Abstract
Purpose
The purpose of this review article is to provide a comprehensive review of the current applications of intravitreal DEX implant (Ozurdex®, Allergan Inc, Irvine, CA) for a variety of ophthalmic conditions – ranging from FDA approved indications to off-label uses. We have attempted to provide relevant evidence from the literature to help a reader develop an understanding of the biological and pharmacokinetic properties of DEX implant, its uses, and potential side effects.
Methods
PubMed searches were performed using the terms “Ozurdex”, or “intravitreal DEX implant”, AND “retinal vein occlusion”, or “diabetic macular edema”, or “uveitis”. The search was performed in July of 2021, with an additional search in October 2021. All original English language articles were considered for this review.
Results
DEX implant has evidence of efficacy in a variety of clinical situations including macular edema associated with retinal vein occlusion, diabetes, uveitis, and others. Safety concerns include cataract formation and progression, intraocular pressure elevation, complications related to intravitreal injection, and opportunistic infections secondary to steroid-induced immune suppression.
Conclusion
DEX implant is a useful tool in the management of several retinal disorders. Further studies are needed for head-to-head comparison with other treatment modalities and to determine its precise place in clinical practice.
Keywords: Ozurdex, intravitreal DEX implant, retinal vein occlusion, diabetic macular edema, uveitis, uveitic macular edema
Introduction
Glucocorticoids bind steroid receptors in the cytoplasm, alter DNA expression and inhibit formation of inflammatory mediators such as interleukin-6 (IL-6), IL-8, prostaglandins, and vascular endothelial growth factor (VEGF).1 They also stabilize endothelial and retinal pigment epithelial tight junctions and restore the integrity of blood retinal barrier.2–5 These mechanisms reduce inflammatory cellular response, vascular permeability, fibrin exudation, and scar formation. Dexamethasone is a potent water-soluble glucocorticoid that was first administered by intravitreal injection as an adjunct in experimental endophthalmitis as early as in 1974 with beneficial results.6 It is a suitable candidate for ocular inflammatory conditions and other disorders where inflammation plays a key role. However, it has a short half-life of 3.5 hours after intravitreal administration.7 Therefore, a sustained-delivery system is needed for the management of chronic conditions.
Intravitreal dexamethasone implant (DEX implant, Ozurdex®, Allergan Inc, Irvine, CA) is a rod-shaped implant made of a solid biodegradable polymer (Novadur™, Allergan Inc, Irvine, CA) designed to release 700 micrograms of preservative-free dexamethasone over a period of 180 days. It has dual-phase pharmacokinetics, initially releasing high dose of dexamethasone peaking at day 60, followed by rapid decline in concentration between day 60 and 90 followed by a steady state at a much lower concentration that is maintained through day 180 in primate eyes.8 The byproducts of Novadur™ degradation are lactic acid and glycolic acid which are subsequently converted to carbon dioxide and water. The pharmacokinetics of dexamethasone was similar in vitrectomized and nonvitrectomized rabbit eyes, with peak concentration of dexamethasone at day 22 following the injection of 0.7 mg DEX implant.9
DEX implant was approved by the Food and Drug Administration (FDA) for the treatment of macular edema caused by retinal vein occlusion in 2009, noninfectious intermediate, posterior and panuveitis in 2010, and diabetic macular edema in 2014. It is also used off-label for a variety of other ocular conditions.
Methods
Multiple PubMed searches were performed using the terms “Intravitreal dexamethasone implant”, “Ozurdex” AND “retinal vein occlusion”, “uveitis”, “macular edema” in July 2021, and again in October 2021. All original English language articles were considered for this review, along with additional articles revealed from those articles. IRB approval was not required for this review article of existing literature.
Injection Technique
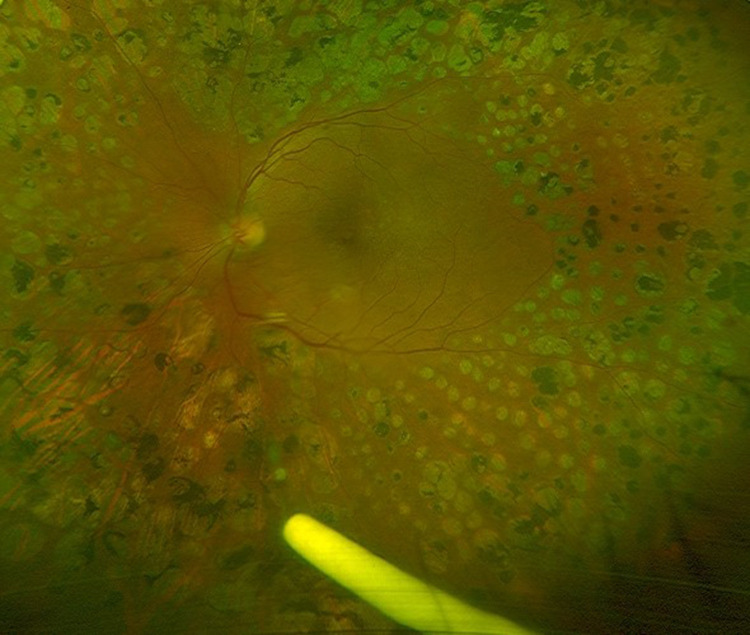
The implant is injected into the vitreous of the eye in an office-based setting in the US.10 After ensuring adequate asepsis and anesthesia preferably by subconjunctival injection of 2% lidocaine, the applicator is held parallel to the limbus, pointing the bevel of the 22-g needle away from the sclera at an oblique angle. After conjunctival displacement, a shelved scleral path is initially created for 1 mm parallel to the limbus at a distance of 3 mm in pseudophakic and 4 mm in phakic eyes, and then the applicator is redirected toward the center of the eye until the vitreous cavity is entered and the sleeve around the needle touches the conjunctiva. The actuator button is fully depressed until there is an audible click. The needle is removed in the same direction used to enter the vitreous.11 Pressure is applied at injection site for about ten seconds to prevent reflux (See video). The eye may be patched for six to eight hours after the injections. The implant is visible in the vitreous cavity as a solid white rod-shaped structure (Figure 1).
Figure 1.
Ultra-wide field image showing dexamethasone implant in the inferior vitreous in a patient with lasered proliferative diabetic retinopathy and recurrent macular edema.
DEX Implant for Macular Edema from Retinal Vein Occlusion
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy. A pooled analysis of patients in the United States, Europe, Asia and Australia estimated an overall global prevalence of 16.4 million adults, 2.5 million (15%) with central retinal vein occlusion (CRVO), and 13.9 million (85%) with branch retinal vein occlusion (BRVO).12 Incidence of RVO increases with age – from 0.7% in younger than 60 years to 4.6% in those 80 years or older,13 with more than 50% cases occurring in individuals older than 65 years.14 Common systemic risk factors associated with RVO include hypertension (48%), hyperlipidemia (20%), and diabetes mellitus (5%).15 Young patients, those with bilateral RVO, and those with past or family history of thrombosis should be evaluated for thrombophilic disorders such as hyperhomocysteinemia, anticardiolipin antibodies, deficiency of antithrombin, proteins C and S, oral contraceptive use, factor V Leiden mutation, prothrombin mutation, methylene tetrahydrofolate (MTHFR) mutation, and increased blood viscosity.16,17 Open angle glaucoma and ocular hypertension are well-recognized ocular associations with RVO.18
Classification
RVO is classified based on anatomical location and degree of retinal ischemia. Branch retinal vein occlusion (BRVO) occurs at arteriovenous crossing and affects one quadrant of the retina. Central retinal vein occlusion (CRVO) occurs at the lamina cribrosa and results in retinal hemorrhages in all four quadrants. Hemi-retinal vein occlusions (HRVO) are the rarest, affecting one hemifield of the retina due to anatomical variation where superior and inferior venous trunks merge together prior to forming central retinal vein.
CRVO can be further classified into ischemic, perfused or indeterminate. Ischemic CRVO has been defined as ≥10 disk areas of retinal capillary nonperfusion by Central Retinal Vein Study Group (CVOS).19 Another set of visual function criteria to define CRVO as ischemic, reported by Hayreh et al include visual acuity ≤20/200, afferent pupillary defect, reduced b wave amplitude on electroretinography, and visual field defect on kinetic perimetry.20 For BRVO, capillary nonperfusion ≥5 disk diameters is considered ischemic. These patients benefit from sectoral panretinal photocoagulation which should be performed if retinal neovascularization develops.21
Macular Edema in RVO
In acute phase of RVO, vision is affected by macular edema, macular hemorrhages, macular ischemia, or a combination of all three. Macular hemorrhages tend to resolve over time. As of now there is no treatment for macular ischemia. Etiology of macular edema in RVO is likely multifactorial. Increase in hydrostatic pressure in the obstructed vein results in extravasation of intravascular fluid across the capillary wall. Reduced venous flow results in impaired capillary perfusion, leading to hypoxia-inducible factor 1 α (HIF-1 α), which in turn upregulates the expression of endothelin-1 and vascular endothelial growth factor (VEGF), which increases endothelial permeability. Inflammation is believed to contribute to increased capillary permeability as well. Elevated levels of pro-inflammatory cytokines, including IL-6 and IL-8 have been detected in the vitreous in eyes affected by RVO.22,23 Macular edema in BRVO can be treated with grid laser, anti-VEGF injections, and intravitreal steroids. Grid laser is not recommended for macular edema caused by CRVO (CRVO Study Group Report M),24 but intravitreal steroids and anti-VEGF intravitreal injections are effective.
Clinical Studies of DEX Implant in RVO
Sham-Controlled Trials
In a sham-controlled Phase 2 randomized controlled trial involving 315 patients with persistent macular edema caused by retinal vein occlusion, diabetes, uveitis or Irvine-Gass syndrome, Kupperman et al showed ≥10 letter best-corrected visual acuity (BCVA) gain in 35%, 24% and 13% patients in 700 mcg, 350 mcg and sham groups respectively at 90-day time point with similar results across all etiologies of macular edema (Table 1). Elevation of intraocular pressure (IOP) was observed in 11% of treated and 2% of observed patients.25 Global Evaluation of Implantable Dexamethasone in Retinal Vein Occlusion (GENEVA) trial consisted of two identical, multicenter, multinational, masked, randomized sham-controlled Phase 3 clinical trials that compared single injection of DEX implant 0.7 mg (n=427), DEX implant 0.35 mg (n=414) with sham injection (n=426) over a period of six months.26 Improvement in mean best-corrected visual acuity (BCVA) was greater in both DEX implant groups compared with sham at all time points. The percentage of eyes with ≥15 letter vision gain was greater in both DEX implant groups compared to sham. 16% of the eyes showed IOP ≥25 mm Hg at day 60 for both doses which returned to baseline by day 180. An open-label extension study of the same population in which second DEX implant 0.7 mg was injected at six months if indicated showed similar vision gains at two months after the first and second injection (30% ≥15 letter gain after the first, and 32% after the second). IOP increase ≥ 10 mm Hg was seen in 15.4% after the second injection, similar to the incidence after the first.27 A post hoc analysis revealed that each one-month increase in the duration of macular edema was associated with significantly lower likelihood of achieving a BCVA improvement of ≥15 letters or central retinal thickness (CRT) reduction of ≥200 microns at both 6 and 12 months, particularly in BRVO patients.28 Another post hoc analysis demonstrated that improvement in BCVA was noted as early as seven days after 0.7 mg DEX implant (10.3% DEX versus 4% sham) and duration of ≥15 letter improvement persisted for two to three months.29
Table 1.
Summary of Studies of DEX Implant in RVO with Macular Edema
| Study (Year) | Design | n | Intervention | Outcome Measure(s) | Time Point(s) | Results | Comments |
|---|---|---|---|---|---|---|---|
| Kuppermann et al. (2007)25 | Phase 2, six-month, sham-controlled RCT, persistent macular edema, various etiologies | 315 | Single injection of DEX implant (350 mcg or 700 mcg) versus sham | ≥10 letter BCVA gain | Day 90 | 35% (700 mcg), 24% (350 mcg) and 13% sham gained ≥ 10 letters | 11% IOP increase ≥10 mm Hg Similar result in DME, RVO, uveitis, and Irvine Gass syndrome |
| Haller et al. (2010) GENEVA Study Group26 | Phase 3, six-month, sham-controlled RCT, RVO with ME | 1267 | Single injection of DEX implant (350 mcg or 700 mcg) versus sham | ≥ 15 letter BCVA gain | Various time points. Peak benefit at day 60 | 29% gainers at day 60, same for both doses. No difference from sham at 180 | IOP ≥25 mm Hg in 16% at day 60 for both doses. Returned to baseline by day 180 |
| Haller et al. (2011)27 | Six-month open label extension of GENEVA trial | 1256 | Single DEX implant 0.7 mg at day 180 if indicated (n=997) | ≥ 15 letter BCVA gain | Day 60 | 32% gainers after second injection (30% after first) | ≥10 mm Hg IOP elevation in 12.6% after first, and 15.4 after the second. 30% showed cataract progression after two injections |
| Li et al. (2018)30 | Phase 3, six-month, sham-controlled RCT followed by two month open-label extension for eyes with ME from RVO | DEX 129, sham 130 | Single injection of 0.7 mg DEX implant versus sham. DEX implant at 6 months for pre-specified criteria | ≥ 15 letter BCVA gain | Various time points. Peak effect at day 60 | VA gainers. 35% DEX versus 12% sham. Mean change +10.6 letters (DEX) versus +1.7 letters (sham) | Duration of benefit 3–4 months. IOP normalized by 4 months |
| Gado and Macky (2014)31 | Six-month, RCT, eyes with perfused CRVO with ME | 60 (30 in each group) | 0.7 mg DEX implant versus PRN bevacizumab | BCVA change between the groups | Monthly for six months | No difference in BCVA between the two groups. Bevacizumab group had thinner CST at one month, no difference thereafter | Higher IOP in DEX group. All eyes in DEX group needed second implant around 4 months. Mean 4.3 injections in bevacizumab group |
| Hoerauf et al. (2016) COMRADE-C32 | Six-month RCT, RZB versus DEX implant for ME secondary to CRVO | 124 RZB, 119 DEX | Monthly RZB for three months followed by PRN versus single DEX implant | Change in BCVA | Monthly for six months | No difference in BCVA at month 1 and 2. RZB superior from month 3–6. +12.86 letters in RZB, +3 in DEX at 6 months | 4.52 injections in RZB versus single in DEX |
| Hattenbach et al. (2018) COMRADE-B33 | Six-month RCT, RZB versus DEX implant for ME secondary to BRVO | 126 RZB, 118 DEX | Monthly RZB for three months followed by PRN versus single DEX implant | Change in BCVA | Monthly for six months | No difference in BCVA at month 1 and 2. RZB superior from month 3–6. +17.3 letters in RZB, +9.2 in DEX at 6 months | 4.68 RZB injections versus single DEX |
| Bandello et al. (2018)34 | Twelve-month RCT, DEX v RZB in ME caused by BRVO | DEX 154, RZB 153 | DEX at baseline and 5 months, optional at month 10 or 11, RZB 5 monthly injections followed by PRN | Change in BCVA | 12 months | +7.4 letters in DEX, +17.4 in RZB at 12 months | 2.5 DEX v 8 RZB injections. RZB superior to DEX regarding visual outcomes |
| Chatziralli et al. (2017) RANIDEX35 | Retrospective Chart review, RZB v DEX for ME from CRVO | RZB 25, DEX 17 | RZB, or DEX monotherapy | Change in BCVA and CST | Various time points over one year | CST. RZB superior at month 5 and 6. No difference at month 12 BCVA. +8.4 letters in RZB and +6.9 in DEX (NS) |
5.1 injections in RZB, 2.1 in DEX. RZB group was likely undertreated |
| Gu et al. (2017)36 | Six-month retrospective comparative Study. RZB v DEX for ME from RVO | RZB 32, DEX 32 | RZB, or DEX monotherapy | BCVA and CST | Various time points up to six months | No difference between DEX and RZB | Only 3.4 and 3.5 injections of RZB in CRVO and BRVO respectively, likely undertreatment |
| Yuksel et al. (2018)37 | Retrospective study. RZB v DEX. ME from BRVO | 44 eyes. RZB 14, DEX 15, Laser 15 | RZB, DEX, or laser | BCVA change, percent gaining ≥10 letters, and CST | 6 months | No difference at six months in all three groups. +13.5 letters DEX, +7.1 RZB | Only 2.4 injections of RZB over 14 months, likely undertreatment |
| De Salles et al. (2021)38 | Retrospective study of eyes with ME from RVO | 492 | Anti-VEGF versus DEX | BCVA and CST change | Final follow-up (variable time) | Superior visual and anatomical gains in anti-VEGF group for both BRVO and CRVO | Patients received about 4.5 anti-VEGF injections, and 1.8 DEX injections annually |
| Gale et al. (2021)39 | Retrospective study of BRVO ME patients | 5661 | Laser, anti-VEGF or DEX implant | Change in BCVA | Up to 3 years | Anti-VEGF superior to DEX at 12 and 18 months. (+9.6 v +4.5 letters) | 5.1 anti-VEGF v 1.5 DEX injections at 12 months. |
| Gale et al. (2021)40 | Retrospective study of CRVO ME patients | 4626 | Anti-VEGF or DEX implant | Change in BCVA | 12, 18 and 36 months | Anti-VEGF superior to DEX at all time points (+10 v +8.4 letters at 12 months, +10.4 v +1.6 at 18 month, +11.5 v +5.7 at 36 months) | Injections at 12 months (anti-VEGF/DEX)=5.6/1.6 At 18 months. 6/1.7 At 36 months 7/1.8 Higher endophthalmitis in DEX (0.09%) v anti-VEGF (0.003%) |
| Manoursaridis et al. (2017)41 | Retrospective chart review of RZB resistant ME secondary to RVO switched to DEX implant | 11 | Single DEX implant for eyes resistant to ≥3 RZB injections | Change in BCVA and CST | Various time points up to 6 months | 0.2 logMAR gain at 2 and 3 months. Return to baseline by month 6. CST improvement mainly at 2 and 3 months | DEX implant was effective in RZB resistant eyes for about 3 months |
| Georgalas et al. (2019)42 | Prospective nonrandomized. Switch to DEX for persistent ME from RVO after ≥ 5 anti-VEGF injections | 23 (13 BRVO, 10 CRVO) | DEX implant baseline, repeated as needed at six month | Change in BCVA and CST | 2,4,6,8,10 and 12 month visits | BRVO. Best VA at 4 months after first and second injections. CRVO. Best VA and CST at 2 months after first and second injections |
Reinjections sooner than 6 months are needed especially in CRVO |
| Mayer et al. (2012)43 | Prospective, consecutive, nonrandomized | 64 | 38 DEX, 26 3 monthly BCB followed by DEX | BCVA and CST change. | 6 months | No benefit from BCB loading | Recurrence of ME 3–4 months after DEX injection in various groups |
| Singer et al. (2012)44 | Prospective, nonrandomized | 34 | BCB followed 2 weeks later by DEX, retreatment as needed with same regimen | Time to reinject, BCVA gain, CST change | Monthly visits up to six months | +16.8 letter BCVA gain at six months, 64% gained ≥15 letters at six months | Retreatment in 82% at a mean of 126 days. No comparison group |
| Maturi et al. (2014)45 | Prospective randomized | 30 | BCB monotherapy PRN versus PRN BCB+ single DEX one week later. Repeat DEX at 4 months PRN | Primary: Change in BCVA Secondary: CST, number of injections |
6 months | No difference in BCVA, Fewer BCB (2 versus 3) and greater CST reduction in combination group, | The primary endpoint was not met. One less BCB injection in combination group balanced by DEX injection |
| Giuffre et al. (2020)46 | Prospective, nonrandomized. ME secondary to RVO unresponsive to aflibercept and DEX monotherapies | 30 | Same day DEX+ aflibercept. Retreatment allowed after 4 months | BCVA and CST changes | 12 months | No change in BCVA but CST reduction by 226 microns at 12 months. | Good anatomical response but not visual. Mean duration of ME was 25 months prior to combination therapy. |
| Harb et al. (2021)47 | Prospective nonrandomized | 74 | DEX monotherapy versus Aflibercept followed by DEX two weeks later combination | BCVA and CST change, number of retreatments | 12 months | Better BCVA gain in combination group compared to DEX monotherapy | No difference in CST or number of retreatments between the groups. Superior BCVA gain in combination group is hard to explain. |
| Bezatis et al. (2013)48 | Retrospective, noncomparative | 102 | Single DEX implant for ME from RVO | BCVA and CST change | Up to 6 months | Peak effect at two months (about 0.3 logMAR gain) | Retreatment in 50% around 4 months |
| Korobelnik et al. (2016) LOUVRE49 | Prospective, multicenter, real-world study in France | 375 | DEX monotherapy at baseline, retreatment at physician’s discretion | Primary. Change in BCVA Secondary. Other therapies, side effects, IOP |
2 years | At 6 months: DEX +5.5, DEX + other therapies +4.2 letters. At 2 yrs: DEX: +20.7 single DEX, +4.9 multiple DEX, +2.3 DEX plus others |
2.6 DEX injections over two years. Mean interval 6.6 months between injections, 55% received other therapy (laser or anti-VEGF). Cataract progression in 40%, increased IOP in 34.4%. Switch to other therapies did not improve outcomes |
| Capone et al. (2014) SHASTA50 | Retrospective, multicenter chart review | 289 | DEX implant at baseline, other therapies per physician's discretion | Change in BCVA, CST, side effects | Variable (3–6 months after last DEX) | Peak change in BCVA from 4–20 weeks after DEX +1 line. 60% BRVO and 66% CRVO gained ≥2 lines | Mean reinjection interval 5.6 months, Increased IOP (≥10) in 32.6%. DEX monotherapy in 29.1%, adjunctive therapy in others |
| Eter et al. (2017)52 | Prospective, multi-center, real-world, Germany | 573 | Single DEX at baseline. Additional treatment per physician's discretion | Change in BCVA at 12 weeks | 12 weeks. Duration of study 6 months | At 12 weeks, +7.8 letters gain, 34% gained ≥3 lines. | ≤90 days duration of ME had the best visual outcomes. 19.9% received other adjunctive treatments |
| Horner et al. (2020)53 | Retrospective, real-world, single site, UK | 66 | Initial RZB, later DEX/laser | Change in BCVA and CST | Year 1 and 3 | 40% gained ≥3 lines at year 3 | Diminished need for injections in year 3 compared to year 1 (2.5 v 5.5) |
| Kim et al. (2021)54 | Prospective, multicenter, real-world, S Korea | 700 | DEX implant at baseline. Subsequent treatment per physician's discretion | Change in BCVA, responder rate, side effects | Month 1, 2, 4 and 6 | Peak benefit at 2 months (approx. 2 lines). | Better vision gains in younger patients, worse presenting vision and treatment naïve status |
| Garay-Aramburu et al. (2021)55 | Retrospective, short-term, real-world study | 111 | DEX implant at baseline | Visual and anatomical improvement | Unspecified | Worse presenting vision (<60 ETDRS letters), first injection, and non-chronic ME predictor of better visual outcomes. | Baseline CST ≥400 microns and central macular volume >12 mm3 predictors of ≥50% CST reduction |
Abbreviations: RVO, retinal vein occlusion; RCT, randomized controlled trial; BCVA, best-corrected visual acuity; IOP, intraocular pressure; ME, macular edema; CRVO, central retinal vein occlusion; CST, central subfield thickness; BCB, bevacizumab; RZB, ranibizumab; BRVO, branch retinal vein occlusion; NS, non-significant; VEGF, vascular endothelial growth factor.
Another sham-controlled study of single injection of 0.7 mg DEX implant in Chinese patients showed similar results. At 2 months (peak effect) ≥15 letter improvement in BCVA was noted in 35% DEX treated eyes compared to 12% sham, with a mean change from baseline +10.6 letters for DEX versus +1.7 for sham, and mean change in CRT from baseline −407 microns for DEX versus −62 microns for sham (p<0.001 for all). The duration of anatomical and visual improvement was 3–4 months.30
Comparative Studies of DEX Implant versus Anti-VEGF Therapy
Gado and Macky (2014) performed a randomized clinical trial of 60 eyes with macular edema from perfused CRVO comparing 0.7 mg DEX implant with as needed bevacizumab injections over a six-month period. All eyes in DEX group needed another implant around the 4 month visit due to decrease in BCVA and increase in CRT. Eyes in bevacizumab group received a mean of 4.3 injections over the six-month study period. They found no significant difference in BCVA between the groups at all time points. The bevacizumab group showed slightly thinner CRT at one month, but no difference thereafter. They concluded that both DEX implant and intravitreal bevacizumab were about equally effective in managing macular edema from CRVO. As expected, DEX treated eyes showed statistically significant higher IOP.31
COMRADE-C (2016) was a multicenter, double blind, randomized clinical trial to compare ranibizumab 0.5 mg given monthly for three injections followed by PRN (n=124) with a single injection of 0.7 mg DEX implant (n=119) in patients with macular edema secondary to CRVO over a six-month period.32 Mean number of ranibizumab injections was 4.52. There was no difference in BCVA at months 1 and 2. However, there was significant difference in visual gain in favor of ranibizumab from months 3 to 6. At month 6, patients in ranibizumab group gained 12.86 letters compared to 2.96 in DEX group. The authors attributed this difference to single injection of DEX over the study period and concluded that DEX retreatment was likely needed sooner than six months.
COMRADE-B trial (2018) compared monthly ranibizumab 0.5 mg given monthly for three injections followed by PRN treatment (n=126) with a single injection of 0.7 mg DEX implant (n=118) over a six-month period in a randomized clinical trial. Similar to COMRADE-C, there was no difference between the groups at month 1 and 2, but BCVA gains were superior in ranibizumab group from month 3 through 6. At six months, patients in ranibizumab group gained 17.3 letters versus 9.2 letters in DEX group.33 Both COMRADE-B and COMRADE-C recognized that retreatment with DEX implant would be required sooner than six months in clinical practice.
A multicenter, randomized noninferiority trial compared 0.5 mg ranibizumab monthly for five injections followed by PRN from month 6 through month 11 (n=153) versus DEX implant at day 1 and month 5 with the option of retreatment at month 10 or 11 (n=154) in patients with macular edema secondary to BRVO. Unlike COMRADE-B, patients in DEX group received mandatory retreatment at month 5 and optional at month 10 or 11. However, at 12 months, mean BCVA gain was 7.4 letters for DEX versus 17.4 letters for ranibizumab. A mean of 2.5 injections were administered in the DEX group compared to 8 for ranibizumab. Dexamethasone did not show noninferiority to ranibizumab in this study.34
A multicenter, retrospective real-life comparative study of 42 eyes with macular edema secondary to CRVO treated with either intravitreal ranibizumab 0.5 mg given monthly for three injections followed by PRN (n=25) or 0.7 mg DEX implant given at baseline and at six months if needed (n=17) showed no statistically significant difference in BCVA or central subfield thickness (CST) change at 12 months between the two groups. However, recurrence of macular edema was noted at 5 months in DEX group suggesting that re-treatment with DEX implant is usually needed prior to six months.35 Both ranibizumab given monthly on PRN basis and DEX implant were comparable regarding BCVA and CRT reduction in a six-month retrospective study from China. Repeat injection of DEX implant was permitted after 4 months in this study.36
A real-world study showed comparable visual gain in DEX group compared to low frequency ranibizumab group (+13.5 letter in DEX versus +7.1 in ranibizumab, NS) in patients with macular edema secondary to BRVO. However, patients received only 2.4 ± 1.4 injections of ranibizumab over the follow-up period of 13.9±10.7 months, which is clearly undertreatment.37
In a real-life retrospective chart review of 492 patients with macular edema from RVO, de Salles and Epstein (2021) found superior visual gain with anti-VEGF therapy compared to monotherapy with DEX implant for both BRVO (+9.8±20.4 for anti-VEGF versus −2.1±23.4 for DEX) and CRVO (anti-VEGF +0.2±27.6 versus −9.7±32.6 letters for DEX) at final follow up visit. Anti-VEGF group also had less CRT compared to DEX.38
A recent retrospective chart review of 5661 patients with macular edema from BRVO treated with a single modality (laser, anti-VEGF or DEX) showed superior visual gain at 12 months for anti-VEGF compared to DEX (+9.6 versus +4.5 letters).39
A similar real-world retrospective chart review of 4626 patients with macular edema from CRVO treated with anti-VEGF injections or DEX implant showed superior visual results for anti-VEGF at 12, 18 and 36 months (+10 versus +8.4 letters at 12 months, +10.4 v +1.6 at 18 months, and +11.5 v +5.7 at 36 months). Patients in DEX group received only 1.8 injections over 36-month period compared to 7 anti-VEGF injections over the same period.40
Switch Studies
Manoursaridis et al (2017) treated 11 eyes with macular edema secondary to RVO that was resistant to at least three monthly injections of ranibizumab with a single DEX implant and noted favorable anatomical and visual results at two and three months with regression to baseline by month six.41
A 12-month prospective nonrandomized interventional study treated 23 eyes with macular edema caused by BRVO (13 eyes) or CRVO (10 eyes) persistent after at least 5 anti-VEGF injections with intraviteal DEX implant monotherapy, repeated as needed after at least six months. They showed peak therapeutic effect of DEX implant on BCVA at 4 months in BRVO (0.3 logMAR improvement from baseline) and at 2 months in CRVO (0.4 logMAR improvement).42
Combination Therapy with DEX Implant and Anti-VEGF Injections
Mayer et al (2012) evaluated DEX monotherapy versus 3 monthly bevacizumab injections followed by a DEX implant in patients with macular edema secondary to RVO in a prospective consecutive, nonrandomized study. Recurrence was treated with DEX implants in both groups. The primary endpoint was change in BCVA at six months. They found superior visual gain in DEX group for BRVO (+13 letters for DEX versus +3.8 for combination) and no statistically significant difference for CRVO. The authors concluded that there was no benefit from loading bevacizumab injections. The study design did not permit comparison between DEX implant and anti-VEGF injections.43
Singer et al (2012) treated 34 eyes with RVO related macular edema with intravitreal bevacizumab followed two weeks later by intravitreal DEX implant in a prospective 6-month study.44 Retreatment with the same combination regimen was performed if needed according to prespecified criteria (increase in CST by ≥50 microns or VA decrease by ≥6 letters). 82% eyes needed retreatment, after a mean of 126 days from initial treatment. BCVA gain at 6 month was +16.8 letters with 64% gaining ≥15 letters. Although there was no comparison group, the authors claimed that combination therapy with bevacizumab and DEX implant was synergistic and superior to monotherapy with either agent.44
Maturi et al (2014) compared intravitreal bevacizumab with intravitreal bevacizumab plus DEX implant one week later in a prospective randomized study of 30 eyes with macular edema secondary to RVO. Bevacizumab was administered monthly as needed in both groups with optional DEX implant at month 4 or 5 in combination group for CST ≥250 microns. At six months, mean change in BCVA was similar in both groups, but eyes receiving combination therapy required fewer bevacizumab reinjections (2 versus 3) and experienced greater mean reduction in CST (−56 microns versus +45 microns).45
Giuffre et al (2020) evaluated combination therapy of intravitreal aflibercept 2.0 mg with DEX implant on the same day in a prospective case series involving 30 eyes with macular edema refractory to each of the two drugs administered previously as monotherapy. Retreatment with combination therapy was permitted at least four months after initial therapy. At 12 months, there was no statistically significant change in BCVA, but CRT decreased to 352.5 from the baseline of 578 microns (p=0.003). The authors concluded that combination therapy was effective in resolving macular edema and perhaps earlier combined treatment might have led to better functional outcomes.46
Harb et al (2021) compared combination therapy of intravitreal aflibercept plus DEX implant two weeks later with DEX monotherapy in a prospective, nonrandomized study involving 74 treatment naïve eyes with macular edema secondary to RVO. The treatment was repeated as needed. At 12 months, eyes in the combination group showed better BCVA gain compared to monotherapy with DEX implant. There was no significant difference in CST or number of retreatments (1.75±1.13 versus 1.42±0.64) between the groups.47
Real-World Studies
The SOLO Study, a retrospective real-world study of 102 eyes with macular edema from BRVO (n=54) or CRVO (n=48) evaluated the results of monotherapy with DEX implant. Patients were followed monthly and retreated with DEX implant or anti-VEGF injections for an increase in CRT by 150 microns or decrease in BCVA by one line. Similar to the GENEVA trial, peak effect on CRT reduction and improvement in BCVA was observed at 2 months. Retreatment around 16 weeks was required in approximately 50% of eyes.48
A 24-month, prospective, multicenter, longitudinal, observation study in France (LOUVRE) enrolled adult patients with macular edema caused by BRVO or CRVO (n=375) treated at baseline with DEX implant.49 Retreatments with DEX implants and other treatments for RVO were performed at treating physician’s discretion. Patients received a mean of 2.6 DEX implant injections over two years, with a mean of 6.6 months between the injections. Approximately 55% patients received other therapy (laser and/or intravitreal ranibizumab). Mean change in BCVA from baseline to month 6 was +5.5 letters in patients who received only DEX compared to +4.2 letters in those who received additional RVO treatment during six months. Cataract progression and elevated IOP were noted in 40% and 34% respectively. No incisional glaucoma surgery was needed. Patients who received additional treatments with laser or ranibizumab did not have improved outcomes.
In the Shasta study, a retrospective multicenter real-world study involving 289 patients with RVO-associated macular edema, DEX implant led to one-line gain at around one month which was maintained for about one month. Patients received DEX implant as monotherapy (29%) or in conjunction with other modalities. BCVA and CRT outcomes were similar in DEX monotherapy and combination groups, although mean interval between DEX implants was longer in the combination group compared to DEX monotherapy (177 days versus 151 days).50,51
A German multi-center, open label, six-month observational real-world study involving eyes with macular edema from RVO (n=573) showed improved outcomes in patients with macular edema of less than 90 days duration compared to those with longer duration of macular edema suggesting the benefit of early treatment.52
Another real-world study from the UK showed that the need for injections diminished in year 3 compared to year 1 for both ranibizumab and DEX implant while maintaining visual gain and CRT improvement.53
A Korean prospective, six-month, observational, post-marketing surveillance study involving 700 patients with RVO-related macular edema showed peak vision gain at two months, with decline from month two to six. Vision gain was positively associated with younger age, worse presenting vision, and treatment naïve status.54
A recent retrospective study (2021) showed superior visual gains in patients presenting with worse presenting visual acuity, treatment naïve status and non-chronic ME, and superior anatomical outcomes in patients with presenting CRT ≥400 microns.55
Summary
Monotherapy with DEX implant is a reasonable treatment option for macular edema from RVO. The duration of therapeutic effect tends to be limited to three to four months and those treated earlier may have better outcomes. High intraocular pressure and cataract progression are well-known side effects of steroids. Most studies are of relatively short (1–2 years duration). A long-term study with a mean follow-up of 50.5 months showed superior results in BRVO compared to CRVO-associated macular edema.56 Studies comparing anti-VEGF therapy with DEX implants have shown superior results with anti-VEGF injections compared to DEX implants. The only advantage of DEX implant is fewer injections but at the cost of increased side effects of cataract and steroid-induced glaucoma. Therefore, DEX implant should be considered as second-line therapy in eyes with persistent macular edema despite prior anti-VEGF injections preferably in pseudophakic eyes (Figure 2). Combination therapy of anti-VEGF plus DEX implant is most likely not superior to anti-VEGF injections alone,45 but may be useful in some eyes refractory to both anti-VEGF injections and DEX implant.46 Loading injections with an anti-VEGF agent followed by DEX was not superior to DEX alone.43
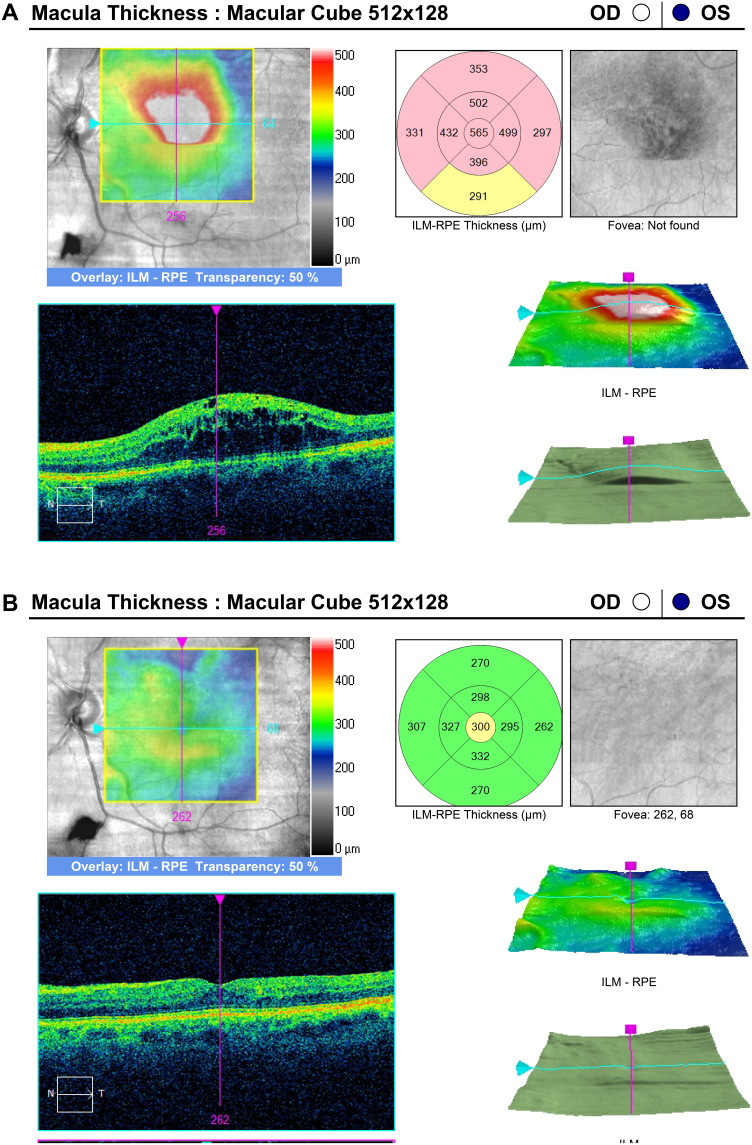
Figure 2.
(A) Persistent macular edema in an eye with superotemporal branch retinal vein occlusion despite 9 intravitreal bevacizumab, 17 ranibizumab, and 3 grid laser treatments over 61 months. (B) Resolution of macular edema one month after DEX implant.
DEX Implant for Uveitis and Uveitis-Associated Macular Edema
Uveitis is a group of ocular disorders characterized by inflammation within the eye, systemic disease association in some cases and potential for vision impairment. In the United States (US), there are 30,000 new cases of blindness caused by uveitis each year, and non-infectious uveitis is responsible for about 10% of legal blindness within the US. Uveitis can be classified based on the anatomic location of the inflammation, as anterior, intermediate, posterior or panuveitis. Uveitis can also be classified as either infectious or noninfectious, with noninfectious uveitis being the most common type in the US.57
Disease Burden
There have been several population-based studies evaluating the incidence and prevalence of uveitis within the US, but each studying different populations, at different times and with variable estimates.58–61 The most recent population-based study, the Pacific Ocular Inflammation study, evaluated the incidence and prevalence of uveitis from a racially diverse population representative of all age groups, within a health system in Hawaii. The study found an overall incidence rate of uveitis of 24.9 per 100,000 person-years and a prevalence rate of 58.0 per 100,000 persons for the year 2007. Anterior uveitis was the most common, with an incidence of 20.3 per 100,000 person-years, followed by posterior/panuveitis (3.9 per 100,000 person-years), and intermediate uveitis (0.7 per 100,000 person years). Incidence rates increased significantly with age (P < 0.001) and when further broken down by anatomic location of uveitis, rates of anterior uveitis increased with age (P < 0.001) but rates of intermediate (P = 0.41) and posterior/panuveitis (P = 0.96) did not.61
However, another claims-based analysis study, utilizing a large insurance claims database for the year 2012, evaluated the prevalence of non-infectious uveitis (NIU) within the US and found that the overall prevalence of all types of uveitis within a combined adult and pediatric population was 113.5 per 100,000.60 This prevalence rate is significantly higher than that reported in the Pacific Ocular Inflammation Study, probably due to higher number of Pacific Highlanders in that study cohort who have overall lower prevalence of uveitis but is similar to the prevalence of 115.3 per 100,000 found in a previous, older, large population-based study, the Northern California Epidemiology of Uveitis Study.58 The findings from the claims-based study also revealed that the prevalence of NIU increased with patient age. Across all age groups, the most common location of the uveitis was anterior. For the oldest patients (age 65 years and older), the most common non anterior location of inflammation was posterior uveitis.60
Uveitic Macular Edema
Macular edema is a common structural ocular complication of uveitis and uveitic macular edema (UME) is responsible for a substantial amount of visual impairment among patients with uveitis. UME is caused by a break down in the blood-retinal barrier, with resultant fluid accumulation within the retina, intra- and extracellularly. There is an influx of inflammatory cells, cytokines, growth factors and intracellular adhesion molecules with fluid accumulation within the macula as either cystoid spaces, or diffuse retinal thickening or as fluid within the subretinal space.62,63
A study by Durrani et al stratified the degree, duration and causes of visual loss in uveitis patients from a tertiary care uveitis referral center and found that not only did presence of intraocular inflammation contribute to moderate and severe vision loss in these patients, but 26.8% of the patients had cystoid macular edema (CME) which also contributed to the vision loss.64 In the Multicenter Uveitis Steroid Treatment (MUST) Trial, among 481 eyes with uveitis, 37% had intermediate uveitis, and 63% had posterior/panuveitis.65 Approximately half of the eyes with uveitis had low vision (best-corrected visual acuity worse than 20/40), and approximately 16% were legally blind (best corrected visual acuity of 20/200 or worse), with a similar distribution across intermediate uveitis vs posterior or panuveitis cases.65 CME was present in 38% of eyes, and this rate was found to be similar in intermediate uveitis and posterior/panuveitis cases. Another recent study,66 evaluating spectral domain optical coherence tomography (SD-OCT) findings on 500 consecutive uveitis patients, found that the anatomic location of inflammation affected significantly the central retinal thickness and macular volume, with the highest values in intermediate and panuveitis. CME was seen in 25% of all uveitic eyes and was most frequent in intermediate (40%) and panuveitis (36%).66
Rationale for Use of Intravitreal Drug Delivery Systems
The approach to the treatment of UME begins with control of the underlying uveitis.67 Corticosteroids remain the mainstay of treatment for non-infectious uveitis, at least in the short term, and may be used topically for anterior segment disease and locally with periocular and intraocular injections, for posterior segment disease, which in turn may help resolve UME. Local steroid therapy may be preferred in cases of unilateral disease to avoid the systemic side effects of steroids. In severe disease, systemic corticosteroid therapy is often required. If long-term systemic therapy is anticipated, steroid-sparing immunomodulatory therapy is used, and all these measures may help UME.67
Intravitreal drug delivery systems (DDSs) have several advantages over systemic delivery in that they bypass the blood-retinal barrier, thus allowing the drug to reach the retina in high concentrations, with little or no systemic exposure.68 They have an advantage over topical application as most topically administered drugs do not achieve therapeutic concentration in the vitreous cavity. Intravitreal drug delivery can be performed through direct injection, or with a sustained release implant. Direct intravitreal injections of steroids such as triamcinolone, although easy to perform carry the risk of endophthalmitis, retinal detachment and vitreous hemorrhage and provide uneven intravitreal concentration of the drug. With an intravitreal sustained DDS there is steady delivery of medication to the target tissue, limiting toxicity to non-target tissues. It may also reduce the need for repeated injections and allows for longer intervals between treatments, improving patient compliance (Figure 3). 68
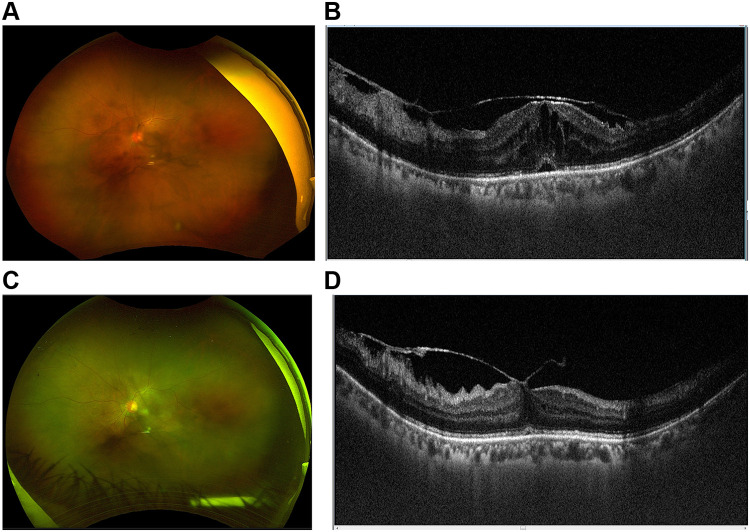
Figure 3.
(A) Fundus photo of an eye with intermediate uveitis showing marked vitreous haze and membranes. (B) Marked macular edema and subretinal fluid on OCT. (C) Three days after DEX implant showing marked improvement in vitreous haze and membranes. (D) Resolution of macular edema.
DEX Implant for Uveitic Macular Edema
The HURON Study Group in 2011 evaluated the safety and efficacy of two different doses (0.7 and 0.35 mg) of DEX implant for the treatment of noninfectious intermediate or posterior uveitis as a randomized controlled Phase III trial (Table 2).69 Patients enrolled in this study had a vitreous haze score of at least +1.5 and an Early Treatment Diabetic Retinopathy Study (ETDRS) best corrected visual acuity (BCVA) of 10–75 letters (20/630 - 20/32). The main outcome measure was the proportion of eyes with a vitreous haze score of 0 at week 8. Key exclusion criteria included the use of IOP-lowering medications within the last 1 month, history of glaucoma, or ocular hypertension including exclusion of patients with IOP of ≥ 21 mmHg at baseline. In this 26-week trial, 229 patients were enrolled, and majority (81%) had intermediate uveitis. The eyes were randomized to a single treatment with a 0.7-mg DEX implant (n = 77), 0.35-mg DEX implant (n = 76), or a sham procedure (n = 76). The results of the study were encouraging. The proportion of eyes with a vitreous haze score of 0 at week 8 was 47% with the 0.7 mg DEX implant, 36% with the 0.35 mg DEX implant and 12% with the sham. The percentage of eyes achieving a vitreous haze score of zero was significantly greater in the dexamethasone implant 0.7 mg group than the sham group from weeks 6 through 26 (p <0.014). The study also evaluated CST, and found that, although there was a statistically significant mean decrease from baseline at week 8 in the 0.7 mg DEX implant group compared with sham, this effect was not sustained through week 26. But despite the lack of significant reduction in CME by week 26, a gain of 15 or more letters from baseline BCVA was seen significantly more frequently in eyes in the DEX implant groups than the sham group at all study visits, including the one at 26 weeks.69
Table 2.
Summary of Studies of DEX Implant in Uveitis and Uveitic Macular Edema
| Study (Year) | Design | n | Intervention | Outcome Measure(s) | Time Point(s) | Results | Comments |
|---|---|---|---|---|---|---|---|
| Lowder et al. (2011) The HURON Study Group69 | 26-week, sham-controlled RCT | 229 eyes with noninfectious posterior or intermediate uveitis | Single injection of DEX implant (350 mcg or 700 mcg) versus sham | Eyes with vitreous haze score of 0 | 8 weeks | 47% with DEX 0.7, 36% with DEX 0.35, 12% sham achieved primary end point. Visual benefit persisted through week 26. CST benefit at 8 weeks, but not at 26 | Superior visual results in DEX treated eyes at all visits. IOP≥25 mm Hg in 7.1% DEX 0.7, 8.7% DEX 0.35 and 4.2% for sham. |
| Pelegrin et al. (2015)70 | Retrospective | 42 eyes of 32 patients with UME | DEX implant, retreatment as needed | BCVA, CST reduction, side effects | Variable | Peak CST reduction at 1 month. Peak VA benefit at 3 months. No difference between vitrectomized and nonvitrectomized eyes | 45.2% needed reinjection after a median of 5 months. Ocular hypertension in 47.6%. |
| Tsang et al. (2017)71 | Retrospective | 25 eyes of 15 patients with UME | DEX implant, repeated as needed | Primary CST, secondary BCVA, time to reinjection | Variable | 91.4% had reduction in CST (−220 microns at 3 months). Approx 2 line VA gain at 3 months | 11 patients on concurrent immunesuppression. No difference in effect between first implant and repeat implant. |
| Khurana et al. (2017)10 | Prospective, noncomparative, single center | 10 eyes with UME and quiescent uveitis | DEX implant at baseline, retreatment as needed after day 90 | BCVA, CST | Day 90, and one year | +14.4 letter gain, and −140 microns CST reduction at 3 months. | 60% needed retreatment. Visual and anatomical benefits maintained through one year with retreatments. |
| Nobre-Cardoso et al. (2017)72 | Retrospective | 41 eyes of 31 patients with UME | DEX implant at baseline, retreatment as needed | BCVA, CST, complications | Variable | At one month, CST improved in most eyes, but 31.7% shows no improvement in VA | Ocular hypertension in 36.2%. Small and short-term effect of DEX implant. |
| Fabiani et al. (2017)73 | Retrospective | 22 eyes with UME | Single DEX implant | Systemic steroid sparing effect | 3 and six months | Reduced dose of systemic prednisone at 3 and 6 months. CST and BCVA improved at 1,3, and 6 month visits. | 13.6% developed ocular hypertension. Returned to baseline by 6 months. |
| Ratra et al. (2018)74 | Retrospective | 42 eyes of 34 patients with UME | DEX implant as needed | BCVA, CST | Variable | Visual, anatomical and steroid sparing effect of DEX implant. 10 eyes (24%) needed no other treatment. Oral steroids stopped in 40% | Only 11 eyes (26%) needed repeat injection after 12.6–20.9 months. Prolonged effect of DEX implant hard to explain. |
| Thorne et al. (2019) The POINT Trial75 | Six month, multicenter, randomized, clinical trial | 235 eyes of 192 patients with UME | PTA (40 mg), IVTA (4mg), DEX (0.7 mg)1:1:1 randomization. Retreatment permitted at 8 weeks in PTA and IVTA, and at 12 weeks in DEX | CST, BCVA, side effects | CST at 8 weeks. BCVA and IOP over six months | Both IVTA and DEX superior to PTA regarding CST reduction and BCVA, DEX noninferior to IVTA. Repeat injection 38 (48%) IVTA, 44 (56%) DEX. | Higher IOP in intravitreal groups compared to PTA, no difference between DEX and IVTA. |
Abbreviations: UME, uveitic macular edema; PTA, periocular triamcinolone acetonide; IVTA, intravitreal triamcinolone acetonide; CST, central subfield thickness.
The findings of reduction in UME within the HURON trial spurred several other studies evaluating the DEX implant specifically for UME. A retrospective study by Pelegrin et al (2015) showed peak effect on CST reduction at one month, on BCVA at three months without any difference between vitrectomized and nonvitrectomized eyes. Reinjection was performed in 45.2% eyes after a median time of 5 months. Ocular hypertension was seen in 47.6% eyes, with nonvitrecomized eyes showing higher IOP.70 A retrospective study in 2017 by Tsang et al from Canada evaluated the use of DEX implant in 15 consecutive patients with non-infectious posterior segment uveitis for the treatment of their macular edema.71 Eleven patients were receiving concomitant systemic immunomodulatory therapy during treatment with the DEX implant which included methotrexate, adalimumab, mycophenolate mofetil, cyclosporine, infliximab. A total of 35 implants on 25 eyes of 15 patients were included in the analysis. Of these, 91.4% had a reduction in CST and 80% had improved BCVA. After the first DEX implant, CST decreased from 590 microns at baseline to 370 microns at 3 months (p < 0.001). The logMAR VA was 0.614 at baseline and improved to 0.35 (p = 0.002), reaching a statistically significant difference at 3 months. A repeat implant led to VA improvement of –0.184 logMAR and CST reduction of –291 microns. There was no significant difference in effect between the first repeat implant and the initial implant. Kaplan–Meier estimates of treatment success were 72% between 3 and 6 months. 18 eyes received only one implant and seven eyes required treatment with multiple implants. The authors concluded that the DEX implant is an effective adjunct treatment to systemic corticosteroid or immunomodulatory therapy and additional research was required to determine the efficacy of DEX implant as monotherapy for controlling chronic uveitic macular edema.71
That same year, Khurana et al prospectively evaluated the use of the DEX implant for 10 patients with persistent UME for 3 months or longer (mean 8.4 months) in the absence of intraocular inflammation.10 The inclusion criteria included patients with noninfectious uveitis, central sub-foveal retinal thickness (CST) ≥350 microns, and visual acuity worse than 20/32. The inflammation from their uveitis had to be well controlled with anterior chamber cell ≤0.5+ and vitreous haze ≤1+. All patients were treated with DEX implant at study entry and were eligible for retreatment with DEX implant at any time after Day 90 if there was any sign of recurrence of CME. Recurrence of CME was defined as the presence of intraretinal cysts on SD-OCT. The authors reported that after a single DEX implant, there was complete resolution of CME in 90% of eyes at 1 month posttreatment and 70% of eyes at 3 months posttreatment. At day 90, mean improvement from baseline BCVA was 14.4 letters (P = 0.0003), 70% of patients had a ≥10 letter BCVA improvement, 50% of patients had a ≥15 letter BCVA improvement, and the mean decrease from baseline central subfield retinal thickness was 140 microns (P = 0.008). Improvements were maintained through day 360 with retreatment as needed. Forty percent of patients received 1, 30% of patients received 2, 20% of patients received 3, and 10% of patients received 4 DEX implant treatments. At day 360, mean improvement in BCVA was 16.5 letters (P = 0.006) and the mean decrease in central subfield retinal thickness was 158 microns (P = 0.002).10
While most studies reported favorable results with DEX implants for uveitis and UME, Nobre-Cardoso et al evaluated the long-term outcomes of DEX implant, need for re-injection, duration of action and relapse rate, as well as the safety profile in patients with uveitis and found that while the DEX implant can be used to treat UME, there was a limited and short-term effect on BCVA.72 The authors studied 41 eyes of 31 patients with UME and non- infectious uveitis of which 21 patients (67.7%) were receiving systemic treatment. Eighteen eyes (43.9%) had a persistent UME for at least 6 months before implantation and in four eyes (9.8%), the edema was present for at least 12 months before implantation. Follow-up time was on average 13.4 ± 5.9 months after the first implant. The authors found that one month after the first implant, even as CRT improved significantly in most eyes (p<0.001), 31.7% showed no improvement in BCVA. At 6 months post-implantation, CRT and BCVA had deteriorated in up to 70% of patients. Thirteen eyes were re-implanted, with a similar effect to that of the first implant. Ocular hypertension developed in 36.2% of eyes, and three eyes had cataract surgery, all in eyes with repeated implants. The percentage of eyes that developed ocular hypertension (intraocular pressure (IOP) ≥21 mmHg) was higher than reported in the HURON trial (23% versus 36%).
Fabiani et al retrospectively analyzed 22 eyes treated with DEX implant for UME related to non-infectious uveitis.73 The mean systemic prednisone (or equivalent) dosage significantly decreased at 3- and 6-month follow-up evaluations compared to baseline (P=0.002 and P=0.01, respectively). Compared to baseline, CRT values significantly decreased at 1-, 3-, and 6-month evaluations after the implantation (P < 0.0001). The mean best corrected visual acuity (BCVA) value also gradually improved at 1-, 3-, and 6- month visits compared to baseline (P=0.009, P=0.0004, and P=0.0001, respectively). The authors concluded that treatment with intravitreal DEX implant in noninfectious uveitis allowed a significant corticosteroid sparing effect, a significant improvement in BCVA, and a prompt resolution of UME without any safety issues observed in any of the patients.73
Ratra et al retrospectively analyzed the efficacy of DEX implant in 42 eyes of 34 adult and pediatric patients with UME from noninfectious intermediate or posterior uveitis over a follow-up period of 19.2+2.2 months, and showed significant visual, anatomical and systemic steroid sparing effect. Only 11 eyes needed repeat implant after a period of 12.6 to 20.9 months.74
The PeriOcular versus INTravitreal corticosteroids for uveitic macular edema (POINT) Trial was a pivotal multicenter randomized clinical trial that evaluated the comparative effectiveness of three regional corticosteroid injections for UME: periocular triamcinolone acetonide (PTA), intravitreal triamcinolone acetonide (ITA), and the intravitreal DEX implant, where patients were randomized to receive 1:1:1 of the three therapies.75 The primary outcome was the proportion of baseline CRT at 8 weeks assessed with optical coherence tomography (OCT) by masked readers. Secondary outcomes included ≥20% improvement and resolution of macular edema, BCVA, and IOP events over 24 weeks. At 8 weeks, each group had clinically meaningful reductions in CRT relative to baseline. Intravitreal triamcinolone acetonide and DEX implant had larger reductions in CRT than PTA (p <0.0001). Intravitreal DEX implant was non-inferior to ITA at 8 weeks. Both ITA and intravitreal DEX implant treatments also were superior to PTA treatment in improving and resolving UME. All treatment groups demonstrated BCVA improvement throughout follow-up. Both ITA and intravitreal DEX implant groups had improvements in BCVA that was 5 letters greater than the PTA group at 8 weeks (p <0.004). The risk of having IOP ≥24 mmHg was higher in the intravitreal treatment groups compared with the periocular group, however, there was no significant difference between the two intravitreal treatment groups. The authors concluded that the intravitreal therapies were superior to PTA for treating UME with modest rise in IOP which was not different between the 2 intravitreal therapies.75 However, despite extensive evidence for the safety and efficacy of DEX implant, its use is currently off-label in UME.
DEX Implant for Diabetic Macular Edema
Diabetic macular edema (DME) is likely due to multifactorial pathologic mechanisms. Other than VEGF, alterations in hydrostatic pressure, inflammatory cytokines and changes in intercellular adhesion molecule have also been shown to incite and perpetuate increased capillary permeability.76–81 This is the rationale for using corticosteroids in the management of diabetic macular edema. Corticosteroids also have additional benefits such as inhibiting leukostasis and inhibiting VEGF signaling at the synthesis level.
Studies on DEX implant in patients with DME have been reported under varied clinical situations- in treatment naïve DME, in anti-VEGF resistant DME, in combination with anti-VEGF [2 RCTs], in combination with macular laser photocoagulation, in non-vitrectomized eyes and in vitrectomized eyes (Table 3). Comparative treatments in studies using DEX implant for DME has included sham injection (1 RCT with 1048 eyes), intravitreal anti-VEGF injection (3 RCTs), and laser photocoagulation (1 study, 253 eyes). In addition, some studies have focused on the short-term safety and efficacy of a single implant while others using multiple implants. The duration of follow-up has varied from 6 months to 3 years.
Table 3.
Summary of Studies of DEX Implant in Diabetic Macular Edema
| Study (Year) | Design | n | Intervention | Outcome Measure(s) | Time Point(s) | Results | Comments |
|---|---|---|---|---|---|---|---|
| Haller JA et al. (2010)83 | Sham-controlled RCT | 171 | Single injection DEX 0.7 mg, DEX 0.35 mg, or sham | ≥10 letter BCVA gain | 90 and 180 days | Day 90. Gainers 33.3% DEX 0.7, 21.1% DEX 0.35 Day 180. Gainers 30% DEX 0.7, 19% DEX 0.35 |
Phase II study |
| Pacella et al. (2013)84 | Prospective, nonrandomized, interventional | 20 eyes of 17 patients | Single DEX implant | BCVA and CST change | Various time points over 6 months | BCVA and CST gain from 3rd day until 3 months. (−.14 logMAR, −226 microns at 3 months) | Regression of BCVA and CST at month 4 and 6. Two eyes re-injected prior to six months |
| Callanan et al. (2013) PLACID93 | RCT | 253 eyes with diffuse DME | Combination. DEX baseline laser 1 month monotherapy. Sham baseline laser 1 month. Up to 3 additional laser and one DEX permitted |
BCVA (≥ 10 letter gain) and area of vascular leakage by angiography. | 12 months | Combination group better at 1 and 9 months, but no difference at 12 months | DEX implant offered no visual benefit to focal laser alone at 12 months. Combinatin group had greater decrease in vascular leakage area |
| Lazic et al. (2014)85 | Prospective, nonrandomized, interventional | 16 eyes of 15 patients | Single DEX, eyes with “unresponsive” DME after ≥3 monthly anti-VEGF injections | BCVA and CST change | Monthly for six months | Peak VA gain at 2 months, CST improvement at 1,2,3 months | Increased IOP at 1,2,3 months. Duration of therapeutic effect 3–4 months. |
| Boyer et al. (2011)86 | Prospective, nonrandomized, interventional | 55 eyes with DME and history of prior PPV | Single DEX implant | Primary: Change in CST and Secondary: change in BCVA | Week 8 and 26 | Week 8. −156 micron CST, +6 letters BCVA Week 26. −65 microns CST, +3 letters BCVA |
30% gained ≥10 letters at week 8 |
| Medeiros et al. (2014)87 | Retrospective, comparative | 58 eyes. 24 vitrectomized, 34 nonvitrectomized | Single DEX | BCVA and CST | Month 1, 3, 6 | Improved BCVA and CST at each visit. | No difference between vitrectomized and nonvitrectomized eyes |
| Medeiros et al. (2014)101 | Retrospective, noncomparative | 58 eyes with “refractory” DME | Single DEX | BCVA and CST | Month 1, 3, 6 | Reduction in CST at 1 and 3 month, some regression at month 6. Peak VA benefit at 3 months (about 2 lines) | Refractory not defined. |
| Boyer et al. (2014) The MEAD Study82 | 3-year, sham-controlled RCT | 1048 | 1:1:1 randomization DEX 0.7 mg, DEX 0.35 mg, sham. Retreatment allowed ≥ 6 months | ≥ 15 letter BCVA gain, CST | 3 years | Gainers: 22.2% (DEX 0.7), 18.4% (DEX 0.35) and 12% (sham). Mean CST reduction −111.6 microns (DEX 0.7), −107.9 (DEX 0.35), −41.9 (sham) | Cataract 67.9% phakic eyes (DEX 0.7), 64% (DEX 0.35). IOP elevation in about 25%, Overall mean improvement in BCVA only 3 to 4 letters |
| Maturi et al. (2015)97 | RCT | 40 eyes with “incomplete response to anti-VEGF injections” | BCB monotherapy versus combination (BCB baseline, DEX at month 1, and 5.9 PRN) | Change in BCVA, and CST | 12 months | Similar BCVA gain in both groups (+4.9 v +5.4 letters), Greater CST reduction in CST group (−45 microns v −30 microns) | 3 fewer BCB injections in combination group but required 2.1 DEX injections (no real benefit) |
| Totan et al. (2016)102 | Prospective, interventional, nonrandomized (Switch study) |
30 | Single DEX for eyes “resistant” to prior BCB | Change in BCVA and CST | 1, 3, and 6 months | BCVA gain at 1 and 3 months (about −0.14 logMAR), Improved CST at all three time points | Regression of effect between 3 and 6 months. |
| Shah S et al. (2016)103 | RCT | 50 | DEX q 3 months, v BCB monotherapy for persistent DME despite ≥3 anti-VEGF injections within 5 months | BCVA, CST reduction | 7 months | Similar BCVA gain (BCB +5.6, DEX +5.8 letters), greater CST reduction in DEX (−122 v −13 microns) | Eyes with “persistent” DME show improvement in vision with continued anti-VEGF injections |
| Chhablani et al. (2016)90 | Retrospective, comparative | 79 eyes (15 treatment naïve, 64 previously treated “recalcitrant”) | DEX at baseline, repeated as needed | BCVA, CST | Variable | Similar BCVA gain in both groups (about 0.15 logMAR) | “Real-life” study. Reinjection after mean 6.5 months |
| Callanan et al. (2017)94 | RCT, multi-center, noninferiority | DEX 181, RZB 182 | DEX every 5 months, versus RZB monthly PRN | BCVA, CST, percentage gainer ≥15 letters | 12 months | DEX +4.34, RZB +7.6 letters | DEX noninferior to RZB, greater decrease in fluorescein leakage area in DEX group |
| Castro-Navarro et al. (2019)89 | Retrospective, comparative | 84 eyes (29 treatment naïve and 55 previously treated “refractory”) | Single DEX implant | BCVA, CST | 2, 4 and 6 months | Similar CST improvement in both groups. Naïve eyes more likely than treated eyes to gain ≥10 letters | “Refractory” not defined. |
| Maturi et al. (2018) DRCR.net Protocol U99 | Six-month, multicenter, RCT | 236 patients with persistent DME despite ≥ 3 anti-VEGF injections over the previous 20 weeks enrolled. All given RZB every month for 3 more injections. Those with still persistent DME (n=129) were randomized. | Combination: DEX every 3 months plus monthly RZB v monthly RZB monotherapy | BCVA gain, proportion of eyes gaining ≥15 letters, CST change | 24 weeks | No difference in BCVA gain (+3 letters) between the two groups. 11% v 2% gained ≥15 letters in combination v monotherapy respectively. Greater CST reduction in combination v monotherapy (110 μm v 62 μm) | 30% developed increased IOP in combination group. No BCVA benefit from combination either in phakic or pseudophakic eyes (subgroup analysis). No difference between short duration (6 months) or longer duration of DME |
Abbreviations: BCB, bevacizumab; PPV, pars plana vitrectomy; RZB, ranibizumab.
MEAD study was a pivotal phase III prospective randomized sham-controlled study to compare the safety and efficacy of two doses of DEX implant (0.35 mg- 347 patients and 0.7 mg- 351 patients) with sham injection (350 patients).82 At a follow-up period of 3 years, beneficial anatomical (about 100 µm versus 40 µm CRT reduction) and functional outcomes were observed for both doses. On average, 4–5 injections were needed over the 3-year period. Unlike with anti-VEGF injections, monthly follow-up over the study period was not necessary. Fifteen letter or greater improvement in BCVA was noted in approximately 12%, 18% and 22% of patients with the sham, 0.35 mg and 0.7 mg groups respectively. However, the mean overall improvement in visual acuity was between 3 and 4 letters in both treatment groups. At 1 year follow-up interval, decline in visual acuity was observed due to progression of cataract but vision was restored following intraocular lens implantation. Cataract surgery was required in 20%, 64% and 68% of phakic eyes in sham, 0.35 mg DEX, and 0.7 mg DEX groups, respectively. Other serious adverse events noted were IOP elevation in every 4th patient in the implant group with 0.1% needing glaucoma surgery and 4–7% risk of vitreous hemorrhage that cleared spontaneously. Prior to the MEAD study, the efficacy of DEX implant had been demonstrated in DME by Haller et al and Pacella et al, in DME not responding to anti-VEGF by Lazic et al, and DME in vitrectomized eyes by Boyer et al and Medeiros et al.83–87 A subsequent subgroup analysis of patients enrolled in the MEAD trial who had been previously treated with laser, anti-VEGF, intravitreal triamcinolone, or a combination of these, were also found to have favorable outcome.88 Castro-Navarro et al (2019) evaluated the safety and efficacy of a single DEX implant in 69 patients (84 eyes) with treatment naïve DME (29 eyes) and DME that was resistant to prior treatment (55 eyes) in a retrospective chart review.89 Over a 6-month follow-up period, the CST improved respectively by about 120 µm and 90 µm in the two groups. Although both groups showed visual and anatomical improvement, treatment naïve did better than previously treated eyes regarding BCVA gain ≥10 letters. Other authors have reported similar effects in both previously treated and untreated DME patients.90 Functional benefits have been reported as early as 4 weeks after the injection.91 In the MEAD study, implant reinjection was carried out after more than 6 months follow-up. More recent analysis suggests that reinjection at 4–5 months may have better outcomes and hence it is possible that MEAD results were an underestimate of the actual potential of the DEX implant.92
In a 12-month prospective randomized, double masked, controlled study by Callanan et al (2013), the percentage of patients who gained ≥ 10 letters were not different in the two study groups (dexamethasone implant followed 1 month later by focal/grid laser photocoagulation versus sham implant followed by laser). Patients with diffuse macular leak on fluorescein angiography however fared better with the implant up to 9 months.93 In a subsequent 12-month, multi-center, open-label, randomized noninferiority study, Callanan et al compared the safety and efficacy of DEX implant given every five months versus ranibizumab in patients with treated but persisting DME (mean duration of 3 years). The mean change in BCVA was about 4 ETDRS letters in the implant group versus 7 letters in the ranibizumab group which was within the prespecified noninferiority margins. Improvement of ≥ 15 letters at different time points was also comparable (7.2–17.7% versus 4.4–26.9% respectively). Noninferiority was obtained with 2.85 DEX implants versus 8.7 Ranibizumab injections.94 In a recently reported meta-analysis, involving 10 studies and 362 eyes, it was found that a single injection of DEX implant was beneficial in lowering the CST and improving visual acuity over 6 months in patients not responding to anti-VEGF therapy (Figure 4).95 However, terms such as “anti-VEGF nonresponders”, “partial or incomplete responders”, and “refractory macular edema” are not clearly defined both in the setting of DME and RVO-associated macular edema.
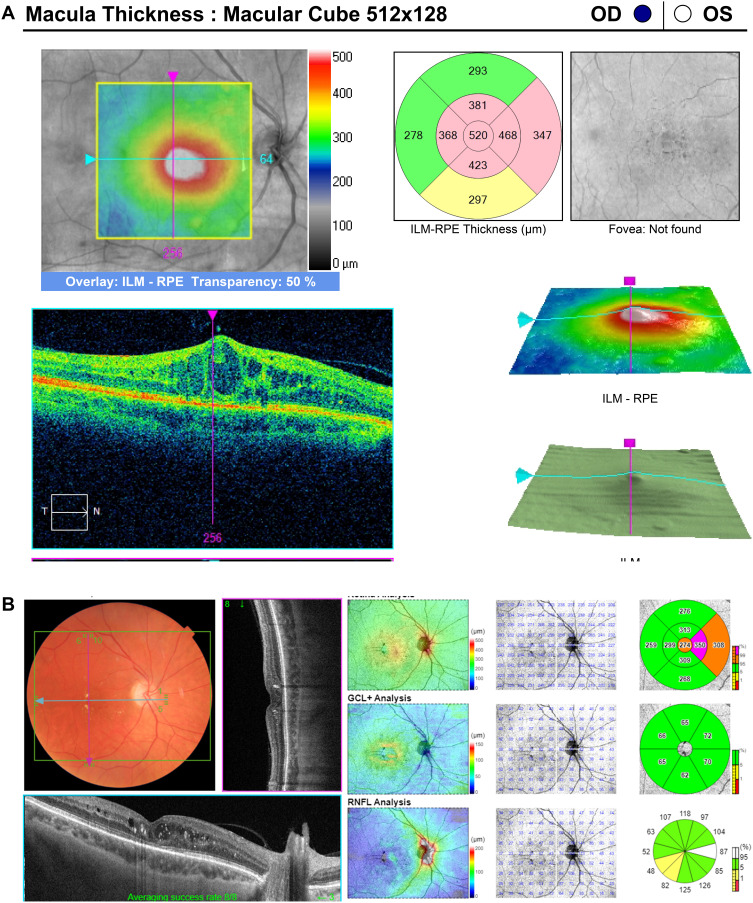
Figure 4.
(A) Diabetic macular edema persistent after 10 intravitreal bevacizumab and 13 aflibercept injections over 40 months. (B) Resolution of macular edema one month after DEX implant.
Another meta-analysis of 4 RCTs and 521 eyes has shown DEX implant to have superior anatomical benefits in terms of reduction in CST at 6 and 12 months compared to anti-VEGF injections, but lower functional results due to progression of cataract.96 Maturi et al evaluated combination therapy with intravitreal bevacizumab at baseline followed by DEX implant at 1 month, and at 5 and 9 months if needed against continued bevacizumab monotherapy in a randomized clinical trial and noted similar visual gains in both groups, although the combination group had a slightly greater CST reduction. The combination group received 3 fewer bevacizumab injections but required 2.1 DEX injections over a period of 12 months.97 Therefore, combination treatment with DEX implant and anti-VEGF injections for the management of DME is generally not recommended because no additional functional benefit has been demonstrated and instead, patients were exposed to the risks of both injections.98,99 Major concerns with the implant, as with other corticosteroid treatments remains a fourfold increase in the risk of IOP elevation and progression of cataract.100 Owing to these concerns, corticosteroid implants, including DEX implant, are generally recommended as second-line therapy in phakic eyes in most guidelines. A retrospective study showed about 2-line visual gain at three months followed by regression over the next three months in 58 eyes with “refractory” DME treated with a single DEX implant.101 Totan et al showed some visual benefit at 1 and 3 months with DEX implant in a nonrandomized prospective study in eyes “resistant” to prior intravireal bevacizumab.102 However, Shah et al showed similar visual gain in “persistent DME despite ≥3 anti-VEGF injections” who continued with intravitreal bevacizumab monotherapy and those who were switched to DEX implant reinjected every three months (+5.6 v +5.8 letters), despite greater CST reduction in the DEX group.103 A recent consensus statement suggests that it may be inappropriate to wait for more than 3 months before consideration of a switch to second-line therapy.104 However, as shown by the DRCR.net Protocol U,99 eyes with persistent DME after >6 anti-VEGF injections can still expect significant visual gain with continued anti-VEGF monotherapy, which, unlike DEX implant, has the added benefit of causing improvement of diabetic retinopathy severity. Whether DEX-implant offers any benefit over anti-VEGF injections in special situations, such as postvitrectomy or pseudophakic eyes is currently not known.
Other off Label Uses of DEX Implant
Neovascular Age-Related Macular Degeneration
A single injection of DEX implant as an adjunct to monthly ranibizumab was evaluated in a small noncomparative interventional study by Calvo et al.105 Although BCVA did not improve, statistically significant reduction in CRT was noted as early as two weeks after DEX implant (248 microns from 273 microns), with maximum reduction in CRT occurring at 3 months.105 Similar results (anatomical benefit but no visual benefit) were noted by Barikian et al in 2017 and Giancipoli et al in 2018.106,107 Rezar-Dreindl et al (2017) conducted a one-year randomized controlled trial involving 40 eyes with neovascular AMD treated with intravitreal ranibizumab PRN alone versus combination of DEX implant with PRN ranibizumab. Reinjection with DEX implant was permitted after six months. No difference in BCVA was noted. But the eyes in combination group needed fewer ranibizumab injections and median time to first re-injection was significantly longer in the combination group.108 However, no anatomical or visual benefits were noted in a randomized multicenter trial comparing adjunct DEX implant versus intravitreal ranibizumab alone.109 In a retrospective study, Mallikarjun et al showed that an adjunct intravitreal DEX implant prolonged treatment-free interval in eyes with polypoidal choroidal vasculopathy without offering any visual benefit.110
Miscellaneous Conditions
DEX implant has been used off-label for macular edema associated with retinitis pigmentosa,111–114 hydroxychloroquine retinopathy,115 persistent macular edema after pars plana vitrectomy,116 and various other indications.117 However, a prospective randomized controlled trial of 140 eyes undergoing vitrectomy surgery with silicone oil for proliferative vitreo-retinopathy failed to show any benefit of DEX implants injected at the time of surgery and silicone oil removal.118
Serious Side Effects and Complications
Potential adverse effects associated with DEX implant can be subdivided into four categories:
Steroid-related complications. Cataract progression and steroid-induced ocular hypertension are well-known complications associated with all steroids including DEX implant and should be managed appropriately. A recent study showed that selective laser trabeculoplasty (SLT) is an effective treatment for elevated IOP after DEX implant. After SLT, mean IOP dropped by 31% at one month and 34% in three months. Eyes that received second DEX implant (31%) after SLT did not experience IOP rise after subsequent injection.119
Opportunistic infections such as CMV retinitis,120–123 and toxoplasmic retinochoroiditis have been reported after DEX implant.124
Injection related complications. Lens injury, vitreous hemorrhage, retinal detachment, impaction in the sclera, endophthalmitis, and hypotony likely due to a relatively larger 22-g applicator needle.125 Retinal and vitreous hemorrhage due to traumatic impact in a vitrectomized eye has been reported.126 As the implant can act as nidus for bacteria, it should be removed if vitrectomy is performed for endophthalmitis in an eye containing DEX implant.
Implant related complications. Migration of implant into the anterior chamber in eyes with open disrupted capsule, eyes with prior vitrectomy, aphakia, ACIOL, scleral fixated IOL, iris fixated PCIOL, large peripheral iridectomies, insufficient zonular support can cause corneal decompensation which may not resolve despite removal of the implant.127–129 For these reasons, it is contraindicated in eyes with open or absent capsule. However, a prior YAG capsulotomy is not a contraindication. Accidental intralenticular implant injection,130–132 iatrogenic macular hole,133 and extramacular retinal hole formation have also been reported.134
Conclusion
DEX implant is a useful tool in the management of several retinal conditions. Side effects are few and easily manageable in most patients. The duration of therapeutic effect is relatively short-3–4 months in most cases. Further studies are needed to determine its precise place alongside other available treatment options.
Funding Statement
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nauck M, Karakiulakis G, Perruchoud AP, et al. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341(2–3):309–315. doi: 10.1016/S0014-2999(97)01464-7 [DOI] [PubMed] [Google Scholar]
- 2.Penfold PL, Wen L, Madigan MC, et al. Triamcinolone acetonide modulates permeability and intercellular adhesion molecule-1 (ICAM-1) expression of the ECV304 cell line: implications for macular degeneration. Clin Exp Immunol. 2000;121(3):458–465. doi: 10.1046/j.1365-2249.2000.01316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer KL, Stevenson BR, Woo PL, et al. Relationship of serine/threonine phosphorylation/dephosphorylation signaling to glucocorticoid regulation of tight junction permeability and ZO-1 distribution in nontransformed mammary epithelial cells. J Biol Chem. 1994;269(23):16108–16115. [PubMed] [Google Scholar]
- 4.Antonetti DA, Wolpert EB, DeMaio L, et al. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80(4):667–677. doi: 10.1046/j.0022-3042.2001.00740.x [DOI] [PubMed] [Google Scholar]
- 5.Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80(2):249–258. doi: 10.1016/j.exer.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 6.Graham RO, Peyman GA. Intravitreal injection of dexamethasone. treatment of experimentally induced endophthalmitis. Arch Ophthalmol. 1974;92(2):149–154. doi: 10.1001/archopht.1974.01010010155016 [DOI] [PubMed] [Google Scholar]
- 7.Kwak HW, D’Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992;110(2):259–266. doi: 10.1001/archopht.1992.01080140115038 [DOI] [PubMed] [Google Scholar]
- 8.Chang-Lin J, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–86. doi: 10.1167/iovs.10-5285 [DOI] [PubMed] [Google Scholar]
- 9.Chang-Lin J, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52(7):4605–4609. doi: 10.1167/iovs.10-6387 [DOI] [PubMed] [Google Scholar]
- 10.Khurana RN, Bansal AS, Chang LK, et al. Prospective evaluation of a sustained-release dexamethasone intravitreal implant for cystoid macular edema in quiescent uveitis. Retina. 2017;37(9):1692–1699. doi: 10.1097/IAE.0000000000001406 [DOI] [PubMed] [Google Scholar]
- 11.Available from: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20180515-OZURDEX-USPI-v1-0USPI3348.pdf. Accessed March 10, 2022.
- 12.Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313,319.e1. doi: 10.1016/j.ophtha.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: the blue mountains eye study . Arch Ophthalmol. 1996;114(10):1243–1247. [DOI] [PubMed] [Google Scholar]
- 14.Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56(4):281–299. doi: 10.1016/j.survophthal.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 15.O’Mahoney PRA, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126(5):692–699. doi: 10.1001/archopht.126.5.692 [DOI] [PubMed] [Google Scholar]
- 16.Janssen MCH, den Heijer M, Cruysberg JRM, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93(6):1021–1026. doi: 10.1160/TH04-11-0768 [DOI] [PubMed] [Google Scholar]
- 17.Bucciarelli P, Passamonti SM, Gianniello F, et al. Thrombophilic and cardiovascular risk factors for retinal vein occlusion. Eur J Intern Med. 2017;44:44–48. [DOI] [PubMed] [Google Scholar]
- 18.Sperduto RD, Hiller R, Chew E, et al. Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: the eye disease case-control study. Ophthalmology. 1998;105(5):765–771. doi: 10.1016/S0161-6420(98)95012-6 [DOI] [PubMed] [Google Scholar]
- 19.CVO Study Group. Baseline and early natural history report. the central vein occlusion study. Arch Ophthalmol. 1993;111(8):1087–1095. doi: 10.1001/archopht.1993.01090080083022 [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228(3):201–217. doi: 10.1007/BF00920022 [DOI] [PubMed] [Google Scholar]
- 21.Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. branch vein occlusion study group. Arch Ophthalmol. 1986;104(1):34–41. doi: 10.1001/archopht.1986.01050130044017 [DOI] [PubMed] [Google Scholar]
- 22.Jaulim A, Ahmed B, Khanam T, et al. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. an update of the literature. Retina. 2013;33(5):901–910. doi: 10.1097/IAE.0b013e3182870c15 [DOI] [PubMed] [Google Scholar]
- 23.Ascaso FJ, Huerva V, Grzybowski A. The role of inflammation in the pathogenesis of macular edema secondary to retinal vascular diseases. Mediators Inflamm. 2014;2014:432685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CVO Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. the central vein occlusion study group M report. Ophthalmology. 1995;102(10):1425–1433. doi: 10.1016/S0161-6420(95)30849-4 [DOI] [PubMed] [Google Scholar]
- 25.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125(3):309–317. doi: 10.1001/archopht.125.3.309 [DOI] [PubMed] [Google Scholar]
- 26.Haller JA, Bandello F, Belfort R, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134–1146. doi: 10.1016/j.ophtha.2010.03.032 [DOI] [PubMed] [Google Scholar]
- 27.Haller JA, Bandello F, Belfort R, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453–2460. doi: 10.1016/j.ophtha.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 28.Yeh W, Haller JA, Lanzetta P, et al. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology. 2012;119(6):1190–1198. doi: 10.1016/j.ophtha.2011.12.028 [DOI] [PubMed] [Google Scholar]
- 29.Kuppermann BD, Haller JA, Bandello F, et al. Onset and duration of visual acuity improvement after dexamethasone intravitreal implant in eyes with macular edema due to retinal vein occlusion. Retina. 2014;34(9):1743–1749. doi: 10.1097/IAE.0000000000000167 [DOI] [PubMed] [Google Scholar]
- 30.Li X, Wang N, Liang X, et al. Safety and efficacy of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in Chinese patients: randomized, sham-controlled, multicenter study. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):59–69. doi: 10.1007/s00417-017-3831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gado AS, Macky TA. Dexamethasone intravitreous implant versus bevacizumab for central retinal vein occlusion-related macular oedema: a prospective randomized comparison. Clin Exp Ophthalmol. 2014;42(7):650–655. doi: 10.1111/ceo.12311 [DOI] [PubMed] [Google Scholar]
- 32.Hoerauf H, Feltgen N, Weiss C, et al. Clinical efficacy and safety of ranibizumab versus dexamethasone for central retinal vein occlusion (COMRADE C): a European label study. Am J Ophthalmol. 2016;169:258–267. [DOI] [PubMed] [Google Scholar]
- 33.Hattenbach L, Feltgen N, Bertelmann T, et al. Head-to-head comparison of ranibizumab PRN versus single-dose dexamethasone for branch retinal vein occlusion (COMRADE-B). Acta Ophthalmol. 2018;96(1):e10–18. doi: 10.1111/aos.13381 [DOI] [PubMed] [Google Scholar]
- 34.Bandello F, Augustin A, Tufail A, et al. A 12-month, multicenter, parallel group comparison of dexamethasone intravitreal implant versus ranibizumab in branch retinal vein occlusion. Eur J Ophthalmol. 2018;28(6):697–705. doi: 10.1177/1120672117750058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatziralli I, Theodossiadis G, Kabanarou SA, et al. Ranibizumab versus dexamethasone implant for central retinal vein occlusion: the RANIDEX study. Graefes Arch Clin Exp Ophthalmol. 2017;255(10):1899–1905. doi: 10.1007/s00417-017-3719-5 [DOI] [PubMed] [Google Scholar]
- 36.Gu X, Yu X, Song S, et al. Intravitreal dexamethasone implant versus intravitreal ranibizumab for the treatment of macular edema secondary to retinal vein occlusion in a Chinese population. Ophthalmic Res. 2017;58(1):8–14. doi: 10.1159/000458534 [DOI] [PubMed] [Google Scholar]
- 37.Yuksel B, Karti O, Celik O, et al. Low frequency ranibizumab versus dexamethasone implant for macular oedema secondary to branch retinal vein occlusion. Clin Exp Optom. 2018;101(1):116–122. doi: 10.1111/cxo.12586 [DOI] [PubMed] [Google Scholar]
- 38.de Salles MC, Epstein D. Real-life study of the use of anti-VEGF therapy versus dexamethasone implant for treatment of macular edema in retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2021;259(9):2653–2660. doi: 10.1007/s00417-021-05146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gale R, Pikoula M, Lee AY, et al. Real world evidence on 5661 patients treated for macular oedema secondary to branch retinal vein occlusion with intravitreal anti-vascular endothelial growth factor, intravitreal dexamethasone or macular laser. Br J Ophthalmol. 2021;105(4):549–554. doi: 10.1136/bjophthalmol-2020-315836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gale R, Gill C, Pikoula M, et al. Multicentre study of 4626 patients assesses the effectiveness, safety and burden of two categories of treatments for central retinal vein occlusion: intravitreal anti-vascular endothelial growth factor injections and intravitreal ozurdex injections. Br J Ophthalmol. 2021;105(11):1571–1576. doi: 10.1136/bjophthalmol-2020-317306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manousaridis K, Peter S, Mennel S. Outcome of intravitreal dexamethasone implant for the treatment of ranibizumab-resistant macular edema secondary to retinal vein occlusion. Int Ophthalmol. 2017;37(1):47–53. doi: 10.1007/s10792-016-0226-3 [DOI] [PubMed] [Google Scholar]
- 42.Georgalas L, Tservakis I, Kiskira EE, et al. Efficacy and safety of dexamethasone intravitreal implant in patients with retinal vein occlusion resistant to anti-VEGF therapy: a 12-month prospective study. Cutan Ocul Toxicol. 2019;38(4):330–337. doi: 10.1080/15569527.2019.1614020 [DOI] [PubMed] [Google Scholar]
- 43.Mayer WJ, Remy M, Wolf A, et al. Comparison of intravitreal bevacizumab upload followed by a dexamethasone implant versus dexamethasone implant monotherapy for retinal vein occlusion with macular edema. Ophthalmologica. 2012;228(2):110–116. doi: 10.1159/000338732 [DOI] [PubMed] [Google Scholar]
- 44.Singer MA, Bell DJ, Woods P, et al. Effect of combination therapy with bevacizumab and dexamethasone intravitreal implant in patients with retinal vein occlusion. Retina. 2012;32(7):1289–1294. doi: 10.1097/IAE.0b013e318242b838 [DOI] [PubMed] [Google Scholar]
- 45.Maturi RK, Chen V, Raghinaru D, et al. A 6-month, subject-masked, randomized controlled study to assess efficacy of dexamethasone as an adjunct to bevacizumab compared with bevacizumab alone in the treatment of patients with macular edema due to central or branch retinal vein occlusion. Clin Ophthalmol. 2014;810:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giuffrè C, Cicinelli MV, Marchese A, et al. Simultaneous intravitreal dexamethasone and aflibercept for refractory macular edema secondary to retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):787–793. doi: 10.1007/s00417-019-04577-8 [DOI] [PubMed] [Google Scholar]
- 47.Harb W, Chidiac G, Harb G. Outcomes of combination therapy using aflibercept and dexamethasone intravitreal implant versus dexamethasone monotherapy for macular edema secondary to retinal vein occlusion. Middle East Afr J Ophthalmol. 2021;28(1):18–22. doi: 10.4103/meajo.MEAJO_297_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bezatis A, Spital G, Höhn F, et al. Functional and anatomical results after a single intravitreal ozurdex injection in retinal vein occlusion: a 6-month follow-up – the SOLO study. Acta Ophthalmol. 2013;91(5):340. doi: 10.1111/aos.12020 [DOI] [PubMed] [Google Scholar]
- 49.Korobelnik J, Kodjikian L, Delcourt C, et al. Two-year, prospective, multicenter study of the use of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in France. Graefes Arch Clin Exp Ophthalmol. 2016;254(12):2307–2318. doi: 10.1007/s00417-016-3394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capone A, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina. 2014;34(2):342–351. doi: 10.1097/IAE.0b013e318297f842 [DOI] [PubMed] [Google Scholar]
- 51.Singer MA, Capone A, Dugel PU, et al. Two or more dexamethasone intravitreal implants as monotherapy or in combination therapy for macular edema in retinal vein occlusion: subgroup analysis of a retrospective chart review study. BMC Ophthalmol. 2015;15(1):118. doi: 10.1186/s12886-015-0106-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eter N, Mohr A, Wachtlin J, et al. Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trial. Graefes Arch Clin Exp Ophthalmol. 2017;255(1):77–87. doi: 10.1007/s00417-016-3431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horner F, Lip PL, Mushtaq B, et al. Combination therapy for macular oedema in retinal vein occlusions: 3-year results from a real-world clinical practice. Clin Ophthalmol. 2020;149:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim MS, Choi J, Lee HD, et al. Dexamethasone intravitreal implant for the treatment of macular edema following retinal vein occlusion: post hoc analysis of post-marketing surveillance data in the real-world setting in Korea. Clin Ophthalmol. 2021;153:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garay-Aramburu G, Gómez-Moreno Á, Urcola A. Short-term effectiveness prognostic factors after dexamethasone intravitreal implant in macular edema due to retinal vein occlusion. Eur J Ophthalmol. 2021:11206721211032520. doi: 10.1177/11206721211032520 [DOI] [PubMed] [Google Scholar]
- 56.Moisseiev E, Goldstein M, Waisbourd M, et al. Long-term evaluation of patients treated with dexamethasone intravitreal implant for macular edema due to retinal vein occlusion. Eye. 2013;27(1):65–71. doi: 10.1038/eye.2012.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5–6):303–308. doi: 10.1007/BF00163549 [DOI] [PubMed] [Google Scholar]
- 58.Gritz DC, Wong IG. Incidence and prevalence of uveitis in northern California; the northern California epidemiology of uveitis study. Ophthalmology. 2004;111(3):491–500; discussion 500. doi: 10.1016/j.ophtha.2003.06.014 [DOI] [PubMed] [Google Scholar]
- 59.Suhler EB, Lloyd MJ, Choi D, et al. Incidence and prevalence of uveitis in veterans affairs medical centers of the pacific northwest. Am J Ophthalmol. 2008;146(6):890,896.e8. doi: 10.1016/j.ajo.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 60.Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237–1245. doi: 10.1001/jamaophthalmol.2016.3229 [DOI] [PubMed] [Google Scholar]
- 61.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2013;131(11):1405–1412. doi: 10.1001/jamaophthalmol.2013.4237 [DOI] [PubMed] [Google Scholar]
- 62.Okhravi N, Lightman S. Cystoid macular edema in uveitis. Ocul Immunol Inflamm. 2003;11(1):29–38. doi: 10.1076/ocii.11.1.29.15582 [DOI] [PubMed] [Google Scholar]
- 63.Ossewaarde-van Norel A, Rothova A. Clinical review: update on treatment of inflammatory macular edema. Ocul Immunol Inflamm. 2011;19(1):75–83. doi: 10.3109/09273948.2010.509530 [DOI] [PubMed] [Google Scholar]
- 64.Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159–1162. doi: 10.1136/bjo.2003.037226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kempen JH, Altaweel MM, Holbrook JT, et al. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149(4):550–561.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grajewski RS, Boelke AC, Adler W, et al. Spectral-domain optical coherence tomography findings of the macula in 500 consecutive patients with uveitis. Eye. 2016;30(11):1415–1423. doi: 10.1038/eye.2016.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dick AD, Rosenbaum JT, Al-Dhibi H, et al. Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: fundamentals of care for UveitiS (FOCUS) initiative. Ophthalmology. 2018;125(5):757–773. doi: 10.1016/j.ophtha.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 68.Yasukawa T, Ogura Y, Sakurai E, et al. Intraocular sustained drug delivery using implantable polymeric devices. Adv Drug Deliv Rev. 2005;57(14):2033–2046. doi: 10.1016/j.addr.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 69.Lowder C, Belfort R, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. doi: 10.1001/archophthalmol.2010.339 [DOI] [PubMed] [Google Scholar]
- 70.Pelegrín L, de la Maza MS, Molins B, et al. Long-term evaluation of dexamethasone intravitreal implant in vitrectomized and non-vitrectomized eyes with macular edema secondary to non-infectious uveitis. Eye. 2015;29(7):943–950. doi: 10.1038/eye.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsang AC, Virgili G, Abtahi M, et al. Intravitreal dexamethasone implant for the treatment of macular edema in chronic non-infectious uveitis. Ocul Immunol Inflamm. 2017;25(5):685–692. doi: 10.3109/09273948.2016.1160130 [DOI] [PubMed] [Google Scholar]
- 72.Nobre-Cardoso J, Champion E, Darugar A, et al. Treatment of non-infectious uveitic macular edema with the intravitreal dexamethasone implant. Ocul Immunol Inflamm. 2017;25(4):447–454. doi: 10.3109/09273948.2015.1132738 [DOI] [PubMed] [Google Scholar]
- 73.Fabiani C, Vitale A, Emmi G, et al. Systemic steroid sparing effect of intravitreal dexamethasone implant in chronic noninfectious uveitic macular edema. J Ocul Pharmacol Ther. 2017;33(7):549–555. doi: 10.1089/jop.2017.0034 [DOI] [PubMed] [Google Scholar]
- 74.Ratra D, Barh A, Banerjee M, et al. Safety and efficacy of intravitreal dexamethasone implant for refractory uveitic macular edema in adults and children. Ocul Immunol Inflamm. 2018;26(7):1034–1040. doi: 10.1080/09273948.2018.1424342 [DOI] [PubMed] [Google Scholar]
- 75.Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283–295. doi: 10.1016/j.ophtha.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyamoto K, Khosrof S, Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96(19):10836–10841. doi: 10.1073/pnas.96.19.10836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehrlich R, Harris A, Ciulla TA, et al. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol. 2010;88(3):279–291. doi: 10.1111/j.1755-3768.2008.01501.x [DOI] [PubMed] [Google Scholar]
- 78.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62(4):231–236. doi: 10.1159/000499540 [DOI] [PubMed] [Google Scholar]
- 80.Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73–79. doi: 10.1016/j.ophtha.2008.09.037 [DOI] [PubMed] [Google Scholar]
- 81.Wang K, Wang Y, Gao L, et al. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull. 2008;31(8):1541–1546. doi: 10.1248/bpb.31.1541 [DOI] [PubMed] [Google Scholar]
- 82.Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 83.Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289–296. doi: 10.1001/archophthalmol.2010.21 [DOI] [PubMed] [Google Scholar]
- 84.Pacella E, Vestri AR, Muscella R, et al. Preliminary results of an intravitreal dexamethasone implant (Ozurdex®) in patients with persistent diabetic macular edema. Clin Ophthalmol. 2013;714:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazic R, Lukic M, Boras I, et al. Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina. 2014;34(4):719–724. doi: 10.1097/IAE.0b013e3182a48958 [DOI] [PubMed] [Google Scholar]
- 86.Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):915–923. doi: 10.1097/IAE.0b013e318206d18c [DOI] [PubMed] [Google Scholar]
- 87.Medeiros MD, Alkabes M, Navarro R, et al. Dexamethasone intravitreal implant in vitrectomized versus nonvitrectomized eyes for treatment of patients with persistent diabetic macular edema. J Ocul Pharmacol Ther. 2014;30(9):709–716. doi: 10.1089/jop.2014.0010 [DOI] [PubMed] [Google Scholar]
- 88.Augustin AJ, Kuppermann BD, Lanzetta P, et al. Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: subgroup analysis of the MEAD study. BMC Ophthalmol. 2015;15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castro-Navarro V, Cervera-Taulet E, Navarro-Palop C, et al. Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema. BMC Ophthalmol. 2019;19(1):15. doi: 10.1186/s12886-018-1022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chhablani J, Bansal P, Veritti D, et al. Dexamethasone implant in diabetic macular edema in real-life situations. Eye. 2016;30(3):426–430. doi: 10.1038/eye.2015.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mastropasqua R, Toto L, Borrelli E, et al. Morphology and function over a one-year follow up period after intravitreal dexamethasone implant (ozurdex) in patients with diabetic macular edema. PLoS One. 2015;10(12):e0145663. doi: 10.1371/journal.pone.0145663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathew R, Pearce E, Muniraju R, et al. Monthly OCT monitoring of ozurdex for macular oedema related to retinal vascular diseases: re-treatment strategy (OCTOME report 1). Eye. 2014;28(3):318–326. doi: 10.1038/eye.2013.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120(9):1843–1851. doi: 10.1016/j.ophtha.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 94.Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):463–473. doi: 10.1007/s00417-016-3472-1 [DOI] [PubMed] [Google Scholar]
- 95.Yuan Q, Liu Y, Xu H, et al. Efficacy and safety of single-dose dexamethasone implantation for patients with persistent diabetic macular edema: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2021;260(2):405–413. doi: 10.1007/s00417-021-05369-9 [DOI] [PubMed] [Google Scholar]
- 96.He Y, Ren X, Hu B, et al. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. 2018;18(1):121. doi: 10.1186/s12886-018-0779-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maturi RK, Bleau L, Saunders J, et al. A 12-month, single-masked, randomized controlled study of eyes with persistent diabetic macular edema after multiple anti-VEGF injections to assess the efficacy of the dexamethasone-delayed delivery system as an adjunct to bevacizumab compared with continued bevacizumab monotherapy. Retina. 2015;35(8):1604–1614. doi: 10.1097/IAE.0000000000000533 [DOI] [PubMed] [Google Scholar]
- 98.Mehta H, Hennings C, Gillies MC, et al. Anti-vascular endothelial growth factor combined with intravitreal steroids for diabetic macular oedema. Cochrane Database Syst Rev. 2018;4:CD011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136(1):29–38. doi: 10.1001/jamaophthalmol.2017.4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rittiphairoj T, Mir TA, Li T, et al. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2020;11:CD005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Medeiros DM, Postorino M, Navarro R, et al. Dexamethasone intravitreal implant for treatment of patients with persistent diabetic macular edema. Ophthalmologica. 2014;231(3):141–146. doi: 10.1159/000356413 [DOI] [PubMed] [Google Scholar]
- 102.Totan Y, Güler E, Gürağaç FB. Dexamethasone intravitreal implant for chronic diabetic macular edema resistant to intravitreal bevacizumab treatment. Curr Eye Res. 2016;41(1):107–113. doi: 10.3109/02713683.2014.1002048 [DOI] [PubMed] [Google Scholar]
- 103.Shah SU, Harless A, Bleau L, et al. Prospective randomized subject-masked study of intravitreal bevacizumab monotherapy versus dexamethasone implant monotherapy in the treatment of persistent diabetic macular edema. Retina. 2016;36(10):1986–1996. doi: 10.1097/IAE.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 104.Downey L, Acharya N, Devonport H, et al. Treatment choices for diabetic macular oedema: a guideline for when to consider an intravitreal corticosteroid, including adaptations for the COVID-19 era. BMJ Open Ophthalmol. 2021;6(1):e000696. doi: 10.1136/bmjophth-2020-000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calvo P, Ferreras A, Al Adel F, et al. Dexamethasone intravitreal implant as adjunct therapy for patients with wet age-related macular degeneration with incomplete response to ranibizumab. Br J Ophthalmol. 2015;99(6):723–726. doi: 10.1136/bjophthalmol-2014-305684 [DOI] [PubMed] [Google Scholar]
- 106.Barikian A, Salti H, Safar A, et al. Intravitreal dexamethasone implant as adjuvant treatment for bevacizumab- and ranibizumab-resistant neovascular age-related macular degeneration: a prospective pilot study. Retina. 2017;37(7):1337–1344. doi: 10.1097/IAE.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 107.Giancipoli E, Pinna A, Boscia F, et al. Intravitreal dexamethasone in patients with wet age-related macular degeneration resistant to anti-VEGF: a prospective pilot study. J Ophthalmol. 2018;2018:5612342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rezar-Dreindl S, Eibenberger K, Buehl W, et al. Role of additional dexamethasone for the management of persistent or recurrent neovascular age-related macular degeneration under ranibizumab treatment. Retina. 2017;37(5):962–970. doi: 10.1097/IAE.0000000000001264 [DOI] [PubMed] [Google Scholar]
- 109.Chaudhary V, Barbosa J, Lam W, et al. Ozurdex in age-related macular degeneration as adjunct to ranibizumab (the OARA study). Can J Ophthalmol. 2016;51(4):302–305. doi: 10.1016/j.jcjo.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 110.Mallikarjun K, Narayanan R, Raman R, et al. Dexamethasone implant improves anatomic response to anti-VEGF therapy in treatment-resistant polypoidal choroidal vasculopathy. Int Ophthalmol. 2021. doi: 10.1007/s10792-021-02113-4 [DOI] [PubMed] [Google Scholar]
- 111.Ahn SJ, Kim KE, Woo SJ, et al. The effect of an intravitreal dexamethasone implant for cystoid macular edema in retinitis pigmentosa: a case report and literature review. Ophthalmic Surg Lasers Imaging Retina. 2014;45(2):160–164. doi: 10.3928/23258160-20140131-03 [DOI] [PubMed] [Google Scholar]
- 112.Sudhalkar A, Kodjikian L, Borse N. Intravitreal dexamethasone implant for recalcitrant cystoid macular edema secondary to retinitis pigmentosa: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1369–1374. doi: 10.1007/s00417-017-3660-7 [DOI] [PubMed] [Google Scholar]
- 113.Park UC, Park JH, Ma DJ, et al. A randomized paired-eye trial of intravitreal dexamethasone implant for cystoid macular edema in retinitis pigmentosa. Retina. 2020;40(7):1359–1366. doi: 10.1097/IAE.0000000000002589 [DOI] [PubMed] [Google Scholar]
- 114.Veritti D, Sarao V, De Nadai K, et al. Dexamethasone implant produces better outcomes than oral Acetazolamide in patients with cystoid macular edema secondary to retinitis pigmentosa. J Ocul Pharmacol Ther. 2020;36(3):190–197. doi: 10.1089/jop.2018.0153 [DOI] [PubMed] [Google Scholar]
- 115.Ahn SJ, Joung J, Lee SH, et al. Intravitreal dexamethasone implant therapy for the treatment of cystoid macular oedema due to hydroxychloroquine retinopathy: a case report and literature review. BMC Ophthalmol. 2018;18(1):310. doi: 10.1186/s12886-018-0985-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hattenbach L, Kuhli-Hattenbach C, Springer C, et al. [Intravitreal dexamethasone implant for treatment of persistent postoperative macular edema after vitrectomy]. Ophthalmologe. 2016;113(7):581–588. German. doi: 10.1007/s00347-016-0223-y [DOI] [PubMed] [Google Scholar]
- 117.Bonfiglio V, Reibaldi M, Fallico M, et al. Widening use of dexamethasone implant for the treatment of macular edema. Drug Des Devel Ther. 2017;112:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Banerjee PJ, Bunce C, Charteris DG. Ozurdex (a slow-release dexamethasone implant) in proliferative vitreoretinopathy: study protocol for a randomised controlled trial. Trials. 2013;14:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bennedjai A, Theillac V, Akesbi J, et al. The effect of selective laser trabeculoplasty on intraocular pressure in patients with dexamethasone intravitreal implant-induced elevated intraocular pressure. J Ophthalmol. 2020;2020:3439182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaudhry SG, Fung AT. Cytomegalovirus retinitis following dexamethasone intravitreal implant. Am J Ophthalmol Case Rep. 2021;22:101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Witmer MT, Connolly BP. Cytomegalovirus retinitis after an intravitreal dexamethasone implant in an immunocompetent patient. Retin Cases Brief Rep. 2021;15(6):670–672. doi: 10.1097/ICB.0000000000000904 [DOI] [PubMed] [Google Scholar]
- 122.Vannozzi L, Bacherini D, Sodi A, et al. Cytomegalovirus retinitis following intravitreal dexamethasone implant in a patient with central retinal vein occlusion. Acta Ophthalmol. 2016;94(2):158. doi: 10.1111/aos.12783 [DOI] [PubMed] [Google Scholar]
- 123.Dogra M, Rohilla V, Dogra M, et al. Macular cytomegalovirus retinitis following dexamethasone intravitreal implant combined with phacoemulsification. Indian J Ophthalmol. 2018;66(9):1361–1363. doi: 10.4103/ijo.IJO_171_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olson DJ, Parhiz AT, Wirthlin RS. Reactivation of latent toxoplasmosis following dexamethasone implant injection. Ophthalmic Surg Lasers Imaging Retina. 2016;47(11):1050–1052. doi: 10.3928/23258160-20161031-10 [DOI] [PubMed] [Google Scholar]
- 125.Celik N, Khoramnia R, Auffarth GU, et al. Complications of dexamethasone implants: risk factors, prevention, and clinical management. Int J Ophthalmol. 2020;13(10):1612–1620. doi: 10.18240/ijo.2020.10.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Casati S, Bruni E, Marchini G. Retinal and vitreous hemorrhage after traumatic impact of dexamethasone implant in a vitrectomized eye. Eur J Ophthalmol. 2016;26(3):55. doi: 10.5301/ejo.5000716 [DOI] [PubMed] [Google Scholar]
- 127.Stelton CR, Townsend J, Peterson LT, et al. Surgical management of anterior chamber migration of a dexamethasone intravitreal implant. Ophthalmic Surg Lasers Imaging Retina. 2015;46(7):756–759. doi: 10.3928/23258160-20150730-11 [DOI] [PubMed] [Google Scholar]
- 128.Khurana RN, Appa SN, McCannel CA, et al. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121(1):67–71. doi: 10.1016/j.ophtha.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 129.Röck D, Bartz-Schmidt KU, Röck T. Risk factors for and management of anterior chamber intravitreal dexamethasone implant migration. BMC Ophthalmol. 2019;19(1):120. doi: 10.1186/s12886-019-1122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clemente-Tomás R, Hernández-Pérez D, Neira-Ibáñez P, et al. Intracrystalline Ozurdex®: therapeutic effect maintained for 18 months. Int Ophthalmol. 2019;39(1):207–211. doi: 10.1007/s10792-017-0780-3 [DOI] [PubMed] [Google Scholar]
- 131.Kurt A, Durukan AH, Küçükevcilioğlu M. Accidental intralenticular injection of Ozurdex® for branch retinal vein occlusion: intact posterior capsule and resolution of macular edema. Case Rep Ophthalmol Med. 2019;2019:8630504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fasce F, Battaglia Parodi M, Knutsson KA, et al. Accidental injection of dexamethasone intravitreal implant in the crystalline lens. Acta Ophthalmol. 2014;92(4):330. doi: 10.1111/aos.12332 [DOI] [PubMed] [Google Scholar]
- 133.De Benedetto U, Battaglia Parodi M, Knutsson KA, et al. Macular hole after injection of dexamethasone intravitreal implant for macular oedema due to central retinal vein occlusion. Acta Ophthalmol. 2013;91(1):75. doi: 10.1111/j.1755-3768.2012.02484.x [DOI] [PubMed] [Google Scholar]
- 134.Ekinci C, Kayıran A, Özdemir H. Extramacular retinal hole following intravitreal dexamethasone implant: case report. Turk J Ophthalmol. 2019;49(3):175–177. doi: 10.4274/tjo.galenos.2019.98975 [DOI] [PMC free article] [PubMed] [Google Scholar]