | 9 | 14.0 (8.6–20.6) | <.001 | 94% |
| 592–673 | 9 | 11.3 (7.8–15.4) | <.001 | 80% |
| Nadir CD4 (Cells/μL) | ||||
| 50–159 | 7 | 11.4 (6.4–17.6) | < 0.001 | 91% |
| 161–208 | 7 | 11.7 (8.7–15.0) | 0.02 | 60% |
| Undetectable VL (%) | ||||
| 69.5–92.6 | 10 | 11.7 (6.4–18.4) | < 0.001 | 97% |
| 93.3–100 | 9 | 10.1 (7.3–13.3) | < 0.001 | 74% |
| Years Since HIV Diagnosis | ||||
| 11.1–17.5 | 7 | 12.5 (7.6–18.3) | < 0.001 | 85% |
| 19.1–23.2 | 6 | 9.7 (7.7–12.0) | 0.66 | 0% |
| Treatment Duration (Years) | ||||
| 2.2–10.0 | 4 | 14.6 (5.0–28.0) | < 0.001 | 98% |
| 11.3–18.3 | 4 | 12.5 (6.5–20.0) | < 0.001 | 85% |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; VL, viral load.
DISCUSSION
We conducted a systematic review of the literature to collect the currently available evidence on the prevalence of frailty in PWH aged 50 or older and identified 26 studies for meta-analysis. The pooled prevalence of frailty and prefrailty defined by the Fried frailty phenotype was 10.9% and 47.2%, respectively. These figures are comparable to the pooled prevalence of frailty and prefrailty among community-dwelling older adults aged 65 or older previously reported in a systematic review [48]. However, it is important to note that the prevalence in each study was quite variable.
The studies identified by our systematic review demonstrated a wide range of frailty prevalence among PWH aged 50 or older (2.9% to 28.6%). For example, Zamudio-Rodríguez et al [29] reported a prevalence of 2.9% at a university-affiliated tertiary care center in Mexico City. Meanwhile, Althoff et al [41] reported a prevalence of 28.6%, which only included men who had sex with men in the United States. These differences are likely multifactorial, including age difference, time since HIV diagnosis, use and adherence to ART, differences in comorbidities among the study population, and the locations of studies. There is also considerable variability reported in how the Fried frailty phenotype was measured depending on local conditions, the experience of the personnel performing the measurements, or the availability of specialized equipment [49]. For example, in the study conducted by Sandkovsky et al [34], exhaustion, grip strength, gait speed, and physical activity were all measured differently from what Fried et al [7] proposed in their study. In addition, Theou et al [49] revealed the differences in the measured prevalence of frailty even within the same cohort due to the variability of Fried frailty phenotype measurement. Several studies had also compared the prevalence of frailty between PWH and people without HIV. For example, Desquilbet et al [50] examined 245 patients infected with HIV including uncontrolled HIV patients and 1977 non-HIV-infected patients, and they demonstrated that the prevalence of frailty among patients with HIV was higher than that of those without HIV. However, Piggott et al [15] reported that the risk of frailty among people with well controlled HIV was similar to that in controls without HIV. Several potential risk factors specific for PWH have been identified, including lower nadir and lower current CD4 cell counts and detectable viremia [19, 51].
Patients with HIV seem to develop frailty earlier than those without HIV [52]. Although the etiology of earlier onset frailty in people with HIV is unclear, a potential mechanism could be the immune deficiency and/or immune activation [53] observed during the clinical course of HIV. Changes to the immune system, such as T-cell telomere length or reduced naive T-cell generation, suggest accelerated immune senescence [54]. In addition, a strong association between inflammatory biomarkers and frailty has been established in previous studies of the general population [55, 56]. Furthermore, chronic inflammation has increasingly been recognized as playing a critical role in frailty in PWH [57]. It is notable that the relatively younger age when frailty presents in HIV cohorts highlights the importance of early frailty screening, evaluation, and intervention in this higher-risk population using a more standardized approach. Future studies should compare frailty prevalence in patients with or without HIV to determine whether frailty has an earlier onset in those with HIV.
Our study also showed that the prevalence of prefrailty was comparable between PWH aged 50 or older and community-dwelling older adults aged 65 or older. Although data are lacking in PWH, several studies have shown that prefrailty in older adults is associated with a worse survival rate and a higher number of impairments to their daily living and instrumental activities compared to those who are nonfrail [58]. Given that prior studies have shown that prefrailty predicts future frailty [7], further research in prefrailty in PWH is needed to characterize transitions between prefrailty and frailty in this vulnerable population.
There was a high degree of heterogeneity across the variables examined; nevertheless, heterogeneity disappeared in the group with the longest duration (19.1–23.2 years) since HIV diagnosis (I2 = 0%). This finding indicates that frailty prevalence in PWH of a longer duration since diagnosis is less variable than in PWH of a shorter duration, or PWH of all lengths duration. The survivorship bias is a possible explanation of this finding.
Frailty assessment is widely implemented in geriatric clinical practice [59], and early recognition of frailty can likely lead to meaningful interventions such as exercise and nutritional support to avoid adverse outcomes [60]. However, care for PWH is often provided in a context that prioritizes goals specific to HIV infection, whereas frailty in PWH may go underrecognized due to a lack of clinical guidelines or evidence [61]. This indicates a need for proper assessment and effective interventions to improve the assessment of frailty in HIV primary care management. This could include HIV provider education on frailty and team-based approaches, such as HIV-geriatric collaborative practice models [62]. Comprehensive geriatric assessment is useful for managing older patients and might be of benefit in patients with HIV with suspected frailty, even if they are younger than the usual age cutoff for geriatric clinics. Evidence surrounding interventions that prevent frailty [63] varies. Epidemiological evidence suggests that interventions to assess and manage comorbidities, such as reducing cardiovascular risk factors, increasing exercise, optimizing body mass index, and improving personal and community resources, may reduce the risk of frailty [64–67]. In patients with HIV, frailty management is likely to require an even more organized and multidimensional approach. For example, the 6M approach, focusing on mind, mobility, medications, multicomplexity, matter most, and modifiable, has been proposed for the management of older patients with HIV [61]. The geriatric 5Ms [68] plus the additional 6th M (modifiable), which targets contributing factors that are modifiable, such as substance use counseling or healthy diet, could be a useful model for caring for frail patients with HIV. Future studies should evaluate these interventions to establish their effectiveness at managing frailty in PWH.
Our study has several strengths. First, the systematic review and meta-analysis were performed using the PRISMA protocol statements, with a comprehensive and reproducible search strategy. Screening was conducted by 2 researchers independently, and all the studies included were evaluated for methodological quality. Second, the large number of studies included enabled subgroup analyses to be conducted. Third, we analyzed international data, including from the United States, Canada, Europe, and Asia. Fourth, to the best of our knowledge, this is the first systematic review-based meta-analysis that has evaluated the pooled prevalence of frailty and prefrailty in PWH aged 50 or over.
Some limitations must also be noted. First, only the PubMed database was used to identify studies in this review; therefore, some relevant studies may not have been included. However, we minimized this risk by thoroughly reviewing the reference lists of all the relevant studies. We also contacted the studies’ corresponding authors to inquire about other potential studies or cohorts for additional data. Second, whereas we conducted subgroup analyses, some may be underpowered due to the limited number of studies available. Third, our analysis only included studies that used the Fried frailty phenotype to define frailty. In addition, in some of these studies of PWH, variations of Fried frailty phenotype measurements were used. Therefore, our findings should be interpreted in the context of this specific frailty phenotype as well as variations in its measurement.
CONCLUSIONS
The pooled prevalence of frailty and prefrailty in PWH aged 50 or older was 10.9% and 47.2%, respectively. Given the comparative prevalence of frailty in older adults without HIV, future steps to identify effective strategies for frailty screening and intervention are needed for HIV-infected patients. Clinicians caring for HIV patients need to be aware of the risk of frailty, and more collaboration between HIV care providers and geriatricians is required for integrated HIV management.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. Jo Nash (Edanz) for editing a draft of this manuscript.
Author contributions. Y. Y. and G. K. had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. F. K. and G. K. supervised the study. Y. Y. and G. K. also designed the study. Y. Y., F. K., A. C., Y. T., and G. K. participated in the acquisition, analysis, and interpretation of data. Y. T. and G. K. did the statistical analysis. Y. Y., T. K., and G. K. prepared the first draft of the manuscript. All authors were involved in the critical revision of the manuscript for important intellectual content and approved the final version for submission. Y. Y. was responsible for administrative, technical, and material support.
Disclaimer. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guaraldi G, Milic J, Mussini C.. Aging with HIV. Curr HIV/AIDS Rep 2019; 16:475–81. [DOI] [PubMed] [Google Scholar]
- 3. Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG.. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med 2017; 18:256–66. [DOI] [PubMed] [Google Scholar]
- 4. Greene M, Justice AC, Lampiris HW, Valcour V.. Management of human immunodeficiency virus infection in advanced age. JAMA 2013; 309:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet 2019; 394:1376–86. [DOI] [PubMed] [Google Scholar]
- 6. Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; M146–M56. [DOI] [PubMed] [Google Scholar]
- 8. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016; 26:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howlett SE, Rutenberg AD, Rockwood K.. The degree of frailty as a translational measure of health in aging. Nat Aging 2021; 1:651–65. [DOI] [PubMed] [Google Scholar]
- 10. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Theou O, Brothers TD, Mitnitski A, Rockwood K.. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–51. [DOI] [PubMed] [Google Scholar]
- 12. Rothman MD, Leo-Summers L, Gill TM.. Prognostic significance of potential frailty criteria. J Am Geriatr Soc 2008; 56:2211–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 2016; 17:1163.e1–17. [DOI] [PubMed] [Google Scholar]
- 14. Desquilbet L, Jacobson LP, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci 2011; 66:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013; 8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erlandson KM, Allshouse AA, Jankowski CM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012; 13:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajasuriar R, Chong ML, Ahmad Bashah NS, et al. Major health impact of accelerated aging in young HIV-infected individuals on antiretroviral therapy. AIDS 2017; 31:1393–403. [DOI] [PubMed] [Google Scholar]
- 18. Levett TJ, Cresswell FV, Malik MA, et al. Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. J Am Geriatr Soc 2016; 64:1006–14. [DOI] [PubMed] [Google Scholar]
- 19. Greene M, Covinsky KE, Valcour V, et al. Geriatric syndromes in older HIV-infected adults. J Acquir Immune Defic Syndr 2015; 69:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dávila-De la Llave G, Parra-Guerra H, Tamez-Rivera O, Ávila-Funes JA.. Frailty in patients aged 50 and older with HIV/AIDS in Mexico. Eur Geriatr Med 2013; 4:S75. [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017; 186:420–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joanna Briggs Institute. Critical Appraisal Tools. Available at: https://jbi.global/critical-appraisal-tools. Accessed 3 June 2021.
- 24. Bregigeon S, Galinier A, Zaegel-Faucher O, et al. Frailty in HIV infected people: a new risk factor for bone mineral density loss. AIDS 2017; 31:1573–7. [DOI] [PubMed] [Google Scholar]
- 25. Ding Y, Lin H, Liu X, et al. Higher prevalence of frailty among a sample of HIV-infected middle-aged and older Chinese adults is associated with neurocognitive impairment and depressive symptoms. J Infect Dis 2017; 215:687–92. [DOI] [PubMed] [Google Scholar]
- 26. Paul RH, Cooley SA, Garcia-Egan PM, Ances BM.. Cognitive performance and frailty in older HIV-positive adults. J Acquir Immune Defic Syndr 2018; 79:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petit N, Enel P, Ravaux I, et al. Frail and pre-frail phenotype is associated with pain in older HIV-infected patients. Medicine (Baltimore) 2018; 97:e9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeoh H-L, Cheng A, Palmer C, Crowe SM, Hoy JF.. Frailty in men living with HIV: a cross-sectional comparison of three frailty instruments. Antivir Ther 2018; 23:117–27. [DOI] [PubMed] [Google Scholar]
- 29. Zamudio-Rodríguez A, Belaunzarán-Zamudio PF, Sierra-Madero JG, et al. Association between frailty and HIV-associated neurodegenerative disorders among older adults living with HIV. AIDS Res Hum Retroviruses 2018; 34:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blanco J-R, Barrio I, Ramalle-Gómara E, et al. Gender differences for frailty in HIV-infected patients on stable antiretroviral therapy and with an undetectable viral load. PLoS One 2019; 14:e0215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fatukasi TV, Edmonds A, Gustafson DR, et al. Prevalence and 1-year incidence of frailty among women with and without HIV in the Women’s Interagency HIV Study. AIDS 2019; 33:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ.. Frailty is an independent risk factor for mortality, cardiovascular disease, bone disease, and diabetes among aging adults with human immunodeficiency virus. Clin Infect Dis 2019; 69:1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morgello S, Gensler G, Sherman S, et al. Frailty in medically complex individuals with chronic HIV. AIDS 2019; 33:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandkovsky U, Robertson KR, Meza JL, et al. Pilot study of younger and older HIV-infected adults using traditional and novel functional assessments. HIV Clin Trials 2013; 14:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wulunggono W, Yunihastuti E, Shatri H, Wahyudi ER, Ophinni Y.. Frailty among HIV-1 infected adults under antiretroviral therapy in Indonesia. Curr HIV Res 2019; 17:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeballos D, Lins L, Brites C.. Frailty and its association with health related quality of life in older HIV patients, in Salvador, Brazil. AIDS Res Hum Retroviruses 2019; 35:1074–81. [DOI] [PubMed] [Google Scholar]
- 37. Chow DC, Bernas MA, Gangcuangco LM, et al. Frailty is associated with insulin resistance in chronic human immunodeficiency virus infection. Clin Infect Dis 2020; 71:1127–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubtsova AA, Sabbag S, Sundermann E, et al. Frailty and neurocognitive impairment: impacts on quality of life in HIV. J Assoc Nurses AIDS Care 2020; 31:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun-Suslow N, Paolillo EW, Morgan EE, Letendre S, Iudicello J, Moore DJ.. Brief report: frailty and HIV disease severity synergistically increase risk of HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr 2020; 84:522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walmsley SL, Ren M, Simon C, et al. Pilot study assessing the Rotterdam Healthy Aging Score in a cohort of HIV-positive adults in Toronto, Canada. AIDS 2020; 34:859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Önen NF, Patel P, Baker J, et al. Frailty and pre-frailty in a contemporary cohort of HIV-infected adults. J Frailty Aging 2014; 3:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smit E, Wanke C, Dong K, et al. Frailty, food insecurity, and nutritional status in people living with HIV. J Frailty Aging 2015; 4:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kooij KW, Wit FW, Schouten J, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS 2016; 30:241–50. [DOI] [PubMed] [Google Scholar]
- 45. Rees HC, Meister E, Mohler MJ, Klotz SA.. HIV-related frailty is not characterized by sarcopenia. J Int Assoc Provid AIDS Care 2016; 15:131–4. [DOI] [PubMed] [Google Scholar]
- 46. Young P, Shah J, Zhang C, et al. Frailty in postmenopausal African American and Hispanic HIV-infected women. J Frailty Aging 2016; 5:242–6. [DOI] [PubMed] [Google Scholar]
- 47. Brañas F, Jiménez Z, Sánchez-Conde M, et al. Frailty and physical function in older HIV-infected adults. Age Ageing 2017; 46:522–6. [DOI] [PubMed] [Google Scholar]
- 48. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC.. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–92. [DOI] [PubMed] [Google Scholar]
- 49. Theou O, Cann L, Blodgett J, Wallace LMK, Brothers TD, Rockwood K.. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev 2015; 21:78–94. [DOI] [PubMed] [Google Scholar]
- 50. Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007; 62:1279–86. [DOI] [PubMed] [Google Scholar]
- 51. Brothers TD, Kirkland S, Guaraldi G, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis 2014; 210:1170–9. [DOI] [PubMed] [Google Scholar]
- 52. Bloch M. Frailty in people living with HIV. AIDS Res Ther 2018; 15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Álvarez S, Brañas F, Sánchez-Conde M, et al. Frailty, markers of immune activation and oxidative stress in HIV infected elderly. PLoS One 2020; 15:e0230339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pathai S, Bajillan H, Landay AL, High KP.. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2014; 69:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 2016; 31:1–8. [DOI] [PubMed] [Google Scholar]
- 56. Marcos-Pérez D, Sánchez-Flores M, Proietti S, et al. Association of inflammatory mediators with frailty status in older adults: results from a systematic review and meta-analysis. GeroScience 2020; 42:1451–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leng SX, Margolick JB.. Aging, sex, inflammation, frailty, and CMV and HIV infections. Cell Immunol 2020; 348:104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fernández-Garrido J, Ruiz-Ros V, Buigues C, et al. Clinical features of prefrail older individuals and emerging peripheral biomarkers: a systematic review. Arch Gerontol Geriatr 2014; 59:7–17. [DOI] [PubMed] [Google Scholar]
- 59. Walston J, Buta B, Xue QL.. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med 2018; 34:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database Syst Rev Implement Rep 2018; 16:140–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Erlandson KM, Karris MY.. HIV and aging: reconsidering the approach to management of comorbidities. Infect Dis Clin North Am 2019; 33:769–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guaraldi G, Palella FJ.. Clinical implications of aging with HIV infection: perspectives and the future medical care agenda. AIDS 2017; 31(Suppl 2):S129–35. [DOI] [PubMed] [Google Scholar]
- 63. Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 2017; 46:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hubbard RE, Searle SD, Mitnitski A, Rockwood K.. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian study of health and aging. J Nutr Heal Aging 2009; 13:468–72. [DOI] [PubMed] [Google Scholar]
- 65. Kojima G, Iliffe S, Walters K.. Smoking as a predictor of frailty: a systematic review. BMC Geriatr 2015; 15:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hubbard RE, Fallah N, Searle SD, Mitnitski A, Rockwood K.. Impact of exercise in community-dwelling older adults. PLoS One 2009; 4:e6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K.. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci 2010; 65:377–81. [DOI] [PubMed] [Google Scholar]
- 68. Tinetti M, Huang A, Molnar F.. The geriatrics 5M’s: a new way of communicating what we do. J Am Geriatr Soc 2017; 65:2115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.