ABSTRACT
Mutations in SPAG1, a dynein axonemal assembly factor (DNAAF) that facilitates the assembly of dynein arms in the cytoplasm before their transport into the cilium, result in primary ciliary dyskinesia (PCD), a genetically heterogenous disorder characterized by chronic oto-sino-pulmonary disease, infertility and laterality defects. To further elucidate the role of SPAG1 in dynein assembly, we examined its expression, interactions and ciliary defects in control and PCD human airway epithelia. Immunoprecipitations showed that SPAG1 interacts with multiple DNAAFs, dynein chains and canonical components of the R2TP complex. Protein levels of dynein heavy chains (DHCs) and interactions between DHCs and dynein intermediate chains (DICs) were reduced in SPAG1 mutants. We also identified a previously uncharacterized 60 kDa SPAG1 isoform, through examination of PCD subjects with an atypical ultrastructural defect for SPAG1 variants, that can partially compensate for the absence of full-length SPAG1 to assemble a reduced number of outer dynein arms. In summary, our data show that SPAG1 is necessary for axonemal dynein arm assembly by scaffolding R2TP-like complexes composed of several DNAAFs that facilitate the folding and/or binding of the DHCs to the DIC complex.
KEY WORDS: SPAG1, Dynein arm assembly, Motile cilia, Primary ciliary dyskinesia, R2TP complex
Summary: SPAG1 scaffolds R2TP-like complexes to assist formation of dynein subunit complexes in dynein assembly; however, a 60-kDa isoform can assemble outer dynein arms in the absence of full-length SPAG1.
INTRODUCTION
Primary ciliary dyskinesia (PCD) is a rare genetically heterogenous disorder caused by the dysfunction of proteins involved in the biogenesis, structure or regulation of motile cilia. Owing to impaired mucociliary clearance, sperm motility and left-right symmetry breaking, the typical clinical characteristics of PCD include neonatal respiratory distress, chronic sinusitis, otitis media, recurrent respiratory infections, bronchiectasis, impaired fertility and situs abnormalities (Leigh et al., 2019; Wallmeier et al., 2020). Currently, genetic variants in at least 50 genes are associated with causing PCD, with most resulting in axonemal ultrastructural defects, although ∼30% of cases show no visible defect. The most common axonemal abnormality seen in PCD is absent or defective axonemal dynein arms (Zariwala et al., 2007; Wallmeier et al., 2020). The outer dynein arms (ODAs) and inner dynein arms (IDAs) are 1–2-MDa multiprotein motor complexes, which are present along the doublet microtubules and are responsible for ATP-dependent ciliary movement (King, 2016). In humans, the ODAs are composed of two dynein heavy chains (DHCs; type 1 ODA, DNAH5 and DNAH11; or type 2 ODA, DNAH5 and DNAH9), two dynein intermediate chains (DICs) (DNAI1 and DNAI2) and a number of dynein light chains (DLCs), including DNAL1. IDAs include a double-headed dynein and six single-headed dyneins, with each of these containing their own distinct DHCs and various DICs and DLCs (Kamiya, 2002; Fliegauf et al., 2005; Pazour et al., 2006; Kollmar, 2016). Although the protein composition of dynein arms has been extensively studied, how these structures are assembled and transported to the ciliary axoneme in mammalian systems is only beginning to be understood.
Studies with the unicellular biflagellate green alga Chlamydomonas reinhardtii have shown that axonemal dynein arms are assembled into discrete subunit complexes in the cytoplasm that later interact before their transport into the ciliary axoneme by intraflagellar transport (IFT) (Fowkes and Mitchell, 1998; Desai et al., 2017). For example, in ODA assembly, a complex composed of intermediate chains and light chains is formed first, followed by the addition of the heavy chains and regulatory light chains to form a stable dynein arm (Tang et al., 1982; Pfister and Witman, 1984). Some of these subcomplexes have been shown to be stable in the cytoplasm; however, without all the necessary components, axonemal dyneins are prevented from transporting through the ciliary gate at the transition zone into the ciliary axoneme, and are degraded (Omran et al., 2008; Mitchison et al., 2012). In humans, insights into the players and mechanisms of dynein arm assembly emerged from the identification of genetic variants in PCD-affected individuals carrying defects in both ODAs and IDAs. These genes include DNAAF1 (also known as LRRC50) (Loges et al., 2009), DNAAF2 (also known as KTU) (Omran et al., 2008), DNAAF3 (Mitchison et al., 2012), DNAAF4 (also known as DYX1C1) (Tarkar et al., 2013), HEATR2 (also known as DNAAF5) (Diggle et al., 2014), CFAP298 (also known as C21ORF59) (Austin-Tse et al., 2013), LRRC6 (also known as DNAAF11) (Kott et al., 2012), ZMYND10 (Moore et al., 2013), DNAAF6 (also known as PIH1D3) (Olcese et al., 2017) and CFAP300 (also known as C11ORF70) (Höben et al., 2018), which are termed axonemal dynein arm assembly factors (DNAAFs). However, the mechanisms behind how these proteins associate with each other and with dynein chains, and their distinct roles in forming fully-assembled dynein arms, are undefined.
Previously, Knowles et al. (2013) discovered biallelic mutations in sperm-associated antigen 1 (SPAG1, also known as DNAAF13) in individuals diagnosed with PCD, with completely immotile cilia and absent/defective ODAs and IDAs. SPAG1 expression correlates with ciliogenesis in differentiating primary human airway epithelial cells, and SPAG1 is present strictly in the cytoplasm (Knowles et al., 2013). Thus, it is hypothesized that SPAG1 plays a role in cytoplasmic axonemal dynein arm assembly but the mechanism by which it facilitates this process is largely unexplored.
SPAG1 has 18 coding exons that translate into at least three distinct isoforms. The 104-kDa full-length isoform of SPAG1 has 926 amino acids, contains nine tetratricopeptide repeat (TPR) motifs organized into three TPR domains, and also has a RNA polymerase II-associated protein 3-like C-terminal (RPAP3_C) domain (Knowles et al., 2013; Maurizy et al., 2018). Another 94-kDa isoform of SPAG1 may be produced by exon skipping of exons 11 and 12, and a 48-kDa isoform is predicted to contain the first 11 exons (Takaishi and Huh, 1999; Neesse et al., 2007). TPRs are 34-amino acid motifs repeated to produce a helix-turn-helix three-dimensional structure that is involved in forming scaffolds to mediate protein-protein interactions and assemble multiprotein complexes (Perez-Riba and Itzhaki, 2019). The RPAP3_C domain represents the binding domain for RUVBL2 and WDR92 (also known as DNAAF10 or Monad) in RPAP3 in the R2TP/Prefoldin-like complex, a heat shock protein 90 (HSP90) co-chaperone complex (Itsuki et al., 2008; Maurizy et al., 2018; Martino et al., 2018).
Here, we studied three PCD subjects with SPAG1 mutations, with two of them presenting with an atypical ultrastructural defect of partial loss of ODAs and almost absent IDAs compared to a typical SPAG1 subject with completely defective or absent ODAs and IDAs. Examination of cultured nasal cells from these individuals with novel SPAG1 variants revealed the presence of a previously uncharacterized 60-kDa isoform of SPAG1 that partially compensates for the absence of full-length SPAG1, leading to some ODA assembly. Our studies using physiologically relevant human airway epithelial cells provide new evidence that SPAG1 plays an essential role in ciliary axonemal dynein arm assembly in humans, likely by forming an R2TP-like complex with other DNAAFs and participating in the formation, folding and/or binding of the DHCs to the DIC complex.
RESULTS
An atypical axonemal ultrastructure defect in PCD subjects with SPAG1 mutations
Previous studies have suggested that SPAG1 is involved in axonemal dynein arm assembly, but its exact role in this process has yet to be determined (Knowles et al., 2013; Horani et al., 2018). To better understand the role of SPAG1 in this process, we explored the effect of mutations in SPAG1 in PCD subjects. We identified and studied three PCD-affected individuals with genetic variants in the SPAG1 gene but differing ultrastructural changes (Fig. 1A; Tables S1, S2). Transmission electron micrographs (TEMs) of nasal cilia revealed that proband III-1 from family UNC-372 had totally absent and/or defective ODA and IDA (Fig. 1A), which is typical for defects in axonemal dynein assembly (Omran et al., 2008; Knowles et al., 2013). Interestingly, both proband III-2 from family UNC-68 and II-1 from family UNC-1231 had few, if any, IDA but many normal ODA present, which is an atypical ciliary ultrastructural defect for PCD subjects with SPAG1 mutations (Fig. 1A). Quantification of these TEMs showed an average of 5.3±3.7 and 5.4±3.3 (mean±s.d.) normal ODA visible in proximal and distal axonemes, respectively, in subject UNC-68 III-2, and 4.9±1.1 and 5.3±2.1 normal ODA visible in proximal and distal axonemes in subject UNC-1231 II-1, compared to 8.5±0.3 in 62 normal controls (de longh and Rutland, 1995; Shapiro and Leigh, 2017). There was no significant difference between the numbers of normal ODA in proximal and distal ciliary axonemes, indicating that there was not a specific type 1 or type 2 ODA defect in these individuals (Fig. 1B; Fliegauf et al., 2005).
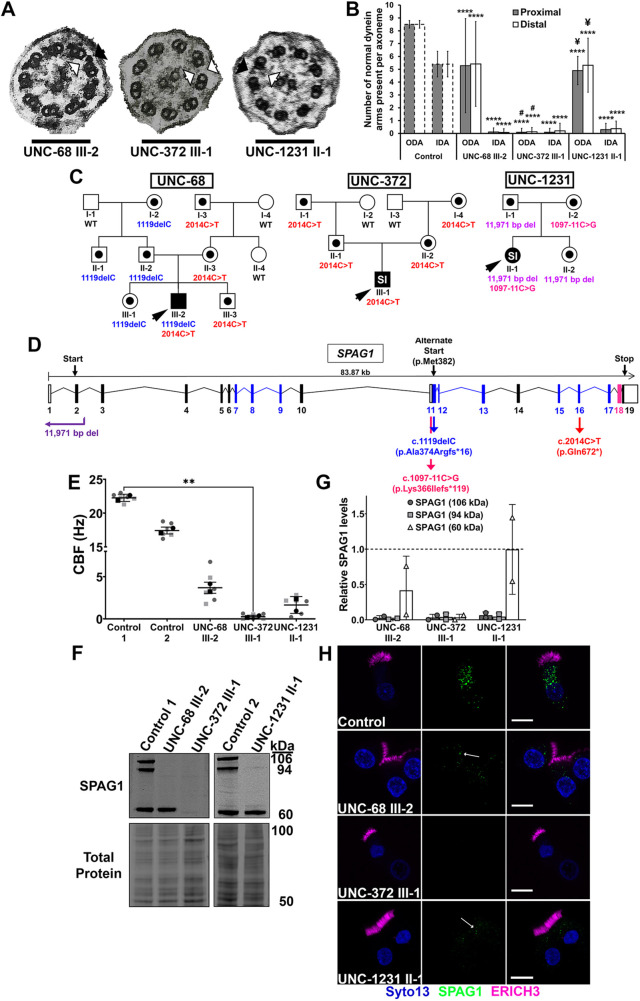
Fig. 1.
Expression of 60-kDa SPAG1 results in an atypical ciliary ultrastructural defect. (A) TEM of nasal cilia cross-sections from probands UNC-68 III-2 (left), UNC-372 III-1 (middle) and UNC-1231 II-1 (right). UNC-372 III-1 has absent IDAs/ODAs (white arrows), whereas UNC-68 III-2 and UNC-1231 II-1 have normal ODAs (black arrows). Scale bars: 150 nm. (B) Quantification of normal ODAs and IDAs present per proximal (gray bars) and distal (white bars) ciliary axoneme examined by TEM. Control values were based on averages of normal ODAs and IDAs scored on TEM of 62 healthy individuals (dashed bars) (Shapiro and Leigh, 2017). UNC-68 III-2, UNC-372 III-1 and UNC-1231 II-1, n=18, 31 and 10 proximal axonemes and 24, 24 and 19 distal axonemes, respectively. ****P<0.0001 compared to Control; #P<0.0001 compared to UNC-68 III-2; ¥P<0.0001 compared to UNC-371 III-1 (ANOVA with Tukey's multiple comparisons). (C) Pedigrees of genetic variants identified in families UNC-68, UNC-372 and UNC-1231. Symbols with black dots indicate carrier status, and black symbols indicate affected individuals with PCD. Probands are designated by black arrowheads, and SI indicates individuals with situs inversus. WT, wild type. (D) Diagram of the SPAG1 gene (NCBI, NG_033834.2). Blue and pink boxes represent the exons that translate into tetratricopeptide domains and the RPAP3-like C-terminal domain, respectively. The positions of all genetic variants found in families UNC-68 (blue and red arrows), UNC-372 (red arrow) and UNC-1231 (purple and magenta arrows) are shown. (E) CBF measurements of HNEC cultures from control and PCD-affected subjects. Six cultures were measured across n=2 independent experiments. **P<0.01 (Kruskal–Wallis with uncorrected Dunn's multiple comparisons). (F) Representative immunoblots for SPAG1, and corresponding total protein stain, on HNEC lysates from normal and PCD-affected individuals. (G) Quantification of immunoblots probing for SPAG1 protein levels. Raw signals were normalized to a total protein stain, then a separate differentiated HBEC control to control for variation across immunoblots, and then the control means were normalized to 1.0 (dashed line). n=2 independent experiments. (H) Representative isolated cell immunofluorescence images of multiciliated HNECs from normal and PCD-affected individuals stained for SPAG1 (green) and ERICH3 (magenta), a cilia marker. SYTO13 (blue) was used to stain the nuclei. Arrows indicate SPAG1 puncta that are still present in UNC-68 III-2 and UNC-1231 II-1 nasal cells. Images are representative of a total of 20 fields across n=2 independent experiments. Scale bars: 10 μm. Data are mean±s.d.
Using whole-exome sequencing (WES), proband III-2 from family UNC-68 was discovered to have compound heterozygous mutations in SPAG1: a deletion resulting in a frameshift in exon 11, c.1119delC (p.Ala374Argfs*16), and a substitution resulting in a premature stop in exon 16, c.2014C>T (p.Gln672*). We identified this same nonsense mutation in exon 16 in proband III-1 from family UNC-372 as homozygous variants. A previously identified large 11,971-bp deletion (c.61+201_POLR2K:c.140+1169_SPAG1del) encompassing exons 1 and 2 of SPAG1 was discovered in proband II-1 from family UNC-1231. This large deletion included the translation start codon and was predicted to abrogate SPAG1 translation (Knowles et al., 2013). Additionally, a variant of unknown significance (VUS) was identified in intron IVS10, c.1097-11C>G, in proband UNC-1231 II-1 (Fig. 1C,D; Fig. S1A-D). These genetic variants follow the autosomal-recessive inheritance model in these three families and are expected to result in the loss of the full-length SPAG1 isoform.
To determine whether the identified novel intronic VUS (c.1097-11C>G) in proband UNC-1231 II-1 is pathogenic, reverse-transcription PCR (RT-PCR) was performed on RNA from cultured human nasal epithelial cells (HNECs) for SPAG1 exons 9-13. The proband sample produced a major transcript similar in size to the normal control (Fig. S1E). Gel isolation, cloning and sequencing of this transcript showed that ∼50% of the plasmids contained a 10-nucleotide insertion (TTCCTCACAG) at the start of exon 11 that matches the ten nucleotides in intron IVS10 immediately downstream of the VUS. This suggests that this substitution shifts the exon 11 splice donor site ten nucleotides upstream and leads to a premature stop codon at the start of exon 12 (p.Lys366Ilefs*119) (Fig. S1F). Thus, these data support the conclusion that this VUS is a pathogenetic variant that can lead to PCD.
All three subjects shared common clinical characteristics of PCD, including neonatal respiratory distress, rhinosinusitis, otitis media and low nasal nitric oxide (Table S2; Leigh et al., 2013). Subjects UNC-68 III-2 and UNC-372 III-1 had bronchiectasis, whereas there was no computed tomography imaging available for UNC-1231 II-1. However, the phenotypic severity could be considered milder in UNC-68 III-2, having not developed bronchiectasis until 20 years of age compared to 5 years of age in UNC-372 III-1. UNC-372 III-1 also had worse lung function, with a forced expiratory volume (FEV)1 of 80% predicted at 15 years of age, whereas UNC-68 III-2 had an FEV1 of 98% predicted at 27 years of age. UNC-1231 II-1 had a FEV1 of 89% predicted at 6 years of age.
To further explore the effect of these SPAG1 variants on ciliary function, we cultured primary HNECs from these three probands and two healthy controls at the air-liquid interface (ALI) to differentiate into mucociliary airway epithelia (Suprynowicz et al., 2012; Müller et al., 2013; Fulcher and Randell, 2013). The ciliary beat frequencies (CBFs) of cultured HNECs from all PCD subjects (∼<4 Hz) were significantly reduced compared to their corresponding controls (22.3±0.5 Hz, 17.4±0.5 Hz; mean±s.d.) (Fig. 1E). Although SPAG1-deficient PCD subjects have previously been reported to have essentially immotile cilia (Knowles et al., 2013), ciliated cells from UNC-68 III-2 and UNC-1231 II-1 showed some ciliary motility, with a CBF of 3.7±0.7 Hz and 1.7±1.0 Hz, respectively. In contrast, the cilia of UNC-372 III-1 HNECs were immotile (0.3±0.2 Hz) (Fig. 1E). Taken together, subjects UNC-68 III-2 and UNC-1231 II-1 have an atypical ciliary phenotype, with normal ODA present and some ciliary motility, compared to other PCD subjects with SPAG1 variants.
Expression of a 60-kDa SPAG1 isoform in PCD results in an atypical axonemal ultrastructure defect
To determine the amount, if any, of SPAG1 proteins produced in these subjects, we performed immunoblots on whole-cell lysates using an antibody that targeted the C terminus of SPAG1 (Fig. 1F). Notably, the full-length and 94-kDa isoforms of SPAG1 were absent in all three subjects. However, a strong 60-kDa band was present in subjects UNC-68 III-2 and UNC-1231 II-1, and in control protein lysates from non-PCD subjects (Fig. 1F). The antibody epitope sequence of SPAG1 was not present in the predicted 48-kDa SPAG1 isoform described in the UniProt Database (Q07617-2); therefore, this 60-kDa band likely represents another SPAG1 isoform. Quantification of these immunoblots revealed that similar levels of the 60-kDa protein were present in UNC-1231 II-1 compared to a control, whereas the protein levels of UNC-68 III-2 were reduced to 42% (Fig. 1G). Immunoblots using a SPAG1 antibody that targeted the amino-terminus only detected the full-length and 94-kDa isoforms in a control protein lysate and no isoforms in the UNC-68 III-2 and UNC-372 III-1 protein lysates, which suggests that the predicted 48-kDa SPAG1 isoform is not expressed in this cell type (Fig. S1G). Immunofluorescent staining for SPAG1 and ERICH3, a proximal cilia marker (Blackburn et al., 2017), on isolated multiciliated cells confirmed the absence of any SPAG1 protein in UNC-372 III-1, whereas some SPAG1 staining was still detected in ciliated cells from UNC-68 III-2 and UNC-1231 II-1 (Fig. 1H). Of note, in control nasal multiciliated cells, SPAG1 was present only in the cytoplasm as large puncta (Fig. 1H, top panel), recently referred to as dynein arm assembly particles (DynAPs) that are hypothesized to be the location of cytoplasmic dynein arm assembly (Huizar et al., 2018; Lee et al., 2020). Previously, SPAG1 has been shown to colocalize with other DNAAFs and DynAP-resident proteins, including DNAAF2, HEATR2, ZYMND10 and RUVLB2, in these large puncta (Horani et al., 2018; Huizar et al., 2018; Lee et al., 2020).
Immunoblots on whole-cell lysates from undifferentiated and differentiated human bronchial epithelial cells (HBECs) and isolated ciliary axonemes detected the full-length and the 94-kDa isoforms of SPAG1 in differentiated HBECs, whereas the 60-kDa band was present in both undifferentiated and differentiated HBECs (Fig. S2A, Fig. S3A). No SPAG1 protein was detected in isolated axonemes, as was shown in previous studies (Knowles et al., 2013). To determine whether the 60-kDa band is a truncated isoform of SPAG1 that contains the C terminus, we performed targeted proteomics for ten peptides present in SPAG1 on gel-isolated 60-kDa bands from whole-cell lysates. In undifferentiated HBEC lysates, all peptides detected in this 60-kDa band corresponded to the C terminus half of SPAG1, whereas no peptides corresponding to the N terminus half were detected (Fig. S2, Table S3). These data demonstrate that the 60-kDa band is a SPAG1 isoform that contains the C-terminal half of the protein but is missing the N-terminal half.
To characterize the transcript of the 60-kDa SPAG1 isoform, we performed 5′ rapid amplification of cDNA ends (5′ RACE) on total RNA isolated from undifferentiated and differentiated HBECs using reverse primers for SPAG1 exons 15 and 19. The exon 19 reverse primer amplified major transcripts that were ∼1700 bp and ∼2800 bp long in undifferentiated and differentiated HBEC samples, respectively. The exon 15 reverse primer amplified ∼1000-bp and ∼2000-bp transcripts in undifferentiated and differentiated HBEC samples, respectively (Fig. S3B). As there was very little full-length SPAG1 expressed in undifferentiated HBECs, the shorter transcript was predicted to be responsible for the 60-kDa SPAG1 isoform detected on immunoblots. Subsequent cloning and sequencing of these transcripts revealed that undifferentiated HBECs expressed a SPAG1 transcript that contained exons 11-19. Interestingly, this transcript initiated 79 bp upstream of the start of exon 11 in intron IVS10 (NG_033834.2, base 59,977). Kozaq sequence analysis predicts the next most likely start codon for SPAG1 to be the methionine in exon 11 at amino acid 382 (NG_033834.2, bases 60,103–60,105) with a reliability of 0.38, compared to 0.62 for the canonical start codon of full-length SPAG1 (Table S4). Thus, the ATG at bases 127-129 of this transcript is likely to be the start codon for the 60-kDa isoform. When translated, this transcript remains in-frame with the full-length isoform of SPAG1, and produces a 545-amino acid protein (60 kDa) that is identical to amino acids 382-926 of full-length SPAG1 (Fig. 2A; Fig. S3C). As this 60-kDa isoform still contains two TPR domains and the RPAP3_C domain (Fig. 2A), it is possible that it can retain some dynein arm assembly function and partially compensate for the loss of full-length SPAG1, which could explain the presence of some ODAs and ciliary motility in probands UNC-68 III-2 and UNC-1231 II-1.
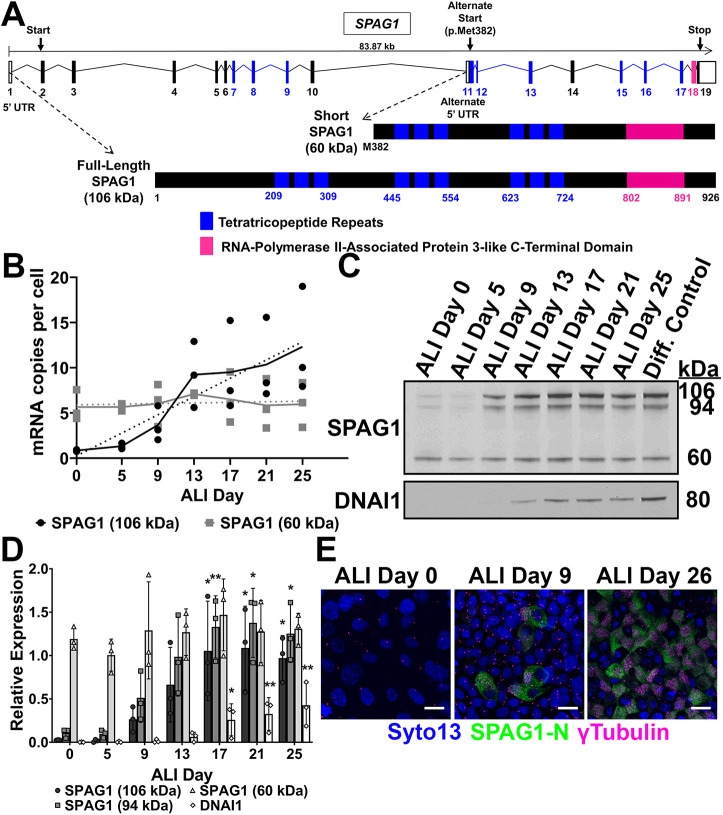
Fig. 2.
Different SPAG1 isoforms have distinct expression patterns. (A) Schematic of the canonical and alternate start sites of SPAG1 and the two different SPAG1 isoforms that are produced. The full-length SPAG1 is a 926-amino acid protein (106 kDa) with three TPR domains (blue boxes) and a RPAP3-like C-terminal domain (pink box). The 60-kDa SPAG1 isoform, or the short isoform, starts at the methionine at amino acid 382 of the full-length SPAG1 and produces a 545-amino acid protein (60 kDa) that has only two of the TPR domains and the RPAP3-like C-terminal domain. (B) Quantification of mRNA expression of 106-kDa and 94-kDa (black) or 60-kDa (gray) SPAG1 isoforms in differentiating HBEC cultures using ddPCR. Calreticulin (CALR) was used as a reference gene. Linear regression analysis (dashed lines) determined that the slope of 60-kDa SPAG1 was not significantly non-zero (P=0.603) compared to the slope of 106-kDa and 94-kDa SPAG1 (P<0.001). Data shown are means across timepoints (solid lines) and individual data points; n=3 independent experiments using three distinct cell codes. (C) Representative immunoblot for SPAG1 and DNAI1 on differentiating HBEC lysates. DNAI1 was used as a ciliogenesis control. (D) Quantification of SPAG1 isoforms and DNAI1 protein levels analyzed by immunoblots on differentiating HBEC lysates. Raw signals were normalized to a total protein stain and then a separate differentiated HBEC lysate control. Data are mean±s.d. n=3 independent experiments using three distinct cell codes. *P<0.05, **P<0.01 compared to ALI day 0 (Kruskal–Wallis with uncorrected Dunn's multiple comparisons). (E) Whole-mount immunofluorescence images on differentiating HBEC cultures stained for the N terminus of SPAG1-N (green), γ-tubulin (magenta) to mark basal bodies, and the nuclei (blue). Images are representative of a total of six fields of view across two cultures per timepoint per independent experiment. Scale bars: 10 μm. n=3 independent experiments using three distinct cell codes.
The expression pattern of full-length SPAG1 correlates with ciliogenesis, and the 60-kDa isoform is constitutively expressed
To begin to elucidate the functions of the full-length and uncharacterized 60-kDa isoforms of SPAG1, we examined their expression throughout HBEC differentiation. The mRNA levels of full-length and 60-kDa isoforms of SPAG1 were measured using droplet digital PCR (ddPCR) and isoform-specific primers in HBEC cultures during differentiation. We detected very low levels of full-length and 94-kDa SPAG1 transcripts in undifferentiated HBECs (ALI day 0). The expression of these transcripts was induced by ALI day 9, which correlated with the initiation of ciliogenesis, and increased throughout differentiation (Fulcher and Randell, 2013). Interestingly, the 60-kDa SPAG1 isoform was expressed at similar levels throughout airway cell differentiation (ALI day 0-28) (Fig. 2B), which could indicate a cilia-independent function. Immunoblots for SPAG1 with whole-cell lysates of differentiating HBECs confirmed these findings at the protein level (Fig. 2C,D).
We performed immunofluorescent staining of whole-mount HBEC cultures at different stages of differentiation using an antibody against the N terminus of SPAG1 to detect the full-length isoforms, and an antibody against γ-tubulin to mark basal bodies (Vertii et al., 2016). In undifferentiated HBEC cultures (ALI day 0), no SPAG1-specific staining was observed. When ciliogenesis started to occur (ALI day 5-9), the number of cells co-expressing SPAG1 and basal bodies increased. In fully differentiated HBEC cultures (ALI day 26), a large number of cells co-expressed SPAG1 and basal bodies, and no cells expressed only basal bodies throughout differentiation (Fig. 2E; Fig. S4). Thus, the expression of full-length and 94-kDa SPAG1 suggests it is primarily involved in ciliogenesis, and the constitutive expression of the 60-kDa isoform indicates that it possibly has a cilia-independent function.
SPAG1 interacts with DNAAFs, dynein chains and components of the R2TP/Prefoldin-like complex
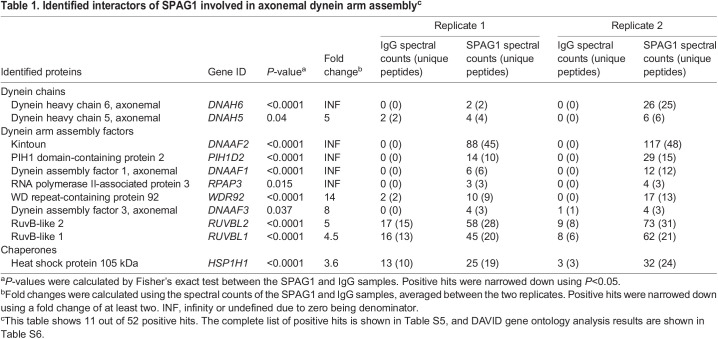
Immunoprecipitations for endogenous SPAG1 in HBECs were performed using a SPAG1 antibody targeting the N terminus, and were analyzed by mass spectrometry and immunoblotting (Table 1, Fig. 3A). A total of 52 positive hits were identified (Tables S5, S6). Intriguingly, two DHCs, DNAH6 and DNAH5, were co-precipitated with SPAG1. A number of previously identified DNAAFs also co-precipitated with SPAG1, including DNAAF2, PIH1D2, DNAAF1, RPAP3, WDR92, DNAAF3, RUVBL1 and RUVBL2 (Table 1). Interestingly, RPAP3, WDR92, RUVBL1 and RUVBL2 are canonical components of the R2TP/Prefoldin-like complex, a heat shock protein 90 (HSP90) co-chaperone complex (Houry et al., 2018).
Table 1.
Identified interactors of SPAG1 involved in axonemal dynein arm assemblyc
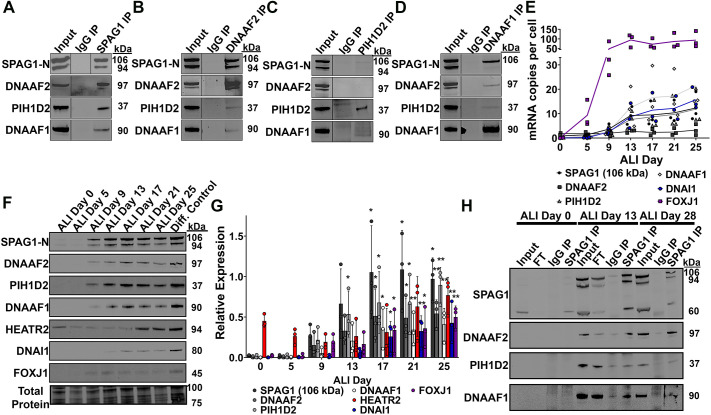
Fig. 3.
SPAG1 interacts with other known DNAAFs. (A) Representative immunoblots for SPAG1, DNAAF2, PIH1D2 and DNAAF1 analyzing co-immunoprecipitation samples for endogenous SPAG1 in HBEC cultures. n=3 independent experiments using three distinct cell codes. (B-D) Representative immunoblots for SPAG1, DNAAF2, PIH1D2 and DNAAF1 analyzing reverse co-immunoprecipitation samples for endogenous DNAAF2 (B), PIH1D2 (C) and DNAAF1 (D) in HBEC cultures. n=2 independent experiments using two distinct cell codes. (E) Quantification of mRNA co-expression between SPAG1, FOXJ1, DNAI1, DNAAF1, DNAAF2 and PIH1D2 in differentiating HBEC cultures. FOXJ1 and DNAI1 were used as ciliogenesis controls. Calreticulin (CALR) was used as a reference gene. Data shown are means across timepoints (lines) and individual data points. n=3 independent experiments using three distinct cell codes. (F,G) Representative immunoblots (F) and quantification (G) of protein co-expression of SPAG1, DNAAF2, PIH1D2, DNAAF1, HEATR2, DNAI1 and FOXJ1 in differentiating HBEC culture lysates. DNAI1 and FOXJ1 were used as ciliogenesis controls. Total protein stain was used as a loading control. Raw signals were normalized to a total protein stain and then a separate differentiated HBEC lysate control. Data are mean±s.d. n=3 independent experiments using three distinct cell codes. *P<0.05, **P<0.01 compared to ALI day 0 (Kruskal–Wallis with uncorrected Dunn's multiple comparisons). (H) Representative immunoblots for SPAG1, DNAAF2, PIH1D2 and DNAAF1 in time-course co-immunoprecipitation for endogenous SPAG1 samples. HBEC cultures were lysed at various days (ALI days 0, 13, 18 and 28) throughout differentiation, and lysates were used to immunoprecipitate endogenous SPAG1 and interactors. n=2 independent experiments using two distinct cell codes. IP, immunoprecipitation. FT, flow through. Input lanes represent 5% of the total lysate.
As DNAAF2, PIH1D2 and DNAAF1 were the most abundant DNAAFs identified in the co-immunoprecipitations, we characterized their interactions with SPAG1. Immunoblots of the immunoprecipitated eluates confirmed that DNAAF2, PIH1D2 and DNAAF1 co-precipitate with SPAG1 (Fig. 3A). Reverse co-immunoprecipitation for DNAAF2 co-precipitated the full-length and 94-kDa SPAG1 with small amounts of PIH1D2 and DNAAF1. Reverse co-immunoprecipitation for DNAAF1 co-precipitated full-length SPAG1 with small amounts of PIH1D2 and DNAAF2 but not the 94-kDa SPAG1 isoform, suggesting that the binding site for DNAAF1 is absent in this isoform. Reverse co-immunoprecipitation for PIH1D2 only co-precipitated minimal amounts of SPAG1 and DNAAF1, but this may be because of the low binding affinity of the PIH1D2 antibody and less than optimal immunoprecipitation conditions (Fig. 3B-D). These results suggest that SPAG1 specifically interacts with these three proteins, either individually or in a complex.
There are limited studies examining PIH1D2 and its functions, especially in human airway epithelia. Therefore, we examined and compared its expression in differentiating HBECs with other known ciliogenesis-related genes and expected interactors of SPAG1. mRNA levels of full-length SPAG1, DNAAF2, PIH1D2, DNAAF1, DNAI1 and FOXJ1, a major transcription factor controlling ciliogenesis, were measured in differentiating HBECs using ddPCR. Full-length SPAG1, DNAAF2, PIH1D2 and DNAAF1 were induced by ALI day 9, after the expression of the transcription factor FOXJ1, and increased throughout differentiation of HBECs (Fig. 3E). Immunoblots of SPAG1, DNAAF2, PIH1D2, DNAAF1, DNAI1 and FOXJ1 revealed that the expression of these proteins during differentiation corresponds with their respective mRNA levels (Fig. 3F,G). Previously, it was suggested that SPAG1, DNAAF2 and HEATR2 are co-expressed, and form a complex at an earlier stage in dynein arm assembly than other DNAAFs (Horani et al., 2018). Here, we show that only 60-kDa SPAG1 and HEATR2 are highly expressed at early stages of differentiation (ALI day 0-5), and HEATR2 was not a positive hit in any of the immunoprecipitation studies for endogenous SPAG1, which is different from previous results. Therefore, it is possible that the earlier expression of HEATR2 in undifferentiated cells (ALI day 0-5) is due to an additional function other than dynein arm assembly, similar to the 60-kDa isoform of SPAG1. However, these differences in results could be due to differences in methods, antibodies used and cell culture conditions. Importantly, PIH1D2 follows expression patterns common with DNAAFs, suggesting PIH1D2 is involved in ciliogenesis in human airway epithelium.
To determine whether there were proteins more likely to interact with the 60 kDa isoform instead of other isoforms of SPAG1, and to further characterize its interactions with DNAAF1, DNAAF2, and PIH1D2, we performed a time course of co-immunoprecipitations using a SPAG1 antibody targeting the C terminus in differentiating HBECs on ALI days 0, 13, 18 and 28, and analyzed the eluates by immunoblotting and mass spectrometry (Fig. 3H; Tables S7-S10). On ALI day 0, the 60-kDa SPAG1 co-precipitated with MISP, DBN1, IMPDH2, RPN2 and SVIL: proteins associated with actin binding according to Database for Annotation, Visualization and Integrated Discovery (DAVID) gene ontology analysis. DNAAF2, DNAAF1 and PIH1D2 co-precipitated with SPAG1 by ALI day 13 or 18, and maintained interactions with SPAG1 on ALI day 28. WDR92 co-precipitated with SPAG1, detected by proteomic analysis, starting on ALI day 13. Interestingly, proteomic analysis revealed that a number of dynein chains co-precipitated with SPAG1 on ALI day 18, including DNAH6, DNAH9, DNAH5 and DNAI1. In addition, multiple chaperones co-precipitated with SPAG1, starting at ALI day 13 or 18, including HSPA4, HSPH1 and several components of the chaperonin CCT. In summary, SPAG1 interacts with DNAAF1, DNAAF2, PIH1D2 and several other DNAAFs, dynein chains and multiple components of chaperone complexes, including the R2TP/Prefoldin-like and CCT complexes.
SPAG1 is required for the interaction of DHCs with the DIC complex
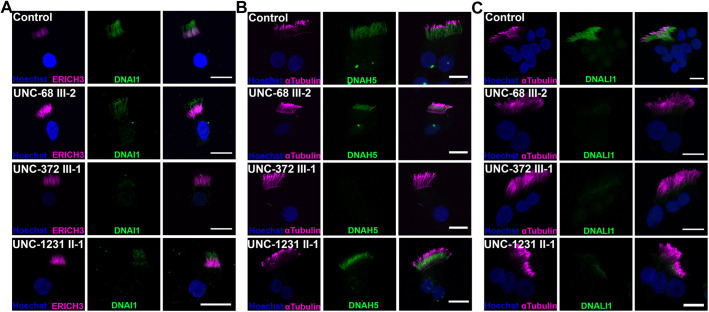
To determine which step(s) in dynein arm assembly requires functional SPAG1, we investigated the axonemal dynein defects found in the three PCD subjects with SPAG1 genetic variants. Immunofluorescent staining for DNAI1, DNAH5, DNALI1 and a cilia marker were performed on HNECs from control and PCD subjects. DNAI1 and DNAH5, ODA dynein chains, were present along the full length of the ciliary axoneme in subjects UNC-68 III-2 and UNC-1231 II-1, albeit at a reduced level compared to control cilia. There was no signal for DNAI1 or DNAH5 in ciliary axonemes of UNC-372 III-1 (Fig. 4A,B). Additionally, there was no signal for DNALI1, an IDA dynein chain, in ciliary axonemes in all three subjects (Fig. 4C). These results corroborate the TEM findings from nasal cilia from these SPAG1-deficient PCD individuals.
Fig. 4.
UNC-372 III-1 has defective ODAs and IDAs, whereas UNC-68 III-2 and UNC-1231 II-1 have normal ODAs. Single-cell immunofluorescence images of cultured multiciliated nasal cells from normal and PCD-affected individuals stained for ODAs, using DNAI1 (A) and DNAH5 (B), and IDAs, using DNALI1 (C) (green). Cilia were labeled with either ERICH3 or acetylated α-tubulin (magenta), and nuclei (blue) were stained using Hoechst 33342. Images are representative of a total of 20 fields of view across n=2 independent experiments. Scale bars: 10 μm.
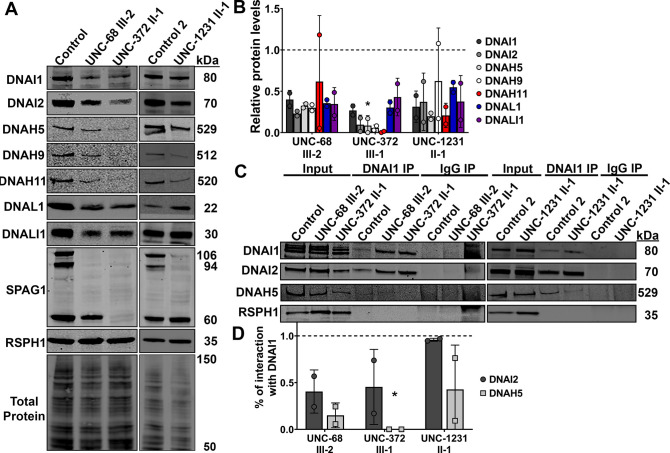
Owing to the instability of dynein chains when not interacting in dynein arm complexes, we hypothesized that distinct dynein chains in SPAG1-deficient PCD individuals would be reduced at a greater level than others based on the stage of dynein assembly SPAG1 is involved in (Omran et al., 2008; Mitchison et al., 2012). We performed immunoblots for various dynein chains in ODAs and IDAs, including DNAI1, DNAI2, DNAH5, DNAH9, DNAH11, DNAL1 and DNALI1, on whole-cell lysates of cultured HNECs. All dynein chains examined were reduced in all three subjects. Intriguingly, in subject UNC-372 III-1, in which no SPAG1 isoforms are expressed, there was a substantial reduction in the levels of ODA DHCs, DNAH5, DNAH9 and DNAH11, with DNAH5 being statistically significant (Fig. 5A,B). This suggests, in combination with the data showing that SPAG1 interacts with various DHCs and chaperone complexes, that SPAG1 facilitates the formation, folding and/or interaction of the DHCs.
Fig. 5.
SPAG1 facilitates the addition of DHCs to the intermediate chain complex. (A) Immunoblots for various ODA dyneins (DNAI1, DNAI2, DNAH5, DNAH9, DNAH11 and DNAL1), an IDA dynein chain (DNALI1), SPAG1, a cilia control (RSPH1) and total protein stain on the HNEC lysates of normal and PCD-affected individuals. (B) Quantification of dynein chain protein expression measured by immunoblots on the HNEC lysates of normal and PCD-affected individuals. Background-subtracted signals were normalized to a total protein stain and RSPH1, then a separate differentiated HBEC lysate to control for variations across immunoblots, and then the nasal control means were set to 1.0 (dashed line). n=2 independent experiments. *P<0.05 compared to control (Kruskal–Wallis test with uncorrected Dunn's multiple comparisons). (C) Immunoblots for DNAI1, DNAI2, DNAH5 and a cilia control (RSPH1) analyzing co-immunoprecipitation for endogenous DNAI1 samples from HNECs of normal and PCD-affected individuals. (D) Quantification of the amount of DNAI2 and DNAH5 protein interacting with DNAI1 in PCD-affected HNECs compared to normal HNECs. Background-subtracted DNAI2 or DNAH5 signals were divided by DNAI1 signals per each eluate sample, and then the control means were set to 1.0 (dashed line). Data shown are mean±s.d. n=2 independent experiments. *P<0.05 compared to control (Kruskal–Wallis test with uncorrected Dunn's multiple comparisons). IP, immunoprecipitation. Input lanes represent 5% of the total lysate.
To determine whether SPAG1 is required for the interaction of the ODA DHCs to the intermediate chains, co-immunoprecipitations for DNAI1 were performed on whole-cell lysates from differentiated cultures of control and PCD nasal cells. Immunoblots for DNAI1, DNAI2, DNAH5 and RSPH1 (a cilia control) were performed and quantified to determine the level of interaction between DNAI2 and DNAH5 with DNAI1. The interaction between DNAI1 with DNAI2 or DNAH5 was reduced in subjects UNC-68 III-2 and UNC-1231 II-1 according to quantification of the immunoblots. Surprisingly, there was no further reduction in the levels of interaction between DNAI1 with DNAI2 in UNC-372 III-1, suggesting that SPAG1 facilitates the interaction, formation and/or stabilization of the ODA DIC complex but is not absolutely required. However, there was no interaction detected between DNAI1 with DNAH5 in subject UNC-372 III-1 (Fig. 5C,D). This result suggests that SPAG1 is necessary for the interaction between DNAI1/DNAI2 and DNAH5. Thus, SPAG1 could be involved in associating and binding the DHCs to the previously formed DIC-DLC complexes. It is also possible that this reduction in the DNAH5 and DNAI1/DNAI2 interaction was due to reduced DNAH5 formation, as shown by reduced total protein levels (Fig. 5A,B), suggesting SPAG1 could be involved in the formation and folding of DHCs. In conclusion, SPAG1 plays a crucial role in cytoplasmic axonemal dynein arm assembly by facilitating the formation of DIC-DHC complexes, either by forming and folding the DHCs, and/or binding the DHCs to previously formed DIC complexes. Further studies are required to determine whether SPAG1 is necessary for one function, the other or both.
The 60-kDa SPAG1 isoform can interact with DNAAF2 and partially compensate for the lack of full-length SPAG1
The above results suggested that the 60-kDa SPAG1 can partially compensate for the lack of full-length SPAG1 and help assemble some normal ODAs. To further test this hypothesis, we explored whether the 60-kDa isoform could still interact with another DNAAF that interacts with SPAG1. DNAAF2 was the most abundant DNAAF that co-precipitated with SPAG1 in our proteomic studies. Therefore, FLAG-tagged full-length SPAG1 or a FLAG-tagged 60-kDa SPAG1 and HA-tagged DNAAF2 were co-expressed in HEK293T cells. Immunoprecipitations for the FLAG-tag were performed, and the levels of HA-tagged DNAAF2 that co-precipitated were analyzed and quantified by immunoblotting. FLAG-tagged full-length SPAG1 co-precipitated the HA-tagged DNAAF2, suggesting that the addition of the epitope tags to the C terminus did not interfere with the function of the protein or its interactions. Interestingly, the FLAG-tagged 60-kDa isoform of SPAG1 also co-precipitated HA-tagged DNAAF2, albeit at a level of 7.7±5.8% (mean±s.d.) of interaction compared to full-length SPAG1 (Fig. 6A,B). Thus, the 60-kDa SPAG1 isoform likely compensates for the absence of full-length SPAG1 and facilitates the assembly of some ODAs, possibly through a pathway that involves DNAAF2. However, a caveat to this experiment is that this interaction may be driven through the overexpression of constructs, and more known interactors of SPAG1 should be tested to determine whether the 60-kDa isoform of SPAG1 can still interact with them as well.
Fig. 6.
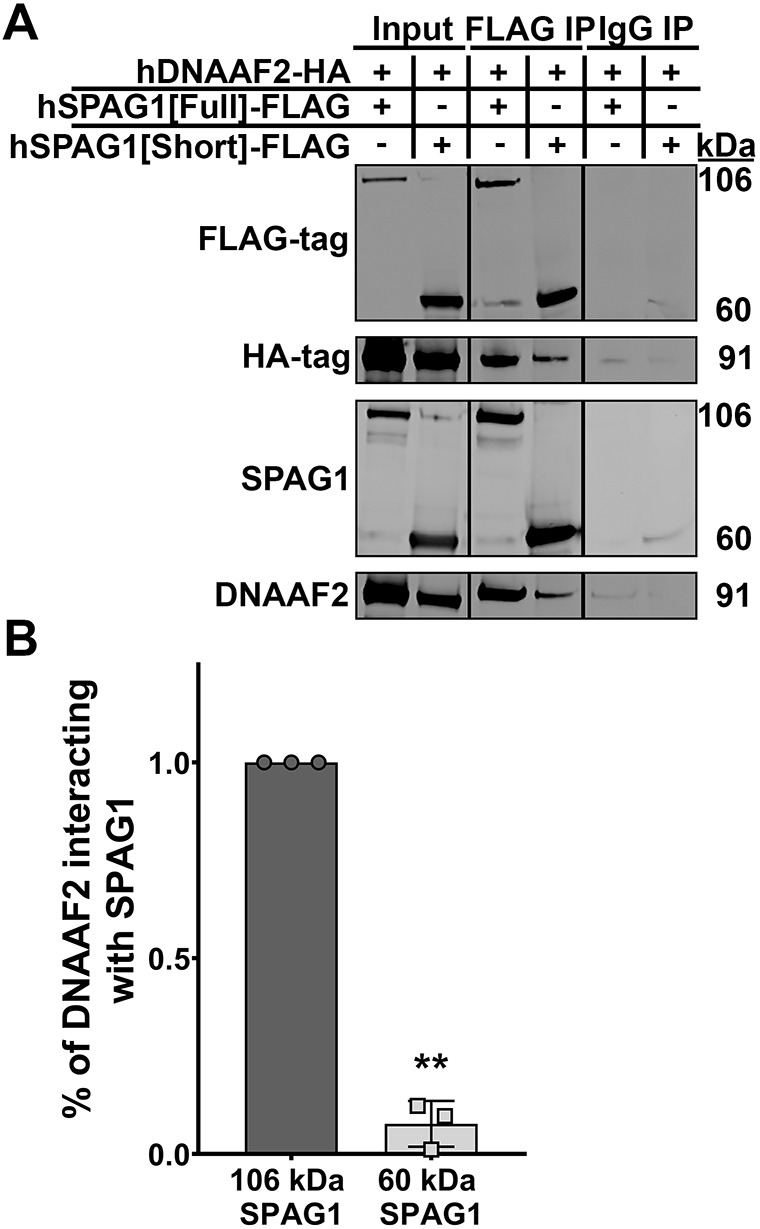
The 60-kDa SPAG1 isoform can still interact with DNAAF2. (A) Immunoblots for SPAG1, DNAAF2, FLAG-tag and HA-tag to analyze co-immunoprecipitations of FLAG-tagged full-length or 60-kDa SPAG1 with HA-tag DNAAF2 in HEK293T cells. IP, immunoprecipitations. Input lanes represent 5% of the total lysate. (B) Quantification of the amount of DNAAF2 interacting with 106-kDa or 60-kDa SPAG1 isoforms. Background-subtracted HA-tag signals were divided by FLAG-tag signals per each sample, and then the 106-kDa SPAG1 ratio was set to 1.0. Data are mean±s.d. n=3 independent experiments. **P<0.01 compared to 106-kDa SPAG1 (two-tailed one-sample t-test).
DISCUSSION
Cilia are complex structures, composed of over 500 proteins that are precisely assembled into different substructures, each playing unique roles in ciliary function (Blackburn et al., 2017; Wallmeier et al., 2020). Defects in many of these substructures (e.g. ODAs, IDAs, radial spokes and the central pair) have been shown to affect ciliary function and cause PCD (Shapiro et al., 2016; Davis et al., 2019; Wallmeier et al., 2020). PCD is a genetically heterogenous disorder, with mutations currently identified in ∼50 different genes that result in PCD, but many more are expected to be revealed (Zariwala et al., 2007; Shapiro et al., 2016; Davis et al., 2019). Ciliary ultrastructural defects, the presence of laterality defects and the severity and progression of lung disease can vary in PCD based on the specific dysfunctional gene (Leigh et al., 2019; Davis et al., 2019). Here, we show that distinct genetic variants within the same gene can lead to variations in axonemal ultrastructural defects, with impacts on ciliary function and possibly disease severity, which can further complicate the diagnosis of PCD.
Three individuals with clinical features compatible with PCD were identified as having genetic variants in SPAG1, but only subject UNC-372 III-1 had a typical mutated SPAG1 PCD presentation, with completely defective ODAs/IDAs and completely immotile cilia (Knowles et al., 2013). In contrast, two individuals (UNC-68 III-2 and UNC-1231 II-1) showed atypical ciliary defects, with the presence of some normal ODAs and ciliary motility. Here, we show that this atypical PCD ciliary phenotype is due to the expression of a previously uncharacterized 60-kDa isoform of SPAG1. Co-immunoprecipitations for FLAG-tagged full-length or 60-kDa SPAG1 showed that both were able to co-precipitate HA-tagged DNAAF2 in HEK293T cells, although the interaction with the 60-kDa SPAG1 was not as strong as with full-length SPAG1. Thus, it is likely that the 60-kDa isoform of SPAG1 can partially compensate for the lack of full-length SPAG1 and assemble some normal ODAs, possibly through a pathway involving DNAAF2. The PIH1D2-binding motif (amino acids 732-784) is still present in the 60-kDa isoform, and it is possible that PIH1D2 can also play a role in assembling ODAs in the absence of full-length SPAG1 (Maurizy et al., 2018). However, one of the binding sites for HSP70 and/or HSP90, the first TPR domain, is absent in this isoform. Interestingly, these subjects only assemble a large number of normal ODAs, but not IDA. This difference suggests that assembly of the IDAs that involve SPAG1 requires the first TPR domain, possibly due to facilitating the recruitment and interaction of multiple chaperones, or exchange of clients between chaperones, specifically HSP70 and HSP90 (zur Lage et al., 2018; Chagot et al., 2019; Dermouche et al., 2021). Interestingly, the homolog of SPAG1 (CG18472/Spag1) has recently been shown to have a conserved function in dynein arm assembly in Drosophila, but only contains the first TPR domain, suggesting this first TPR domain is required for efficient dynein arm assembly of ODAs and IDAs (zur Lage et al., 2018). Further studies are needed to corroborate that the 60-kDa SPAG1 can partially compensate for the lack of full-length SPAG1, specifically determining which interactors and/or complexes are involved in dynein assembly, and why only ODAs are assembled.
The expression of full-length 106-kDa SPAG1, along with the 94-kDa isoform, correlates with ciliogenesis. In contrast, the 60-kDa isoform is constitutively expressed, suggesting a possible motile cilia-independent function in normal epithelium. This isoform has previously been shown to be expressed in human testis extracts and HBEC lysates by immunoblotting, but was not fully characterized until now (Lin et al., 2001; Neesse et al., 2007; Knowles et al., 2013). The mouse homolog Spag1 (also known as Tpis) also has a second isoform transcribed from an alternative start site at exon 11 that translates to a 529-amino-acid protein (59 kDa) with five TPR motifs (GenBank, AF181253; UniProt, Q80ZX8-2), suggesting that the 60-kDa SPAG1 protein is a conserved isoform transcribed from a conserved alternative start site (Takaishi and Huh, 1999). Through proteomic analysis, we identified five proteins (MISP, DBN1, IMPDH2, RPN2 and SVIL) as possible interactors of 60-kDa SPAG1. The majority of these proteins are involved in regulating the actin cytoskeleton and/or the interaction of actin with microtubules, either during mitotic/meiotic spindle assembly, cytokinesis, focal adhesion initiation or cell migration (Smith et al., 2010; Maier et al., 2013; Kasioulis et al., 2017; Duan et al., 2018). Interestingly, the 60-kDa Spag1 isoform in mice has been implicated in spindle morphogenesis (Huang et al., 2016). In addition, SPAG1 expression is upregulated in multiple cancers (Neesse et al., 2007; Biermann et al., 2007; Silina et al., 2011; Lin et al., 2021). Several studies have reported that knockdown of SPAG1 causes impairment of cell proliferation and/or cell motility (Neesse et al., 2007; Hu et al., 2017; Lin et al., 2021). Taken together, the 60-kDa isoform of SPAG1 is hypothesized to function as a regulator of microtubule and actin dynamics to facilitate cell motility and proliferation, but further studies are required. Additionally, it is also possible that the 60-kDa SPAG1 plays a role in the assembly of a subset of ODAs in normal airway epithelium.
Intriguingly, several dynein chains (DNAH6, DNAH5, DNAH9 and DNAI1), previously identified DNAAFs (DNAAF2, PIH1D2, DNAAF1, RPAP3, WDR92, DNAAF3, RUVBL1 and RUVBL2) and chaperones (HSPH1, HSP70 and CCT complex) co-precipitated with endogenous SPAG1 in differentiating HBECs. DNAAF1/LRRC50, DNAAF2/KTU and DNAAF3 are previously identified DNAAFs, and mutations in these genes cause PCD with defects in ODAs and IDAs (Omran et al., 2008; Loges et al., 2009; Mitchison et al., 2012). RPAP3, RUVBL1, RUVBL2 and WDR92 are canonical components of the HSP90 co-chaperone R2TP/Prefoldin-like complex. The R2TP complex contains a hexamer of the AAA+ ATPases RUVBL1 and RUVBL2 bound to a heterodimer of RPAP3 and PIH1D1, and WDR92 interacts with PIH1D1 and RPAP3. The R2TP/Prefoldin-like complex facilitates the formation and stabilization of multiprotein complexes by acting as a platform between chaperones, including HSP90, HSP70 and CCT, and their clients, either directly or through interaction with specificity factors or adaptors (Houry et al., 2018). All of these components have been previously associated with defects in dynein arm assembly (Zhao et al., 2013; Li et al., 2017; zur Lage et al., 2018; Dafinger et al., 2018; Liu et al., 2019; Patel-King et al., 2019). PIH1D2 is a protein in the PIH1-domain-containing protein family, which includes PIH1D1, PIH1D3 and DNAAF2, all of which have been found to cause dynein arm defects in zebrafish (Omran et al., 2008; Olcese et al., 2017; Yamaguchi et al., 2018). PIH1D2 follows the same expression pattern as other DNAAFs in human bronchial epithelial cells, suggesting that the function of PIH1D2 in dynein arm assembly is conserved in humans.
Interestingly, it has been theorized that SPAG1 is a paralog of RPAP3 (Maurizy et al., 2018). Previous studies have shown interactions between SPAG1, DNAAF2, PIH1D2 and a RUVBL1-RUVBL2 subcomplex, in which the interaction between SPAG1 and RUVBL1-RUVBL2 is further stabilized by the addition of PIH1D2 (Maurizy et al., 2018; Horani et al., 2018). In Drosophila S2 cells, Wdr92 was also shown to interact with Spag1, with their association increased by overexpression of Reptin and Pontin (zur Lage et al., 2018). Thus, it has been suggested that SPAG1 forms an R2TP-like complex, called R2SP, containing a hexamer of RUVBL1-RUVBL2 with a heterodimer of SPAG1 and PIH1D2. Studies in vitro hypothesized the existence of another R2TP-like complex, called R2SD, that replaces PIH1D2 with DNAAF2; however, this complex was not detected in proteomic experiments in vivo (Maurizy et al., 2018). Here, we provide the first evidence that the R2SP complex, and possibly the R2SD complex, occur endogenously in human airway epithelia by co-immunoprecipitating all components of these complexes with SPAG1. Further studies are required to determine whether there are two or three complexes occurring separately, which proteins are included in these complexes, and which complex is necessary for which dynein arm subtype.
The R2TP-like complexes are hypothesized to further interact with other DNAAFs, possibly as adaptors or specificity factors for dynein chain clients. In this study, DNAAF1 and DNAAF3 were co-immunoprecipitated with endogenous SPAG1 in HBECs. Although interactions with DNAAF3 have not been described previously, DNAAF1 has been shown to interact with RUVBL1 and RUVBL2 (Hartill et al., 2018). Additionally, WDR92 has been suggested to be a specificity factor that associates dynein clients to an R2TP-like complex and HSP90 through its interaction with SPAG1 (zur Lage et al., 2018). Here, we identified various chaperones in endogenous SPAG1 co-immunoprecipitations in HBECs, including HSPA4 (HSP70), HSPH1 and several components of the CCT chaperonin complex. Previously, SPAG1 has been shown to interact with HSP70 and HSP90 through its first and third TPR domains (Chagot et al., 2019; Dermouche et al., 2021). RPAP3 is known to interact with both HSP70 and HSP90, possibly to facilitate the exchange of clients between chaperones, and recently, this function has also been suggested for SPAG1 (Benbahouche et al., 2014; Martino et al., 2018; Dermouche et al., 2021). Components of the CCT chaperonin complex, which associates with the canonical R2TP/Prefoldin-like complex through WDR92, have previously been immunoprecipitated with WDR92 and DYX1C1 (Tarkar et al., 2013; Liu et al., 2019). Taken together, SPAG1 likely forms R2TP-like complexes with RUVBL1-RUVBL2, PIH1D2 and/or DNAAF2 that scaffold the interactions of various chaperones, including HSP70, HSP90, HSPH1 and CCT, adaptors, including WDR92, DNAAF1 or DNAAF3, and dynein chain clients (Fig. 7A).
Fig. 7.
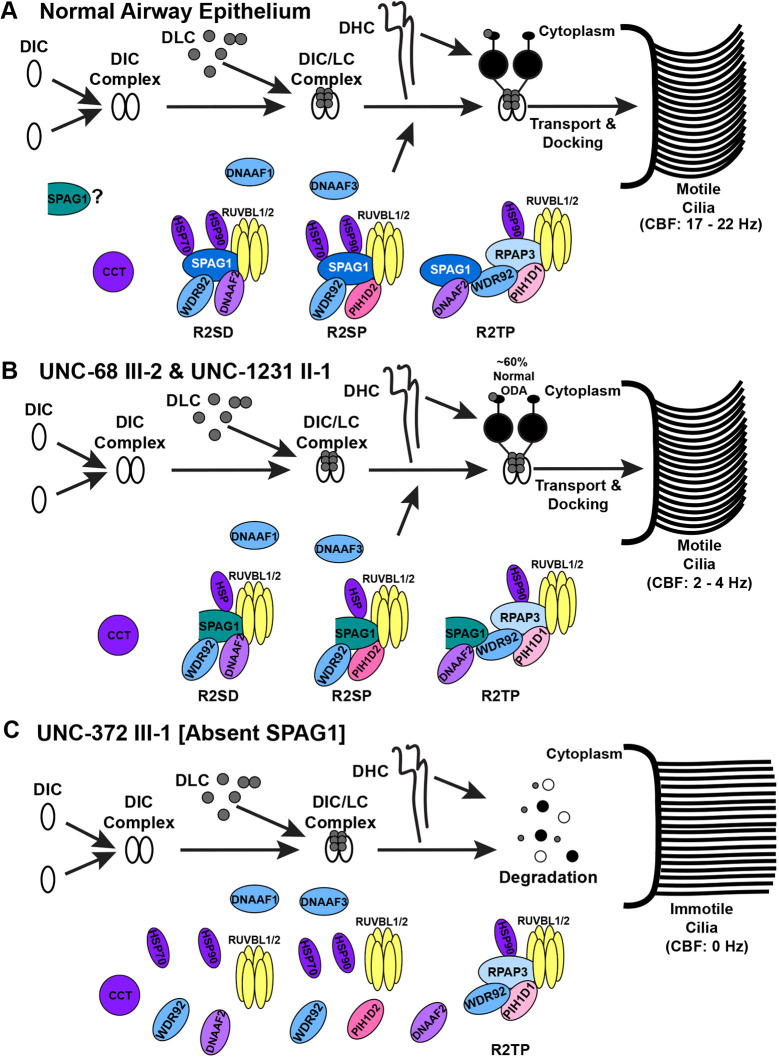
Function of full-length SPAG1 and compensation of 60-kDa SPAG1 in axonemal dynein arm assembly. (A) In normal human airway multiciliated cells, full-length SPAG1 (blue with white text) interacts with RUVBL1/2, DNAAF2 or PIH1D2, and WDR92, to form potential R2TP-like complexes that facilitate the folding and binding of the DHCs to the DIC-DLC (DIC/LC) complex. It is also possible that SPAG1 interacts with the canonical R2TP complex through WDR92 to facilitate dynein arm assembly. These R2TP-like complexes can further interact with different chaperones (purple), such as HSP70, HSP90 and CCT, and other DNAAFs (light blue), including DNAAF1 and DNAAF3, to recruit dynein chains to chaperones for formation into dynein arms. The dynein arms are then transported into the ciliary axoneme by intraflagellar transport, leading to motile cilia (CBF, 17-22 Hz). The 60-kDa SPAG1 isoform (teal) could play a role in dynein arm assembly to assemble a subset of ODAs, or it has a cilia-independent function. (B) In cells that are lacking full-length SPAG1 but still express the 60-kDa isoform of SPAG1 (UNC-68 III-2 and UNC-1231 II-1), some assembly of ODAs occurs even though SPAG1 is lacking one of its HSP binding sites. About 60% of normal ODAs leads to some ciliary motility (CBF, 2-4 Hz). (C) If SPAG1 is completely absent in airway epithelium (UNC-372 III-1), these R2TP-like complexes are unlikely to form, and thus, the folding and binding of DHCs to the DIC/LC complex is impaired. The cytoplasmic assembly process of dynein arms is inhibited, and the partially assembled dynein complex is degraded, instead of being transported into the ciliary axoneme. With this, cilia are completely immotile (CBF, 0 Hz).
To determine which step in the axonemal dynein assembly process required SPAG1, we performed immunoblots to measure levels of dynein chains and immunoprecipitations for DNAI1 on normal and mutant HNECs. In HNEC lysates in which all isoforms of SPAG1 were absent (UNC-372 III-1), there was a substantial reduction in the levels of ODA heavy chains DNAH5, DNAH9 and DNAH11. Surprisingly, co-immunoprecipitations for DNAI1 showed that the interaction between DNAI1 and DNAI2 occurs in the absence of SPAG1. However, DNAH5 was not present in this dynein complex in the absence of SPAG1, which suggests that SPAG1 is required for the binding of DHCs to the DIC complex. However, the significant reduction in this interaction could be due to the decrease in total DHC protein levels, which could suggest that SPAG1 plays a role in dynein heavy chain formation and folding. If SPAG1 is instead only required for the binding of DHCs to the DIC complex, the reduction in total protein DHC levels could be attributed to the degradation of unstable DHCs not present in dynein complexes. Further new evidence for this was obtained by co-immunoprecipitations of endogenous SPAG1 indicating interactions between SPAG1 and ODA DHCs, including DNAH5 and DNAH9, IDA g heavy chain, DNAH6 and ODA dynein intermediate chain, DNAI1. It was previously suggested that DNAAF1 and DNAAF2 were involved in the folding of DHCs, with DNAAF3 facilitating the stabilization or interaction of DHCs to the DICs (Fowkes and Mitchell, 1998; Omran et al., 2008; Mitchison et al., 2012). In Wdr92 mutant strains of Chlamydomonas reinhardtii, a significant decrease in the presence of ODA and IDA heavy chains was observed, and the intermediate chain complex was still present (Liu et al., 2019; Patel-King et al., 2019). As SPAG1 has potential interactions with DNAAF1, DNAAF2, DNAAF3 and WDR92, these previous studies further suggest that SPAG1 plays a role in the formation, folding, stabilization and/or interaction of DHCs to the DIC complex.
In summary, in normal airway epithelium, full-length SPAG1 likely forms R2TP-like complexes with RUVBL1-RUVBL2, PIH1D2 and/or DNAAF2. SPAG1 can then interact further with chaperones HSP70, HSP90 and possibly CCT through WDR92, and other DNAAFs, such as DNAAF1, DNAAF3 and WDR92, as client adaptors. This co-chaperone complex then facilitates the formation of axonemal dynein arms by folding, stabilizing and/or binding DHCs to the DIC complex (Fig. 7A). In PCD cells lacking the full-length and 94-kDa isoforms of SPAG1, the expressed 60-kDa isoform partially compensates for the lack of full-length SPAG1 and results in the assembly of some ODAs (Fig. 7B). In PCD cells that lack all isoforms of SPAG1, the R2TP-like complexes cannot form. This leads to inhibition of the folding and interaction of DHCs, causing impairment of dynein arm assembly, and the unstable dynein complex components are eventually degraded (Fig. 7C).
MATERIALS AND METHODS
Subjects
Protocols involving human studies were approved by the Institutional Review Board at the University of North Carolina and were performed in compliance with ethical regulations. Written informed consent was acquired from the PCD-affected individuals, their parents and other participants. All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki.
Genetic analysis
Using WES as described previously (Knowles et al., 2013), genetic variants in SPAG1 were identified in family UNC-372. WES was carried out for the probands in families UNC-68 and UNC-1231 at the Yale Center for Genome Analysis. Briefly, 1 μg of genomic DNA was sheared at ∼140 or ∼200 bp, captured with Nimblegen SEqCapEZ Exome Library v2 (Roche, Pleasanton, CA, USA) or with a modified Integrated DNA Technologies xGen Exome Research Panel v1.0 (Roche), for proband UNC-68 III-2 or UNC-1231 II-1, respectively. For proband UNC-68 III-2 or UNC-1231 II-1, respectively, captured fragments were sequenced using 76-bp or 101-bp paired-end sequencing reads with a NovaSeq 2000 according to the manufacturer's instructions or with an Illumina NovaSeq 6000 with a S4 flowcell according to Illumina protocols. Sequencing reads were aligned to human genome build 37 (GRCh37/hg19) using the Burrows–Wheeler aligner-Maximal Exact Match (BWA-MEM) (Li, 2013 preprint), aggregated into a BAM file, and further processed to produce variants using the Genome Analysis Toolkit (GATK) v3.4 (McKenna et al., 2010) following the GATK best practices workflow (van der Auwera et al., 2013). Variants were annotated using ANNOVAR (Wang et al., 2010), and MetaSVM (Dong et al., 2015) was used to predict the deleteriousness of non-synonymous variants. For UNC-68 III-2, an average 61.8% mean fold coverage was achieved, with 90.4% covered at ≥8×. For UNC-1231 II-1, an average 54.6% mean fold coverage was achieved, with 98.5% covered at ≥8×. After the removal of duplicates and common variants [minor allele frequency of <0.1% in the Genome Aggregation Database (gnomAD, https://gnomad.broadinstitute.org/)], a manual review of the WES dataset was performed for all currently known PCD-associated genes (Table S1). Exonic variants including missense, nonsense, frameshift, in-frame, 1 bp at the start and end of an exon, and intronic variants ∼15 bp on either side of an exon, including canonical splice-site, were reviewed. Candidate variants were confirmed by PCR amplification followed by Sanger sequencing.
For UNC-68 III-2, a single pathogenic variant in the SPAG1 gene was identified. Manual inspection of BAM files revealed certain gaps in WES, and hence, Sanger sequencing was carried out for SPAG1 as reported previously (Knowles et al., 2013). This led to the identification of another likely pathogenic variant. For UNC-1231 II-1, WES analysis identified a single VUS in an intron, close to the extended splice site in SPAG1. PCR to confirm the POLR2K and SPAG1 deletion in family UNC-1231 was performed as described previously (Knowles et al., 2013). Primers used for the POLR2K and SPAG1 deletion confirmation sequencing are listed in Table S12. Segregation analysis revealed that genetic variants identified in all three families were inherited in trans (Fig. 1C).
Cell cultures
All cells and cell lines were acquired from the Marsico Lung Institute Tissue and Procurement and Cell Culture Core, unless otherwise stated. HBECs were acquired using protocols approved by the University of North Carolina Institutional Review Board. Normal HBECs obtained from non-smoking male and female donors were cultured at an ALI as described previously (Fulcher and Randell, 2013). HBEC cultures are considered fully differentiated after 28 days in culture (ALI day 26).
HNECs were obtained from probands UNC-68 III-2, UNC-372 III-1, UNC-1231 II-1 and controls through nasal scrapes as described previously (Müller et al., 2013). HNECs were processed using Accutase (Sigma-Aldrich, St Louis, MO, USA) and were expanded in conditionally reprogrammed cell conditions, as described previously, or using Pneumacult medium (Stem Cell Technologies, Vancouver, BC, Canada) (Suprynowicz et al., 2012; Bustamante-Marin et al., 2019). Briefly, irradiated 3T3-J2 fibroblasts were seeded on PureCol-coated culture dishes 12-24 h before co-culture with HNECs. Cells were expanded in conditionally reprogrammed cell (CRC) medium supplemented with 5 μM of the Rho-associated kinase (ROCK) inhibitor Y-27632 (Sigma-Aldrich), which is referred to as CRC+Y medium. Base CRC medium is composed of a 3:1 volume ratio of Dulbecco's modified Eagle's medium supplemented with 4.5 g/l D-glucose, L-glutamine, 110 mg/l sodium pyruvate (Gibco, Waltham, MA, USA) and Ham's F12 Nutrient Mix (Gibco, Waltham, MA, USA), with 1× penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA), 0.025 μg/ml hydrocortizone (Sigma-AldrichA), 0.125 ng/ml epidermal growth factor (Invitrogen, Waltham, MA, USA), 5 μg/ml insulin (Sigma-Aldrich), 0.25 μg/ml amphotericin B (Thermo Fisher Scientific, Waltham, MA, USA), 10 μg/ml gentamicin (Gibco), 0.1 nM cholera toxin (Sigma-Aldrich) and 10% fetal bovine serum (FBS) (Corning, Corning, NY, USA). Once colonies were formed and almost confluent, HNECs were passaged and seeded onto collagen-coated Millicell inserts (Millipore, Burlington, MA, USA). Once the cultures became confluent, the basolateral medium was changed to ALI medium, and the cultures were differentiated under ALI conditions as described previously. Using PneumaCult medium according to manufacturer's protocols (Stem Cell Technologies, Vancouver, BC, Canada), PneumaCult-Expand Plus medium replaced the CRC+Y medium, and PneumaCult-ALI medium substituted the ALI medium in these procedures. HNECs were cultured at least twice at separate times for each experiment using HNECs.
HEK293T cells were cultured in Dulbecco's modified Eagle medium (Gibco, Waltham, MA, USA) supplemented with 4.5 g/l D-glucose, L-glutamine, 110 mg/l sodium pyruvate, 10% fetal bovine serum (FBS) (Corning, Corning, NY, USA) and 1× penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA).
RT-PCR, cloning and sequencing
RT-PCR was performed to determine the effect of the VUS (c.1097-11C>G) identified in IVS10 in family UNC-1231 on SPAG1 RNA transcripts. In brief, total RNA was isolated using an RNeasy Kit (Qiagen, Germantown, MD, USA) according to manufacturer's protocols. Using SuperScript II Reverse Transcriptase (Thermo Fisher Scientific), first-strand cDNA was synthesized. PCR was performed using Terra PCR Direct Red Dye Premix (TaKaRa, San Jose, CA, USA). PCR products were electrophoresed on a 1% agarose gel, and isolated using a QIAquick Gel Extraction Kit (Qiagen) according to manufacturer's protocols. Isolated PCR product from proband UNC-1231 II-1 was cloned into a pCR 2.1-TOPO TA vector using a TOPO TA Cloning Kit (Thermo Fisher Scientific). Cloned vectors were transformed into One Shot Chemically TOP10 Competent Escherichia coli cells using heat shock, and were cultured at 37°C overnight. Colonies were selected and subjected to colony PCR, and ten positive colonies were liquid cultured overnight at 37°C. Plasmids were isolated using a QIAprep Spin Midiprep Kit (Qiagen) and were Sanger sequenced. The acquired sequences were compared to SPAG1 mRNA reference sequence [National Center for Biotechnology Information (NCBI), NM_003114.5] using BLAST and SnapGene analysis. The primers used are listed in Table S12.
Ciliary beat frequency measurements
Ciliary beat frequency was measured as described previously (Sears et al., 2015). In brief, three cultures of each experimental group were individually visualized using a Nikon Eclipse TE2000 inverted microscope with phase optics and a 20× objective (NA, 0.45). Using a Tokai HIT controller (model INU-TIZ-F1) and a microscope stage-heater block, the temperature was maintained at 37°C. High-speed videos (60 frames/s) were recorded using a Basler acA1300-200 μm camera controlled by SAVA software (Ammons Engineering). CBF videos were analyzed using SAVA whole-field analysis.
5′ RACE
The 5′ RACE assay was performed on undifferentiated and differentiated HBEC total RNA using a SMARTer 5′/3/RACE Kit according to manufacturer's protocols (TaKaRa, San Jose, CA, USA). In brief, total RNA was isolated using an RNeasy Kit (Qiagen). The 5′ RACE-ready cDNA was synthesized using SMARTScribe Reverse Transcriptase (TaKaRa). Using two different gene-specific reverse primers (GSP1 and GSP2) and the Universal Primer Mix, 5′ RACE was performed following the manufacturer's touchdown PCR cycling conditions. The 5′ RACE products were electrophoresed on a 1% agarose gel, and isolated using a NucleoSpin Gel and PCR Clean-Up Kit (TaKaRa). Isolated 5′ RACE products were cloned into the provided pRACE vector using an In-Fusion Cloning Kit (TaKaRa). Stellar Competent Cells (TaKaRa) were transformed with 5′ RACE product vectors using heat shock and cultured overnight at 37°C. Colonies were selected and subjected to colony PCR, and positive colonies were liquid cultured overnight at 37°C. Plasmids were isolated using a QIAprep Spin Midiprep Kit (Qiagen) and Sanger sequenced. The 5′ RACE sequences were compared to SPAG1 genomic sequence (NCBI, NG_033834.2) using BLAST and SnapGene analysis. Sequences of the primers used are listed in Table S12.
Droplet digital PCR
ddPCR was used to measure mRNA expression as described previously (van Heetvelde et al., 2017). Briefly, total RNA was isolated from differentiating HNEC cultures on various days in culture using an RNeasy Kit (Qiagen). Using SuperScript II Reverse Transcriptase (Thermo Fisher Scientific), first-strand cDNA was synthesized. At least 10,000 droplets were generated, using a Bio-Rad QX200 Automated Droplet Generator, from 20 μl of ddPCR reaction mixes consisting of 10 ng of cDNA, 250 nM primers, 900 nM probes and 1× ddPCR Supermix for Probes (No dUTP) (Bio-Rad, Hercules, CA, USA). Target gene probes were fluorescently labeled with FAM, whereas the reference gene (calreticulin) probe was labeled with HEX. All primers and probes were obtained from Bio-Rad, and their relative sequences are listed in Table S12. PCR reactions were performed using the cycling conditions provided in the manufacturer's protocols. After PCR, the fluorescent positive and negative droplets were identified using a Bio-Rad QX200 Droplet Reader (Bio-Rad). Calculation of the target concentration was completed using Quantasoft software (Bio-Rad), after normalization to the concentration of the reference gene calreticulin. The specific primer/probe assay for the full-length and 94-kDa SPAG1 isoform was generated using Primer3Plus, and custom ordered from Bio-Rad. Validation of this custom assay was performed by running ddPCR on undifferentiated and differentiated HBEC cDNA samples, but instead of reading the droplets using a Bio-Rad QX200 Droplet Reader, amplicons were isolated using chloroform, as described previously, and Sanger sequenced (Bio-Rad, 2018). Quantification of the levels of the 60-kDa SPAG1 isoform was calculated by subtracting the levels of the full-length and 94-kDa isoforms measured by Bio-Rad assay dHsaCPE5033992 from the levels of all SPAG1 isoforms measured by our custom Bio-Rad assay for exons 14 and 15.
Immunoblots
HBECs and HNECs were lysed in RIPA buffer (Thermo Fisher Scientific) supplemented with protease inhibitor cocktail (Sigma-Aldrich). Cilia were isolated from HBECs using methods described previously (Ostrowski et al., 2002). Using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific), the protein concentration of lysates was determined. Samples (5-20 µg) of either whole-cell lysates, isolated cilia or co-immunoprecipitation eluates were electrophoresed on Novex NuPAGE 4-12% Bis-Tris gels in 1× NuPAGE MOPS-SDS Running Buffer (Thermo Fisher, Waltham, MA, USA) and then transferred to 0.45-μm nitrocellulose membranes using 1× NuPAGE Transfer Buffer. For immunoblotting for DHCs, samples were electrophoresed on Novex NuPAGE 3–8% Tris-Acetate gels in 1× NuPAGE Tris-Acetate Running Buffer (Thermo Fisher Scientific). After transfer, membranes were stained with REVERT Total Protein Stain (LI-COR, Lincoln, NE, USA), imaged using a LI-COR Odyssey Scanner, destained and then blocked in 5% non-fat milk in 1× TBS solution for 1 h at room temperature. Membranes were then incubated in primary and secondary antibody solutions in 5% non-fat milk in 1× TBS plus 0.1% Tween 20 (TBST) for 1 h at room temperature or 4°C overnight, with washes with 1× TBST after each incubation. Fluorescent membranes were then scanned using a LI-COR Odyssey Scanner, or chemiluminescent membranes were probed using Amersham ECL Plus reagents (GE Healthcare, Chicago, IL, USA) according to manufacturer's protocol. All antibodies used and their dilutions are listed in Table S11.
Quantitative analysis was performed on fluorescent immunoblots scanned by a LI-COR Odyssey Scanner using ImageStudio Lite software (LI-COR). For time-course expression studies on HBECs, background-subtracted signals were normalized to a total protein stain, and then normalized to a positive control (diff. control). For immunoblots examining levels of SPAG1 and dynein chains in HNECs, background-subtracted signals were normalized to a total protein stain, followed by normalization to levels of RSPH1 as a cilia control if the protein is a dynein chain, before their normalization to a positive HBEC control to control for variations across immunoblots. Normal control means were then set to 1.0. Immunoblot images were processed and their brightness/contrast adjusted for publication with FIJI software. Experiments involving HNECs were repeated in whole beginning from cell culture, twice (n=2), and depending on the immunoblot and availability of lysates, at least two technical replicates were averaged for each n. Full immunoblot images are shown in Fig. S5.
Immunofluorescence and confocal microscopy
Immunofluorescent staining of isolated human airway epithelial cells was performed as described previously (Blackburn et al., 2017). Briefly, isolated cells from HBEC or HNEC cultures in 1× PBS were placed on 1% Alcian Blue (Sigma-Aldrich)-treated slides/cover slips and allowed to settle on the slides/cover slips for 20 min on ice. Cells were fixed for 10 min with 2% paraformaldehyde or methanol. After washing with 1× PBS twice, cells were permeabilized with 0.2% Triton X-100 in PBS for 15 min. Cells were then incubated in blocking solution [5% donkey serum, 3% bovine serum albumin (BSA) and 0.1% Triton X-100 in 1× PBS] for 1 h at room temperature. Samples were incubated in primary antibody diluted in blocking solution either for 1 h at room temperature or overnight at 4°C. After washing with 1× PBS three times, cells were incubated in secondary antibody solutions for 2 h at room temperature, and then washed with 1× PBS four times.
Whole-mount staining of ALI cultures was performed as described previously (Bustamante-Marin et al., 2019). Briefly, washed ALI cultures were fixed with 4% paraformaldehyde or methanol for 20 min, and permeabilized with 0.2% Triton X-100 in 1× TBS for 30 min at room temperature. Cultures were then incubated in blocking solution (1% BSA, 1% fish gelatin, 0.1% Triton X-100 and 5% donkey serum in 1× TBS) for 1 h at room temperature. Cultures were incubated in primary and secondary antibody solutions (antibodies diluted in blocking solution) for 1 h each at room temperature, with three washes (15 min each) with washing solution (0.25% BSA, 0.25% fish gelatin, 0.025% Triton X-100 and 1.25% donkey serum in 1× TBS) or 1× TBS after each antibody incubation.
For both protocols, nuclei were stained using DNA dyes SYTO13 or Hoechst 33342 (Thermo Fisher Scientific). Isolated cell or whole-mount membranes were mounted on slides with ProLong Gold or Diamond Antifade Reagent (Thermo Fisher Scientific). Slides were imaged using a Leica SP5 inverted confocal microscope or a Zeiss 800 upright confocal microscope with a 63×/1.4 oil objective. No detectable staining was observed for isotype-matched antibody or no primary antibody control slides. All images were processed using FIJI software and pseudocolored, and brightness/contrast was adjusted evenly across subjects per experiment for publication. All antibodies used and their dilutions are listed in Table S11.
Immunoprecipitation
Immunoprecipitations for endogenous SPAG1, DNAAF1, DNAAF2, PIH1D2, DNAI1 and exogenous FLAG-tag were performed using a Pierce Crosslink IP Kit (Thermo Fisher Scientific) according to manufacturer's protocols. In essence, 5-10 µg of bait antibody or control IgG was coupled and crosslinked to protein A/G plus agarose resin in spin columns by incubating with Coupling Buffer and 2.5 mM disuccinimidyl suberate in DMSO for 45 min each at room temperature. HBEC and HNEC cultures were lysed using IP Lysis/Wash Buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich), and protein concentration was determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Cell lysates were precleared by incubating with control agarose resin for 1 h at 4°C. Similar amounts of protein from precleared lysates were added to columns containing resin crosslinked to bait antibody or IgG control, and these columns were incubated overnight at 4°C. Columns were washed multiple times with IP Lysis/Wash Buffer and 1× Conditioning Buffer, followed by elution of eluates using Elution Buffer or 4× LDS Sample Buffer. Eluates were examined by immunoblotting as described above or by using mass spectrometry as described below.
Immunoprecipitation for exogenous FLAG-tagged SPAG1 in HEK293T co-expressing HA-tagged DNAAF2 were also performed using the Pierce Crosslink IP Kit (Thermo Fisher Scientific). Before immunoprecipitation, HEK293T cells were transfected with FLAG-tagged SPAG1 and HA-tagged constructs using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific). Next, 24 h after transfection, HEK293T cells were lysed using RIPA Buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich), followed by immunoprecipitation for the FLAG-tag.
Quantitative analysis of protein interactions was performed by analyzing the level of protein co-precipitated with the bait protein compared to a control on immunoblots using ImageStudio Lite (LI-COR). Specifically, background-subtracted signals were normalized to the amount of bait protein that was eluted in each sample, and then compared with the control, which was normalized to 1.0.
Mass spectrometry
For targeted proteomics on 60-kDa SPAG1, undifferentiated and differentiated HBEC cultures were lysed using RIPA buffer supplemented with protease inhibitor cocktail. Protein lysates or co-immunoprecipitation eluates were loaded and electrophoresed on Novex 4-12% Bis-Tris Nu-PAGE gradient gels, and stained with GelCode Coomassie Blue Stain Reagent (Thermo Fisher Scientific). For targeted proteomics, gel sections were excised corresponding to proteins between 50-75 kDa. Gel-isolated proteins were reduced, alkylated and in-gel digested with trypsin overnight at 37°C. Peptides were extracted, desalted with C18 spin columns (Pierce, Appleton, WI, USA) and dried via vacuum centrifugation.
Peptide samples were analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS) using an Easy nLC 1000 coupled to a QExactive HF (Thermo Scientific, Waltham, MA, USA) as described previously (Sun et al., 2020). Samples were injected onto an Easy Spray PepMap C18 column (75 μm id×25 cm, 2 μm particle size; Thermo Scientific) and separated over a 120 min method. The gradient for separation consisted of 5-35% mobile phase B at a 250 nl/min flow rate, where mobile phase A was 0.1% formic acid in water and mobile phase B consisted of 0.1% formic acid in acetonitrile. For untargeted analysis, the QExactive HF was operated in data-dependent mode, where the 15 most intense precursors were selected for subsequent higher-energy C-trap dissociation (HCD) fragmentation. Resolution for the precursor scan (m/z 400-1600) was set to 120,000 with an automatic gain control (AGC) target value of 3×106 ions, 100 ms inject time. MS/MS scans resolution was set to 15,000 with an AGC target value of 5×104 ions, 50 ms inject time. The normalized collision energy was set to 27% for HCD, with an isolation window of 1.6 m/z. Peptide match was set to preferred, and precursors with unknown charge or a charge state of 1 and ≥7 were excluded. Targeted analysis of SPAG1 peptides was conducted on the same LC-MS system as above using parallel reaction monitoring (PRM). Peptides were eluted over a 65 or 120 min method, and the QExactive was operated in PRM mode; an inclusion list was used to target ten previously identified SPAG1 peptides. MS1 scans (350-1600 m/z) were collected at a 120,000 resolution, a 2×106 AGC target value and a 200-ms injection time. MS/MS (PRM) scans resolution was set to 15,000, with an isolation window of 2 m/z, a 1×106 AGC target value and a 100-ms injection time.
Raw data files were processed using Proteome Discoverer version 1.4 or 2.1 (Thermo Scientific). Peak lists were searched against a reviewed UniProt human database, appended with a common contaminants database, using Sequest. The following parameters were used to identify tryptic peptides for protein identification: 10 ppm precursor ion mass tolerance; 0.02 Da product ion mass tolerance; up to two missed trypsin cleavage sites; carbamidomethylation of Cys was set as a fixed modification; and oxidation of Met and acetylation of the N terminus were set as variable modifications. Scaffold (version 4.7.3, Proteome Software) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95% probability to achieve a false discovery rate (FDR) less than 0.1% by the Scaffold Local FDR algorithm. Protein identifications were accepted if they could be established at greater than 99% probability and contained at least two identified peptides. Relative quantitation was performed using the calculated quantitative values (spectral counts) within Scaffold. Positive hits for co-immunoprecipitations were narrowed down using the following parameters: a significant P-value (P<0.05) calculated by a Fisher's exact test; a fold change of at least 2 between the target and IgG eluates; and at least two unique peptides per protein in each replicate.
Creation of pcDNA3.1(+)-hSPAG1[60 kDa]-FLAG plasmid
The pcDNA3.1(+)-hSPAG1[wt]-FLAG and pcDNA3.1(+)-hDNAAF2-HA vectors were designed and purchased from GenScript Biotech (Piscataway, NJ, USA). The pcDNA3.1(+)-hSPAG1[60 kDa]-FLAG vector was created using primers that flank nucleotides that correspond to amino acids 1-381 in full-length SPAG1 and a Q5 Site-Directed Mutagenesis Kit Protocol (New England Biolabs, Ipswich, MA, USA) according to the manufacturer's protocols. Briefly, PCR using Q5 Hot Start High-Fidelity 2× Master Mix and the primers that flank the region of SPAG1 absent in the 60-kDa SPAG1 on the pcDNA3.1(+)-hSPAG1[wt]-FLAG construct led to a linearized vector containing a SPAG1 insert that only contained a transcript for the 60-kDA isoform. Incubation of this product with the KLD Enzyme and Reaction Buffer led to the ligation of this construct, and depletion of the original construct. The ligated construct was transformed into NEB 5-α Competent E. coli cells using heat shock, and transformed cells were cultured on selective Luria–Bertani agar plates overnight at 37°C. Colonies were picked and subjected to colony PCR, and positive colonies with expected transcript size were incubated in liquid cultures overnight at 37°C. Plasmids were isolated using a Qiagen Plasmid Maxiprep Kit. Isolated plasmids had their sequences confirmed by Sanger sequencing. Transfection and expression of this construct in HEK293T cells using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific) was optimized and confirmed by immunoblotting. Primers that were used in the creation and confirmation of this construct are listed in Table S12.
Statistics
Data are expressed as the mean±s.d. in all experiments. Experiments involving HBECs and HEK293T cells were performed with at least three independent biological replicates, except proteomic studies, which were performed twice. Experiments involving HNECs were performed as at least two independent replicates, in whole beginning with cell culture at separate times due to limits on the availability of nasal cells. Owing to the small sample size and the possibility of a non-normal distribution, non-parametric statistical tests were used for the HNECs experiments. The following statistical methods were calculated using Prism (GraphPad) or Scaffold software: ANOVA with Tukey's multiple comparisons; Kruskal–Wallis test with uncorrected Dunn's multiple comparisons; linear regression analysis; Fisher's exact test; and a two-tailed one-sample t-test. Figure legends specify the statistical method and P-values for each experiment. P<0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank the PCD subjects and family members for their participation in this study; the investigators and coordinators, especially Kelli Sullivan, of the Genetic Disorders of Mucociliary Clearance Consortium, part of the Rare Disease Clinical Research Network, and the US PCD Foundation; Kimberly Burns for histology and electron microscopy of nasal cilia; Jaclyn Stonebraker and Whitney Wolf for technical help with genetic studies; Hong Dang for bioinformatics assistance; Agathe Ceppe for biostatistics assistance; Robert Currin and the University of North Carolina (UNC) Hooker Imaging Core for providing the Zeiss 800 confocal microscope; Michael Chua and Robert Currin for confocal imaging assistance; Wanda O'Neal, Lisa C. Morton, Rodney Gilmore and the Marisco Lung Institute Molecular Biology Core for assistance with, and providing reagents for, ddPCR and cloning; and Scott H. Randell and the Marsico Lung Institute Tissue Procurement and Cell Culture Core for providing the human bronchial epithelial cells, HEK293T cells and 3T3-J2 cells.
The Yale Center for Mendelian Genomics is funded by the National Human Genome Research Institute (UM1HG006504). The Genome Sequencing Program Coordinating Center (U24 HG008956) contributed to cross-program scientific initiatives and provided logistical and general study coordination. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Genetic Disorders of Mucociliary Clearance Consortium (U54HL096458) is part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research funded through a collaboration between NCATS and NHLBI. This research is based in part upon work conducted using the University of North Carolina Proteomics Core Facility, which is supported in part by a National Cancer Institute Cancer Center Core Support Grant to the University of North Carolina Lineberger Comprehensive Cancer Center (P30 CA016086). The University of North Carolina Marsico Lung Institute Molecular Biology Core is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; P30-DK065988) and the Cystic Fibrosis Foundation (BOUCHE15R0). The University of North Carolina Marsico Lung Institute Tissue Procurement and Cell Culture Core is supported by the Cystic Fibrosis Foundation (BOUCHE19R0) and the NIH (P30-DK065988).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.J.S., X.M.B.-M., W.Y., P.R.S., L.E.O.; Methodology: A.J.S., X.M.B.-M., W.Y., P.R.S., L.E.H., F.L.-G., S.M., M.A.Z., L.E.O.; Software: R.T.; Validation: A.J.S.; Formal analysis: A.J.S., L.E.H., F.L.-G., S.M., M.A.Z., L.E.O.; Investigation: A.J.S., L.E.H., N.N.D., F.L.-G., S.M., M.A.Z.; Resources: W.Y., L.E.H., R.T., M.W.L., M.R.K., M.A.Z., L.E.O.; Data curation: A.J.S., L.E.H., N.N.D., F.L.-G., S.M., M.W.L., M.R.K., M.A.Z.; Writing - original draft: A.J.S.; Writing - review & editing: A.J.S., X.M.B.-M., W.Y., P.R.S., L.E.H., N.N.D., F.L.-G., S.M., R.T., M.W.L., M.R.K., M.A.Z., L.E.O.; Visualization: A.J.S.; Supervision: L.E.O.; Funding acquisition: A.J.S., M.R.K., L.E.O.
Funding
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH; F31HL142170 to A.J.S., R01HL117836 to L.E.O. and R01HL071798 to M.R.K.). Deposited in PMC for release after 12 months.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD032349.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.259512.
References
- Austin-Tse, C., Halbritter, J., Zariwala, M. A., Gilberti, R. M., Gee, H. Y., Hellman, N., Pathak, N., Liu, Y., Panizzi, J. R., Patel-King, R. S.et al. (2013). Zebrafish ciliopathy screen plus human mutational analysis identifies C21orf59 and CCDC65 defects as causing primary ciliary dyskinesia. Am. J. Hum. Genet. 93, 672-686. 10.1016/j.ajhg.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbahouche, N. E. H., Iliopoulos, I., Török, I., Marhold, J., Henri, J., Kajava, A. V., Farkaš, R., Kempf, T., Schnölzer, M., Meyer, P.et al. (2014). Drosophila Spag is the homolog of RNA polymerase II-associated protein 3 (RPAP3) and recruits the heat shock proteins 70 and 90 (Hsp70 and Hsp90) during the assembly of cellular machineries. J. Biol. Chem. 289, 6236-6247. 10.1074/jbc.M113.499608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann, K., Heukamp, L. C., Steger, K., Zhou, H., Franke, F. E., Sonnack, V., Brehm, R., Berg, J., Bastian, P. J. and Müller, S. C. (2007). Genome-wide expression profiling reveals new insights into pathogenesis and progression of testicular germ cell tumors. Cancer Genomics Proteomics 4, 359-367. [PubMed] [Google Scholar]
- Bio-Rad. (2018). Droplet DigitalTM PCR Droplet DigitalTM PCR Applications Guide. Bulletin 6407, 1-145. [Google Scholar]
- Blackburn, K., Bustamante-Marin, X., Yin, W., Goshe, M. B. and Ostrowski, L. E. (2017). Quantitative proteomic analysis of human airway cilia identifies previously uncharacterized proteins of high abundance. J. Proteome Res. 16, 1579-1592. 10.1021/acs.jproteome.6b00972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante-Marin, X. M., Yin, W.-N., Sears, P. R., Werner, M. E., Brotslaw, E. J., Mitchell, B. J., Jania, C. M., Zeman, K. L., Rogers, T. D., Herring, L. E.et al. (2019). Lack of GAS2L2 causes PCD by impairing cilia orientation and mucociliary clearance. Am. J. Hum. Genet. 104, 229-245. 10.1016/j.ajhg.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagot, M.-E., Santos Morais, R. D., Dermouche, S., Lefebvre, D., Manival, X., Chipot, C., Dehez, F. and Quinternet, M. (2019). Binding properties of the quaternary assembly protein SPAG1. Biochem. J. 476, 1679-1694. 10.1042/BCJ20190198 [DOI] [PubMed] [Google Scholar]
- Dafinger, C., Rinschen, M. M., Borgal, L., Ehrenberg, C., Basten, S. G., Franke, M., Höhne, M., Rauh, M., Göbel, H., Bloch, W.et al. (2018). Targeted deletion of the AAA-ATPase Ruvbl1 in mice disrupts ciliary integrity and causes renal disease and hydrocephalus. Exp. Mol. Med. 50, 1-17. 10.1038/s12276-018-0108-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. D., Rosenfeld, M., Lee, H.-S., Ferkol, T. W., Sagel, S. D., Dell, S. D., Milla, C., Pittman, J. E., Shapiro, A. J., Sullivan, K. M.et al. (2019). Primary ciliary dyskinesia: longitudinal study of lung disease by ultrastructure defect and genotype. Am. J. Respir. Crit. Care Med. 199, 190. 10.1164/rccm.201803-0548OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- de longh, R. U. and Rutland, J. (1995). Ciliary defects in healthy subjects, bronchiectasis, and primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 151, 1559-1567. 10.1164/ajrccm.151.5.7735615 [DOI] [PubMed] [Google Scholar]
- Dermouche, S., Chagot, M.-E., Manival, X. and Quinternet, M. (2021). Optimizing the first TPR domain of the human SPAG1 protein provides insight into the HSP70 and HSP90 binding properties. Biochemistry 60, 2349-2363. 10.1021/acs.biochem.1c00052 [DOI] [PubMed] [Google Scholar]
- Desai, P. B., Dean, A. B. and Mitchell, D. R. (2017). Cytoplasmic preassembly and trafficking of axonemal dyneins. In The Biology of Dynein Motors (ed. King S.). Cambridge, MA: Elsevier, Inc. [Google Scholar]
- Diggle, C. P., Moore, D. J., Mali, G., Zur Lage, P., Ait-Lounis, A., Schmidts, M., Shoemark, A., Munoz, A. G., Halachev, M. R., Gautier, P.et al. (2014). HEATR2 plays a conserved role in assembly of the ciliary motile apparatus. PLoS Genet. 10, e1004577. 10.1371/journal.pgen.1004577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C., Wei, P., Jian, X., Gibbs, R., Boerwinkle, E., Wang, K. and Liu, X. (2015). Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 24, 2125-2137. 10.1093/hmg/ddu733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, S., Huang, W., Liu, X., Liu, X., Chen, N., Xu, Q., Hu, Y., Song, W. and Zhou, J. (2018). IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J. Exp. Clin. Cancer Res. 37, 304. 10.1186/s13046-018-0980-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf, M., Olbrich, H., Horvath, J., Wildhaber, J. H., Zariwala, M. A., Kennedy, M., Knowles, M. R. and Omran, H. (2005). Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 171, 1343-1349. 10.1164/rccm.200411-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes, M. E. and Mitchell, D. R. (1998). The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol. Biol. Cell 9, 2337-2347. 10.1091/mbc.9.9.2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher, M. L. and Randell, S. H. (2013). Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 945, 109-121. 10.1007/978-1-62703-125-7_8 [DOI] [PubMed] [Google Scholar]
- Hartill, V. L., van de Hoek, G., Patel, M. P., Little, R., Watson, C. M., Berry, I. R., Shoemark, A., Abdelmottaleb, D., Parkes, E., Bacchelli, C.et al. (2018). DNAAF1 links heart laterality with the AAA 1 ATPase RUVBL1 and ciliary intraflagellar transport. Hum. Mol. Genet. 27, 529-545. 10.1093/hmg/ddx422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höben, I. M., Hjeij, R., Olbrich, H., Dougherty, G. W., Nöthe-Menchen, T., Aprea, I., Frank, D., Pennekamp, P., Dworniczak, B., Wallmeier, J.et al. (2018). Mutations in C11orf70 cause primary ciliary dyskinesia with randomization of left/right body asymmetry due to defects of outer and inner dynein arms. Am. J. Hum. Genet. 102, 973-984. 10.1016/j.ajhg.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horani, A., Ustione, A., Huang, T., Firth, A. L., Pan, J., Gunsten, S. P., Haspel, J. A., Piston, D. W. and Brody, S. L. (2018). Establishment of the early cilia preassembly protein complex during motile ciliogenesis. Proc. Natl. Acad. Sci. USA 115, E1221-E1228. 10.1073/pnas.1715915115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houry, W. A., Bertrand, E. and Coulombe, B. (2018). The PAQosome, an R2TP-based chaperone for quaternary structure formation. Trends Biochem. Sci. 43, 4-9. 10.1016/j.tibs.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Hu, P., Guan, K., Feng, Y., Ma, C., Song, H., Li, Y., Xia, X., Li, J. and Li, F. (2017). miR-638 Inhibits immature Sertoli cell growth by indirectly inactivating PI3K/AKT pathway via SPAG1 gene. Cell Cycle 16, 2290-2300. 10.1080/15384101.2017.1380130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C., Wu, D., Khan, F. A., Jiao, X., Guan, K. and Huo, L. (2016). The GTPase SPAG-1 orchestrates meiotic program by dictating meiotic resumption and cytoskeleton architecture in mouse oocytes. Mol. Biol. Cell 27, 1776-1785. 10.1091/mbc.e16-02-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar, R. L., Lee, C., Boulgakov, A. A., Horani, A., Tu, F., Marcotte, E. M., Brody, S. L. and Wallingford, J. B. (2018). A liquid-like organelle at the root of motile ciliopathy. eLife 7, e38497. 10.7554/eLife.38497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsuki, Y., Saeki, M., Nakahara, H., Egusa, H., Irie, Y., Terao, Y., Kawabata, S., Yatani, H. and Kamisaki, Y. (2008). Molecular cloning of novel Monad binding protein containing tetratricopeptide repeat domains. FEBS Lett. 582, 2365-2370. 10.1016/j.febslet.2008.05.041 [DOI] [PubMed] [Google Scholar]
- Kamiya, R. (2002). Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int. Rev. Cytol. 219, 115-155. 10.1016/S0074-7696(02)19012-7 [DOI] [PubMed] [Google Scholar]
- Kasioulis, I., Das, R. M. and Storey, K. G. (2017). Inter-dependent apical microtubule and actin dynamics orchestrate centrosome retention and neuronal delamination. eLife 6, e26215. 10.7554/eLife.26215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S. M. (2016). Axonemal dynein arms. Cold Spring Harb. Perspect. Biol. 8, a028100. 10.1101/cshperspect.a028100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles, M. R., Ostrowski, L. E., Loges, N. T., Hurd, T., Leigh, M. W., Huang, L., Wolf, W. E., Carson, J. L., Hazucha, M. J., Yin, W.et al. (2013). Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am. J. Hum. Genet. 93, 711-720. 10.1016/j.ajhg.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmar, M. (2016). Fine-tuning motile cilia and flagella: Evolution of the dynein motor proteins from plants to humans at high resolution. Mol. Biol. Evol. 33, 3249-3267. 10.1093/molbev/msw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott, E., Duquesnoy, P., Copin, B., Legendre, M., Moal, F. D., Montantin, G., Jeanson, L., Tamalet, A., Papon, J. F., Siffroi, J.-P.et al. (2012). Loss-of-function mutations in LRRC6, a gene essential for proper axonemal assembly of inner and outer dynein arms, cause primary ciliary dyskinesia. Am. J. Hum. Genet. 91, 958-964. 10.1016/j.ajhg.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C., Cox, R. M., Papoulas, O., Horani, A., Drew, K., Devitt, C. C., Brody, S. L., Marcotte, E. M. and Wallingford, J. B. (2020). Functional partitioning of a liquid-like organelle during assembly of axonemal dyneins. Elife 9, e58662. 10.7554/eLife.58662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh, M. W., Hazucha, M. J., Chawla, K. K., Baker, B. R., Shapiro, A. J., Brown, D. E., LaVange, L. M., Horton, B. J., Qaqish, B., Carson, J. L.et al. (2013). Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann. Am. Thorac. Soc. 10, 574-581. 10.1513/AnnalsATS.201305-110OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh, M. W., Horani, A., Kinghorn, B., O'Connor, M. G., Zariwala, M. A. and Knowles, M. R. (2019). Primary ciliary dyskinesia (PCD): a genetic disorder of motile cilia. Transl. Sci. Rare Dis. 4, 51-75. 10.3233/TRD-190036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv [Preprint]. arXiv:1303.3997. [Google Scholar]
- Li, Y., Zhao, L., Yuan, S., Zhang, J. and Sun, Z. (2017). Axonemal dynein assembly requires the R2TP complex component Pontin. Development 144, 4684-4693. 10.1242/dev.152314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W., Zhou, X., Zhang, M., Li, Y., Miao, S., Wang, L., Zong, S. and Koide, S. S. (2001). Expression and function of the HSD-3.8 gene encoding a testis-specific protein. Mol. Hum. Reprod. 7, 811-818. 10.1093/molehr/7.9.811 [DOI] [PubMed] [Google Scholar]
- Lin, S., Lv, Y., Zheng, L., Mao, G. and Peng, F. (2021). Expression and prognosis of sperm-associated antigen 1 in human breast cancer. Onco. Targets. Ther. 14, 2689-2698. 10.2147/OTT.S288484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G., Wang, L., Pan, J. and Yao, X. (2019). Chlamydomonas WDR92 in association with R2TP-like complex and multiple DNAAFs to regulate ciliary dynein preassembly. J. Mol. Cell Biol. 11, 770-780. 10.1093/jmcb/mjy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loges, N. T., Olbrich, H., Becker-Heck, A., Häffner, K., Heer, A., Reinhard, C., Schmidts, M., Kispert, A., Zariwala, M. A., Leigh, M. W.et al. (2009). Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am. J. Hum. Genet. 85, 883-889. 10.1016/j.ajhg.2009.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, B., Kirsch, M., Anderhub, S., Zentgraf, H. and Krämer, A. (2013). The novel actin/focal adhesion-associated protein MISP is involved in mitotic spindle positioning in human cells. Cell Cycle 12, 1457-1471. 10.4161/cc.24602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, F., Pal, M., Muñoz-Hernández, H., Rodríguez, C. F., Núñez-Ramírez, R., Gil-Carton, D., Degliesposti, G., Skehel, J. M., Roe, S. M., Prodromou, C.et al. (2018). RPAP3 provides a flexible scaffold for coupling HSP90 to the human R2TP co-chaperone complex. Nat. Commun. 9, 1501. 10.1038/s41467-018-03942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizy, C., Quinternet, M., Abel, Y., Verheggen, C., Santo, P. E., Bourguet, M., Paiva, A. C. F., Bragantini, B., Chagot, M.-E., Robert, M. C.et al. (2018). The RPAP3-Cterminal domain identifies R2TP-like quaternary chaperones. Nat. Commun. 9, 2093. 10.1038/s41467-018-04431-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., Garimella, K., Altshuler, D., Gabriel, S., Daly, M.et al. (2010). The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297-1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison, H. M., Schmidts, M., Loges, N. T., Freshour, J., Dritsoula, A., Hirst, R. A., O'Callaghan, C., Blau, H., Al Dabbagh, M., Olbrich, H.et al. (2012). Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat. Genet. 44, 381-389. 10.1038/ng.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, D. J., Onoufriadis, A., Shoemark, A., Simpson, M. A., zur Lage, P. I., de Castro, S. C., Bartoloni, L., Gallone, G., Petridi, S., Woollard, W. J.et al. (2013). Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am. J. Hum. Genet. 93, 346-356. 10.1016/j.ajhg.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, L., Brighton, L. E., Carson, J. L., Fischer, W. A., II and Jaspers, I. (2013). Culturing of human nasal epithelial cells at the air liquid interface. J. Vis. Exp. 80, 50646. 10.3791/50646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neesse, A., Gangeswaran, R., Luettges, J., Feakins, R., Weeks, M. E., Lemoine, N. R. and Crnogorac-Jurcevic, T. (2007). Sperm-associated antigen 1 is expressed early in pancreatic tumorigenesis and promotes motility of cancer cells. Oncogene 26, 1533-1545. 10.1038/sj.onc.1209961 [DOI] [PubMed] [Google Scholar]
- Olcese, C., Patel, M. P., Shoemark, A., Kiviluoto, S., Legendre, M., Williams, H. J., Vaughan, C. K., Hayward, J., Goldenberg, A., Emes, R. D.et al. (2017). X-linked primary ciliary dyskinesia due to mutations in the cytoplasmic axonemal dynein assembly factor PIH1D3. Nat. Commun. 8, 14279. 10.1038/ncomms14279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran, H., Kobayashi, D., Olbrich, H., Tsukahara, T., Loges, N. T., Hagiwara, H., Zhang, Q., Leblond, G., O'Toole, E., Hara, C.et al. (2008). Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 456, 611-616. 10.1038/nature07471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski, L. E., Blackburn, K., Radde, K. M., Moyer, M. B., Schlatzer, D. M., Moseley, A. and Boucher, R. C. (2002). A proteomic analysis of human cilia: identification of novel components. Mol. Cell Proteomics 1, 451-465. 10.1074/mcp.M200037-MCP200 [DOI] [PubMed] [Google Scholar]
- Patel-King, R. S., Sakato-antoku, M., Yankova, M. and King, S. M. (2019). WDR92 is required for axonemal dynein heavy chain stability in cytoplasm. Mol. Biol. Cell 30, 1834-1845. 10.1091/mbc.E19-03-0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G. J., Agrin, N., Walker, B. L. and Witman, G. B. (2006). Identification of predicted human outer dynein arm genes: Candidates for primary ciliary dyskinesia genes. J. Med. Genet. 43, 62-73. 10.1136/jmg.2005.033001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riba, A. and Itzhaki, L. S. (2019). The tetratricopeptide-repeat motif is a versatile platform that enables diverse modes of molecular recognition. Curr. Opin. Struct. Biol. 54, 43-49. 10.1016/j.sbi.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Pfister, K. K. and Witman, G. B. (1984). Subfractionation of Chlamydomonas 18 S dynein into two unique subunits containing ATPase activity. J. Biol. Chem. 259, 12072-12080. 10.1016/S0021-9258(20)71321-9 [DOI] [PubMed] [Google Scholar]
- Sears, P. R., Yin, W.-N. and Ostrowski, L. E. (2015). Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L99-L108. 10.1152/ajplung.00024.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, A. J. and Leigh, M. W. (2017). Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: genetic defects with normal and non-diagnositc ciliary ultrastructure. Ultrastruct. Pathol. 41, 373-385. 10.1080/01913123.2017.1362088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, A. J., Zariwala, M. A., Ferkol, T., Davis, S. D., Sagel, S. D., Dell, S. D., Rosenfeld, M., Olivier, K. N., Milla, C., Daniel, S. J.et al. (2016). Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr. Pulmonol. 51, 115-132. 10.1002/ppul.23304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silina, K., Zayakin, P., Kalnina, Z., Ivanova, L., Meistere, I., Endzelins, E., Abols, A., Stengrēvics, A., Leja, M., Ducena, K.et al. (2011). Sperm-associated antigens as targets for cancer immunotherapy: expression pattern and humoral immune response in cancer patients. J. Immunother. 34, 28-44. 10.1097/CJI.0b013e3181fb64fa [DOI] [PubMed] [Google Scholar]
- Smith, T. C., Fang, Z. and Luna, E. J. (2010). Novel interactors and a role for Supervillin in early cytokinesis. Cytoskeleton 67, 346-364. 10.1002/cm.20481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C., Shou, P., Du, H., Hirabayashi, K., Chen, Y., Herring, L. E., Ahn, S., Xu, Y., Suzuki, K., Li, G.et al. (2020). THEMIS-SHP1 recruitment by 4-1BB tunes LCK-mediated priming of chimeric antigen receptor-redirected T cells. Cancer Cell 37, 216-225. 10.1016/j.ccell.2019.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprynowicz, F. A., Upadhyay, G., Krawczyk, E., Kramer, S. C., Hebert, J. D., Liu, X., Yuan, H., Cheluvaraju, C., Clapp, P. W., Boucher, R. C.et al. (2012). Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc. Natl. Acad. Sci. USA 109, 20035-20040. 10.1073/pnas.1213241109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi, M. and Huh, N.-H. (1999). A tetratricopeptide repeat-containing protein gene, tpis, whose expression is induced with differentiation of spermatogenic cells. Biochem. Biophys. Res. Commun. 264, 81-85. 10.1006/bbrc.1999.1477 [DOI] [PubMed] [Google Scholar]
- Tang, W. J. Y., Bell, C. W., Sale, W. S. and Gibbons, I. R. (1982). Structure of the dynein-1 outer arm in sea urchin sperm flagella. I. Analysis by separation of subunits. J. Biol. Chem. 257, 508-515. 10.1016/S0021-9258(19)68393-6 [DOI] [PubMed] [Google Scholar]
- Tarkar, A., Loges, N. T., Slagle, C. E., Francis, R., Dougherty, G. W., Tamayo, J. V., Shook, B., Cantino, M., Schwartz, D., Jahnke, C.et al. (2013). DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat. Genet. 45, 995-1003. 10.1038/ng.2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Auwera, G. A., Carneiro, M. O., Hartl, C., Poplin, R., del Angel, G., Levy-Moonshine, A., Jordan, T., Shakir, K., Roazen, D., Thibault, J.et al. (2013). From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1-11.10.33. 10.1002/0471250953.bi1110s43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heetvelde, M. V., van Loocke, W. V., Trypsteen, W., Baert, A., Vanderheyden, K., Crombez, B., Vandesompele, J., Leeneer, K. D. and Claes, K. B. M. (2017). Evaluation of relative quantification of alternatively spliced transcripts using droplet digital PCR. Biomol. Detect. Quantif. 13, 40-48. 10.1016/j.bdq.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertii, A., Hung, H. F., Hehnly, H. and Doxsey, S. (2016). Human basal body basics. Cilia 5, 13. 10.1186/s13630-016-0030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmeier, J., Nielsen, K. G., Kuehni, C. E., Lucas, J. S., Leigh, M. W., Zariwala, M. A. and Omran, H. (2020). Motile ciliopathies. Nat. Rev. Dis. Prim. 6, 77. 10.1038/s41572-020-0209-6 [DOI] [PubMed] [Google Scholar]
- Wang, K., Li, M. and Hakonarson, H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164. 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, H., Oda, T., Kikkawa, M. and Takeda, H. (2018). Systematic studies of all PIH proteins in zebrafish reveal their distinct roles in axonemal dynein assembly. eLife 7, e36979. 10.7554/eLife.36979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala, M. A., Knowles, M. R. and Leigh, M. W. (2007. [Updated 2019]). Primary Ciliary Dyskinesia. In GeneReviews [Internet]. (Eds Adam, M. P., Ardinger, H. H., Pagon, R. A., et al.). University of Washington, Seattle, WA, USA. https://www.ncbi.nlm.nih.gov/books/NBK1122/ [PubMed] [Google Scholar]
- Zhao, L., Yuan, S., Cao, Y., Kallakuri, S., Li, Y., Kishimoto, N., DiBella, L. and Sun, Z. (2013). Reptin/Ruvbl2 is a Lrrc6/Seahorse interactor essential for cilia motility. Proc. Natl. Acad. Sci. USA. 110, 12697-12702. 10.1073/pnas.1300968110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Lage, P., Stefanopoulou, P., Styczynska-Soczka, K., Quinn, N., Mali, G., von Kriegsheim, A., Mill, P. and Jarman, A. P. (2018). Ciliary dynein motor preassembly is regulated by Wdr92 in association with HSP90 co-chaperone, R2TP. J. Cell Biol. 217, 2583-2598. 10.1083/jcb.201709026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.