Abstract
TAFRO syndrome is a rare disorder that manifests as thrombocytopenia, anasarca, fever, reticulin myelofibrosis, renal dysfunction, and organomegaly. Although this disease often follows a severe clinical course, the cause remains unknown. The coronavirus disease 2019 (COVID-19) pandemic is a major global problem. Vaccination against COVID-19 has been successful; however, there are concerns about severe adverse events. Herein, we report a rare presentation of TAFRO syndrome triggered by the COVID-19 vaccine with a fatal clinical course. A 42-year-old Japanese man presented to our hospital complaining of fever lasting for 2 weeks that occurred a day after receiving the BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine. The patient had a low platelet count, ascites, reticulin myelofibrosis, renal failure, and lymphadenopathy and was diagnosed with TAFRO syndrome. Despite administering several immunosuppressive drugs, the condition did not improve. The patient repetitively developed and eventually died of bacteremia caused by multidrug-resistant Klebsiella pneumoniae. We highlight the first reported case of TAFRO syndrome after COVID-19 vaccination.
Keywords: TAFRO syndrome, COVIID-19, Vaccination, Side effect
1. Introduction
TAFRO syndrome, first reported in 2010 [1], is a rare disease characterized by thrombocytopenia (T), anasarca (A), fever (F), reticulin myelofibrosis, renal dysfunction (R), and organomegaly (O), including hepatosplenomegaly and lymphadenopathy [2]. Initially, TAFRO syndrome was thought to be a subtype of idiopathic multicentric Castleman disease, which is a rare heterogeneous systemic disorder characterized by systemic inflammation, multicentric lymphadenopathy with characteristic histopathological features, and organ dysfunction due to elevated pro-inflammatory cytokines, including interleukin-6 (IL-6) [3,4].
The coronavirus disease 2019 (COVID-19) pandemic has been a worldwide concern since 2019. Vaccines including BNT162b2 mRNA COVID-19 (Pfizer-BioNTech) have been administered worldwide to prevent this pandemic. Although vaccination is usually effective and safe, some people experience severe side effects. Side effects such as local injection site reaction, anaphylaxis, fever, and fatigue are common; however, those caused by autoantibodies, such as thrombosis with thrombocytopenia syndrome, Guillain-Barre syndrome, and idiopathic thrombocytopenic purpura (ITP) [5], are rare but severe. Moreover, cytokine release syndrome, including multisystem inflammatory syndrome in adults (MIS-A) [6] and systemic capillary leak syndrome (SCLS) [7], have also been reported as rare side effects of COVID-19 vaccination.
Here, we report a case of TAFRO syndrome following BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccination.
2. Case report
A 42-year-old Japanese man with no relevant medical history presented to our hospital with a chief complaint of persistent fever after the second dose of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine. The patient was in the usual state of health until the first vaccination, but the patient noticed a loss of appetite and general malaise the next day. From the day after the second vaccination, high fever (>38 °C) occurred. Fever, loss of appetite, and general malaise persisted for 2 weeks, followed by abdominal fullness and weight gain; hence, the patient presented to our hospital.
On admission, the patient's temperature was 37.2 °C, blood pressure was 125/86 mmHg, pulse rate was 98 beats/min, respiratory rate was 16 breaths/min, and oxygen saturation was 98% on room air. Initial laboratory investigations revealed the following: white blood cell count, 8.06 × 103/mm3 (71.5% neutrophils, 8.5% lymphocytes, and 9.5% monocytes); hemoglobin level, 14.5 g/dL; platelet count, 10 × 103/mm3; C-reactive protein level, 23.9 mg/dL; albumin level, 2.8 g/dL; ferritin level, 539 ng/mL, erythrocyte sedimentation rate, 42 mm/1 hour; and soluble interleukin 2 receptor level, 1400 U/mL. There were no electrolyte or aminotransferase abnormalities, and renal function was normal. Human immunodeficiency virus antibody, hepatitis B surface antigen, and hepatitis C virus antibody were negative. Urine dipstick examination results were normal. Additional data showed immunoglobulin G level of 553 mg/dL, immunoglobulin G4 level of 24.2 mg/dL, immunoglobulin A level of 48 mg/dL, and immunoglobulin M level of 30 mg/dL. The results of immunoelectrophoresis showed α1-globulin levels of 12.0%, α2-globulin levels of 14.9%, β-globulin levels of 11.7%, and γ-globulin levels of 9.5%. Rheumatoid factor level of <5.0 IU/mL, antinuclear antibody level of <40, antineutrophil cytoplasmic antibody level of <1.0 U/mL, double-stranded DNA level of <10 IU/mL, anti-Sm antibody level of <1.0 U/mL, anti SS-A/B antibody level of <1.0 U/mL, anti-ribonucleoprotein antibody level of <2.0 U/mL. Contrast-enhanced computed tomography (CT) revealed pleural effusion (Fig. 1a and Fig. 1b ), ascites, periportal edema, mild hepatosplenomegaly, and mild lymphadenopathy (mediastinal and retroperitoneal, approximately 6 mm in short-axis diameter). Blood, sputum and urine cultures and interferon-gamma release assays were negative. Echocardiography did not reveal vegetation on the valves, and positron emission tomography-CT scan showed no lymphadenopathy that could be biopsied. Moreover, random skin biopsy was negative, while bone marrow examination showed reticulin myelofibrosis. Additional laboratory tests revealed an IL-6 level of 47.5 pg/mL, vascular endothelial growth factor of 5520 pg/mL, and a positive result for platelet-associated IgG (PAIgG). Based on these clinical features and examination results, the patient was diagnosed with TAFRO syndrome.
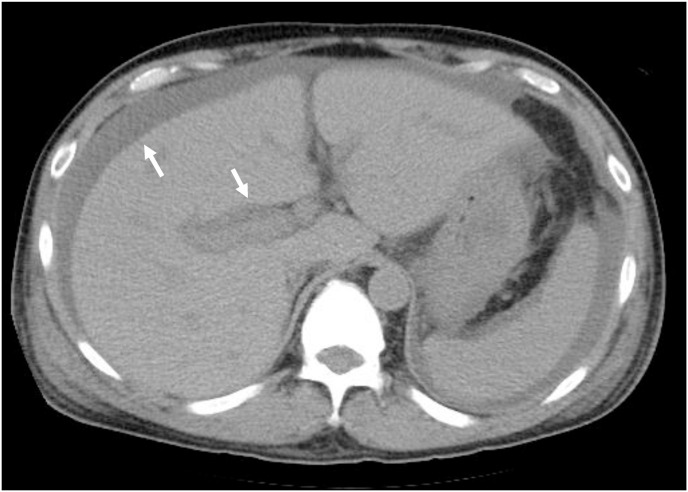
Fig. 1a.
CT on the 17th day of admission showed hepatosplenomegaly, ascites and periportal collar sign.
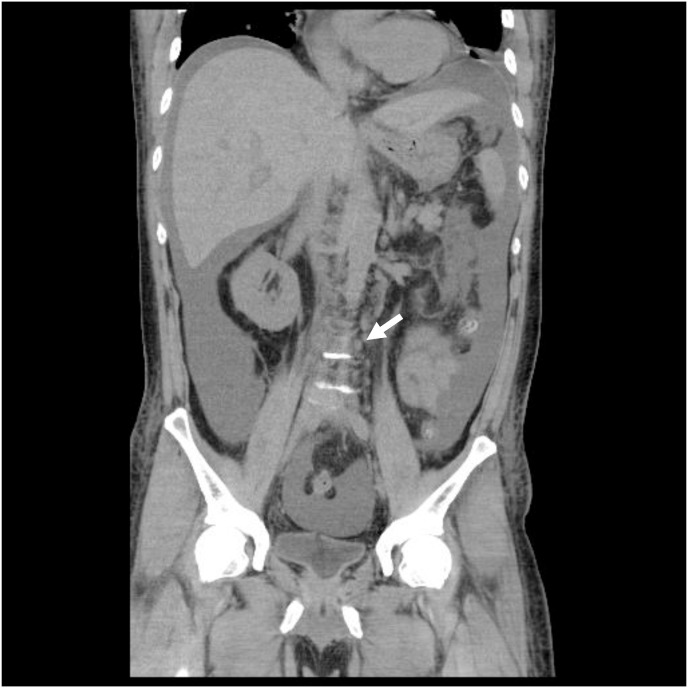
Fig. 1b.
Coronal view of CT scan revealed mild retroperitoneal lymphadenopathy (about 6 mm in short-axis diameter).
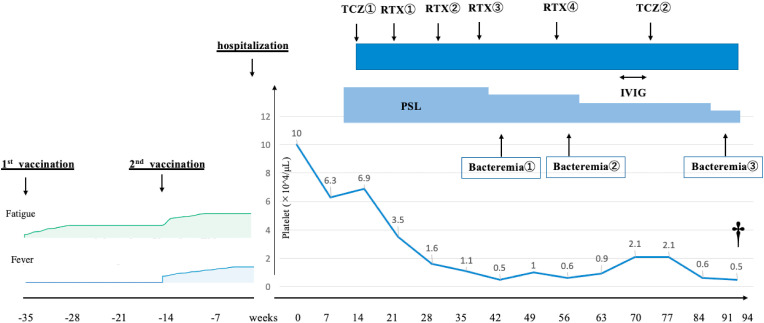
Twelve days after admission, we started intravenous methylprednisolone pulse therapy (1000 mg/day) for 3 days, followed by prednisolone 1 mg/kg daily (70 mg/day). Despite starting steroids, laboratory abnormalities and the patient's condition continued to worsen. Even after adding tocilizumab 8 mg/kg on the 16th day, anasarca increased, while platelet count and renal function decreased. On the 22nd, 32nd, 40th, and 55th days, we treated the patient with four doses of weekly rituximab 375 mg/m2. Since daily platelet transfusion was required, we added intravenous immunoglobulin therapy from the 68th day to the 72nd day and tocilizumab 8 mg/kg again on the 75th day. While platelet count gradually improved to around 1.0 × 103/mm3 without daily transfusion, anasarca did not decrease. We performed abdominal paracentesis and removed 5 L of ascites every other day. Fetal bacteremia caused by multidrug-resistant bacteria occurred several times. On the 91st day, the patient developed fever, and blood culture revealed bacteremia caused by multidrug-resistant Klebsiella pneumoniae. Despite using susceptibility-based antibiotics including Cefmetazole, levofloxacin, imipenem, tigecycline, and colistin, the patient's condition did not improve, and eventually, the patient died on the 94th day of hospitalization (Fig. 2 ). An autopsy was performed the day after the death. The autopsy revealed ascites, pleural effusion, hepatomegaly, splenomegaly, and myelofibrosis in the bone marrow. Retroperitoneal lymphadenopathy showed only inflammatory changes and no atypical lymphocytes, and it did not show any depositions of amyloid proteins.
Fig. 2.
Dagger indicates patient death. PSL:prednisolone. TCZ:tocilizumab. RTX:rituximab. IVIG:intravenous immunoglobrin Bacteremia① caused by Bacteroides fragilis and Klebsiella pneumoniae treated with Cefmetazole. Bacteremia② caused by Enterobacter cloacae (Metallo-β-Lactamase producing bacteria) treated with Cefmetazole and Levofloxacin Bacteremia③ caused by multidrug-resistant Klebsiella pneumoniae treated with Cefmetazole, levofloxacin, imipenem, tigecycline, and colistin.
3. Discussion
To the best of our knowledge, this is the first case of TAFRO syndrome in a healthy person associated with BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccines, as described in the scientific literature. Unfortunately, the patient had an extremely fatal clinical course despite multiple immunosuppressive treatments.
We diagnosed the patient with TAFRO syndrome because three major and two minor criteria of the 2015 diagnostic criteria for TAFRO syndrome [2] were met. Major criteria include anasarca, thrombocytopenia, and systemic inflammation such as fever of unknown etiology or serum C-reactive protein concentration >2 mg/dL or both. Minor criteria include reticulin myelofibrosis, mild organomegaly, and progressive renal insufficiency. Even if the patient fulfills these characteristics, several diseases need to be ruled out to diagnose TAFRO syndrome, such as malignancies including lymphoma, autoimmune disorders including systemic lupus erythematosus and ANCA-associated vasculitis, and infectious disorders including acid-fast bacterial infection, rickettsial disease, Lyme disease, and severe fever with thrombocytopenia syndrome. Moreover, it is necessary to rule out POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, IgG4-related disease, hepatic cirrhosis, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome before the diagnosis of TAFRO syndrome [2], which we have conducted and confirmed through the examination results during hospitalization. In addition, autopsy examination of the retroperitoneal lymphadenopathy did not show Castleman's disease-like features such as hyaline vascular, hypervascular, and/or plasmacytic pathology [8], as described in the diagnostic criteria for TAFRO syndrome [2]. We speculate that the biopsy results may have been masked by the administration of steroids and immunosuppressive drugs, which interfered with the expression of the typical pathological characteristic of TAFRO syndrome. Although the patient's symptoms occurred immediately after the administration of the vaccine, it is difficult to establish a causal relationship between the vaccine and TAFRO syndrome. We considered that the hyperinflammatory condition after the vaccine might have contributed to the syndrome and provide three reasons for this hypothesis.
First, the patient experienced symptoms that mimicked MIS-A. MIS-A has recently been recognized among persons who received COVID-19 vaccines and patients diagnosed with COVID-19. MIS-A was originally recognized as a severe illness in a person aged over 21 years, with laboratory evidence of severe inflammation and current or previous COVID-19 infection, crucial organ dysfunction, and absence of severe respiratory symptoms [6]. There was a previous case report of MIS-A that occurred after COVID-19 vaccination without a diagnosis of COVID-19 infection [9]. According to the criteria by the Brighton Collaboration network, to diagnose COVID-19 vaccination-induced MIS-A, patients should have exhibited symptoms within 4–6 weeks of vaccination [9], and the patient, in this case, fulfilled the criteria within at least 2 weeks after the vaccine. Second, this case had some characteristics of SCLS, and some reports indicate an association between the COVID-19 vaccine and SCLS, which is characterized by severe hypotension, hypoalbuminemia, and hemoconcentration [7]. This syndrome can occur in many medical conditions, such as sepsis, systemic inflammatory response syndrome, and infectious diseases. The mechanism of SCLS is thought to be the extravasation of fluid and protein from the intravascular to the interstitial space [10]. Some studies have reported that hypercytokinemia and increased intra-abdominal pressure due to massive ascites caused SCLS in patients with TAFRO syndrome [11]. Third, PAIgG was positive in this case. According to some reports, the COVID-19 vaccine could trigger ITP [12], which is an immune-mediated disorder that causes thrombocytopenia and bleeding and is sometimes caused by infectious diseases, autoimmune diseases, vaccines, and drugs [13]. PAIgG, an antiplatelet antibody, is often positive in ITP but could also be positive in TAFRO syndrome, as similarly observed in this case [14]. Moreover, in this case, immunoglobulin infusion was probably effective against thrombocytopenia, which suggested that the patient might have an aspect of ITP. Based on these reasons, we believe that MIS-A, SCLS, and ITP occurred simultaneously in this case, and the hyperinflammatory state caused by COVID-19 vaccination could contribute to such a condition.
In conclusion, a previously healthy man developed TAFRO syndrome a day after COVID-19 vaccination. Therefore, physicians should be aware that COVID-19 vaccines may cause various hyperinflammatory states, leading to TAFRO syndrome.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Treatment of the patient: MY, RS, AM. Diagnosis and treatment of the patient: GO. Drafting the manuscript: MY, RS, EK. Revising the manuscript critically for important intellectual content: SM, ST, HA, HM, KH. All authors contributed to the writing of the final manuscript and approved the version of the manuscript to be submitted.
Consent to publish
We obtained written informed consent from the patient to publish the case report.
Declaration of competing interest
None.
Acknowledgements
None.
Footnotes
All authors meet the ICMJE authorship criteria.
References
- 1.Takai K., Nikkuni K., Shibuya H., Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites, and hepatosplenomegaly. Rinsho Ketsueki. 2010;51:320–325. doi: 10.11406/rinketsu.51.320. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 2.Masaki Y., Kawabata H., Takai K., Kojima M., Tsukamoto N., Ishigaki Y., et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol. 2016;103:686–692. doi: 10.11406/rinketsu.57.2029. [DOI] [PubMed] [Google Scholar]
- 3.Tu K.H., Fan P.Y., Chuang W.Y., Wu C.Y., Ku C.L., Tian Y.C., et al. TAFRO Syndrome with renal thrombotic microangiopathy: insights into the molecular mechanism and treatment opportunities. Int J Mol Sci. 2021;22:6286. doi: 10.3390/ijms22126286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawabata H., Takai K., Kojima M., Nakamura N., Aoki S., Nakamura S., et al. Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012) J Clin Exp Hematop. 2013;53:57–61. doi: 10.3960/jslrt.53.57. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention: CDC Information about reported adverse events of COVID-19 vaccine. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
- 6.Vogel T.P., Top K.A., Karatzios C., Hilmers D.C., Tapia L.I., Moceri P., et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037–3049. doi: 10.1016/j.vaccine.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robichaud J., Côté C., Côté F. Systemic capillary leak syndrome after ChAdOx1 nCOV-19 (Oxford–AstraZeneca) vaccination. CMAJ (Can Med Assoc J) 2021;193:E1341–E1344. doi: 10.1503/cmaj.211212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fajgenbaum D.C., Wu D., Goodman A., Wong R., Chadburn A., et al. Insufficient evidence exists to use histopathologic subtype to guide treatment of idiopathic multicentric Castleman disease. Am J Hematol. 2020;95:1553–1561. doi: 10.1002/ajh.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nune N., Iyengar K.P., Goddard C., Ahmed A.E. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V) BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddall Eric, Khatri Minesh, Radhakrishnan Jai. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92:37–46. doi: 10.1016/j.kint.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 11.Hibi A., Mizuguchi K., Yoneyama A., Kasugai T., Kamiya K., Kamiya K., et al. Severe refractory TAFRO syndrome requiring continuous renal replacement therapy complicated with Tricosporon asahii infection in the lungs and myocardial infarction: an autopsy case report and literature review. Ren Replace Ther. 2018;4:16. doi: 10.1186/s41100-018-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyama H., Kakiuchi S., Rikitake J., Matsuba H., Sekinada D., Kozuki Y., et al. Immune thrombocytopenia associated with Pfizer-BioNTech's BNT162b2 mRNA COVID-19 vaccine. ID Cases. 2021;25 doi: 10.1016/j.idcr.2021.e01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clines D.B., Bussel J.B., Liebman H.A., Prak E.T.L. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwaki N., Fajgenbaum D.C., Nabel C.S., Gion Y., Kondo E., Kawano M., et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91:220–226. doi: 10.1002/ajh.24242. [DOI] [PubMed] [Google Scholar]