Abstract
Background aims
The acute respiratory distress syndrome (ARDS) resulting from coronavirus disease 2019 (COVID-19) is associated with a massive release of inflammatory cytokines and high mortality. Mesenchymal stromal cells (MSCs) have anti-inflammatory properties and have shown activity in treating acute lung injury. Here the authors report a case series of 11 patients with COVID-19-associated ARDS (CARDS) requiring mechanical ventilation who were treated with remestemcel-L, an allogeneic MSC product, under individual patient emergency investigational new drug applications.
Methods
Patients were eligible if they were mechanically ventilated for less than 72 h prior to the first infusion. Patients with pre-existing lung disease requiring supplemental oxygen or severe liver or kidney injury were excluded. Each patient received two infusions of remestemcel-L at a dose of 2 million cells/kg per infusion given 48–120 h apart.
Results
Remestemcel-L infusions were well tolerated in all 11 patients. At the end of the 28-day follow-up period, 10 (91%, 95% confidence interval [CI], 59–100%) patients were extubated, nine (82%, 95% CI, 48–97%) patients remained liberated from mechanical ventilation and were discharged from the intensive care unit and two (18%, 95 CI%, 2–52%) patients died. The median time to extubation was 10 days. Eight (73%, 95% CI, 34–100%) patients were discharged from the hospital. C-reactive protein levels significantly declined within 5 days of MSC infusion.
Conclusions
The authors demonstrate in this case series that remestemcel-L infusions to treat moderate to severe CARDS were safe and well tolerated and resulted in improved clinical outcomes.
Key Words: mesenchymal stromal cells, COVID-19, acute respiratory distress syndrome, Remestemcel-L
Introduction
The novel severe acute respiratory syndrome coronavirus 2 with its associated coronavirus disease 2019 (COVID-19) is a global public health emergency, with over 69 million confirmed cases and 860 386 deaths in the United States [1]. Although most affected patients recover from COVID-19 infection without sequelae, reports in the early days of the pandemic demonstrated that up to 42% of hospitalized patients developed acute respiratory distress syndrome (ARDS) [2], [3], [4], [5]. Patients with COVID-19-associated ARDS (CARDS) have unique features, including relatively high lung compliance in the early phase; however, there may be progression to a more typical presentation of ARDS, with poorly compliant lungs, worsening alveolar infiltrates and high mortality [6]. In a retrospective cohort of 201 Chinese patients with COVID-19, mortality was 66% among those who received mechanical ventilation [3]; however, more recent reports suggest that mortality rate may vary [7]. There are no currently approved therapies for CARDS. The current standard of care includes lung-protective ventilation and conservative fluid management [8]. In the early phase of CARDS, targeting a lower positive end-expiratory pressure and reducing rigorous spontaneous inspiratory efforts may lower the shear forces on the hyperpermeable microvasculature [6]. Given the burden of this pandemic on health care resources, there is an urgent need to improve the morbidity and mortality of patients and to reduce ventilator days and intensive care unit (ICU) length of stay.
Recent publications have identified the “cytokine storm,” a massive inflammatory cytokine release, as an underlying insult leading to lung injury in COVID-19 [9]. The authors reasoned that infusions of allogeneic mesenchymal stromal cells (MSCs), which exert broad, anti-inflammatory effects, may be of benefit in patients with this condition, which is characterized by pathological immune activation and inflammation. MSCs suppress T-cell proliferation in response to alloantigenic and mitogenic challenge, decrease the secretion of inflammatory cytokines such as interferon gamma and tumor necrosis factor alpha (TNF-α) and increase the secretion of anti-inflammatory cytokines such as IL-4 and IL-10 via multiple immune cell subsets [10], [11], [12]. MSCs are also characterized by a hypoimmunogenic phenotype that decreases rejection of these allogeneic cells by the host.
The promising efficacy of MSC infusions has been demonstrated in clinical trials of steroid-refractory acute graft-versus-host disease (GVHD) [13], [14], [15] and in pre-clinical models of acute lung injury [10,16]. MSCs have demonstrated safety in phase 1/2a trials of ARDS [17,18]. A small study in China reported that seven patients with COVID-19 pneumonia who were not mechanically ventilated recovered quickly with improved inflammatory markers after being treated with MSCs [19]. In this study, the authors used remestemcel-L, an investigational, allogeneic, bone marrow-derived, off-the-shelf, cryopreserved MSC product candidate, to treat patients with moderate to severe CARDS requiring mechanical ventilation.
Methods
This study involved a series of consecutive cases of patients with CARDS treated at a single academic center between March 2020 and April 2020. Patients were observed, at a minimum, until death or ICU discharge, whichever came first. Each patient was treated under individual emergency investigational new drug (eIND) applications approved by the United States Food and Drug Administration and the institutional review board of the Icahn School of Medicine at Mount Sinai. Informed consent was obtained from patients or their legally authorized representative. CARDS was defined according to the modified Berlin criteria as the acute onset of hypoxemic respiratory failure manifested by bilateral opacities on chest imaging not fully explained by pleural effusions, lobar collapse or lung nodules [20]. Furthermore, the clinical deterioration could not be fully explained by cardiac failure or fluid overload. The degree of oxygen impairment was characterized by the ratio of partial pressure of oxygen in arterial blood to the fraction of inspired supplemental oxygen (P:F) on a minimum positive end-expiratory pressure ≥5 cm of water. Moderate ARDS was defined as a P:F ratio between 101 mmHg and 200 mmHg, and severe ARDS was defined as a P:F ratio ≤100 mmHg.
Patients were eligible if they had severe acute respiratory syndrome coronavirus 2 infection confirmed by real-time reverse transcription polymerase chain reaction assay on nasopharyngeal sampling with moderate to severe ARDS. The severity of ARDS was determined by the lowest P:F ratio within the first 24 h of intubation.
Additional inclusion criteria were invasive mechanical ventilation for less than 72 h prior to infusion, C-reactive protein (CRP) level (a non-specific marker of inflammation) ≥4.0 mg/dL (or 40 mg/L), baseline Acute Physiology and Chronic Health Evaluation (APACHE) II score (a measure of ICU mortality risk assessed by the worst physiological parameters within 24 h of ICU admission) [21] ≥5 and absence of severe liver injury (alanine and aspartate aminotransferase less than five times the upper limit of normal) or renal dysfunction (serum creatinine <2.0 mg/dL or creatinine clearance ≥30 mL/min) (see supplementary Table 1). Patients were excluded from participation if they were currently receiving extracorporeal membrane oxygenation, had known hypersensitivity to dimethyl sulfoxide or porcine or bovine proteins, had severe baseline pulmonary disease requiring oxygen therapy prior to diagnosis of ARDS due to COVID-19 infection or had any end-stage organ disease.
Cellular therapy product and treatment
Remestemcel-L is an investigational allogeneic cell product composed of ex vivo-expanded MSCs isolated from the bone marrow of adult healthy donors. The cells express mesenchymal lineage markers, including CD73, CD90, CD105 and CD166, and lack expression of hematopoietic cell surface antigens such as CD45 and CD31 [22]. The cells also express low levels of major histocompatibility complex class I, are negative for major histocompatibility complex class II molecules and are negative for co-stimulatory molecules CD40, CD80 and CD86.
MSCs were harvested after five passages and then formulated in a cryopreservation medium composed of Plasma-Lyte A, 10% dimethyl sulfoxide and human serum albumin solution. The cryopreserved remestemcel-L product for administration was stored in the vapor phase of liquid nitrogen (≤–135°C) until use. Each dose was thawed and reconstituted in Plasma-Lyte A immediately prior to administration. No manipulation occurred between thawing and administration.
Each patient received two intravenous remestemcel-L infusions at a dose of 2 × 106 cells/kg per infusion given 48–120 h apart. This dose was chosen based on the safety and efficacy data in steroid-refractory acute GVHD studies, in which this dose was given twice weekly for 4 weeks [13], [14], [15]. Cell viability ranged from 78% to 90%. Patients were pre-medicated with hydrocortisone and diphenhydramine at least 30 min prior to each remestemcel-L infusion as per institutional standard practice for cellular therapy infusions and manufacturer instructions. Patients were monitored for infusion-associated reactions by vital sign assessment 15 min prior to the infusion, every 15 min for the first hour after the start of the infusion and at 120 min.
Data collection
Clinical assessments included baseline APACHE II score, daily Sequential Organ Failure Assessment (SOFA) score (which represents a composite measure of organ failure) [23], P:F ratio, days in the ICU, days on mechanical ventilation and discharge disposition. Laboratory assessments included hematology, chemistry, CRP and ferritin, the latter two representing non-specific markers of inflammation. A pre-infusion baseline was established as the values on the day of intubation or the following 24-h period, not including the day of the first remestemcel-L infusion, if data were missing. No imputations were made for data missing at day 0, day 3 or day 5. Chest radiography was performed at baseline and only as needed thereafter by standard of care protocols of the ICU.
Outcomes
The primary outcome was ICU mortality, and patients were followed, at a minimum, until death or discharge from the ICU. Secondary outcomes included the proportion of patients who were extubated for greater than 48 h, time to extubation after the initial remestemcel-L infusion, total duration of mechanical ventilation, proportion of patients discharged from the ICU and proportion of patients discharged from the hospital.
Statistical analysis
Given the nature of eINDs, where the number of patients is small and a control group is lacking, standard descriptive statistics were used. Categorical variables were reported as proportions with corresponding Clopper–Pearson 95% confidence intervals (CIs) for outcome variables. Continuous, non-normally distributed variables were reported as medians and interquartile ranges (IQRs). Time to extubation was calculated using the Kaplan–Meier method. Changes in physiological parameters were assessed for significance using the Friedman test, with post-hoc analysis with Wilcoxon signed-rank test (comparing day 0 with day 3 and day 5) of parameters that were significantly changed overall from pre-infusion baseline to day 5 post-infusion. The Bonferroni correction method was applied to account for multiple comparisons.
Results
Eleven patients were treated with remestemcel-L at Mount Sinai Hospital per eIND treatment plan and were included in this analysis. All patients were screened within 24 h of intubation, and none demonstrated clinical improvement prior to treatment (see supplementary Table 2). Individual patient characteristics are provided in Table 1 . The median age was 51 years (IQR, 39–60), and patients were followed for a median of 22 days (IQR, 16–30) (see supplementary Table 2). The most frequent comorbid conditions were obesity (73%), diabetes mellitus (27%), hypertension (27%) and asthma (18%), and patients had a median baseline APACHE II score of 14 (IQR, 12–16). The median P:F ratio on day 0 was 120 mmHg (IQR, 103–205). All patients had bilateral opacities on baseline chest radiograph, and one patient had a post-intubation pneumothorax that subsequently improved with chest tube drainage.
Table 1.
Demographic and pre-treatment disease characteristics on ICU admission.
| Patient ID | Sex | Age, years | Coexisting conditions | Body mass index, kg/m2 | APACHE II score | P:F nadir on admission, mmHga |
|---|---|---|---|---|---|---|
| 1 | Male | 60 | Obesity | 32.0 | 14 | 78 |
| 2 | Male | 39 | Obesity | 38.9 | 15 | 132 |
| 3 | Male | 35 | Obesity | 37.7 | 16 | 91 |
| 4 | Male | 60 | Obesity, asthma | 32.9 | 12 | 80 |
| 5 | Male | 51 | Obesity | 34.4 | 13 | 133 |
| 6 | Female | 67 | Obesity, diabetes mellitus, hypertension | 37.8 | 14 | 106 |
| 7 | Male | 48 | Diabetes mellitus | 23.0 | 11 | 127 |
| 8 | Male | 58 | Hypertension, asthma | 25.2 | 14 | 144 |
| 9 | Female | 34 | Obesity | 36.4 | 8 | 134 |
| 10 | Male | 50 | Obesity, diabetes mellitus, hypertension | 35.6 | 16 | 151 |
| 11 | Male | 60 | – | 23.6 | 17 | 71 |
ID, identifier.
Values within 24 h of intubation used for screening.
All patients received vasopressor therapy (norepinephrine and/or vasopressin) before remestemcel-L administration (i.e., before day 0) (see supplementary Table 4). Although the dose of vasopressors fluctuated, the number of patients on vasopressors decreased from six on day 0 to two on day 5 (see supplementary Table 5). In addition, the P:F ratio, which is a measure of hypoxemia, improved from 120 on day 0 to 200 on day 5, and the SOFA score, which is a composite score of total organ failure, improved from 6.0 on day 0 to 3.5 on day 5 (see supplementary Table 5). Of note, none of the patients required renal replacement therapy during follow-up. All patients were treated with hydroxychloroquine, and 45% received tocilizumab (an IL-6 receptor inhibitor), with two (18%) receiving tocilizumab alone and three (27%) receiving tocilizumab and glucocorticoids (see supplementary Table 3). A total of 73% of patients received glucocorticoids alone. Of note, steroids were administered as part of standard pre-medication when administering cellular therapy products and were not given as COVID-19-related therapy. One patient did not receive steroids or tocilizumab, and because of lack of availability at such an early time in the pandemic, none of the patients received anti-viral therapy.
Clinical outcomes
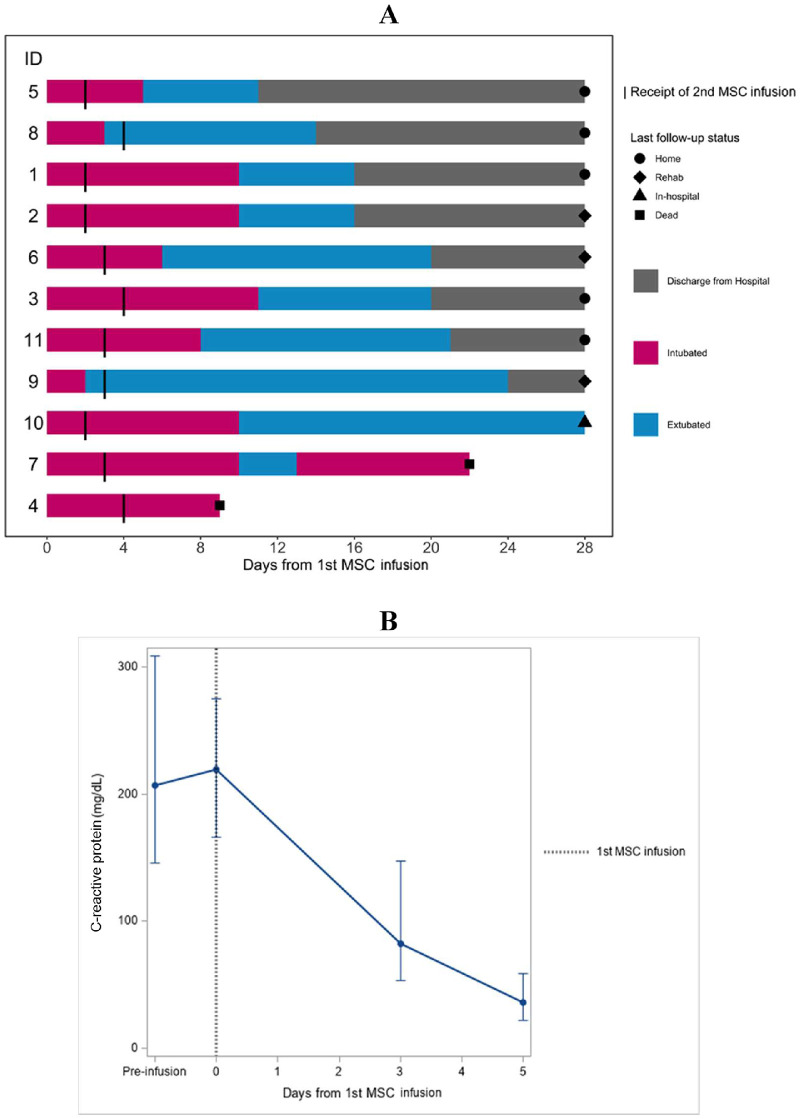
Clinical outcomes are shown in Figure 1 A. All patients were followed, at a minimum, until death or ICU discharge, and ICU mortality was 18% (95% CI, 2–52%). Over the period of observation, 10 (91%, 95% CI, 59–100%) patients were extubated.
Figure 1.
Effects of remestemcel-L infusions on clinical outcomes. (A) Swimmer plot of events according to time from remestemcel-L infusion. Numbers on the left represent individual patient IDs as indicated in Table 1. (B) CRP levels. Median and IQR of CRP were plotted according to time from remestemcel-L infusion. IDs, identifiers. (Color version of figure is available online).
One patient (patient 7) was reintubated because of worsening ARDS and possible pulmonary embolism. The patient was treated empirically with thrombolytic therapy and improved. However, the patient ultimately died at day 23 post-infusion of multi-organ failure. At the end of the study period, nine (82%, 95% CI, 48–97) patients were liberated from mechanical ventilation, nine (82%, 95% CI, 48–97) patients were discharged from the ICU and eight (73%, 95% CI, 34–100) patients were discharged from the hospital. Two remained hospitalized in stable condition on the medical floor. The median time to extubation from first remestemcel-L infusion was 10 days (IQR, 3–10). The median duration of mechanical ventilation was 12 days (IQR, 7–12) (Table 2 ).
Table 2.
Outcomes and adverse events.
| Primary outcome | |
|---|---|
| ICU mortality, n (%, 95% CI) | 2 (18, 2–52) |
| Secondary outcomes | |
| Successful extubation, n (%, 95% CI) | 10 (91, 59–100) |
| Time to successful extubation, days, median (IQR) | 10 (3–10) |
| Duration of mechanical ventilation, days, median (IQR) | 12 (7–12) |
| ICU length of stay, days, median (IQR) | 13 (10–14) |
| Proportion discharged from ICU, n (%, 95% CI) | 9 (82, 48–97) |
| Proportion discharged from hospital, n (%, 95% CI) | 8 (73, 34–100) |
| Discharge disposition | |
| Home, n (%, 95% CI) | 5 (45, 13–86) |
| Rehabilitation facility, n (%, 95% CI) | 3 (27, 6–61) |
| Long-term acute care facility, n (%) | 0 (0) |
| Adverse events | |
| Possible thromboembolic disease, n (%, 95% CI) | 1 (9, 0–45) |
| Secondary infections, n (%, 95% CI) | 5 (45, 21–96) |
Physiological data
There was an improvement in oxygenation, as assessed by the daily P:F ratio (median, +78 mmHg, P = 0.002), from day 0 to day 3 (see supplementary Figure 1; see supplementary Tables 4, 6). CRP levels decreased from day 0 to day 5 (median, 36.5–219.2 mg/L, P = 0.002) (Figure 1B; also see supplementary Tables 4, 6).
There was no change in fever curve or white blood cell count (see supplementary Table 4). Ferritin levels and SOFA scores did not change significantly from day 0 to day 3 or day 5. One patient (patient 2) had similar improvement in CRP following remestemcel-L infusion without exposure to tocilizumab or glucocorticoids (see supplementary Figure 2).
In addition to CRP and ferritin (Figure 1; also see supplementary Table 4), inflammatory cytokines such as IL-6, IL-1β, IL-8 and TNF-α were also measured. Serum levels of IL-1β were within the normal range in all evaluated patients at all times, and serum levels of IL-6, IL-8 and TNF-α are summarized in supplementary Figure 3 and supplementary Table 7. However, because of the nature of the eIND, cytokine levels were not systematically measured. As a result, a statistical analysis could not be performed to draw a meaningful conclusion.
Safety
There were no infusion-related adverse events. During the 28-day follow-up period, five patients had secondary infections and two patients died (Table 2). No adverse events were attributed to remestemcel-L infusions.
Discussion
Here the authors have reported the outcomes in a series of 11 critically ill patients with moderate to severe CARDS who were treated with remestemcel-L, an investigational, allogeneic, bone marrow-derived, off-the-shelf MSC product candidate. All but one patient were extubated shortly after remestemcel-L infusion, and the authors observed an 82% rate of discharge from the ICU and a 28-day mortality rate of 18%. None of the authors’ patients required renal replacement therapy. The authors had an adequate period to observe that recovery was sustained in survivors. Although markers of inflammation were not comprehensively analyzed, the authors did observe a significant and sustained decrease in the inflammatory marker CRP coincident with clinical improvement. Although the authors cannot conclude that these improvements were the direct result of treatment with remestemcel-L, the results are consistent with a previous report of recovery in seven patients with mild to severe hypoxemia treated with a different MSC product in China (albeit none were mechanically ventilated at the time of treatment) [19].
The pathophysiology of ARDS is marked by increased inflammation leading to destruction of the alveolar capillary barrier, increased protein leakage and hyaline membrane formation in the alveoli with resultant oncotic pulmonary edema. Ex vivo-generated MSCs have demonstrated biological efficacy in treating acute lung injury in pre-clinical studies [24,25]. In animal models, bone marrow-derived MSCs have been shown to migrate to the lungs, where they remain for several weeks without engraftment, during which time they release anti-inflammatory paracrine factors such as IL-1 receptor antagonist and prostaglandin E2, improve alveolar fluid clearance and reduce bacterial overgrowth by releasing anti-microbial peptides [26], [27], [28], [29], [30]. These pre-clinical data are consistent with the observed decrease in CRP and TNF-α levels and the decreased number of pro-inflammatory CD4+ and CD8+ T cells and natural killer cells in the Chinese patients treated with MSCs for CARDS [19].
The utility of MSCs in ARDS is an active area of inquiry. A phase 1/2 trial of MSCs in moderate to severe ARDS in 30 participants randomized to a single infusion of 900 million cells or placebo demonstrated that MSCs were well tolerated, with only a single adverse reaction [31]. Although it was not powered to determine improvement in mortality, favorable trends were noted in the subgroup of patients with P:F ratio <150 mmHg, and a larger randomized controlled trial is underway (NCT04367077). Similarly, a phase 2a trial investigating a single infusion of 10 million MSCs/kg in patients with ARDS met its primary endpoint of safety but showed no improvement in mortality [18]. However, this study was limited by low cell viability (range, 36–85%), and efficacy is being investigated in a phase 2b trial (NCT03818854). It is possible that the efficacy of treatment with MSCs may vary in ARDS resulting from different etiologies. Further clinical and laboratory phenotyping of CARDS will be needed to understand how this novel disease differs from other causes of lung injury.
Remestemcel-L has been studied across a wide variety of demographic, exposure and disease states, and there is similar safety in both pediatric and adult patients. Reports on more than 500 adult and pediatric patients with steroid-refractory acute GVHD and adult patients with moderate to severe chronic obstructive pulmonary disease support a favorable safety profile for remestemcel-L [[13], [14], [15],32]. An updated systematic review and meta-analysis on the safety of MSCs by Thompson et al. [32] reported no significant safety signals, which further supports the favorable safety profile of MSC administration.
Remestemcel-L infusions were well tolerated in this case series. Thromboembolism is a serious complication in COVID-19 patients [33]. The possible pulmonary embolism reported in a single patient in this case series was an expected event given the high incidence of such events in CARDS patients generally. Although a hypercoagulable state may theoretically occur in relation to MSC products that are either placenta- or adipose-derived, because of their high level of tissue factor expression, bone marrow-derived MSCs express much lower levels of tissue factor and have not been reported to portend risks for hypercoagulability [34].
As a case series, the authors’ study has important limitations. First, the small size and lack of an untreated control group prohibit any inference about efficacy. Second, the authors’ patients were selected based on those with severe lung injury who had been intubated for less than 72 h, and the authors excluded patients who had pre-existing severe lung disease or concomitant significant kidney and liver injury or who had been intubated for more than 72 h. As a result, the authors cannot generalize the results to patients who were at greater risk of death due to comorbid conditions or in the later phase of CARDS. Third, the authors’ patients also received several concurrent therapies—namely, hydroxychloroquine, tocilizumab and corticosteroids. Prior administration of tocilizumab or steroids may have facilitated the anti-inflammatory effect of remestemcel-L, although the authors note anecdotally that one patient recovered without receiving either agent. It is important to note that none of the patients in this series received remdesivir, a nucleotide analog that inhibits viral RNA polymerase, which has been shown to shorten the time to recovery in patients with COVID-19 [35]. Finally, clinical-grade MSCs can be produced from different cell sources and under a variety of conditions. It is not yet clear how these different production methods affect biological activity in vivo. Thus, the outcomes the authors observed with remestemcel-L may not be reproduced with different MSCs.
Nonetheless, the results reported here demonstrate that administration of remestemcel-L was well tolerated and resulted in improved clinical outcomes, including liberation from mechanical ventilation and discharge from the ICU and/or hospital. Although this eIND case series of 11 critically ill adult patients was not structured to assess efficacy, it demonstrated promising pilot data in support of a larger randomized controlled trial in CARDS (NCT 04371393). During preparation of this article, the randomized controlled trial was halted by the trial's Data and Safety Monitoring Board after the third interim analysis because the primary endpoint of 30-day all-cause mortality would not be attained with the planned enrollment schedule. Evaluation of secondary endpoints and subgroup analysis are ongoing. Importantly, the Data and Safety Monitoring Board noted no safety concerns.
Funding
No funding was received.
Declaration of Competing Interest
JL reports grant support and personal fees from Mesoblast Ltd and personal fees from bluebird bio and Novartis. SI, FG and EB are employees of Mesoblast Ltd. SI and FG have patents pending to Mesoblast Ltd.
Author Contributions
Conception and design of the study: S-AWB, CI-R, FG, SI, JL and KO. Acquisition of data: S-AWB, CI-R, AA, AA, EB, TD, FG, SI, JL, J-YL, AM, JEL and KO. Analysis and interpretation of data: S-AWB, CI-R, AA, AA, EB, TD, FG, SI, JL, J-YL, AM, JEL and KO. Drafting or revising the manuscript: S-AWB, CI-R, FG, SI, JL and KO. All authors have approved the final article.
Acknowledgments
Remestemcel-L was provided by Mesoblast Ltd. The authors would like to thank nurse practitioners Clare Kearney, Keiko Wahlin, Ariffa Santana, Stefon Deallie, Christopher Wilkinson and Mary McLelland for administering the infusions. The authors would also like to thank Svitlana Shpontak and Yelena Sinitsyn from the Cellular Therapy Laboratory at Mount Sinai Hospital, New York, New York, USA.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jcyt.2022.03.006.
Appendix. Supplementary materials
References
- 1.Centers for Disease Control and Prevention. COVID data tracker. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#2
- 2.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;323(13):1239–1242. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 7.Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT, Jabaley CS, Carpenter D, Kaplow R, Hernandez-Romieu AC, et al. ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019. Crit Care Med. 2020;48(9):e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 9.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay MA, Pati S, Lee JW. Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells. 2017;35(2):316–324. doi: 10.1002/stem.2551. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 12.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 13.Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, Waller EK, Burke E, Skerrett D, Shpall E, et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020;26(5):835–844. doi: 10.1016/j.bbmt.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, Nemecek E, Neudorf S, Prasad V, Prockop S, et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020;26(5):845–854. doi: 10.1016/j.bbmt.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtzberg J, Prockop S, Chaudhury S, Horn B, Nemecek E, Prasad V, Satwani P, Teira P, Hayes J, Burke E. Study 275: Updated Expanded Access Program for Remestemcel-L in Steroid-Refractory Acute Graft-versus-Host Disease in Children. Biol Blood Marrow Transplant. 2020;26(5):845–854. doi: 10.1016/j.bbmt.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, Nicholls JM, Fang X, Guan Y, Lee JW, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A. 2016;113(13):3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. The Lancet Respiratory medicine. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 22.Locatelli F, Algeri M, Trevisan V, Bertaina A. Remestemcel-L for the treatment of graft versus host disease. Expert Rev Clin Immunol. 2017;13(1):43–56. doi: 10.1080/1744666X.2016.1208086. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 24.Cruz FF, Weiss DJ, Rocco PR. Prospects and progress in cell therapy for acute respiratory distress syndrome. Expert Opin Biol Ther. 2016;16(11):1353–1360. doi: 10.1080/14712598.2016.1218845. [DOI] [PubMed] [Google Scholar]
- 25.Boyle AJ, O'Kane CM, McAuley DF. Where next for cell-based therapy in ARDS. Thorax. 2019;74(1):13–15. doi: 10.1136/thoraxjnl-2018-212272. [DOI] [PubMed] [Google Scholar]
- 26.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, et al. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol. 2011;45(4):809–816. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33(4):328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33(4):335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19(9):1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellingan G, Jacono F, Bannard-Smith G, Brealey D, Meyer N, Thickett D, Young D, Bentley A, McVerry B, Wunderink R.G., et al. Primary Analysis of a Phase 1/2 Study to Assess MultiStem® Cell Therapy, a Regenerative Advanced Therapy Medicinal Product (ATMP), in Acute Respiratory Distress Syndrome (MUST-ARDS) American Journal of Respiratory and Critical Care Medicine. 2019;199:A7353. [Google Scholar]
- 32.Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, Sullivan KJ, Doxtator E, Lalu M, English SW, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;19 doi: 10.1016/j.eclinm.2019.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava R, Parveen R, Mishra P, Saha N, Bajpai R, Agarwal NB. Venous thromboembolism is linked to severity of disease in COVID-19 patients: A systematic literature review and exploratory meta-analysis. Int J Clin Pract. 2021:e14910. doi: 10.1111/ijcp.14910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moll G, Hoogduijn MJ, Ankrum JA. Editorial: Safety, Efficacy and Mechanisms of Action of Mesenchymal Stem Cell Therapies. Front Immunol. 2020;11:243. doi: 10.3389/fimmu.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JS, Ferreira D, Denholm JT, Tong SY. Clinical trials for the prevention and treatment of COVID-19: current state of play. Med J Aust. 2020;213(2):86–93. doi: 10.5694/mja2.50673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.