Abstract
Purpose
Spontaneous oocyte activation (SOA) is a recently classified phenomenon characterized by the presence of a single pronucleus immediately following oocyte retrieval, without the apparent involvement of sperm. SOA currently remains poorly understood in humans, with no clear genetic or pathological factor(s). Herein, we report two separate cases of recurrent spontaneous oocyte activation, investigating potential avenues to identify causative etiology.
Methods
Two patients with several cycles with SOA have undergone further genetic and embryologic investigation to reveal underlying causes for SOA and provide a treatment if possible.
Results
One case was a patient with recurrent pregnancy loss and the other was diagnosed as unexplained infertility. In the first case, 61 out of 69 oocytes retrieved exhibited SOA in five cycles while in the second case 44 out of 49 oocytes exhibited SOA in five cycles. Oocytes were injected with sperm; embryo development and presence of paternal contribution were investigated. No pregnancy is ensued following embryo transfer in both patients. Time-lapse imaging of embryogenesis from the second case did not reveal even momentary second pronucleus appearance. We also performed clinical whole exome sequencing for both patients but did not identify any disease-causing variant.
Conclusion
Patients with SOA suffer from infertility. Our results indicate that more investigation is required to understand the etiology of SOA in humans concentrating on the molecular mechanisms that underpin regulation of oocyte activation and calcium dynamics need to be investigated to fully understand, and perhaps in the future rectify, recurrent SOA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02435-x.
Keywords: Spontaneous oocyte activation, Genetic analysis, Unexplained infertility, Recurrent pregnancy loss, Paternal contribution
Introduction
Assisted reproductive technology (ART) requires mature oocytes arrested at metaphase II, having extruded the first polar body for the normal fertilization process to occur. Oocytes arrested at this stage require sperm entry to trigger resumption of meiosis followed by several concurrent and sequential events including second polar body extrusion, the formation of female and male pronuclei and the start of cleavage divisions [1, 2]. Collectively termed oocyte activation, these concurrent events are initiated by elevations in levels of intracellular calcium (Ca2+) with a series of repetitive Ca2+ oscillations [3]. In mammals, Ca2+ oscillations are mediated by inositol trisphosphate (IP3), the patterns of which seem species specific and essential in all species studied to date [3, 4]. While much work is still required to fully elucidate the molecular mechanisms underlying meiotic resumption in humans, it is generally well accepted that the fertilizing sperm delivers a soluble sperm-specific factor termed phospholipase C zeta (PLCζ), which initiates signaling cascades resulting in Ca2+ release and oocyte activation [3, 5].
The achievement of meiotic competency is a complex process regulated by stabilization/destabilization of maturation promoting factor (MPF), in turn modulated by mitogen-activated protein kinase3/1 (MAPK3/1) in oocytes. MAPK3/1 interacts with other signal molecules, transcription and post-transcription factors in cumulus cells or cytostatic factors in oocyte [6]. In addition to physiological meiotic resumption from MII arrest during fertilization, exit from MII can also occur in other circumstances such as parthenogenesis, or with no particularly obvious reason; a scenario dubbed spontaneous oocyte activation (SOA) [7]. SOA has been reported to result from abnormalities in cell cycle regulators controlling meiotic arrest/progression [8]. Rat oocytes are well-known examples of cells susceptible to SOA in vitro [9]. Following deposition in culture media, oocytes spontaneously enter anaphase II and extrude second polar bodies in SOA [10, 11]. Postovulatory oocyte aging and temperature changes during oocyte collection might be contributing factors toward SOA [7, 11]. Genetic abnormalities may also play a role as observed in c-mos knockout mice [12], which spontaneously extrude second polar bodies, form pronuclei and undergo cleavage divisions and could form ovarian teratomas [12].
In humans, however, there is a paucity of literature reporting SOA as a pathological condition characterized by the presence of a single pronucleus upon removal of cumulus cell after oocyte retrieval without any sperm exposure [13–15]. Indeed such studies and descriptions are necessary for us to begin to understand causative factors underlying this phenomenon. Herein, we report two cases of recurrent SOA, examining at multiple levels for potential causative factors underlying SOA in these cases.
Case Report
Table 1 summarizes the cycle details of both patients.
Table 1.
Cycle details of two patients with SOA. PGT-A, preimplantation genetic testing for aneuploidy screening; PGT-STR, preimplantation genetic testing with short tandem repeats; IVM, in vitro maturation; IVF, in vitro fertilization; ICSI intracytoplasmic sperm injection
| Patient (diagnosis) | Cycle (year) | ART | Oocytes | MII | Activated | 2PN | PGT | Embryo | Paternal contribution |
|---|---|---|---|---|---|---|---|---|---|
| 1 (Recurrent pregnancy loss) | 1 (2004) | PGT-A | 23 | 22 | 22 | 1 | Not performed | Arrested | Not performed |
| 2 (2005) | PGT-A | 30 | 26 | 26 | 2 | Not performed | Arrested | Not performed | |
| 3 (2005) | IVM | 2 | 0 | 0 | Not performed | Not performed | |||
| 4 (2006) | IVM | 2 | 1 | 1 | 0 | Not performed | Not performed | ||
| 5 (2009) | PGT-STR | 12 | 12 | 12 | 0 | 10 biopsied | 1 transferred no pregnancy | 2 with paternal contribution | |
| 2 (unexplained infertility) | 1 (2015) | Split IVF/ICSI | 16 | 8 ICSI | 8 | 2 | Not performed | 1 transferred no pregnancy | Not performed |
| 8 IVF | 0 | Not performed | Not performed | ||||||
| 2 (2015) | PGT-A | 8 | 6 | 6 | 0 | 5 biopsied, all abnormal, no transfer | One blastocyst with nullisomy 21 and sex chromosomes | Not performed | |
| 3 (2016) | PGT-STR | 19 | 18 | 18 | 0 | Analysis of 9 whole embryos for paternal contribution | No blastocyst by day 5, no biopsy | 6 with paternal contribution | |
| 4 (2017) | PGT-STR | 5 | 4 | 4 | 0 | 3 embryos biopsied on day 3 | All embryos were arrested by day 5 | No paternal contribution | |
| 5 (2021) | PGT-STR | 9 | 8 | 8 | 0 | 8 embryos biopsied on day 3 | 2 transferred on day 5 at cleavage stage, no pregnancy | 2 with paternal contribution |
Case 1
The first patient was a 30-year-old female presenting with recurrent pregnancy loss (RPL) with four preclinical losses during the last 7 years. Further investigations for RPL revealed high lipoprotein a (1.17 g/L; normal 0.00–0.30), heterozygosity for MTHFR C677T mutation, high homocysteine (21 µmol/L; normal 5–15) and high fibrinogen (5.20 g/L; normal 1.50–4.00). The patient and her husband exhibited normal chromosomal karyotype, while the husband exhibited normal sperm parameters except sperm morphology (2% normal sperm). The couple were referred for preimplantation genetic testing for aneuploidy (PGT-A) and underwent a total of five ART cycles. In her first two cycles, PGT-A was not performed due to the absence of normal fertilization. A total of 69 oocytes were obtained (Table 1) of which 61 were at MII stage all with an unusual presence of one pronucleus. These were injected with the husband’s sperm with only three showing two pronuclei with early embryonic arrest. No further action was taken, and subsequent cycles were canceled. The couple was also counseled and underwent two in vitro maturation (IVM) cycles to eliminate exposure to hCG/LH and hormonal stimulation. Out of two cycles, a total of four oocytes were obtained with only one at the MII stage again with one pronucleus following 40 h culture, resulting in cycle cancellation. The last cycle was performed with the intention of checking paternal contributions toward any divided embryos. Twelve mature oocytes were obtained which were immediately injected. However, none displayed two pronuclei. 10/12 continued dividing and were subject to biopsy on day 3. Two embryos were diploid with clear paternal contribution. One had arrested and the other one was transferred. No pregnancy ensued.
Case 2
The second patient was a 35-year-old lady referred for ART with unexplained infertility for 7 years. She had a history of eight failed ovulation induction cycles with timed intercourse, as well as five failed intrauterine insemination elsewhere. No RPL investigation was performed since she presented with unexplained infertility. Whole exome data revealed that she is a wild type genotype for MHTFR gene. In her first cycle, 16 oocytes were obtained and 8 were allocated to in vitro fertilization (IVF) and the other 8 to intracytoplasmic sperm injection (ICSI). All oocytes for ICSI were mature, exhibiting one pronucleus, all of which were injected with the husband’s sperm. Two of these showed two pronuclei, one displayed cleavage arrest, with the others reaching the 5-cell stage but with heavy fragmentation on day 3. These were transferred, but no pregnancy ensued. No normal fertilization was observed in the oocytes allocated to IVF. In the second cycle, eight oocytes were obtained and six were at MII with one pronucleus. These were injected with the husband’s sperm. However, none of the injected oocytes exhibited signs of normal fertilization, but five were observed to continue to divide. Biopsies were performed on day 3 and analyzed by 5-chromosome FISH analysis, yielding abnormal results. Only one embryo reached the blastocyst stage but was not transferred due to nullisomic sex chromosomes.
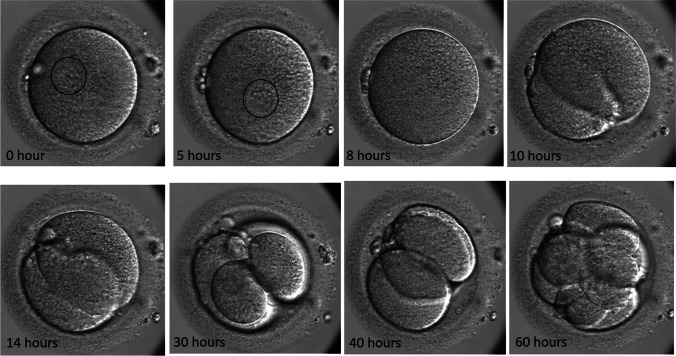
In the next cycle, PGT was also performed to examine for paternal contributions of the dividing embryos. A total of 19 oocytes were obtained, 18 of which were at MII with one pronucleus. All were injected with husband’s sperm and cultured until day 5. None of the embryos reached the blastocyst stage. The cycle was canceled, and nine embryos were tested as whole embryos for paternal contributions, with six exhibiting paternal alleles. Two more cycles were performed with day 3 biopsies and testing for paternal contributions. In the first cycle, five oocytes were obtained, with four at MII and one pronucleus. All were injected with a single sperm each. However, none of the injected oocytes showed two pronuclei, and none had paternal contributions following day 3 biopsy. In the following cycle, nine oocytes were retrieved, with eight at the MII stage with one pronucleus. All eight were injected with a single sperm each, none of which showed two pronuclei. However, all continued to divide and were biopsied on day 3. While two showed a paternal contribution, they did not reach the blastocyst stage but were transferred on day 5. No pregnancy ensued. Oocytes in the last four cycles were cultured in time-lapse incubators to continuously monitor even momentary presence of the second pronucleus that never appeared (Fig. 1, Supplementary Video 1).
Fig. 1.
The sequential development of one of the embryos from the second patient’s last cycle which showed paternal contribution. At the beginning (0 h) and 5 h, a single pronucleus can be clearly seen as marked in a circle. By 8 h, the pronucleus was faded and cleavage division started around 10 h. Two and four cells were seen by 14 and 30 h, respectively. However, the embryo has reverse cleavage and became 3-cell around 40 h and reached to 8-cell by 60 h where it was biopsied
In order to confirm paternal contribution, biopsied samples were first amplified by whole-genome amplification (WGA) using REPLI-g Single Cell Kit [16]. Then, the AmpFLSTR™ Identifiler™ Plus PCR Amplification Kit was used [17].
Both patients were counseled for genetic studies with clinical exome sequencing. Following informed written consent, venous blood was collected for genomic DNA from the affected patients. The study was approved by the local IRB (KFSHRC RAC# 2121053). Standard clinical exome sequencing was performed on index patient and the protocol is described in Monies et al. [18] while variant identification and interpretation were performed as described before [19]. The American College of Medical Genetics and Genomic (ACMG) guidelines were applied for the variant interpretation and classification. No disease-causing variants were identified following a detailed analysis (Figure S1).
Discussion
Mature oocytes at metaphase II stage are the main requirement for a successful ART cycle. Metaphase II oocytes resume meiosis during fertilization upon activation by the sperm entry [1]. Here, we present two patients with an unusual presence of activated MII oocytes before they were introduced to sperm in repeated cycles. Spontaneous oocyte activation is a rare occurrence in human and these two cases were among 18,484 patients treated during last 25 years in our unit. In the literature, we could find only three case reports describing SOA [13–15]. Only the patients described by Osman et al. [15] had repeated SOA in multiple cycles as observed in our two cases. The case in Combelles et al. [13] also had four miscarriages similar to our first case. Combelles et al. [13] speculated that LH signaling might prematurely initiate calcium transients which could result in spontaneous activation. An evaluation of in vitro matured oocytes retrieved prior to any LH surge may shed light on understanding SOA in such patients.
Each patient in this report had different clinical presentations; the first one had RPL and the second one was diagnosed as unexplained infertility. Due to failure in responding to RPL therapies, we aimed to perform PGT-A, which we were unable to perform since no normal fertilization occurred. The first patient was counseled and agreed to undergo IVM cycle without any hormonal stimulation. Although only few oocytes were obtained, only one mature oocyte again displayed the presence of a single pronucleus indicating SOA in the absence of hormonal stimulation annulling the speculation that LH might be responsible for SOA. In the last cycle, it was decided to check for paternal contributions since there was embryo development following ICSI. Two out of ten biopsied embryos displayed paternal short tandem repeats (STR). Following transfer of one embryo, no pregnancy occurred. The presence of paternal STR in only two embryos might be the results of remnant of sperm DNA in the biopsied cells. Ye et al. [14] also reported the spontaneous development of 8-cell embryo from stimulated oocytes without sperm injection.
The second patient exhibited unexplained infertility with many unsuccessful trials. In her first cycle in our unit, split ICSI and IVF were performed per policy [20], without any normal fertilization resulting from IVF. All oocytes allocated to ICSI had one pronucleus following denudation and only two displayed two pronuclei following ICSI. Of these one arrested, and one was transferred without any resultant pregnancy. After patient counseling, subsequent cycles were performed with PGT. In the first PGT cycle with FISH analysis, no normal embryos were present. In the following three cycles, oocytes were cultured in time-lapse incubators with PGT with the intention of checking paternal contribution. One cycle was started with the intension of trophectoderm biopsy which had to be canceled due to the absence of blastocyst formation. STR analysis among the nine whole embryos analyzed, six revealed paternal contribution. This again could be due to remnant of injected sperm DNA. In the last two cycles, all retrieved oocytes showed activation following denudation apparent with the presence of one pronucleus. They were injected anyway and cultured in time-lapse incubator followed by day 3 biopsy to check paternal contribution. Only two out of eight in the last cycle had paternal contribution. They were transferred without resulting in a pregnancy. Although there was paternal contribution in these two embryos, careful review of time-lapse videos did not show any second pronuclei forming (Supplementary Video 1). Again, the presence of paternal alleles might represent remnant of injected sperm DNA. Similarly, Combelles et al. [13] had also conflicting results between FISH and PCR analysis for the presence of Y chromosome probably due to remnant of sperm DNA.
The etiology of SOA in human is not clear and not well studied. Combeles et al. [13] and Osman et al. [15] speculated that SOA might result from molecular defects in the cell cycle regulators involved in MII arrest. In fact, there are several animal models supporting this hypothesis. For example, oocytes from c-mos knockout mice do not stay at MII arrest and undergo parthenogenetic activation [12]. Moreover, extracellular regulated kinase-1 and -2 (ERK1/2) cascades play important roles in regulating MII arrest maintenance and male pronuclear formation in mouse oocytes [21]. Repeated nature of the activation in our patients raises the possibility that the phenotype could be related to genetic factors. In fact, whole exome sequencing is shedding light on the genetic bases of many infertility phenotypes [22, 23] making it plausible to investigate possible genetic contribution to SOA. However, clinical exome sequencing in our current study revealed no disease-causing variants following very detailed analysis. As with other negative exome studies, we are careful to point out the usual limitations of this otherwise very powerful molecular tool. In other words, the cause of SOA in these two patients may have indeed been monogenic but escaped detection by exome sequencing because of the nature of the variant, e.g., deep intronic, regulatory element or genomic rearrangement. We plan to pursue whole-genome sequencing to address these limitations.
The potential causative molecular factors underlying SOA are quite diverse, and as such, investigations of the factors underlying SOA would require a thorough evaluation of all such factors. Indeed, a number of oocyte-specific factors could be playing a role in SOA, including the Protein Kinase C (PKC) family of proteins, membrane channels regulating store operated calcium entry (SOCE) such as STIM1 and ORAI1, Ca2+-ATPases or Ca2+-dependent proteins such as CAMKII or MAPK [24]. Furthermore, Cui et al. [25] reported that premature decline of MAPK and activation of spindle assembly checkpoint proteins also could lead to SOA. However, the findings of our current study indicated a lack of mutations/polymorphisms in any gene(s) involved in Ca2+-regulation at fertilization suggesting other factors might be involved. Recent advances allowed the RNA sequencing and transcriptomic profiling at the single oocyte level [26]. Therefore, it will be also interesting to study single human oocyte RNA profiles to understand whether they play any role in SOA.
An avenue that has yet only been scarcely investigated in relation to SOA is the regulation of zinc (Zn2+) in relation to spontaneous Ca2+ release. Indeed, Ca2+ oscillations trigger a coordinated profile of Zn2+ release, termed Zn2+ ‘sparks’ at human oocyte activation, a meiotic-stage dependent phenomenon [27, 28]. Interestingly, however, chelation of intracellular Zn2+ alone sufficiently induced cell cycle resumption in human oocytes [28]. Indeed, Zn2+ regulates the activity of CDC25 which in turn activates MPF [29], while EMI2 (required to maintain high MPF activity) is also Zn2+-dependent [30]. Intriguingly, this requirement of Zn2+ depletion has been utilized as a mechanism of assisted oocyte activation (AOA) using the Zn2+ chelator TPEN (N,N,N’,N’-tetrakis (2-pyridylmethyl)ethane-1,2-diamine), whereby incubation of oocytes with this substance results in effective MII resumption [31, 32]. Indeed, similar inhibitors have been used clinically to reduce levels of MPF as opposed to artificially eliciting Ca2+ release in oocytes. Such examples include protein synthesis or kinase inhibitors such as puromycin or 6-dimethylaminopurine (6-DMAP) that block cyclin B synthesis or inhibit CDK1 activity, respectively [32]. To this degree, perhaps examining the occurrence of similar phenomenon in cases of SOA may be merited.
In conclusion, recurrent spontaneous oocyte activation is a very rare phenomenon with a poorly understood underlying mechanism in human causing infertility. Negative clinical exome sequencing and sporadic paternal contribution without identifying second pronucleus require further investigation at the cellular level which might be difficult to perform in human due to ethical concerns and limited availability of oocytes. Patients with such cases should be counseled for negative cycle outcome.
Supplementary Information
Below is the link to the electronic supplementary material.
Figure S1. Exome was performed on index patients from case 1 and 2. Filtering strategy starting from larger to smaller circles; Total number of variants=>Homozygous variants=>Coding/Splicing=>Absent in gnomAD and local database=>Predicted deleterious in silico=>Relevant biology (JPG 110 KB)
Supplementary Video 1. Development of embryo generated from the spontaneously activated oocyte following ICSI. Although STR analysis showed paternal contribution, no other pronucleus was seen. (AVI 18404 KB)
Author’s contributions
SC and FA conceived the study. SC and JK wrote the manuscript. KA and MA recruited the patients and collected data. WQ and SM performed the experiments. All authors contributed to the article and approved the submitted version.
The authors declare no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 21(4):427–54. 10.1093/humupd/dmv011. [DOI] [PubMed]
- 2.Okabe M. The cell biology of mammalian fertilization. Development. 2013;140(22):4471–4479. doi: 10.1242/dev.090613. [DOI] [PubMed] [Google Scholar]
- 3.Kashir J. Increasing associations between defects in phospholipase C zeta and conditions of male infertility: not just ICSI failure? J Assist Reprod Genet. 2020;37(6):1273–1293. doi: 10.1007/s10815-020-01748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleh A, Kashir J, Thanassoulas A, Safieh-Garabedian B, Lai FA, Nomikos M. Essential role of sperm-specific PLC-Zeta in egg activation and male factor infertility: an update. Front Cell Dev Biol. 2020;29(8):28. doi: 10.3389/fcell.2020.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen A, Caiazza F. Oocyte maturation: a story of arrest and release. Front Biosci (Schol Ed) 2013;1(5):451–477. doi: 10.2741/s383. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari M, Gupta A, Sharma A, Prasad S, Pandey AN, Yadav PK, et al. Role of mitogen activated protein kinase and maturation promoting factor during the achievement of meiotic competency in mammalian oocytes. J Cell Biochem. 2018;119:123–129. doi: 10.1002/jcb.26184. [DOI] [PubMed] [Google Scholar]
- 7.Cui W. Oocyte spontaneous activation: an overlooked cellular event that impairs female fertility in mammals. Front Cell Dev Biol. 2021;8(9):648057. doi: 10.3389/fcell.2021.648057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madgwick S, Jones KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div. 2007;26(2):4. doi: 10.1186/1747-1028-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kito S, Yano H, Ohta Y, Tsukamoto S. Superovulatory response, oocyte spontaneous activation, and embryo development in WMN/Nrs inbred rats. Exp Anim. 2010;59:35–45. doi: 10.1538/expanim.59.35. [DOI] [PubMed] [Google Scholar]
- 10.Zernicka-Goetz M. Spontaneous and induced activation of rat oocytes. Mol Reprod Dev. 1991;28(2):169–176. doi: 10.1002/mrd.1080280210. [DOI] [PubMed] [Google Scholar]
- 11.Chebotareva T, Taylor J, Mullins JJ, Wilmut I. Rat eggs cannot wait: Spontaneous exit from meiotic metaphase-II arrest. Mol Reprod Dev. 2011;78(10–11):795–807. doi: 10.1002/mrd.21385. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370(6484):68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- 13.Combelles CM, Kearns WG, Fox JH, Racowsky C. Cellular and genetic analysis of oocytes and embryos in a human case of spontaneous oocyte activation. Hum Reprod. 2011;26(3):545–552. doi: 10.1093/humrep/deq363. [DOI] [PubMed] [Google Scholar]
- 14.Ye Y, Li N, Yan X, Wu R, Zhou W, Cheng L, et al. Genetic analysis of embryo in a human case of spontaneous oocyte activation: a case report. Gynecol Endocrinol. 2020;36:294–296. doi: 10.1080/09513590.2019. [DOI] [PubMed] [Google Scholar]
- 15.Osman EK, Hong KH, Scott RT. A case of recurrent spontaneous parthenogenetic oocyte activation. Reprod Biomed Online. 2019;39(Suppl. 2):e7–e8. doi: 10.1016/j.rbmo.2019.07.018. [DOI] [Google Scholar]
- 16.Qubbaj W, Al-Swaid A, Al-Hassan S, Awartani K, Deek H, Coskun S. First successful application of preimplantation genetic diagnosis and haplotyping for congenital hyperinsulinism. Reprod Biomed Online. 2011;22(1):72–79. doi: 10.1016/j.rbmo.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Alghofaili L, Almubarak H, Gassem K, Islam SS, Coskun S, Kaya N, et al. Cell-free DNA levels of twins and sibling pairs indicate individuality and possible use as a personalized biomarker. PLoS ONE. 2019;14(10):e0223470. doi: 10.1371/journal.pone.0223470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monies D, Abouelhoda M, AlSayed M, Alhassnan Z, Alotaibi M, Kayyali H, et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum Genet. 2017;136(8):921–939. doi: 10.1007/s00439-017-1821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monies D, Abouelhoda M, Assoum M, Moghrabi N, Rafiullah R, Almontashiri N, et al. Lessons learned from large-scale, first-tier clinical exome sequencing in a highly consanguineous population. Am J Hum Genet. 2019;104(6):1182–1201. doi: 10.1016/j.ajhg.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaroudi K, Al-Hassan S, Al-Sufayan H, Al-Mayman H, Qeba M, Coskun S. Intracytoplasmic sperm injection and conventional in vitro fertilization are complementary techniques in management of unexplained infertility. J Assist Reprod Genet. 2003;20(9):377–381. doi: 10.1023/a:1025433128518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YL, Liu XM, Ji SY, Sha QQ, Zhang J, Fan HY. ERK1/2 activities are dispensable for oocyte growth but are required for meiotic maturation and pronuclear formation in mouse. J Genet Genomics. 2015;42(9):477–485. doi: 10.1016/j.jgg.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Altaf S, Bao J. Exome sequencing shines in empty follicle syndrome: zona pellucida gene mutations manifest genuine empty follicle syndrome. Fertil Steril. 2021;115(5):1170–1171. doi: 10.1016/j.fertnstert.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Maddirevula S, Awartani K, Coskun S, AlNaim LF, Ibrahim N, Abdulwahab F, et al. A genomics approach to females with infertility and recurrent pregnancy loss. Hum Genet. 2020;139(5):605–613. doi: 10.1007/s00439-020-02143-5. [DOI] [PubMed] [Google Scholar]
- 24.Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016;22(1):23–47. doi: 10.1093/humupd/dmv040. [DOI] [PubMed] [Google Scholar]
- 25.Cui W, Zhang J, Lian HY, Wang HL, Miao DQ, Zhang CX, et al. Roles of MAPK and spindle assembly checkpoint in spontaneous activation and MIII arrest of rat oocytes. PLoS ONE. 2012;7(2):e32044. doi: 10.1371/journal.pone.0032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asami M, Lam BYH, Ma MK, Rainbow K, Braun S, VerMilyea MD, et al. Human embryonic genome activation initiates at the one-cell stage. Cell Stem Cell. 2022;29(2):209–216.e4. doi: 10.1016/j.stem.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, et al. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol. 2011;6(7):716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan FE, Que EL, Zhang N, Feinberg EC, O'Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep. 2016;26(6):24737. doi: 10.1038/srep24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Chai Y, Hannigan R, Bhogaraju VK, Machaca K. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J Cell Physiol. 2007;213(1):98–104. doi: 10.1002/jcp.21090. [DOI] [PubMed] [Google Scholar]
- 30.Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol. 2006;174(6):791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K, Davis A, Zhang L, Ryu J, Spate LD, Park KW, Samuel MS, Walters EM, Murphy CN, Machaty Z, Prather RS. Pig oocyte activation using a Zn2+ chelator, TPEN. Theriogenology. 2015;84(6):1024–32. doi: 10.1016/j.theriogenology.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashir & Swann, Chapter 14: Assisted oocyte activation: Current understanding, practice, and future perspectives, 219–234. In: Textbook of Assisted Reproductive Techniques, David K. Gardner, Ariel Weissman, Colin M. Howles, Zeev Shoham (Eds), 5th edition, CRC Press, Boca Raton.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Exome was performed on index patients from case 1 and 2. Filtering strategy starting from larger to smaller circles; Total number of variants=>Homozygous variants=>Coding/Splicing=>Absent in gnomAD and local database=>Predicted deleterious in silico=>Relevant biology (JPG 110 KB)
Supplementary Video 1. Development of embryo generated from the spontaneously activated oocyte following ICSI. Although STR analysis showed paternal contribution, no other pronucleus was seen. (AVI 18404 KB)