Abstract
A recent study reported that patients with interstitial lung disease (ILD) are at increased risk of death from coronavirus disease 2019 (COVID-19). However, there are no studies on the outcome of COVID-19 patients with preexisting ILD treated with corticosteroids or antiviral drugs. We extracted 26 patients with preexisting ILD by medical records and HRCT pattern. Of 503 patients with COVID-19, we selected 52 patients as control matched for age and sex. Twenty out of the 26 ILD patients (76.9%) received corticosteroid therapy, and 23 patients (88.5%) also received antiviral treatment with remdesivir or favipiravir. Although no statistical difference was found, the proportion of severe patients in ILD group tended to be higher than in non-ILD group (23.1% vs. 42.3%; p = 0.114). Also, mortality rate in ILD group tended to be higher than in non-ILD patients (11.5% vs. 3.8%; p = 0.326). In univariate analysis to evaluate risk factors for severe condition, diagnosis of idiopathic pulmonary fibrosis, usual interstitial pneumonia pattern, and honeycomb lung were not risk factors of severe disease. Treatment with corticosteroids, antiviral drugs, and immunosuppressive agents may affect the outcome of COVID-19 patients with ILD.
Keywords: COVID-19, Interstitial lung disease, Corticosteroid, Antiviral drug
Coronavirus disease 2019 (COVID-19) has spread globally. Although over two years have passed since the outbreak was declared a pandemic by the World Health Organization, the number of infections is still increasing. Recent studies have reported that patients with interstitial lung disease (ILD) are at increased risk of death from COVID-19 [[1], [2], [3], [4]]. However, there are few data on the effectiveness of drug treatments against COVID-19, including corticosteroids as well as antiviral and immunosuppressive drugs in patients with ILD. These treatments may influence the outcome of patient with ILD who contract COVID-19. In this retrospective study, we investigated the outcome of COVID-19 patients with preexisting ILD and factors that may predict the severity of disease.
We retrospectively included 503 Japanese patients who were admitted to Kanagawa Cardiovascular and Respiratory Center between February 1, 2020 and March 31, 2021. All patients underwent reverse transcription-polymerase chain reaction test and were confirmed to be positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The diagnosis of COVID-19 pneumonia was determined by chest computed tomography (CT) on admission. The following information on patient characteristics and laboratory data was obtained at the time of admission or as the physician determined necessary: age, sex, vital signs, body mass index, smoking history, comorbidities, treatment before and after COVID-19, blood test results, use of high-flow nasal cannula or noninvasive positive-pressure ventilation, and outcome. When patients were transferred to other hospitals to receive treatment with mechanical ventilation or extracorporeal membrane oxygenation (ECMO), we contacted the hospitals to collect information on the use of mechanical ventilation or ECMO and the patients' outcomes. The saturation of percutaneous oxygen/fraction of inspiratory oxygen ratio was used to assess the patients’ oxygenation status. Some patients did not receive oxygen therapy and no patient received mechanical ventilation at our hospital. Severe illness was defined as “worsening of respiratory status requiring oxygen administration of 5 L/min or more” [5]. Time to recovery was defined as “improvement from a baseline score of 2–5 to a score of 6–7 or from a baseline score of 6 to a score of 7” using the 7-point ordinal scale [6].
We identified ILD patients from their medical records. If the COVID-19 patients had not previously visited our hospital and there were no medical records, we identified ILD from features on the CT, such as the presence of honeycombing or traction bronchiectasis, performed upon the first visit.
All analyses were performed using the statistical analysis software EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Continuous variables were compared using the Mann-Whitney test, and categorical variables were compared using the Fisher's exact test. Risk factors associated with severity of disease were analyzed by the logistic regression model. The optimal cutoff level that can discriminate non-severe groups from severe groups was determined from previous studies [5,[7], [8], [9]]. Statistical significance was set at p < 0.05. The Ethics Committee of the Kanagawa Cardiovascular and Respiratory Center approved the study protocol (approval no. KCRC-21-0014; August 2, 2021). The requirement for informed consent was waived due to the retrospective nature of the study.
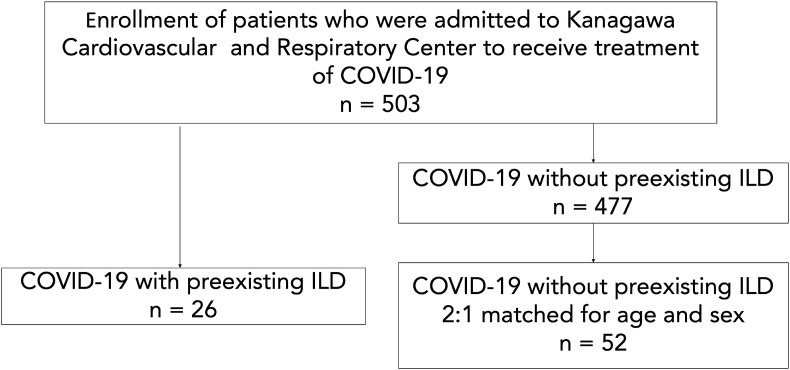
Twenty-six out of the 503 patients had preexisting ILD diagnosed before the COVID-19 infection, 12 of whom had not been previously reviewed at the hospital and had no medical records. For the control group, we randomly selected 52 COVID-19 patients without ILD, matched for age and sex (Fig. 1 ). The clinical characteristics of COVID-19 patients diagnosed as ILD before and on admission are listed in Table 1 . The mean age was 74.5 years, and 76.9% patients were male. Twenty-one patients in ILD group had COVID-19 pneumonia, and 47 patients in non-ILD group had COVID-19 pneumonia (p = 0.287). Six patients (23.1%) had a diagnosis of idiopathic pulmonary fibrosis (IPF), connective-tissue disease associated ILD, and unclassifiable idiopathic interstitial pneumonias.
Fig. 1.
We retrospectively enrolled 503 COVID-19 patients who were hospitalized at our hospital between February 1, 2020 and March 31, 2021. Twenty-six out of the 503 patients had preexisting ILD diagnosed before the COVID-19 infection. As a control, 52 COVID-19 patients without preexisting ILD were randomly selected.
Table 1.
Clinical characteristics of COVID-19 patients with and without ILD.
| ILD group (n = 26) | Non-ILD group (n = 52) | p-value | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years), median (IQR) | 74.5 (67.3–82.5) | 74.5 (67.0–83.0) | 1.000 |
| Male, n (%) | 20 (76.9) | 40 (76.9) | 1.000 |
| BMI (kg/m2), median (IQR) | 22.7 (20.3–25.0) | 23.3 (21.4–26.1) | 0.450 |
| Smoker, n (%) | 19 (73.1) | 34 (65.3) | 0.082 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 8 (30.8) | 15 (28.8) | 1.000 |
| Cardiovascular disease | 6 (23.1) | 14 (26.9) | 0.789 |
| Hypertension | 12 (46.2) | 26 (50.0) | 0.813 |
| COPD | 9 (34.6) | 8 (15.4) | 0.080 |
| SpO2/FiO2 on admission, median (IQR) | 453 (351–457) | 452 (438–457) | 0.265 |
| SOFA score, median (IQR) | 1 (0–2) | 1 (0–1) | 0.369 |
| COVID-19 pneumonia, n (%) | 21 (80.8) | 47 (90.4) | 0.287 |
| ILD, n(%) | |||
| IPF | 6 (23.1) | - | - |
| Unclassifiable IIPs | 6 (23.1) | - | - |
| CTD-ILD | 6 (23.1) | - | - |
| ILA | 3 (11.5) | - | - |
| Other ILD | 5 (19.2) | - | - |
| Treatment before COVID-19, n(%) | |||
| Corticosteroids | 7 (26.9) | 1 (1.9) | 0.002 |
| Immunosuppressants | 4 (15.4) | 1 (1.9) | 0.040 |
| Antifibrotics | 3 (11.5) | 0 (0) | - |
| LTOT before admission | 4 (15.4) | 0 (0) | - |
| Lung function test | |||
| Subject number, n | 15 | 0 | |
| FVC (L), mean (SD) | 2.4 (2.0–3.4) | - | - |
| FVC (percentage of predicted), mean (SD) | 88.4 (65.3–105.9) | - | - |
| DLCO, (ml/min/kPa), mean (SD) | 12.1 (8.7–14.0) | - | - |
| DLCO, (percentage of predicted), mean (SD) | 66.5 (56.6–92.3) | - | - |
| Admission blood results, median (IQR) | |||
| WBC (/μL) | 6025 (4420–8315) | 5430 (4400–6820) | 0.316 |
| Lymphocyte (/μL) | 911 (618–1318) | 1071 (710–1278) | 0.398 |
| LDH (U/L) | 251 (204–347) | 249 (194–294) | 0.262 |
| CRP (mg/dL) | 4.5 (2.0–9.5) | 4.5 (2.0–10.3) | 0.621 |
| Ferritin (ng/mL) | 487 (245–646) | 466 (159–760) | 0.898 |
| KL-6 (U/mL) | 719 (462–1022) | 275 (228–362) | <0.001 |
| D-dimer (μg/mL) | 1.1 (0.80–2.79) | 1.07 (0.73–1.49) | 0.431 |
| Treatment, n (%) | |||
| Corticosteroid | 20 (76.9) | 40 (76.9) | 1.000 |
| Favipiravir | 16 (61.5) | 32 (61.5) | 1.000 |
| Remdesivir | 11 (42.3) | 17 (32.7) | 0.458 |
| Tocilizumab | 7 (26.9) | 4 (7.7) | 0.036 |
| Baricitinib | 3 (11.5) | 4 (7.7) | 0.680 |
| Oxygen therapy | 18 (69.2) | 28 (53.8) | 0.229 |
| NIPPV/HFNC | 8 (30.8) | 1 (1.9) | <0.001 |
| Intubation | 1 (3.8) | 5 (9.6) | 0.657 |
| ECMO | 0 (0) | 1 (1.9) | - |
| Outcome | |||
| Severe disease, n(%) | 11 (42.3) | 12 (23.1) | 0.114 |
| Death, n(%) | 3 (11.5) | 2 (3.8) | 0.326 |
| Time to recovery, median (IQR) | 20 (11.5–34.5) | 12 (9.5–19.5) | 0.029 |
| LTOT started after discharge from hospital, n(%) | 6 (23.1) | 4 (7.7) | 0.065 |
Continuous variables are shown as medians (interquartile ranges).
Fisher's exact tests were performed on categorical variables.
Mann-Whitney U tests were used to compare the non-ILD group and ILD groups.
BMI: body mass index, COPD: chronic obstructive pulmonary disease, COVID-19: coronavirus disease 2019, CRP: C-reactive protein, CTD: connective-tissue disease, DLCO: diffusing capacity for carbon monoxide, ECMO: extracorporeal membrane oxygenation, FiO2: fraction of inspiratory oxygen, FVC: forced vital capacity, HFNC: high-flow nasal cannula, IIPs: idiopathic interstitial pneumonias, ILA: interstitial lung abnormalities, ILD: interstitial lung disease, IPF: idiopathic pulmonary fibrosis, IQR: interquartile range, KL-6: Krebs von den Lungen-6, LDH: lactate dehydrogenase, LTOT: long-term oxygen therapy, NIPPV: noninvasive positive pressure ventilation, SOFA: sequential organ failure assessment, SpO2: saturation of percutaneous oxygen, WBC: white blood cell.
Twenty out of the 26 ILD patients (76.9%) received corticosteroid therapy, and 23 patients (88.5%) also received antiviral treatment with remdesivir or favipiravir. The proportion of ILD patients treated with tocilizumab was higher than non-ILD patients; however, the proportion of patients treated with other drugs did not differ between the ILD and non-ILD groups (Table 1). Serum lactate dehydrogenase levels and admission Sequential Organ Failure Assessment score, reported risk factors of lung fibrosis after COVID-19 [10], were not differ between two groups. The proportion of patients receiving oxygen therapy did not differ between the ILD and non-ILD groups (69.2% vs. 53.8%; p = 0.229). The proportion of ILD patients receiving noninvasive positive pressure ventilation or high-flow nasal oxygen was higher than the non-ILD group (30.8% vs. 1.9%; p < 0.001).
Although the proportion of severe patients in ILD group tended to be higher than in non-ILD group, there was no statistical difference between the two groups (23.1% vs. 42.3%; p = 0.114). On the other hand, time to recovery in ILD group was longer than that in non-ILD group (20 days vs. 12 days; p = 0.029). Three patients with preexisting ILD died. The mortality rate of COVID-19 patients with preexisting ILD tended to be higher than that of non-ILD patients, although there was no statistical difference between the two groups (11.5% vs. 3.8%; p = 0.326). More patients with preexisting ILD received long-term oxygen therapy after being discharged from our hospital compared with non-ILD patients, but this did not reach statistical significance (23.1% vs. 7.7%; p = 0.065).
In the univariate analysis, the presence of diabetes mellitus was the only risk factor associated with more severe COVID-19 in patients with preexisting ILD (Table 2 ). The presence of IPF, usual interstitial pneumonia pattern and honeycombing features on chest CT were not associated with severity of COVID-19 disease in patients with preexisting ILD.
Table 2.
Univariate analysis of risk factors for severe disease in COVID-19 patients with ILD.
| Variable | Odds ratio | Univariate model |
p-value |
|---|---|---|---|
| 95% CI | |||
| Age | 1.000 | 0.157-6.388 | 1.000 |
| Sex | 1.319 | 0.145-17.88 | 1.000 |
| BMI | 2.765 | 0.346-25.46 | 0.369 |
| Smoking | 1.778 | 0.216-23.25 | 0.668 |
| Cardiovascular disease | 1.812 | 0.190-17.52 | 0.644 |
| COPD | 1.445 | 0.203-10.07 | 0.692 |
| Diabetes mellitus | 9.367 | 1.133-131.2 | 0.026 |
| LDH | 1.634 | 0.254-10.89 | 0.689 |
| CRP | 1.774 | 0.267-14.65 | 0.683 |
| D-dimer | 4.067 | 0.551-37.21 | 0.189 |
| KL-6 | 0.692 | 0.099-4.938 | 0.692 |
| %FVC | 0.524 | 0.038-6.566 | 0.622 |
| %DLCO | 0.142 | 0.002–2.215 | 0.138 |
| IPF | 1.811 | 0.190-17.52 | 0.644 |
| UIP pattern | 2.867 | 0.263-41.99 | 0.340 |
| Honeycombing | 0.562 | 0.04–4.636 | 0.668 |
Risk factors associated with severity of disease were analyzed by the logistic regression model. A p value of <0.05 was considered statistically significant.
Cutoffs: Age, BMI, LDH, CRP, D-dimer, KL-6, %FVC, %DLCO were 75 years, 25 kg/m2, 305 U/L, 3.15 mg/dL, 2.0 mg/dL, 495 mg/dL, 80%, and 70%, respectively.
BMI: body mass index, COPD: chronic obstructive pulmonary disease, CRP: C-reactive protein, DLCO: diffusing capacity for carbon monoxide, FVC: forced vital capacity, IPF: interstitial pulmonary fibrosis, KL-6: Krebs von den Lungen-6, LDH: lactate dehydrogenase, UIP: usual interstitial pneumonia.
Although some studies have reported on COVID-19 patients with ILD [[1], [2], [3], [4]], effective treatments with anti-inflammatory and antiviral drugs have not been indicated. The mortality rate in ILD patients with COVID-19 (11.5%) was tended to be higher than that in non-ILD COVID-19 patients (3.8%, p = 0.326) in the present study and was lower than what has been previously reported (49.1%) [1]. In the previous study, a low proportion (27.8%) of COVID-19 patients received treatment with corticosteroids [1]; however, the study did not provide information about the proportion of patients receiving antiviral drugs. Other study reported that patients with ILD were more likely to develop severe disease and showed higher mortality rate compared with patients without ILD [2]. However, the study did not report information about treatment with corticosteroids or antiviral drugs. Other multicentered observational study reported a low proportion of COVID-19 patients received treatment with corticosteroids and antiviral drug (11%, 7%, relatively) [3]. In the study, the mortality rate of COVID-19 patients with preexisting ILD (25%) was higher than that of this study. To our knowledge, this is the first case series of the use of corticosteroids and antivirals at a high frequency in COVID-19 patients with preexisting ILD.
A previous study has reported the efficacy of corticosteroid therapy in COVID-19 patients [11]; corticosteroid therapy might be also effective in ILD patients with COVID-19. In this study, 76.9% of patients with COVID-19 were treated with corticosteroids. Furthermore, the proportion of patients treated with corticosteroids, remdesivir and baricitinib was approximately matched between the ILD and non-ILD groups. Treatment with remdesivir and baricitinib has been shown to be effective in COVID-19 patients [6,12]. Although the effectiveness of tocilizumab is still unclear [13], treatment with these drugs may improve outcome, especially in patients with ILD.
This study had some limitations. First, this was a single-center retrospective study, and the number of enrolled patients was relatively small. Although there was no statistical difference between the two groups, the mortality rate in ILD patients with COVID-19 was about three times as much as that in non-ILD COVID-19 patients. This suggests statistically low power of this study. Also, as our hospital is designated to treat patients with mild to severe illness only; critically ill patients were not admitted. This may have influenced the small number of deaths in our hospital. Second, the number of patients with IPF was relatively low. This may affect the outcome of COVID-19 patients with ILD. Third, it was sometimes difficult to discriminate preexisting ILD if patients had not visited the hospital prior to the COVID-19 admission. Further studies are needed to establish the optimum treatment for COVID-19 patients with ILD.
In conclusion, the mortality rate was not statistically different between ILD group and non-ILD group. Treatment with corticosteroids, antiviral drugs, and immunosuppressive agents may affect the outcome of COVID-19 patients with ILD.
I certify that a part of this study was presented at European Respiratory Society International Congress 2021 (Online, September 6, 2021).
Authorship statement
Concept and design: T.Y, E.H, T.O, Acquisition, analysis, or interpretation of data: T.Y, Data collection: T.Y, T.B, T.I, Drafting of the manuscript: T.Y, Statistical analysis: T.Y, All the authors meet the ICMJE authorship criteria.
Funding
None.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We thank the patients who consented to participate in this study.
References
- 1.Drake T.M., Docherty A.B., Harrison E.M., Quint J.K., Adamali H., Agnew S., et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease: an international multicenter study. Am J Respir Crit Care Med. 2020;202:1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee H., Choi H., Yang B., Lee S.K., Park T.S., Park D.W., et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur Respir J. 2021:2004125. doi: 10.1183/13993003.04125-2020. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallay L., Uzunhan Y., Borie R., Lazor R., Rigaud P., Marchand-Adam S., et al. Risk factors for mortality after COVID-19 in patients with preexisting interstitial lung disease. Am J Respir Crit Care Med. 2021;203:245–249. doi: 10.1164/rccm.202007-2638LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esposito A.J., Menon A.A., Ghosh A.J., Putman R.K., Fredenburgh L.E., El-Chemaly S.Y., et al. Increased odds of death for patients with interstitial lung disease and COVID-19: a case-control study. Am J Respir Crit Care Med. 2020;202:1710–1713. doi: 10.1164/rccm.202006-2441LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otoshi R., Hagiwara E., Kitayama T., Yamaya T., Higa K., Murohashi K., et al. Clinical characteristics of Japanese patients with moderate to severe COVID-19. J Infect Chemother. 2021;27:895–901. doi: 10.1016/j.jiac.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., et al. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020 15;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awano N., Inomata M., Kuse N., Tone M., Takada K., Muto Y., et al. Serum KL-6 level is a useful biomarker for evaluating the severity of coronavirus disease 2019. Respir Investig. 2020;58:440–447. doi: 10.1016/j.resinv.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Y., Cao J., Wang Q., Shi Q., Liu K., Luo Z., et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaya T., Hagiwara E., Baba T., Kitayama T., Murohashi K., Higa K., et al. Serum Krebs von den Lungen-6 levels are associated with mortality and severity in patients with coronavirus disease 2019. Respir Investig. 2021;59:596–601. doi: 10.1016/j.resinv.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGroder C.F., Zhang D., Choudhury M.A., Salvatore M.M., D'Souza B.M., Hoffman E.A., et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021;76:1242–1245. doi: 10.1136/thoraxjnl-2021-217031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]