Abstract
Background.
Real-time monitoring of treatment response with a liquid biomarker has potential to inform treatment decisions for patients with rectal adenocarcinoma (RAC), esophageal adenocarcinoma (EAC), and colorectal liver metastasis (CRLM). Circulating hybrid cells (CHCs), which have both immune and tumor cell phenotypes, are detectable in the peripheral blood of patients with gastrointestinal cancers, but their potential as an indicator of treatment response is unexplored.
Methods.
Peripheral blood specimens were collected from RAC and EAC patients after neoadjuvant therapy (NAT) or longitudinally during therapy and evaluated for CHC levels by immunostaining. Receiver operating characteristics (ROCs) and the Kaplan-Meier method were used to analyze the CHC level as a predictor of pathologic response to NAT and disease-specific survival (DSS), respectively.
Results.
Patients with RAC (n = 23) and EAC (n = 34) were sampled on the day of resection, and 11 patients (32%) demonstrated a pathologic complete response (pCR) to NAT. On ROC analysis, CHC levels successfully discriminated pCR from non-pCR with an area under the curve of 0.82 (95% confidence interval [CI], 0.71–0.92; (P<0.001). Additionally, CHC levels in the EAC patients correlated with residual nodal involvement (P = 0.026) and 1-year DSS (P = 0.029). The patients with RAC who were followed longitudinally during NAT (n = 2) and hepatic arterial infusion therapy for CRLM (n = 2) had CHC levels that decreased with therapy response and increased before clinical evidence of disease progression.
Conclusion.
Circulating hybrid cells are a novel blood-based biomarker with potential for monitoring treatment response and disease progression to help guide decisions for further systemic therapy, definitive resection, and post-therapy surveillance. Additional validation studies of CHCs are warranted.
Approximately 215,800 new cases of gastrointestinal (GI) cancers are diagnosed each year in the United States and account for more than 86,500 deaths.1 Surgical resection is a mainstay of therapy for localized disease, but the invention of new therapeutics and multimodal approaches has resulted in continued evolution toward more personalized cancer therapy.
Despite these advancements, determining the optimal treatment for individual patients remains a challenge. Thus, improved markers of treatment response are needed to guide therapeutic decisions. Circulating hybrid cells (CHCs) are a novel cell population found in the peripheral blood of patients across myriad cancer types and have translational potential as a noninvasive analyte in GI malignancies.2–5
Neoadjuvant therapy (NAT) with radiation and systemic chemotherapy has multiple benefits for patients with rectal adenocarcinoma (RAC),6–8 resulting in a pathologic complete response (pCR) in upwards of 16–27% of patients. Not only does pCR portend improved survival,9 but patients also can potentially avoid surgical resection with a clinical complete response (cCR) to NAT,10 sparing them the morbidity of pelvic surgery and the risk of complications.11 Neoadjuvant chemoradiotherapy (CXRT) combined with resection also has demonstrated improved outcomes for patients with esophageal adenocarcinoma (EAC) compared with surgery alone.12 Although 25% of EAC patients achieve pCR, organ-sparing strategies have garnished less enthusiasm than RAC because retrospective studies have indicated worse outcomes with non-operative management. Therefore, total neoadjuvant therapy (TNT) for EAC generally is reserved for surgically unfit patients until better methods of determining cCR are developed.13,14
Although cCR currently is assessed with a combination of endoscopic procedures, biopsies, and imaging studies,15–17 reports have demonstrated that these methods have low sensitivity for identifying foci of persistent disease.18 Additionally, intensive surveillance regimens are required, with watch-and-wait approaches, because 25% to 30% of RAC patients with cCR and up to 53% of EAC patients with cCR experience tumor relapse.19–21 Current clinically available blood-based biomarkers, including carcinoembryonic antigen (CEA), have shown little utility in cancer surveillance or in guiding therapy decisions after NAT.22 Development of sensitive noninvasive biomarkers of treatment response would greatly improve patient care and provide an optimal method for closely monitoring patients managed with TNT.
Treatment decisions for patients with unresectable and metastatic cancers pose discrete challenges. Assessing the chemotherapy response of patients with colorectal liver metastasis (CRLM) by imaging often is insensitive and can be further influenced by the addition of liver-directed therapies, including thermal ablation, hepatic arterial infusion (HAI), and transarterial procedures.23–25 During liver-directed therapies, patients remain at risk of extrahepatic progression and require standard surveillance imaging in addition to dedicated liver evaluation.26,27 However, radiographic detection of progression often is delayed, which can risk exclusion of patients from salvage therapies due to advanced disease or a decline in functional status.28 A noninvasive analyte of both intra- and extrahepatic disease status could provide earlier recognition of progression and prompt a switch to next-line therapy.
Circulating hybrid cells are detectable in the peripheral blood of patients with GI malignancies by their dual tumor and immune cell phenotypes.2,3 Murine and in vitro studies have demonstrated that spontaneous cellular fusion between bone marrow-derived cells and tumor cells generates hybrid cells with retained attributes from both parent cell types.2,29,30
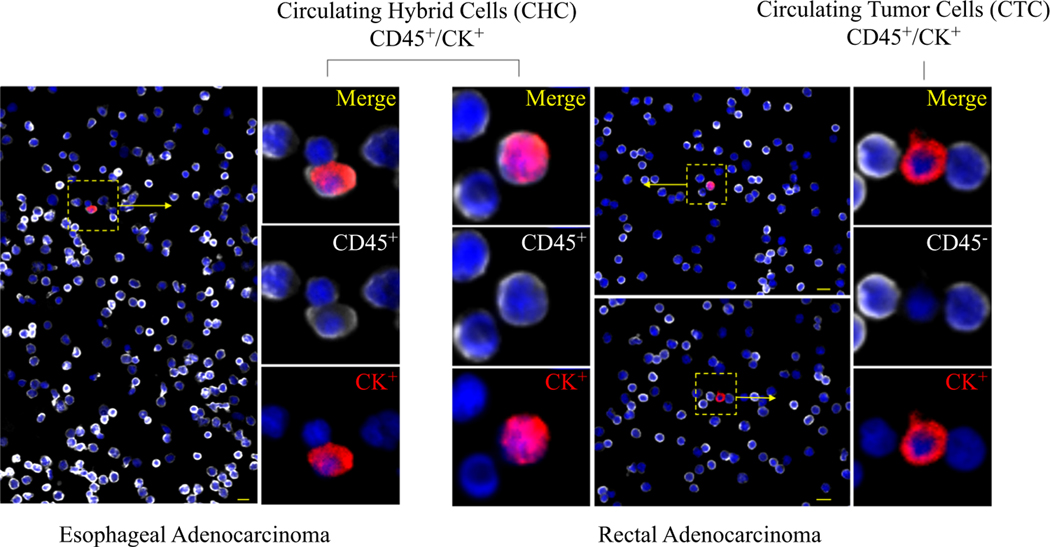
Further supporting that CHCs are a disseminated tumor-derived cell population, our prior studies have demonstrated that CHCs from patients with pancreatic adenocarcinoma (PDAC) harbor common KRAS mutations found in primary tumors.2,5 Although other mechanisms whereby tumor cells can gain immune cell phenotypes have been described, including exosome-mediated transfer,31 immune mimicry,32,33 and developmental gene expression,34 no direct evidence links them to hybrid cells detected in the circulation of human patients. However, these could account for a portion of CHCs identified. Identified by the co-expression of pan-leukocyte antigen CD45 and tumor marker cytokeratin (CK), CHCs (CD45+/ CK+) are found at much higher levels than conventionally defined circulating tumor cells (CTC, CD45–/CK+; Fig. 1). In patients with PDAC, CHCs expressing CK correlate with cancer stage and are an indicator of overall survival.2
FIG. 1.
Tumor-derived cells detected in peripheral blood samples of patients with esophageal (EAC) and rectal adenocarcinoma (RAC).Circulating hybrid cells (CHC)are identified by the coexpressionof CD45 (white) and cytokeratin (CK; red), whereas a conventionally defined circulating tumor cell (CTC;CD45−/CK+) detected in a patient with RAC lacks CD45expression. Nuclear DAPI staining is blue. Scale bar: 10μm
Furthermore, CHCs detected by E-cadherin expression correlate with pancreatic cancer nodal stage.35 As a cell-based biomarker with tumor-derived protein and genetics,4 CHCs may hold translational value for providing insight on tumor response in real time and guiding management decisions without the need for invasive procedures.
METHODS
Human Patient Samples
All human peripheral blood samples were collected and analyzed under approved protocols in accordance with the ethical requirements and regulations of the institutional review board. Informed consent was obtained from all subjects. Peripheral blood was obtained from patients with RAC and EAC immediately before surgical resection, as well as longitudinally during NAT for two patients with RAC. Longitudinal samples were collected from two patients receiving HAI therapy for unresectable CRLM on the first day of therapy cycles as well as during clinic visits while off therapy. Neoadjuvant and HAI therapies were delivered as part of a multidisciplinary team approach and not standardized across patients. Additionally, blood samples also were collected from self-reported healthy volunteers.
Sample Processing and Immunohistochemistry
Peripheral blood (10–20 mL) collected into heparinized vacutainer tubes was subjected to isolation of peripheral blood mononuclear cells (PBMCs) using standard density centrifugation with Ficoll-Paque (GE). PBMCs, then was adhered to poly-D-lysine-coated glass microscope slides permeabilized with Triton-X and fixed with 4% paraformaldehyde.
For evaluation of CHCs, samples were first treated with 5% bovine serum albumin, followed by TrueBlack Lipofuscin Autofluorescence Quencher (Biotium), and Image-iT FX Signal Enhancer (Invitrogen). Samples were stained with fluorescent-conjugated antibodies for pan-cytokeratin (CK; eBioscience, clone: AE1/AE), and CD45 (Biolegend, clone:HI30), then finally counterstained with the nuclear dye, DAPI. Each sample was processed with unstained and isotype controls (eBioscience). Additionally, slides of PBMCs isolated from healthy donors spiked in cultured Capan-2 cells were processed and analyzed with each batch of patient samples as positive controls (Fig. S1). Specimens were digitally imaged with the Axio Scan.Z1 (Zeiss, NY).
Image Capture and Data Processing
Manual quantification blinded to the clinical status of the specimen was performed. Zeiss Efficient Navigation (ZEN) software was used to quantify the total number of PBMCs and CHCs (CK+/CD45+). Histograms were established for each sample based on control-stained cells. For each specimen, CHC counts were normalized to 50,000 PBMCs.
Flow Cytometric Analyses of CHCs and CTCs in Human Peripheral Blood
For evaluation of CHCs by flow cytometry, PBMCs were isolated as described earlier and resuspended to a concentration of 5 × 107 cells/mL. The cells were incubated in phosphate-buffered saline (PBS) containing Live Dead Aqua (Thermo Fisher Scientific) with Fc Receptor Binding Inhibitor (Thermo Fisher Scientific), then incubated with antibodies to CD45 (Biolegend, clone:HI30). The cells were fixed and permeabilized with the eBioscience Intracellular Fixation & Permeabilization Buffer Set before incubation with pan-CK antibodies (Abcam, Clone C-11). The samples were analyzed on the BD LSRFortessa (Becton Dickinson, NJ, USA) together with single-color and unstained controls. Gates were established for CHC quantification based on sample controls, as we have previously described,2 and normalized to 100,000 live cells.
Definitions
Patient records were reviewed, and clinicopathologic data related to cancer diagnosis, staging, treatment, and outcomes were collected. Clinical and pathologic staging was defined by the American Joint Committee on Cancer (AJCC) Cancer Staging Manuel, eighth edition.36 The response to NAT was determined from pathology reports and graded according to the AJCC tumor regression grade (TRG)37 as complete (TRG-0), incomplete (TRG 1–2), or no response (TRG-3). The clinical response to NAT was assessed by exam, rectal magnetic resonance imaging (MRI), and endoscopy findings and graded as complete, near-complete, or incomplete response according to the schema published by Memorial Sloan Kettering Cancer Center (MSKCC).15
Statistical Analysis
Descriptive statistics of EAC and RAC patients were compiled, displaying categorical variables as number with percentage and as median with interquartile range (IQR). Levels of CHC were compared in patients with pCR, incomplete, and no response. Normalized CHC counts per 50,000 PBMCs were treated as patient-specific values, and one-way analysis of variance (ANOVA) with Games-Howell post hoc testing was performed for inter-group comparisons. Additionally, the total number of counted CHCs and peripheral blood cells were pooled within response groups, then analyzed with z test of proportions, which found the difference between response groups to be more statistically pronounced but ultimately not different from the ANOVA testing results. Receiver operating characteristics (ROC) analysis was performed for the outcome of pCR using normalized CHC counts as the predictive variable. Finally, Kaplan-Meier analysis of 1-year disease-specific survival (measured from the date of diagnosis) was performed via the log-rank test using recursive partitioning to identify the CHC cutoff yielding the greatest disparity in oncologic outcomes between groups.
jdmOnly patients receiving NAT and with nonmetastatic disease at final pathology were included in analyses of oncologic outcomes. All statistical analyses were performed in SPSS version 26 (IBM Corp., NY, USA).
RESULTS
CHC Levels are Associated with Treatment Response to Neoadjuvant Therapy
The study evaluated 23 patients with RAC and 34 patients with EAC before surgical resection (Table 1). Additionally, blood samples were analyzed from 20 healthy control subjects, 50% of whom were men, with a median age of 63 years (IQR, 57–74 years).
TABLE 1.
Clinicopathologic and treatment characteristics of patients analyzed for circulating hybrid cell counts
| Characteristic | Rectal adenocarcinoma (n = 23) n (%) | Esophageal adenocarcinoma (n = 34) n (%) |
|---|---|---|
|
| ||
| Median age: years (IQR) | 66 (52–69) | 69 (62–77) |
| Sex | ||
| Male | ||
| Female | ||
| Clinical tumor (T) stage | ||
| T1–T2 | 2 (9) | 6 (18) |
| T3–T4 | 21 (91) | 28 (82) |
| Clinical node (N) stage | ||
| N− | 7 (30) | 9 (27) |
| N+ | 16 (70) | 25 (74) |
| Median tumor size pre-NAT: cm (IQR) | 4.9 (4.1–6.3) | 5 (3.0–5.5) |
| Neoadjuvant 4egimen | ||
| Carboplatin/taxol + XRT | 0 (0) | 32 (94) |
| FOLFOX/FOLFIRI + XRT | 18 (78) | 0 (0) |
| Other + XRT | 1 (4) | 2 (6) |
| None | 4 (17) | 0 (0) |
| Pathologic response to NAT | ||
| Complete (TRG 0) | 6 (26) | 5 (15) |
| Incomplete (TRG 1–2) | 11 (48) | 27 (80) |
| None/no treatment (TRG 3) | 6 (26) | 2 (6) |
| Pathologic tumor (T) stage | ||
| T0 | 6 (26) | 5 (15) |
| Tis/T1 | 1 (4) | 9 (27) |
| T2 | 3 (13) | 4 (12) |
| T3 | 10 (44) | 15 (44) |
| T4 | 3 (13) | 1 (3) |
| Pathologic node (N) stage | ||
| N0 | 15 (65) | 19 (56) |
| N1 | 8 (35) | 7 (21) |
| N2 | 0 (0) | 4 (12) |
| N3 | 0 (0) | 4 (12) |
| Pathologic metastasis (M) stage | ||
| M0 | 21 (91) | 33 (97) |
| M1 | 2 (9) | 1 (3) |
| Median tumor size post-NAT: cm (IQR) | 2.3 (0–4.3) | 1.2 (0.1–2.9) |
| Downstaged after NAT | 10 (53)a | 18 (53) |
IQR interquartile range; NAT neoadjuvant therapy; XRT radiotherapy; FOLFOX folinic acid, fluorouracil, oxaliplatin; FOLFIRI folinic acid, fluorouracil, irinotecan; TRG tumor regression grade
Of 19 patients receiving NAT
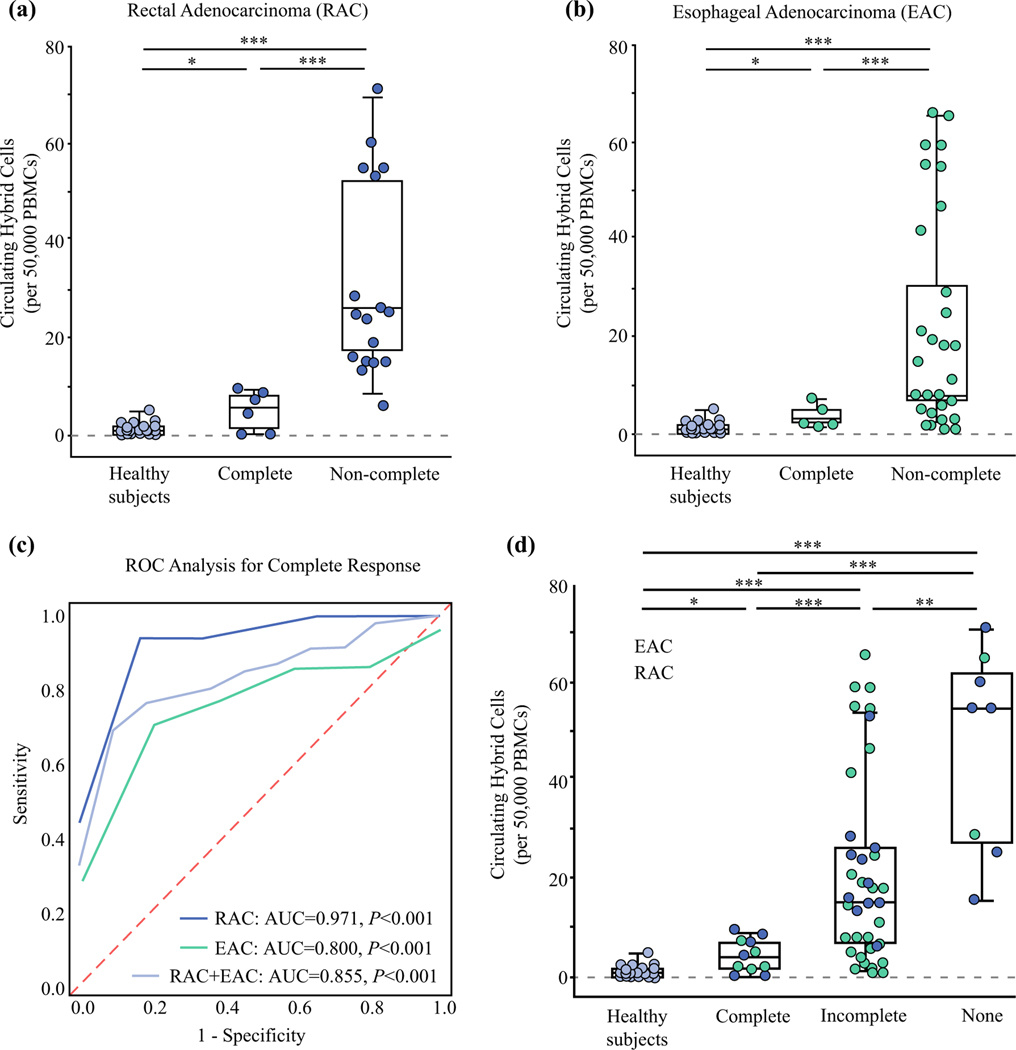
Analysis of patient PBMCs collected before resection identified at least one CHC in 50,000 PBMCs from 91% of the RAC patients (n = 21) and 100% of the EAC patients (Fig. 2a, b). Both patients without CHCs detected had a pCR. The RAC patients with a pCR had a mean of 4.7 ± 4 CHCs compared with a mean of 31 ± 19.9 CHCs in the non-pCR group (with incomplete or no response; P\0.001). The pCR group among the patients with EAC had a mean of 3.8 ± 2.1 CHCs, whereas the non-pCR patients had a mean of 21 ± 21.2 CHCs (P<0.001). The healthy control subjects had a mean CHC count of 1.4 ± 1.4, which did not differ significantly from that of the EAC or RAC patients with pCR (both P>0.1). In the ROC analysis, CHC levels successfully differentiated pCR and non-pCR patients with an area under the curve (AUC) of 0.971 (95% confidence interval [CI], 0.9–1.0; P<0.001) for the RAC cohort and an AUC of 0.800 (95% CI, 0.65–0.95; P<0.001) for the EAC cohort (Fig. 2C).
FIG. 2.
Levels of circulating hybrid cells (CHCs) are a marker of neoadjuvant treatment response in patients with esophageal and rectal adenocarcinoma. a Levels of circulating hybrid cells (CHCs) per 50,000 peripheral mononuclear cells (PBMCs) in rectal adenocarcinoma (RAC) patients with pathologic complete response (pCR) after neoadjuvant therapy (NAT) compared with non-complete response (non-pCR) and healthy control subjects. The patients with pCR have significantly fewer CHCs than the non-pCR patients (P<0.001). b CHC levels in the patients with esophageal adenocarcinoma (EAC) after NAT differ significantly between the patients with pCR and those with non-pCR (P = 0.001). c Random operating characteristics (ROC) analysis for detecting patients with pCR. The RAC cohort had an area under the curve (AUC) of 0.971, whereas the EAC patients had an AUC of 0.800 (both P<0.001). Analysis with both groups combined showed an AUC of 0.855 (P<0.001). d Analysis of the RAC and EAC patients combined demonstrated a significantly higher level of CHCs in the patients with no response to NAT than in the incomplete responders (P = 0.031) or the complete responders (P = 0.003). The pCR group also had significantly lower CHC levels than the incomplete response group (P<0.001). *P>0.05. ** P = 0.031. ***P<0.01.
To evaluate CHC levels as a potential marker of non-responsive disease, the EAC and RAC cohorts were combined to increase group sizes (Fig. 2D). The patients with no response to NAT had a mean of 47 ± 20.9 CHCs compared with 20 ± 18.1 CHCs for the patients with an incomplete response (P = 0.031). Combined, the patients with pCR had a mean of 4.3 ± 3.2 CHCs, which was significantly lower than for either the incomplete responders (P<0.001) or the non-responders (P = 0.003). Pooling of the raw CHC and PBMC counts demonstrated even greater significance between all the response groups (all P<0.001). The AUC for detecting patients with a pCR after NAT in the combined analysis was 0.855 (95% CI, 0.76–0.95; P<0.001).
CHC Levels Correlate with Residual Nodal Involvement in Patients with Esophageal Adenocarcinoma
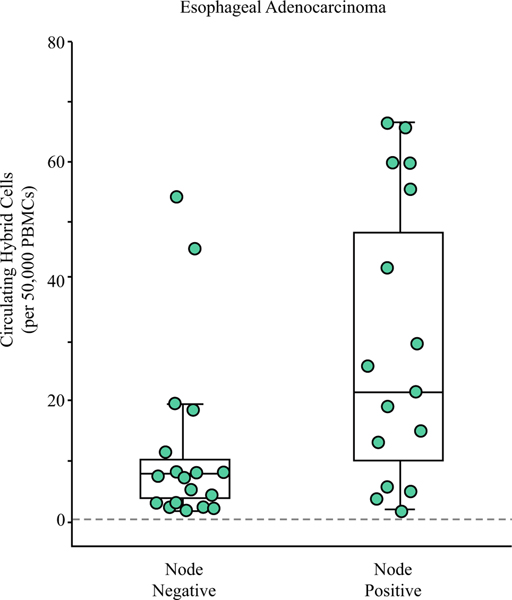
Of the 34 EAC patients, 44% (n = 15) had at least one positive lymph node (N+) found on pathology. The node-negative (N0) patients had a mean of 11.9 ± 14.8 CHCs after NAT compared with a mean of 27.8 ± 23.4 CHCs for the N+ patients (P = 0.026; Fig. 3). Of interest, the highest outlier in the N0 group had unencapsulated tumor deposits in the peri-esophagogastric adipose tissue, which in contrast to RAC staging, is not considered N+ disease in EAC but may be associated with a worse prognosis.38 Analysis of the RAC cohort did not identify a significant association between nodal status and CHC levels (P = 0.245).
FIG. 3.
Circulating hybrid cells (CHCs) correlate with residual nodal disease in the patients with esophageal adenocarcinoma after neoadjuvant therapy. The patients without lymph node metastasis on pathology had lower CHC levels than the patients with at least one positive node (P = 0.026). The patient with the highest CHC level in the node-negative group had extra-nodal tumor deposits identified on pathology, which may have prognostic implications similar to node positivity.
Post-treatment CHC Levels Are Associated With 1-Year Disease-Specific Survival in Esophageal Adenocarcinoma
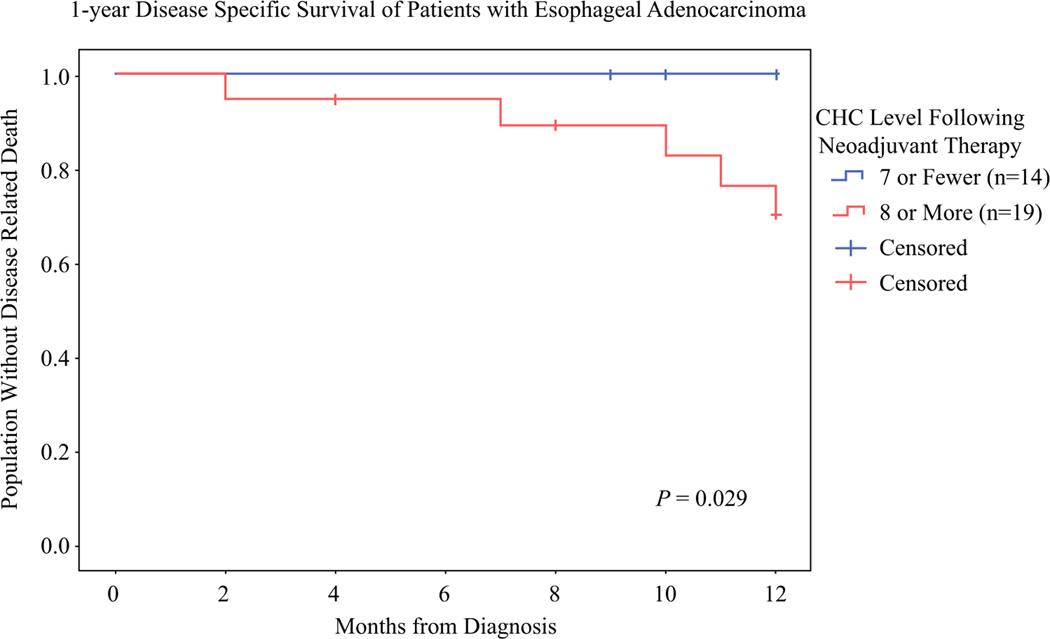
The median oncologic follow-up period was 18 months for the EAC group and 23 months for the RAC group. The EAC group had 13 recurrences and 7 deaths from recurrent EAC. The patients with seven or fewer CHCs (n = 14) had significantly better disease-specific survival (DSS) at 1 year than those with more than seven CHCs (n = 19) (100% vs 70%; P = 0.029; Fig. 4). A cutoff of seven CHCs captured all five patients with pCR and nine patients with an incomplete response. This may indicate that regardless of the degree of pathologic response, lower levels of CHCs may portray improved survival for patients with EAC. The RAC cohort had insufficient recurrences (n = 4) and deaths (n = 0) for analysis of oncologic outcomes by CHC levels.
FIG. 4.
Level of circulating hybrid cells (CHCs) after 1-year Disease Specific Survival of Patients with Esophageal Adenocarcinoma (EAC) are associated with disease-specific survival (DSS) at 1 year. The Kaplan-Meier curve of 1-year DSS for the EAC patients with 7 or fewer CHCs and the patients with more than 7 CHCs per 50,000 peripheral blood mononuclear cells shows that only 70% of the patients with more than 7 CHCs were alive at 1 year compared with 100% of those who had 7 or fewer CHCs (P = 0.029).
CHCs Provide Insight Into Treatment Response to NAT for Rectal Cancer in Real Time
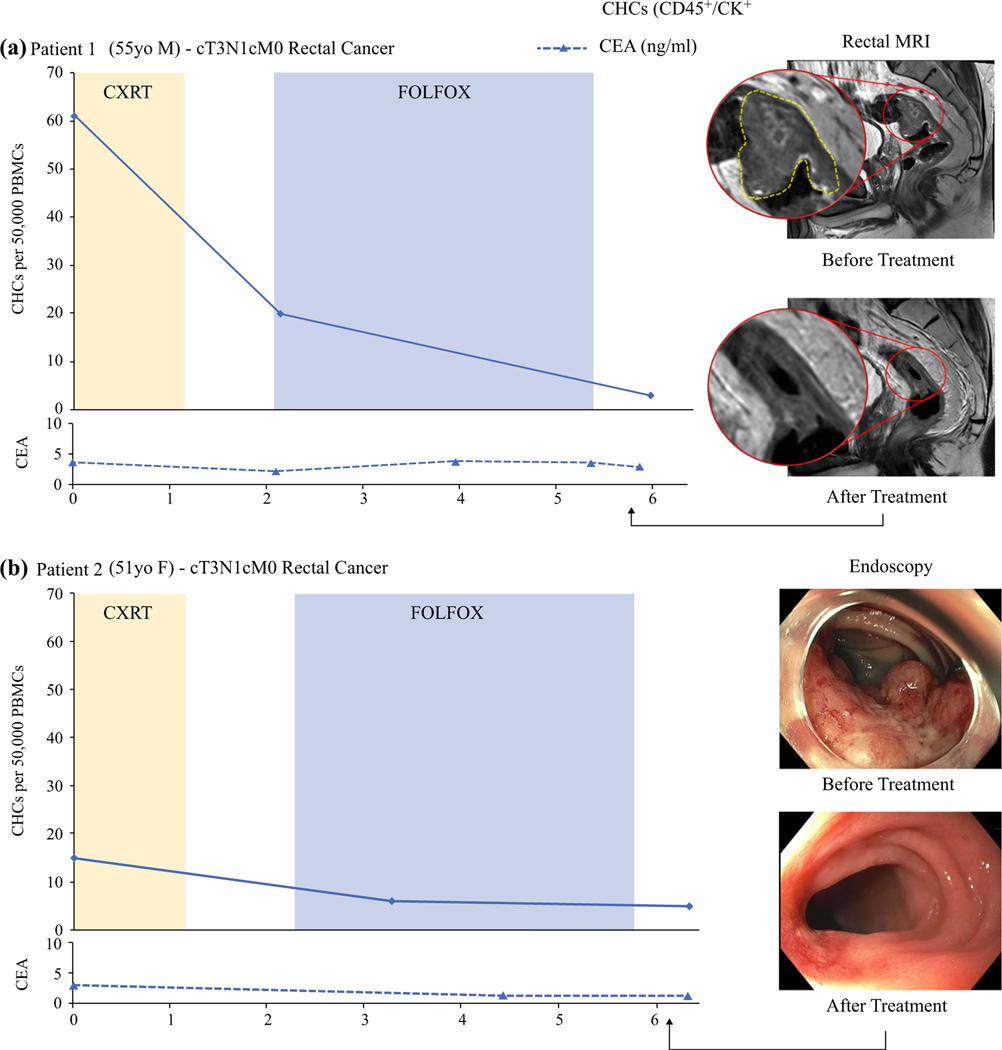
To evaluate how CHC levels change in response to NAT, we longitudinally evaluated two RAC patients for CHC levels and clinical measures of treatment response including endoscopy, rectal MRI, and CEA levels (Fig. 5). Both patients had a diagnosis of clinical stage 3 (cT3N1M0) rectal cancer and received upfront radiation therapy with concurrent trifluridine and tipiracil (TAS-102), followed by eight cycles of fluorouracil-leucovorinoxaliplatin (FOLFOX) chemotherapy as part of an ongoing clinical trial (NCT04104139).
FIG. 5.
Longitudinal evaluation showing that circulating hybrid cells (CHCs) decrease during neoadjuvant therapy (NAT) for rectal adenocarcinoma. a Patient 1 had clinical stage 3 rectal cancer treated with total neoadjuvant therapy (TNT) and rectal MRI demonstrating a near-complete clinical response (cCR). The CHC levels decreased 67% with the initiation of chemoradiotherapy (CXRT). At the end of leucovorin-fluorouracil-oxaliplatin (FOLFOX) chemotherapy, the CHCs were 95% of their pre-therapy levels. The carcinoembryonic antigen (CEA) level had no clinically relevant changes with NAT. b Patient 2 had clinical stage 3 rectal cancer that demonstrated findings of a cCR by endoscopy. The CHC level decreased with CXRT and two FOLFOX cycles but remained stable at the end of NAT. The CEA levels were not associated with treatment response.
Patient 1 (PT1) had a 67% drop in CHC levels after CXRT, at which time rectal MRI demonstrated a reduction in both tumor burden and size of the mesorectal lymph nodes. After completion of chemotherapy, the number of detectable CHCs had decreased by 85% and was similar to the levels found in pCR patients. Rectal MRI demonstrated minimal residual enhancement, and superficial ulceration with erythematous mucosa was found on endoscopy (near-complete response). Follow-up studies after 2 months did not identify any evidence of tumor (cCR), and at this writing, the patient is clinically disease free after 6 months.
Patient 2 (PT2) had a partial response to CXRT according to rectal MRI and a more dramatic response after chemotherapy, with complete tumor resolution on MRI and only a flat scar with telangiectasias found on endoscopy (cCR). Although PT2 had a lower baseline CHC level than PT1 (15 vs 61), the CHCs of PT2 decreased by 60% after CXRT and two cycles of FOLFOX, similar to that seen in PT1. At the time of the final sample collection, PT2 had evidence of a cCR and a CHC level that remained stable. After 2 months, regrowth of the tumor developed, and PT2 underwent resection, but no additional blood samples were collected.
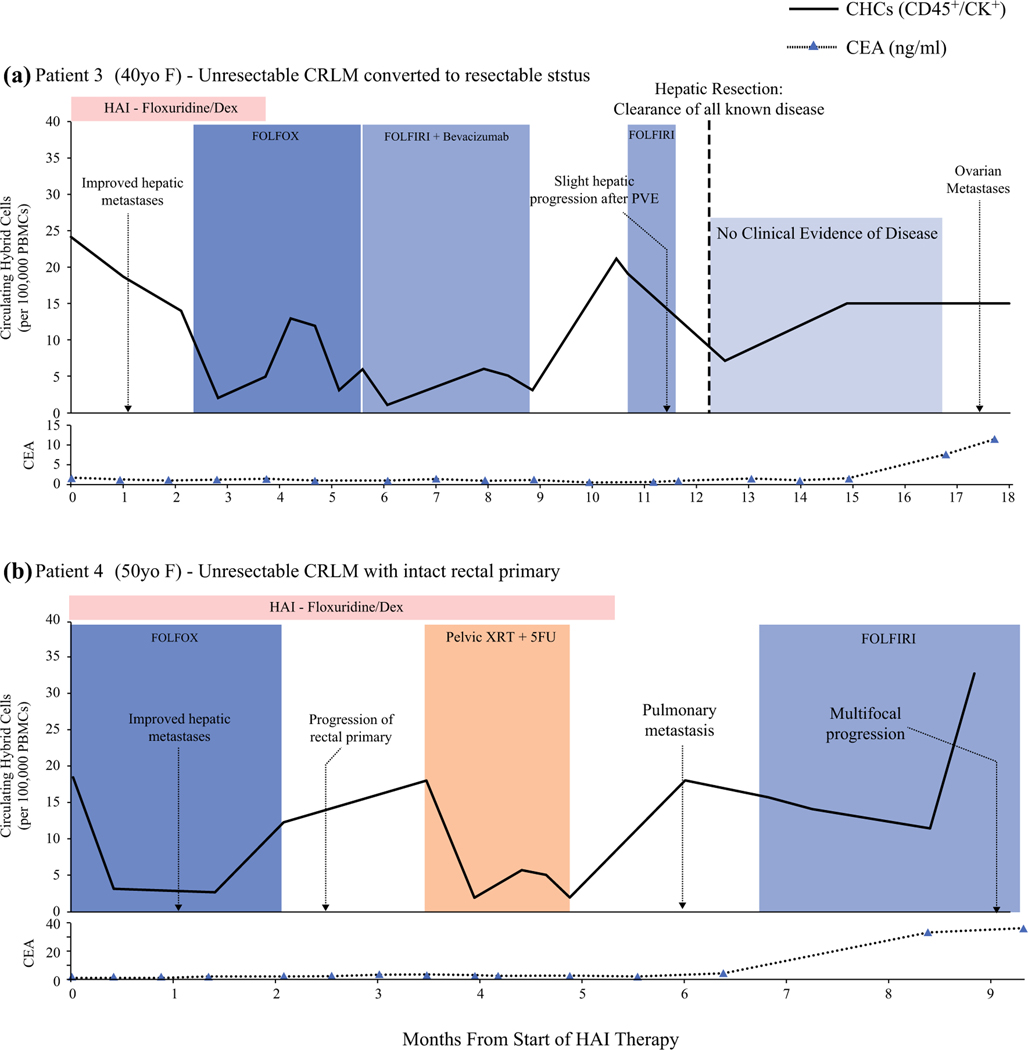
CHC Levels Correlate with Therapeutic Response and Disease Progression in Advanced CRLM
To evaluate the utility of CHCs as a biomarker of treatment response in patients with complex disease, we longitudinally evaluated CHC levels in two patients with advanced, initially unresectable CRLM at the start of HAI pump therapy with floxuridine and dexamethasone (dex). Analysis was performed by two separate established approaches, immunohistochemistry for patient 3 (PT3) and flow cytometry for patient 4 (PT4), to demonstrate that CHC analysis is not isolated to a single platform (Fig. 6). For both patients, synchronous CRLM was diagnosed without evidence of extrahepatic metastasis 6 months after the start of HAI therapy and reception of preoperative FOLFOX (7 and 10 cycles).
FIG. 6.
Circulating hybrid cell (CHC) levels correlate with treatment response and disease progression in patients who had advanced colorectal liver metastases (CRLM) treated with hepatic arterial infusion (HAI) therapy. a Patient 3 had unresectable CRLM converted to resectable status after HAI and leucovorin-fluorouraciloxaliplatin (FOLFOX) chemotherapy. The CHC levels decreased in response to HAI therapy and increased with slight CRLM progression after portal vein embolization (PVE) and therapy hold. After hepatic resection, a downtrend in CHC level occurred before increasing despite no clinical evidence of disease. Ovarian metastases were later identified by imaging. The carcinoembryonic antigen (CEA) levels were unresponsive to disease status until late in the patient’s course. b Patient 4 had unresectable CRLM that responded to HAI therapy, evident by decreased CHC levels and imaging. The CHC levels increased before imaging findings of local progression at the rectal primary, which responded to pelvic radiotherapy (XRT) and 5-fluorouracil (5-FU). The CHC levels also increased with the development of pulmonary metastases and mildly decreased after leucovorin-fluorouracil-irinotecan (FOLFIRI) was initiated before spiking later with multifocal disease progression. The CEA increased only when the patient had widespread progression.
Initially, PT3 demonstrated a strong response to HAI floxuridine/dex but had mostly stable, necrotic-appearing hepatic disease for the remainder of her course. A year after starting HAI, the patient’s initially unresectable CRLM was converted to resectable CRLM, and she was cleared of all known disease. Pathology showed a 95% treatment response and that most tumors had more than 80% necrosis. No clinical evidence of hepatic or extrahepatic disease was observed until bilateral ovarian metastases were discovered after 7.5 months.
Similar to PT3, the intrahepatic disease of PT4 demonstrated a good response to combined HAI floxuridine and FOLFOX. After six cycles of HAI therapy with continued intrahepatic response, the patient was found to have pulmonary metastases and was switched to leucovorin-fluorouracil-irinotecan (FOLFIRI) chemotherapy, but later experienced widespread progressive disease.
Although the two patients had differing clinical courses, we found similar trends in CHC levels that corresponded to their disease burden and treatment response on imaging. After the initiation of HAI therapy, the CHC levels for PT3 decreased by 42% during 2 months and then decreased by 85% with the addition of systemic therapy. Patient 4, who was immediately started on combined HAI and systemic therapy, had an initial 83% decrease in CHC levels after a single treatment cycle. Additionally, CHCs were elevated to more than 66% of the baseline level before clinical evidence of disease progression. Although the CHC levels of PT4 decreased to near normal with CXRT, only a 35% decrease was observed after FOLFIRI was initiated, and the levels then increased with evidence of widespread progression. Additionally, after PT3 restarted FOLFIRI and had clearance of all known disease, the CHC levels remained elevated and further increased despite no clinical evidence of disease, possibly reflecting the undiscovered ovarian metastases. Notably, PT3 had an increase in CHC count after stopping HAI therapy, but although no obvious evidence of disease progression was identified, it could possibly have been related to the biliary inflammation from floxuridine toxicity reported on imaging.
DISCUSSION
As new therapeutic strategies are added to our arsenal against GI malignancies, an increasing need exists for better informed management decisions for individual patients. Although resection has historically been the foundation of curative treatment, a personalized approach could spare select patients from unnecessary surgery and prioritize more effective therapies. However, to achieve this goal, better biomarkers are needed to provide this evolving care more effectively and safely. Because CHCs are a disseminated tumor-derived cell population, the CHC level is influenced by the extent of viable tumor cells and therefore associated with therapeutic response. Our data demonstrated the potential of CHCs to provide a noninvasive analyte for evaluating treatment responsiveness in real time, which could have important clinical implications for patients with esophageal and rectal cancers.
The CHC level in RAC patients at the time of resection provides a sensitive marker of response to NAT treatment and could have additional clinical utility for early identification of disease progression in patients with advanced CRLM. With an AUC of 0.971 in the ROC analysis, the CHC biomarker may outperform the current standards for predicting pCR,18,39 as well as those of other circulating biomarkers such as CEA and circulating tumor DNA.40,41 However, combining CHC analysis with established disease markers likely would increase sensitivity, and additional studies with larger cohorts are needed. Whereas pre-surgical levels provide a robust indictor of treatment response, longitudinal evaluation offers an important context to the evolving tumor because pre-therapy levels could have an impact on the interpretation of treatment-related changes. For example, despite similar CHC levels after NAT, PT1 had a 95% decrease in CHCs from baseline, whereas PT2 only had a 66% decrease and later experienced recurrence. Possibly a greater change from the baseline CHC level grants greater insight into a tumor’s response to NAT than a single time point. Future studies will need to include pretreatment sampling from more patients for a better understanding of this relationship and to determine whether the rate or degree of change in CHC levels is reflective of treatment response.
Our findings support the potential clinical utility of CHCs for assessing RAC patients with cCR under consideration for organ preservation strategies and for identifying patients with residual disease needing additional therapy. Although only two patients were analyzed throughout NAT, their CHC levels reflected the clinical response identified by both endoscopy and rectal MRI. This was further supported by our findings in patients with advanced unresectable CRLM, whose CHC levels decreased after new therapies were initiated, with evidence of treatment response on imaging. Conversely, elevations in CHC levels were associated with disease progression in both CRLM patients and occurred before clinically detectable changes on imaging or CEA testing.
Frequent CHC evaluation could benefit patients with advanced disease and those pursing watch-and-wait strategies by detecting progression or recurrence earlier.
Additionally, the CHC level provided insight concerning the burden of residual disease after therapy. At the time of hepatic resection, PT3 likely had clinically undetectable ovarian metastases, and her persistently elevated CHC levels could have indicated a continued need for systemic therapy. In addition, PT3 had stable-appearing hepatic disease by imaging, but mostly necrotic tumors on pathology, highlighting the observation that classic imaging techniques rely heavily on changes in tumor size to determine treatment effect28 and are poorly suited for assessing a cancer’s viability.24 Alternatively, circulating biomarkers can provide a more complete assessment of cancer status of patients with complex disease and better inform therapy decisions.
For patients with EAC, pCR from NAT is associated with improved overall survival and recurrence-free survival after resection.42 Similar to RAC, our analysis of EAC patients at the time of resection showed that CHC levels were associated with the degree of treatment response, predicting pCR with an AUC of 0.800. Studies have indicated that imaging techniques have poor accuracy for identifying pCR in patients with EAC,43 and current strategies for assessing clinical response require endoscopic ultrasound with multiple biopsies, which are subject to sampling error.16,17
For select patients, TNT remains an option because the benefit of resection has been debated for both patients with high-risk disease and patients with cCR,16,17,44 and favorable results have been reported for salvage esophagectomy after close surveillance.21 As a noninvasive method of assessing treatment response and disease burden after TNT, a clinical CHC assay could more frequently test for possible treatment failures among patients with EAC.
In addition to treatment response, CHC levels were associated with residual nodal disease in this study, which has previously been reported to have an impact on survival and is poorly assessed by imaging.45 Finally, although larger studies with longer patient follow-up evaluation are needed for a better understanding of the hybrid cell biomarker’s prognostic importance, our finding that CHC level is associated with 1-year DSS suggests that its value may extend beyond assessing treatment response. Together, these data support the potential clinical utility of CHCs for better informing therapy decisions for patients with EAC across the continuum of their care.
Our study had limitations, including the small number of patients in each treatment response group. We analyzed CHC levels in the EAC and RAC groups both individually and combined to provide increased statistical power and further validate our findings. Additionally, we did not include a designated validation cohort to establish CHC thresholds for predicting response or oncologic outcomes in a testing cohort. Larger prospective studies will more rigorously evaluate the CHC biomarker in EAC, in RAC, and across the spectrum of GI malignancies. Although potential bias exists in the quantification of CHCs, all efforts were made to remain blinded to patient outcomes during CHC analysis. Finally, the timing of sample collection from the longitudinal patients was influenced by availability and thus not standardized across patients.
In conclusion, CHCs are a novel noninvasive biomarker with great potential for monitoring treatment response and disease progression in gastrointestinal malignancies. With the ability to predict pCR accurately in patients treated with NAT, CHCs could help guide clinical decision-making for individual patients.
Supplementary Material
Acknowledgments
FUNDING BW: Newton Esophageal Cancer Foundation, Medical Research Foundation (MRF), Collins Medical Trust. MW: DOD W81XWH-18-1-0621, NIH/NCI P30 CA069533. TLS: MRF
Footnotes
DISCLOSURES EC: Investigator-initiated trial sponsored by Taiho Oncology, Inc. MW: Intellectual Property—Patents & Copyrights—circulating hybrid cell as a biomarker for disease status
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1245/s10434021-10379-2.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics,2021. CA Cancer J Clin. 2021;71:7–33. 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Gast CE, et al. Cell fusion potentiates tumor heterogeneity andreveals circulating hybrid cells that correlate with stage and survival. Science Adv. 2018;4:7828. 10.1126/sciadv.aat7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton TL, Walker BS, Wong MH. Digesting the importance ofcell fusion in the intestine. Cell Mol Gastroenterol Hepatol. 2021;11:299–302. 10.1016/j.jcmgh.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton TL, Walker BS, Wong MH. Circulating hybrid cells jointhe fray of circulating cellular biomarkers. Cell Mol Gastroenterol Hepatol. 2019;8:595–607. 10.1016/j.jcmgh.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietz MS, et al. Relevance of circulating hybrid cells as a noninvasive biomarker for myriad solid tumors. Biorxiv. 2021.2003.2011.434896. doi: 10.1101/2021.03.11.434896 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theodoropoulos G, et al. T-level downstaging and completepathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895–903. 10.1007/s10350-004-6325-7. [DOI] [PubMed] [Google Scholar]
- 7.Allal AS, et al. Sphincter-sparing surgery after preoperative radiotherapy for low rectal cancers: feasibility, oncologic results, and quality-of-life outcomes. Br J Cancer. 2000;82:1131–7. 10.1054/bjoc.1999.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma B, et al. What has preoperative radio(chemo)therapy broughtto localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int J Cancer. 2017; 141:1052–65. 10.1002/ijc.30805. [DOI] [PubMed] [Google Scholar]
- 9.Martin ST, Heneghan HM, Winter DC. Systematic review andmeta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28. 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 10.Benson AB, et al. NCCN Guidelines Insights: Rectal Cancer,Version 6.2020. 18, 806. doi: 10.6004/jnccn.2020.0032 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Konanz J, Herrle F, Weiss C, Post S, Kienle P. Quality of life ofpatients after low anterior, intersphincteric, and abdominoperineal resection for rectal cancer: a matched-pair analysis. Int J Colorectal Dis. 2013;28:679–88. 10.1007/s00384013-1683-z. [DOI] [PubMed] [Google Scholar]
- 12.Chan KKW, et al. Neoadjuvant treatments for locally advanced,resectable esophageal cancer: a network meta-analysis. Int J Cancer. 2018;143:430–7. 10.1002/ijc.31312. [DOI] [PubMed] [Google Scholar]
- 13.Tougeron D, et al. Definitive chemoradiotherapy in patients withesophageal adenocarcinoma: an alternative to surgery? J Surg Oncol. 2012;105:761–6. 10.1002/jso.22157. [DOI] [PubMed] [Google Scholar]
- 14.Shridhar R, et al. Single-institution retrospective comparison ofpreoperative versus definitive chemoradiotherapy for adenocarcinoma of the esophagus. Ann Surg Oncol. 2014;21:3744–50. 10.1245/s10434-014-3795-2. [DOI] [PubMed] [Google Scholar]
- 15.Smith JJ, et al. Organ preservation in rectal adenocarcinoma: aphase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. 10.1186/s12885-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noordman BJ, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19:965–74. 10.1016/s1470-2045(18)302018. [DOI] [PubMed] [Google Scholar]
- 17.van der Wilk BJ, et al. Active surveillance versus immediatesurgery in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter propensity-matched study. Ann Surg. 2019. 10.1097/sla.0000000000003636. [DOI] [PubMed] [Google Scholar]
- 18.Hiotis SP, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131–5. 10.1016/s1072-7515(01)01159-0. [DOI] [PubMed] [Google Scholar]
- 19.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501–13. 10.1016/s2468-1253(17)30074-2. [DOI] [PubMed] [Google Scholar]
- 20.van der Valk MJM, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–45. 10.1016/s0140-6736(18)31078-x. [DOI] [PubMed] [Google Scholar]
- 21.Sudo K, et al. Importance of surveillance and success of salvagestrategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol. 2014;32:3400–5. 10.1200/jco.2014.56.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarour LR, et al. Colorectal cancer liver metastasis: evolvingparadigms and future directions. Cell Mol Gastroenterol Hepatol. 2017;3:163–73. 10.1016/j.jcmgh.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckers RCJ, et al. Advanced imaging to predict response tochemotherapy in colorectal liver metastases: a systematic review. HPB. 2018;20:120–7. 10.1016/j.hpb.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Vera R, et al. Correlation of RECIST, Computed tomographymorphological response, and pathological regression in hepatic metastasis secondary to colorectal cancer: the AVAMET Study. Cancers Basel. 2020;12. doi: 10.3390/cancers12082259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maas M, et al. Follow-up after radiological intervention inoncology: ECIO-ESOI evidence and consensus-based recommendations for clinical practice. Insights Imaging. 2020;11:83. 10.1186/s13244-020-00884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemeny NE, et al. Hepatic arterial infusion versus systemictherapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395–403. 10.1200/jco.2005.03.8166. [DOI] [PubMed] [Google Scholar]
- 27.Zhang GQ, et al. Aggressive multimodal treatment and metastaticcolorectal cancer survival. J Am Coll Surg. 2020;230:689–98. 10.1016/j.jamcollsurg.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Hazhirkarzar B, et al. Current state of the art imaging approachesfor colorectal liver metastasis. Hepatobil Surg Nutr. 2020;9:35–48. 10.21037/hbsn.2019.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shabo I, et al. Roles of cell fusion, hybridization, and polyploidcell formation in cancer metastasis. World J Clin Oncol.2020;11:121–35. 10.5306/wjco.v11.i3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiler J, Dittmar T. Cell fusion in human cancer: the dark matterhypothesis. Cells. 2019;8. doi: 10.3390/cells8020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–41. 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakhoum SF, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–72. 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell.2016;164:1233–47. 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laughney AM, et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat Med. 2020;26:259–69. 10.1038/s41591-019-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng KS, et al. ALICE: a hybrid AI paradigm with enhanced connectivity and cybersecurity for a serendipitous encounter with circulating hybrid cells. Theranostics. 2020;10:11026–48. 10.7150/thno.44053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin MB, Edge SB. AJCC Cancer Staging Manual. Springer, 2017. [Google Scholar]
- 37.Westerhoff M, Osecky M, Langer R. Varying practices in tumor regression grading of gastrointestinal carcinomas after neoadjuvant therapy: results of an international survey. Mod Pathol. 2020;33:676–89. 10.1038/s41379-019-0393-7. [DOI] [PubMed] [Google Scholar]
- 38.Shang QX, et al. Prognostic significance and role in TNM stage of tumor deposits in esophageal cancer. J Thorac Dis. 2017;9:4461–76. 10.21037/jtd.2017.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho MS, et al. Endoscopy and magnetic resonance imaging based prediction of ypT stage in patients with rectal cancer who received chemoradiotherapy: results from a prospective study of 110 patients. Medicine Baltimore. 2019;98:e16614. 10.1097/md.0000000000016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dayde D, Tanaka I, Jain R, Tai MC, Taguchi A. Predictive and prognostic molecular biomarkers for response to neoadjuvant chemoradiation in rectal cancer. Int J Mol Sci. 2017;18. 10.3390/ijms18030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheong C, Shin JS, Suh KW. Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer. World J Gastroenterol. 2020;26:7022–35. 10.3748/wjg.v26.i44.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum Murphy M, et al. Pathological complete response inpatients with esophageal cancer after the trimodality approach: the association with baseline variables and survival: the University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123:4106–13. 10.1002/cncr.30953. [DOI] [PubMed] [Google Scholar]
- 43.de Gouw D, et al. Detecting pathological complete response inesophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta-analysis. J Thorac Oncol. 2019;14:1156–71. 10.1016/j.jtho.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Goense L, et al. Preoperative nomogram to risk stratify patientsfor the benefit of trimodality therapy in esophageal adenocarcinoma. Ann Surg Oncol. 2018;25:1598–607. 10.1245/s10434-018-6435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanoni A, et al. ypN0: does it matter how you get there? Nodaldownstaging in esophageal cancer. Ann Surg Oncol. 2016;23:998–1004. 10.1245/s10434-016-5440-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.