ABSTRACT
Establishing a functional circulatory system is required for post-implantation development during murine embryogenesis. Previous studies in loss-of-function mouse models showed that FOXO1, a Forkhead family transcription factor, is required for yolk sac (YS) vascular remodeling and survival beyond embryonic day (E) 11. Here, we demonstrate that at E8.25, loss of Foxo1 in Tie2-cre expressing cells resulted in increased sprouty 2 (Spry2) and Spry4 expression, reduced arterial gene expression and reduced Kdr (also known as Vegfr2 and Flk1) transcripts without affecting overall endothelial cell identity, survival or proliferation. Using a Dll4-BAC-nlacZ reporter line, we found that one of the earliest expressed arterial genes, delta like 4, is significantly reduced in Foxo1 mutant YS without being substantially affected in the embryo proper. We show that FOXO1 binds directly to previously identified Spry2 gene regulatory elements (GREs) and newly identified, evolutionarily conserved Spry4 GREs to repress their expression. Furthermore, overexpression of Spry4 in transient transgenic embryos largely recapitulates the reduced expression of arterial genes seen in conditional Foxo1 mutants. Together, these data reveal a novel role for FOXO1 as a key transcriptional repressor regulating both pre-flow arterial specification and subsequent vessel remodeling within the murine YS.
KEY WORDS: Arterial specification, Dll4, Foxo1, Sprouty
Summary: A previously unreported role for FOXO1 as a key transcriptional repressor regulating both pre-flow arterial specification and subsequent vessel remodeling within the murine yolk sac.
INTRODUCTION
During early development, the mammalian embryo requires a functional circulatory system to distribute oxygen, nutrients and hormones. The mammalian heart is the first organ to form and function within this early embryo, along with the first arteries and veins that arise de novo via vasculogenesis (Fish and Wythe, 2015; Risau, 1994; Risau and Flamme, 1995; Chong et al., 2011). Primitive erythrocytes form in the blood islands of the extra-embryonic yolk sac (YS), and are drawn into circulation as the heart begins to beat around embryonic day (E) 8 in the mouse embryo (Lucitti et al., 2007; Palis, 2014; Ji et al., 2003). A complete circulatory loop between the embryo and the extra-embryonic YS is evident shortly after the onset of cardiac contractions, with blood flowing through the dorsal aorta to the vitelline (omphalomesenteric) artery (VA), through the YS capillary plexus, and back through the vitelline (omphalomesenteric) vein (VV) to the sinus venosus of the heart. Circulation through the YS is the main circulatory loop until the chorio-allantoic placenta connections develop later, around E9.
A key finding several years ago showed that arterial-venous (AV) identity is established before the onset of blood flow in the early mouse embryo (Herzog et al., 2005; Chong et al., 2011; Aitsebaomo et al., 2008; Wang et al., 1998; Adams et al., 1999). Others have since shown that some aspects of AV identity are plastic, as they can be influenced by changes in blood flow and hemodynamics (le Noble et al., 2004, 2005; Wragg et al., 2014). Extensive work in zebrafish showed that AV specification depends on differential responses to VEGF signaling through the tyrosine kinase receptor KDR (also known as VEGFR2 and FLK1), high levels of which activate the MEK/ERK kinase cascade in arterial cells and PI3 K/AKT signaling in venous cells (Fish and Wythe, 2015; Covassin et al., 2006; Weinstein and Lawson, 2002). In the arterial endothelium, VEGFR2 signaling stimulates the Notch pathway, which in turn promotes an arterial identity while simultaneously repressing a venous fate (Weinstein and Lawson, 2002; Krebs et al., 2010, 2000; Lawson et al., 2001, 2002; Liu et al., 2003; Shutter et al., 2000; Swift and Weinstein, 2009; Siekmann and Lawson, 2007; Duarte et al., 2004; Lobov et al., 2007). VEGF upregulates expression of delta-like 4 (Dll4), which encodes a ligand for the Notch family of transmembrane receptors. Dll4 is the earliest Notch ligand expressed in arterial cells in the early mouse embryo (Shutter et al., 2000; Mailhos et al., 2001; Wythe et al., 2013; Cleaver and Krieg, 1998; Chong et al., 2011), and is essential for AV patterning (Krebs et al., 2000; Gale et al., 2004; Duarte et al., 2004). Expression of Dll4 depends on the activation of ETS transcription factors, which are downstream of VEGF signaling (Wythe et al., 2013; Fish et al., 2017), and Dll4 mRNA expression can be increased by shear stress (Masumura et al., 2009; Obi et al., 2009). However, the expression of Dll4 and a few other select arterial markers before, and independently of, the onset of blood flow (Chong et al., 2011; Wang et al., 1998) suggests flow-independent mechanisms regulate arterial specification. VEGFR2 also upregulates expression of the main endothelial cell surface receptor for DLL4, NOTCH1 (Lawson et al., 2002). NOTCH1 itself is activated by blood flow, and the strength of this signal depends on the magnitude of shear stress (Masumura et al., 2009; Mack et al., 2017). NOTCH1 regulates cell junctions and cell cycle arrest, and induces an arterial gene expression program via a connexin 37 (Gja4) and Cdkn1b (previously known as p27Kip1) pathway (Mack et al., 2017; Su et al., 2018; Fang et al., 2017). Crucially, both VEGFR2 and NOTCH1 are thought to act as mechanosensors, likely linking arterial specification of ECs and hemodynamic feedback via blood flow to re-enforce and solidify their arterial identity (Mack et al., 2017; Tzima et al., 2005; Mack and Iruela-Arispe, 2018; Shay-Salit et al., 2002).
Forces exerted by blood flow play a clear role in AV specification, and also influence vessel morphogenesis and remodeling in the early embryo (Fang et al., 2017; Masumura et al., 2009; le Noble et al., 2004; Chong et al., 2011; Hwa et al., 2017). Hemodynamic force is both necessary and sufficient to remodel the high-resistance mouse YS capillary plexus into a more complex hierarchical network with a large caliber VA and VV, which progressively leads to smaller diameter vessels (Lucitti et al., 2007). Our lab has shown that murine YS vessel remodeling depends on both vessel fusion and EC migration (Udan et al., 2013). Interestingly, live-imaging studies have shown distinct differences in how arterial and venous cells respond to changes in hemodynamic force (Udan et al., 2013; Kondrychyn et al., 2020; Goetz et al., 2014). These data suggest that pathways that control AV identity, which can be regulated by blood flow, may also influence physical responses of ECs to blood flow, such as migration and motility (which facilitate vessel remodeling).
Despite the knowledge gained regarding the mechanisms regulating AV identity and the discovery of mechanosensors that are required for ECs to sense blood flow, a full understanding of these mechanistic pathways has yet to be realized. A recent analysis estimates that ∼6% of genes in the genome (∼1200) may be required during early cardiovascular development (E9.5-E12.5) (Dickinson et al., 2016). One such gene, and the focus of this study, is Foxo1 (forkhead box protein O1). Forkhead domain class O transcription factors (FOXOs) integrate different cellular signaling pathways to regulate cellular homeostasis (Paik et al., 2007; Huang and Tindall, 2007; Jiramongkol and Lam, 2020). daf-16/FoxO was originally identified as a regulator of dauer formation in C. elegans (Albert et al., 1981) and was later shown to control longevity by sensing environmental cues such as hormones, nutrient availability, oxidative stress and energy metabolism via signaling through the insulin, AKT/mTor, JNK and AMPK pathways (Sun et al., 2017). Several studies have since established that Foxo1 is also required for normal embryonic development in mice. Homozygous null Foxo1 embryos display a primitive YS vasculature, pericardial edema and disorganized embryonic vessels by E9.5, resulting in lethality by E11.5 (Furuyama et al., 2004; Dharaneeswaran et al., 2014; Hosaka et al., 2004; Sengupta et al., 2012; Wilhelm et al., 2016; Ferdous et al., 2011). Further analysis showed that Tie2-cre-mediated loss of Foxo1 in the endothelium, but not the myocardium, phenocopied germline loss of Foxo1 (Sengupta et al., 2012), demonstrating the requirement for FOXO1 in the embryonic vasculature. Follow-up studies have since found that FOXO1 controls a variety of different processes in endothelial cells (ECs), including, but not limited to EC proliferation and metabolism (Wilhelm et al., 2016), endothelial barrier function (Beard et al., 2020), sprouting angiogenesis (Kim et al., 2019; Fukumoto et al., 2018; Dang et al., 2017), autophagy (Zhang et al., 2019), EC growth (Riddell et al., 2018; Rudnicki et al., 2018) and migration (Niimi et al., 2017). Despite these studies, the exact function that FOXO1 plays in the early vasculature remains elusive. Given that FOXO1 activity can be modulated in response to fluid shear stress (Chlench et al., 2007; Dixit et al., 2008), combined with our studies showing that hemodynamic force is necessary and sufficient for early vascular remodeling (Lucitti et al., 2007; Udan et al., 2013), and the growing evidence for a role for FOXO1 in cell migration and sprouting angiogenesis (Fosbrink et al., 2006; Niimi et al., 2017; Kim et al., 2019), we sought to define the requirement for FOXO1 in the remodeling vasculature of the early embryonic YS.
Herein, we demonstrate a previously unreported role for FOXO1 in regulating AV identity in the murine YS vasculature. From conditional loss-of-function mutants, where Foxo1 is deleted specifically in Tie2-cre-expressing cells (predominantly ECs) (Eklund and Olsen, 2006; Schlaeger et al., 1997; Kisanuki et al., 2001; Chu et al., 2016), we identified a significant downregulation of arterial gene expression in the mouse YS prior to the onset of blood flow. We also detected a significant reduction in Flk1 (also known as Vegfr2) transcripts, but normal expression levels for other pan-endothelial genes, such as Pecam1, indicating that the formation of ECs is not disrupted but rather VEGF signaling is affected. Using a recently developed Dll4 arterial reporter line (Herman et al., 2018), we showed that Foxo1 is required for Dll4 expression in the murine YS, but not in the embryo proper. Further analysis showed that FOXO1 represses expression of sprouty (Spry) genes, which encode inhibitors of Raf/MEK/ERK signaling downstream of FGF and VEGF receptor activation. sprouty factors also modulate angiogenesis by negatively regulating small vessel branching, as well as repressing EC migration (Gong et al., 2013; Wietecha et al., 2011; Lee et al., 2001). Although some researchers have shown that FOXO1 positively regulates sprouty gene expression in the liver (Paik et al., 2007), our studies demonstrate that Foxo1 loss increased sprouty 2 (Spry2) and Spry4 mRNA levels, suggesting that FOXO1 represses Spry2 and/or Spry4 in the murine YS. We went on to find that Spry4 overexpression throughout the YS and embryo profoundly altered arterial gene expression in the YS but, similar to early FOXO1 loss, had an insignificant effect on these transcripts in the embryo proper. Taken together, these data highlight a novel role for FOXO1 in regulating arteriovenous specification in the early YS and reveal a new mechanism whereby FOXO1 represses sprouty gene expression and downstream signaling in the endothelium.
RESULTS
YS vascular remodeling defects in Foxo1ECKO embryos are not due to abnormalities in hemodynamic force or allantois defects
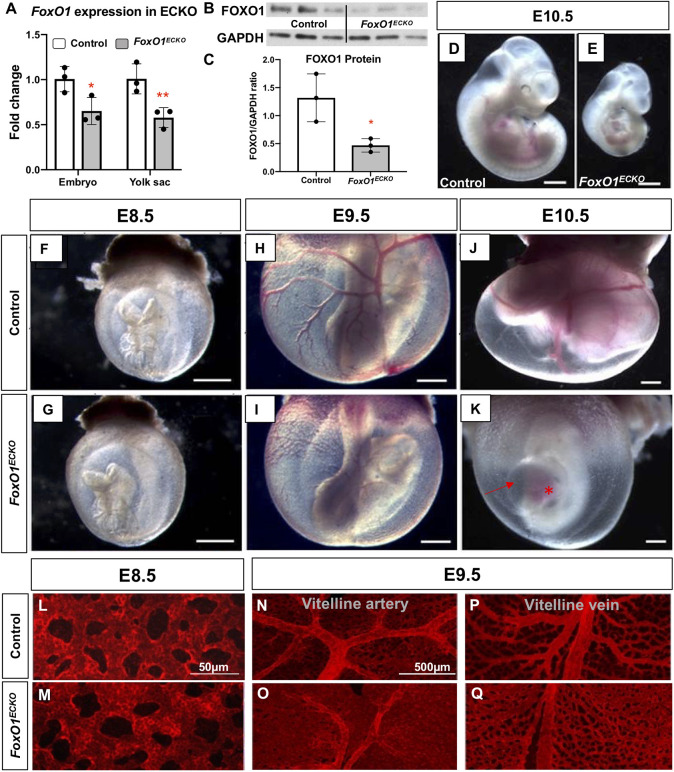
To define the role of FOXO1 in ECs within the early embryo, we conditionally ablated Foxo1 in the endothelium by crossing female Foxo1flox mice (Paik et al., 2007) with male Tie2-Cre transgenic mice, producing control (Tie2-Cre+/+;FoxO+/flox) and CKO (Tie2-Cre+/tg;Foxo1flox/flox, hereafter referred to as Foxo1ECKO) embryos, in which Cre recombinase is expressed in endothelial and in hematopoietic cells and progenitors, starting at E7.5 (Kisanuki et al., 2001). The efficiency of the Tie2-Cre-mediated recombination of the Foxo1flox allele was confirmed at the transcript level by qRT-PCR and at the protein level using western blots. At E8.5, Foxo1 mRNA was significantly reduced in the conditional knockout YSs compared with control littermates, in contrast to the germline knockouts where Foxo1 transcripts were undetectable (Fig. 1A, Fig. S1C). FOXO1 protein levels were also significantly reduced at E8.5 (Fig. 1B,C). We observed gross phenotypes in Foxo1 conditional and global knockout mutants, similar to previous reports (Fig. 1D,E; Fig. S1A,B) (Sengupta et al., 2012; Furuyama et al., 2004; Hosaka et al., 2004). We did not detect any visible differences in vascular morphology or embryo size between control and Foxo1ECKO at E8.5 (Fig. 1F,G). However, at E9.5 and E10.5, although the primitive vascular plexus of the control YS remodeled into a hierarchy of large caliber vessels that iteratively branched into smaller diameter capillaries, Foxo1ECKO YSs retained a primitive vascular plexus (Fig. 1H-K). Foxo1ECKO YSs labeled using anti-PECAM1 (CD31) antibodies, a pan EC marker, showed normal plexus at E8.5 but thinner and less branched vitelline vein and artery compared with controls by E9.5 (Fig. 1L-Q). At E9.5, mutant embryos were reduced in overall size compared with control littermates (Fig. 1H,I), which became more evident at E10.5 (Fig. 1D,E,J,K). Additionally, in both E9.5 (not shown) and E10.5 Foxo1ECKO embryos (Fig. 1K), the pericardial sac was enlarged, and blood was abnormally pooled in the heart. Overall, phenotypes in Foxo1 conditional and global mutant embryos are highly reproducible and our observations align well with previously published studies (Sengupta et al., 2012; Furuyama et al., 2004; Hosaka et al., 2004), supporting the conclusion that loss of Foxo1 in ECs and other Tie2-expressing cells is sufficient to cause defects in vessel remodeling.
Fig. 1.
Foxo1ECKO results in vascular remodeling defects and lethality. (A) qRT-PCR for Foxo1 expression in E8.5 control and Foxo1ECKO YSs (*P<0.05, **P<0.01). (B,C) FOXO1 protein expression in E8.5 control and Foxo1ECKO YS (n=3) by western and quantification (*P<0.05). (D,E) Bright-field images of E10.5 littermate control and Foxo1ECKO embryos. Scale bars: 500 µm. (F-K) Control and Foxo1ECKO embryos within the YS at E8.5 (F,G), E9.5 (H,I) and E10.5 (J,K). Scale bars: 500 µm. (K) Pericardial edema (arrow); blood pooling in the heart (asterisk). (L-Q) Pecam1 staining in E8.5 and E9.5 control and CKO YSs. Data are mean±s.d.
The appearance of pericardial edema in Foxo1ECKO embryos suggested heart failure and compromised circulation. To determine if and when blood flow was impaired, we crossed a primitive erythrocyte transgenic fluorescent reporter line, ε-globin-KGFP (Dyer et al., 2001), into the Foxo1ECKO background. Live imaging of cultured embryos and high-speed confocal microscopy were used to track individual KGFP-labeled erythroblasts to determine blood velocity (Fig. 2) (Jones et al., 2004). At E8.5, both control and Foxo1ECKO embryonic vessels were filled with blood and erythrocytes moved in a steady directional flow with similar periodicity and velocity (Fig. 2A,B,E,G). By E9.5, control embryos had clearly remodeled vessels, blood flow velocity greater than 700 μm/s and a defined wave pattern with periodicity of 400 ms (Fig. 2C,F). However, Foxo1ECKO embryos vessels were not remodeled, and blood flow had a significantly lower velocity with a poorly defined wave pattern (Fig. 2D,H). Quantification of blood velocity (Fig. 2I,J) and heart rate (Fig. 2K,L) revealed no statistical difference in E8.5 control and Foxo1ECKO embryos (Fig. 2I,K). However, by E9.5, blood velocity and heart rate were significantly decreased in Foxo1ECKO embryos (Fig. 2J,L), indicating heart failure was occurring in embryos with un-remodeled vessels. Thus, flow initiates normally in Foxo1ECKO embryos, but defects in both vascular remodeling and blood flow abnormalities were observed by E9.5. Given these results, we restricted our analysis, whenever possible, to E8.25 (the 4- to 7-somite range) embryos so that poor blood flow did not influence our data.
Fig. 2.
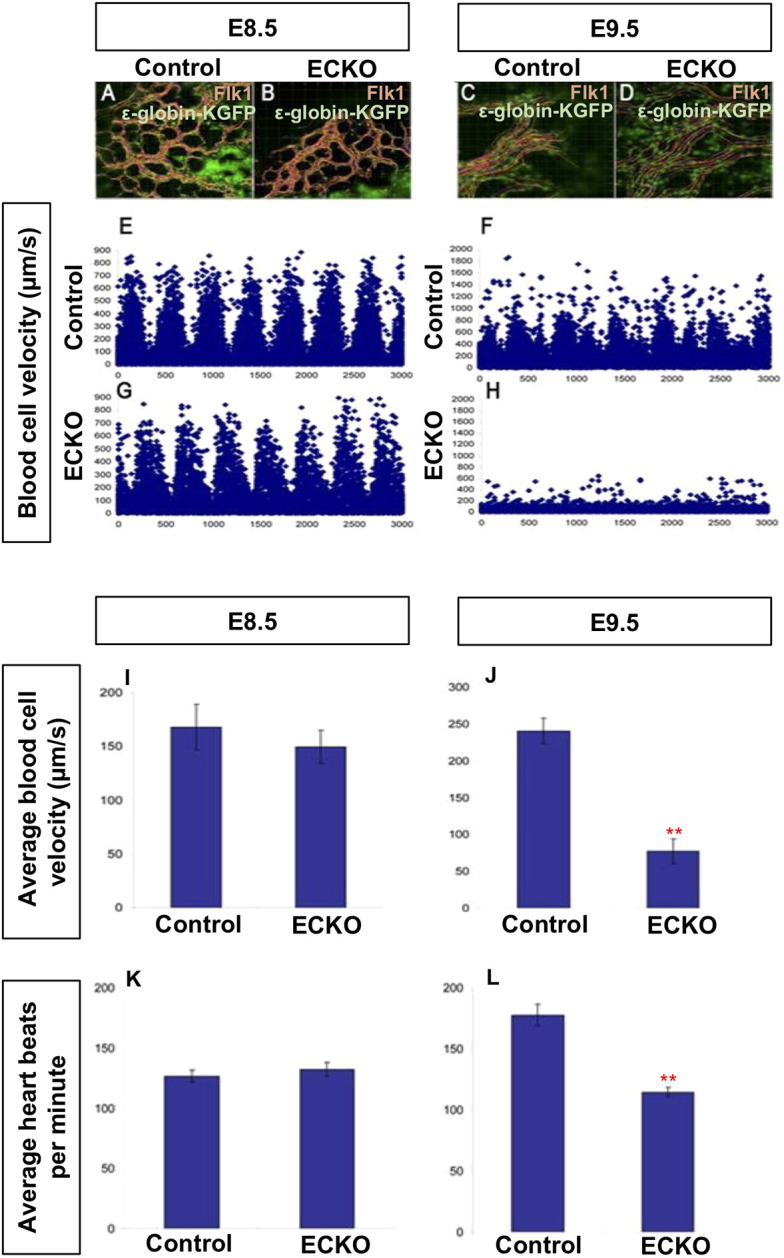
Vascular remodeling defects in Foxo1ECKO embryos do not result from reduced blood flow. Primitive erythroblasts in circulation in wild-type and CKO embryos were marked by crossing to an ε-globin-GFP transgenic reporter. Representative still images of E8.5 wild-type (A) and Foxo1ECKO (B), and E9.5 wild type (C) and Foxo1ECKO (D) embryos. (E-H) Individual blood cells from A-D were tracked and velocity profiles are plotted. (I,J) Quantification of the average blood velocity (Mann–Whitney U-test, **P=0.005). Average heart rates quantified in wild-type and Foxo1ECKO embryos at E8.5 (K) and E9.5 (L) (Kruskal–Wallis test, **P=0.003). Data are mean±s.e.m.
A previous report on the role of Foxo1 in placental development described phenotypes including swollen or hydropic allantois, failed chorion-allantoic fusion, and increased cell death in the allantois (Ferdous et al., 2011). Since the previous study was conducted in germline Foxo1 null embryos, we examined the allantois in global null and Foxo1ECKO mutants. While germline Foxo1 mutants exhibited partially penetrant defects in allantois formation and fusion, these phenotypes were not evident in Foxo1ECKO embryos at E9.5 (Table 1). However, both germline and Foxo1ECKO embryos show defects in YS vascular remodeling, heart failure phenotypes, and lethality by E11.5. Taken together, these results support a cell autonomous requirement for FOXO1 in YS vessel remodeling and suggest that published cardiac and blood flow defects are secondary to the impaired remodeling of YS vasculature.
Table 1.
Allantois phenotype analysis in control, null and Foxo1ECKO embryos at E9.5
FOXO1 is necessary to maintain Fkl1 expression in E8.25 YSs
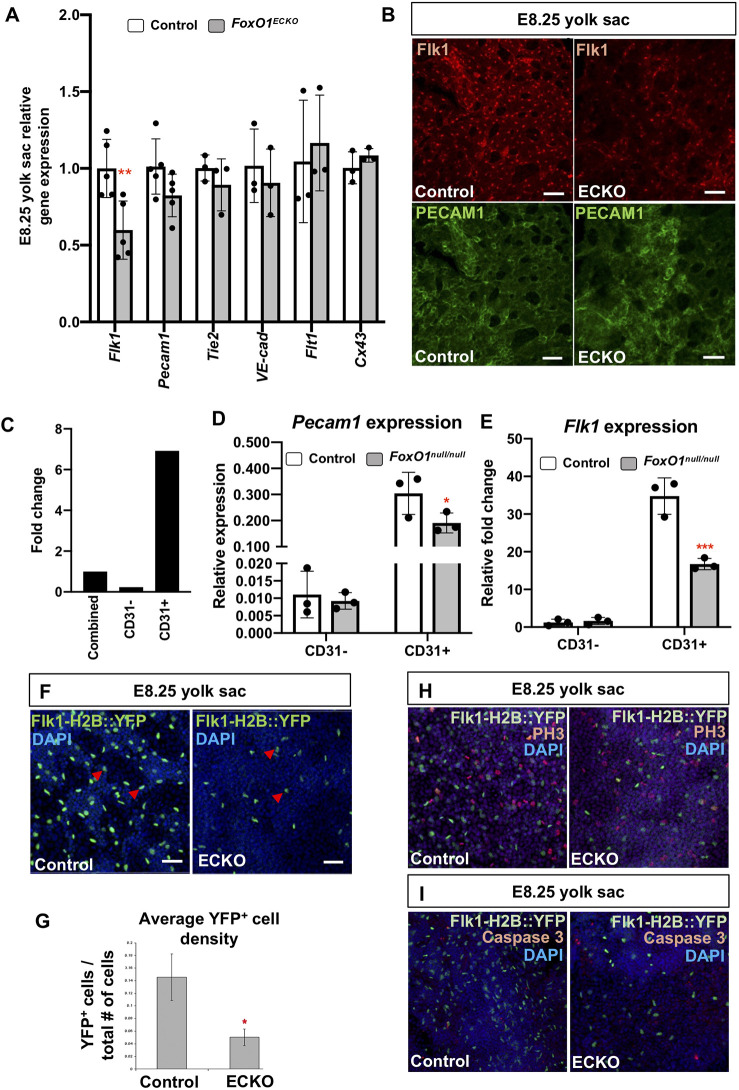
To assess mechanisms disrupted in Foxo1ECKO, we examined transcript levels of genes normally expressed in blood vessels by qRT-PCR within Foxo1ECKO E8.25 YSs. We focused these experiments at E8.25 prior to vessel remodeling and to avoid potential complications of later stage heart failure. We observed a significant decrease in Flk1 expression in Foxo1ECKO YSs, while other pan-endothelial markers, such as Pecam1, Tie2, VE-cadherin (Cdh5), Flt1 (also known as Vegfr1) and Cx43 (Gja1) were not significantly affected (Fig. 3A). Consistent downregulation of Flk1 was also seen in the Foxo1 global knockout YSs and no change in Flk1 expression was detected in the embryo proper (Fig. S2A). Immunolabeling of endogenous Flk1 showed a similar reduction in expression in Foxo1ECKO YS ECs at E8.25, while PECAM1 (CD31) expression appeared unaffected (Fig. 3B). We further confirmed the reduction in Flk1 expression using magnetic-activated cell sorting (MACS) to isolate CD31+ cells (primarily ECs) from E8.25 wild-type and Foxo1 germline deletion mutant YSs. CD31+ YS cells showed sevenfold higher Pecam1 (CD31) expression than whole YSs (sorted then recombined) and ∼30-fold higher than the CD31− population, demonstrating high-fidelity enrichment via MACS (Fig. 3C). To compare wild-type and mutant YS ECs, we assayed transcript levels of both Pecam1 and Flk1 in the CD31+ populations. Pecam1 levels were comparable between wild-type and mutant CD31+ cells, whereas Flk1 was significantly reduced in the null CD31+ population (Fig. 3D,E). Finally, we examined Flk1 expression using a transgenic reporter, Flk1-H2B::YFP (Fraser et al., 2005), that labels EC nuclei. At E8.25, YFP+ endothelial nuclei were evenly dispersed throughout the vascular plexus of control YSs; however, the number of YFP+ nuclei within the Foxo1ECKO YSs were significantly reduced (Fig. 3F). The total number of DAPI+ nuclei was unchanged. Nuclear segmentation and quantification of the average YFP+ cell density revealed a significant reduction in the number of YFP+ cells in Foxo1ECKO YSs compared with controls (Fig. 3G). To determine whether the reduction in YFP+ cells was due to a difference in apoptosis or proliferation, Foxo1ECKO litters positive for the Flk1-H2B-YFP reporter were immunostained for phospho-histone 3 (PH3) or caspase 3 (Fig. 3H,I). We found that neither cell proliferation nor apoptosis within the YS differed significantly between control and Foxo1ECKO embryos (Fig. 3H,I, Fig. S2B-E). As the mRNA expression of pan-endothelial markers was not decreased, but FLK1 transcripts and protein were reduced, we concluded that the low density in YFP+ cells is not due to reduced EC numbers, but rather to reduced expression of the Flk1 reporter. Collectively, these data indicate that FOXO1 is required to maintain cell autonomous Flk1 expression in ECs, but not required for the formation, proliferation or survival of ECs, or the expression of other pan-endothelial markers.
Fig. 3.
FOXO1 regulates FLK1 expression without affecting other endothelial genes or EC viability prior to blood flow. (A) Expression levels of endothelial genes by quantitative RT-PCR. (B) Immunolabeling for endogenous FLK1 and PECAM1 in control and Foxo1ECKO YSs. Scale bars: 50 µm. (C) Comparison of Pecam1 expression in MACS-sorted CD31−, CD31+ and combined control E8.25 YS cells by qPCR. (D,E) Relative Pecam1 (D) and Flk1 (E) expression between wild-type and Foxo1null E8.25 MACS-sorted CD31− and CD31+ YS cells. (F) YSs from control and Foxo1ECKO at E8.25 DAPI stained and positive for Flk1-H2B::YFP transgene, which marks the EC nuclei (arrowheads). (G) Quantification of YFP+ cells relative to total number of cells labeled with DAPI from F. (H,I) Whole-mount phosphor-Histone-H3 (PH3) (H) or activated caspase 3 (I) staining of control and Foxo1ECKO E8.25 YSs co-labeled with Flk1-H2B::YFP transgene and DAPI. Data are mean±s.d. (n=3). *P<0.05, **P<0.01, ***P<0.001.
FOXO1 is required to regulate arterial gene expression in the YS vasculature
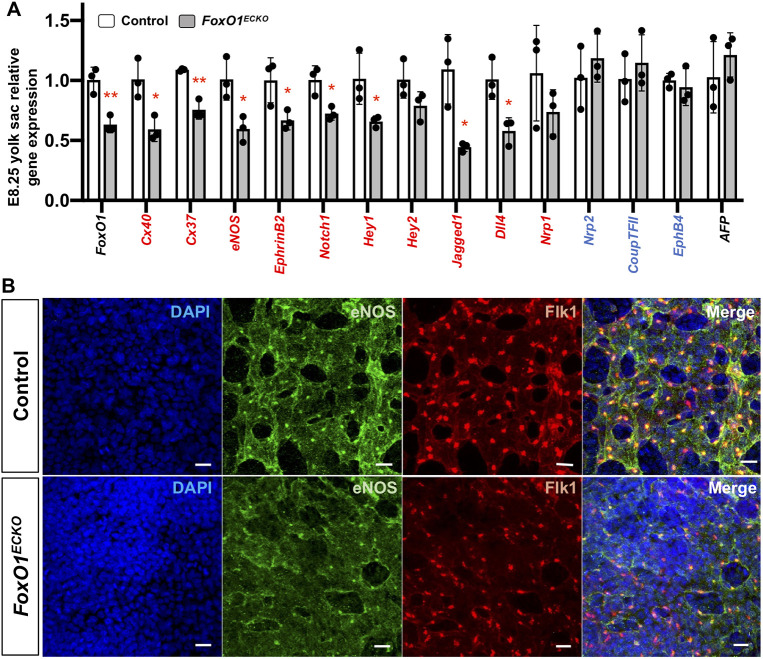
Given the reduced Flk1 expression in E8.25 YSs, and the crucial role of VEGF-VEGFR2 signaling in establishing arteriovenous identity in the early embryonic endothelium, we next examined other AV specification markers (Fig. 4A). Previously, Furuyama et al. showed reduced arterial-enriched transcripts Cx40 (Gja5), Cx37 (Gja5), eNOS (Nos3) and ephrin B2 (Efnb2) in E9.5 Foxo1ECKO YSs compared with control littermates (Furuyama et al., 2004), but given the changes in blood flow that we observed in E9.5 Foxo1ECKO embryos, we were interested to determine whether expression of these markers was affected earlier. Indeed, these genes were significantly reduced in Foxo1ECKO YSs at E8.25 compared with controls (Fig. 4A). In addition, we found a significant reduction in Notch family member transcript levels, including Notch1, Hey1, jagged 1 (Jag1) and Dll4, which are required for arterial specification (Gridley, 2010; Duarte et al., 2004; Xue et al., 1999; Fischer et al., 2004). Venous markers, neuropilin 2 (Nrp2), Coup-TFII (Nr2f2) and Ephb4 showed no significant change (Wang et al., 1998; You et al., 2005). The endodermal marker Afp was also unchanged (Fig. 4A) (Dziadek and Adamson, 1978). These results demonstrate that FOXO1 is required for normal early arterial gene expression in the murine YS.
Fig. 4.
Arterial marker expression is reduced in Foxo1ECKO YS. (A) qRT-PCR expression analysis in control and Foxo1ECKO YSs; arterial (red), venous (blue) and endoderm markers at E8.25. *P<0.05, **P<0.01; data are mean±s.d. (n=3). (B) Co-immunolabeling of eNOS, FLK1 and DAPI in control and Foxo1ECKO YSs at E8.25. Scale bars: 20 µm.
To determine whether reduced arterial-specific gene expression correlated with decreased expression of their respective proteins in Foxo1ECKO YSs, immunofluorescence was performed on sectioned YSs at E8.5 and E9.5. Confocal imaging of Cx37 and Cx40 revealed an overall reduction in the number of connexin-positive puncta in Foxo1ECKO YSs when compared with controls at both stages (Fig. S3A and B). Similarly, we observed decreased eNOS expression within the vascular plexus in Foxo1ECKO yolks sacs compared with controls (Fig. 4B). These data, in addition to previous gene expression analysis, indicate that FOXO1 within the developing endothelium is necessary for the regulation of arterial identity.
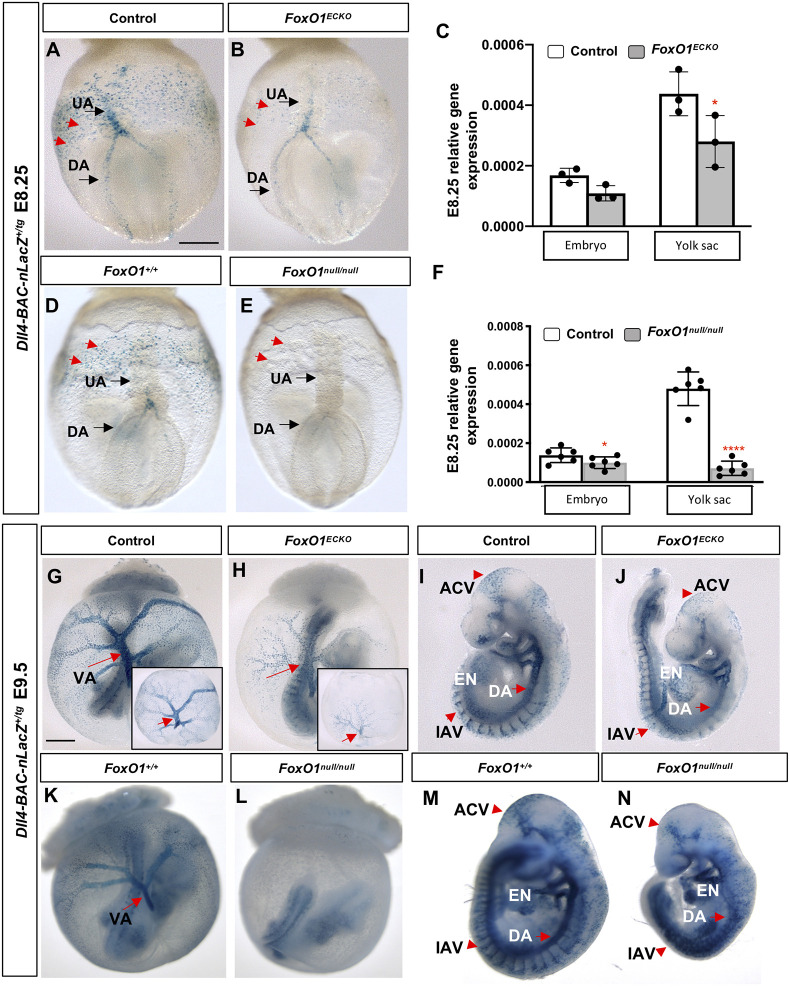
Characterization of arterial defects in Foxo1ECKO and germline mutants using the Dll4-BAC-nlacZ reporter
Thus far, our phenotypic, transcriptional and immunolabeling studies support a role for FOXO1 in regulating vascular remodeling, Flk1 expression and arterial specification of ECs within the YS. To further analyze the arterial specification defects in Foxo1ECKO mutants, we examined the spatial expression of one of the earliest markers of arterial identity (Chong et al., 2011; Wythe et al., 2013), Dll4, using a transgenic reporter line, Dll4-BAC-nlacZ, that faithfully recapitulates endogenous Dll4 expression (Herman et al., 2018). E8.25 Foxo1ECKO embryos carrying the nuclear-localized β-galactosidase reporter were compared with control littermates (Fig. 5 and Fig. S4). In E8.25 control embryos, Dll4-BAC-nlacZ reporter activity was observed in the dorsal aorta (DA), endocardium (EN) and nascent umbilical artery (UA) within the allantois (Fig. 5A and Fig. S4A). LacZ-positive nuclei were also detected within the YS in and around the vitelline artery and arterioles (Fig. 5A, red arrows). Foxo1ECKO YSs exhibited a similar LacZ expression pattern spatially, albeit with reduced intensity (Fig. 5B), consistent with our transcript analysis showing reduced Dll4 mRNA expression in the Foxo1ECKO YSs (Figs 4A and 5C). However, unlike in the YS, nLacZ expression was only slightly reduced in the embryo proper of Foxo1ECKO mutants (Fig. S4B compared with Fig. S4A, Fig. 5C). To determine whether the differences in nLacZ reporter expression between the YS and embryo was influenced by Cre-mediated recombination in our conditional knockout studies, we examined the activity of the Dll4 LacZ reporter in germline Foxo1 mutants. Fig. S4E,F show representative images of anterior views of the embryos, while Fig. 5D,E show posterior views. Foxo1null/null showed even greater decreases in reporter expression compared with control YSs and embryos (Fig. 5D,E, red arrows). A small but significant decrease in endogenous Dll4 expression was seen between wild-type and Foxo1null/null embryos (Fig. 5F). Whereas, a dramatic reduction in Dll4 expression was observed in the Foxo1 null YSs compared with wild-type controls (Fig. 5F).
Fig. 5.
Characterization of arterial defects in Foxo1ECKO and germline mutants. (A,B,D,E) Using the Dll4-BAC-nlacZ reporter, nlacZ reporter activity was detected in the dorsal aorta (DA) and umbilical artery (UA) in E8.25 littermate control, Foxo1ECKO and Foxo1-null embryos. Red arrows indicate YS ECs in the posterior region of the YS plexus. (C,F) qRT-PCR for Dll4 mRNA expression in littermate control, Foxo1ECKO and Foxo1-null embryos and YSs (n>3; *P<0.05, ****P<0.0001). Data are mean±s.d. (G,H,K,L) nlacZ reporter activity in E9.5 littermate control, Foxo1ECKO and Foxo1-null YS and embryo. VA, vitelline artery (arrows); insets in G and H show YSs only. (I,J,M,N) nlacZ reporter activity in E9.5 littermate control, Foxo1ECKO and Foxo1-null embryos. EN, endocardium; DA, dorsal aorta; IAV, intersomitic arterial vessels; ACV, arterial cranial vasculature. Scale bars: 200 µm (E8.25); 500 µm (E9.5).
By E9.5, nLacZ reporter expression was detected in the arterial tree in the YS, particularly in the VA and within the arterioles (Fig. 5G). Consistent with the E8.25 results, we found Dll4 expression reduced in E9.5 Foxo1ECKO YSs (Fig. 5H) but strong expression in vessels within control and Foxo1ECKO embryos (Fig. 5I,J). Germline null embryos showed similar nLacZ activity compared with controls (Fig. 5K), but reporter expression was not detectable in null YSs, despite the strong expression seen in the embryo (Fig. 5L). Isolated embryos confirmed LacZ expression in both control (Fig. 5M) and null embryos (Fig. 5N). Additional analysis of Dll4 reporter expression confirmed previous reports of vascular defects in Foxo1 mutants, including within the intersomitic vessels, cranial vessels and dorsal aorta (Fig. 5I,J,M,N, red arrows) (Hosaka et al., 2004; Ferdous et al., 2011; Dharaneeswaran et al., 2014). Collectively, our results indicate that FOXO1 plays a crucial and early role in the regulation of Dll4 expression, a key factor in determining arterial identity, within the extra-embryonic arteries of the YS, but does not appear to be required for Dll4 expression within the embryo proper.
Conditional Foxo1 deletion upregulates Spry2 and Spry4 expression
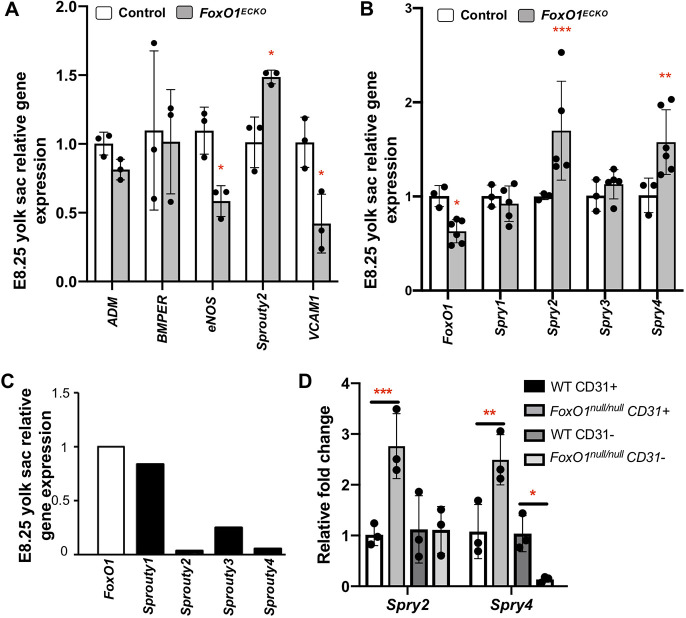
We uncovered a novel role for FOXO1 in the establishment of arterial identity, but neither Flk1 nor Dll4 contains known binding sites for FOXO1; thus, we examined expression levels of previously validated direct transcriptional targets of FOXO1 in the endothelium: adrenomedullin (Adm), BMP binding endothelial regulator (Bmper), Nos3, Spry2 and Vcam1 (Potente et al., 2005; Ferdous et al., 2011). qRT-PCR analysis in Foxo1ECKO YSs at E8.25 (Fig. 6A) revealed no significant reduction in Adm or Bmper, but a significant downregulation in Nos3 and Vcam1 compared with controls, as previously described in other tissues (Potente et al., 2005; Ferdous et al., 2011). Interestingly, Foxo1ECKO YSs showed significantly increased Spry2 expression (Fig. 6A). Subsequent analysis showed that in addition to Spry2, Spry4 was also upregulated in mutant YSs compared with controls, while Spry1 and Spry3 levels were unchanged (Fig. 6B). The upregulation in Spry2 and Spry4 is specific to the YS as no significant changes in sprout gene expression were seen in the embryo proper (Fig. S5). We focused our subsequent analysis on the Spry factors because Spry4 overexpression has been shown to inhibit angiogenesis in the YS (Lee et al., 2001) and the upregulation of sprouty transcripts in Foxo1ECKO led us to hypothesize that FOXO1 may act as a direct repressor of sprouty gene expression.
Fig. 6.
FOXO1 regulates Spry2 and Spry4 expression in the YS vasculature. (A,B) qRT-PCR analysis in littermate E8.25 control and Foxo1ECKO YSs for (A) known FOXO1 targets and (B) Spry family members. (C) qRT-PCR of endogenous Spry1, Spry2, Spry3 and Spry4 expression relative to Foxo1. (D) Quantitative RT-PCR of Spry2 and Spry4 in MACS-sorted E8.25 CD31+ and CD31− control and Foxo1null YS cells. Data are mean±s.d. (n=3). *P<0.05, **P<0.01, ***P<0.001.
In keeping with the idea that FOXO1 may normally repress Spry 2 and Spry4 transcription, we examined endogenous mRNA levels of Foxo1, Spry1, Spry2, Spry3 and Spry4 in wild-type E8.25-8.5 YSs. This analysis revealed that Spry2 and Spry4 expression is much lower than Foxo1, Spry1 or Spry3 (Fig. 6C). To expand and confirm endothelial specificity of our previous Spry2 and Spry4 expression analysis, we examined Spry2 and Spry4 transcripts in CD31+ and CD31− MACS-sorted cells from germline Foxo1 mutants and wild-type YSs (Fig. 3D). For Spry2, we observed increased expression in YS CD31+ cells, but there was no change in CD31− cells from null mutant YSs (Fig. 6D). Surprisingly, although Spry4 transcripts were increased in CD31+ cells of null YSs, transcripts were decreased in the CD31− population. These data suggest that FOXO1 may act as both a transcriptional repressor or activator in adjacent tissues in the YS, depending on the cell identity or transcriptional target.
FOXO1 directly binds to endogenous Spry2 and Spry4 promoters and represses Spry2 and Spry4 transcription
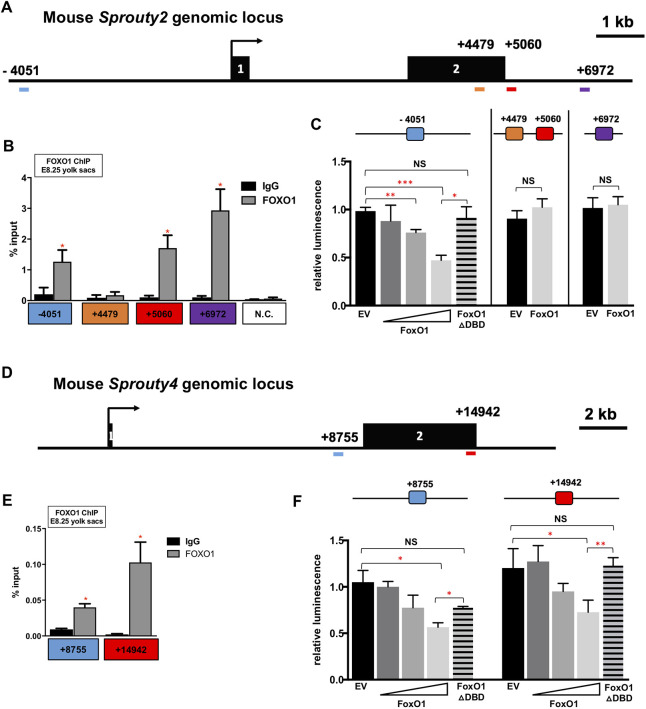
FOXO1 is known to regulate Spry2 mRNA expression in liver ECs, and in vivo chromatin immunoprecipitation (ChIP) experiments confirmed that FOXO1 occupies four conserved FOXO-binding elements within the murine Spry2 locus (Paik et al., 2007). The first FOXO1-binding site (Fig. S5A) is located ∼4 kb upstream of the transcriptional start site (TSS) of murine Spry2; the second DNA-binding site is within exon 2 (Fig. 7A). The third and fourth FOXO1 binding sites are located ∼5 kb and 7 kb downstream of the TSS, respectively (Fig. 7A). Paik et al. showed that FOXO1 interacts with these loci to activate Spry2 in the liver, but it was unknown whether FOXO1 uses the same binding sites to repress Spry2 in the YS. To determine whether FOXO1 occupies any of these four identified binding sites in the murine YS, we performed ChIP-PCR using pooled wild-type E8.25 YSs. As shown in Fig. 7B, FOXO1 occupancy was significantly enriched at the −4051, +5060 and +6972 regions compared with IgG control. FOXO1 enrichment was not observed at the +4479 region. This demonstrates that, during early YS vessel development, FOXO1 binds Spry2 at regulatory regions −4051, +5060 and +6972, and supports the context-dependent function of FOXO1.
Fig. 7.
FOXO1 directly binds to endogenous Spry2 and Spry4 promoters, and represses Spry2 and Spry4 transcription. (A) Genomic locus of mouse Spry2 gene with the FOXO1-binding sites in blue, orange, red and purple. (B,E) FOXO1 ChIP-PCR using E8.25 YS chromatin. (C) Luciferase activity of FOXO1 on the Spry2 promoter in H1299 cells. (D) Genomic locus of mouse Spry4 gene with the FOXO1-binding sites in blue and red. (F) Luciferase activity of FOXO1 on the Spry4 promoter in H1299 cells. *P<0.05, **P<0.01, ***P<0.001. EV, empty vector. Data are mean±s.d.
Next, we generated luciferase reporter constructs containing ∼2 kb of the murine Spry2 promoter or specific regulatory regions harboring FOXO1-binding sites, and measured transcriptional activity in cultured mammalian cells (Fig. S6B). To avoid potential confounds in our analysis from endogenous FOXO1, the human lung cancer cell line H1299 was chosen as FOXO1 protein expression is undetectable in this line (Zhao et al., 2010). Overexpression of Foxo1 (FLAG::Foxo1) significantly repressed luciferase activity of the promoter construct containing the −4051 FOXO1-binding site in a dose-dependent manner. Furthermore, co-transfecting the same reporter construct along with a FOXO1 cDNA without a DNA-binding domain abolished this transcriptional repression (Fig. 7C). In contrast, FOXO1 did not significantly repress luciferase activity in the constructs containing either the +4479/5060 combined or +6972 Spry2 regulatory regions (Fig. 7C). These results suggest that FOXO1 directly downregulates Spry2 expression via the −4051 site in its promoter.
To determine whether this role for FOXO1 is evolutionarily conserved, we examined the Spry4 locus for conserved FOXO1 DNA-binding motifs (Fig. S6A). Two putative binding sites, which were conserved in at least three vertebrate genomes (mammalian and non-mammalian), were identified +8755 bp and +14,942 bp downstream of the Spry4 TSS (Fig. 7D). FOXO1 ChIP-PCR using E8.25 YS chromatin showed a significant enrichment of FOXO1 occupancy in both regulatory regions (Fig. 7E). Luciferase assays in H1299 cells also showed that these same sites were required for wild-type FOXO1 dose-dependent repression of reporter activity (Fig. 5F and Fig. S6C). Taken together, data from the Spry mRNA expression analysis, as well as ChIP and luciferase assays, demonstrated that FOXO1 directly repressed Spry2 and Spry4 transcription in the E8.25 murine YS via known and newly identified conserved DNA-binding sites.
Transient overexpression of Spry4 in ECs partially phenocopies conditional loss-of-function Foxo1 mutants
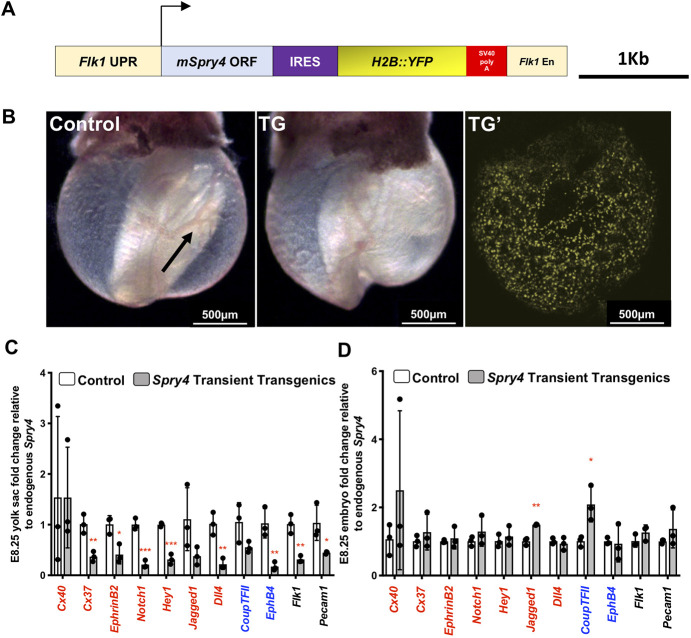
Spry2 and Spry4 are known to have anti-angiogenic functions (Taniguchi et al., 2009; Wietecha et al., 2011; Lee et al., 2001), and our data here show that FOXO1 directly represses Spry2 and Spry4 expression in the YS, we hypothesized that FOXO1 promotes arterial gene expression by repressing Spry2 and Spry4. It had previously been shown that adenovirus-mediated overexpression of Spry4 in developing embryos inhibited sprouting and branching of small vessels in the embryo proper and vessel remodeling in the YS (Lee et al., 2001). To test whether Spry4 overexpression could recapitulate the Foxo1 loss-of-function phenotype, we used a well-characterized Flk1 promoter-enhancer construct that is expressed in YS and embryonic ECs (Kappel et al., 1999; Rönicke et al., 1996; Fraser et al., 2005) to transiently overexpress Spry4 beginning at E7.5. To track transgene expression, a H2B::YFP reporter was inserted downstream to enable YFP+ transgenic embryo identification (schematized in Fig. 8A). At E9.5, YSs of YFP+ transgenic embryos (n=3) showed poorly remodeled vasculature, as their vessels remained as a primitive vascular plexus, while non-transgenic embryos had a normally developed vitelline artery with large caliber vessels branching into smaller diameter capillaries (Fig. 8B). The lack of YS vascular remodeling in the transient transgenic Flk1-Spry4 embryos phenocopied the vascular remodeling defects in Foxo1ECKO embryos and was similar to previous loss-of-function data (Lee et al., 2001). YSs harvested from both transgenic and control embryos confirmed the expression of YFP transcripts and exogenous Spry4 only in transgenic embryos (Fig. S7).
Fig. 8.
Transient overexpression of Spry4 in ECs partially phenocopies Foxo1ECKO mutants. (A) Schematic of Spry4 overexpression construct for pro-nuclei injection. (B) Bright-field image of E9.5 non-transgenic (control) and a transgenic embryo (TG). Confocal imaging of a TG embryo (TG′) showing YFP fluorescence in YS. Vessel remodeling in the control YS (arrow). (C,D) qRT-PCR of arterial markers in E8.25 control and TG YSs (C) and embryos (D) (n=3). *P<0.05, **P<0.01 and ***P<0.001. Data are mean±s.d.
Next, we used total RNA from transgenic YFP+ and control YSs and embryos that were within E8.25 4-7 somite stage for analysis. The relative expression level of each arterial marker was first normalized Gapdh, the internal control, then to endogenous Spry4 in order to compare the effect of exogenous Spry4 overexpression. Transcript levels were compared between control and YFP+ transgenic groups. Arterial markers, such as Cx37, Efnb2, Notch1, Hey1, Jag1 and Dll4 were significantly downregulated in the YSs of transgenic embryos compared with controls (Fig. 8C), whereas expression within the embryo proper of these markers was not significantly changed, apart from Jag1 (Fig. 8D). Cx40 expression was not significantly different between the control and transgenic groups in either the YS or embryos (Fig. 8C,D). Additionally, unlike in Foxo1ECKO YSs, expression of venous marker Ephb4 was significantly downregulated in the YS and Nr2f2 expression was significantly increased in the transgenic embryos. The broader effects produced by Spry4 overexpression directed by the Flk1 promoter/enhancer could indicate that SPRY4 has FOXO1-independent functions, or that abnormally high levels of Spry4 may affect other processes. These data, combined with our results showing that FOXO1 represses Spry2 and Spry4 transcription, indicates that FOXO1 acts as a key transcriptional regulator in AV specification by repressing an antagonist of arterial specification.
DISCUSSION
Previously, using either germline mutations or conditional approaches, several groups demonstrated a requirement for FOXO1 in the early embryo, as these mutants featured failed YS remodeling and mid-gestation lethality (Sengupta et al., 2012; Furuyama et al., 2004; Hosaka et al., 2004). In this paper, we investigated the role of FOXO1 within the YS vascular plexus prior to the onset of consistent circulation and overt vascular remodeling. It is well known that arteriovenous specification and the arterial gene expression program are influenced by hemodynamic forces (le Noble et al., 2004, 2005; Wragg et al., 2014), but our goal here was to determine whether FOXO1 functions before the onset of hemodynamic signaling to affect arteriovenous patterning. Herein, we demonstrate that blood flow is normal in Foxo1ECKO mutants at these early stages, although heart failure and poor circulation are evident by E9.5 (Fig. 2). Others have shown that FOXO1 is not required for heart development (Sengupta et al., 2012), and it is possible that heart failure in these embryos is caused by the increased resistance of blood flow encountered in the unremodeled vitelline vessels. Additionally, loss of FOXO1 causes allantois defects, preventing normal allantois fusion and circulation to the placenta (Ferdous et al., 2011). We did not observe overt defects in allantois fusion in Foxo1ECKO embryos (0/10 Foxo1flox/flox;Tie2-creTg/+) (Table 1) and observed only low penetrance of allantois fusion defects (2/15) in the germline Foxo1 knockout embryos, whereas all fully null or Foxo1ECKO embryos examined showed defects in YS remodeling, heart failure and mid-gestation lethality. Thus, it is likely that the heart failure and lethality are caused by increased resistance to blood flow in vitelline vessels; however, the reduction in Dll4 expression that we observed using the Dll4-BAC-nlacZ reporter in Foxo1ECKO and germline null mutants suggests that further investigation of the consequence Dll4 loss in allantois development is warranted.
In this study, we report that FOXO1 plays a previously unidentified role in regulating arterial-specific gene expression prior to the onset of blood flow. Based on transcript expression analyses, immunostaining and transgenic reporter experiments, we have concluded that loss of Foxo1 causes a significant downregulation in Flk1 and crucial arterial markers, including Dll4, in the YS without affecting cell proliferation or cell death. Furthermore, our data demonstrate that FOXO1 directly binds to Spry 2 and Spry4 regulatory regions in the YS, and that FOXO1 acts as a direct repressor of Spry 2 and Spry4 in YS cells. Finally, we show that overexpression of Spry4 in ECs in vivo was sufficient to recapitulate impairments in both the vascular remodeling at E9.5 and arterial cell fate specification at E8.25 seen in Foxo1 mutants. These data, combined with our results showing that FOXO1 represses Spry2 and Spry4 transcription, indicate that FOXO1 acts as a key transcriptional regulator in AV specification by repressing an antagonist of arterial specification. However, given the caveat that we also observed disruptions to venous marker expression in Spry 4 overexpression embryos, it is not yet clear whether sprouty factors participate only in limiting arterial gene expression or whether they play a broader role in early EC specification.
Our studies support a model where FOXO1 represses Spry2 and Spry4 in YS ECs to promote early arterial specification in the YS prior to the onset of robust embryonic circulation. Interestingly, although we observed elevated Spry2 and Spry4 transcripts in YS CD31+ cells of Foxo1 null embryos, we found reduced Spry 4 mRNA expression in CD31− cells in the YS, suggesting FOXO1 may act as activator for Spry4 in other cell types of the YS. Recent studies showed that FOXO1 functions as a transcriptional repressor in hepatocytes (Langlet et al., 2017) and pancreatic progenitor cells (Jiang et al., 2017). In some instances, different co-factors have been identified that enable FOXO1 to act as an activator or a repressor in different cell types of the same tissues (Langlet et al., 2017). A similar mechanism may explain the observed differences between endothelial and non-ECs within the YS. It is not yet known whether a transcriptional co-factor in YS ECs is required for FOXO1 to act as a repressor, or whether another mechanism accounts for the opposite regulation of Spry4 in adjacent cell layers. It is also not yet clear whether the downregulation of Spry4 in the CD31− cells has a functional consequence. Further work is needed to determine whether Sprouty factors play multiple roles in early yolk sac development.
Foxo1 loss did not appear to affect normal expression of pan-endothelial markers Pecam1 and Tie2, and other genes, but Flk1 was significantly reduced in both Foxo1ECKO YS and sorted germline mutant YS CD31+ cells. We also found that despite the reduction in Flk1, we did not observe changes in cell proliferation or cell viability, two process that are directly regulated by VEGF-VEGFR signaling (Bernatchez et al., 1999). Sprouty factors inhibit receptor tyrosine kinase signaling, and sprouty overexpression could cause a reduction in Flk1 that is normally promoted by FLK1 or FGF receptor activation (Lee et al., 2001; Casci et al., 1999). Indeed, we observed a reduction in Flk1 transcripts when Spry4 was overexpressed in transgenic embryos, but also observed a strong effect on the expression of other endothelial markers such as Pecam1. It is also possible that reduction of Flk1 expression seen in Foxo1 mutants is a secondary consequence of disrupted arterial specification, rather than a primary driver of this defect.
Dll4 is among the earliest markers of arterial gene expression (Chong et al., 2011; Wythe et al., 2013), but precisely how Dll4 expression is initiated within the early YS and embryo remains poorly understood. While Dll4 transcription was suggested to be regulated by 5′ binding of FOXC1, FOXC2 and β-catenin in its proximal promoter (Corada et al., 2010; Hayashi and Kume, 2008; Seo et al., 2006), subsequent in vivo analysis showed that this region is not sufficient to mediate expression (Wythe et al., 2013). Additionally, endothelial-specific loss of β-catenin failed to alter Dll4 expression in mice or to produce arteriovenous patterning defects (Wythe et al., 2013). Furthermore, functional enhancers were found within intron 3 and upstream at −12 and −16 kb that recapitulated the pattern of endogenous Dll4 expression (Sacilotto et al., 2013; Wythe et al., 2013), and these regions lacked conserved FOXC1- and FOXC2-binding sites, as well as TCF- and LEF-binding sites. In these current studies, we used a nuclear localized LacZ reporter that recapitulates the normal expression pattern of Dll4 (Herman et al., 2018). The data presented here clearly show that Foxo1 is required to sustain Dll4 and arterial gene expression through the repression of Spry 2 and Spry4, but there are likely other mechanisms involved in activation of Dll4 and arterial gene expression.
Our data clearly showed that the reduction in Dll4 expression was far more severe in the Foxo1ECKO or null YS than within the embryo proper. In addition to Dll4, reduced Flk1 expression (Fig. S2A) and alterations to Spry 2 and Spry4 expression (Fig. S5A) also appeared to be confined to the YS. Similarly, Spry4 overexpression throughout the embryo and YS using an endothelial-specific Flk1 promoter (Kappel et al., 1999; Rönicke et al., 1996; Fraser et al., 2005) indicated that Spry4 overexpression did not alter arterial gene expression in the embryo, but suppressed arterial transcripts (and altered some venous marker expression) in the YS. The mechanism that explains the differential activity of FOXO1, SPRY2 and SPRY4 within the vasculature of the YS versus the embryo proper remains unclear. Future experiments will be required to address numerous possible explanations, including differences in mesodermal cell lineages, differential binding to co-factors and/or differences in post-translational modifications regulated by local cell-cell signaling.
One unresolved issue from these studies is the relationship between abnormal arteriovenous specification and failed vessel remodeling. Both arteriovenous identity and vessel remodeling are regulated by hemodynamic forces, and AV specification relies on pathways that respond to VEGF signaling (Fish and Wythe, 2015; Covassin et al., 2006; Weinstein and Lawson, 2002; Fang et al., 2017). In Foxo1ECKO, we detected downregulation of VEGFR2. VEGFR2 and other VEGF receptors have been shown to act as shear stress mechanosensors, signaling through downstream pathways such as the MEK-ERK kinase cascade in response to changes in blood flow (Tzima et al., 2005; Baeyens and Schwartz, 2016). Thus, the downregulation of VEGFR2 in Foxo1 mutant embryos could prevent ECs from responding to normal blood flow signaling needed for vessel remodeling. Previously, our lab showed that ECs within the vitelline arteries, but not the vitelline veins, migrate directionally in response to hemodynamic changes in YS vasculature (Udan et al., 2013) so it is possible that the loss of FOXO1 and/or the overexpression of Spry2 and Spry4 interferes not only with initial Dll4 specification, but with the ability of the cell to sense mechanical signaling that is necessary to direct cell migration required for remodeling. Further work will be needed to better understand the mechanisms leading both to early Dll4 expression and those that regulate the cellular responses needed for vessels to adapt to changes in blood flow.
MATERIALS AND METHODS
Animals and genotyping
All animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. Ella-Cre and Tie2-Cre transgenic mice were purchased from Jackson Labs (003724 and 008863, respectively). ε-globin-KGFP mice (Dyer et al., 2001), Foxo1flox/flox mice (Paik et al., 2007) and Dll4-BAC-nLacZ mice (Herman et al., 2018) were maintained and genotyped as previously described. Foxo1 germline knockout mice were generated by crossing the Foxo1flox/flox mice to Ella-Cre mice (Lakso et al., 1996). Flk1-H2B::YFP reporter mice were kindly provided by Dr K. Hadjantonakis (Memorial Sloan Kettering Cancer Center, NY, USA) (Fraser et al., 2005).
Immunostaining of whole or sectioned YSs
E8.25 YSs were fixed in 4% PFA, rinsed in PBS, permeabilized with 0.1% TritonX-100 for 1 h and blocked in 2% normal donkey serum/1% BSA for 5 h. YSs were then incubated with anti-PECAM1 antibody (BD Pharmingen, #550274; 1:100) overnight at 4°C. After several PBS washes, YSs were incubated with goat anti-rabbit antibody (Molecular Probes, AlexaFluor 633, 1:500) and DAPI (1:500) overnight at 4°C. Finally, YSs were rinsed in PBS and imaged using the Zeiss LSM510 META confocal microscope. Dissected YSs were cryosectioned at 20 μm and sections were permeabilized and blocked, and incubated with antibodies to caspase 3 (Cell Signaling, 9661, 1:50); connexin 37 (ThermoFisher Scientific, 404200, 1:50), connexin 40 (ThermoFisher Scientific, 364900, 1:50), eNOS (Santa Cruz, sc-654, 1:50), FLK1 (Sigma, V1014, 1:100) or pHistone H3 (Millipore, 06-570, 1:50) overnight at 4°C. The secondary antibody incubation and image acquisition were performed as described previously.
LacZ staining
Dll4-BAC-nLacZ transgenic reporter were examined on Foxo1 germline or Foxo1ECKO (Tie2-Cre+/tg;Foxo1floxfloxl) backgrounds. E8.5 and E9.5 embryos were dissected in ice-cold PBS and fixed in 4% PFA. Embryos were then washed in X-gal rinse buffer (0.02% NP40 and 0.01% sodium deoxycholate; four times for 15 min) and thereafter stained in X-gal solution [5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 0.01% sodium deoxycholate, 0.02% NP40, 2 mM MgCl2, 5 mM EGTA and 1 mg/ml X-gal] at 37°C overnight. Embryos were then post-fixed in 4% PFA and then cleared in 50% and 70% glycerol. Embryos from the same litter were processed and stained in a 20 ml scintillation vial. Stained embryos were photographed using the Axio ZoomV16 (Zeiss) stereo microscope and thereafter genotyped.
Protein isolation and western blotting
Yolk sacs from three E8.25 wild-type or Foxo1ECKO embryos were pooled and lysed in RIPA buffer [10 mM Tris-HCl (pH 8), 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl and 1 mM PMSF] supplemented with protease inhibitors. Protein lysates were quantitated using Bradford assay. Twenty micrograms of lysates were electrophoresed on 4-15% protein gels (BioRad), transferred to a PVDF membrane and immunoblotted with antibodies to either FOXO1 or GAPDH (Cell Signaling Technology, 2880 and 97166; 1:2000) overnight at 4°C. After incubation with HRP-conjugated secondary antibodies (Thermo Fisher, 65-6120, 1:5000) for 1 h at room temperature, protein bands were detected via chemiluminescence with Clarity ECL western substrate (BioRad) on film. Western bands were quantified using Fiji gel analyzer and FOXO1 was normalized to GAPDH.
Quantification of Flk1-H2B::YFP+ cell density, proliferation index and apoptotic index in whole-mount YSs
Acquired wild-type and Foxo1ECKO YS whole-mount images (n>3 YSs per genotype, n>3 regions of interest per YS) were made into maximum intensity projections and separated into individual RGB images: red (pHistone-H3/Caspase 3), green (Flk1-H2B::YFP) and blue (DAPI). Individual nuclei for RGB channels were segmented and quantified using FARSIGHT. FARSIGHT is an open source image analysis tool box for segmentation and quantitation of biological features courtesy of Badri Roysam (University of Houston, TX, USA; PMID: 18294697; https://github.com/RoysamLab/Farsight-toolkit). We used this to generate both intensity and volume thresholds to distinguish two nuclei as separate. YFP+ cell density was defined as the ratio of YFP+ nuclei to DAPI+ nuclei within that same field of view. Proliferative/apoptotic index was defined as the ratio of PH3+/caspase 3+ nuclei to the number of DAPI+ nuclei. EC proliferative/apoptotic index was defined as the ratio of YFP+;PH3+/caspase 3+ double-positive nuclei to the number of YFP+ nuclei. The ratios were then averaged over the various wild-type and Foxo1ECKO YS images.
Live imaging and analysis of blood flow in Foxo1 conditional knockout embryos
The ε-globin-KGFP reporter expressing GFP in primitive erythroblasts was examined in control and Foxo1ECKO background, and litters were dissected at E8.5 or E9.5 for blood velocity analysis. Embryos were dissected under a heated (37°C) dissection stage with warm dissection media (DMEM/F-12, 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin). Embryos with an intact ectoplacental cone were placed in a glass-bottomed culture chamber with culture medium (1:1 DMEM/F-12: rat serum, 100 U/ml penicillin and 100 μg/ml streptomycin) and allowed to recover in a 37°C incubator for 20 min. Embryos were then placed on a heated confocal microscope stage (37°C) and imaged using the Zeiss LSM 5 LIVE laser scanning confocal microscope, using the Achroplan 20×/0.45 NA objective. A 200-frame time lapse (in a 512×512 pixel frame) was acquired at 30-50 frames per second. Blood flow time lapse images were acquired at three different locations throughout the YS per embryo, and at least three embryos of each genotype were used for data collection. Individual blood cell velocities in each track were determined from time-lapse movies using Imaris. Individual blood cell velocities from three different locations per embryo were averaged. Average heart beats per minute were calculated by measuring the average time interval between peak velocities during the course of five cardiac cycles in individual velocity profiles for each embryo imaged. Embryos were genotyped after imaging, and blood velocities and heart beats per minute were averaged in wild type and Foxo1ECKO.
Magnetic-activated cell sorting of YS ECs
To isolate E8.25 YS ECs, fresh YSs were dissected in ice-cold DMEM/F12 media without Phenol Red (ThermoFisher Scientific, 21041025), individually placed in 100 µl of cold TrypsinLE (Fisher Scientific, 12605010) and kept on ice until all YSs were harvested. Embryos were used for genotyping. To dissociate YSs into a single cell suspension, they were gently triturated with a p200 pipette and incubated on ice for 5 min. This was repeated four times. To inhibit the enzyme, 1 ml of stop solution media and 10% FBS (ThermoFisher Scientific, 26140079) was added. The YS cell suspensions were pelleted at 800 g for 5 min at 4°C. The pellets were resuspended in 90 µl of cell suspension buffer (PBS, 2% FBS and 2 mM EDTA). 10 µl of CD31 MicroBeads (Miltenyi Biotec, 130-097-418) was added to each YS cell suspension and samples were incubated on ice for 15 min in the dark. Cell mixtures were pelleted at 800 g for 5 min at 4°C and washed with 1 ml of cell suspension buffer. Cell mixtures were once again pelleted at 800 g for 5 min at 4°C and resuspended in 200 μl of cell suspension buffer. Cell mixtures were passed through 40 µm cell strainers (Fisher Scientific, 352340) into FACS tubes and strainers were washed with 300 µl of cell suspension buffer. MS columns (Miltenyi Biotec, 130-041-301) were placed on OctoMACS separator and prepared according to the manufacturer's instructions. Cell mixtures were individually passed through columns and the flow through was reapplied through columns to maximize EC retention (CD31+ population). Columns were washed three times with 500 µl cell suspension buffer and all flow through was collected (CD31− population). Bound cells were released from the columns by removal from magnetic separator. 1 ml of cell suspension buffer was applied to the columns and cells were flushed using a plunger into a 1.5 ml Eppendorf tube. Collected cells were then pelleted at 800 g for 5 min at 4°C and resuspended in Trizol (Thermo Fisher, 15596018). After genotyping, CD31+ and CD31− populations from two YSs were combined and processed for RNA isolation (QIAGEN RNeasy Micro Kit, 74004), cDNA synthesis and qRT-qPCR as described below.
RNA isolation and qRT-PCR analysis
Total RNA was isolated from pooled (2-8 YS/sample) E8.25 YSs dissected from either Tie2-Cre+/tg; Foxo1+/flox or Tie2-Cre+/tg; Foxo1flox/flox embryos. Purified RNA was reverse transcribed (ThermoFisher Scientific, 11752-050) and gene expression analysis was performed using TaqMan real-time assays for Foxo1, Adm, Bmper, Vcam1 and a panel of endothelial, arterial and venous markers (see Table S2 for list). The data were normalized to Gapdh (Pfaffl, 2001) and relative expression ratios between control and Foxo1ECKO embryos were determined. Endogenous Foxo1, Spry1, Spry2, Spry3 and Spry4 expression from pooled E8.25 YSs (CD1 strain) was also probed by TaqMan real-time assay, but expression was calculated as fold change relative to Foxo1.
Endogenous Dll4 expression was measured in either germline Foxo1 knockouts or Foxo1ECKO embryos at E8.25. The allantois was used for genotyping and total RNA was extracted from individual embryos and YSs (n≥3) and probed for Dll4 expression using a TaqMan assay to measure fold change of expression between controls and homozygous Foxo1ECKO mutants. An unpaired Student's t-test was used to assess statistical significance and P<0.05 was considered statistically significant.
Chromatin immunoprecipitation (ChIP) and qPCR
To determine endogenous FOXO1 chromatin occupancy, E8.25 YSs from CD1 embryos were used for chromatin extraction. Freshly dissected YSs were dissociated in ice cold PBS with protease inhibitors and the tissue was then crosslinked with 1.5% formaldehyde, followed by incubation with 125 mM glycine and washed with PBS. After centrifugation, the pellet was resuspended in cell lysis buffer [5 mM PIPES (pH 8), 85 mM KCl and 0.5% NP40]. The samples were spun and the pellet was resuspended in nuclear lysis buffer [50 mM Tris-HCl (pH 8.1), 10 mM EDTA; 1% SDS] and then sonicated on ice using a Bioruptor (Diagenode) to obtain sheared chromatin ranging between 100 and 500 bp. ChIP was performed according to the instructions for Magna ChIP kit (Millipore, 17-10085) using 5 μg of anti-FOXO1 antibody (Abcam, ab39670) or rabbit IgG (Millipore, 12370). The crosslinks were then reversed, and the purified DNA was then analyzed by qPCR in technical triplicates using SYBR green master mix and the primers listed in Table S1 to measure the percentage of co-precipitating DNA relative to input (% input) in Spry2 and Spry4 genomic regions.
Cloning of murine Spry2 and Spry4 promoter constructs and luciferase assay
Genomic regions of ∼2 kb in length of murine Spry2 and Spry4 were PCR amplified using primers listed in Table S1 and using BAC clones of C57BL6 genomic DNA as template DNA (CH29-611D15 and CH29-100M12, respectively; CHORI BAC/PAC resources). PCR fragments were ligated into pCRII-TOPO vector, sequenced and then subcloned into pGL3-Promoter vector (Promega). H1299 cells (ATCC, CRL-5803) were maintained in DMEM media supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. For transient transfections, 50,000 cells were plated (48-well plate) and after 24 h, each well was co-transfected with 200 ng of Spry2 and Spry4 promoter construct, 10 ng of pRL-TK and 125 ng expression plasmid (pcDNA3-FOXO::FLAG; Addgene 13507) using manufacturer's recommendations for lipofectamine 3000. The total amount of expression plasmid transfected per well was kept constant with varying amounts of pcDNA3.1 vector. As a negative control, a Foxo1 plasmid encoding a deleted DNA-binding domain (amino acids 208-220) was used (Addgene 10694). After 24 h, cells were lysed and analyzed for firefly and Renilla luciferase activities according to the procedure outlined in the Dual-Glo luciferase assay system (Promega, E2920). All luciferase assays were performed in triplicate and repeated at least three times. An unpaired Student's t-test was used to assess statistical significance (P<0.05 was considered statistically significant) and the averages and standard deviation from triplicate samples from representative assays were shown.
Transient endothelial-specific Sprouty expression in embryos
A mouse Spry4 cDNA clone (TransOmics clone BC057005) was used as a template to PCR amplify the coding sequence with 5′ SacI and 3′ PmeI restriction sites (5′ GAGCTCCCAGCCTCATGGAGCCC 3′ and 5′ GTTTAAACTCAGAAAGGCTTGTCAGAC 3′), and subcloned into pCRIITOPO vector (S4-2). The internal ribosome entry site (IRES) sequence was amplified from pIRES-hrGFP1a vector (Agilent Technologies) with 5′ EcoRV and 3′ EcoRI restriction sites using primers 5′ CTATAGATATCACCCCCCTCTCCCTA 3′ and 5′ GCATGAATTCGGTTGTGGCCATTATCATCGTG 3′ and subcloned into pCRIITOPO vector (IRES-4). To assemble the final transgenic construct, clones S4-2 and IRES-4 were excised with 5′ Ecl136II and 3′ NotI, and 5′ NotI and 3′ Ecl136II, respectively, and co-ligated into Flk1-H2B/EYFP vector (kindly provided by Dr K. Hadjantonakis, Memorial Sloan Kettering Cancer Center, NY, USA) via a blunt-ended HindIII site. The Flk1-H2B/EYFP vector has a well characterized Flk1 promoter and intronic enhancer sequences that drive YFP expression in ECs (Fraser et al., 2005). All clones were verified by DNA sequencing and the final transgenic construct was excised with 5′ SalI and 3′ XbaI to purify a 4.5 kb fragment for pronuclear microinjection, which was performed at the BCM Genetically Engineered Mouse Core. Transient transgenic embryos were dissected with the YS intact and initially screened for YFP expression using confocal microscopy. Gross morphology of the embryo and YS vasculature was examined at E9.5 while arterial marker analysis (connexin 37, connexin 40, Dll4, Efnb2 and Hey1) was performed at E8.25. Embryos and YSs were individually lysed in Trizol for RNA extraction and screened for YFP-positive and -negative samples (n=3 each) that were stage matched at 4-7 somites (Fig. S7A). Additionally, the ratio of exogenous over endogenous murine Spry4 expression was quantitated for YFP-positive samples using transcript-specific primers (Fig. S7B). Detection was via the Sybr-green or Taqman assay (for arterial markers) and fold change of expression between YFP-positive and -negative samples, and statistical analysis were performed as previously described.
Supplementary Material
Acknowledgements
We thank Tegy J. Vadakkan in the Optical imaging and vital microscopy core facility.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: N.L.-V., R.L.Y.W., M.D.G., R.A.P., T.L.R., J.D.W., M.E.D.; Methodology: A.M.R.; Validation: N.L.-V.; Data curation: N.L.-V., R.L.Y.W., M.D.G., R.S.U., T.L.R., A.M.R.; Writing - original draft: N.L.-V., R.L.Y.W.; Writing - review & editing: N.L.-V., R.L.Y.W., M.D.G., R.S.U., R.A.P., T.L.R., J.D.W., M.E.D.; Supervision: M.E.D.; Funding acquisition: M.E.D.
Funding
The authors’ research was funded by the National Institutes of Health (R01 HD099026). Deposited in PMC for release after 12 months.
Peer review history
The peer review history is available online at https://journals.biologists.com/dev/article-lookup/doi/10.1242/dev.200131.
References
- Adams, R. H., Wilkinson, G. A., Weiss, C., Diella, F., Gale, N. W., Deutsch, U., Risau, W. and Klein, R. (1999). Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13, 295-306. 10.1101/gad.13.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitsebaomo, J., Portbury, A. L., Schisler, J. C. and Patterson, C. (2008). Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circ. Res. 103, 929-939. 10.1161/CIRCRESAHA.108.184937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, P. S., Brown, S. J. and Riddle, D. L. (1981). Sensory control of dauer larva formation in Caenorhabditis elegans. J. Comp. Neurol. 198, 435-451. 10.1002/cne.901980305 [DOI] [PubMed] [Google Scholar]
- Baeyens, N. and Schwartz, M. A. (2016). Biomechanics of vascular mechanosensation and remodeling. Mol. Biol. Cell 27, 7-11. 10.1091/mbc.E14-11-1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard, R. S., Jr, Hoettels, B. A., Meegan, J. E., Wertz, T. S., Cha, B. J., Yang, X., Oxford, J. T., Wu, M. H. and Yuan, S. Y. (2020). AKT2 maintains brain endothelial claudin-5 expression and selective activation of IR/AKT2/FOXO1-signaling reverses barrier dysfunction. J. Cereb. Blood Flow Metab. 40, 374-391. 10.1177/0271678X18817512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatchez, P. N., Soker, S. and Sirois, M. G. (1999). Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J. Biol. Chem. 274, 31047-31054. 10.1074/jbc.274.43.31047 [DOI] [PubMed] [Google Scholar]
- Casci, T., Vinos, J. and Freeman, M. (1999). Sprouty, an intracellular inhibitor of Ras signaling. Cell 96, 655-665. 10.1016/S0092-8674(00)80576-0 [DOI] [PubMed] [Google Scholar]
- Chlench, S., Mecha Disassa, N., Hohberg, M., Hoffmann, C., Pohlkamp, T., Beyer, G., Bongrazio, M., da Silva-Azevedo, L., Baum, O., Pries, A. R.et al. (2007). Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett. 581, 673-680. 10.1016/j.febslet.2007.01.028 [DOI] [PubMed] [Google Scholar]
- Chong, D. C., Koo, Y., Xu, K., Fu, S. and Cleaver, O. (2011). Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev. Dyn. 240, 2153-2165. 10.1002/dvdy.22706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, M., Li, T., Shen, B., Cao, X., Zhong, H., Zhang, L., Zhou, F., Ma, W., Jiang, H., Xie, P.et al. (2016). Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII. eLife 5, e21032. 10.7554/eLife.21032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver, O. and Krieg, P. A. (1998). VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development 125, 3905-3914. 10.1242/dev.125.19.3905 [DOI] [PubMed] [Google Scholar]
- Corada, M., Nyqvist, D., Orsenigo, F., Caprini, A., Giampietro, C., Taketo, M. M., Iruela-Arispe, M. L., Adams, R. H. and Dejana, E. (2010). The Wnt/β-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev. Cell 18, 938-949. 10.1016/j.devcel.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin, L. D., Villefranc, J. A., Kacergis, M. C., Weinstein, B. M. and Lawson, N. D. (2006). Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. USA 103, 6554-6559. 10.1073/pnas.0506886103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, L. T. H., Aburatani, T., Marsh, G. A., Johnson, B. G., Alimperti, S., Yoon, C. J., Huang, A., Szak, S., Nakagawa, N., Gomez, I.et al. (2017). Hyperactive FOXO1 results in lack of tip stalk identity and deficient microvascular regeneration during kidney injury. Biomaterials 141, 314-329. 10.1016/j.biomaterials.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharaneeswaran, H., Abid, M. R., Yuan, L., Dupuis, D., Beeler, D., Spokes, K. C., Janes, L., Sciuto, T., Kang, P. M., Jaminet, S.-C. S.et al. (2014). FOXO1-mediated activation of Akt plays a critical role in vascular homeostasis. Circ. Res. 115, 238-251. 10.1161/CIRCRESAHA.115.303227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, M. E., Flenniken, A. M., Ji, X., Teboul, L., Wong, M. D., White, J. K., Meehan, T. F., Weninger, W. J., Westerberg, H., Adissu, H.et al. (2016). High-throughput discovery of novel developmental phenotypes. Nature 537, 508-514. 10.1038/nature19356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit, M., Bess, E., Fisslthaler, B., Hartel, F. V., Noll, T., Busse, R. and Fleming, I. (2008). Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc. Res. 77, 160-168. 10.1093/cvr/cvm017 [DOI] [PubMed] [Google Scholar]
- Duarte, A., Hirashima, M., Benedito, R., Trindade, A., Diniz, P., Bekman, E., Costa, L., Henrique, D. and Rossant, J. (2004). Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 18, 2474-2478. 10.1101/gad.1239004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer, M. A., Farrington, S. M., Mohn, D., Munday, J. R. and Baron, M. H. (2001). Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development 128, 1717-1730. 10.1242/dev.128.10.1717 [DOI] [PubMed] [Google Scholar]
- Dziadek, M. and Adamson, E. (1978). Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. J. Embryol. Exp. Morphol. 43, 289-313. 10.1242/dev.43.1.289 [DOI] [PubMed] [Google Scholar]
- Eklund, L. and Olsen, B. R. (2006). Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 312, 630-641. 10.1016/j.yexcr.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Fang, J. S., Coon, B. G., Gillis, N., Chen, Z., Qiu, J., Chittenden, T. W., Burt, J. M., Schwartz, M. A. and Hirschi, K. K. (2017). Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat. Commun. 8, 2149. 10.1038/s41467-017-01742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous, A., Morris, J., Abedin, M. J., Collins, S., Richardson, J. A. and Hill, J. A. (2011). Forkhead factor FoxO1 is essential for placental morphogenesis in the developing embryo. Proc. Natl. Acad. Sci. USA 108, 16307-16312. 10.1073/pnas.1107341108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A., Schumacher, N., Maier, M., Sendtner, M. and Gessler, M. (2004). The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 18, 901-911. 10.1101/gad.291004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish, J. E. and Wythe, J. D. (2015). The molecular regulation of arteriovenous specification and maintenance. Dev. Dyn. 244, 391-409. 10.1002/dvdy.24252 [DOI] [PubMed] [Google Scholar]
- Fish, J. E., Cantu Gutierrez, M., Dang, L. T., Khyzha, N., Chen, Z., Veitch, S., Cheng, H. S., Khor, M., Antounians, L., Njock, M.-S.et al. (2017). Dynamic regulation of VEGF-inducible genes by an ERK/ERG/p300 transcriptional network. Development 144, 2428-2444. 10.1242/dev.146050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosbrink, M., Niculescu, F., Rus, V., Shin, M. L. and Rus, H. (2006). C5b-9-induced endothelial cell proliferation and migration are dependent on Akt inactivation of forkhead transcription factor FOXO1. J. Biol. Chem. 281, 19009-19018. 10.1074/jbc.M602055200 [DOI] [PubMed] [Google Scholar]
- Fraser, S. T., Hadjantonakis, A.-K., Sahr, K. E., Willey, S., Kelly, O. G., Jones, E. A., Dickinson, M. E. and Baron, M. H. (2005). Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis 42, 162-171. 10.1002/gene.20139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto, M., Kondo, K., Uni, K., Ishiguro, T., Hayashi, M., Ueda, S., Mori, I., Niimi, K., Tashiro, F., Miyazaki, S.et al. (2018). Tip-cell behavior is regulated by transcription factor FoxO1 under hypoxic conditions in developing mouse retinas. Angiogenesis 21, 203-214. 10.1007/s10456-017-9588-z [DOI] [PubMed] [Google Scholar]
- Furuyama, T., Kitayama, K., Shimoda, Y., Ogawa, M., Sone, K., Yoshida-Araki, K., Hisatsune, H., Nishikawa, S., Nakayama, K., Nakayama, K.et al. (2004). Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J. Biol. Chem. 279, 34741-34749. 10.1074/jbc.M314214200 [DOI] [PubMed] [Google Scholar]
- Gale, N. W., Dominguez, M. G., Noguera, I., Pan, L., Hughes, V., Valenzuela, D. M., Murphy, A. J., Adams, N. C., Lin, H. C., Holash, J.et al. (2004). Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc. Natl. Acad. Sci. USA 101, 15949-15954. 10.1073/pnas.0407290101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, J. G., Steed, E., Ferreira, R. R., Roth, S., Ramspacher, C., Boselli, F., Charvin, G., Liebling, M., Wyart, C., Schwab, Y.et al. (2014). Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep 6, 799-808. 10.1016/j.celrep.2014.01.032 [DOI] [PubMed] [Google Scholar]
- Gong, Y., Yang, X., He, Q., Gower, L., Prudovsky, I., Vary, C. P. H., Brooks, P. C. and Friesel, R. E. (2013). Sprouty4 regulates endothelial cell migration via modulating integrin beta3 stability through c-Src. Angiogenesis 16, 861-875. 10.1007/s10456-013-9361-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley, T. (2010). Notch signaling in the vasculature. Curr. Top. Dev. Biol. 92, 277-309. 10.1016/S0070-2153(10)92009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, H. and Kume, T. (2008). Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS ONE 3, e2401. 10.1371/journal.pone.0002401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, A. M., Rhyner, A. M., Devine, W. P., Marrelli, S. P., Bruneau, B. G. and Wythe, J. D. (2018). A novel reporter allele for monitoring Dll4 expression within the embryonic and adult mouse. Biol. Open 7, bio026799. 10.1242/bio.026799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog, Y., Guttmann-Raviv, N. and Neufeld, G. (2005). Segregation of arterial and venous markers in subpopulations of blood islands before vessel formation. Dev. Dyn. 232, 1047-1055. 10.1002/dvdy.20257 [DOI] [PubMed] [Google Scholar]
- Hosaka, T., Biggs, W. H., III, Tieu, D., Boyer, A. D., Varki, N. M., Cavenee, W. K. and Arden, K. C. (2004). Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA 101, 2975-2980. 10.1073/pnas.0400093101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. and Tindall, D. J. (2007). Dynamic FoxO transcription factors. J. Cell Sci. 120, 2479-2487. 10.1242/jcs.001222 [DOI] [PubMed] [Google Scholar]
- Hwa, J. J., Beckouche, N., Huang, L., Kram, Y., Lindskog, H. and Wang, R. A. (2017). Abnormal arterial-venous fusions and fate specification in mouse embryos lacking blood flow. Sci. Rep. 7, 11965. 10.1038/s41598-017-12353-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, R. P., Phoon, C. K., Aristizabal, O., Mcgrath, K. E., Palis, J. and Turnbull, D. H. (2003). Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 92, 133-135. 10.1161/01.RES.0000056532.18710.C0 [DOI] [PubMed] [Google Scholar]
- Jiang, Z., Tian, J., Zhang, W., Yan, H., Liu, L., Huang, Z., Lou, J. and Ma, X. (2017). Forkhead protein FoxO1 acts as a repressor to inhibit cell differentiation in human fetal pancreatic progenitor cells. J. Diabetes Res. 2017, 6726901. 10.1155/2017/6726901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiramongkol, Y. and Lam, E. W.-F. (2020). FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 39, 681-709. 10.1007/s10555-020-09883-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. A. V., Baron, M. H., Fraser, S. E. and Dickinson, M. E. (2004). Measuring hemodynamic changes during mammalian development. Am. J. Physiol. Heart Circ. Physiol. 287, H1561-H1569. 10.1152/ajpheart.00081.2004 [DOI] [PubMed] [Google Scholar]
- Kappel, A., Rönicke, V., Damert, A., Flamme, I., Risau, W. and Breier, G. (1999). Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood 93, 4284-4292. 10.1182/blood.V93.12.4284 [DOI] [PubMed] [Google Scholar]
- Kim, Y. H., Choi, J., Yang, M. J., Hong, S. P., Lee, C.-K., Kubota, Y., Lim, D.-S. and Koh, G. Y. (2019). A MST1-FOXO1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nat. Commun. 10, 838. 10.1038/s41467-019-08773-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki, Y. Y., Hammer, R. E., Miyazaki, J.-I., Williams, S. C., Richardson, J. A. and Yanagisawa, M. (2001). Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230, 230-242. 10.1006/dbio.2000.0106 [DOI] [PubMed] [Google Scholar]
- Kondrychyn, I., Kelly, D. J., Carretero, N. T., Nomori, A., Kato, K., Chong, J., Nakajima, H., Okuda, S., Mochizuki, N. and Phng, L.-K. (2020). Marcksl1 modulates endothelial cell mechanoresponse to haemodynamic forces to control blood vessel shape and size. Nat. Commun. 11, 5476. 10.1038/s41467-020-19308-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs, L. T., Xue, Y., Norton, C. R., Shutter, J. R., Maguire, M., Sundberg, J. P., Gallahan, D., Closson, V., Kitajewski, J., Callahan, R.et al. (2000). Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 14, 1343-1352. 10.1101/gad.14.11.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs, L. T., Starling, C., Chervonsky, A. V. and Gridley, T. (2010). Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis 48, 146-150. 10.1002/dvg.20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso, M., Pichel, J. G., Gorman, J. R., Sauer, B., Okamoto, Y., Lee, E., Alt, F. W. and Westphal, H. (1996). Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93, 5860-5865. 10.1073/pnas.93.12.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet, F., Haeusler, R. A., Lindén, D., Ericson, E., Norris, T., Johansson, A., Cook, J. R., Aizawa, K., Wang, L., Buettner, C.et al. (2017). Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell 171, 824-835.e18. 10.1016/j.cell.2017.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, N. D., Scheer, N., Pham, V. N., Kim, C.-H., Chitnis, A. B., Campos-Ortega, J. A. and Weinstein, B. M. (2001). Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128, 3675-3683. 10.1242/dev.128.19.3675 [DOI] [PubMed] [Google Scholar]
- Lawson, N. D., Vogel, A. M. and Weinstein, B. M. (2002). sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell 3, 127-136. 10.1016/S1534-5807(02)00198-3 [DOI] [PubMed] [Google Scholar]
- le Noble, F., Moyon, D., Pardanaud, L., Yuan, L., Djonov, V., Matthijsen, R., Bréant, C., Fleury, V. and Eichmann, A. (2004). Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 131, 361-375. 10.1242/dev.00929 [DOI] [PubMed] [Google Scholar]
- le Noble, F., Fleury, V., Pries, A., Corvol, P., Eichmann, A. and Reneman, R. S. (2005). Control of arterial branching morphogenesis in embryogenesis: go with the flow. Cardiovasc. Res. 65, 619-628. 10.1016/j.cardiores.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Lee, S. H., Schloss, D. J., Jarvis, L., Krasnow, M. A. and Swain, J. L. (2001). Inhibition of angiogenesis by a mouse sprouty protein. J. Biol. Chem. 276, 4128-4133. 10.1074/jbc.M006922200 [DOI] [PubMed] [Google Scholar]
- Liu, Z.-J., Shirakawa, T., Li, Y., Soma, A., Oka, M., Dotto, G. P., Fairman, R. M., Velazquez, O. C. and Herlyn, M. (2003). Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol. Cell. Biol. 23, 14-25. 10.1128/MCB.23.1.14-25.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov, I. B., Renard, R. A., Papadopoulos, N., Gale, N. W., Thurston, G., Yancopoulos, G. D. and Wiegand, S. J. (2007). Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 104, 3219-3224. 10.1073/pnas.0611206104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti, J. L., Jones, E. A. V., Huang, C., Chen, J., Fraser, S. E. and Dickinson, M. E. (2007). Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134, 3317-3326. 10.1242/dev.02883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, J. J. and Iruela-Arispe, M. L. (2018). NOTCH regulation of the endothelial cell phenotype. Curr. Opin Hematol. 25, 212-218. 10.1097/MOH.0000000000000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, J. J., Mosqueiro, T. S., Archer, B. J., Jones, W. M., Sunshine, H., Faas, G. C., Briot, A., Aragón, R. L., Su, T., Romay, M. C.et al. (2017). NOTCH1 is a mechanosensor in adult arteries. Nat. Commun. 8, 1620. 10.1038/s41467-017-01741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhos, C., Modlich, U., Lewis, J., Harris, A., Bicknell, R. and Ish-Horowicz, D. (2001). Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation 69, 135-144. 10.1046/j.1432-0436.2001.690207.x [DOI] [PubMed] [Google Scholar]
- Masumura, T., Yamamoto, K., Shimizu, N., Obi, S. and Ando, J. (2009). Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler. Thromb. Vasc. Biol. 29, 2125-2131. 10.1161/ATVBAHA.109.193185 [DOI] [PubMed] [Google Scholar]
- Niimi, K., Ueda, M., Fukumoto, M., Kohara, M., Sawano, T., Tsuchihashi, R., Shibata, S., Inagaki, S. and Furuyama, T. (2017). Transcription factor FOXO1 promotes cell migration toward exogenous ATP via controlling P2Y1 receptor expression in lymphatic endothelial cells. Biochem. Biophys. Res. Commun. 489, 413-419. 10.1016/j.bbrc.2017.05.156 [DOI] [PubMed] [Google Scholar]
- Obi, S., Yamamoto, K., Shimizu, N., Kumagaya, S., Masumura, T., Sokabe, T., Asahara, T. and Ando, J. (2009). Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J. Appl. Physiol. (1985) 106, 203-211. 10.1152/japplphysiol.00197.2008 [DOI] [PubMed] [Google Scholar]
- Paik, J.-H., Kollipara, R., Chu, G., Ji, H., Xiao, Y., Ding, Z., Miao, L., Tothova, Z., Horner, J. W., Carrasco, D. R.et al. (2007). FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309-323. 10.1016/j.cell.2006.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis, J. (2014). Primitive and definitive erythropoiesis in mammals. Front. Physiol. 5, 3. 10.3389/fphys.2014.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente, M., Urbich, C., Sasaki, K.-I., Hofmann, W. K., Heeschen, C., Aicher, A., Kollipara, R., Depinho, R. A., Zeiher, A. M. and Dimmeler, S. (2005). Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Invest. 115, 2382-2392. 10.1172/JCI23126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell, M., Nakayama, A., Hikita, T., Mirzapourshafiyi, F., Kawamura, T., Pasha, A., Li, M., Masuzawa, M., Looso, M., Steinbacher, T.et al. (2018). aPKC controls endothelial growth by modulating c-Myc via FoxO1 DNA-binding ability. Nat. Commun. 9, 5357. 10.1038/s41467-018-07739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau, W. (1994). Angiogenesis and endothelial cell function. Arzneimittelforschung 44, 416-417. [PubMed] [Google Scholar]
- Risau, W. and Flamme, I. (1995). Vasculogenesis. Annu. Rev. Cell Dev. Biol. 11, 73-91. 10.1146/annurev.cb.11.110195.000445 [DOI] [PubMed] [Google Scholar]
- Rönicke, V., Risau, W. and Breier, G. (1996). Characterization of the endothelium-specific murine vascular endothelial growth factor receptor-2 (Flk-1) promoter. Circ. Res. 79, 277-285. 10.1161/01.RES.79.2.277 [DOI] [PubMed] [Google Scholar]
- Rudnicki, M., Abdifarkosh, G., Nwadozi, E., Ramos, S. V., Makki, A., Sepa-Kishi, D. M., Ceddia, R. B., Perry, C. G., Roudier, E. and Haas, T. L. (2018). Endothelial-specific FoxO1 depletion prevents obesity-related disorders by increasing vascular metabolism and growth. eLife 7, e39780. 10.7554/eLife.39780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacilotto, N., Monteiro, R., Fritzsche, M., Becker, P. W., Sanchez-del-Campo, L., Liu, K., Pinheiro, P., Ratnayaka, I., Davies, B., Goding, C. R.et al. (2013). Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc. Natl. Acad. Sci. USA 110, 11893-11898. 10.1073/pnas.1300805110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeger, T. M., Bartunkova, S., Lawitts, J. A., Teichmann, G., Risau, W., Deutsch, U. and Sato, T. N. (1997). Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. USA 94, 3058-3063. 10.1073/pnas.94.7.3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta, A., Chakraborty, S., Paik, J., Yutzey, K. E. and Evans-Anderson, H. J. (2012). FoxO1 is required in endothelial but not myocardial cell lineages during cardiovascular development. Dev. Dyn. 241, 803-813. 10.1002/dvdy.23759 [DOI] [PubMed] [Google Scholar]
- Seo, S., Fujita, H., Nakano, A., Kang, M., Duarte, A. and Kume, T. (2006). The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev. Biol. 294, 458-470. 10.1016/j.ydbio.2006.03.035 [DOI] [PubMed] [Google Scholar]
- Shay-Salit, A., Shushy, M., Wolfovitz, E., Yahav, H., Breviario, F., Dejana, E. and Resnick, N. (2002). VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 99, 9462-9467. 10.1073/pnas.142224299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter, J. R., Scully, S., Fan, W., Richards, W. G., Kitajewski, J., Deblandre, G. A., Kintner, C. R. and Stark, K. L. (2000). Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 14, 1313-1318. 10.1101/gad.14.11.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann, A. F. and Lawson, N. D. (2007). Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781-784. 10.1038/nature05577 [DOI] [PubMed] [Google Scholar]
- Su, T., Stanley, G., Sinha, R., D'Amato, G., Das, S., Rhee, S., Chang, A. H., Poduri, A., Raftrey, B., Dinh, T. T.et al. (2018). Single-cell analysis of early progenitor cells that build coronary arteries. Nature 559, 356-362. 10.1038/s41586-018-0288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Chen, W.-D. and Wang, Y.-D. (2017). DAF-16/FOXO transcription factor in aging and longevity. Front. Pharmacol. 8, 548. 10.3389/fphar.2017.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift, M. R. and Weinstein, B. M. (2009). Arterial-venous specification during development. Circ. Res. 104, 576-588. 10.1161/CIRCRESAHA.108.188805 [DOI] [PubMed] [Google Scholar]
- Taniguchi, K., Ishizaki, T., Ayada, T., Sugiyama, Y., Wakabayashi, Y., Sekiya, T., Nakagawa, R. and Yoshimura, A. (2009). Sprouty4 deficiency potentiates Ras-independent angiogenic signals and tumor growth. Cancer Sci. 100, 1648-1654. 10.1111/j.1349-7006.2009.01214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima, E., Irani-Tehrani, M., Kiosses, W. B., Dejana, E., Schultz, D. A., Engelhardt, B., Cao, G., Delisser, H. and Schwartz, M. A. (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426-431. 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- Udan, R. S., Vadakkan, T. J. and Dickinson, M. E. (2013). Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac. Development 140, 4041-4050. 10.1242/dev.096255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. U., Chen, Z.-F. and Anderson, D. J. (1998). Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741-753. 10.1016/S0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- Weinstein, B. M. and Lawson, N. D. (2002). Arteries, veins, Notch, and VEGF. Cold Spring Harb. Symp. Quant. Biol. 67, 155-162. 10.1101/sqb.2002.67.155 [DOI] [PubMed] [Google Scholar]
- Wietecha, M. S., Chen, L., Ranzer, M. J., Anderson, K., Ying, C., Patel, T. B. and Dipietro, L. A. (2011). Sprouty2 downregulates angiogenesis during mouse skin wound healing. Am. J. Physiol. Heart Circ. Physiol. 300, H459-H467. 10.1152/ajpheart.00244.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm, K., Happel, K., Eelen, G., Schoors, S., Oellerich, M. F., Lim, R., Zimmermann, B., Aspalter, I. M., Franco, C. A., Boettger, T.et al. (2016). FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 529, 216-220. 10.1038/nature16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wragg, J. W., Durant, S., Mcgettrick, H. M., Sample, K. M., Egginton, S. and Bicknell, R. (2014). Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation 21, 290-300. 10.1111/micc.12119 [DOI] [PubMed] [Google Scholar]
- Wythe, J. D., Dang, L. T. H., Devine, W. P., Boudreau, E., Artap, S. T., He, D., Schachterle, W., Stainier, D. Y. R., Oettgen, P., Black, B. L.et al. (2013). ETS factors regulate Vegf-dependent arterial specification. Dev. Cell 26, 45-58. 10.1016/j.devcel.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Y., Gao, X., Lindsell, C. E., Norton, C. R., Chang, B., Hicks, C., Gendron-Maguire, M., Rand, E. B., Weinmaster, G. and Gridley, T. (1999). Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 8, 723-730. 10.1093/hmg/8.5.723 [DOI] [PubMed] [Google Scholar]
- You, L.-R., Lin, F.-J., Lee, C. T., Demayo, F. J., Tsai, M.-J. and Tsai, S. Y. (2005). Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435, 98-104. 10.1038/nature03511 [DOI] [PubMed] [Google Scholar]
- Zhang, H., Ge, S., He, K., Zhao, X., Wu, Y., Shao, Y. and Wu, X. (2019). FoxO1 inhibits autophagosome-lysosome fusion leading to endothelial autophagic-apoptosis in diabetes. Cardiovasc. Res. 115, 2008-2020. 10.1093/cvr/cvz014 [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Yang, J., Liao, W., Liu, X., Zhang, H., Wang, S., Wang, D., Feng, J., Yu, L. and Zhu, W.-G. (2010). Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat. Cell Biol. 12, 665-675. 10.1038/ncb2069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.