Abstract
Pars plana vitrectomy has become the standard procedure for primary macular holes (MHs) repair, including the removal of the posterior cortical vitreous, the stripping of eventual epiretinal membranes, and finally an intraocular gas tamponade. During this procedure, peeling the internal limiting membrane (ILM) has been proven to increase closure rates and avoid postoperative reopening in several researches. In fact, even in large MHs more than 400 µm, the advantage of peeling off the ILM was highlighted by better anatomical closure rates. Nevertheless, some authors suggested that ILM peeling is not always essential, because it generates various side effects in retinal structure and function. Furthermore, the ideal amount of ILM peeling and the most effective strategies for removing the ILM are still subject of research. Different surgical modifications have been reported as alternatives to traditional peeling in certain clinical settings, including ILM flaps, ILM scraping, and foveal sparing ILM peeling. As regards large MHs, the introduction of ILM inverted flap appeared as a game changer, offering a significantly higher >90% closure rate when compared to traditional ILM peeling. Modifications to inverted ILM flap procedures have been claimed in recent years, in order to define the best area and direction of ILM peeling and its correlation with functional outcomes. Moreover, several innovations saw the light in the setting of recurrent MHs, such as ILM free flap transposition, inverted ILM flap combined autologous blood clot technique, neurosensory retinal flap, and human amniotic membrane (HAM) plug, claiming higher anatomical success rate also in those complex settings. In conclusion, the aim of this review is to report how the success rate of contemporary macular surgery has grown since the turn of the century, especially for big and chronic MHs, analyzing in which way ILM management became a crucial point of this kind of surgery.
Keywords: macular hole, vital dyes, internal limiting membrane, inverted flap, peeling, human amniotic membrane
Introduction
Idiopathic macular holes (MH) is a vitreomacular interface disorder with a complex and not fully understood mechanical pathogenesis.1 His prevalence is estimated in 3.3 cases every 1000 individuals, in the over-55 aged subgroup, with a female-to-male ratio to be around 3:1. The population-based incidence of MH is 8.7 eyes per 100,000 individuals per year.2
Gass classified four stages of MH evolution, based on careful ophthalmoscopic exam.3,4 Stage I corresponds to a central yellow spot observed at the foveal center during fundoscopy, with loss of the foveal depression (Stage Ia). This can be followed by the formation of a yellowish reflection, which assumes a ring shape (Stage Ib), without a full thickness defect. Stage II indicates a small Full Thickness Macular Hole (FTMH, <400 µm), often associated with a visible operculum, which is the roof of the cysts seen in stage I. Stage III is characterized by the widening of the FTMH to more than 400 um in diameter, but complete posterior vitreous detachment (PVD) has not yet occurred, with the vitreous still adhering to the MH edges and to the papilla. Stage IV is the same as Stage III after complete vitreous separation from the disk.
With the introduction of spectral domain optical coherence tomography (SD-OCT), the studies about the relationship between the vitreous cortex and the macula in vitreoretinal interface disorder has been deepened. In 2013, the International Vitreomacular Traction Study Group directed by Duker proposed an anatomical classification of vitreoretinal interface disorders using SD-OCT, introducing three different disorders: vitreo-macular adhesion (VMA), vitreo-macular traction (VMT), corresponding to Gass’ stage 1 MH, and full thickness macular holes (FMTH). The latter is defined as “interruption of all retinal layers extending from internal limiting membrane (ILM) to the retinal pigment epithelium (RPE)”. It is sub-classified according to the size of the hole measured with OCT and by the presence or absence of VMT. The diameter was used to define small (<250 µm), medium (250–400 µm), and large (>400 µm) MHs.5
The first description of vitrectomy surgery for macular hole repair dates back to 1991 with the seminal report by Kelly and Wendel.6 From that moment, numerous changes to the procedure contributed to the evolution of macular hole surgery, optimizing success rates from the initially reported 58%6 to more than 90% in most recent series.7–16 In 1997 Eckardt et al firstly reported Internal Limiting Membrane (ILM) peeling for MH surgery, initially encountering great skepticism in the ophthalmologic community.17 Nevertheless, this procedure gradually gained widespread acceptance thanks to the improvement of closure rates and prevention of late post-operative re-opening of MH, one of the most important described complications during follow-up.7,17,18 As of today, pars plana vitrectomy (PPV) combined with ILM removal and gas tamponade is considered as the standard procedure in the treatment of MH.
However, debates still remain as ILM peeling may cause iatrogenic damage and determine changes in retinal structure and visual function.19–21 Moreover, several approaches of ILM peeling has been described, offering the surgeon a range of options as regards the extent of ILM peeled during surgery and how the ILM is peeled.22
Structural ILM Role
The ILM forms the innermost layer of the retina, composed of the internal expansion of Muller cell footplates and a fibrous layer,23 but it also guests proteins shed into the vitreous cavity from the lens and ciliary body during embryogenesis.24 Its composition mostly consists of type IV collagen, but also of laminin, fibronectin, glycoproteins, and glycosaminoglycans.23,25 Many of these molecules permit both the adhesion of the ILM to the retina but also the adhesion of the cortical vitreous to the ILM.22
This membrane has a thickness of 400 nm at the peripheral retina, increasing to about 1400 nm in the macular area. His structure offers much more mechanical strength than retinal cell layers, contributes for at least 50% of the retinal rigidity.26,27 From this assumption, the rationale of its removal is to increase retinal compliance, aiding hole closure.27,28
The ILM could also act as a scaffold for myofibroblasts, fibrocytes, and RPE cells proliferation, and the migration of glial cells onto the surface of the ILM can determine a tangential contractile force, hence contributing to MH advancement. On one side, surgical peeling of ILM offers many advantages, blocking this mechanism of residual tangential traction removing the remaining macular cortical vitreous, and moreover inhibiting the formation of post-operative epiretinal membranes, which could lead to a secondary tangential traction. Moreover, because of the stress to the Muller cell and feet, ILM removal may trigger a retinal glial cell proliferation response, which could accelerate MH contraction and healing.27,29,30 In fact, Müller cell proliferation is an inflammatory response that shields the neuro-retinal layers from mechanical stimuli and heals the retina after a mechanical or inflammatory injury. For example, in the instance of posterior pole vitreal traction, it protects photoreceptors against apoptosis caused by passive retinal motions.31
On the other side, the responsiveness of Müller cells may have a deleterious impact on vision. Changes in Müller cells during ILM peeling surgery could create intraretinal fibrosis, which may lead to changes in neuronal intraretinal connections and macular photoreceptor metabolism. Furthermore, epiretinal fibrosis may exacerbate this occurrence because the ILM serves as a scaffold for glial cell attachment and subsequent pathological proliferation.28,32–34 Müller cells, among other features, feed nutrition to neural cells and eliminate waste materials from them, as well as protecting them from neurotoxic neurotransmitters (eg, glutamate) and electrolytes (eg, potassium). Müller cells are thought to make retinoic acid from retinol as well.
After ILM peeling, studies show incomplete recovery of Müller cells although it is unclear if a full restoration can occur over time. Furthermore, the clinical significance of this anomaly has yet to be determined.20,35
Indications for ILM Peeling
The Cochrane Database of Systemic Reviews stated in 2013 that there was adequate evidence to suggest the favorable benefits of ILM peeling for stages 2–4 idiopathic FTMHs to enhance the primary anatomical hole closure rate.36 A randomized clinical trial by Lois et al reported an 84% closure rate of MHs in eyes that underwent ILM peeling compared with 48% of eyes without ILM peeling. There were also fewer reoperations (12% vs 48%, respectively).37 ILM peeling also helps FTMH patients with extreme myopia, notably those with posterior pole staphyloma, myopic retinoschisis, and localized retinal detachment.38 The necessity for ILM peeling on smaller MHs, especially those smaller than 250 µm, is more debatable. Some authors did not evidence benefit from ILM peeling for FTMH smaller than 400 µm.39 It’s also widely known that small MHs may close spontaneously with vitreomacular adhesion release significantly more frequently than bigger ones. Smaller holes are assumed to have lower degrees of tangential traction, and investigations have revealed that the level of ILM vitreous side debris (residual vitreous and ERM) is proportional to hole size and stage, with larger quantity of debris for stage 4 MHs.40
Moreover, the hole shape may be relevant to determine its closure rate. MHs having a minor difference in diameter between the midway and the base, showing a rectangular shape, have a greater rate of closure rather than holes, which are triangular-shaped. Thus, this subcategory of small rectangular MHs may be considered for pars plana vitrectomy without ILM peeling.40,41
ILM Staining
Adjuvant dyes, also known as chromo-vitrectomy, are reportedly useful in MH surgery to improve visibility of the pre-retinal membranes and/or ILM, allowing for more precise peeling and reducing the risk of iatrogenic mechanical stress to the retina. ILM peeling is frequently aided with intraoperative dyes.
In addition, vital dyes determine an increase of retinal stiffness, through an illumination-dependent mechanism. Raised ILM rigidity aids peeling and the initial formation of an ILM flap, but may also cause a variation of the ILM cleavage plane from the retina, resulting in a deeper plane of separation.42,43 Whichever of the dye employed, dye concentration and contact time with the retina should be kept to a minimum, with contact periods of 5 to 10 seconds providing sufficient staining contrast.40
Indocyanine green (ICG),44 triamcinolone acetonide,45 brilliant blue G (BBG)46 and trypan blue (TB) are some of the dyes used to stain the ERM and ILM. Triamcinolone acetonide is indeed not a vital dye that can be washed away by irrigation. Triamcinolone acetonide attaches to the retina and is subsequently removed during ILM peeling, contrasting the area of ILM remaining where its particles are still adhered.45
ICG is reported to have a strong affinity with the ILM, offering a good contrast between stained and unstained retina.44 However, there were reports of deleterious consequences in individuals who had received ICG-assisted surgery shortly following the first report. In vitro studies revealed an extremely dose-dependent toxicity,47–49 with noticeable damage to the inner retina50 and other alterations, such as low visual outcomes and optic nerve injury.51,52 ICG may be harmful to the retina due to its osmolarity, concentration, and presence of iodine.53
Based on these concerns, TB and BBG have been developed as iso-osmolar solutions. BBG shows a preferential affinity for ILM,46 while TB not only stains the ILM but also the ERM.54 A comparison among BBG TB, and ICG during ILM peeling in MH patients showed that BBG outperformed the other two dyes in terms of operation facilitation, ease of preparation and removal, and ILM staining. It was also better for the retina because it did not contain harmful iodine, providing it an edge over TB and ICG.55
Recent studies have introduced acid violet 17 (AV) as a new vital dye, successfully stain the ILM resulting in less retinal debris, thus offering a different cleavage plane from the retina, but with lowered staining contrast than brilliant blue.56 AV seems to determine no functional or morphologic retinal toxicity either in rabbit models and in human eyes but further studies are required to assess AV safety pattern and effectiveness.56,57
ILM Peeling Technique
Peel Initiation
The formation of an ILM flap, which allows the peel to begin, is a crucial stage in ILM peeling. The ideal starting point of ILM peeling has not been defined yet. Its thickness varies across the fovea reaching its maximum thickness at a point approximately 1000 um from the foveal center, which correlates with the location of greatest ILM rigidity.58 The temporal retina, on one side, has the thinnest ILM, while the nasal retina carries the papillo-macular nerve-fiber bundle. In conclusion, the ideal starting point may localize 1000 µm above or below the foveola.
After sufficient staining of the ILM, picks and micro-vitreoretinal blades, among other devices, have been utilized to create a small cleft on the retinal surface. Alternatively, a flap can be made by gently scraping the ILM surface with a diamond-dusted membrane scraper or, much recently, using a micro-serrated nitinol loop (Finesse Flex Loop, Alcon, Ft. Worth, TX) of variable length and stiffness to create an edge on the ILM.59
Despite all, many surgeons utilize a direct “pinch” approach with custom-designed forceps to begin a flap. In this technique, the ILM is gripped with forceps, gently elevated off the retinal surface, and then pulled tangentially to create a flap with the rip point 180 degrees from the pull direction. To prevent capturing retinal tissue in the initial “pinch,” forceps design as well as hand control are crucial.
Peeling Propagation and Extension
Both vitreoretinal forceps and diamond-dusted membrane are capable for ILM peeling propagation.60 The former is most usually used to remove the required region of ILM utilizing circular motions around the fovea once the flap is produced, similar to capsulorhexis in cataract surgery.61 The optimization of this procedure aims to reduce the shear stress on the retinal layers. Intraoperative OCT may provide useful information for advising surgeons in this area,62 but also computer models have been developed to study the best angle for peeling. Drogamaci et al developed a simulation software which evidenced that peeling at 165 degrees to the retinal plane gives the minimum shear stress to retinal layers.63
Regarding ILM peeling extent for MHs, there are not global guidelines among surgeons. The majority tends to peel an ILM radius of around one disk diameter around the foveal center, although reports range from 0.5 to 3 disk diameters.64 Theoretically, for every macular hole and depending on its shape and dimension, there may be a customized ILM-peel area that allows for enough decreased retinal compliance to facilitate anatomical closure. Debate is still on to determine which extent of ILM peeling may ensure that this threshold dimension is matched but at the same time minimizing inner retinal alteration.
Some studies found out an improvement in metamorphopsia with a bigger ILM peeling extent (1.5 vs 0.75 diameter disks).65 On the other side, Modi et al Argued for better visual results with less thinning of nerve-fiber layer if the peel radii is kept at 1 diameter disks against 1.5 diameter, but with no difference in MH closure rates between the two subgroups.66 Meantime, some authors have claimed for large ILM peel regions in all instances.67
Side Effects of ILM Peeling
ILM peeling is a difficult surgical treatment with a number of possible drawbacks, either immediate and late.
Instrument stress at the initiation and ILM pickup locations can result in focal retinal hemorrhages, Nerve Fiber Layer (NFL) damage, and whole thickness retinal abnormalities, while iatrogenic eccentric holes have also been described.68–70 As the ILM is peeled off the retinal surface and separated from instrument stress, it is also seen that superficial retinal hemorrhages, mostly located nasally, can develop. This occurs as a consequence of Muller cells traction above retinal capillaries.71
A distinct alteration reported from 1 to 6 months after MHs surgery, firstly described by Tadayoni et al, is known as Disassociated Optic Nerve Fiber Layer (DONFL) and appears as a defect of inner retinal layers.72–74 This alteration was initially noticed after ERM peeling surgery, but it was later discovered to be unique and limited to the region where the ILM had been peeled.72,73,75,76 The exact process is unknown, although it has been suggested by a number of scientists that it is linked to damage to the Muller cell end plates, which abut and partially constitute the retinal side of the ILM.72,77 DONFL extent is indeed linked with Muller cell debris found on the ILM side after peeling and may be visible on SD OCT as apparent depressions in the nerve fiber layer that can extend into the inner plexiform layer. DONFL visualization can also be enhanced in red-free and in blue reflectance imaging.78–81 Steel et al60 described a statistical difference in DONFL degree comparing forceps pinch–peel technique against diamond dusted membrane scraper (DDMS), with more retinal debris found in the ILM after DDMS peel rather than forceps one, suggesting that surgical instrument characteristics are important to define the plane of retinal separation of the ILM. Functionally speaking, this alteration may lead to microperimetry defects and loss of macular sensitivity,71,73 but generally without affection on visual acuity.
ILM peeling may also lead to retinal morphologic changes, such as retinal displacement. Ishida et al82 indeed described a shifting of the fovea toward the optic nerve head, which has been linked to nasal retina thickening and temporal retina thinning.83 A centripetal but asymmetrical retinal shifting has been highlighted, because the horizontal meridian moves more than the vertical, and the temporal retina moves more toward the disk than the nasal.82,84
Paracentral scotomas and reduced central retinal sensitivity have been reported after ILM peeling, but with controversial clinical significance.73,85,86 The lower inner retinal volume in the macular area following ILM peeling may affect postoperative visual result, leading to functional defect.87 Despite this, the analysis of the Cochrane Database of Systemic Reviews showed no difference in post-operative visual acuity between peeling and no-peeling groups. Furthermore, a statistically significantly higher rate of additional surgery was required in participants in whom the ILM was not peeled.36
ILM Peeling Technique Variants
Foveolar Sparing ILM Peeling
Reducing ILM damage was the aim Ho et al pursued introducing foveal sparing ILM peeling.88 Using a combination peeling method with forceps and scissors if needed, the ILM is peeled off saving a center ring spanning 400 um around the MH rim.88 The flap can be cut down with scissors, similarly to the ILM flap, with the exception that the area where the ILM is left connected to the retina is wider. It was hypothesized that by retaining the ILM in its central location, the foveal microstructure would be retained better. The umbo of the fovea is believed to be necessary for optimal visual function. Muller cells in the fovea, with their expanded funnel shape, greater refractive index than surrounding tissue, and orientation parallel to light propagation, are hypothesized to operate as optical fibers, maximizing light transmission through the mesh-like inner retina to the photoreceptors.89 In the process of MH formation, the foveal center cones are avulsed from the retinal pigment epithelium and extend along the sides of the MH, but they keep their Muller cell sheaths and are therefore attached to the peri-MH ILM.90,91 By avoiding peeling this critical part of the ILM, Muller cell integrity is preserved, allowing for improved foveal shape reconstruction and, as a result, superior postoperative visual acuity.90 Ho et al compared eyes treated with foveal sparing ILM peeling with non-foveal sparing technique. After surgery, all eyes had a closed hole, but the foveal sparing group had much higher visual acuity, a better reconstitution of External Limiting Membrane (ELM) and Ellipsoid Zone (EZ) and a smooth foveal umbo restored appearance.88 Recently, Murphy et al proposed a variant technique of foveal sparing ILM peeling, which did not require the use of scissors. A series of overlapping circles of peeled ILM were created around the hole circumference, leaving a 500-um rim of ILM around the MH edge. If necessary, the ILM peeling region was expanded to 1 to 2 disk diameters surrounding the hole once this operation was completed. In this cohort of 34 eyes, a 100% rate of hole closure was achieved and a thicker foveal floor with less steep was reported.90
ILM Abrasion Technique
Mahajan et al introduced an alternative technique to avoid complete ILM peeling, defining it as ILM abrasion, which aims to remove all ILM surface vitreous and ERM material, without damage subjacent tissues.92 Following the core vitrectomy, a DDMS was scraped convex side down over the macula in both circumferential and radial motions in a region of one-disk diameter encompassing the MH, taking care to prevent unintended ILM rips. This procedure may reduce ILM thickness and loosen its attachment to the retina, without disruption of the underlying RNFL, but meantime still favor glial cell activation, increasing MH closure rate.92,93 This study reported a 94% closure rate with a single treatment in 100 eyes with Stage 2 to 4 holes utilizing the aforesaid approach, with visual outcomes comparable to ILM peeling group.92,94
Large Macular Hole Approach
Even with ILM peeling, MH with a diameter of 400 µm or higher has inadequate surgical results.95,96 In a comprehensive MH repair trial, the Manchester Large Macular Hole Study, Ch’ng et al used ILM peeling and gas tamponade, evidencing significantly different success rates for MH between 400 and 649 µm against those bigger than 650 µm (90 vs 76% closure rates). This research suggested that big FTMH should be reclassified, with novel surgical procedures, such as inverted ILM flaps, to be reserved for MH larger than 650 µm, since similar closure rates have been reported in smaller diameters.97
Various procedures, such as ILM flaps or retinal expansion techniques, have been observed to boost success rates when used in conjunction with ILM peelings.
Inverted ILM Flap
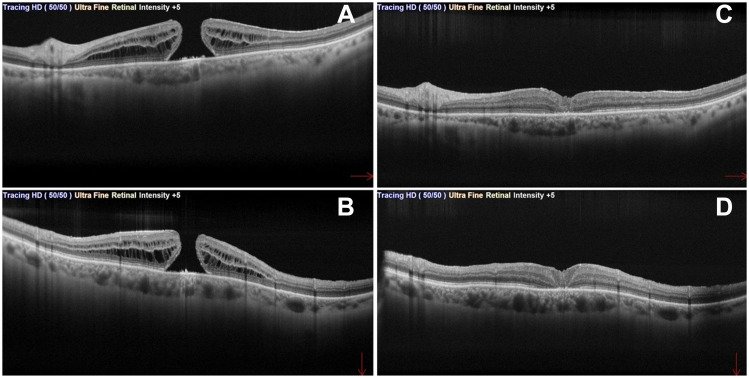
Michalewska et al established the inverted ILM-flap approach in 2010 for the treatment of large Stage 4 MHs.98 This approach ideally leaves a piece of ILM that adheres to the edge of the MH and then inverts to cover the MH [Figures 1 and 2].
Figure 1.
Full thickness macular hole (FTMH) in pre-operative (A and B) macula-cross OCT (red arrows indicates horizontal and vertical directions of the B-scan). Post-operative macula-cross (C and D) after PPV with inverted ILM flap technique.
Abbreviations: FTMH, Full-thickness macular hole; OCT, Optical coherence tomography; PPV, pars plana vitrectomy; ILM, internal limiting membrane.
Figure 2.
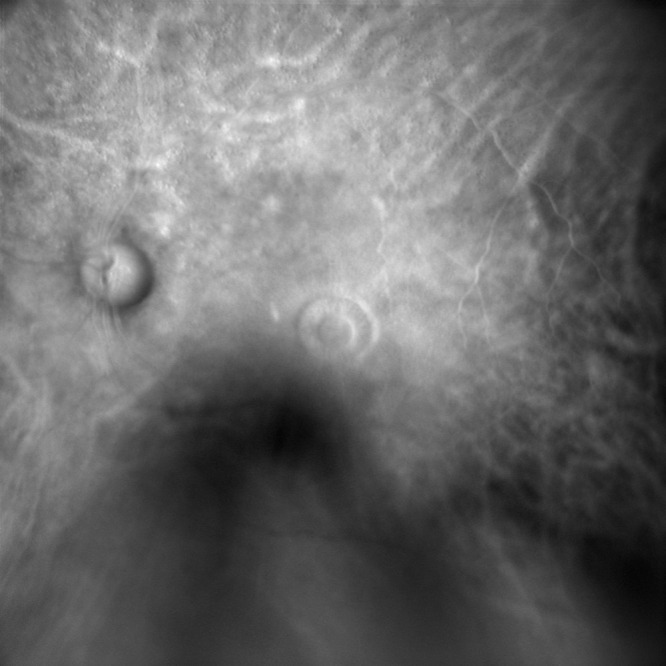
Retro mode pre-operative imaging of a FTMH, captured employing confocal scanning laser ophthalmoscopy, and showing alterations in RPE elevation at the hole’s boundaries.
Abbreviations: FTMH, Full-thickness macular hole; RPE, retinal pigmented epithelium.
According to the original description, the ILM was gripped with forceps and peeled off in a circular pattern for around 2 disc diameters around the macular hole. The ILM was not entirely removed from the retina during circumferential peeling, but a frill of ILM was left connected to the borders of the macular hole. A peripheral segment of the ILM was then clipped with a vitreous cutter or vitreous scissors to 0.5 mm to 1 mm in length, leaving the center component of the ILM in situ.98,99 As a result, minor portions of the ILM remained in the area of the macular hole. The ILM was then carefully rubbed across the macular hole from all sides until it inverted, or turned upside down, so that the surface that was ordinarily facing the vitreous body was directed towards the retinal pigment epithelium. The vitreous cavity, at the end of the surgery, was filled with air. SD-OCT could be conducted soon after surgery using air tamponade, which is not achievable with long-acting gas tamponade. This allowed for early postoperative visualization of macular holes.98
The specific method through which the inverted ILM flap approach improves surgical outcomes is unknown. According to one idea, the ILM flap acts as a scaffold or a sealant for glial cell growth, causing the MH to fill with proliferating cells, which improves MH closure.100 Another possibility is that the ILM acts as a barrier, preventing fluid from the vitreous cavity from entering the MH.101
Micro-structurally speaking, ILM peeling can cause gliosis because the peeled-off ILM contains Müller cell fragments. If a segment of peeled-off ILM is left connected, it may cause gliosis inside the retina as well as on the ILM’s surface. Based on this assumption, the ILM might potentially act as a scaffold for tissue growth. This theory is based on histopathologic evidence that cells always require a basement membrane in order to proliferate.102 As per Michalewska et al, SD-OCT showed improved foveal shape and increased macular hole covering tissue in the first several months after macular hole surgery with the inverted ILM flap method, even if the histology of this tissue was unknown.103,104 Glial cells were believed multiply, creating a favorable environment in which photoreceptors could take up new locations in close proximity to the fovea. These findings could explain why stimulating Müller cell proliferation enhances postoperative visual acuity as well as macular hole closure rate.98
The analysis of MHs closure patterns highlighted four categories: U and V shaped, irregular, and flat open,105 the first one being the more functionally favorable. Michalewska et al found out that after inverted-flap procedure, the creation of a U-shape closure type was the most common and had a better functional prognosis than other kinds of closure. Patients with U-shape closure exhibited fewer photoreceptor layer defects and normal retinal thickness at the end of the follow-up period, according to postoperative structural examination of the fovea.106,107
A thorough study of surgical outcomes of inverted ILM flap techniques for large MHs was published by Gu et al.108 This meta-analysis demonstrated that inverted ILM flap procedures are a successful operation for large MHs, with an anatomical closure rate of up to 95% and a substantial improvement in BCVA in 75% of eyes.108 Rizzo et al found out that the traditional ILM peeling approach had 78.6% success when compared to 95.6% for the ILM flap procedure in a large MHs cohort study.109 Other studies confirmed that inverted ILM flap technique is more effective than the classic ILM peeling for the closure of large stage 4 MHs > 400 μm, improving both anatomical and functional outcomes for both idiopathic and myopic MHs.110–113
By the way, this approach has several downsides, such as the ILM flap easily detaching from the MH’s edge during fluid–air exchange.114 Many academics have worked to improve this approach in order to address these drawbacks.115,116 MH closure was not accomplished in some cases, presumably because the ILM flap detached spontaneously following the fluid–air exchange.98
By the time, several variants of ILM inverted flap procedure have been proposed, in order to to lessen surgical time and the danger of retinal damage. Moreover, it has now been expanded to include MHs associated with severe myopia with and without accompanying perifoveal retinal detachment.99
Temporal Inverted ILM Flap
Michalewska again devised a modified “temporal” inverted ILM flap method in 2015, this time only inverting the temporal side of ILM flap to cover MHs bigger than 400 μm.117 The goal was to reduce the trauma generated by peeling to the ILM, limiting the shear stress to a more localized zone. In an area of roughly two optic nerve diameters, ILM forceps were utilized to grip and pull the ILM away from the temporal side of the macular hole. During this peeling, the ILM was not completely removed from the retina, but rather left attached to the temporal boundary of the macular hole, inverted, and progressively directed over the macular hole until sufficient coverage was achieved. The results revealed that MH closure was effective in 93% of 44 eyes, which was similar to the rate of closure with the initial inverted ILM flap.117
“Cover” and “Fill” Techniques for Inverted Flap
Rossi et al recently analyzed the differences among two different techniques for ILM inverted flap surgery.118 On one side, they defined to the original surgical procedure as the “Cover” technique, indicating the procedure in which the ILM flap is everted over the MH gap in a single layer. On the other side, the “Fill” technique refers to when the ILM flap is folded into many layers within the MH.118
In the Cover technique group, there was a rapid restoration of foveal depression and internal retinal layers. The ILM, which was occasionally duplicated, covered on top the closed MH, but it did not intrude into the prior MH. In this case, the outer retina receives less benefit and takes longer to heal, and indeed outer retinal cysts can persist for up to three months following surgery. Differently, in the eyes of the Fill group, the MH closed in the early post-operative period, with no hypo-reflective patches detectable at 1 or 3 months, while numerous and folded ILM layers perpendicular to the stacked retinal surface were visible in all the eyes throughout the follow-up period. In the Fill method, the ILM acts as a filler, adhesive, and scaffold at the same time.118
Both groups obtained a very high success rate and significant vision improvement, except for extremely large MHs exceeding 700 um, which closed more frequently when the Fill approach was used. In these cases, the simple creation of a roof on top of the hole might not be enough to start endogenous sealing mechanism in MHs exceeding a critical span.98
Other Variants of ILM Inverted Flap Technique
In order to lessen the risk of inadvertent ILM-flap avulsion after inverted flap surgery, Hussain et al proposed a zero vacuum-trimming technique.119 The ILM was peeled toward the fovea and trimmed with zero suction to leave a residual island of ILM attached at the fovea.120
Andrew et al presented a folded inverted ILM flap as another inverted ILM flap-related approach.121 In this technique, the mobilized ILM was placed into the bed of the MH and folded on itself in any orientation. Successively, a viscoelastic cover on the MH was injected in order to keep the ILM flap in place. The 24 eyes that received this procedure showed anatomical improvement, with a 100% closure rate and better BCVA.121
Ghassemi et al recently compared three inverted ILM flap techniques for treating large MHs, using 24 eyes with hemi-circular ILM peel with temporally hinged inverted flap, 23 eyes with circular ILM peel with temporally hinged inverted flap, and 25 eyes with circular ILM peel with superior inverted flap.122 Inverted flap procedures had a non-statistically significant closure rate difference, from 87.5% to 100%, but circular ILM peel with superiorly hinged inverted flap was the only technique to reach anatomical closure in all yes. BCVA was comparable among all subgroups.122
A novel technique, called ILM dragging and peeling, was proposed by Peng et al in 2020.123 Two horizontal ILM strips were peeled off in the inferior and superior quadrant of macula. A rectangle ILM flap was peeled from the superior to inferior area in a centripetal way to drag the superior edge of the MH with the assistance of ILM-retina adhesive force. A rectangle ILM flap in the inferior part was peeled in the same way from the opposite direction. After centripetally jointing the MH edges with ILM-retina adhesive forces, ILM was peeled in a traditional circular fashion.123 This technique derives from the idea that MH margins’ mobility is one of the most critical requirements for MH closure. ILM peeling may not give adequate mobility in some cases, particularly in chronic or big MH.124 The novelty of this approach relies on the fact that the retinal margins were narrowed and joined just taking advantage of the pre-existing ILM-retina adhesive force, thus reducing retinal damage induced by DDMS125,126 and forceps.124,127 There were no other harmful alterations in this group and the overall group’s MH closure rate was 96.2%.
One of the most recent variation of the ILM peeling and inverted flap technique consists in inverting the ILM flap under air, for large idiopathic MHs.128 The ILM flap was trimmed with a vitreous cutter after ILM peeling, leaving the central part attached to the hole’s edge. After that, a thorough fluid-gas exchange was accomplished, and the air pressure was reduced to around 20 mmHg to avoid ILM flap vanishing into the backflush needle. Finally, the ILM flap was inverted. MH closure was accomplished in all 20 eyes during follow-up.128
A review of large MH closure rates with different ILM approaches among several studies is reported in Table 1.
Table 1.
Large Macular Holes: Surgical Techniques with Various ILM Approaches and Outcomes Among Different Studies
| Study (Year) | Surgical Technique | Eyes (Number) | MH Size (μm) | Pre-Operative VA (LogMAR) | Post-Operative VA (LogMAR) | Closure Rate | Mean f-up (Months) |
|---|---|---|---|---|---|---|---|
| Michalewska et al (2010)98 | Inverted ILM flap | 50 | Range 415–1618 | 1.11 | 0.55 | 98% | 12 |
| Michalewska et al (2015)117 | Inverted ILM flap | 43 | Range 400–841 | 1.01 | 0.40 | 93% | 12 |
| Temporal inverted ILM flap | 44 | 1.03 | 0.45 | 93% | |||
| Andrew et al (2016)121 | Folded inverted ILM flap | 24 | Mean 528 | 0.90 | 0.48 | 100 | 12 |
| Mete et al (2017)111 | ILM peeling | 36 | Myopic MHs ≥ 400 | 0.60 | 0.58 | 61% | 6 |
| Inverted ILM flap | 34 | 0.70 | 0.39 | 94% | |||
| Ota et al (2018)113 | ILM peeling | 44 | Mean 465.9–491.5 | 0.70 | 0.30 | 93% | 6 |
| Inverted ILM flap | 46 | 0.80 | 0.40 | 100% | |||
| Ch’ng et al (2018)97 | ILM peel with gas tamponade | 258 | Range: 400–1416 | 0.95 | 0.62 | 90% | 3 |
| Rizzo et al (2018)109 | ILM peeling | 300 | MH ≥ 400 | 0.79 | 0.56 | 79% | 9 |
| Inverted ILM flap | 320 | 0.81 | 0.49 | 96% | |||
| Ghassemi et al (2019)122 | Hemicircular ILM peel with temporally hinged inverted flap | 24 | Mean 553–548 | 0.91 | 0.52 | 87% | 6 |
| Circular ILM peel with temporally hinged inverted flap | 23 | 0.90 | 0.53 | 91% | |||
| Circular ILM peel with superior inverted flap | 25 | 0.91 | 0.55 | 100% | |||
| Ramtohul et al (2020)110 | ILM peeling | 23 | Range 400–1159 | 1.04 | 0.70 | 70% | 6 |
| Inverted ILM flap | 23 | 0.92 | 0.45 | 96% | |||
| Peng et al (2020)123 | ILM dragging and peeling | 26 | Mean 524 | 1.20 | 0.70 | 96% | 21 |
Abbreviations: MH, idiopathic full-thickness macular hole; ILM, internal limiting membrane; VA, visual acuity.
ILM Role in Persistent/Recurrent Macular Hole
Failure of primary MH surgery or persistent MH is the commonest complication of MH repairing, accounting for 10–12%.129 The persistence of vitreomacular traction, either due to an inadequate removal of the ILM during past operations or a regeneration of the ERM, is assumed to be linked to the failure of primary surgery.17 A recent report by Baumann et al, the Manchester Revisional Macular Hole Study, claimed that a second PPV with ILM peel extension could guarantee a 89% closure rate for persistent MHs, and highlighted the crucial prognostic value of pre-operative OCT parameters.130
Furthermore, for these challenging conditions of unsuccessful closure or recurrence, several special techniques have been developed, such as internal limiting membrane transposition and tuck technique,131,132 inverted ILM flap combined autologous blood clot technique,133 neurosensory retinal flap,134 perfluorocarbon liquid-assisted inverted limiting membrane flap technique,116,135 and human amniotic membrane (HAM) plug.136–139
ILM Free Flap
In patients who have a persistent MH hole following earlier surgery with ILM peeling, a comparable method is the use of an ILM-free flap. A free patch of peripheral peeling ILM is put above or in the FTMH during redo surgery. Morizane et al reported a closure rate of large macular holes of 90% after insertion of a free ILM flap created at the peripheral macula.140 Despite the high closure rate for big MHs, the insertion of an ILM within hole raises numerous problems. Damage to the retinal pigment epithelium (RPE) in the fovea is a distinct possibility, leading to microstructural fovea alterations such as inadequate healing of the photoreceptor layers.141 Contact between the BBG-stained ILM and the RPE for an extended period of time may cause chemical damage to the RPE, as detected by De Novelli et al.142
Moreover, the appropriate position of the free flap during the air-fluid exchange and or throughout the postoperative days has been a recurring problem with this procedure, due to its frequent displacement. Whereas Morizane et al placed viscoelastic material over the free flap for stabilization,140 De Novelli et al slowly removed the fluid to prevent dislodging of the ILM sheet from the hole,142 while Wang et al proposed a tiled transplantation ILM pedicle flap technique, to provide a bridge for retinal gliosis.143 As time went by, small variations to the original procedure have been presented,144,145 with similar levels of anatomical success, employing various agents such as perfluorocarbon solutions for free flap implantation,146,147 viscoelastic plugs,148,149 or autologous serum or blood as tissue adhesives.133
ILM Inverted Flap with Autologous Blood Technique
For a long time, blood components have been employed to aid hole closure in MH surgery.150–153 Hu et al recently claimed the association of ILM inverted flap technique with the autologous blood clot (ABC) as an excellent alternative for recurrent large macular hole.133 During surgery, the superior ILM was not removed completely from the retina, but left attached to the MH margin, forming an ILM flap of about 1 disk diameter, which was flipped to cover the whole MH and then massaged to flatten it. After this, about 0.1 mL fresh autologous blood was injected gently to cover the macula, soon becoming a clot on the surface of the macula. The ILM and ABC mixture became a macular plug that sealed the hole within minutes. In addition, the components and growth factors in the blood might help complete the hole closing following blood clot formation. The hastened repair of the macular structure and the recovery of microvascular blood may eventually improve visual recovery.133
Superior Wide-Base ILM Flap Transposition (SWIFT)
A recent study conducted by Tabandeh et al focused on a new technique for persistent MHs with previously removed ILM. In their superior wide-base ILM flap transposition (SWIFT) the ILM harvest site was above the previously peeled area: a flap was created, but leaving an attached ILM edge, and was successively everted to cover the MH. This technique was useful to limit intraoperative displacement of ILM flaps, with a reported 82% coverage of the MH by the flap, and ensured a 94% MH closure rate.154 Moreover, they proposed a post-operative investigation of flap position and integrity using “en face” imaging of the ILM flap obtained by ICG fluorescence, overtaking the OCT difficulties in analyzing the entire flap.154,155
Conclusion
Peeling of the ILM has become a pivotal point of macular hole surgery and is now considered as the “gold standard”. ILM peeling, however, is a delicate procedure which carries the risk of structural and functional retinal damages. Several studies have been conducted in order to adequately exploit ILM characteristics, especially in challenging conditions such as large, myopic or recurrent MHs. ILM inverted flap approach was a gear change in this panorama and drastically improved closure rates in large MHs. With recent advancements in modified surgical procedures and microsurgical equipment, even better results are possible. On the other hand, the treatment of recurrent or chronic MHs is more difficult and several surgical procedures have been offered to increase the success rate of this complicated organism, but further comparative clinical studies are needed to reach a solid judgment about which surgical option is the best.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Freeman WR. Vitrectomy surgery for full-thickness macular holes. Am J Ophthalmol. 1993;116(2):233–235. doi: 10.1016/s0002-9394(14)71292-9 [DOI] [PubMed] [Google Scholar]
- 2.McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology. 2009;116(7):1366–1369. doi: 10.1016/j.ophtha.2009.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106(5):629–639. doi: 10.1001/archopht.1988.01060130683026 [DOI] [PubMed] [Google Scholar]
- 4.Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119(6):752–759. doi: 10.1016/s0002-9394(14 [DOI] [PubMed] [Google Scholar]
- 5.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi: 10.1016/j.ophtha.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 6.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031 [DOI] [PubMed] [Google Scholar]
- 7.Brooks HL Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107(10):1939–1948. doi: 10.1016/S0161-6420(00)00331-6 [DOI] [PubMed] [Google Scholar]
- 8.Da Mata AP, Burk SE, Foster RE, et al. Long-term follow-up of indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for idiopathic macular hole repair. Ophthalmology. 2004;111(12):2246–2253. doi: 10.1016/j.ophtha.2004.05.037 [DOI] [PubMed] [Google Scholar]
- 9.Haritoglou C, Gass CA, Schaumberger M, Gandorfer A, Ulbig MW, Kampik A. Long-term follow-up after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol. 2002;134(5):661–666. doi: 10.1016/s0002-9394(02)01751-8 [DOI] [PubMed] [Google Scholar]
- 10.Chang E, Garg P, Capone A Jr. Outcomes and predictive factors in bilateral macular holes. Ophthalmology. 2013;120(9):1814–1819. doi: 10.1016/j.ophtha.2013.01.051 [DOI] [PubMed] [Google Scholar]
- 11.Krishnan R, Tossounis C, Fung Yang Y. 20-gauge and 23-gauge phacovitrectomy for idiopathic macular holes: comparison of complications and long-term outcomes. Eye. 2013;27(1):72–77. doi: 10.1038/eye.2012.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai MM, Williams GA. Anatomical and visual outcomes of idiopathic macular hole surgery with internal limiting membrane removal using low-concentration indocyanine green. Retina. 2007;27(4):477–482. doi: 10.1097/01.iae.0000247166.11120.21 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Kondo M, Asami T, Terasaki H. Comparison of macular hole surgery without internal limiting membrane peeling to eyes with internal limiting membrane peeling with and without indocyanine green staining. Ophthalmic Res. 2009;41(3):136–141. doi: 10.1159/000209666 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Kishi S. Pathogenesis of macular hole recurrence and its prevention by internal limiting membrane peeling. Retina. 2007;27(2):169–173. doi: 10.1097/01.iae.0000224940.79223.fb [DOI] [PubMed] [Google Scholar]
- 15.Benson WE, Cruickshanks KC, Fong DS, et al. Surgical management of macular holes: a report by the American Academy of Ophthalmology. Ophthalmology. 2001;108(7):1328–1335. doi: 10.1016/s0161-6420(01 [DOI] [PubMed] [Google Scholar]
- 16.Passemard M, Yakoubi Y, Muselier A, et al. Long-term outcome of idiopathic macular hole surgery. Am J Ophthalmol. 2010;149(1):120–126. doi: 10.1016/j.ajo.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 17.Eckardt C, Eckardt U, Groos S, Luciano L, Reale E. [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings] Entfernung der Membrana limitans interna bei Makulalochern. Klinische und morphologische Befunde. Ophthalmologe. 1997;94(8):545–551. doi: 10.1007/s003470050156 [DOI] [PubMed] [Google Scholar]
- 18.Morris R, Kuhn F, Witherspoon CD. Hemorrhagic macular cysts. Ophthalmology. 1994;101(1):1. doi: 10.1016/s0161-6420(13)31237-8 [DOI] [PubMed] [Google Scholar]
- 19.Haritoglou C, Gass CA, Schaumberger M, Ehrt O, Gandorfer A, Kampik A. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol. 2001;132(3):363–368. doi: 10.1016/s0002-9394(01 [DOI] [PubMed] [Google Scholar]
- 20.Terasaki H, Miyake Y, Nomura R, et al. Focal macular ERGs in eyes after removal of macular ILM during macular hole surgery. Invest Ophthalmol Vis Sci. 2001;42(1):229–234. [PubMed] [Google Scholar]
- 21.Uemura A, Kanda S, Sakamoto Y, Kita H. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136(2):252–257. doi: 10.1016/s0002-9394(03)00157-0 [DOI] [PubMed] [Google Scholar]
- 22.Chatziralli IP, Theodossiadis PG, Steel DHW. Internal limiting membrane peeling in macular hole surgery; why, when, and how? Retina. 2018;38(5):870–882. doi: 10.1097/IAE.0000000000001959 [DOI] [PubMed] [Google Scholar]
- 23.Fine BS. Limiting membranes of the sensory retina and pigment epithelium. An electron microscopic study. Arch Ophthalmol. 1961;66(6):847–860. doi: 10.1001/archopht.1961.00960010849012 [DOI] [PubMed] [Google Scholar]
- 24.Halfter W, Dong S, Dong A, Eller AW, Nischt R. Origin and turnover of ECM proteins from the inner limiting membrane and vitreous body. Eye. 2008;22(10):1207–1213. doi: 10.1038/eye.2008.19 [DOI] [PubMed] [Google Scholar]
- 25.Keenan TD, Clark SJ, Unwin RD, Ridge LA, Day AJ, Bishop PN. Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera. Invest Ophthalmol Vis Sci. 2012;53(12):7528–7538. doi: 10.1167/iovs.12-10797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Candiello J, Balasubramani M, Schreiber EM, et al. Biomechanical properties of native basement membranes. FEBS J. 2007;274(11):2897–2908. doi: 10.1111/j.1742-4658.2007.05823.x [DOI] [PubMed] [Google Scholar]
- 27.Wollensak G, Spoerl E, Grosse G, Wirbelauer C. Biomechanical significance of the human internal limiting lamina. Retina. 2006;26(8):965–968. doi: 10.1097/01.iae.0000250001.45661.95 [DOI] [PubMed] [Google Scholar]
- 28.Morescalchi F, Costagliola C, Gambicorti E, Duse S, Romano MR, Semeraro F. Controversies over the role of internal limiting membrane peeling during vitrectomy in macular hole surgery. Surv Ophthalmol. 2017;62(1):58–69. doi: 10.1016/j.survophthal.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 29.Smiddy WE, Flynn HW Jr. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004;137(3):525–537. doi: 10.1016/j.ajo.2003.12.011 [DOI] [PubMed] [Google Scholar]
- 30.Schumann RG, Rohleder M, Schaumberger MM, Haritoglou C, Kampik A, Gandorfer A. Idiopathic macular holes: ultrastructural aspects of surgical failure. Retina. 2008;28(2):340–349. doi: 10.1097/IAE.0b013e31814cef23 [DOI] [PubMed] [Google Scholar]
- 31.Reichelt W, Pannicke T, Biedermann B, Francke M, Faude F. Comparison between functional characteristics of healthy and pathological human retinal Müller glial cells. Surv Ophthalmol. 1997;42:S105–S117. doi: 10.1016/S0039-6257(97)80033-1 [DOI] [PubMed] [Google Scholar]
- 32.Iandiev I, Uckermann O, Pannicke T, et al. Glial cell reactivity in a porcine model of retinal detachment. Invest Ophthalmol Vis Sci. 2006;47(5):2161–2171. doi: 10.1167/iovs.05-0595 [DOI] [PubMed] [Google Scholar]
- 33.Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24(1):75–86. doi: 10.1016/j.preteyeres.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Morescalchi F, Duse S, Gambicorti E, Romano MR, Costagliola C, Semeraro F. Proliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloid. Mediators Inflamm. 2013;2013:269787. doi: 10.1155/2013/269787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornish KS, Lois N, Scott NW, et al. Vitrectomy with internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole. Ophthalmology. 2014;121(3):649–655. doi: 10.1016/j.ophtha.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 36.Cornish KS, Lois N, Scott N, et al. Vitrectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full‐thickness macular hole (FTMH). Cochrane Database Syst Rev. 2013;6:456. [DOI] [PubMed] [Google Scholar]
- 37.Lois N, Burr J, Norrie J, et al. Full-thickness macular hole and internal limiting membrane peeling study (FILMS) Group Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: a pragmatic randomized controlled trial. Invest Ophthalmol Vis Sci. 2011;52(3):1586–1592. doi: 10.1167/iovs.10-6287 [DOI] [PubMed] [Google Scholar]
- 38.Gao X, Guo J, Meng X, Wang J, Peng X, Ikuno Y. A meta-analysis of vitrectomy with or without internal limiting membrane peeling for macular hole retinal detachment in the highly myopic eyes. BMC Ophthalmol. 2016;16(1):1–8. doi: 10.1186/s12886-016-0266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tadayoni R, Gaudric A, Haouchine B, Massin P. Relationship between macular hole size and the potential benefit of internal limiting membrane peeling. Br J Ophthalmol. 2006;90(10):1239–1241. doi: 10.1136/bjo.2006.091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steel DHW, Dinah C, Magdi HA, White K, Rees J. The staining pattern of brilliant blue G during macular hole surgery: a clinicopathologic study. Invest Ophthalmol Vis Sci. 2014;55(9):5924–5931. doi: 10.1167/iovs.14-14809 [DOI] [PubMed] [Google Scholar]
- 41.Tornambe PE. Macular hole genesis: the hydration theory. Retina. 2003;23(3):421–424. doi: 10.1097/00006982-200306000-00028 [DOI] [PubMed] [Google Scholar]
- 42.Wollensak G, Spoerl E, Wirbelauer C, Pham DT. Influence of indocyanine green staining on the biomechanical strength of porcine internal limiting membrane. Ophthalmologica. 2004;218(4):278–282. doi: 10.1159/000078621 [DOI] [PubMed] [Google Scholar]
- 43.Haritoglou C, Mauell S, Schumann RG, et al. Increase in lens capsule stiffness caused by vital dyes. J Cataract Refract Surg. 2013;39(11):1749–1752. doi: 10.1016/j.jcrs.2013.02.057 [DOI] [PubMed] [Google Scholar]
- 44.Kadonosono K, Itoh N, Uchio E, Nakamura S, Ohno S. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118(8):1116–1118. doi: 10.1001/archopht.118.8.1116 [DOI] [PubMed] [Google Scholar]
- 45.Kimura H, Kuroda S, Nagata M. Triamcinolone acetonide-assisted peeling of the internal limiting membrane. Am J Ophthalmol. 2004;137(1):172–173. doi: 10.1016/s0002-9394(03 [DOI] [PubMed] [Google Scholar]
- 46.Enaida H, Hisatomi T, Hata Y, et al. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26(6):631–636. doi: 10.1097/01.iae.0000236469.71443.aa [DOI] [PubMed] [Google Scholar]
- 47.Iriyama A, Uchida S, Yanagi Y, et al. Effects of indocyanine green on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45(3):943–947. doi: 10.1167/iovs.03-1026 [DOI] [PubMed] [Google Scholar]
- 48.Maia M, Kellner L, de Juan E Jr, et al. Effects of indocyanine green injection on the retinal surface and into the subretinal space in rabbits. Retina. 2004;24(1):80–91. doi: 10.1097/00006982-200402000-00012 [DOI] [PubMed] [Google Scholar]
- 49.Sato Y, Tomita H, Sugano E, Isago H, Yoshida M, Tamai M. Evaluation of indocyanine green toxicity to rat retinas. Ophthalmologica. 2006;220(3):153–158. doi: 10.1159/000091757 [DOI] [PubMed] [Google Scholar]
- 50.Gandorfer A, Haritoglou C, Gandorfer A, Kampik A. Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci. 2003;44(1):316–323. doi: 10.1167/iovs.02-0545 [DOI] [PubMed] [Google Scholar]
- 51.Ando F, Yasui O, Hirose H, Ohba N. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Adverse effect of ICG-assisted ILM peeling. Graefes Arch Clin Exp Ophthalmol. 2004;242(12):995–999. doi: 10.1007/s00417-004-0864-4 [DOI] [PubMed] [Google Scholar]
- 52.Haritoglou C, Gandorfer A, Gass CA, Schaumberger M, Ulbig MW, Kampik A. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: a clinicopathologic correlation. Am J Ophthalmol. 2002;134(6):836–841. doi: 10.1016/s0002-9394(02)01816-0 [DOI] [PubMed] [Google Scholar]
- 53.Engelbrecht NE, Freeman J, Sternberg P Jr, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002;133(1):89–94. doi: 10.1016/s0002-9394(01)01293-4 [DOI] [PubMed] [Google Scholar]
- 54.Oberstein SL, De Smet M. Use of heavy Trypan blue in macular hole surgery. Eye. 2010;24(7):1177–1181. doi: 10.1038/eye.2010.3 [DOI] [PubMed] [Google Scholar]
- 55.Shukla D, Kalliath J, Neelakantan N, Naresh KB, Ramasamy K. A comparison of brilliant blue G, trypan blue, and indocyanine green dyes to assist internal limiting membrane peeling during macular hole surgery. Retina. 2011;31(10):2021–2025. doi: 10.1097/IAE.0b013e318213618c [DOI] [PubMed] [Google Scholar]
- 56.Steel DH, Karimi AA, White K. An evaluation of two heavier-than-water internal limiting membrane-specific dyes during macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2016;254(7):1289–1295. doi: 10.1007/s00417-015-3193-x [DOI] [PubMed] [Google Scholar]
- 57.Cardoso EB, Moraes-Filho M, Rodrigues EB, et al. Investigation of the retinal biocompatibility of acid violet for chromovitrectomy. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1115–1121. doi: 10.1007/s00417-013-2258-y [DOI] [PubMed] [Google Scholar]
- 58.Henrich PB, Monnier CA, Halfter W, et al. Nanoscale topographic and biomechanical studies of the human internal limiting membrane. Invest Ophthalmol Vis Sci. 2012;53(6):2561–2570. doi: 10.1167/iovs.11-8502 [DOI] [PubMed] [Google Scholar]
- 59.Matoba R, Morizane Y, Kimura S, Toshima S, Shiraga F. Retinal nerve fiber layer defect and paracentral scotoma after internal limiting membrane peeling with a nitinol loop. Acta Med Okayama. 2017;71(6):539–542. doi: 10.18926/AMO/55592 [DOI] [PubMed] [Google Scholar]
- 60.Steel DH, Dinah C, Habib M, White K. ILM peeling technique influences the degree of a dissociated optic nerve fibre layer appearance after macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2015;253(5):691–698. doi: 10.1007/s00417-014-2734-z [DOI] [PubMed] [Google Scholar]
- 61.Almony A, Nudleman E, Shah GK, et al. Techniques, rationale, and outcomes of internal limiting membrane peeling. Retina. 2012;32(5):877–891. doi: 10.1097/IAE.0b013e318227ab39 [DOI] [PubMed] [Google Scholar]
- 62.Ehlers JP, Han J, Petkovsek D, Kaiser PK, Singh RP, Srivastava SK. Membrane peeling-induced retinal alterations on intraoperative OCT in vitreomacular interface disorders from the PIONEER Study. Invest Ophthalmol Vis Sci. 2015;56(12):7324–7330. doi: 10.1167/iovs.15-17526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dogramaci M, Williamson TH. Dynamics of epiretinal membrane removal off the retinal surface: a computer simulation project. Br J Ophthalmol. 2013;97(9):1202–1207. doi: 10.1136/bjophthalmol-2013-303598 [DOI] [PubMed] [Google Scholar]
- 64.Abdelkader E, Lois N. Internal limiting membrane peeling in vitreo-retinal surgery. Surv Ophthalmol. 2008;53(4):368–396. doi: 10.1016/j.survophthal.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 65.Bae K, Kang SW, Kim JH, Kim SJ, Kim JM, Yoon JM. Extent of internal limiting membrane peeling and its impact on macular hole surgery outcomes: a Randomized Trial. Am J Ophthalmol. 2016;169:179–188. doi: 10.1016/j.ajo.2016.06.041 [DOI] [PubMed] [Google Scholar]
- 66.Modi A, Giridhar A, Gopalakrishnan M. Comparative analysis of outcomes with variable diameter internal limiting membrane peeling in surgery for idiopathic macular hole repair. Retina. 2017;37(2):265–273. doi: 10.1097/IAE.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 67.Goker YS, Koc M, Yuksel K, et al. Relationship between peeled internal limiting membrane area and anatomic outcomes following macular hole surgery: a quantitative analysis. J Ophthalmol. 2016;2016. doi: 10.1155/2016/5641273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuiki E, Fujikawa A, Miyamura N, Yamada K, Mishima K, Kitaoka T. Visual field defects after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2007;143(4):704–705. doi: 10.1016/j.ajo.2006.10.051 [DOI] [PubMed] [Google Scholar]
- 69.Mason III JO, Feist RM, Michael A, Albert J. Eccentric macular holes after vitrectomy with peeling of epimacular proliferation. Retina. 2007;27(1):45–48. doi: 10.1097/01.iae.0000256661.56617.69 [DOI] [PubMed] [Google Scholar]
- 70.Rubinstein A, Bates R, Benjamin L, Shaikh A. Iatrogenic eccentric full thickness macular holes following vitrectomy with ILM peeling for idiopathic macular holes. Eye. 2005;19(12):1333–1335. doi: 10.1038/sj.eye.6701771 [DOI] [PubMed] [Google Scholar]
- 71.Steel DH, Dinah C, White K, Avery P. The relationship between a dissociated optic nerve fibre layer appearance after macular hole surgery and M uller cell debris on peeled internal limiting membrane. J Acta ophthalmologica. 2017;95(2):153–157. doi: 10.1111/aos.13195 [DOI] [PubMed] [Google Scholar]
- 72.Tadayoni R, Paques M, Massin P, Mouki-Benani S, Mikol J, Gaudric A. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology. 2001;108(12):2279–2283. doi: 10.1016/s0161-6420(01)00856-9 [DOI] [PubMed] [Google Scholar]
- 73.Ito Y, Terasaki H, Takahashi A, Yamakoshi T, Kondo M, Nakamura M. Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holes. Ophthalmology. 2005;112(8):1415–1420. doi: 10.1016/j.ophtha.2005.02.023 [DOI] [PubMed] [Google Scholar]
- 74.Brazitikos PD, Katsimpris JM, Tsironi E, Androudi S. Retinal nerve fiber layer thickness evaluation after trypan blue-assisted macular surgery. Retina. 2010;30(4):640–647. doi: 10.1097/IAE.0b013e3181c085ab [DOI] [PubMed] [Google Scholar]
- 75.Miura M, Elsner AE, Osako M, Iwasaki T, Okano T, Usui M. Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular hole. Retina. 2003;23(4):561–563. doi: 10.1097/00006982-200308000-00024 [DOI] [PubMed] [Google Scholar]
- 76.Mitamura Y, Ohtsuka K. Relationship of dissociated optic nerve fiber layer appearance to internal limiting membrane peeling. Ophthalmology. 2005;112(10):1766–1770. doi: 10.1016/j.ophtha.2005.04.026 [DOI] [PubMed] [Google Scholar]
- 77.Spaide RF. Dissociated optic nerve fiber layer appearance” after internal limiting membrane removal is inner retinal dimpling. Retina. 2012;32(9):1719–1726. doi: 10.1097/IAE.0b013e3182671191 [DOI] [PubMed] [Google Scholar]
- 78.Nukada K, Hangai M, Ooto S, Yoshikawa M, Yoshimura N. Tomographic features of macula after successful macular hole surgery. Invest Ophthalmol Vis Sci. 2013;54(4):2417–2428. doi: 10.1167/iovs.12-10838 [DOI] [PubMed] [Google Scholar]
- 79.Miura M, Elsner AE, Osako M, et al. Spectral imaging of the area of internal limiting membrane peeling. Retina. 2005;25(4):468–472. doi: 10.1097/00006982-200506000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kishimoto H, Kusuhara S, Matsumiya W, Nagai T, Negi A. Retinal surface imaging provided by Cirrus high-definition optical coherence tomography prominently visualizes a dissociated optic nerve fiber layer appearance after macular hole surgery. Int Ophthalmol. 2011;31(5):385–392. doi: 10.1007/s10792-011-9474-4 [DOI] [PubMed] [Google Scholar]
- 81.Alkabes M, Salinas C, Vitale L, Bures-Jelstrup A, Nucci P, Mateo C. En face optical coherence tomography of inner retinal defects after internal limiting membrane peeling for idiopathic macular hole. Invest Ophthalmol Vis Sci. 2011;52(11):8349–8355. doi: 10.1167/iovs.11-8043 [DOI] [PubMed] [Google Scholar]
- 82.Ishida M, Ichikawa Y, Higashida R, Tsutsumi Y, Ishikawa A, Imamura Y. Retinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular hole. Am J Ophthalmol. 2014;157(5):971–977. doi: 10.1016/j.ajo.2014.01.026 [DOI] [PubMed] [Google Scholar]
- 83.Ohta K, Sato A, Fukui E. Asymmetrical thickness of parafoveal retina around surgically closed macular hole. Br J Ophthalmol. 2010;94(11):1545–1546. doi: 10.1136/bjo.2009.176693 [DOI] [PubMed] [Google Scholar]
- 84.Rodrigues IA, Lee EJ, Williamson TH. Measurement of retinal displacement and metamorphopsia after epiretinal membrane or macular hole surgery. Retina. 2016;36(4):695–702. doi: 10.1097/IAE.0000000000000768 [DOI] [PubMed] [Google Scholar]
- 85.Ripandelli G, Scarinci F, Piaggi P, et al. Macular pucker: to peel or not to peel the internal limiting membrane? A microperimetric response. Retina. 2015;35(3):498–507. doi: 10.1097/IAE.0000000000000330 [DOI] [PubMed] [Google Scholar]
- 86.Tadayoni R, Svorenova I, Erginay A, Gaudric A, Massin P. Decreased retinal sensitivity after internal limiting membrane peeling for macular hole surgery. Br J Ophthalmol. 2012;96(12):1513–1516. doi: 10.1136/bjophthalmol-2012-302035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pilli S, Zawadzki RJ, Werner JS, Park SS. Visual outcome correlates with inner macular volume in eyes with surgically closed macular hole. Retina. 2012;32(10):2085–2095. doi: 10.1097/IAE.0b013e31825c1c0c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho TC, Yang CM, Huang JS, Yang CH, Chen MS. Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1553–1560. doi: 10.1007/s00417-014-2613-7 [DOI] [PubMed] [Google Scholar]
- 89.Franze K, Grosche J, Skatchkov SN, et al. Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci U S A. 2007;104(20):8287–8292. doi: 10.1073/pnas.0611180104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy DC, Fostier W, Rees J, Steel DH. Foveal sparing internal limiting membrane peeling for idiopathic macular holes: effects on anatomical restoration of the fovea and visual function. Retina. 2020;40(11):2127–2133. doi: 10.1097/IAE.0000000000002724 [DOI] [PubMed] [Google Scholar]
- 91.Geenen C, Murphy DC, Sandinha MT, Rees J, Steel DHW. Significance of preoperative external limiting membrane height on visual prognosis in patients undergoing macular hole surgery. Retina. 2019;39(7):1392–1398. doi: 10.1097/IAE.0000000000002137 [DOI] [PubMed] [Google Scholar]
- 92.Mahajan VB, Chin EK, Tarantola RM, et al. Macular hole closure with internal limiting membrane abrasion technique. JAMA Ophthalmol. 2015;133(6):635–641. doi: 10.1001/jamaophthalmol.2015.204 [DOI] [PubMed] [Google Scholar]
- 93.Almeida DR, Chin EK, Tarantola RM, et al. Effect of internal limiting membrane abrasion on retinal tissues in macular holes. Invest Ophthalmol Vis Sci. 2015;56(5):2783–2789. doi: 10.1167/iovs.14-16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Essex RW, Kingston ZS, Moreno-Betancur M, et al. The effect of postoperative face-down positioning and of long- versus short-acting gas in macular hole surgery: results of a registry-based study. Ophthalmology. 2016;123(5):1129–3116. doi: 10.1016/j.ophtha.2015.12.039 [DOI] [PubMed] [Google Scholar]
- 95.Freeman WR, Azen SP, Kim JW, el-Haig W, Mishell DR 3rd, Bailey I. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch Ophthalmol. 1997;115(1):11–21. doi: 10.1001/archopht.1997.01100150013002 [DOI] [PubMed] [Google Scholar]
- 96.Gupta B, Laidlaw DA, Williamson TH, Shah SP, Wong R, Wren S. Predicting visual success in macular hole surgery. Br J Ophthalmol. 2009;93(11):1488–1491. doi: 10.1136/bjo.2008.153189 [DOI] [PubMed] [Google Scholar]
- 97.Ch’ng SW, Patton N, Ahmed M, et al. The Manchester large macular hole study: is it time to reclassify large macular holes? Am J Ophthalmol. 2018;195:36–42. doi: 10.1016/j.ajo.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 98.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117(10):2018–2025. doi: 10.1016/j.ophtha.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 99.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 2014;34(4):664–669. doi: 10.1097/IAE.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 100.Hayashi H, Kuriyama S. Foveal microstructure in macular holes surgically closed by inverted internal limiting membrane flap technique. Retina. 2014;34(12):2444–2450. doi: 10.1097/IAE.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 101.Shiode Y, Morizane Y, Matoba R, et al. The role of inverted internal limiting membrane flap in macular hole closure. Invest Ophthalmol Vis Sci. 2017;58(11):4847–4855. doi: 10.1167/iovs.17-21756 [DOI] [PubMed] [Google Scholar]
- 102.Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthalmol. 1969;82(2):151–159. doi: 10.1001/archopht.1969.00990020153002 [DOI] [PubMed] [Google Scholar]
- 103.Nawrocki J, Michalewska Z. Spectral domain optical coherence tomography for macular holes. Medical Retina. 2010;5:141–155. [DOI] [PubMed] [Google Scholar]
- 104.Michalewska Z, Michalewski J, Nawrocki J. Macular hole closure after vitrectomy: the inverted flap technique. Br J Ophthalmol. 2003;87:1015–1019. doi: 10.1136/bjo.87.8.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Michalewska Z, Michalewski J, Cisiecki S, Adelman R, Nawrocki J. Correlation between foveal structure and visual outcome following macular hole surgery: a spectral optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2008;246(6):823–830. doi: 10.1007/s00417-007-0764-5 [DOI] [PubMed] [Google Scholar]
- 106.Boninska K, Nawrocki J, Michalewska Z. Mechanism of “flap closure” after the inverted internal limiting membrane flap technique. Retina. 2018;38(11):2184–2189. doi: 10.1097/IAE.0000000000001861 [DOI] [PubMed] [Google Scholar]
- 107.Michalewska Z, Michalewski J, Nawrocki J. Continuous changes in macular morphology after macular hole closure visualized with spectral optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1249–1255. doi: 10.1007/s00417-010-1370-5 [DOI] [PubMed] [Google Scholar]
- 108.Gu C, Qiu Q. Inverted internal limiting membrane flap technique for large macular holes: a systematic review and single-arm meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2018;256(6):1041–1049. doi: 10.1007/s00417-018-3956-2 [DOI] [PubMed] [Google Scholar]
- 109.Rizzo S, Tartaro R, Barca F, Caporossi T, Bacherini D, Giansanti F. Internal limiting membrane peeling versus inverted flap technique for treatment of full-thickness macular holes: a comparative study in a large series of patients. Retina. 2018;38(Suppl 1):S73–S78. doi: 10.1097/IAE.0000000000001985 [DOI] [PubMed] [Google Scholar]
- 110.Ramtohul P, Parrat E, Denis D, Lorenzi U. Inverted internal limiting membrane flap technique versus complete internal limiting membrane peeling in large macular hole surgery: a comparative study. BMC Ophthalmol. 2020;20(1):11. doi: 10.1186/s12886-019-1294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mete M, Alfano A, Guerriero M, et al. Inverted internal limiting membrane flap technique versus complete internal limiting membrane removal in myopic macular hole surgery: a Comparative Study. Retina. 2017;37(10):1923–1930. doi: 10.1097/IAE.0000000000001446 [DOI] [PubMed] [Google Scholar]
- 112.Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, Matsumoto M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 2013;156(1):125–131 e1. doi: 10.1016/j.ajo.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 113.Ota H, Kunikata H, Aizawa N, Nakazawa T. Surgical results of internal limiting membrane flap inversion and internal limiting membrane peeling for macular hole. PLoS One. 2018;13(9):e0203789. doi: 10.1371/journal.pone.0203789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chatziralli I, Machairoudia G, Kazantzis D, Theodossiadis G, Theodossiadis P. Inverted internal limiting membrane flap technique for myopic macular hole: a meta-analysis. Surv Ophthalmol. 2021;66(5):771–780. doi: 10.1016/j.survophthal.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 115.Hirata Y, Yuda K, Odontuya D, Hayashi T, Suzuki Y. A viscoelastic aspiration technique for autologous transplantation of the free-flap inner limiting membrane during macular hole surgery. Retina. 2019;39(Suppl 1):S87–S91. doi: 10.1097/IAE.0000000000001968 [DOI] [PubMed] [Google Scholar]
- 116.Shin MK, Park KH, Park SW, Byon IS, Lee JE. Perfluoro-n-octane-assisted single-layered inverted internal limiting membrane flap technique for macular hole surgery. Retina. 2014;34(9):1905–1910. doi: 10.1097/IAE.0000000000000339 [DOI] [PubMed] [Google Scholar]
- 117.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Adelman RA, Nawrocki J. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: a Comparative Study. Retina. 2015;35(9):1844–1850. doi: 10.1097/IAE.0000000000000555 [DOI] [PubMed] [Google Scholar]
- 118.Rossi T, Gelso A, Costagliola C, et al. Macular hole closure patterns associated with different internal limiting membrane flap techniques. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1073–1078. doi: 10.1007/s00417-017-3598-9 [DOI] [PubMed] [Google Scholar]
- 119.Hussain RN, Steel DHW, Sandinha T, Stappler T, Heimann H, Wong D. Cutting the internal limiting membrane with zero aspiration technique: a clinical audit. Retina. 2019;39(Suppl 1):S133–S136. doi: 10.1097/IAE.0000000000001641 [DOI] [PubMed] [Google Scholar]
- 120.Ho TC, Chen MS, Huang JS, Shih YF, Ho H, Huang YH. Foveola nonpeeling technique in internal limiting membrane peeling of myopic foveoschisis surgery. Retina. 2012;32(3):631–634. doi: 10.1097/IAE.0B013E31824D0A4B [DOI] [PubMed] [Google Scholar]
- 121.Andrew N, Chan WO, Tan M, Ebneter A, Gilhotra JS. Modification of the inverted internal limiting membrane flap technique for the treatment of chronic and large macular holes. Retina. 2016;36(4):834–837. doi: 10.1097/IAE.0000000000000931 [DOI] [PubMed] [Google Scholar]
- 122.Ghassemi F, Khojasteh H, Khodabande A, et al. Comparison of three different techniques of inverted internal limiting membrane flap in treatment of large idiopathic full-thickness macular hole. Clin Ophthalmol. 2019;13:2599–2606. doi: 10.2147/OPTH.S236169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peng J, Zhang LH, Chen CL, Liu JJ, Zhu XY, Zhao PQ. Internal limiting membrane dragging and peeling: a modified technique for macular holes closure surgery. Int J Ophthalmol. 2020;13(5):755–760. doi: 10.18240/ijo.2020.05.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alpatov S, Shchuko A, Malyshev V. A new method of treating macular holes. Eur J Ophthalmol. 2007;17(2):246–252. doi: 10.1177/112067210701700215 [DOI] [PubMed] [Google Scholar]
- 125.Kumar A, Tinwala SI, Gogia V, Sehra SV. Tapping of macular hole edges: the outcomes of a novel technique for large macular holes. Asia Pac J Ophthalmol. 2013;2(5):305–309. doi: 10.1097/APO.0b013e31829a1919 [DOI] [PubMed] [Google Scholar]
- 126.Manasa S, Kakkar P, Kumar A, Chandra P, Kumar V, Ravani R. Comparative evaluation of standard ILM peel with inverted ILM flap technique in large macular holes: a Prospective, Randomized Study. Ophthalmic Surg Lasers Imaging Retina. 2018;49(4):236–240. doi: 10.3928/23258160-20180329-04 [DOI] [PubMed] [Google Scholar]
- 127.Ohana O, Barak A, Schwartz S. Internal aspiration under perfluorocarbon liquid for the management of large macular holes. Retina. 2017;37(11):2145–2150. doi: 10.1097/IAE.0000000000001449 [DOI] [PubMed] [Google Scholar]
- 128.Zong Y, Wu K, Yu J, Zhou C, Jiang C. Internal limiting membrane peeling and flap inverting under air in large idiopathic macular hole surgery. J Ophthalmol. 2021;2021:2003001. doi: 10.1155/2021/2003001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D’Souza MJ, Chaudhary V, Devenyi R, Kertes PJ, Lam WC. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br J Ophthalmol. 2011;95(11):1564–1567. doi: 10.1136/bjo.2010.195826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baumann C, El-Faouri M, Ivanova T, et al. Manchester revisional macular hole study: predictive value of optical coherence tomography parameters on outcomes of repeat vitrectomy, extension of internal limiting membrane peel, and gas tamponade for persistent macular holes. Retina. 2021;41(5):908–914. doi: 10.1097/IAE.0000000000002959 [DOI] [PubMed] [Google Scholar]
- 131.Fung NSK, Mak AKH, Yiu R, Wong IYH, Lam WC. Treatment of large, chronic and persistent macular hole with internal limiting membrane transposition and tuck technique. Int J Retina Vitreous. 2020;6(1):3. doi: 10.1186/s40942-019-0206-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Giansanti F, Tartaro R, Caporossi T, et al. An internal limiting membrane plug and gas endotamponade for recurrent or persistent macular hole. J Ophthalmol. 2019;2019:6051724. doi: 10.1155/2019/6051724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu Z, Lin H, Liang Q, Wu R. Comparing the inverted internal limiting membrane flap with autologous blood technique to internal limiting membrane insertion for the repair of refractory macular hole. Int Ophthalmol. 2020;40(1):141–149. doi: 10.1007/s10792-019-01162-0 [DOI] [PubMed] [Google Scholar]
- 134.Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016;134(2):229–230. doi: 10.1001/jamaophthalmol.2015.5237 [DOI] [PubMed] [Google Scholar]
- 135.Gu X, Hu Z, Qian H, et al. Perfluorocarbon liquid-assisted inverted limiting membrane flap technique combined with subretinal fluid drainage for macular hole retinal detachment in highly myopic eyes. Retina. 2021;41:317–323. doi: 10.1097/IAE.0000000000002853 [DOI] [PubMed] [Google Scholar]
- 136.Rizzo S, Caporossi T, Tartaro R, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39(Suppl 1):S95–S103. doi: 10.1097/IAE.0000000000002320 [DOI] [PubMed] [Google Scholar]
- 137.Caporossi T, Pacini B, Bacherini D, Barca F, Faraldi F, Rizzo S. Human amniotic membrane plug to promote failed macular hole closure. Sci Rep. 2020;10(1):18264. doi: 10.1038/s41598-020-75292-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caporossi T, De Angelis L, Pacini B, et al. A human amniotic membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol. 2020;98(2):e252–e256. doi: 10.1111/aos.14174 [DOI] [PubMed] [Google Scholar]
- 139.Caporossi T, Pacini B, De Angelis L, Barca F, Peiretti E, Rizzo S. Human amniotic membrane to close recurrent, high myopic macular holes in pathologic myopia with axial length of ≥ 30 mm. Retina. 2020;40(10):1946–1954. doi: 10.1097/Iae.0000000000002699 [DOI] [PubMed] [Google Scholar]
- 140.Morizane Y, Shiraga F, Kimura S, et al. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157(4):861–869 e1. doi: 10.1016/j.ajo.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 141.Lee SM, Kwon HJ, Park SW, Lee JE, Byon IS. Microstructural changes in the fovea following autologous internal limiting membrane transplantation surgery for large macular holes. Acta Ophthalmol. 2018;96(3):e406–e408. doi: 10.1111/aos.13504 [DOI] [PubMed] [Google Scholar]
- 142.De Novelli FJ, Preti RC, Monteiro MLR, Pelayes DE, Nóbrega MJ, Takahashi WY. Autologous internal limiting membrane fragment transplantation for large, chronic, and refractory macular holes. Ophthalmic Res. 2016;55(1):45–52. doi: 10.1159/000440767 [DOI] [PubMed] [Google Scholar]
- 143.Wang L-P, Sun W-T, Lei C-L, Deng J. Clinical outcomes with large macular holes using the tiled transplantation internal limiting membrane pedicle flap technique. Int J Ophthalmol. 2019;12(2):246. doi: 10.18240/ijo.2019.02.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hernandez-da Mota SE, Bejar-Cornejo F. [Modified technique of autologous transplantation of internal limiting membrane for macular hole] Tecnica modificada de trasplante autologo de membrana limitante interna en agujero macular. Cir Cir. 2016;84(6):454–458. doi: 10.1016/j.circir.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 145.Wolfensberger T. Autologous internal limiting membrane (ILM) recycling to close very large refractory macular holes. Klinische Monatsblätter für Augenheilkun. 2015;232(04):585–586. doi: 10.1055/s-0035-1545681 [DOI] [PubMed] [Google Scholar]
- 146.Ozdek S, Baskaran P, Karabas L, Neves PP. A modified perfluoro-n-octane-assisted autologous internal limiting membrane transplant for failed macular hole reintervention: a case series. Ophthalmic Surg Lasers Imaging Retina. 2017;48(5):416–420. doi: 10.3928/23258160-20170428-08 [DOI] [PubMed] [Google Scholar]
- 147.Park SW, Pak KY, Park KH, Kim KH, Byon IS, Lee JE. Perfluoro-n-octane assisted free internal limiting membrane flap technique for recurrent macular hole. Retina. 2015;35(12):2652–2656. doi: 10.1097/IAE.0000000000000754 [DOI] [PubMed] [Google Scholar]
- 148.Song Z, Li M, Liu J, Hu X, Hu Z, Chen D. Viscoat assisted inverted internal limiting membrane flap technique for large macular holes associated with high myopia. J Ophthalmol. 2016;2016:8283062. doi: 10.1155/2016/8283062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Romano MR, Borgia A, Raimondi R. Viscoelastic-assisted inverted cover and free ILM flap techniques. Eur J Ophthalmol. 2021;11206721211016976. doi: 10.1177/11206721211016976 [DOI] [PubMed] [Google Scholar]
- 150.Lai CC, Chen YP, Wang NK, et al. Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 2015;122(9):1889–1898. doi: 10.1016/j.ophtha.2015.05.040 [DOI] [PubMed] [Google Scholar]
- 151.Lai CC, Hwang YS, Liu L, et al. Blood-assisted internal limiting membrane peeling for macular hole repair. Ophthalmology. 2009;116(8):1525–1530. doi: 10.1016/j.ophtha.2009.02.025 [DOI] [PubMed] [Google Scholar]
- 152.Korobelnik J-F, Hannouche D, Belayachi N, Branger M, Guez JE, Hoang-Xuan T. Autologous platelet concentrate as an adjunct in macular hole healing: a pilot study. Ophthalmology. 1996;103(4):590–594. doi: 10.1016/S0161-6420(96)30648-9 [DOI] [PubMed] [Google Scholar]
- 153.Ryan EA, Lee S, Chern S. Use of intravitreal autologous blood to identify posterior cortical vitreous in macular hole surgery. Arch Ophthalmol. 1995;113(6):822–823. doi: 10.1001/archopht.1995.01100060148050 [DOI] [PubMed] [Google Scholar]
- 154.Tabandeh H, Morozov A, Rezaei KA, Boyer DS. Superior wide-base internal limiting membrane flap transposition for macular holes: flap status and outcomes. Ophthalmol Retina. 2021;5(4):317–323. doi: 10.1016/j.oret.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 155.Tabandeh H. Fluorescence imaging of the ILM flap following MH surgery. Am J Ophthalmol Case Rep. 2021;24:101203. doi: 10.1016/j.ajoc.2021.101203 [DOI] [PMC free article] [PubMed] [Google Scholar]