Abstract
With the emergence of big data and more personalized approaches to spine care and predictive modeling, data science and deep analytics are taking center-stage. Although current techniques in machine learning and artificial intelligence have gained attention, their applications remain limited by their reliance on traditional analytic platforms. Quantum computing has the ability to circumvent such constraints by attending to the various complexities of big data while minimizing space and time dimensions within computational algorithms. In doing so, quantum computing may one day address research and clinical objectives that currently cannot be tackled. Understanding quantum computing and its potential to improve patient management and outcomes is therefore imperative to drive further advancements in the spine field for the next several decades.
Keywords: quantum, computing, spine, disc degeneration, pain, artificial intelligence, big data
Introduction
With the massive and ever-growing spine registries that house the healthcare data of millions of patients, along with advancements in technology that support increasingly complex data analytics, real-time patient monitoring, and artificial intelligence (AI)-enhanced clinical decision making, there has been a strong push to personalize spine care. The Intelligence-Based Spine Care (IBSC) model, an approach proposed by Mallow et al.1) at Rush University in 2020, seeks to provide such a personalized treatment by interfacing AI, augmented-reality, three-dimensional printing, robotics, wearable technologies, computational methods for predictive modeling, and automated phenotype recognition. Prior to the advent of these technologies, more traditional statistical methods were leveraged to identify and describe adverse events, complications, and relevant risk factors concerning spine pathology. As our understanding of the spine deepens, the adoption of advanced computational technologies, such as machine learning and deep learning, is becoming a necessity. Clinicians and researchers now have greater opportunity to tailor prognostication and risk stratification to each patient, leading many institutions to join in collaborative efforts to expand datasets targeted toward various domains of spine disorders, conditions, and management2-4). One of the more prominent examples of such registries is the National Surgical Quality Improvement Program (NSQIP), which includes more than 700 hospitals nationally and more than 100 hospitals internationally5). Large, global datasets like NSQIP have facilitated the development of personalized AI algorithms to detect adverse events, complications, establish preventative measures, assist in refined patient phenotyping, and addressing risk management of spine patients possible.
The term “Big Data” is often construed as synonymous with “Artificial Intelligence” and thus misinterpreted as a solution rather than a problem. Within medicine specifically, “Big Data” may pose a computational obstacle wherein the size of a given dataset exceeds the computational power of the modern computer. Genetic data, for instance, include millions of single-nucleotide polymorphisms and other biomarker data, requiring significant storage space and computational aptitude to run analyses with a modicum of efficiency. The growing body of multidimensional data available to analyze complex phenotypes, risk factors, and outcomes only further highlights this issue. Limitations of classical computing are contributing to a tremendous lag in pioneering genetic and/or molecular epidemiology research on spine disorders6,7). Fortunately, advanced computing techniques, such as parallel processing and supercomputing methods, are being designed to circumvent the constraints of today's modern computers. Research investigating optimization theory and quantum phenomena has also promoted theoretical advancements in computing that are beginning to be realized today. As the spine community continues to drive the development and processing of Big Data, familiarization and understanding of emerging technologies is of great importance.
Classical Computing
Modern transistors and their limitations
An understanding of how our current computing technology functions is relevant to any discussion of quantum computing. The most basic unit of data processing in modern microchips is the transistor, which is nothing more than a switch that either permits or blocks the flow of information in the form of an electrical current. A current flowing through a transistor is interpreted as “on,” or 1, and the absence of current is read as “off,” or 0 (i.e., binary operators). Combinations of 1's and 0's, referred to as “bits,” are used to represent more complicated information, forming the basis of Classical Computing. The arrangement of transistors into higher order structures, called logic gates (Fig. 1), is what confers the ability to perform complex computations.
Figure 1.
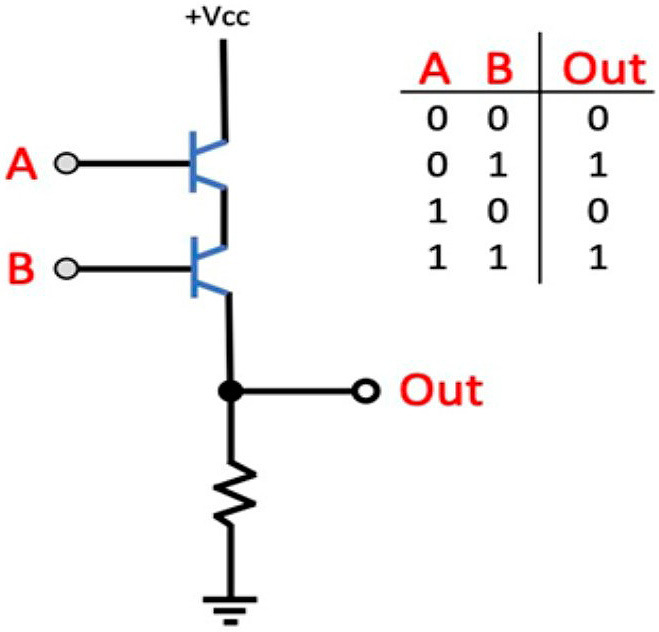
AND Logic Gate. The arrangement of transistors (blue) in the AND logic gate is such that both transistors must be open to allow current originating from the positive voltage at common collected (+Vcc) to flow through them for the output (Out) bit to read “1.” If at least one of the transistors is closed the output will instead read “0.” “A” and “B” are the gates, or input bits, that control their respective transistors.
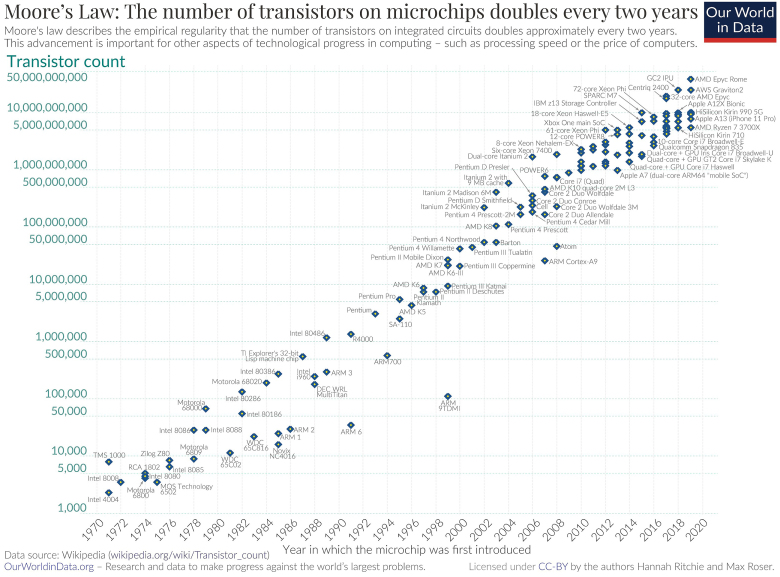
Since their initial development, transistors have become vastly smaller through time. In 1965, Gordon Moore, co-founder of Intel, predicted that the density of transistors on microchips would double every two years. By 1975, his prediction held true and has since been referred to as Moore's Law (Fig. 2). Numerous technological innovations made over the past 70 years have miniaturized transistors from 40 micrometers in 1947 down to the roughly 5-nanometer transistors of today, representing a reduction in size of four orders of magnitude without compromising processing power. One of the first fully transistorized computers developed in the late 1950s, the IBM 7090, contained over 50,000 transistors and could perform roughly 229,000 operations per second8). Alternatively, Apple's new high-performance M1 processor holds 16 billion transistors, each measuring a mere 5 nanometers in length, and performs more than 11 trillion operations each second9). Despite the great superiority in capability, the M1 processor costs only $50 and is easily housed within the modern laptop, whereas the IBM 7090, which sold for $2.9 million in 1960 (equivalent to $20 million in 2020), required an entire room to contain the computer, electrical, and cooling equipment. As transistors continue to shrink, however, the behavior of fundamental particles on such microscopic scales begins violating the very principles of classic mechanics.
Figure 2.
Roser M., Ritchie H. Moore’s Law: the number of transistors on a microchip double every two years. Available at: https://ourworldindata.org/uploads/2020/11/Transistor-Count-over-time.png (Accessed 15 August 2021).
Determining the exact location of incredibly small particles, such as the an electron, is problematic. Over small enough scales, subatomic particles may appear anywhere within a probability distribution, allowing them to behave in a manner not in accordance with classical mechanics. One phenomenon, called quantum tunneling, occurs when waveforms, like the electron that is both a particle and wave, propagate through potential barriers such as a closed tiny transistor, making their binary outputs unreliable. As transistors approach the size of the atom, the likelihood of an electron passing through a closed transistor per unit time ultimately increases. Effects of quantum tunneling likely indicate that the size limit of modern transistors has been reached, and increasing the density of transistors per unit size, which has been our main method for improving CPU performance for nearly 70 years, will not be possible. As such, current technology will not be able to meet the processing needs required to incorporate the wealth of patient data necessary to personalize spine care, despite the incredible computation speed of modern transistorized computers.
Quantum Computing
A brief history
The conception of quantum computing is often incorrectly attributed to the early 1980s, either to Paul Benioff10)―for his quantum mechanical model of the infamous Turing machine―or Richard Feynman11)―for his paper that postulated future problems that classical computers may not be able to solve. However, the first publication involving a computational device based on quantum mechanics can be traced back to Alexander Holevo in 197312). The 1980s provided many of the well-known theoretical additions to the quantum computing domain, including those by Benioff10), Deutch13), and Feynman11). Despite the progress in the beginning of the 1980s, nearly 10 years passed before the first quantum algorithms, or algorithms that use features of quantum phenomena for computation, were developed, the Deutsch-Josza algorithm14) and Simon's algorithm15), respectively. These algorithms paved the wave for Dr. Peter Shor to create one of the most widely recognized quantum algorithms in 199416). This algorithm provided for a provocative approach to factoring integers, which many postulate holds the potential to decrypt one of the most widely utilized cryptosystems for secure data transfer, the Rivest-Shamir-Adleman security encryption17,18). Since the early 2000s, novel algorithms, such as measurement-based algorithms19,20) or topological-quantum-field algorithms21,22), compounded by advancements in modern physics, have led to unprecedented growth in the field of quantum computing17). As of last year, organizations such as Google, IBM Microsoft, and Rigetti have all built quantum computers.
How does it work and what are the properties?
At the intersection between Computer Science and Quantum Physics, quantum computing is a budding field of science used to perform complex computations by exploiting quantum phenomena such as superposition and quantum entanglement. Unlike traditional computers that determine the presence or absence of an electron current as bits, quantum computers use two-state quantum systems, called qubits, as their fundamental unit of information. Examples of qubits are the orientation of polarization of a photon, either vertical or horizontal, and the direction of spin, either up or down, of an electron. Whereas bits are binary and must have a state of either 0 or 1, qubits may exist as 0, 1, or in any proportion of both. This combination of states that would ordinarily exist independently of one another is called superposition. Consequently, because they can exist simultaneously in combinations of states, qubits can store much more information than traditional bits. However, once a qubit is measured, it must collapse into a single, discrete state.
Another departure from classical computing in quantum computers is the use of quantum gates instead of logic gates. Quantum gates “entangle” qubits. When a member of an entangled pair's state is observed, the other member qubit's state reacts instantaneously and may therefore be deduced regardless of how far apart (even across billions of light-years) the entangled qubits are. This unintuitive and very science fiction-sounding phenomenon that Albert Einstein called “spooky action at a distance” is known as quantum entanglement. The implications of these two phenomena, superposition and quantum entanglement, are that we can store exponentially more information in the same number of qubits and can access that stored information much more efficiently than in traditional circuitry.
Superposition, quantum entanglement, and quantum mechanics are complex and difficult theoretical topics to understand. Therefore, interdisciplinary collaborative efforts among physicists, computer scientists, and spine researchers are necessary to drive innovation in neuroradiological applications, clinical simulations, computational optimization, and the IBSC approach.
Applications in non-spine fields
As the transition from bits to qubits heralds a new era in computing, the potential implications largely remain to be elucidated. While scientists continue to grapple with scientific and engineering challenges associated with quantum computing, numerous large companies, such as Google, IBM, Microsoft, and Amazon, in addition to several startups, have begun exploring this emerging field and have made significant progress toward establishing commercial applications23). One field particularly shaken by the rise of quantum computing is Cybersecurity. Because quantum algorithms can be applied to many cryptographic calculations, modern encryption algorithms can effectively be reduced from exponential complexities to polynomial problems, significantly decreasing the time and computational cost required to break them16). Recognizing the possibility that modern cybersecurity may soon be obsolete, the U.S. government passed the Quantum Initiative Act24) in 2018 to allocate funding and effectively join the quantum computing race. Addressing combinatoric challenges through quantum computing has also demonstrated value in the financial sector by optimizing derivatives pricing and risk assessment25), as well as in advanced manufacturing, where identifying faults in systems despite a paucity of data from prior failures can reduce the likelihood of future failure and translate to enormous savings23).
Healthcare represents a particular field of promise for applications of quantum computing. One of its main advantages is the theorized ability to simulate molecular systems with a high degree of fidelity. In 2019, Google's quantum computer (Fig. 3) accurately simulated the isomerization of diazene, which is a molecule consisting of two nitrogen and two hydrogen atoms. Although relatively simple, this achievement demonstrates that simulating higher order structures, as is currently done on classical computers with extensive applications in biochemical research, is feasible with quantum computing. However, there are limits to what can be studied on classical computers. For instance, simulating protein folding is too computationally expensive for processors that rely on current technology26). Quantum computing may allow us to simulate molecular structures as it leverages the same tenets of physics that we attribute to biological structures27). Cheng et al.27) recently detailed why three biochemical systems―histone demethylase, telomerase, and biotin-avidin binding―are challenging to simulate for current computational methods. A quantum computer may therefore one day be able to simulate the entire inner workings of a cell, a tissue colony of those cells, an organ comprised of multiple types of tissues, and even complete organ systems, all based on variations of that original simulated cell.
Figure 3.

Stearns F. Artist’s rendition of the Sycamore processor mounted in the cryostat. Available at: https://ai.googleblog.com/2019/ 10/quantum-supremacy-using-programmable.html (Accessed December 10, 2021).
Whereas drug discovery has a history filled with luck and chance, the application of quantum computing to modern R&D may allow scientists to better understand the structure and properties of their products while simultaneously minimizing cost and maximizing efficacy. Perhaps even more impressive is the speed with which new drugs can be discovered. During the height of the Ebola outbreak in 2015, Atomwise, a company using supercomputers to discover novel drug therapies from an extensive database of molecular structures, conducted a search using its deep convolutional network and successfully identified two drug candidates to treat the virus in less than one day, a feat that would otherwise take several months28). Translated directly to patient care, quantum computing can assist physicians in their clinical practices. By improving precision and interpretation of imaging, guiding radiation therapy and targeting of malignant cells in the treatment of various cancers, and interpreting large amounts of patient data, healthcare providers will be better able to determine truly personalized approaches to the preventative and therapeutic management of their patients29-31). Moreover, the incorporation of quantum computing into implantable and prosthetic devices may directly affect patient quality of life. For example, quantum-enabled neural devices can be used to establish a brain-computer interface that records and decodes brain signaling concerning motor intention and planning and translates this information to movement of prosthetic or robotic limbs32-34). The BrainGate group has already successfully demonstrated the ability of neural interface technology to enable robotic prostheses to perform three-dimensional reach and grasp movements35), indicating profound implications for patients with mobility limitations due to paralysis or stroke.
Applications for spine care and research
The ability of quantum computers to collect and analyze vast amounts of patient data using pattern recognition generates extensive potential applications in the field of spine care, specifically. Koebbe et al.36) demonstrated how quantum-mechanically entangled light augments the utility of imaging in the diagnosis and treatment of spinal conditions when paired with AI and the precision-based spine care approach37,38). In fact, quantum sensors may improve imaging technology by enabling visualization to the molecular level, providing clinicians with substantially more accurate imagery to guide them in identifying the pain source, mapping treatment efficacy, and even identifying high-risk candidates for disease development and progression. The age of Big Data has introduced a wealth of platforms worthy of investigation in relation to spinal disorders, such as those of genomics, metabolomics, transcriptomics, lipidomics, proteomics, interactomics, epigenomics, and so on39-42). A “panomics” or “integrative omics” approach is most likely a necessity considering the complex disease states and conditions that are inherent to human variation and associated with spine disorders/pathologies. Implications of the microbiome and its effects on human health and disease further add to the complexity of understanding why we develop certain spine conditions, their severity, and resolution43,44). Similarly, pain genes have shed light on questions surrounding which patients may develop a symptomatic herniated disc as well as pain severity, resolution, and outcomes among other conditions45).
Integration of the above platforms will surely introduce new dimensions to spine conditions and their management options. Initiatives, such as the multicenter National Institutes of Healthsupported Back Pain Consortium Research Project46), among others, may ultimately address the aforementioned data platforms by using quantum computing, allowing for robust conclusions regarding mechanisms of disease, provide guidelines for treatment options, and thus facilitating more precise approaches for patients with chronic low back pain. Phenotyping clinical profiles and maximizing outcomes are critical and demand intensive analytical scrutiny and applications. Quantum computing will further fuse the cellular, molecular, structural, and overall histopathological sub-tissue elements with various “panomic” domains into high fidelity simulations, allowing us to better understand disease development and progression, develop targeted therapeutics, improve and optimize existing treatments, and discover new markers for prevention. For example, near-exact simulated spines may allow researchers to elaborate upon the natural history of disc degeneration and its severity as well as explain who may develop symptomatic disease, recurrent herniation following discectomy, or adjacent segment disease following fusion. Furthermore, such simulations built upon these integrations will also allow us to identify which patients may develop intra- and/or postoperative complications, improve patient stratifications, develop risk profile calculators, and refine algorithmic modeling of spine conditions to optimize outcomes, inevitably paving the way for translational findings from the bench to the bedside. Moreover, regenerative biologics of the spine is a field that has encountered numerous barriers and challenges, with advances that remain controversial; however, quantum computing may add value to refined patient and dose selection as well as algorithmic modeling of outcomes and the regenerative process from the molecular to structural level with greater certainty. Finally, the interaction of a given reconstituted motion segment with the whole spine and the entire body as a functional, interactive unit may be better understood and incorporated into more elaborative and precise predictive modeling through a quantum computing approach. With the explosion of new data platforms and the need for data-driven science, quantum computing may one day become commonplace and instrumental in every facet of patient care.
Conclusion
Considering the great potential quantum computing carries to provide personalized patient care38), incorporated with the IBSC model1), the traction and support it has gained recently within the spine community is well-warranted. Harnessing advanced computational algorithms in conjunction with extensive patient datasets, optimization of patient care using quantum computing is here to stay. In a time of abundance of data, complexities that may seem impossible to tackle today can be addressed tomorrow with the emergence of quantum computing. To achieve such goals, a team-based approach involving multicenter and multidisciplinary collaboration is mandatory. The data scientist and the clinician must work together to find solutions and map the way forward for the spine field to evolve.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Acknowledgement
The authors wish to thank Zakariah Siyaji for his insights towards this topic.
References
- 1.Mallow GM, Siyaji ZK, Galbusera F, et al. Intelligence-based spine care model: A new era of research and clinical decision-making. Glob Spine J. 2021;11(2):135-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi M, Grob D. SSE Spine tango: a European Spine Registry promoted by the Spine Society of Europe (SSE). Eur Spine J. 2004;13(8):661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breakwell LM, Cole AA, Birch N, et al. Should we all go to the PROM? The first two years of the British Spine Registry. Bone Joint J. 2015;97(7):871-4. [DOI] [PubMed] [Google Scholar]
- 4.Strömqvist B, Fritzell P, Hägg O, et al. The Swedish Spine Register: development, design and utility. Eur Spine J. 2009;18(suppl 3):294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surgeons ACo. ACS National Surgical Quality Improvement Program.
- 6.Eskola PJ, Männikkö M, Samartzis D, et al. Genome-wide association studies of lumbar disc degeneration―are we there yet? Spine J. 2014;14(3):479-82. [DOI] [PubMed] [Google Scholar]
- 7.Freidin MB, Tsepilov YA, Palmer M, et al. Insight into the genetic architecture of back pain and its risk factors from a study of 509,000 individuals. Pain. 2019;160(6):1361-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archives I. 7090 Data Processing System.
- 9.Newsroom A. Apple unleashes M1.
- 10.Benioff P. The computer as a physical system: A microscopic quantum mechanical Hamiltonian model of computers as represented by turing machines. J Stat Phys. 1980;22(5):563-91. [Google Scholar]
- 11.Feynman RP. Simulating physics with computers. Int J Theor Phys. 1982;21(6-7):467-88. [Google Scholar]
- 12.Holeyo AS. Bounds for the quantity of information transmitted by a quantum communication channel. Probl Peredachi Inf. 1973;9:3-11. [Google Scholar]
- 13.Deutsch D. Quantum theory, The Church-Turing principle and the universal quantum computer. Proc R Soc Lond A. 1985;400(1818):97-117. [Google Scholar]
- 14.Deutsch D, Jorza R. Rapid solution of problems by quantum computation. Proc R Soc Lond A. 1992;439(1907):553-8. [Google Scholar]
- 15.Simon DR. On the power of quantum computation. SIAM J Comput. 1997;26(5):1474-83. [Google Scholar]
- 16.Shor PW. Polynomial-time algorithms for prime factorization and discrete logarithms on a quantum computer. SIAM J Comput. 1997;26(5):1484-509. [Google Scholar]
- 17.Hagar AC, Cuffaro M. Quantum computing. The Stanford Encyclopedia of Philosophy (Winter 2019 Edition). Stanford University: Metaphysics Research Lab; 2019. Available from https://plato.stanford.edu/archives/win2020/entries/qt-quantcomp/.
- 18.Rivest RLS, Shamir A, Adleman L. A method for obtaining digital signatures and public-key cryptosystems. Commun ACM. 1978;21(2):120-6. [Google Scholar]
- 19.Raussendorf RB, Browne DE, Briegel HJ. Measurement-based quantum computation on cluster states. Phys Rev A. 2003;68(2):022312. [Google Scholar]
- 20.Briegel HJB, Browne DE, Dür W, et al. Measurement-based quantum computation. Nat Phys. 2009;5(1):19-26. [Google Scholar]
- 21.Alexandrov MS, Schwarz A, Zaboronsky O, et al. The geometry of the master equation and topological quantum field theory. Int J Mod Phys A. 1997;12(7):1405-29. [Google Scholar]
- 22.Witten E. Topological quantum field theory. Commun Math Phys. 1988;117(3):353-86. [Google Scholar]
- 23.Bova F, Goldfarb A, Melko RG. Commercial applications of quantum computing. EPJ Quantum Technol. 2021;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith L. National Quantum Initiative Act 2018. Available from: https://www.congress.gov/bill/115th-congress/house-bill/6227.
- 25.Rebentrost PG, Gupt B, Bromley TR. Quantum computational finance: Monte Carlo pricing of financial derivatives. Phys Rev A. 2018;98(2):022321. [Google Scholar]
- 26.Outeiral CS, Shi J, Morris G, et al. The prospects of quantum computing in computational molecular biology. WIREs Comp Mol Sci. 2020;11(1):e1481. [Google Scholar]
- 27.Cheng HP, Deumens E, Freericks JK, et al. Application of quantum computing to biochemical systems: A look to the future. Front Chem. 2020;8:587143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elizabeth K, Wilson EK. Deep learning to the rescue. C&EN Global Enterp. 2017;95(4):29-30. [Google Scholar]
- 29.Dilsizian SE, Siegel EL. Artificial intelligence in medicine and cardiac imaging: harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr Cardiol Rep. 2014;16(1):441. [DOI] [PubMed] [Google Scholar]
- 30.Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-10. [DOI] [PubMed] [Google Scholar]
- 31.Raudaschl A. Quantum computing and healthcare 2017. Available from: https://blogs.bmj.com/technology/2017/11/03/quantum-computing-and-health-care.
- 32.Andersen RA, Musallam S, Pesaran B. Selecting the signals for a brain-machine interface. Curr Opin Neurobiol. 2004;14(6):720-6. [DOI] [PubMed] [Google Scholar]
- 33.Mulliken GH, Musallam S, Andersen RA. Decoding trajectories from posterior parietal cortex ensembles. J Neurosci. 2008;28(48):12913-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulliken GH, Musallam S, Andersen RA. Forward estimation of movement state in posterior parietal cortex. Proc Natl Acad Sci U S A. 2008;105(24):8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochberg LRB, Bacher D, Jarosiewicz B, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koebbe CJ, Maroon JC, Abla A, et al. Lumbar microdiscectomy: a historical perspective and current technical considerations. Neurosurg Focus. 2002;13(2):E3. [DOI] [PubMed] [Google Scholar]
- 37.Harada GK, Siyaji ZK, Younis S, et al. Imaging in spine surgery: current concepts and future directions. Spine Surg Relat Res. 2020;4(2):99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samartzis D, Alini M, An HS, et al. Precision spine care: A new era of discovery, innovation, and global impact. Glob Spine J. 2018;8(4):321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao PY, Chan D, Samartzis D, et al. Genetics of lumbar disk degeneration: technology, study designs, and risk factors. Orthop Clin North Am. 2011;42(4):479-86. [DOI] [PubMed] [Google Scholar]
- 40.Wong AYL, Karppinen J, Samartzis D. Low back pain in older adults: risk factors, management options and future directions. Scoliosis Spinal Disord. 2017;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung JPY, Kao PYP, Sham P, et al. Etiology of developmental spinal stenosis: A genome-wide association study. J Orthop Res. 2018;36(4):1262-8. [DOI] [PubMed] [Google Scholar]
- 42.Samartzis D, Karppinen J, Cheung JP, et al. Disk degeneration and low back pain: are they fat-related conditions? Glob Spine J. 2013;3(3):133-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabeerdoss J, Sandhya P, Danda D. Gut inflammation and microbiome in spondyloarthritis. Rheumatol Int. 2016;36(4):457-68. [DOI] [PubMed] [Google Scholar]
- 44.Rajasekaran S, Soundararajan DCR, Tangavel C, et al. Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur Spine J. 2020;29(7):1621-40. [DOI] [PubMed] [Google Scholar]
- 45.Samartzis D, Borthakur A, Belfer I, et al. Novel diagnostic and prognostic methods for disc degeneration and low back pain. Spine J. 2015;15(9):1919-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Back Pain Consortium. (BACPAC) Research Program. Available from: https://heal.nih.gov/research/clinical-research/back-pain.