Abstract
Background:
Enhanced error-related negativity (ERN), an event-related potential (ERP) component reflecting neural sensitivity to errors and threat, has been theorized to represent an endophenotype of internalizing psychopathologies (IPs). We tested whether intergenerational transmission of ERN patterns may confer risk for internalizing symptoms. We examined associations among maternal and offspring ERN, and offspring internalizing symptoms. Given the role of parenting in IP risk, we also explored how maternal negative parenting styles related to maternal ERN and offspring internalizing symptoms.
Methods:
Participants included 117 biological mother-child dyads (ages 9-16 years, 70.9% female). Seventy-two mothers had a history of major depression (32 with lifetime anxiety) and 45 had no history of psychiatric illness. Dyads completed psychiatric interviews, parenting questionnaires, and a flanker task to elicit the ERN while electroencephalogram (EEG) was recorded.
Results:
Path analyses revealed that maternal ERN was significantly associated with enhanced offspring ERN and greater negative parenting styles. Enhanced offspring ERN and maternal negative parenting styles were significantly related to greater internalizing symptoms in offspring. Maternal ERN had a significant indirect effect on offspring internalizing symptoms through offspring ERN and maternal negative parenting styles, above the effects of self-reported maternal internalizing symptoms. Maternal IP history did not moderate pathways.
Conclusions:
Study findings suggest enhanced maternal ERN is indirectly associated with greater offspring internalizing symptoms through its relations to offspring ERN and negative parenting styles. Future longitudinal work is needed to evaluate the temporal timing and directionality of these tested pathways and their clinical implications for the prevention of IPs.
Keywords: Depression, Anxiety, Event-Related Negativity, Event-Related Potentials, Youth, Parent-Child Interactions
Internalizing psychopathologies (IPs), such as anxiety and depressive disorders, are characterized by significant mood and emotion regulation challenges that impact psychosocial adjustment (1,2). IP diagnoses are highly comorbid (e.g., 3) and may share common underlying biological and environmental mechanisms (4,5). Although there are multiple etiological pathways for IPs, both biological and environmental risk factors play a substantial role (5,6). Given that IPs typically emerge in childhood and adolescence (e.g., 7), the identification of risk factors associated with IP vulnerability could potentially identify novel, objective targets for preventing IPs in youth.
One promising biomarker implicated in IP risk is the error-related negativity (ERN), an event-related potential (ERP) component measured at frontocentral electrodes 0-100 ms following the commission of an error (8,9), and thought to be generated by activity in the anterior cingulate cortex (ACC;10,11). Exhibiting an enhanced ERN is hypothesized to reflect an overactive performance monitoring system (12,13) as well as heightened defensive reactivity in response to errors (e.g., perception of errors as threatening to the self; 14). Research shows that individual differences in ERN amplitudes are relatively stable overtime and evince trait-like features (15,16). Heightened ERN is an established neural correlate of anxiety (12,14, 17) and predicts the onset of anxiety disorders in children and adolescents (18-19). Although enhanced ERN has also been documented among individuals with depression (20,21), relations have been less consistent. Several studies have demonstrated no association between depression and ERN (22,23) or an attenuated relation between depressive symptoms and ERN, particularly with individuals with severe depression rather than mild-to-moderate depression (24,25). Some have proposed that depression presentations that are characterized by heightened negative affect, worry, and comorbid anxiety are specifically associated with an enhanced ERN (26,27). Additionally, individuals who report greater negative emotionality, a shared characteristic of anxiety and depressive disorders, demonstrate heightened neurophysiological responses to errors (26). Although research on the ERN and depression has been limited, prior work could suggest that the ERN may be a general endophenotype of IP risk.
Toward establishing ERN as a potential endophenotype for IPs, studies have evaluated its heritability and familial aggregation (28-31). Results indicate that ERN amplitudes are moderately heritable, with approximately 50% of variation in the ERN shared between twin pairs (28). Other research shows moderate intra-class ERN correlations between family members, including mothers and children (29,30) and increased ERN amplitudes in patients with anxiety disorders and their unaffected first-degree relatives relative to healthy controls (31). Despite emerging evidence, intergenerational patterns of ERN have not been evaluated in relation to youth IP risk.
To address this gap, our study examined the relation between maternal and offspring ERN in a sample enriched for IP risk (half of the mothers had histories of depression and many had comorbid anxiety). Consistent with prior heritability findings (28-31), we predicted significant intergenerational associations between maternal and offspring ERN, which in turn would confer risk for internalizing symptoms in offspring through the indirect effect of maternal ERN. We also included current maternal internalizing symptoms in our model to evaluate their unique effects on pathways, and tested multi-group mediation models to test the moderating effects of maternal IP history.
A second goal of the current study was to explore how maternal parenting practices potentially influence these associations. Prior research has shown that various negative parenting behaviors (e.g., criticism, disengagement) are associated with increased rates of IPs among youth (32,33), and enhanced ERN in offspring (34-38). Some evidence indicates that offspring ERN mediates the relationship between negative parenting styles and offspring anxiety (39), although these studies have not investigated how maternal ERN vulnerabilities may account for the documented relations between negative parenting styles and mothers with histories of IPs (40). Indeed, neurobiological models of caregiving (e.g., 41) emphasize how difficulties with regulation of physiological, neurocognitive, and affective processes can increase the likelihood of negative parenting styles (e.g., critical, less engaged and responsive parenting). Given links between IPs, ERN, and negative affect (e.g., 26,27), we hypothesized that maternal enhanced ERN may be associated with greater negative parenting styles, which in turn would be related to increased offspring internalizing symptoms.
Methods and Materials
Participants and Procedures
Participants were 117 mothers (Mage= 40.90 years, SD = 6.75, Range = 25-56 years) and their 9-16 year-old biological children (Mage = 12.35, SD = 2.08, 70.9% female). Dyads were enrolled across two studies on the intergenerational transmission of maternal depression. Recruitment strategies, study procedures, and research personnel were identical across studies. Dyads were recruited in a large Midwestern metropolitan city using flyers, community events, and Internet postings (e.g., Facebook).
Psychiatric diagnoses for mothers and offspring were obtained using the Structured Clinical Interview for DSM-5 (42) and Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL; 43), respectively. Seventy-two mothers had a history of major depressive disorder (MDD), and 45 had no psychiatric illness histories. Of the 72 mothers with MDD histories, 13 met criteria for current MDD, 49 for recurrent MDD, 32 had lifetime histories of anxiety disorders (current and past combined), and 27 had a current anxiety disorder. Six youth met criteria for past MDD, 21 had a current anxiety disorder, and two met criteria for a past anxiety disorder. Study exclusions included substance/alcohol dependence within the past 6 months or histories of bipolar disorder, schizophrenia, intellectual disability, serious medical conditions, pervasive developmental disorders, and current active suicidal ideation.
Participants identified as White (47.9% offspring, 49.6% mothers), African American (28.2% offspring, 29.1% mothers), Asian (8.5% offspring, 7.7% mothers), other or unknown race (11.1% offspring, 9.4% mothers), multiracial (4.3% offspring, 3.4% mothers), and American Indian or Alaskan Native (0% offspring, .9% mothers). Approximately 27.4% of offspring and 23.9% of mothers identified as Hispanic/Latinx. Median family income was $65,001- $75,000.
The UIC Institutional Review Board approved study procedures prior to data collection. Informed consent and assent was obtained from mothers and their offspring after study procedures were explained to them.
Measures
Offspring internalizing symptoms.
Youth completed the Youth Self-Report (YSR, Ages 11-18; 44), which is a 112-item measure of emotional and behavioral problems. The anxious/depressed subscale was used to assess internalizing symptoms, and demonstrated adequate internal consistency (α = .80). Raw scores were used in the analyses to account for the full variation in scores. Inspection of T scores indicated that 15.8% of youth in the sample reported clinically relevant anxiety and depression symptoms (T scores > 63).
Maternal internalizing symptoms.
Mothers completed the Beck Anxiety Inventory (BAI; 45) and the Beck Depression Inventory, Second Edition (BDI-II; 46) to assess anxiety and depression symptoms. On the BAI, total scores that are greater than 7 suggest mild anxiety symptoms, while scores above 16 indicate clinically significant anxiety. Sixteen percent of mothers reported clinical levels of anxiety. On the BDI-II, total scores greater than 14 indicate the presence of depressive symptoms, and 24.79% of mothers reported clinically significant depressive symptoms. Both scales demonstrated excellent internal consistency (αs = .94) and were significantly correlated with each other (r = .67, p < .001). Principal components analysis was performed on the two subscales to determine whether they loaded onto a single factor representing internalizing symptoms. Inspection of the eigenvalues (> 1) and the scree plot supported a single-factor solution, with strong positive loadings for BAI (r = .92) and BDI (r = .92). The scales were standardized and averaged to create a composite of maternal internalizing symptoms.
Maternal negative parenting styles.
To assess maternal negative parenting styles, mothers completed two self-report measures. First, mothers completed the Perceived Criticism Scale (PCS; 47), which assesses parental perceptions of how critical they are of their child. The PCS is scored on an item basis, and the item, “How critical do you think you are of your child?” was used in the analysis. Mothers also completed the Parental Bonding Instrument (PBI; 48), and the warmth subscale, which measures engagement and responsiveness to children’s emotional states and interests, was reverse scored and used in the analyses. The scale demonstrated satisfactory internal consistency (α = .81). The single item from the PCS and reverse scored warmth subscale from the PBI were significantly correlated, r = .31, p < .01. Principal components analysis was performed on the two subscales. Inspection of the eigenvalues (> 1) and scree plot supported a single-factor solution, yielding positive loadings for maternal criticism (r = .81) and warmth (reverse; r = .81). The scales were standardized and averaged to create a maternal negative parenting styles composite.
Error monitoring task.
Dyads completed a modified computerized Flanker task (8), which measures performance monitoring in response to errors. The flanker task reliably elicits the ERN in youth and adults and evinces excellent retest reliability (15,16). See Supplemental Material for detailed description of the flanker task, behavioral performance, and data inclusion/exclusion criteria.
EEG data acquisition and preprocessing.
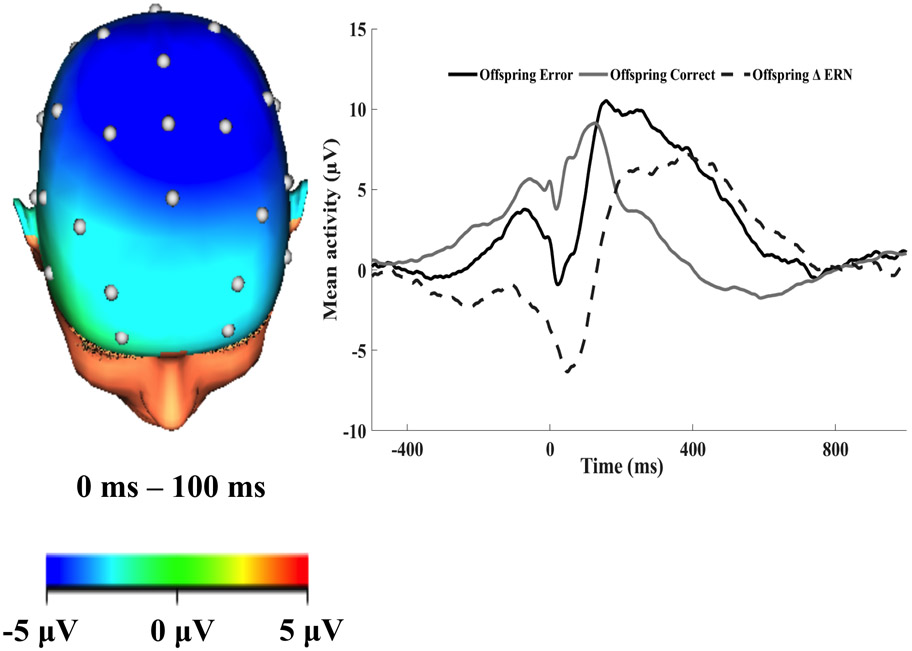
We followed standard procedures of EEG data acquisition and processing steps (see Supplemental Material for detailed description). ERPs to errors and correct responses were averaged separately and baseline corrected −500 – −300 ms before the response. The ERN was scored at FCz 0 to 100 ms after the response (Figures 1-2). The ERP response was more negative following errors versus correct responses for mothers (t (78) = −6.30, p < .01) and offspring (t (90) = −8.13, p < .01). The ERN was scored as the difference between amplitudes on correct and error trials. More negative ERN amplitudes indicate enhanced response to errors relative to correct responses (i.e., increased error monitoring). We computed the internal consistencies for error, correct, and ERN variables for mothers and offspring, and the internal consistencies were adequate (See Supplemental Material). We also re-ran analyses using a residualized ERN score in the models. The results were identical, with the exception of the association between maternal and offspring ERN, which was not significant (p = .10). Please see Supplemental Material for the full results.
Figure 1.
The topographic maps of neural activity (error minus correct) and response-locked event-related potential (ERP) waveforms at FCz 0 to 100 ms after the response for mothers. The raw waveforms for error and correct responses and the error minus correct difference score (ERN; dotted line) are shown in the graph.
Figure 2.
The topographic maps of neural activity (error minus correct) and response-locked event-related potential (ERP) waveforms at FCz 0 to 100 ms after the response for offspring. The raw waveforms for error and correct responses and the error minus correct difference score (ERN; dotted line) are shown in the graph.
Results
Tables 1 and 2 show descriptive statistics and correlations among the study variables. Missing data ranged from 1.7% to 32.5% across the variables due to equipment failure, too few or too many errors on the Flanker Task, insufficient useable EEG segments, and incomplete surveys (See Supplemental Material). To evaluate whether data were missing completely at random (MCAR), we examined the pattern of missingness using Little MCAR’s test (49,50). Results did not support rejecting the null hypothesis (χ2 = 49.29, df = 95, p = 1.0), suggesting data were MCAR. We utilized the missing data estimation function in AMOS (e.g., full information maximum likelihood; 51) and included the full sample in the final analyses. This method is appropriate when the data are MCAR and the amount of missing data is below 50% (51). We inspected the data for outliers. There were outliers (> 2.5 SDs from their respective means) on child ERN (n = 3), mother ERN (n = 1), and maternal internalizing symptoms (n = 3). We winsorized values to ≤ 2.5 SDs from the means for each variable.
Table 1.
Descriptive statistics for primary variables in the analysis.
| Variable | Mean | SD | Range |
|---|---|---|---|
| Maternal FCz Correct | 6.20 | 5.04 | −5.71 – 20.18 |
| Maternal FCz Error | 1.22 | 6.94 | −18.51 – 24.35 |
| Maternal ERN (error minus correct) | −4.98 | 7.02 | −22.03 – 14.54 |
| Maternal Accuracy (% Correct) | 86.87 | 18.87 | 3.63 – .98.18 |
| Maternal Total Errors | 43.33 | 62.28 | 6 – 318 |
| Maternal Average Reaction Time | 393.69 | 74.02 | 289.84 – 643.28 |
| Maternal Negative Parenting | .00 | .81 | −1.36 – 1.77 |
| Maternal Internalizing Symptoms | .02 | .94 | 0 – 34 |
| Offspring FCz Correct | 6.72 | 6.42 | −5.05 – 26.35 |
| Offspring FCz Error | 1.84 | 7.07 | −15.86 – 22.88 |
| Offspring ERN (error minus correct) | −4.88 | 5.74 | −21.56 – 10.51 |
| Offspring Accuracy (% Correct) | 72.35 | 21. 90 | 8.79 – 97.88 |
| Offspring Total Errors | 89.92 | 71.27 | 7 – 301 |
| Offspring Average Reaction Time | 394.48 | 104.85 | 265.78 – 765.12 |
| Offspring Internalizing Symptoms | 5.02 | 4.05 | 0 – 15 |
Table 2.
Bivariate correlations between variables in the analysis.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Offspring Age | -- | ||||||
| 2. Female | .28** | -- | |||||
| 3. Maternal IP History | −.17 | −.08 | -- | ||||
| 4. Maternal ERN | −.03 | .21 | −.24* | -- | |||
| 5. Offspring ERN | −.27** | −.03 | .06 | .28* | --- | ||
| 6. Maternal Negative Parenting | −.08 | .06 | .18† | −.24* | −.13 | -- | |
| 7. Maternal Internalizing Symptoms | −.14 | −.16† | .49** | −.30** | .13 | .25* | -- |
| 8. Offspring Internalizing Symptoms | .12 | .19* | .17† | −.002 | −.28** | .31** | .02 |
Note. IP = Internalizing Psychopathology. ERN = Error-Related Negativity.
p < .10
p < .05
p < .01
Path analyses were performed within a structural equation modeling framework to test the hypothesized model using AMOS, Version 27.0 (SPSS Inc., Chicago, 2007). We calculated three model fit indices. The chi-square statistic tests the null hypothesis that the overidentified model fits the data as well as the fully saturated model. A nonsignificant chi-square (p > .05) indicates that the fit between the specified model and the data is not significantly worse than the fit between the saturated model and the data (52). We also evaluated model fit by computing the comparative fit index (CFI; 53), which is a goodness of fit measure that compares the tested model to the fit of the independence model. CFI values above .90 suggest acceptable fit (53). We examined the root-mean-square error of approximation (RMSEA; 54), which is an absolute measure of fit based on the non-centrality parameter. Values less than .08 suggest acceptable fit (54).
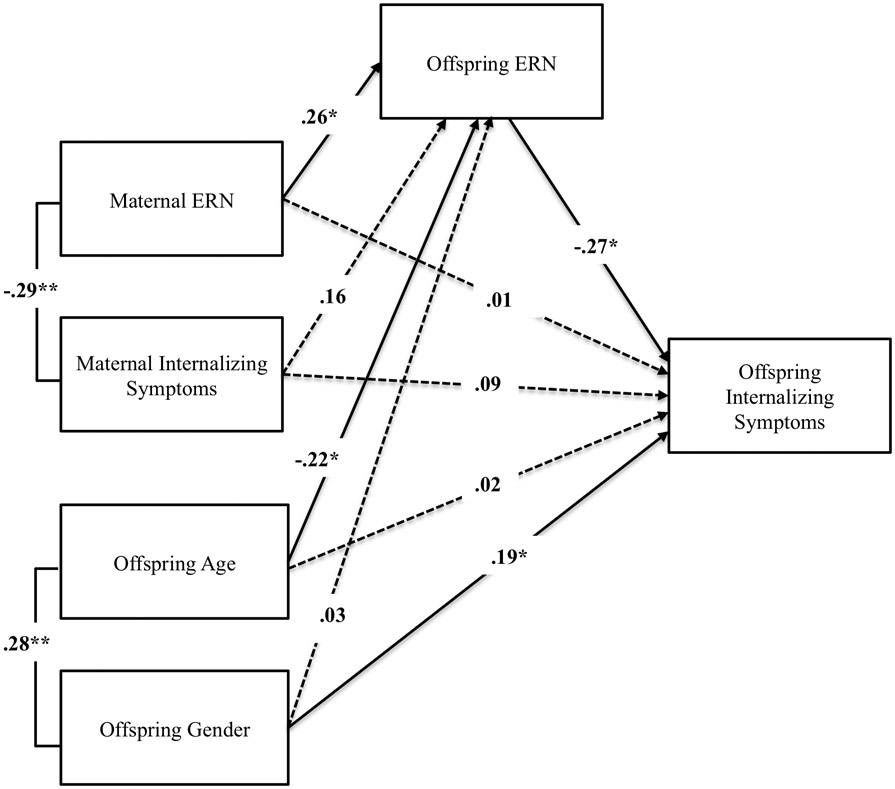
First, we tested associations among maternal ERN, offspring ERN, and offspring internalizing symptoms while adjusting for child age, gender, and maternal internalizing symptoms. In the first model, we did not include the direct path from offspring ERN to offspring internalizing symptoms. The model fit the data well (χ2 (1, N = 117) = .01, p = .93, RMSEA = 0, 90% CI [.08, .94], CFI = 1.0). Results revealed that enhanced maternal ERN (i.e., greater error sensitivity) was significantly associated with heightened offspring ERN (β = .26, SE = .10, p = .04). Maternal ERN (β = −.06, SE = .07, p = .61) and maternal internalizing symptoms (β = .04, SE = .43, p = .66) were not significantly associated with offspring internalizing symptoms. We then included the direct path from offspring ERN to offspring internalizing symptoms, and it was significant. Enhanced offspring ERN (e.g., more negative) was significantly associated with greater internalizing symptoms in offspring (β = −.27, SE = .08, p = .01; Figure 2). We also observed a significant correlation between maternal ERN and maternal internalizing symptoms (r = − .29, p = .01), mirroring the linkage between offspring ERN and offspring internalizing symptoms. Current consensus indicates that a significant direct effect is not required for testing an indirect pathway (e.g., 55,56) Thus, we proceeded to test the significance of the indirect effect of maternal ERN on offspring internalizing symptoms via offspring ERN, which was evaluated with the PRODCLIN program (57) via the RMediation web applet (58). The PRODCLIN program computes the 95% confidence interval for the indirect effect using the distribution of the product of the coefficients method. The results revealed that maternal ERN was indirectly linked with offspring IP symptoms via offspring ERN (z = −.04, SE = .03, 95% CI [−.10, 0]).
Next, we explored the indirect effect of maternal ERN on offspring internalizing symptoms via maternal negative parenting styles while adjusting for child age, gender, and maternal internalizing symptoms. The model fit the data well (χ2 (1, N = 117) = .00, p = .99, RMSEA = 0, 90% CI [.00, .99], CFI = 1.0). Enhanced maternal ERN (i.e., greater error sensitivity) was significantly associated with greater negative parenting styles (β = −.25, SE = .01, p = .03). Maternal internalizing symptoms were also positively linked with greater negative parenting styles (β = .20, SE = .09, p = .04). Next, we included the direct path from maternal negative parenting to offspring internalizing symptoms. Greater negative parenting styles were significantly linked with greater offspring internalizing symptoms (β = .33, SE = .50, p < .01; Figure 3). We examined the significance of the indirect effect of maternal ERN on offspring internalizing symptoms via maternal negative parenting. The indirect effect was significant (z = −.05, SE = .03, 95% CI [−.11, −.003]). We also detected an indirect effect of maternal internalizing symptoms via maternal negative parenting styles on offspring internalizing symptoms (z = .29, SE = .17; 95% CI [.01, .67]).
Figure 3.
Path model of indirect effect of maternal ERN on offspring internalizing symptoms via offspring ERN. Solid lines denote significant effects and dashed lines denote non-significant effects (p > .05). For ease of interpretability, non-significant correlations between predictor and covariate variables are not depicted. ERN values are scored such that negative values = enhanced ERN amplitude (greater error sensitivity). ERN = Error-Related Negativity. *p < .05, **p ≤ .01.
Lastly, we conducted a multi-group analysis for each indirect effect path model to determine if paths significantly differed across the groups of mothers with and without IP histories. Our multi-group models showed that when we compared the chi-square values between the freely estimated model and the model where paths were constrained to equality across maternal IP history groups, model fit did not significantly differ between the respective comparison models (Offspring ERN Model: χdiff2 = 9.55, Δdf = 18, p = .94; Maternal Negative Parenting Model: Xdiff2 = 12,26, Δdf = 18, p = .83), suggesting that maternal IP history did not significantly moderate the effects. However, when we examined the path estimates within each group (mothers with IP histories and mothers without IP histories), the significant associations and indirect effects detected in the full sample model only remained statistically significant in the group of mothers with IP histories. In Supplemental Material we also describe the results of the analyses where we examined anxiety and depression symptoms separately. Findings indicated that effects were not driven by anxiety versus depression symptoms.
Discussion
Consistent with research demonstrating familial aggregation and heritability of ERN (e.g., 28-31), we found that maternal and offspring ERN were significantly associated. Furthermore, maternal ERN was indirectly linked with greater offspring internalizing symptoms through its significant association with offspring ERN. Results also revealed linkages between maternal ERN and their own internalizing symptoms, mirroring the offspring ERN and internalizing symptom association. Findings indicate that ERN may demonstrate heritable intergenerational transmission patterns between mothers and their biological children, which may increase risk for internalizing symptoms in their offspring.
Although the functional significance of the ERN is still debated (13,14), the present findings may be interpreted within the context of both the error detection and defensive reactivity ERN frameworks. Based on the error detection framework, one interpretation could be that overactive monitoring system predispositions toward preoccupation and over-concern with behavioral performance contributes to behavioral symptoms associated with IPs (avoidance, withdrawal; 12,13). From the defensive reactivity model (14), which emphasizes the affective and physiological responses to error, individuals who are biologically predisposed to perceive errors as abnormally aversive and threatening could experience a significant amount of emotional distress and negative affect on a daily basis, which over time contributes to the clinical manifestation of IPs (17, 59-60). Given the cross-sectional study design, these hypotheses are speculative and not mutually exclusive. Longitudinal work is needed to examine individual differences in ERN among offspring at risk for IPs due to maternal IP histories and enhanced ERN patterns. Assessing ERN in early childhood through adulthood and testing causal pathways and predictive associations between youth ERN and the development of IPs could delineate whether ERN is a heritable trait that increases risk for IPs across the lifespan. In addition, experimental randomized control trials could be used to determine whether interventions can directly modify the ERN in at-risk offspring if delivered early in development, prior to IP onset when the ERN might be more malleable, or if individual differences in ERN predict who benefits the most from preventive interventions.
These findings also suggest that enhanced maternal ERN is associated with greater maternal negative parenting styles, defined as parents being insensitive to their child’s needs and emotional states, disengaged, and critical of their children, which in turn was associated with greater offspring internalizing symptoms. Linkages among maternal internalizing symptoms, maternal negative parenting, and offspring internalizing symptoms have been well established (e.g., 33). While there is clear evidence that maternal IPs are associated with more negative parenting styles (32, 61, 62), less work has examined how neurobiological risk factors, such as maternal neural vulnerabilities associated with IPs, may increase risk for problematic parenting styles. Our findings suggest that ERN may be a risk biomarker for negative parenting styles, which could also explain why mothers with IP histories continue to exhibit negative parenting behaviors even after IPs have remitted (62). Drawing upon the defensive reactivity framework of the ERN (14) and psychobiological models of parenting (41), one tentative hypothesis is that mothers who experience errors and mistakes as more aversive and internally threatening could have a tendency to be more critical of their offspring’s mistakes and may be more prone to disengage and withdraw from their children. Future longitudinal studies are needed to provide a greater understanding of whether maternal ERN is a stable versus state dependent neural vulnerability factor for negative parenting styles and how it coincides with maternal and offspring internalizing symptom trajectories
Our multi-group analyses indicated that maternal IP history did not moderate pathways. However, the direct and indirect effects of maternal ERN on youth internalizing symptoms via offspring ERN and maternal negative parenting styles were only significant in the group of mothers with histories of IPs. It is possible that we did not have the statistical power to detect significant differences between the parameter estimates in our multi-group analyses. Future studies should test a multi-group model with larger groups of mothers with and without IP histories, in addition to examining if specific IP diagnoses moderate pathways, which we were underpowered to do in the current study.
There were some study limitations that are important to consider when interpreting the results. We examined indirect effects cross-sectionally; therefore, causal interpretations cannot be inferred. Although these findings provide a blueprint for a potential novel model of youth IP risk, longitudinal and experimental randomized control trials are required for determining temporality and causality. The study only included mothers, while paternal neural vulnerabilities and parenting practices may also influence offspring internalizing symptoms. Parenting was only assessed with subjective maternal reports of parenting styles. Future work should integrate more objective parenting measures and consider other aspects of parenting to determine specificity in associations among maternal ERN, parenting styles, and offspring IP risk. Next, the YSR has been normed for youth between the ages of 11-18 (63), and there were some youth in our study that were 9 and 10 year olds. However, previous work has provided support that the YSR may be completed by younger children (e.g., 7-10 years old; 64, 65), and additional supplemental analyses in the current study indicated that the internal consistency of the anxious/depressed subscale among the 9-10 year olds was adequate. Fifth, our study focused solely on IP risk among youth; and as previously discussed, the ERN has been more heavily implicated in anxiety versus depression risk (14). Future studies with larger sample sizes would benefit from examining whether findings are specific to anxiety and/or depression versus IP risk more broadly among youth. Lastly, it may be beneficial for future work to examine how maternal ERN relates to chronic family stressors, which in turn could contribute to the development of offspring IPs. We did not have these assessments in our study; nonetheless, examining these additional risk processes could add further nuance in developmental models of IPs.
Despite study limitations, the present findings could have important clinical implications. Although research has shown that the ERN demonstrates trait-like features (15,16), some studies have found that the ERN can be modified via varying treatment modalities, such as attention bias modification (66,67), a computerized intervention designed to directly target error monitoring. One promising mechanistic approach to the prevention of IPs in youth could be to conduct randomized two-generation interventions that target mothers and children at heightened risk for IPs due to neural vulnerabilities, and examine whether modifying ERNs in both mothers and their children simultaneously can improve parenting practices and decrease IP symptomatology with greater potency and precision.
Supplementary Material
Figure 4.

Path model of indirect effect of maternal ERN on offspring internalizing symptoms via maternal negative parenting. Solid lines denote significant effects and dashed lines denote non-significant effects (p > .05). For ease of interpretability, non-significant correlations between predictor and covariate variables are not depicted. ERN values are scored such that negative values = enhanced ERN amplitude (greater error sensitivity). ERN = Error-Related Negativity. *p < .05, **p ≤ .01.
Acknowledgements
This research reported in this publication was supported by awards from the National Institute of Mental Health [K23-MH113793 award] to KLB and F32-HD100075 award from the National Institute of Child Health and Human Development [F32-HD100075] to JHS. Research is also supported by grants from the Klingenstein Third Generation Foundation and Brain and Behavior Research Foundation awarded to KLB and by the National Center for Advancing Translational Science, National Institutes of Health, through Grant UL1TR002003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Klingenstein Third Generation Foundation, and Brain and Behavior Foundation. We are much appreciative of the mothers and children who participated in this project. We also thank the students and staff on the project, including: Kaveh Afshar, Sophia Chervak, Nicholas Defelice, Bailey V. Hamner, Shannon Karich, Gabrielle Munoz, and Jaime Sosa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Jennifer Suor reported no biomedical financial interests or potential conflicts of interest. Alison Calentino reported no biomedical financial interests or potential conflicts of interest. Maria Granros reported no biomedical financial interests or potential conflicts of interest. Katie Burkhouse reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Essau CA, Lewinsohn PM, Olaya B, Seeley JR (2014): Anxiety disorders in adolescents and psychosocial outcomes at age 30. J Affect Disord 163: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pettit JW, Lewinsohn PM, Roberts RE, Seeley JR, Monteith L (2009): The long-term course of depression: Development of an empirical index and identification of early adult outcomes. Psychol Med 39: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garber J, Weersing VR (2010): Comorbidity of anxiety and depression in youth: Implications for treatment and prevention. Clin Psychol 17: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosgrove VE, Rhee SH, Gelhorn HL, Boeldt D, Corley RC, Ehringer MA, et al. (2011): Structure and etiology of co-occurring internalizing and externalizing disorders in adolescents. J Abnorm Child Psychol 39: 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hettema JM, Bourdon JL, Sawyers C, Verhulst B, Brotman MA, Leibenluft E, et al. (2020): Genetic and environmental risk structure of internalizing psychopathology in youth. Depress Anxiety 37: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebowitz ER, Leckman JF, Silverman WK, Feldman R (2016): Cross-generational influences on childhood anxiety disorders: Pathways and mechanisms. J Neural Transm 123: 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, Blumberg SJ (2019): Prevalence and treatment of depression, anxiety, and conduct problems in us children. J Pediatr 206: 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L (1991): Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78: 447–455. [DOI] [PubMed] [Google Scholar]
- 9.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E (1993): A neural system for error detection and compensation. Psychol Sci 4: 385–390. [Google Scholar]
- 10.Debener S, Ullsperger M, Siegel M, Fiehler K, Von Cramon DY, Engel AK (2005): Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25:11730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehaene S, Posner MI, Tucker DM (1994): Localization of a neural system for error detection and compensation. Psychol Sci 5: 303–305. [Google Scholar]
- 12.Riesel A, Endrass T, Kaufmann C, Kathmann N (2011): Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: Evidence from unaffected first-degree relatives. Am J Psychiatry 168 :317–24. [DOI] [PubMed] [Google Scholar]
- 13.Yeung N, Botvinick MM, Cohen JD (2004): The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol Rev 111: 931–959. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg A, Riesel A, Hajcak G (2012): Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motiv Emot 36: 84–100. [Google Scholar]
- 15.Foti D, Kotov R, Hajcak G (2013): Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. J Abnorm Psychol 122: 520–531. [DOI] [PubMed] [Google Scholar]
- 16.Olvet DM, Hajcak G (2009): Reliability of error-related brain activity. Brain Res 1284: 89–99. [DOI] [PubMed] [Google Scholar]
- 17.Meyer A (2017): A biomarker of anxiety in children and adolescents: A review focusing on the error-related negativity (ERN) and anxiety across development. Dev Cogn Neurosci 27: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN (2015): Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J Abnorm Psychol 124: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer A, Nelson B, Perlman G, Klein DN, Kotov R (2018): A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. J Child Psychol Psychiatry 59: 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aarts K, Vanderhasselt M-A, Otte G, Baeken C, Pourtois G (2013): Electrical brain imaging reveals the expression and timing of altered error monitoring functions in major depression. J Abnorm Psychol 122: 939–950. [DOI] [PubMed] [Google Scholar]
- 21.Chiu PH, Deldin PJ (2007): Neural evidence for enhanced error detection in major depressive disorder. AJP 164: 608–616. [DOI] [PubMed] [Google Scholar]
- 22.Ruchsow M, Herrnberger B, Beschoner P, Grön G, Spitzer M, Kiefer M (2006): Error processing in major depressive disorder: Evidence from event-related potentials. JPsychiatr Res 40: 37–46. [DOI] [PubMed] [Google Scholar]
- 23.Ruchsow M, Herrnberger B, Wiesend C, Grön G, Spitzer M, Kiefer M (2004): The effect of erroneous responses on response monitoring in patients with major depressive disorder: A study with event-related potentials. Psychophysiology 41: 833–840. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg A, Kotov R, Proudfit GH (2015): Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J Abnorm Psychol 124: 172–185. [DOI] [PubMed] [Google Scholar]
- 25.Olvet DM, Klein DN, Hajcak G (2010): Depression symptom severity and error-related brain activity. Psychiatry Res 179: 30–37. [DOI] [PubMed] [Google Scholar]
- 26.Hajcak G, McDonald N, Simons RF (2004): Error-related psychophysiology and negative affect. Brain Cogn 56: 189–197. [DOI] [PubMed] [Google Scholar]
- 27.Olvet DM, Hajcak G (2008): The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clin Psychol Rev 28: 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anokhin AP, Golosheykin S, Heath AC (2008): Heritability of frontal brain function related to action monitoring. Psychophysiology 45: 524–534. [DOI] [PubMed] [Google Scholar]
- 29.Burwell SJ, Malone SM, Iacono WG (2016): One-year developmental stability and covariance among oddball, novelty, go/no-go, and flanker event-related potentials in adolescence: A monozygotic twin study. Psychophysiology 53: 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser JS, Fisher M, Hicks BM, Zucker RA, Durbin CE (2018): Feedback-related neurophysiology in children and their parents: Developmental differences, familial transmission, and relationship to error-monitoring. Int J Psychophysiol 132: 338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riesel A, Klawohn J, Grützmann R, Kaufmann C, Heinzel S, Bey K, et al. (2019): Error-related brain activity as a transdiagnostic endophenotype for obsessive-compulsive disorder, anxiety and substance use disorder. Psychol Med 49: 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkhouse KL, Uhrlass DJ, Stone LB, Knopik VS, Gibb BE (2012): Expressed emotion-criticism and risk of depression onset in children. J Clin Child Adolesc Psychol 41: 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman SH, Simon HFM, Shamblaw AL, Kim CY (2020): Parenting as a mediator of associations between depression in mothers and children’s functioning: A systematic review and meta-analysis. Clin Child Fam Psychol Rev 23: 427–460. [DOI] [PubMed] [Google Scholar]
- 34.Brooker RJ, Buss KA (2014): Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Dev Cogn Neurosci 9: 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer A, Proudfit GH, Bufferd SJ, Kujawa AJ, Laptook RS, Torpey DC, Klein DN (2015): Self-reported and observed punitive parenting prospectively predicts increased error-related brain activity in six-year-old children. J Abnorm Child Psychol 43: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer A, Wissemann K (2020): Controlling parenting and perfectionism is associated with an increased error-related negativity (ERN) in young adults. Soc Cogn Affect Neurosci 15: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banica I, Sandre A, Weinberg A (2019): Overprotective/authoritarian maternal parenting is associated with an enhanced error-related negativity (ERN) in emerging adult females. Int J Psychophysiol 137: 12–20. [DOI] [PubMed] [Google Scholar]
- 38.Chong LJ, Mirzadegan IA, Meyer A (2020): The association between parenting and the error-related negativity across childhood and adolescence. Dev Cogn Neurosci 45: 100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer A, Carlton C, Chong LJ, Wissemann K (2019): The presence of a controlling parent is related to an increase in the error-related negativity in 5–7 year-old children. J Abnorm Child Psychol 47 :935–45. [DOI] [PubMed] [Google Scholar]
- 40.Goodman SH (2020): Intergenerational transmission of depression. Annu Rev Clin Psychol 16: 213–238. [DOI] [PubMed] [Google Scholar]
- 41.Barrett J, Fleming AS (2011): Annual Research Review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry 52: 368–397. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Williams JBW, Karg RS, Spitzer RL (2015): Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- 43.Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N (2016): The KSADS-PL DSM-5. Baltimore, MD: Kennedy Krieger Institute. [Google Scholar]
- 44.Achenbach TM, Rescorla LA (2001): Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- 45.Beck AT, Epstein N, Brown G, Steer RA (1988): An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56: 893–897. [DOI] [PubMed] [Google Scholar]
- 46.Beck AT, Steer RA, Brown G (1996): Beck Depression Inventory-II. Psychol Assess 67: 588–597. [Google Scholar]
- 47.Hooley JM, Teasdale JD (1989): Predictors of relapse in unipolar depressives: Expressed emotion, marital distress, and perceived criticism. J Abnorm Psychol 98: 229–235. [DOI] [PubMed] [Google Scholar]
- 48.Parker G, Tupling H, Brown LB (1979): A parental bonding instrument. Br J Med Psychol 52: 1–10. [Google Scholar]
- 49.Little RJA (1988): A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 83: 1198–1202. [Google Scholar]
- 50.Schlomer GL, Bauman S, Card NA (2010): Best practices for missing data management in counseling psychology. J Couns Psychol 57: 1–10. [DOI] [PubMed] [Google Scholar]
- 51.Enders CK (2001): The impact of nonnormality on full information maximum-likelihood estimation for structural equation models with missing data. Psychol Methods 6: 352–370. [PubMed] [Google Scholar]
- 52.Hu L, Bentler PM (1999): Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model 6: 1–55. [Google Scholar]
- 53.Bentler PM (1990): Comparative fit indexes in structural models. Psychol Bull 107: 238–246. [DOI] [PubMed] [Google Scholar]
- 54.Steiger JH, Lind J (1980): Statistically based tests for the number of common factors. Paper presented at the annual meeting of the Psychometric Society, IA. [Google Scholar]
- 55.Hayes AF (2009): Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun Monogr 76: 408–420. [Google Scholar]
- 56.MacKinnon DP, Krull JL, Lockwood CM (2000): Equivalence of the mediation, confounding and suppression effect. Prev Sci 1: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacKinnon DP, Fritz MS, Williams J, Lockwood CM (2007): Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behav Res Methods 39: 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tofighi D, MacKinnon DP (2011): RMediation: An R package for mediation analysis confidence intervals. Behav Res 43: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothbart MK, Derryberry D, Posner MI (1994): A psychobiological approach to the development of temperament. In: Bates JE, Wachs TD,editors. Temperament: Individual Differences at the Interface of Biology and Behavior. Washington, DC: American Psychological Association, pp 83–116. [Google Scholar]
- 60.Wachs TD, Pollitt E, Cueto S, Jacoby E (2004). Structure and cross-contextual stability of neonatal temperament. Infant Behav Dev 27: 382–396. [Google Scholar]
- 61.Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D (2011): Maternal depression and child psychopathology: A meta-analytic review. Clin Child Fam Psychol Rev 14: 1–27. [DOI] [PubMed] [Google Scholar]
- 62.Lovejoy MC, Graczyk PA, O'Hare E, Neuman G (2000): Maternal depression and parenting behavior: A meta-analytic review. Clin Psychol Rev 20:561–92. [DOI] [PubMed] [Google Scholar]
- 63.Breslend NL, Parent J, Forehand R, Peisch V, Compas BE (2019): Children of parents with a history of depression: The impact of a preventive intervention on youth social problems through reductions in internalizing problems. Dev Psychopathol 31: 219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chahal R, Kirshenbaum JS, Ho TC, Mastrovito D, Gotlib IH (2021): Greater age-related changes in white matter morphometry following early life stress: Associations with internalizing problems in adolescence. Dev Cogn Neurosci 47: 100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ebesutani C, Bernstein A, Martinez JI, Chorpita BF, Weisz JR (2011): The youth self report: Applicability and validity across younger and older youths. J Clin Child Adolesc Psychol 40: 338–46. [DOI] [PubMed] [Google Scholar]
- 66.Klawohn J, Hajcak G, Amir N, Kathmann N, Riesel A (2020): Application of attentional bias modification training to modulate hyperactive error-monitoring in OCD. Int J Psychophysiol 156: 79–86. [DOI] [PubMed] [Google Scholar]
- 67.Meyer A, Gibby B, Wissemann K, Klawohn J, Hajcak G, Schmidt NB (2020): A brief, computerized intervention targeting error sensitivity reduces the error-related negativity. Cogn Affect Behav Neurosci 20: 172–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.