Abstract
Previous studies investigating the effects of blocking the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis in prostate cancer found no effects of the growth hormone receptor (GHR) antagonist, pegvisomant, on the growth of grafted human prostate cancer cells in vivo. However, human GHR is not activated by mouse GH, so direct actions of GH on prostate cancer cells were not evaluated in this context. The present study addresses the species specificity of GH-GHR activity by investigating GH actions in prostate cancer cell lines derived from a mouse Pten-deletion model. In vitro cell growth was stimulated by GH and reduced by pegvisomant. These in vitro GH effects were mediated at least in part by the activation of JAK2 and STAT5. When Pten-mutant cells were grown as xenografts in mice, pegvisomant treatment dramatically reduced xenograft size, and this was accompanied by decreased proliferation and increased apoptosis. RNA sequencing of xenografts identified 1765 genes upregulated and 953 genes downregulated in response to pegvisomant, including many genes previously implicated as cancer drivers. Further evaluation of a selected subset of these genes via quantitative reverse transcription–polymerase chain reaction determined that some genes exhibited similar regulation by pegvisomant in prostate cancer cells whether treatment was in vivo or in vitro, indicating direct regulation by GH via GHR activation in prostate cancer cells, whereas other genes responded to pegvisomant only in vivo, suggesting indirect regulation by pegvisomant effects on the host endocrine environment. Similar results were observed for a prostate cancer cell line derived from the mouse transgenic adenocarcinoma of the mouse prostate (TRAMP) model.
Keywords: prostate cancer, GH, IGF-1, pegvisomant, RNA-seq
Prostate cancer is the most common noncutaneous cancer affecting men in the United States, making research in this area vital (1).The investigation of the role of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) in promoting diseases including prostate cancer has resulted in the publication of multitudes of peer-reviewed manuscripts over the past 4 decades. Human Laron syndrome is caused by loss-of-function mutations in the growth hormone receptor (GHR) gene and, thus, disruption of the entire GH/IGF-1 axis, resulting in short stature. Humans and mouse models possessing this or similar mutations appear to be protected from experimentally induced cancer (2-4). In a study of patients with Laron syndrome, none of the 169 participants developed cancer while their relatives with a functioning GH/IGF-1 axis developed cancer at the expected rate of the normal population (3). Research focusing on this protective effect has focused on the role of GH in cancer progression. In prostate cancer, higher circulating IGF-1 levels have been associated with worse outcomes (5, 6). Although much of the available evidence suggests that the effects of GH in prostate cancer depend largely on its ability to regulate IGF-1, some actions may be through direct activation of GHRs expressed in normal or cancerous prostate cells (7, 8). When crossed with the mouse model of Laron syndrome (Ghr–/– null mice), the C3 (1)/TAg mouse model of prostate cancer fails to develop prostatic intraepithelial neoplasia lesions as expected, showing that GH signaling disruption can prevent prostate cancer progression, thus mimicking the protection seen in human Laron syndrome individuals (4). One limitation of these studies is that Laron patients and the Laron mouse model lack GH signaling at all stages of development and adulthood so they cannot address the potential ongoing requirement for GH signaling in prostate cancer.
Pegvisomant is a Food and Drug Administration–approved drug that acts as a GHR antagonist originally intended to treat patients with acromegaly and reduce circulating IGF-1 to normal levels by decreasing GH signaling. The original characterization of pegvisomant identified it as being highly specific for GHR without affecting similar hormone receptors such as prolactin receptor (9).The potential anticancer effects of pegvisomant have been investigated for several cancers. For example, pegvisomant-treated mice showed a reduction in mammary tumor cell proliferation in vivo (10). Likewise, endometrial cancer cell xenograft growth was slowed when treated with pegvisomant in combination with radiation therapy (11). The potential roles of GH and GHR in prostate cancer and potential use of pegvisomant have also been investigated using human prostate cancer cell lines. One investigation of GH in human LNCaP prostate cancer cells found no effect on proliferation in GH-treated cells, but did observe GH-induced changes in androgen receptor (AR) expression including a transient increase in expression after 12 hours of GH treatment followed by decreased AR expression after 48 hours of GH treatment (12). Nakonechnaya et al (13) found that autocrine GH overexpression in LNCaP cells resulted in decreased proliferation and increased apoptosis, indicating a specific role of direct GH signaling on prostate cells. A 2017 study described the effects of pegvisomant on xenografts of the human 22Rv1 prostate cancer cell line in mice (14). While the study found that pegvisomant treatment resulted in decreased levels of liver IGF-1 as well as xenograft AR variant 7 (ARv7) and IGF-1, the size of the xenografts was not affected by pegvisomant. However, an important limitation of this study is that GH activation of the GHR is species-specific, meaning only primate GH can activate the human receptor (15). As a consequence, pegvisomant used in the xenografting study could inhibit the activation of host GHR by circulating mouse GH. This had important effects, including decreasing circulating IGF-1 levels. Since human GHR is not activated by mouse GH, possible direct actions of GH on prostate cancer cells were not evaluated in this xenografting study. Another issue that stems from the species-specific nature of GH activation of GHR is that the most widely used human prostate cancer cell lines have all undergone selection to grow robustly in the absence of exogenous GH since standard media formulations lack primate GH.
Mouse models can be used to account for species-specific differences in GH-GHR binding. The PTEN-P2 and PTEN-P8 cell lines are 2 independent, clonal prostate epithelial cell lines heterozygous for Pten deletion derived from a Ptenloxp/loxp;PB-Cre4 + mouse. Subsequent transduction with a CRE expressing retrovirus produced cell lines PTEN-CaP2 and PTEN-CaP8 that are homozygous for Pten deletion and derived from PTEN-P2 and PTEN-P8, respectively (16). These 4 cell lines were also immortalized in and have been maintained in media containing bovine pituitary extract (BPE) that is a rich source of GH. Thus, these cell lines have never been selected for the capacity to grow in the absence of exogenous GH. Renal xenografting of these cells has previously been successful in our laboratory and has shown to be a viable model of in vivo growth of mouse prostate cancer cells in immunocompromised mice (17). The TRAMP-C2 cell line provides an additional mouse cell line model of prostate cancer (18) that was derived from the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse (19) and has a cancer driver gene, SV40 T-antigen, different from the PTEN series of cell lines.
In this study we investigated the role of GH signaling in prostate cancer by using pegvisomant to block GH function in prostate cancer models in vitro and in vivo. The PTEN mouse prostate cancer cell lines provided a viable model to determine that blocking GH decreased viability and migration of prostate cancer cells in culture. In these cells, GH signaled primarily through the JAK2/STAT5 pathway. Use of pegvisomant in renal grafts of the PTEN and TRAMP mouse prostate cancer cells modeled the effects of GHR antagonism in vivo and showed that grafts in pegvisomant-treated mice were smaller in than vehicle-treated mice. In addition, a large set of genes were differentially regulated on GHR antagonism, including dozens linked to cancer. Together, through GH/IGF-1 axis disruption in vitro and in vivo in models of prostate cancer, this study suggests GH activity through the JAK/STAT pathway promotes prostate cancer growth and regulates cancer-related gene expression.
Materials and Methods
Animal Care
Mice were housed in polysulfone cages containing corn cob bedding and maintained on a 12-hour light and dark cycle at 20.5 ± 5 °C and 30% to 70% relative humidity. Mice were fed a 5015 Diet (PMI Nutrition International) from conception through weaning (postnatal day 21) and an 8604 Teklad Rodent Diet thereafter (Harlan Laboratories). Feed and water were available ad libitum. All procedures were approved by the University of Wisconsin–Madison Animal Care and Use Committee (M005521-R01-A03) and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals were humanely killed by CO2 asphyxiation in accordance with guidelines set forth by the American Medical Veterinary Association (20).
Cell Lines and Pharmacologic Agents
Prostate cancer cell lines PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 (courtesy of Dr Hong Wu from the University of California Los Angeles) were cultured in humid conditions at 37 °C and 5% CO2 in media containing 4.5 g/L glucose Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin, 6 ng/mL human epidermal growth factor, 5 μg/mL insulin, and 25 μg/mL BPE (16). TRAMP-C2 cells (courtesy of Dr Barbara Foster from Roswell Park Cancer Institute) were cultured in humid conditions at 37 °C and 5% CO2 in media containing 4.5 g/L glucose Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated FBS, 1% penicillin/streptomycin, 5 μg/mL insulin, and 10–8 dihydrotestosterone (19). Bovine growth hormone (bGH) was purchased from MP Biomedicals (No. 160074) and suspended in 0.1% bovine serum albumin (BSA) at a stock concentration of 1 mg/mL and stored at –20 °C for short-term storage. Pegvisomant was provided by Pfizer and suspended in 0.1% BSA in phosphate-buffered saline (PBS) at a stock concentration of 10 mg/mL and stored at –80 °C. IGF-1 was purchased from Thermo Fisher Scientific (No. PMG0075) and used at a working concentration of 0.1 µg/mL. The STAT inhibitor SH-4-54 was purchased from Selleck Chemicals (No. S7337) and the mitogen-activated protein kinase (MAPK) inhibitor UO126 from Cell Signaling Technologies (No. 9903S) and used at working concentrations of 300 nM and 10 µM, respectively.
In Vitro Assays
Cell viability in culture was assessed using the Promega CellTiter-Blue Cell Viability Assay (No. G808A; Promega). Specifically, cells were plated at 250 cells/well in 96-well plates 24 hours before the addition of vehicle-, pegvisomant-, or bGH-treated media. At 72 hours after treatment, 20 µL of CellTiter-Blue reagent was added to each well and fluorescence signal was read on a FLUOstar Omega fluorescence plate reader (BMG Labtech) at 544/590 nm.
Cell migration in vitro was assessed by scratch assay as described previously (21). In short, cells were plated onto a 60-mm petri dish to create a confluent monolayer and incubated for 6 hours at 37 °C and 5% CO2. A p200 pipette tip was used to scratch a straight line through the monolayer and the media was changed to vehicle- or pegvisomant-treated media. Images were acquired at t = 0 and 6 hours using a Motic AE20 Series inverted microscope (BLE1800371; Boston Lab Co). Wound distance was calculated as the width of the wound in pixels and presented as a percentage of distance at t = 0.
Western Blots
PTEN cells were grown to 70% confluency in 6-well plates, starved of media containing FBS and BPE for 24 hours, and treated with full media containing 0.1 µg/mL of bGH for 30 minutes before collection. One group of cells was additionally pretreated for 30 minutes with 20 µg/mL pegvisomant before bGH supplementation. Cell lysates were prepared using Pierce radioimmunoprecipitation assay (RIPA) Buffer (No. 89900, Thermo Fisher Scientific) with the addition of Halt Protease and Phosphatase Inhibitor Cocktail (No. 7844, Thermo Fisher Scientific) following the manufacturing instructions. A total of 15 µg of denatured protein was loaded on home-prepared 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. Electrophoresis was performed using the Invitrogen XCell SureLock Mini-Cell apparatus followed by X-Cell II Blot Module protein transfer to a Nitrocellulose Hybond-C Extra 45-µm protein transfer membrane (RPN303E, GE) according to the user manual. Antibody-appropriate blocking buffer (5% dry milk or 5% BSA diluted in TBST) was used to block nonspecific membrane binding. To measure the amount of protein expression the following primary antibodies were used: p44/42 MAPK (Erk1/2) (22), Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (23), STAT5 (D2O6Y) (24), Phospho-STAT5 (Tyr694) (25), JAK2 (D2E12) (26), Phospho-JAK2 (Tyr1007/1008) (27), Beta actin horseradish peroxidase/loading control (28), and donkey antirabbit immunoglobulin G H&L secondary (29). Secondary antibody was detected by chemiluminescent (ECL) horseradish peroxidase substrate from Thermo Scientific SuperSignal West Femto Maximum Sensitivity Substrate (34095, Cell Signaling Technologies) and imaged on an Azure C600 Imager.
The density of each Western blot band was measured with ImageJ software and is reported here as a ratio of phosphorylated over native (total) protein band densities and normalized to base media = 1.
Renal Xenografts
PTEN-CaP2, PTEN-CaP8, or TRAMP-C2 cells (3.5 × 105) were combined with collagen mix containing 6 mg/mL rat tail collagen (BD Bioscience), 1X (PBS), 1 N NaOH, and distilled H2O to make 25-μL collagen pellets for xenograft surgery as described previously (17, 30). Pellets were surgically grafted under the kidney capsules of 10-week-old male Balb/C nu/nu mice (Charles River). Xenografts were grown in vivo for 3 weeks at which time mice were treated with 100 mg/kg pegvisomant suspended in sterile saline or a sterile saline vehicle control 3 times per week for 3 weeks. Three days after the final injection, mice were humanely killed for organ and xenograft collection. Graft hemispheres were formalin-fixed and paraffin-embedded or flash-frozen. RNA was extracted from frozen tissue using a NucleoSpin RNA extraction kit (No. 740955, Machery-Nagel) per the manufacturer’s instructions.
Serum Collection and Circulating Insulin-like Growth Factor-1 Measurement
Trunk blood was collected for analysis of serum IGF-1 at the time of tissue collection. The blood samples were centrifuged at 1500g for 15 minutes, and serum was harvested and stored at −20 °C until analysis by enzyme-linked immunosorbent assay using a commercially available kit (MG100; R&D Systems).
Steroid hormone analysis was adapted from previously described methods (31, 32). Briefly, internal standard was added to mouse serum samples (80-220 µL), and they were then extracted twice using methyl tert-butyl ether followed by dichloromethane. Samples were derivatized using dansyl chloride and then analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS; Sciex QTRAP 5500). Individual calibration curves were constructed for each analyte with at least 8 points. The linearity was r greater than 0.9990 and the curve fit was linear with 1/x weighting. None of the compounds of interest were detected in blank or double blank samples. Interassay coefficient of variation was determined by 3 levels of human serum and ranged from 5.6% to 9.7%.
Histology and Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde, dehydrated in alcohol, cleared in CitriSolv, and infiltrated with paraffin. Hematoxylin and eosin stains of 5 µm sections of tissue mounted on Superfrost Plus Gold Slides (Thermo Fisher Scientific) were assessed by a histopathologist (R.H.). Cell proliferation was measured via Ki67 immunohistochemistry (IHC) staining. Sections were dewaxed in CitriSolv and rehydrated through graded ethanol. For antigen retrieval, the sections were boiled in Vector Citrate-based Unmasking Solution (No. H-330) and cooled to room temperature (RT). For Ki67 analysis, slides were incubated in 3% H2O2 to quench the reaction, followed by blocking, overnight incubation at 4 °C with a 1:200 dilution of Ki67 primary antibody (33) in 2.5% goat serum in PBS, a series of PBS + Tween washes, and 1-hour-long incubation at RT in a 1:1000 dilution of biotinylated goat antirabbit immunoglobulin G secondary antibody in 2.5% goat serum in PBS (34). Slides were stained with the Vector Vectastain ABC Elite kit (No. PK6100) and incubated with a DAB Peroxidase Substrate Kit (SK-4100; Vector Laboratories) for 5 to 10 minutes. DAB oxidation was halted by submerging the slides in water. Slides were counterstained in hematoxylin, dehydrated in graded ethanol and CitriSolv (No. 1601; Deacon Labs), then mounted using Permount mounting medium (No. SP15-100; Fisher Scientific). After Ki67 slides were stained, images were captured using a DMLB microscope (Leica Microsystems) and MicroPublisher Color RTV-5.0 CCD Camera (No. 01-MP5.0-RTV-R-CLR-10; QImaging), blinded, and analyzed in the ImageJ Java image processing program via the Cell Counter and counted manually. Positively stained cells were counted as well as a total count.
Immunofluorescence
Xenograft sections were dewaxed, rehydrated, and antigens were retrieved as stated earlier. Tissues were blocked in 1% BSA (No. BP1600; Fisher Scientific) plus 0.3% Triton X-100 (No. IB07100; IBI Scientific) in PBS for 1 hour at RT. Primary antibody in 1:100 dilution in 1% BSA in PBS for AR (35) was incubated for 1 hour at RT. Tissues were then incubated in a combination of immunofluorescent (IF) secondary antibodies including 1:150 in PBS donkey antirabbit 594 (36) and 1:1000 in PBS DAPI (No. D8417; MilliporeSigma) for 1 hour at RT in the dark. Slides were washed in PBS and mounted with Vectashield (No. H-1000; Vector) and nail polish.
Blinded IF staining images were scored using a semiquantitative scale where 0 = no positive cells; 1 = some positive cells (< 5%); 2 = estimated 5% to 15% positive cells; 3 = estimated 16% to 30% positive cells; 4 = estimated 31% to 50% positive cells; 5 = estimated greater than 50% positive cells.
RNA Sequencing
Total RNA submitted to the University of Wisconsin–Madison Biotechnology Center was assayed for purity and integrity via the NanoDrop One Spectrophotometer and Agilent 2100 Bioanalyzer, respectively. RNA libraries were prepared from samples that met the TruSeq Stranded Total RNA Sample Preparation Guide (No. 15031048 E) input guidelines using the Illumina TruSeq Stranded Total (Gold) RNA Sample Preparation kit (Illumina Inc). For each library preparation, cytoplasmic ribosomal RNA was removed using biotinylated target-specific oligos combined with paramagnetic beads tagged with streptavidin. Following purification, the reduced RNA was fragmented using divalent cations at elevated temperature. Fragmented RNA was copied into first-stranded complementary DNA (cDNA) using SuperScript II Reverse Transcriptase (Invitrogen) and random primers. Second-strand cDNA was synthesized using a modified dNTP mix (dTTP replaced with dUTP), DNA polymerase I, and RNase H. Double-stranded cDNA was cleaned up with AMPure XP Beads (1X) (Agencourt, Beckman Coulter).
The cDNA products were incubated with Klenow DNA Polymerase to add a single “A” nucleotide to the 3′ end of the blunt DNA fragments. Unique dual indexes (UDI) were ligated to the DNA fragments and cleaned up with 2 rounds of AMPure XP beads (0.8X). Adapter ligated DNA was amplified by polymerase chain reaction (PCR) and cleaned up with AMPure XP beads (0.8X). Final libraries were assessed for size and quantity using an Agilent DNA1000 chip and Qubit dsDNA HS Assay Kit (Invitrogen), respectively. Libraries were standardized to 2 nM. Paired-end 150 bp sequencing was performed, using standard sequencing by synthesis chemistry on an Illumina NovaSeq6000 sequencer. Images were analyzed using the standard Illumina Pipeline, version 1.8.2. Raw BAM files produced by this sequencing can be found at NCBI Gene Expression Omnibus with accession number GSE188543.
Bioinformatic analysis of transcriptomic data adheres to recommended ENCODE guidelines and best practices for RNA sequencing (37). The NCBI reference accessions used for the reference were NR_0032791 (Rn28s1), NR_003278.3 (Rn18s) and NR_003280.2 (Rs5-8s1). Alignment of adapter-trimmed (Skewer v0.1.123) (38); 2 × 150 (paired-end; PE) bp strand-specific Illumina reads to the Mus musculus GRCm38 genome (assembly accession GCA_000001635.7) was achieved with the Spliced Transcripts Alignment to a Reference (STAR v2.5.3a) software (39), a splice-junction aware aligner, using annotation modifications (v4.3.2) made by Lawson et al (40). Expression estimation was performed with RSEM v1.3.0 (RNASeq by Expectation Maximization) (41). To test for differential gene expression among individual group contrasts, expected read counts obtained from RSEM were used as input into DESeq2 (v1.32.0) (42). Before DESeq2 differential expression analysis, the quality of the read counts between samples was investigated through principal component analysis (PROSTATE CANCER) and correlation plots using the pheatmap R package for visualization (43). We determined that vehicle sample sV0194L2 should be dropped from the analysis because of the lower correlation of gene expression values of this sample to the other samples, including the remaining 2 vehicle samples and the sample’s relative distance from the other samples in the PROSTATE CANCER plots. Statistical P values were adjusted with Benjamini-Hochberg and the significant genes were selected at the 1% false discovery rate level (44). We implemented a custom filter that removed genes with expression level greater than 100 in 1 sample and less than 10 in all other samples. This resulted in the removal of 39 genes. For Cancer Census genes we mapped the human genes to mouse homologues using data available from http://www.informatics.jax.org/.
Functional analysis was carried out with the GSVA R package (45) using the Poisson kernel cumulative distribution function and the c2 (curated) gene sets for mouse, downloaded from http://bioinf.wehi.edu.au/MSigDB/. GSVA calculates an enrichment score matrix for each experimental condition (columns) and gene set (rows). The enrichment scores from GSVA were tested for statistical significance between experiment and control with the limma package using an adjusted P value cutoff of .01 (46). Heat maps were generated with the aheatmap command in the NMF package (47).
Reverse Transcription–Quantitative Polymerase Chain Reaction
cDNA was reverse transcribed from RNA extracted from pegvisomant- or vehicle-treated xenografts. Gene expression was assessed as an inverse function of cycle threshold (Ct) read by StepOnePlus Real-Time PCR System (No. 4376600; Thermo Fisher Scientific). To determine gene expression relative to the housekeeping gene, TATA box-binding protein (TBP), the mean Ct, TBP was subtracted from the mean Ct, gene. Relative gene copy was 2 raised to the negative of that difference, such as the following: .
Statistical Analysis
Experimental groups consisted of 3 to 6 animals for histological, IHC, and quantitative PCR analyses; figure images are representative of each treatment group. For all histological stains, a Welch t test was conducted to identify differences among means. Results are reported as mean ± SEM. A difference of P less than .05 was considered statistically significant. All statistics were performed using GraphPad statistical software (GraphPad Software).
Results
Growth Hormone Stimulates and Pegvisomant Inhibits Cell Growth and Migration of Pten-Mutant Prostate Cancer Cells
PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 were derived from the Pten-PbCre mouse prostate cancer model and chosen for in vitro investigation of GHR antagonism because of their immortalization and maintenance in GH-containing media (16). To determine if these cells were GH dependent, BPE found in their normal media was removed. The 3-day viability of all 4 cell lines was significantly reduced after removal of BPE (Fig. 1A). Viability was fully recovered in a dose-dependent manner when GH was returned to the media, and there was no difference in viability between full media containing BPE and BPE-depleted media containing the highest bGH concentration. A lower concentration of GH was able to restore growth for homozygous Pten-null cell lines PTEN-CaP2 and PTEN-CaP8 compared to heterozygous Pten-null cell lines PTEN-P2 and PTEN-P8. PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 cells were also grown in media containing increasing concentrations of the GHR antagonist, pegvisomant. At 3 days, all 4 cell lines showed a decreased viability when treated with 20 µg/mL pegvisomant (Fig. 1B). In addition, the migration of all 4 cell lines across an artificial wound was inhibited by pegvisomant as determined by a scratch assay (Fig. 1C and 1D). As with viability, the sensitivity of cell migration to pegvisomant varied among cell lines with all 4 cell lines showing inhibition at 20 µg/mL (Fig. 1D).
Figure 1.
Growth and migration of prostate cancer cell lines in respond to growth hormone (GH) and pegvisomant. A, Growth of 72 hours was assayed for PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 cells (n = 3 per condition) using a CellTiter-Blue assay for cells grown in base media without bovine pituitary extract (BPE) as a control, media supplemented with various concentrations of bovine growth hormone (bGH) as indicated by key on the right of the graphs, and in media containing BPE (Full). B, Growth of 72 hours was assayed for PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 cells (n = 3 per condition) using a CellTiter-Blue assay for cells gown in in full media containing BPE (a rich source of bGH) as a control and full media containing BPE supplemented with various concentrations of pegvisomant as indicated by key on the right of the graphs. C, Cell migration was determined using a scratch assay for PTEN cells grown in full media containing BPE with increasing concentrations of pegvisomant. Representative images of PTEN-CaP2 cells at 0 and 6 hours after the initial scratch when treated with vehicle or 20 µg/mL concentration of pegvisomant. The red, dashed line marks the boundary of the cells on either side of the wound. D, The ability of PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 cells (n = 3 per condition) to migrate across the wound is shown at various concentrations of pegvisomant as indicated by key on the right of the graphs. Statistically significant differences from control are indicated by *P less than .05; **P less than .01; and ***P less than .001 as determined by analysis of variance, Tukey multiple comparison test.
Growth Hormone Activates the JAK/STAT Pathway in Pten-mutant Prostate Cancer Cells and Blocking That Pathway Decreases Cell Viability
Phosphorylation of tyrosine 695 of JAK2 (p-JAK2) and of tyrosine 1007/1008 of STAT5 (p-STAT5) are indicative of the activation of these proteins downstream of GHR. GH treatment increased both p-JAK2 and p-STAT5 in PTEN cells, and these GH-induced increases in p-JAK2 and p-STAT5 were blocked by pretreatment with pegvisomant (Fig. 2A and 2B). In contrast, there was no statistically significant change in the activity of ERK1/2 in response to GH for any of the PTEN cell lines as determined by the phosphorylation of threonine 202/tyrosine 204 (see Fig. 2A and 2B). GH activation of JAK/STAT proved to be necessary for GH-induced growth as the small molecule inhibitor of STAT5, SH-4-54, reduced in vitro viability of all 4 PTEN cell lines to base levels (growth without the addition of GH) while the small-molecule inhibitor of MEK1/2, UO126, failed to block GH-induced proliferation (Fig. 2C). This reduction in viability mirrored that seen in cells treated with pegvisomant (see Fig. 1B and 2C). There was no change in Ghr expression in PTEN cells treated with bGH or pegvisomant (Fig. 2D).
Figure 2.
Growth hormone activates growth hormone receptor (GHR) signaling primarily through the JAK2/STAT5 pathway in Pten-mutant prostate cancer cells. PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 cells were starved in fetal bovine serum–free and bovine pituitary extract (BPE)-free medium for 24 hours followed by treatment with 0.1 µg/mL of bovine growth hormone (bGH; with or without a 30-minute pretreatment with pegvisomant). Protein was isolated after 30 minutes and JAK, STAT, and ERK 1/2 activation was assessed by immunoblot to determine relative level of phosphorylation of the tyrosine 694, tyrosine 1007/1008, and threonine 202/tyrosine 204 epitopes, respectively. Immunoblots for total JAK, total STAT, total ERK 1/2, and β-actin were conducted as controls. A, Representative immunoblots for PTEN-P2 and PTEN-CaP2 bands are shown. B, Quantified band densities (n = 3 per condition) are shown as the ratio of the phosphorylated- over total-protein for all 4 PTEN cell lines (statistically significant differences from base media control are indicated as *P < .05, **P < .01, ns = no significance; ANOVA, Tukey multiple comparison test). C, Growth of 72 hours was assayed for PTEN-P2, PTEN-CaP2, PTEN-P8, and PTEN-CaP8 cells (n = 3 per condition) using a CellTiter-Blue assay and is graphed as the increase in growth relative to base media without BPE. Cells were treated with 0.1 µg/mL bGH, 2 µg/mL pegvisomant, 300 nM STAT inhibitor SH-4-54, 10 µM MEK 1/2 inhibitor UO126, or a combination thereof as indicated in the key to the right of the graph. D, Quantitative reverse transcription–polymerase chain reaction was used to measure Ghr messenger RNA expression (shown as relative expression normalized to housekeeping gene Tbp) in untreated, bGH-treated, and bGH + pegvisomant-treated cells, and no significant differences were observed among treatment groups. Statistically significant differences from control are indicated by ***P less than .001 and ****P less than .0001 as determined by analysis of variance, Tukey multiple comparison test.
Pegvisomant Slows Tumor Growth of Pten-mutant Xenografts by Inhibiting Proliferation and Inducing Apoptosis
The PTEN-CaP2 and PTEN-CaP8 cell lines have previously been shown to grow tumors when xenografted under the kidney capsule (17). In the present study, PTEN-CaP2 and PTEN-CaP8 cells were implanted under the renal capsule and allowed to establish tumors for 3 weeks at which time treatment with pegvisomant or vehicle was initiated and continued for 3 weeks before tissue harvest. Both PTEN-CaP2 and PTEN-CaP8 xenografts’ size and weight were significantly reduced when treated with pegvisomant compared to vehicle treatment (Fig. 3A-3E). Serum IGF-1 was also significantly reduced in mice treated with pegvisomant (Fig. 3F). Pegvisomant treatment did not result in statistically significant differences in serum testosterone levels (Fig. 3G), and serum estradiol was undetectable in these samples (data not shown). Animal weight was not affected by pegvisomant treatment (data not shown). Histology review of hematoxylin and eosin–stained sections from the grafts showed an overall increased stroma fibrosis in the pegvisomant-treated group (data not shown). The largest graft from the vehicle group showed prominent geographic necrosis (up to 20% tumor necrosis), most likely due to ischemia. The cytology of the tumor cells was similar in the pegvisomant-treated group and vehicle-treated control group. There was no increased necrosis or hemosiderin deposition in the pegvisomant-treated group compared to the vehicle-treated control group. IHC was used to quantify the labeling index for proliferation marker Ki67, and pegvisomant-treated xenografts had a statistically significant decrease in Ki67 labeling index relative to vehicle controls indicating decreased proliferation in response to pegvisomant (Fig. 4A-4E). Similarly, IHC was used to quantify the labeling index for apoptosis marker cleaved caspase 3 (CC3), and pegvisomant-treated xenografts had a statistically significant increase in CC3 labeling index relative to vehicle controls, indicating increased apoptosis in response to pegvisomant (Fig. 5).
Figure 3.
Growth of Pten mutant prostate cancer cell xenografts is inhibited by pegvisomant. PTEN-CaP2 or PTEN-CaP8 cells (3.5 × 105 cells per graft) suspended in rat collagen pellets were surgically grafted under the kidney capsules of 10-week-old male Balb/C nu/nu mice. Xenografts were grown for 3 weeks at which time treatments with 100 mg/kg pegvisomant suspended in sterile saline or a sterile saline vehicle control began (intraperitoneal injection, 3× per week for 3 weeks). Examples of resultant xenografts are shown for A, PTEN-CaP2 vehicle; B, PTEN-CaP2 pegvisomant; C, PTEN-CaP8 vehicle; and D, PTEN-CaP8 pegvisomant. Photos in A to D show kidneys (dark red) with xenografts (lighter areas on kidney surface inside dashed lines) and a ruler for scale. E, Graft masses are shown for vehicle control (black bars, n = 7 per group) and pegvisomant-treated (gray bars, n = 5 per group) mice. F and G, Serum was also collected at the time of xenograft collection, and F, insulin-like growth factor-1 (IGF-1) was measured by enzyme-linked immunosorbent assay in vehicle control (black bars, n = 7 per group) and pegvisomant-treated (gray bars, n = 5 per group) mice. Testosterone was measured by liquid chromatography–tandem mass spectrometry in vehicle control (black circles, n = 4 per group) and pegvisomant-treated (hollow rhombuses, n = 4 per group) mice. Statistically significant differences from control are indicated as *P less than .05 and **P less than .01; t test.
Figure 4.
Pegvisomant reduces proliferation of prostate cancer xenografts. Representative images of Ki67-stained sections are shown for PTEN-CaP2 cells treated with A, a vehicle control, or B, pegvisomant, and PTEN-CaP8 cells treated with C, a vehicle control or D, pegvisomant. Scale bar for A to D shown in B. Arrows point to examples of Ki67-positive cells (brown stain). E, Ki67 labeling index was determined by counting Ki67-positive cells and total cells based in nuclear hematoxylin stain by an observer (M.L.) blinded to treatment group, pegvisomant (n = 5) vehicle control (n = 7). Statistically significant differences determined by analysis of variance with Tukey multiple comparison test are indicated as *P less than .05; **P less than .01; and ***P less than .001.
Figure 5.
Pegvisomant increases apoptosis of prostate cancer xenografts. Representative images of cleaved caspase 3 (CC3)-stained sections are shown for PTEN-CaP2 cells treated with A, a vehicle control, or B, pegvisomant and PTEN-CaP8 cells treated with C, a vehicle control, or D, pegvisomant. Scale bar for A to D shown in B. Arrows point to examples of CC3-positive cells (brown stain). E, CC3 labeling index was determined by counting CC3-positive cells and total cells based in nuclear hematoxylin stain by an observer (M.L.) blinded to treatment group, pegvisomant (n = 5) vehicle control (n = 7). Statistically significant differences determined by analysis of variance with Tukey multiple comparison test are indicated as *P less than .05 and **P less than .01.
Pegvisomant Treatment Alters Gene Expression in Pten-mutant Xenografts
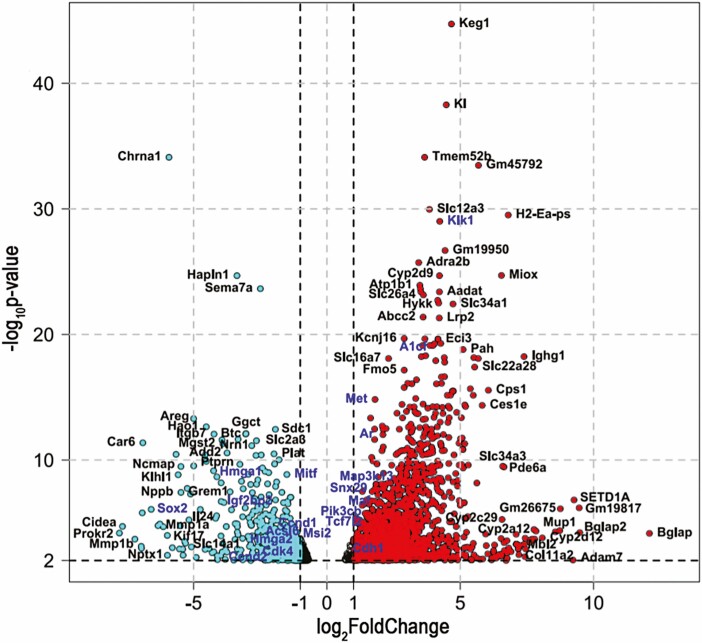
RNA sequencing was performed for pegvisomant-treated and vehicle control PTEN-CaP2 xenografts. Pegvisomant treatment resulted in statistically significant (adjusted P value < .01) expression changes for 2718 genes in PTEN-CaP2 xenografts, of which 953 were downregulated. A volcano plot of these genes’ log2 foldchange vs P value is shown in Fig. 7. Genes with log2 fold change greater than 1 are shown in red, and genes with log2 fold change less than –1 are shown in cyan. Of this set of significantly differentially regulated genes, 99 of them belong to a database of Cancer Census genes implicated as cancer driver genes as curated by the Sanger Institute’s Catalog of Somatic Mutations in Cancer (48), several of which are highlighted in blue in Fig. 7 and 35 of which are downregulated. The top 20 (in terms of log2 fold change) Cancer Census genes downregulated and upregulated by pegvisomant treatment in PTEN-CaP2 xenografts are listed in Tables 1 and 2, respectively. Gene set enrichment analysis of canonical gene sets (C2) identified 480 differentially enriched gene sets, of which 124 were overenriched in PTEN-CaP2 xenografts treated with pegvisomant, and 356 were underenriched. The top 20 overenriched and underenriched C2 gene sets are shown in Tables 3 and 4, respectively. Of particular interest, downregulated gene sets included those involved in protein translation such as KEGG_RIBOSOME, REACTOME_EUKARYOTIC_TRANSLATION_ELONGATION, and REACTOME_EUKARYOTIC_TRANSLATION_INITIATION (Table 3). On the other hand, upregulated gene sets of particular interest included REACTOME_VITAMINS and REACTOME_XENOBIOTICS, which contain numerous cytochrome p450 gene family members (see Table 4). The top 20 upregulated and downregulated genes by fold change are also shown in heat maps in Fig. 7A. Seven of these genes were confirmed to be either upregulated or downregulated in all PTEN-CaP2 xenografts as well in PTEN-CaP8 xenografts (Fig. 7B and 7C). Four of these genes—Grem1, Igf2bp2, Cdh1, and Ar—were differentially regulated by pegvisomant treatment in the same fashion both in vivo and in vitro (see Fig. 7B). However, other genes were altered in response to pegvisomant in vivo but not in vitro (see Fig. 7C). In these cases, treatment with IGF-1 was able to alter gene expression in vitro (see Fig. 7C) suggesting possible indirect regulation in xenografts by pegvisomant-induced changes in the host endocrine environment. In the case of AR, IF staining of xenografts was used to evaluate whether the upregulation of Ar messenger RNA in response to pegvisomant was reflected at the protein level, and a statistically significant increase in AR protein for pegvisomant-treated xenografts was observed in PTEN-CaP2 xenografts but not PTEN-CaP8 xenografts (Fig. 8).
Figure 7.
A number of genes are differentially regulated in PTEN-CaP2 xenografts treated with pegvisomant. (a) Grayscale heatmaps of the top 20 downregulated (top) and upregulated (bottom) genes in response to pegvisomant in terms of log2 fold change (for color figure refer to online version). Expression values are row-normalized. (b–c) Expression of selected genes identified as differentially expressed by RNAseq was assayed using qPCR for PTEN-CaP2 xenografts and PTEN-CaP8 xenografts treated in vivo with vehicle or pegvisomant and for PTEN-CaP2 and PTEN-CaP8 cells treated in vitro with vehicle, pegvisomant, bGH, or IGF-1. Y axis = gene expression relative to the housekeeping gene Tbp and normalized to vehicle = 1 (n = 4 per xenograft group, n = 3 per group). Statistically significant differences from vehicle control were determined by ANOVA with Tukey’s multiple comparison’s test and are indicated as *P < .05; **P < .01; ***P < .001; ****P < .0001 compared to vehicle controls.
Table 1.
Top 20 downregulated Cancer Census genes in pegvisomant-treated PTEN-CaP2 xenografts
| Gene symbol | Gene name | Log2 fold change | Corrected P |
|---|---|---|---|
| Sox2 | SRY (sex determining region Y)-box 2 | –6.592 | 8.511 × 10–7 |
| Hmga1 | High mobility group AT-hook 1 | –4.250 | 7.142 × 10–10 |
| Igf2bp2 | Insulin-like growth factor 2 mRNA binding protein 2 | –3.966 | 2.001 × 10–7 |
| Hmga2 | High mobility group AT-hook 2 | –3.164 | 1.741 × 10−4 |
| Acsl6 | Acyl-CoA synthetase long-chain family member 6 | –2.596 | 5.372 × 10−5 |
| Braf | Braf transforming gene | –2.593 | 3.269 × 10−4 |
| Etv4 | Ets variant 4 | –2.305 | 2.950 × 10−3 |
| Prf1 | Perforin 1 (pore forming protein) | –2.267 | 1.893 × 10−3 |
| Ccnd1 | Cyclin D1 | –2.106 | 1.617 × 10−5 |
| Plag1 | Pleiomorphic adenoma gene 1 | –1.882 | 5.399 × 10−4 |
| Ccnd2 | Cyclin D2 | –1.828 | 4.403 × 10−3 |
| Fance | Fanconi anemia, complementation group E | –1.602 | 3.528 × 10−3 |
| Etv5 | Ets variant 5 | –1.503 | 5.043 × 10−4 |
| Mitf | Melanogenesis-associated transcription factor | –1.503 | 1.421 × 10–9 |
| Pparg | Peroxisome proliferator activated receptor γ | –1.408 | 5.117 × 10−3 |
| Casp3 | Caspase 3 | –1.394 | 6.191 × 10−3 |
| Acsl3 | Acyl-CoA synthetase long-chain family member 3 | –1.364 | 1.484 × 10−3 |
| Ddit3 | DNA-damage inducible transcript 3 | –1.349 | 9.979 × 10−4 |
| Map2k1 | Mitogen-activated protein kinase 1 | –1.251 | 5.297 × 10−3 |
| Msi2 | Musashi RNA-binding protein 2 | –1.186 | 6.001 × 10−5 |
Abbreviation: mRNA, messenger RNA.
Table 2.
Top 20 upregulated Cancer Census genes in pegvisomant-treated PTEN-CaP2 xenografts
| Gene symbol | Gene name | Log2 fold change | Corrected P |
|---|---|---|---|
| Klk1 | Kallikrein 1 | 4.231 | 9.819 × 10–30 |
| Ptprd | Protein tyrosine phosphatase, receptor type, D | 4.131 | 1.768 × 10-8 |
| A1cf | APOBEC1 complementation factor | 3.975 | 7.557 × 10–20 |
| Hnf1a | HNF1 homeobox A | 3.502 | 5.789 × 10–9 |
| Nrg1 | Neuregulin 1 | 3.389 | 3.409 × 10–9 |
| Omd | Osteomodulin | 3.170 | 1.396 × 10–7 |
| Gli1 | GLI-Kruppel family member GLI1 | 3.107 | 4.820 × 10–9 |
| Creb3l1 | cAMP responsive element binding protein 3-like 1 | 3.059 | 4.950 × 10−4 |
| Pax8 | Paired box 8 | 3.013 | 1.611 × 10−3 |
| Bcl11b | B-cell leukemia/lymphoma 11B | 2.973 | 4.048 × 10−3 |
| Hoxc13 | Homeobox C13 | 2.807 | 1.011 × 10−3 |
| Map3k13 | Mitogen-activated protein kinase kinase kinase 13 | 2.779 | 3.061 × 10–9 |
| Il7r | Interleukin 7 receptor | 2.735 | 6.567 × 10−4 |
| Cblc | Casitas B-lineage lymphoma c | 2.719 | 1.056 × 10−3 |
| Tmprss2 | Transmembrane protease, serine 2 | 2.582 | 4.774 × 10–9 |
| Hlf | Hepatic leukemia factor | 2.551 | 1.679 × 10−3 |
| Prdm16 | PR-domain containing 16 | 2.542 | 9.043 × 10−3 |
| Mn1 | Meningioma 1 | 2.454 | 5.333 × 10−4 |
| Cdh1 | Cadherin 1 | 2.435 | 1.258 × 10−3 |
| Esr1 | Estrogen receptor 1 (α) | 2.378 | 5.514 × 10−5 |
Table 3.
Top 20 downregulated C2 gene sets in response to pegvisomant treatment in PTEN-CaP2 xenografts
| Pathway name | Log2 fold change | Corrected P |
|---|---|---|
| KEGG_RIBOSOME | –1.437 | 2.731 × 10−3 |
| REACTOME_EUKARYOTIC_TRANSLATION_ELONGATION | –1.436 | 2.731 × 10−3 |
| REACTOME_RESPONSE_OF_EIF2AK4_GCN2_TO_AMINO_ACID_DEFICIENCY | –1.409 | 2.731 × 10−3 |
| REACTOME_UPTAKE_AND_FUNCTION_OF_DIPHTHERIA_TOXIN | –1.393 | 2.829 × 10−3 |
| REACTOME_SRP_DEPENDENT_COTRANSLATIONAL_PROTEIN_TARGETING_TO_MEMBRANE | –1.387 | 2.731 × 10−3 |
| DER_IFN_ALPHA_RESPONSE_DN | –1.361 | 2.731 × 10−3 |
| REACTOME_EUKARYOTIC_TRANSLATION_INITIATION | –1.324 | 2.731 × 10−3 |
| REACTOME_NONSENSE_MEDIATED_DECAY_NMD | –1.304 | 2.731 × 10−3 |
| REACTOME_FOLDING_OF_ACTIN_BY_CCT_TRIC | –1.303 | 3.152 × 10−3 |
| BILANGES_SERUM_AND_RAPAMYCIN_SENSITIVE_GENES | –1.283 | 2.874 × 10−3 |
| REACTOME_ACTIVATION_OF_THE_MRNA_UPON_BINDING_OF_THE_CAP_BINDING_COMPLEX_AND_EIFS_AND_SUBSEQUENT_BINDING_TO_43S | –1.222 | 2.874 × 10−3 |
| REACTOME_SUMO_IS_CONJUGATED_TO_E1_UBA2_SAE1 | –1.213 | 3.177 × 10−3 |
| BIOCARTA_SUMO_PATHWAY | –1.212 | 3.177 × 10−3 |
| REACTOME_SELENOAMINO_ACID_METABOLISM | –1.211 | 2.874 × 10−3 |
| BIOCARTA_RAN_PATHWAY | –1.209 | 3.177 × 10−3 |
| RODRIGUES_DCC_TARGETS_UP | –1.209 | 3.152 × 10−3 |
| REACTOME_INFLUENZA_INFECTION | –1.189 | 2.874 × 10−3 |
| REACTOME_RECYCLING_OF_EIF2_GDP | –1.187 | 3.152 × 10−3 |
| KEGG_PROTEASOME | –1.180 | 3.129 × 10−3 |
| FU_INTERACT_WITH_ALKBH8 | –1.178 | 3.177 × 10−3 |
Table 4.
Top 20 upregulated C2 gene sets in response to pegvisomant treatment in PTEN-CaP2 xenografts
| Pathway name | Log2 fold change | Corrected P |
|---|---|---|
| REACTOME_CYP2E1_REACTIONS | 1.477 | 2.731 × 10−3 |
| REACTOME_ORGANIC_ANION_TRANSPORT | 1.450 | 2.874 × 10−3 |
| REACTOME_BIOSYNTHESIS_OF_MARESIN_LIKE_SPMS | 1.416 | 3.051 × 10−3 |
| REACTOME_VITAMINS | 1.393 | 2.731 × 10−3 |
| REACTOME_DIGESTION_OF_DIETARY_LIPID | 1.375 | 2.874 × 10−3 |
| REACTOME_BIOSYNTHESIS_OF_MARESINS | 1.373 | 3.152 × 10−3 |
| REACTOME_SYNTHESIS_OF_EPOXY_EET_AND_DIHYDROXYEICOSATRIENOIC_ACIDS_DHET | 1.344 | 3.152 × 10−3 |
| REACTOME_XENOBIOTICS | 1.292 | 2.874 × 10−3 |
| REACTOME_SODIUM_COUPLED_SULPHATE_DI_AND_TRI_CARBOXYLATE_TRANSPORTERS | 1.291 | 3.278 × 10−3 |
| REACTOME_HIGHLY_SODIUM_PERMEABLE_POSTSYNAPTIC_ACETYLCHOLINE_NICOTINIC_RECEPTORS | 1.275 | 2.874 × 10−3 |
| PASTURAL_RIZ1_TARGETS_DN | 1.241 | 3.177 × 10−3 |
| REACTOME_TYROSINE_CATABOLISM | 1.224 | 3.152 × 10−3 |
| TUOMISTO_TUMOR_SUPPRESSION_BY_COL13A1_DN | 1.224 | 2.874 × 10−3 |
| REACTOME_CHOLINE_CATABOLISM | 1.217 | 2.874 × 10−3 |
| REACTOME_SIGNALING_BY_MST1 | 1.211 | 4.614 × 10−3 |
| REACTOME_INTESTINAL_ABSORPTION | 1.204 | 2.874 × 10−3 |
| REACTOME_MET_RECEPTOR_ACTIVATION | 1.194 | 3.430 × 10−3 |
| REACTOME_MULTIFUNCTIONAL_ANION_EXCHANGERS | 1.188 | 3.152 × 10−3 |
| REACTOME_AMINO_ACID_CONJUGATION | 1.176 | 5.263 × 10−3 |
| REACTOME_CONJUGATION_OF_BENZOATE_WITH_GLYCINE | 1.176 | 5.263 × 10−3 |
Figure 8.
Androgen receptor (AR) protein expression is upregulated in PTEN-CaP2 cells treated with pegvisomant. A to F, Immunofluorescent (IF) staining of PTEN-CaP2 xenografts shows AR protein expression in A to C, vehicle-, and D to F, pegvisomant-treated mice. A and D, Nucleic staining of 4′,6-diamidino-2-phenylindole (DAPI) was stained in the blue channel; B and E, AR was stained in the red channel; and C and F, a merger of the 2 channels is presented with a 50-µm scale bar. G, Semiquantitative scoring reveals increases in AR protein expression in PTEN-CaP2 cells treated with pegvisomant (hollow rhombuses) compared to vehicle (black circles). No significant difference in AR protein expression was observed in PTEN-CaP8 xenografts. Statistically significant differences are indicated as *P less than .05; ns = no significance; t test.
Pegvisomant Similarly Alters Graft Size, Proliferation, Apoptosis, and Gene Expression in T-Antigen–Driven Mouse Prostate Cancer Cells
As a first step toward determining whether the effects of pegvisomant in Pten-mutant mouse prostate cancer cells were relevant for prostate cancer cells with different driver genes, a parallel set of xenografting experiments were conducted with TRAMP-C2 cells (Fig. 9). Pegvisomant reduced growth (Fig. 9A-9C) and proliferation (Fig. 9D) of TRAMP-C2 xenografts. Pegvisomant also increased apoptosis (Fig. 9E) in TRAMP-C2 xenografts. Likewise, the pattern of gene expression changes observed in PTEN-CaP2 and PTEN-CaP8 xenografts treated with pegvisomant (see Fig. 7B and 7C) was similar for TRAMP-C2 xenografts (Fig. 9F). In addition, the gene expression changes in vitro in response to pegvisomant, GH, and IGF-1 in TRAMP-C2 cells (see Fig. 9F) were similar to those observed for PTEN cells.
Figure 9.
Pegvisomant alters graft size, proliferation, apoptosis, and gene expression in T-antigen–induced mouse prostate cancer cells. TRAMP-C2 cells (3.5 × 105 cells per graft) suspended in rat collagen pellets were surgically grafted under the kidney capsules of 10-week-old male Balb/C nu/nu mice. Xenografts were grown for 3 weeks at which time treatments with 100 mg/kg pegvisomant suspended in sterile saline or a sterile saline vehicle control began (intraperitoneal injection, 3× per week for 3 weeks). Examples of resultant xenografts are shown for A, TRAMP-C2 vehicle, and B, TRAMP-C2 pegvisomant. Photos in A and B show kidneys (dark red) with xenografts (lighter areas on kidney surface inside the dashed lines) and a ruler for scale. C, Graft masses are shown for vehicle control (black bars, n = 5) and pegvisomant-treated (gray bars, n = 5) mice. D, Ki67 staining was reduced in in pegvisomant-treated grafts. E, Cleaved caspase 3 (CC3) was increased in pegvisomant-treated grafts. F, Expression of selected genes was assayed using quantitative polymerase chain reaction for TRAMP-C2 xenografts treated in vivo with vehicle or pegvisomant and for TRAMP-C2 cells treated in vitro with vehicle, pegvisomant, bovine growth hormone (bGH), or insulin-like growth factor-1 (IGF-1). Y axis = gene expression relative to the housekeeping gene Tbp and normalized to vehicle = 1 (n = 4 per xenograft group, n = 3 per in vitro group). Statistically significant differences from vehicle control were determined by analysis of variance with Tukey multiple comparison test and are indicated as *P less than .05 and **P less than .01.
Discussion
GH/IGF-1 axis disruption can be achieved clinically through a Food and Drug Administration–approved drug, pegvisomant, and has shown to have therapeutic properties in multiple models of disease. Acromegaly shows a positive response to GHR antagonism with pegvisomant, which lowers the unnaturally high levels of serum IGF-1 brought on by abnormally abundant circulating GH in these patients (49). The anticancer potential of pegvisomant has been explored in multiple models including pituitary adenomas and endometrial and mammary cancers (10, 11, 50). The drug has also been used in other preclinical studies of prostate cancer. In a study of the human prostate cancer cell line 22Rv1, pegvisomant decreased AR variant 7 expression and, when combined with the antiandrogen drug enzalutamide, reduced circulating prostate-specific antigen levels in a xenograft model (14). However, subcutaneously xenografted 22Rv1 cells treated with pegvisomant failed to show any decrease in graft size (14). This can be explained in 2 ways. First, 22Rv1 cells express human GHRs that cannot be stimulated by nonprimate GH (15). Briefly, primates possess interacting arginine and aspartate in GHR and GH, respectively, whereas nonprimates possess leucine and histidine in their respective positions; these substitutions prevent nonprimate GH from stimulating primate GHR (15). In the 22Rv1 xenograft model, circulating mouse GH would fail to activate GHR on the grafted cells so growth of 22Rv1 xenografts was independent of direct GH action. Second, 22Rv1 cells have been immortalized and maintained in media lacking primate GH, thus selecting for GH-independent growth (51). In unpublished studies using unselected, primary human prostate epithelial cells, our laboratory has found that human GH was sufficient to induce growth in primary human prostate epithelial cells in vitro. After 3 days in culture, as little as 10 ng/mL human GH was capable of significantly increasing viability of primary cells while 1000 ng/mL human GH resulted in an 86% (±3%) increase in viability (data not shown). To thoroughly investigate how GH signaling contributes to cell proliferation in both in vitro and in vivo models, we must use an experimental system that accounts for the fact that human cells fail to respond to nonprimate GH.
In this study, the Pten-mutant mouse prostate cancer cell lines were chosen because of their immortalization and maintenance in serum containing exogenous GH (in the form of BPE). Although mouse GH fails to stimulate human or bovine GHR, mouse GHR is activated by human and bovine GH (15). Because of GH’s species specificity, these cells were potentially dependent on the exogenous GH to live in culture, and this study showed that growth of these cell lines was indeed at least partially GH dependent (see Fig. 1A). Supplemented bGH was able to fully rescue decreased viability due to BPE depletion, indicating that the GH in BPE is sufficient to drive GH-induced growth. This dependency made these cells susceptible to decreases in viability and migratory behavior when blocking GH by treating with pegvisomant (see Fig. 1B-1D). Additional evidence for species specificity was the activation of the JAK/STAT pathway by bGH (Fig. 2A and 2B). Increased activity of JAK2 and STAT5 in PTEN cells shows that mouse GHR is activated primarily through JAK/STAT signaling and not through MAPK. As expected, pegvisomant blocked GH-induced JAK/STAT activity (see Fig. 2B). The relevance of JAK/STAT activity for the responses to pegvisomant in vivo is uncertain as immunofluorescent staining of xenografts for p-JAK2 and p-STAT5 gave highly variable results independent of the treatment group (data not shown). However, a STAT pathway inhibitor, and not a MAPK pathway inhibitor, successfully blocked GH-induced cell proliferation in vitro to a similar extent as pegvisomant (see Fig. 2C). Pegvisomant had no effect on Ghr expression that could explain any changes in GH activity in these trials (see Fig. 2D). While the importance of JAK2 and STAT5 activity shown here is congruent with what has been observed in human prostate cancer cells, mouse prostate cancer cells in this study did not depend on MAPK activity as has been reported for at least some human prostate cancer models (12).
Renal xenografts of PTEN cells showed that pegvisomant reduced tumor growth in vivo (see Fig. 3). This occurred in spite of achieving only a partial blockade of the GH/IGF-1 axis as serum IGF-1 was decreased by only 18.3% (±3.4%) in pegvisomant-treated animals (see Fig. 3F). By comparison, the original model of Ghr disruption by a germline null mutation in mice characterized in 1997 described a 90% reduction in serum IGF-1 (52). A similar mouse model of global Ghr deletion using a standardized tamoxifen-induced recombination protocol using a Cre-lox system shows an approximately 86% decrease in serum IGF-1 following recombination (53). In our laboratory, progeny of mice following Ghr deletion have an 86.1% (±5.1%) decrease in serum IGF-1 (data not shown). These comparisons indicate that pegvisomant was an incomplete inhibitor of the GH/IGF-1 axis in our prostate cancer model. Nevertheless, partial blockade of the GH/IGF-1 axis reduced xenograft proliferation and increased apoptosis as was shown by a decrease in Ki67-positive tumor cells and an increase in CC3-positive tumor cells when treated with pegvisomant (see Figs. 4 and 5). These data were recapitulated in a separate model of prostate cancer in the immortalized TRAMP series. TRAMP-C2 cells similarly show a negative response to growth accompanied by decreased proliferation and increased apoptosis in vivo on pegvisomant treatment (see Fig. 9A-9E). These data in the T-antigen–transformed TRAMP cells support the use of pegvisomant in models of prostate cancer other than those driven by increased PI3K activity such as the PTEN series.
The treatment of PTEN xenografts with pegvisomant resulted in differential expression of a number of genes and gene sets compared to the vehicle control (see Figs. 6 and 7 and Tables 1-4). It is important to note here that Igf1 did not return as significantly downregulated in these samples. The Catalogue of Somatic Mutations in Cancer has developed a Cancer Gene Census that curates a list of genes involved in driving human cancer (48). The list includes 719 (and counting) cancer-driving genes. This list was cross-referenced during analysis of the gene sequencing data collected from pegvisomant- and vehicle-treated PTEN xenografts. Certain genes highlighted in the Cancer Census database produced within the Catalogue for Somatic Mutations in Cancer appear to be regulated by direct GH action on prostate cancer cell expressed GHR as these genes exhibited similar regulation by pegvisomant in PTEN-CaP2 and PTEN-CaP8 cells whether treatment was in vivo or in vitro, and GH treatment of the cells changed gene expression in the opposite direction relative to pegvisomant (see Fig. 7B). Other genes that were regulated by pegvisomant in vivo but not in vitro responded to IGF-1 in vitro (see Fig. 7C), implying the need of the entire GH/IGF-1 axis in these genes’ regulation. Grem1 is a gene whose protein product, gremlin 1, is implicated in conserving the stem cell niche in mature cells; its expression is positively correlated with poor prognosis in breast cancer (54, 55). The upregulation of certain Igf2bp2 polymorphisms has been connected to breast, pancreatic, and esophageal cancers (56-58). Cdk4, Ccnd1, and Ccnd2 all code for separate proteins in the cell cycle, the upregulation of which can lead to the uncontrolled proliferation of cells associated with cancer. On pegvisomant treatment Grem1, Igfbp2, Cdk4, Ccnd1, and Ccnd2 were downregulated in cells treated with pegvisomant while the same treatment resulted in an upregulation of Ar and Cdh1. IF staining in PTEN-CaP2 cells for AR protein confirmed that the messenger RNA upregulation enhances the translation of the downstream protein and makes this finding functionally relevant (see Fig. 8).The upregulation of Ar by pegvisomant in this study fits with previous data from human LNCaP cells that observed decreased AR on long-term GH treatment but is distinct from previous reports that pegvisomant action in 22Rv1 cells did not affect full-length AR expression and reduced ARv7 at the protein level (14). Further studies should be conducted to identify the gene’s role in tumor growth in this model. E-cadherin, encoded by Cdh1, is a transmembrane protein in epithelial cells that is responsible for cell adhesion, loss of which leads to increased cell motility and can act as a potential promoter of the epithelium-to-mesenchymal transition (59, 60). The upregulation of Cdh1 on pegvisomant treatment suggests that a future investigation of the potential role of GH in the epithelium-to-mesenchymal transition is warranted.
Figure 6.
A volcano plot of differentially expressed genes in PTEN-CaP2 xenografts treated with pegvisomant with log2 fold change on the X axis and the negative log10 of the adjusted P value on the Y-axis. Thresholds for significance are –log10P value > 2 and log2 fold change > 1 or < −1. For a color figure refer to online version which contains: genes upregulated in response to pegvisomant colored red, genes downregulated in response to pegvisomant colored cyan, and selected Cancer Census genes highlighted in blue font.
Other genes exhibited similar regulation both for PTEN-CaP2 and PTEN-CaP8 cells treated with pegvisomant in vivo but were mainly regulated by IGF-1 in vitro (see Fig. 7C), indicating that these genes were likely altered because of pegvisomant effects on the host endocrine environment such as the decrease in serum IGF-1 observed in pegvisomant-treated mice (see Fig. 3F). This pattern of pegvisomant-induced gene expression changes was similar for TRAMP-C2 cells (see Fig. 9), indicating that the genetic alterations observed in this model are not exclusive to Pten-driven cancer models. The differential regulation of Cancer Census genes—briefly described in Tables 1 and 2—indicates that pegvisomant treatment has substantial effects on tumor gene expression aside from reducing proliferation and increasing apoptosis in this model. The GH/IGF-1 axis, in general, promotes the growth and proliferation of tissues while inhibiting apoptosis. In cancerous cells such as the ones used in this study, overall gene regulation varied. Gene set enrichment analysis identified the downregulation of ribosomal, translational, and proteasomal gene sets (see Table 3) and the upregulation of xenobiotic and vitamin metabolism gene sets, among others (see Table 4). The downregulation of genes related to translation directly opposes the cancer-driving mechanism of the PTEN cells. The deletion of Pten drives the activation of AKT and S6 kinase, which results in the initiation of translation (61). Further, the upregulation of xenobiotic and vitamin metabolism gene sets widely covers cytochrome p450 genes, and at least one of those gene products, CYP27B1, acts as a tumor suppressor (62). This novel set of diverse pathways affected by blocking GH treatment provides further exciting hypotheses to follow.
In summary, our in vitro experiments showed that blocking GH signaling with pegvisomant has anticancer effects on mouse prostate cancer cells, including decreased viability and migratory ability of cells in culture. In addition, GH activated JAK2 and STAT5, and blocking the GH-induced activity of JAK2/STAT5 with inhibitors has a similar effect on viability with pegvisomant. One important goal of this study was to assess how prostate cancer cells respond to physiologic levels of GH by using mouse prostate cancer cells in a mouse host and then inhibiting the GH/IGF-1 axis with pegvisomant. Xenografts of both Pten mutant prostate cancer cells and TRAMP-derived prostate cancer cells were growth-inhibited by pegvisomant in this study. A previous study treated xenografts of human 22Rv1 cells with pegvisomant in mice (14) and did not observe changes in the size of xenografts in response to pegvisomant. However, owing to the species specificity of GH-GHR interactions (15), only the host GHR and not the 22Rv1 GHR was inhibited in this context, which may explain differences from our study. Future studies could further investigate the role of GHR actions in human prostate cancer cells by supplementing host mice with human GH in xenografting studies. Such a model of human GH supplementation has been used for other models of cancer, including melanoma (63). Using this approach for future prostate cancer studies would further clarify the relevance of our findings for human prostate cancer. Inhibiting the GH/IGF-1 axis also results in the downregulation of dozens of cancer-related genes. These results build on previous investigations into the therapeutic role of GH antagonism using pegvisomant in prostate cancer (14). Using pegvisomant as a tool to disrupt the GH/IGF-1 axis, these data suggest that GH, via JAK2/STAT5 signaling, is in part responsible for the growth of prostate cancer cells and the regulation of cancer-related genes in those cells. Future studies could investigate changes in these candidate mediators of pegvisomant action in the context of genetically engineered mouse prostate cancer models such as the prostate-specific deletion model (16) from which the PTEN cell lines were derived to further clarify the relevance of the present study for understanding the role of the GH/IGF-1 axis in prostate cancer.
Acknowledgments
Special thanks to Sandra Splinter BonDurant and Molly Zeller at the University of Wisconsin–Madison Biotechnology Center DNA Sequencing Facility, Amita Kapoor and Cody Corbett at the Wisconsin National Primate Research Center, Avan Colah in the Collier Lab at the University of Wisconsin–Madison, and Maryellen Brand at Pfizer’s Global Medical Grants division.
Glossary
Abbreviations
- AR
androgen receptor
- bGH
bovine growth hormone
- BPE
bovine pituitary extract
- BSA
bovine serum albumin
- CC3
cleaved caspase 3
- cDNA
complementary DNA
- Ct
cycle threshold
- FBS
fetal bovine serum
- GH
growth hormone
- GHR
growth hormone receptor
- IF
immunofluorescent
- IGF-1
insulin-like growth factor-1
- IHC
immunohistochemistry
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- RT
room temperature
- TBP
TATA box-binding protein
- TRAMP
transgenic adenocarcinoma of the mouse prostate
Financial Support
This work was supported by the National Cancer Institute (grant No. R21CA238105). Pegvisomant was kindly provided by Pfizer through its Global Medical Grants division. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or Pfizer.
Author Contributions
C.J.U., S.M.S., and P.C.M. conceived the animal study and the experiment design. C.J.U., V.I.M., and M.L. collected the data and carried out the experiments. R.H. assessed tissue histology. C.J.U., S.J.M., and P.K.T. analyzed the data. C.J.U., S.J.M., and P.K.T. organized and created the figures. All authors were involved in the writing of the paper and had final approval of the submitted and published versions.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. RNA-Seq data are available for download from NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) with accession number GSE188543.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. [DOI] [PubMed] [Google Scholar]
- 2. Janecka A, Kołodziej-Rzepa M, Biesaga B. Clinical and molecular features of Laron syndrome, a genetic disorder protecting from cancer. In Vivo. 2016;30(4):375-381. [PubMed] [Google Scholar]
- 3. Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164(4): 485-489. [DOI] [PubMed] [Google Scholar]
- 4. Wang Z, Prins GS, Coschigano KT, et al. Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology. 2005;146(12):5188-5196. [DOI] [PubMed] [Google Scholar]
- 5. Travis RC, Appleby PN, Martin RM, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016;76(8):2288-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watts EL, Fensom GK, Smith Byrne K, et al. Circulating insulin-like growth factor-I, total and free testosterone concentrations and prostate cancer risk in 200 000 men in UK Biobank. Int J Cancer. 2021;148(9):2274-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henry GH, Malewska A, Joseph DB, et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 2018;25(12):3530-3542.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bidosee M, Karry R, Weiss-Messer E, Barkey RJ. Regulation of growth hormone receptors in human prostate cancer cell lines. Mol Cell Endocrinol. 2009;309(1-2):82-92. [DOI] [PubMed] [Google Scholar]
- 9. Goffin V, Bernichtein S, Carrière O, Bennett WF, Kopchick JJ, Kelly PA. The human growth hormone antagonist b2036 does not interact with the prolactin receptor. Endocrinology. 1999;140(8):3853-3856. [DOI] [PubMed] [Google Scholar]
- 10. Divisova J, Kuiatse I, Lazard Z, et al. The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res Treat. 2006;98(3):315-327. [DOI] [PubMed] [Google Scholar]
- 11. Evans A, Jamieson SMF, Liu DX, Wilson WR, Perry JK. Growth hormone receptor antagonism suppresses tumour regrowth after radiotherapy in an endometrial cancer xenograft model. Cancer Lett. 2016;379(1):117-123. [DOI] [PubMed] [Google Scholar]
- 12. Weiss-Messer E, Merom O, Adi A, et al. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol Cell Endocrinol. 2004;220(1-2):109-123. [DOI] [PubMed] [Google Scholar]
- 13. Nakonechnaya AO, Jefferson HS, Chen X, Shewchuk BM. Differential effects of exogenous and autocrine growth hormone on LNCaP prostate cancer cell proliferation and survival. J Cell Biochem. 2013;114(6):1322-1335. [DOI] [PubMed] [Google Scholar]
- 14. Recouvreux MV, Wu JB, Gao AC, et al. Androgen receptor regulation of local growth hormone in prostate cancer cells. Endocrinology. 2017;158(7):2255-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Souza SC, Frick GP, Wang X, Kopchick JJ, Lobo RB, Goodman HM. A single arginine residue determines species specificity of the human growth hormone receptor. Proc Natl Acad Sci U S A. 1995;92(4):959-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiao J, Wang S, Qiao R, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67(13):6083-6091. [DOI] [PubMed] [Google Scholar]
- 17. Le B, Powers GL, Tam YT, et al. Multi-drug loaded micelles delivering chemotherapy and targeted therapies directed against HSP90 and the PI3K/AKT/mTOR pathway in prostate cancer. PLoS One. 2017;12(3):e0174658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57(16): 3325-3330. [PubMed] [Google Scholar]
- 19. Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED. The TRAMP mouse as a model for prostate cancer. Curr Protoc Immunol. 2001;Chapter 20:Unit 20.5. [DOI] [PubMed] [Google Scholar]
- 20. American Veterinary Medical Association (AVMA). AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. AVMA; 2020. [Google Scholar]
- 21. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329-333. [DOI] [PubMed] [Google Scholar]
- 22. RRID:AB_330744, https://scicrunch.org/resolver/AB_330744. [Google Scholar]
- 23. RRID:AB_334646, https://scicrunch.org/resolver/AB_331646. [Google Scholar]
- 24. RRID:AB_2737403, https://scicrunch.org/resolver/AB_2737403. [Google Scholar]
- 25. RRID:AB_2315225, https://scicrunch.org/resolver/AB_2315225. [Google Scholar]
- 26. RRID:AB_2128522, https://scicrunch.org/resolver/AB_2128522. [Google Scholar]
- 27. RRID:AB_330403, https://scicrunch.org/resolver/AB_330403. [Google Scholar]
- 28. RRID:AB_445482, https://scicrunch.org/resolver/AB_445482. [Google Scholar]
- 29. RRID:AB_955387, https://scicrunch.org/resolver/AB_955387. [Google Scholar]
- 30. Nicholson TM, Uchtmann KS, Valdez CD, Theberge AB, Miralem T, Ricke WA. Renal capsule xenografting and subcutaneous pellet implantation for the evaluation of prostate carcinogenesis and benign prostatic hyperplasia. J Vis Exp. 2013;(78):50574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kenealy BP, Kapoor A, Guerriero KA, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33(49):19051-19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kenealy BP, Keen KL, Garcia JP, Kohlenberg LK, Terasawa E. Obligatory role of hypothalamic neuroestradiol during the estrogen-induced LH surge in female ovariectomized rhesus monkeys. Proc Natl Acad Sci U S A. 2017;114(52):13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RRID:AB_302459, https://scicrunch.org/resolver/AB_302459. [Google Scholar]
- 34. RRID:AB_2313606, https://scicrunch.org/resolver/AB_2313606. [Google Scholar]
- 35. RRID:AB_1563391, https://scicrunch.org/resolver/AB_1563391. [Google Scholar]
- 36. RRID:AB_2782993, https://scicrunch.org/resolver/AB_2782993. [Google Scholar]
- 37. Encode Project. ENCODE Guidelines and Best Practices for RNA-Seq. 2016.
- 38. Jiang H, Lei R, Ding SW, Zhu S. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinformatics. 2014;15(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawson ND, Li R, Shin M, et al. An improved zebrafish transcriptome annotation for sensitive and comprehensive detection of cell type-specific genes. Elife. 2020;9:e55792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kolde R. pheatmap: Pretty Heatmaps. R package 2019; version 1.0.12. [Google Scholar]
- 44. Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19(3):368-375. [DOI] [PubMed] [Google Scholar]
- 45. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaujoux R, Seoighe CA. flexible R package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sondka Z, Bamford S, Cole CG, Ward SA, Dunham I, Forbes SA. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat Rev Cancer. 2018;18(11):696-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giustina A, Arnaldi G, Bogazzi F, et al. Pegvisomant in acromegaly: an update. J Endocrinol Invest. 2017;40(6):577-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cuny T, Zeiller C, Bidlingmaier M, et al. In vitro impact of pegvisomant on growth hormone-secreting pituitary adenoma cells. Endocr Relat Cancer. 2016;23(7):509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sramkoski RM, Pretlow TG II, Giaconia JM, et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35(7):403-409. [DOI] [PubMed] [Google Scholar]
- 52. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A. 1997;94(24):13215-13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duran-Ortiz S, Bell S, Kopchick JJ. Standardizing protocols dealing with growth hormone receptor gene disruption in mice using the Cre-lox system. Growth Horm IGF Res. 2018;42-43:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rowan SC, Jahns H, Mthunzi L, et al. Gremlin 1 depletion in vivo causes severe enteropathy and bone marrow failure. J Pathol. 2020;251(2):117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ren J, Smid M, Iaria J, et al. Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression. Breast Cancer Res. 2019;21:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu W, Li Y, Wang B, Dai L, Qian W, Zhang JY. Autoimmune response to IGF2 mRNA-binding protein 2 (IMP2/p62) in breast cancer. Scand J Immunol. 2015;81(6):502-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dahlem C, Barghash A, Puchas P, Haybaeck J, Kessler SM. The insulin-like growth factor 2 mRNA binding protein IMP2/IGF2BP2 is overexpressed and correlates with poor survival in pancreatic cancer. Int J Mol Sci . 2019;20(13):3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qiu H, Wang Y, Kang M, et al. The relationship between IGF2BP2 and PPARG polymorphisms and susceptibility to esophageal squamous-cell carcinomas in the eastern Chinese Han population. Onco Targets Ther. 2017;10:5525-5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deep G, Jain AK, Ramteke A, et al. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol Cancer. 2014;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Veveris-Lowe TL, Lawrence MG, Collard R, et al. Kallikrein 4 (hK4) and prostate-specific antigen (PSA) are associated with the loss of E-cadherin and an epithelial-mesenchymal transition (EMT)-like effect in prostate cancer cells. Endocr Relat Cancer. 2005;12(3):631-643. [DOI] [PubMed] [Google Scholar]
- 61. Raught B, Peiretti F, Gingras AC, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23(8):1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen TC. 25-Hydroxyvitamin D-1 alpha-hydroxylase (CYP27B1) is a new class of tumor suppressor in the prostate. Anticancer Res. 2008;28(4A):2015-2017. [PubMed] [Google Scholar]
- 63. Basu R, Kulkarni P, Qian Y, et al. Growth hormone upregulates melanocyte-inducing transcription factor expression and activity via JAK2-STAT5 and SRC signaling in GH receptor-positive human melanoma. Cancers (Basel). 2019;11(9):1352-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. RNA-Seq data are available for download from NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) with accession number GSE188543.