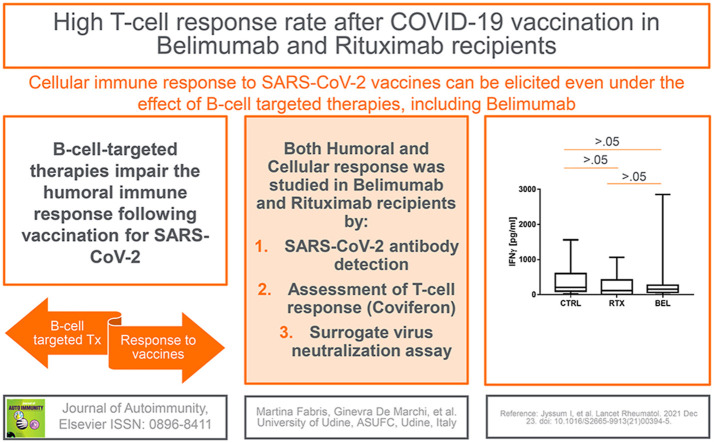
Abstract
Objective
To evaluate B-cell- and T-cell-mediated immune response to SARS-CoV-2 mRNA vaccination in patients with complex or rare systemic autoimmune diseases previously been treated with or under continuous treatment with B-cell-targeted therapies including rituximab (RTX) and belimumab (BEL).
Materials and methods
Twenty-eight consecutive patients receiving RTX (n = 11) or BEL (n = 17) treatment and 13 age-/sex-matched controls (non-rheumatic healthcare personnel) were recruited. None of the patients had detectable anti-SARS-CoV-2 antibodies caused by prior exposure to the virus. All the patients and controls received mRNA vaccines and were tested three to four weeks after completion of vaccination. In all the RTX patients, vaccination was started within 5 months from the last infusion, and B-cell depletion was confirmed in all but one of them. Total anti-SARS-CoV-2 RBD antibodies were analyzed using a diagnostic assay, while T-cell response was evaluated using the interferon-gamma release assay (IGRA). Further, SARS-CoV-2 pseudoviruses were employed to verify the strain-specific neutralizing capacity of the antibodies.
Results
Detectable anti-SARS-CoV-2 antibodies were documented in 1 out of the 11 RTX patients and 16 of the 17 BEL patients. The median concentration in the RTX and BEL patients was significantly lower than that in the controls (39.6 AU/ml vs. 1133 AU/ml, p = 0.002). The result of IGRA was positive in 8 of the 11 (72.7%) RTX patients and 16 of the 17 (94.1%) BEL patients, and interferon release in both the RTX and BEL patients was comparable to that in the control participants.
Conclusion
B-cell-targeted therapies do not preclude SARS-CoV-2 vaccination, since virus-specific cellular immunity can be induced even in the absence of circulating B cells. An important finding was that lupus patients treated with BEL developed immune responses to SARS-CoV-2; this indicates retention of the immunogenicity of the COVID-19 vaccine.
Keywords: COVID-19, Rituximab, Belimumab, B cell, T cell, Vaccine
Graphical abstract
1. Introduction
As part of the worldwide effort to control the SARS-CoV-2 pandemic, an aggressive vaccination program has been implemented, and it is one of the most effective public health measures to deal with this emergency. One of the types of vaccines being administered are novel, mRNA-based vaccines, which have shown an excellent safety profile and have been effective in reducing the incidence of severe clinical manifestations related to the acute respiratory syndrome caused by SARS-CoV-2, i.e., COVID-19 [1,2]. Patients with rheumatic diseases were among the first to be administered the vaccine, as they have systemic conditions that make them more prone to contracting the infection and to potentially developing a severe outcome [3,4]. However, a major concern for these patients is the risk of a reduced response to vaccination as a result of the immunosuppressive therapies they are administered. In order to address their concerns, the ACR 2021 guidelines have suggested precise timings for the suspension of immunosuppressive therapies before vaccination based on the type of drug used [5]. Both humoral and cell-mediated immunity play a key role in the vaccine-induced protective response, but a growing body of literature shows that patients receiving immunosuppressive agents, particularly B-cell-targeted therapies, do not develop an adequate humoral immune response following vaccination [6]. Published data demonstrate that especially in rituximab (RTX)-treated patients, total B-cell depletion impairs the humoral response, and these patients exhibit a negative serological response to vaccination [7]. With regard to the effect of belimumab (BEL) on the immune response to vaccination, however, there is a very little information available. BEL is an add-on therapy for systemic lupus erythematosus (SLE) that inhibits the binding of B-lymphocyte stimulator to its receptor on B lymphocytes and interferes with their survival and activation. To date, it has been observed that, unlike RTX, this drug might not affect humoral response [8].
With regard to the T-cell response to vaccination, several studies have reported that it is conserved in RTX-exposed patients [[9], [10], [11]], but very little information is available about its effectiveness when it is administered early after RTX treatment in patients for whom the therapeutic intervention is non-deferrable for clinical reasons (such as induction or maintenance of treatment for severe diseases). With regard to BEL-exposed patients, more information is required about the T-cell response to vaccination [12]. Based on these gaps in the literature, the main aim of this study was to evaluate both B-cell- and T-cell-mediated immune responses to mRNA vaccination in patients with complex or rare systemic autoimmune diseases, such as vasculitis or systemic connective tissue diseases, who are on treatment with one of two B-cell-targeted therapies—RTX and BEL. We also investigated the possible influence of other concomitant immunosuppressive therapies and assessed the in vitro effective neutralizing capacity of anti-SARS-CoV-2 antibodies elicited by vaccination in patients under treatment with BEL.
2. Materials and methods
2.1. Patients
Consecutive patients with autoimmune inflammatory disease who were receiving B-cell-targeted therapy with RTX or BEL at a single tertiary rheumatology reference center for rare autoimmune systemic disease were recruited between March and July 2021. With regard to the recruitment of patients under RTX treatment, this study included only those who had received the first dose of vaccine within 5 months from the last RTX infusion. Importantly, the RTX infusion needed to have been administered for active disease that had required a prompt therapeutic intervention which could not be deferred. With regard to the patients under BEL treatment, the study included only SLE patients in remission or with low disease activity according to the Lupus Low Disease Activity State (LLDAS) [13], without the need for any changes in the drug schedule due to the vaccination program. Age- and sex-matched volunteers were included in a control group and were selected from among healthcare personnel of the analysis laboratory who had received the BNT162b2 vaccination, did not have any rheumatic conditions, and were not under treatment with immunosuppressive drugs. All patients received the BNT162b2 mRNA vaccine injection on days 0 and 21 or the mRNA-1273 vaccine on days 0 and 28 according to the local guidelines at the time. Participants with a detectable response against the SARS-CoV-2 nucleocapsid at any time due to previous exposure to SARS-CoV-2 were excluded.
Blood samples were collected three to four weeks after complete vaccination. Peripheral B-cell count analysis was performed with flow cytometry (normal level of CD19+ B lymphocytes = 105–560 cells/μl; percentage = 7–14%).
The study was approved by the local Ethics Committee (approval no. CEUR-2019-OS-233), and all the patients and volunteers provided their informed consent.
2.2. SARS-CoV-2 antibody detection
Anti-SARS-CoV-2 antibodies were measured using two commercially available serological tests: iFlash-SARS-CoV-2 (Yhlo, distributed in Italy by Pantec), a paramagnetic particle-based chemiluminescence immunoassay, was used to assess anti-nucleocapsid (N) and anti-spike (S) IgG and IgM antibodies. This assay cannot recognize the SARS-CoV-2 protein RBD and was used to detect previous exposure to SARS-CoV-2 (cut-off, >10 U/ml). The second test used was Elecsys anti-SARS-CoV-2 electrochemiluminescence immunoassay analyzer (ECLIA) by Roche Diagnostics (Mannheim, Germany). This is an immunoassay for quantitative determination of total (IgG/IgM/IgA) antibodies against SARS-CoV-2 spike 1 protein RBD, and is used to assess the serological response to vaccination (cut-off, >0.79 U/ml).
2.3. Assessment of T-cell response (Coviferon)
The T-cell-mediated cellular immune response to SARS-CoV-2, called also Coviferon, was evaluated using the interferon-gamma release assay (IGRA, Euroimmun). Freshly collected lithium-heparin blood (0.5 ml) was added to a stimulation tube coated with a peptide pool based on the S1 domain of the SARS-CoV-2 spike protein (Wuhan strain). Negative (blank) and positive (mitogen) controls were used to define individual background cell activation and to test the viability and responsiveness of T cells.
The samples were incubated at 37 °C for 24 h in a 5% CO2 atmosphere and then centrifuged at 12,000 g for 10 min. The Coviferon test results are considered reliable when the IFNγ released by the blank stimulation tube is < 9 pg/ml and by the mitogen stimulation tube is > 141 pg/ml. The Coviferon test result is positive when the difference between the IFNγ released by spike stimulation tube and blank stimulation tube is > 12 pg/ml.
In the same plasma supernatant, we measured IFNγ and a panel of other pro- and anti-inflammatory cytokines: TNFα, IL-2, IL-4, IL-6, IL-10, and IL-1β. Cytokines were measured by microfluidic ultrasensitive ELISA using the Protein simple plex technology on the ELLA instrument (R&D Systems, Biotechne, USA).
2.4. Surrogate virus neutralization assay
The assay was conducted with the target HEK293 T-cell line overexpressing angiotensin-converting enzyme 2 (ACE2) and two different strains of green fluorescent protein (GFP)-expressing, replication-deficient retroviruses pseudo-typed with the spike protein of either the Wuhan-Hu1 strain or the B.1.1.7 strain (all the reagents were from Creative Diagnostics, Shirley, NY, USA). The pseudoviruses were incubated for 1 h at 37 °C with log dilutions of patients’ sera ranging from pure to 106 before target cell seeding. Pre-pandemic sera (n = 3), sera from vaccinated (n = 4) and apparently healthy individuals, and a neutralizing monoclonal antibody against the spike protein were employed as negative and positive controls, respectively. Fluorescence images were acquired with an inverted microscope (DMI600B; Leica Microsystems, Germany) at 50 × magnification, and the GFP-positive area was computed using ImageJ. Data were normalized, and dose-response curves were computed with Prism9 for MacOS (GraphPad Software, San Diego, CA, USA) based on the number of transducing units obtained with incubation of pre-immune serum and pseudovirus at 100% efficiency. Robust regression was applied to fit the data and to compute IC50.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (Q1 to Q3). Categorical variables were compared using Fisher's exact test, and continuous variables were compared using the Mann-Whitney U test without correction for multiple comparisons. The analyses were performed using SPSS statistics for Windows, version 11.0 (SPSS Inc., Chicago, Ill., USA).
3. Results
3.1. Patients characteristics
This study included 28 patients (11 in the RTX group and 17 in the BEL group) and 13 controls. None of the patients had, at the time of vaccination, detectable anti-SARS-CoV-2 antibodies due to prior exposure to the virus. Details about the demographic features, clinical characteristics, and concurrent medications are provided in Table 1 . All the RTX-treated patients received the first dose of the vaccine 13 ± 8 weeks after the last drug infusion. Seven patients in the RTX group and six in the BEL group received low-dose corticosteroids at the time of vaccination. Fourteen patients were taking hydroxychloroquine, and among them, 13 were in the BEL group. Five patients in the RTX group and 11 in the BEL group received an immunosuppressive agent; this included two patients (one patient each from the RTX and BEL group) who were taking two immunosuppressants. One patient with dermatomyositis from the RTX group received methotrexate with cyclosporin A, and one patient with SLE from the BEL group received methotrexate with tacrolimus (Table 1).
Table 1.
Demographic characteristics, clinical features and co-medications.
| Controls (n = 13) |
Rituximab (n = 11) |
Belimumab (n = 17) |
|
|---|---|---|---|
| Age in years, mean ± SD* | 46 ± 9 | 61 ± 8 | 47 ± 8 |
| Women, n (%) | 8 (60) | 5 (45) | 16 (95) |
| Diagnosis, n (%) | |||
| ANCA-associated vasculitis | – | 6 (54) | – |
| Mixed connective tissue disease | – | 2 (18) | – |
| Anti-synthetase syndrome | – | 2 (18) | – |
| Dermatomyositis | – | 1 (9) | – |
| Systemic lupus erythematosus | – | 0 | 17 (100) |
| Type of vaccine, n (%) | |||
| BNT162b2 | 13 (100) | 11 (100) | 13 (76) |
| mRNA-1273 | 0 (0) | 0 (0) | 4 (14) |
| Time (days) between vaccine doses, mean ± SD* | 21 ± 2 | 21 ± 2 | 22.3 ± 3 |
| Time (weeks) between the last RTX infusion and vaccination, mean ± SD* | – | 13 ± 8 | – |
| Co-medication, n (%) | – | 7 (63) | 4 (36) |
| Prednisone, mg/day (median, range) | – | 3.75 (0–10 | 1 (0–5) |
| Hydroxychloroquine | – | 1 (9) | 13 (76) |
| Methotrexate | – | 2 (18) | 1 (5) |
| Mycophenolate mofetil | – | 0 | 5 (29) |
| Azathioprine | – | 1 (9) | 1 (5) |
| Calcineurin inhibitors | – | 3 (36) | 4 (14) |
| Two immunosuppressive agents | – | 1 (9) | 1 (6) |
| Disease activity, n (%) | |||
| Induction phase | – | 5 (45) | – |
| Maintenance phase | – | 6 (55) | – |
| Remission | – | – | 13 (76) |
| Low disease activity (according to LLDAS) | – | – | 4 (24) |
ANCA, antineutrophil cytoplasmic antibody; LLDAS, Lupus Low Disease Activity State; SD, standard deviation.
The CD19+ B-cell count was significantly different between the two groups, but the total white blood cell count, lymphocyte count, CD4+ and CD8+ T-cell subpopulations, and serum IgG levels did not differ significantly between the RTX and BEL treatment groups (Table 2 ).
Table 2.
Humoral and cellular response to SARS-CoV-2 in patients and controls.
| Controls (n = 13) | RTX or BEL (n = 28) | P value (RTX or BEL vs. controls) | RTX alone (n = 11) |
P value (RTX vs. controls) | BEL alone (n = 17) |
P value (BEL vs. controls) | P value (RTX vs. BEL) | |
|---|---|---|---|---|---|---|---|---|
| Presence of humoral response, n (%) | 13 (100) | 18 (64.3) | 0.0172 | 1 (9) | <0.0001 | 16 (94.1) | 1.0 | <0.0001 |
| Anti-RBD titer, U/ml | 1133 (692.5–2260.5) | 39.65 (0–459.5) | <0.0001 | 0 (0–0) | <0.0001 | 243 (77.55–744.0) | 0.002 | <0.0001 |
| Positive Coviferon result, n (%) | 13 (100) | 24 (85.7) | 0.288 | 8 (72.7) | 0.0815 | 16 (94.1) | 1.0 | 0.269 |
| IFNγ release, pg/ml | 204.01 (96.7–590.7) | 149.85 (50.69–273.19) | 0.249 | 114.9 (3.15–414.7) | 0.277 | 156.14 (61.5–266.9) | 0.363 | 0.746 |
| Total white blood cell count, cells/μl | – | – | – | 5417.0 (4978.0–6413.0) | – | 4764.0 (3617.0–6781.0) | – | 0.305 |
| Lymphocyte count, cells/μl | – | – | – | 1270.0 (925.0–1670.0) | – | 1220.0 (840.0–1950.0) | – | 0.610 |
| CD3+ T cell count, cells/μl | – | – | – | 1070.0 (870.0–1518.0) | – | 1109.0 (701.0–1838.0) | – | 0.683 |
| CD4+ T cell count, cells/μl | – | – | – | 687.0 (577.0–926.0)] | – | 581.0 (351.0–1248.0) | – | 0.721 |
| CD8+ T cell count, cells/mmc | – | – | – | 483.0 (259.0–562.0) | – | 339.0 (250.0–481.0) | – | 0.121 |
| CD19+ B cell count, cells/μl | – | – | – | 0 (0–0) | – | 32.0 (9.0–48.0) | – | <0.0001 |
| Serum IgG, mg/dl | – | – | – | 1130.0 (951.0–1510.0) | – | 887.0 (834.0–1077.5) | – | 0.085 |
BEL: belimumab; IFN: interferon; IL: interleukin; RTX: rituximab.
Results are expressed as median (Q1–Q3 interquartile range) unless indicated otherwise.
3.2. Effect of B-cell-targeted therapy on the immune response to vaccination
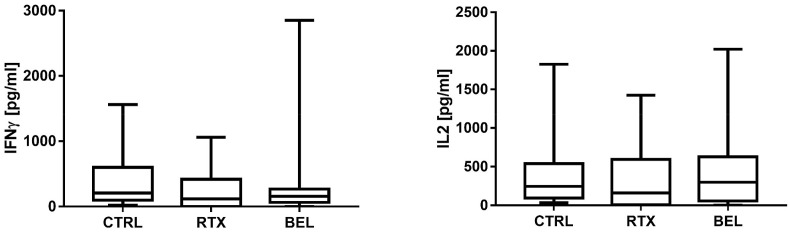
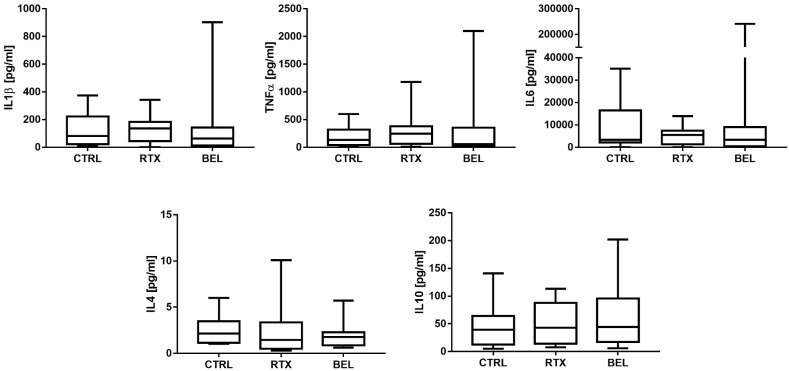
In the whole cohort of patients undergoing B-cell-targeted therapy with RTX or BEL, the median antibody titer was 39.65 (0–459.5) U/ml compared to 1133 (692.5–2260.5) U/ml in the control group (p < 0.0001) (Table 2). T-cell response was detectable in 24 out 28 (85%) patients and in all the control participants (100%) (Table 2). The median IFNγ level (T-cell response) was 149.85 (50.69–273.19) pg/ml in the patients and 204.01 (96.7–590.7) pg/ml in the controls (p > 0.05, Table 2, Fig. 1 ). Further, the median IL-2 level (T-cell response) of the patients was similar to that of the controls (230.28 [52.7–459.0] pg/ml vs. 245.2 [94.9–538.0] pg/ml] (Table 3 , Fig. 1). None of the other cytokines evaluated in the IGRA test were significantly different between the patients and controls (Table 3, Fig. 2 ). These findings indicate that B-cell-targeted therapy with RTX and BEL impaired the humoral response, but did not affect the T-cell response.
Fig. 1.
Box-plot graphs depicting the IFNγ response (panel on the left) and the IL2 response (panel on the right) in RTX-/BEL-treated patients and controls CTRL, controls; RTX, rituximab; BEL, belimumab.
Table 3.
Comparison of pro-inflammatory and anti-inflammatory cytokine release during the IGRA test in RTX- or BEL-treated patients and controls.
| Controls (n = 13) | RTX or BEL (n = 28) | P value (RTX or BEL vs. controls) | RTX alone (n = 11) | P value (RTX vs. controls) | BEL alone (n = 17) | P value (BEL vs. controls) | P value (RTX vs. BEL) | |
|---|---|---|---|---|---|---|---|---|
| TNF-α, pg/ml | 135.3 (42.5–319.75) | 74.9 (18.2–288.4) | 0.69 | 231.8 (37.3–362.7) | 0.776 | 48.3 (14.4–277.25) | 0.398 | 0.422 |
| IL-6, pg/ml | 3425.4 (2083.8–15,043.5) | 3972.5 (47.0–7449.67) | 0.413 | 5026 (264.6–7394.7) | 0.776 | 3104.9 (−6.32 to 8862.7) | 0.320 | 0.458 |
| IL-1β, pg/ml | 73.4 (19.0–187.45) | 65.45 (6.22–141.42) | 0.75 | 121.6 (11.5–173.8) | 0.733 | 58.7 (0.85–129.8) | 0.457 | 0.306 |
| IL-2, pg/ml | 245.2 (94.9–538.0) | 230.28 (52.7–459.0) | 0.588 | 159.9 (7.14–594.0) | 0.459 | 264.2 (57.9–447.46) | 0.812 | 0.368 |
| IL-10, pg/ml | 35.6 (10.65–53.25) | 30.0 (1.72–70.3) | 0.648 | 31.5 (−0.1 to 49.6) | 0.691 | 28.5 (1.95–82.15) | 0.711 | 0.963 |
BEL: belimumab; IL: interleukin; RTX: rituximab; TNF: tumor necrosis factor. Results are expressed as median (Q1–Q3 interquartile range).
Fig. 2.
Box-plot graphs depicting the response to various cytokines (IL1β, TNFα, IL6, IL4, and IL10) in RTX-/BEL-treated patients and controls CTRL, controls; RTX, rituximab; BEL, belimumab.
3.3. Effect of RTX on the immune response to vaccination
A very low titer of anti-RBD antibodies was documented only in 1 out of 11 patients in the RTX subgroup (0.93 U/ml, positive if > 0.79 U/ml), and this patient was the only one who showed an initial B-cell recovery (CD19+ B-cell count: 5 cells/μl) (p < 0.0001) (Table 2). In contrast, 8 of the 11 (72.7%) RTX-treated patients showed a T-cell response that was comparable to that of the control group, in terms of both IFNγ and IL-2 release (Table 2, Table 3, Fig. 1). These findings indicate that anti-CD20 monoclonal antibody therapy with RTX not only impairs humoral response against mRNA vaccination but also affects T-cell response, although to a much lesser extent.
3.4. Effect of BEL on the immune response to vaccination
The majority of BEL patients (16/17, 94.1%) developed anti-RBD antibodies. However, their average titer was significantly lower than that of the controls (median, 243 [77.55–744.0] U/ml) (p = 0.002). Additionally, the result of the Coviferon test was positive in 16 out of 17 (94.1%) patients under BEL treatment. Importantly, the amount of IFNγ and IL-2 released (156.14 [61.5–266.9] pg/ml and 264.2 [57.9–447.46] pg/ml, respectively) did not differ significantly in comparison with the control group (Table 2, Table 3, Fig. 1).
While the serological response to vaccination was almost completely abolished in the RTX-treated patients, in the BEL-treated patients, we recorded only a reduced level of anti-SARS-CoV-2 antibodies as compared to the controls. In contrast, the T-cell response in terms of both IFNγ and IL-2 release was not different between the two treatment groups (Table 2, Table 3, Fig. 1).
Notably, all SLE patients under BEL treatment had CD19+ B-cell levels that were below the lowest normal value, ranging from 4 to 87 cells/μl, with the B-cell count in one patient being below 5 cells/μl. These findings indicate that anti-BAFF monoclonal antibody treatment with BEL only slightly impairs humoral response and T-cell response to the mRNA vaccination.
3.5. SARS-CoV-2-neutralizing capacity of anti-RBD antibodies in BEL patients after vaccination
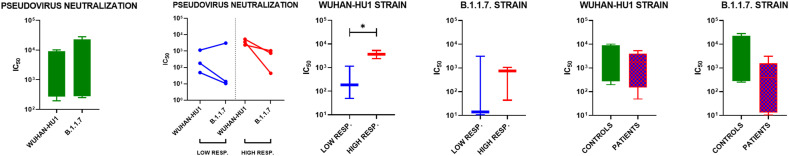
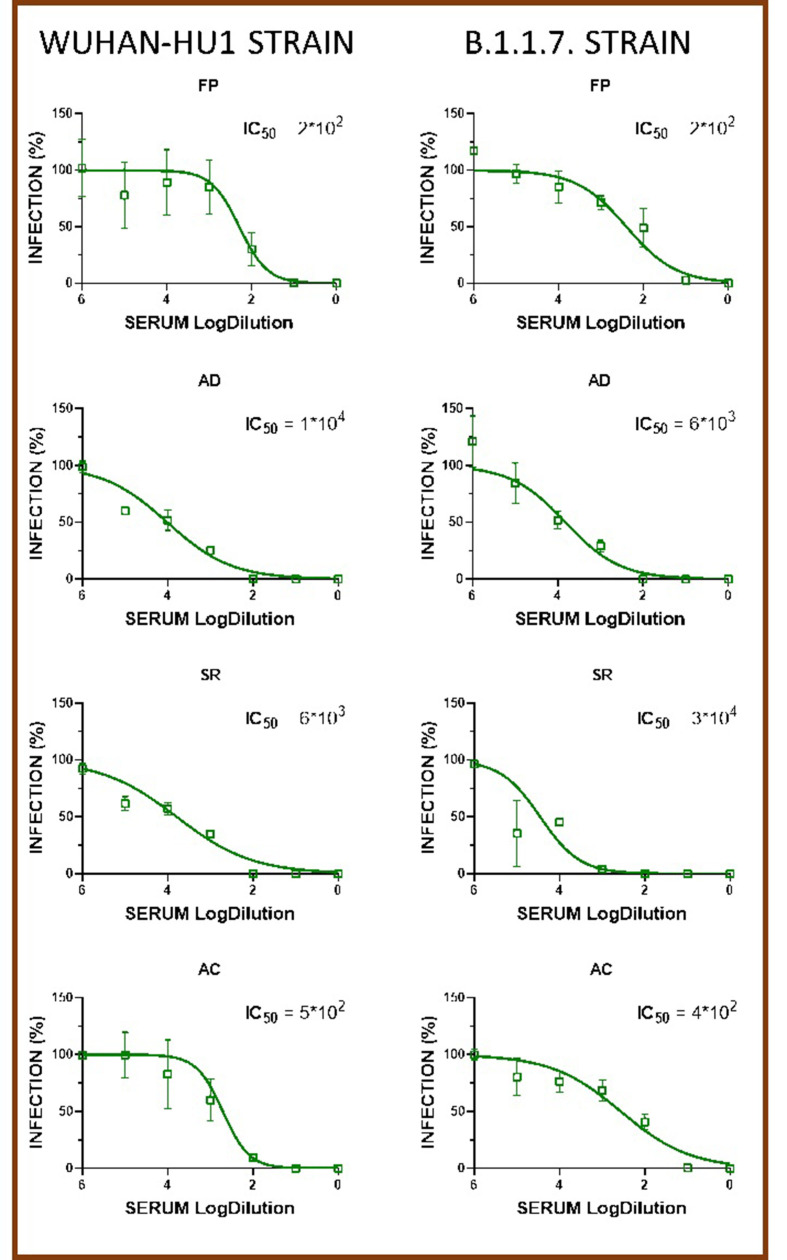
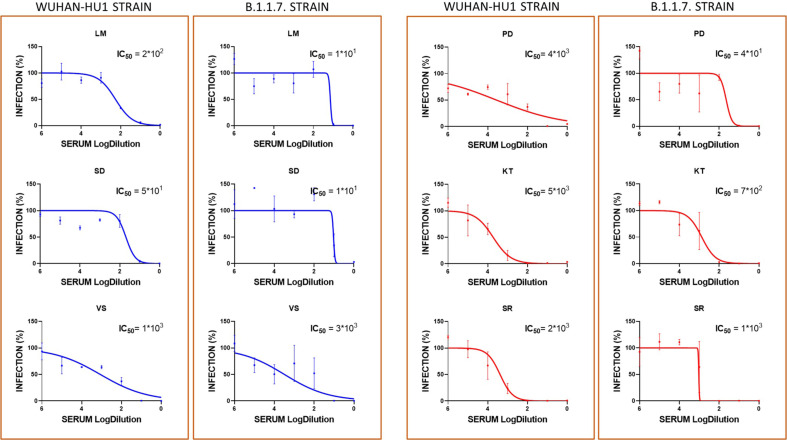
The serum samples of four representative vaccinated controls and six patients under treatment with BEL were tested for their ability to neutralize under in vitro conditions retroviruses pseudotyped with the spike protein of either the Wuhan-Hu1 strain (WT) or the B.1.1.7 strain (that originated in the UK) (Supplemental files, Fig. 1, Fig. 2). Overall, no significant difference was observed in the ability of sera samples from both groups to neutralize either the WT pseudovirus (IC50 = 862.4 [272.5–4872] for controls and 1771 [136.0–4039] for patients) or the UK pseudovirus [IC50 347 [278–22,905] for controls and 395.7 [13.11–1563] for patients] (Fig. 1). A pairwise comparison of the ability of control and patient sera to neutralize the WT and UK pseudoviruses showed no strain-specific significant difference (Fig. 3 ). However, when BEL patients were stratified according to their levels of total anti-RBD antibodies as high responders (>800 AU/mL) and low responders (≤45 AU/mL), strain-specific differences emerged (Fig. 1). Indeed, consistent with the serological data, we observed that high responders neutralized the WT pseudovirus with a significantly higher dilution (IC50 = 165.0 [49.0–1149] and 3606 [2393–5337] in the low and high responders, respectively) (Fig. 3). Conversely, no significant difference was observed when the UK pseudovirus was employed, and the neutralizing capacity was highly heterogenous (IC50 = 747 [44.29–1051] and 13.94 [10.61–3097] in the low and high responders, respectively) (Fig. 3).
Fig. 3.
Graphs depicting the IC50 values of vaccinated controls (green) and belimumab-treated patients after mRNA vaccination, stratified as high responders (in red) and low responders (in blue) *p < 0.05. IC50 corresponds to 50% inhibition of pseudovirus entry into the HEK293T-ACE2 cells at 7 days after exposure to non-diluted serum (101) or diluted serum (>101).
3.6. Effect of co-medications on T-cell activation and response
The result of the IGRA test was negative in 6 of the 28 patients, and 4 of them showed a completely negative IFNγ response to spike stimulation. These four patients were vaccinated with BNT162b2, and three of them were from the RTX-treated group and one was from the BEL-treated group. Importantly, all four were receiving co-medication with drugs that affect T-cell activity: the three RTX-treated patients were taking calcineurin inhibitors, and the BEL-treated patient was taking mycophenolate mofetil. Moreover, all patients who showed an observable T-cell response to mitogen were under treatment with prednisone or its equivalent at a dose ranging from 5 to 10 mg/day.
3.7. Clinical follow-up of the patients
The mean follow-up period was 8.8 ± 0.7 months from the second dose of the vaccine. During the follow-up period, two patients enrolled in this study developed symptomatic COVID-19 infection. Notably, both patients were taking either cyclosporin A or mycophenolate mofetil in combination with BEL, and both showed a humoral response to the vaccine. However, one of them had also shown a cellular response to the vaccine. One patient was treated with molnupiravir, but neither of them required hospitalization for COVID-19. None of the patients in the cohort reported disease relapse after vaccination.
4. Discussion
Patients with autoimmune systemic diseases are more vulnerable to infections and are at an increased risk of developing severe infective complications. Infections, in turn, can reactivate and worsen the autoimmune disease itself in a vicious cycle [14]. Thus, vaccination is the main weapon to prevent these complications and represents an important and safe instrument of care for rheumatic patients [15] that needs to be further promoted [16]. Unfortunately, the immunosuppressive drugs used to treat rheumatic diseases may impair the response to vaccines [15]. In the present study, we have explored the effect of two B-cell targeted therapies on both the humoral and cellular response to mRNA vaccines for SARS-CoV-2 in some systemic autoimmune diseases. Overall, our results show that B-cell-targeted therapies impair the humoral response induced by anti-SARS-CoV-2 mRNA vaccines. However, for the first time, we demonstrated that while RTX abolishes the humoral response when the vaccination is administered early under conditions of complete B-cell depletion, BEL only slightly impairs antibody production and does not compromise the humoral response to the vaccine. In accordance with our findings, the report of Ruddy et al. also showed that there was no evidence of impairment of the humoral response after two-dose mRNA vaccination in 56 patients treated with BEL [17]. In contrast, Medeiros-Ribeiro et al. found seroconversion in 17 out of 30 (56.7%) BEL-treated patients, and 14 out of the 17 (82.3%) patients had neutralizing antibody activity against RBD (measured as the ability to block the interaction of the RBD of the viral spike glycoprotein with ACE2 in cells) [18]. By applying a different and more specific experimental approach to measure the neutralizing capacity in an HEK293 T-cell line overexpressing ACE2 and two different strains of pseudovirus incubated with the patients’ sera, we confirmed that the humoral response in BEL-treated patients is not as efficient as that in the general population. In line with these findings, we observed that, while only 1 out of 6 patients had a very low IC50 (i.e., <100) against the WT strain, 3 out of 6 patients had an IC50 < 100 against the UK strain and none of the healthy controls had an IC50 < 100 against either of the two variants. These results indicate that BEL patients may be vulnerable to more infectious viral variants.
Recent data indicate a reduced or absent humoral response to the COVID-19 vaccine in a large proportion of patients treated with B-cell-targeted therapies, in particular RTX [7,[9], [10], [11]]. Some authors have demonstrated a correlation between circulating anti-spike antibodies and peripheral CD19+ B-cell count, highlighting the absence of a serological response in patients with complete B-cell depletion [7,19]. Further, an important association has been shown between the CD4+ cell count and immune response [19]. Therefore, B-cell reconstitution and CD4+ cell count may be crucial in developing an adequate humoral response to vaccination, and accordingly, it is important to choose the optimal interval after the last exposure to RTX. To date, available data indicate that a mean interval of 6 months is ideal: in patients vaccinated one year after RTX therapy, the serological response rate increases according to the degree of CD19+ B-cell reappearance [7,20]. The updated ACR COVID-19 Vaccine Guidance Task Force guidelines also recommend the administration of COVID-19 vaccination at the latest possible time after the last dose of RTX [5].
With regard to T-cell response, our study confirmed that RTX did not have a strong impact on T-cell response or the production of pro-inflammatory and anti-inflammatory cytokines. Further, this study demonstrates for the first time that BEL therapy has similar effects in terms of T-cell response and inflammatory cytokine production. Several authors have reported the maintenance of T-cell response against the vaccine in patients treated with RTX, regardless of humoral response and time after the last RTX infusion [6,7,[9], [10], [11], [12],21], but there is still some controversy about this [19]. Nonetheless, in keeping with the present findings, the robustness of the inflammatory response has been demonstrated in rheumatic and non-rheumatic patients under treatment with anti-CD20 antibodies, and the T-cell responses were found to be even higher in patients than in controls [22].
Data on immunization early after RTX therapy are substantially lacking in the literature. This poses a big problem for patients with a critical disease that requires non-deferrable therapeutic intervention despite the pandemic or for patients undergoing RTX maintenance for severe diseases. Our results indicate that early exposure to RTX (<5 months after last infusion) does not preclude SARS-CoV-2 vaccination, since a cellular immune response can be elicited even in the absence of circulating B cells. This finding is relevant in light of the increasing evidence of the pivotal role of the T-cell response in protection against hospitalization and death by SARS-CoV-2 infection [23].
In our cohort, despite the lack of significant differences, the levels of proinflammatory cytokines, i.e., TNFα, IL-1β, and IL-6, tended to be higher in the RTX-treated patients than in the controls, as observed in previous studies [22], while the levels of the same cytokines were similar between the BEL-treated patients and controls. These findings underscore the inverse correlation between B-cell depletion and inflammation after vaccination. This is consistent with the poor outcomes caused by a hyperinflammatory state in RTX-treated patients infected with SARS-CoV-2 [4].
Interestingly, the pivotal role of IL-2-secreting T cells in providing protection from COVID-19 has been highlighted by a recent preliminary work [24]. The authors suggested that this effect is probably a result of the priming of these T cells after exposure to other coronaviruses in the past [24]. Therefore, it is reasonable to hypothesize a similar mechanism of priming IL-2-secreting T-cells against SARS-CoV-2 after vaccination in patients who do not show a humoral response.
Concomitant medications, including medium to high doses of glucocorticoids, may have a strong impact on T-cell response [15,18,25,26]. In fact, in our cohort, the patients who did not have T-cell reactivity were on co-medications that impair T-cell response, such as calcineurin inhibitors and mycophenolate mofetil in combination with low to medium doses of prednisone. Indeed, COVID-19 was diagnosed in two patients who were taking BEL plus cyclosporin A or mycophenolate mofetil. Thus, during the present outbreak, concomitant treatment with B-cell-depleting therapies and T-cell inhibitors should be avoided, if possible, and patients who are on such treatment should be considered to be at the highest risk of COVID-19 and serious outcomes unless vaccinated. In these patients, we suggest that mycophenolate be withdrawn for one or two weeks after the vaccination if the disease is stable, according to published data [5]. No data are available regarding the effect of withdrawing calcineurin inhibitors [27], even though this would be advisable based on the immunogenicity observed in solid organ transplant recipients [28].
This study has some limitations, and the main one is the low number of patients recruited. In addition, our cohort of patients was heterogeneous in terms of both their diagnoses and co-medications. Yet, the clinical efficacy of such an immune response remains to be defined, as well as the effect of a booster dose and the impact of new viral variants in these patients. On the other hand, this study presents considerable original data. As far as we know, this is the first report that focuses on very early immunization after RTX treatment, i.e., before 5 months, and almost all the patients had B-cell depletion at the time of vaccination. Moreover, this is the first time two different B-cell therapies are being compared, and this is the largest group of BEL-treated lupus patients to be analyzed for both B-cell and T-cell response to the vaccine up to now. The long-lasting protective effect of vaccination is also highlighted here, since COVID-19 was diagnosed only in two patients during a follow-up period of almost 9 months after vaccination. Finally, the evaluation of the T-cell response also included the measurement of a large panel of pivotal pro-inflammatory and anti-inflammatory cytokines that further support the robustness of T-cell reactivity in this setting.
5. Conclusion
B-cell-targeted therapies are the cornerstone of treatment for many systemic autoimmune diseases, and they often represent non-deferrable treatment for some clinical conditions. It has been suggested that B-cell recovery before vaccination increases the chances of an adequate humoral response to the vaccine [29]. However, our results indicate that B-cell depletion cannot preclude SARS-CoV-2 vaccination, since a cellular immune response can be elicited even in the absence of circulating B cells. Indeed, recent data show that the cellular response, rather than the humoral immune response, can be boosted by a third vaccine dose in RTX-treated patients [30]. The present findings highlight the effects of co-medications on T-cell response. Further, the findings indicate the activation of an immune response following COVID-19 vaccination in SLE patients treated with BEL, although the neutralizing capacity might be lowered.
Author contributions
MF, GDM, LQ, RD, APB, FC: conception and design of the study; RD, SG, NC, FC, CDS: acquisition of data; MF, GDM, LQ, APB, RD, FC: analysis and interpretation of data; all authors: drafting of the article or its critical revision for important intellectual content; all authors: final approval of the version to be submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ statement
All authors have read and approved submission of the final manuscript and have taken due care to ensure the integrity of the work. The work has not been published previously and it is not under consideration for publication elsewhere.No conflict of interest exists for anyone of the authors.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2022.102827.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
Neutralization activity of Wuhan-Hu-1 and Variant B.1.1.7 SARS-CoV-2 pseudoviruses in HEK293T-ACE2 cells at 7 days after exposure to serially diluted serum samples from vaccinated controls.
figs2.
Neutralization activity of Wuhan-Hu-1 and Variant B.1.1.7 SARS-CoV-2 pseudoviruses in HEK293T-ACE2 cells at 7 days after exposure to serially diluted serum samples of belimumab-treated patients after mRNA vaccination, stratified as high (IgG+IgM+IgA) responders (red curves) and low responders (blue curves).
Data availability
Data will be made available on request.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci O., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. C4591001 clinical trial group, safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L., Izadi Z., Jacobsohn L., Katz P., Lawson-Tovey S., Mateus E.F., Rush S., Schmajuk G., Simard J., Strangfeld A., Trupin L., Wysham K.D., Bhana S., Costello W., Grainger R., Hausmann J.S., Liew J.W., Sirotich E., Sufka P., Wallace Z.S., Yazdany J., Machado P.M., Robinson P.C. COVID-19 Global Rheumatology Alliance, Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strangfeld A., Schäfer M., Gianfrancesco M.A., Lawson-Tovey S., Liew J.W., Ljung L., Mateus E.F., Richez C., Santos M.J., Schmajuk G., Scirè C.A., Sirotich E., Sparks J.A., Sufka P., Thomas T., Trupin L., Wallace Z.S., Al-Adely S., Bachiller-Corral J., Bhana S., Cacoub P., Carmona L., Costello R., Costello W., Gossec L., Grainger R., Hachulla E., Hasseli R., Hausmann J.S., Hyrich K.L., Izadi Z., Jacobsohn L., Katz P., Kearsley-Fleet L., Robinson P.C., Yazdany J., Machado P.M. COVID-19 Global Rheumatology Alliance, Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., Calabrese C., Gravallese E.M., Harpaz R., Kroger A., Sadun R.E., Turner A.S., Williams E.A., Mikuls T.R. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73:e60. doi: 10.1002/art.41928. –e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prendecki M., Clarke C., Edwards H., McIntyre S., Mortimer P., Gleeson S., Martin P., Thomson T., Randell P., Shah A., Singanayagam A., Lightstone L., Cox A., Kelleher P., Willicombe M., McAdoo S.P. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS-CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021;80:1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 8.Moyon Q., Sterlin D., Miyara M., Anna F., Mathian A., Lhote R., Ghillani-Dalbin P., Breillat P., Mudumba S., de Alba S., Cohen-Aubart F., Haroche J., Pha M., Boutin T.H.D., Chaieb H., Flores P.M., Charneau P., Gorochov G., Amoura Z. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann. Rheum. Dis. 2021 doi: 10.1136/annrheumdis-2021-221097. Oct 4:annrheumdis-2021-221097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonelli M.M., Mrak D., Perkmann T., Haslacher H., Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021;80:1355–1356. doi: 10.1136/annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- 10.Mrak D., Tobudic S., Koblischke M., Graninger M., Radner H., Sieghart D., Hofer P., Perkmann T., Haslacher H., Thalhammer R., Winkler S., Blüml S., Stiasny K., Aberle J.H., Smolen J.S., Heinz L.X., Aletaha D., Bonelli M. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 11.Benucci M., Damiani A., Infantino M., Manfredi M., Grossi V., Lari B., Gobbi F.L., Sarzi-Puttini P. Presence of specific T cell response after SARS-CoV-2 vaccination in rheumatoid arthritis patients receiving rituximab. Immunol. Res. 2021;69:309–311. doi: 10.1007/s12026-021-09212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieiro Santos C., Calleja Antolin S., Moriano Morales C., Garcia Herrero J., Diez Alvarez E., Ramos Ortega F., Ruiz de Morales J.G. Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklyn K., Lau C.S., Navarra S.V., Louthrenoo W., Lateef A., Hamijoyo L., Wahono C.S., Chen S.L., Jin O., Morton S., Hoi A., Huq M., Nikpour M., Morand E.F. Asia-pacific lupus collaboration, definition and initial validation of a lupus low disease activity state (LLDAS) Ann. Rheum. Dis. 2016;75:1615–1621. doi: 10.1136/annrheumdis-2015-207726. [DOI] [PubMed] [Google Scholar]
- 14.Danza A., Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22:1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 15.Furer V., Rondaan C., Heijstek M.W., Agmon-Levin N., van Assen S., Bijl M., Breedveld F.C., D'Amelio R., Dougados M., Kapetanovic M.C., van Laar J.M., de Thurah A., Landewé R.B., Molto A., Müller-Ladner U., Schreiber K., Smolar L., Walker J., Warnatz K., Wulffraat N.M., Elkayam O O. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 16.Quartuccio L., Zabotti A., Gallo T., De Vita S., Valent F. Influenza vaccination in chronic inflammatory arthritis undergoing immunosuppressive treatments: temporal trend and factors of adherence. Rheumatology. 2021;60:2456–2460. doi: 10.1093/rheumatology/keaa454. [DOI] [PubMed] [Google Scholar]
- 17.Ruddy J.A., Connolly C.M., Boyarsky B.J., Werbel W.A., Christopher-Stine L., Garonzik-Wang J., Segev D.L., Paik J.J. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medeiros-Ribeiro A.C., Aikawa N.E., Saad C.G.S., Yuki E.F.N., Pedrosa T., Fusco S.R.G., Rojo P.T., Pereira R.M.R., Shinjo S.K., Andrade D.C.O., Sampaio-Barros P.D., Ribeiro C.T., Deveza G.B.H., Martins V.A.O., Silva C.A., Lopes M.H., Duarte A.J.S., Antonangelo L., Sabino E.C., Kallas E.G., Pasoto S.G., Bonfa E. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat. Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 19.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., Njue L.M., Medri C., Angelillo-Scherrer A., Borradori L., Radonjic-Hoesli S., Seyed Jafari S.M., Chan A., Hoepner R., Bacher V.U., Mani L.Y., Iype J.M., Hirzel C., Maurer B., Sidler D. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronbichler A., Geetha D., Smith R.M., Egan A.C., Bajema I.M., Schönermarck U., Mahr A., Anders H.J., Bruchfeld A., Cid M.C., Jayne D.R.W. The COVID-19 pandemic and ANCA-associated vasculitis - reports from the EUVAS meeting and EUVAS education forum. Autoimmun. Rev. 2021;20:102986. doi: 10.1016/j.autrev.2021.102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitoun S., Henry J., Desjardins D., Vauloup-Fellous C., Dib N., Belkhir R., Mouna L., Joly C., Bitu M., Ly B., Pascaud J., Seror R., Roque Afonso A.M., Le Grand R., Mariette X. Rituximab impairs B-cell response but not T-cell response to COVID-19 vaccine in auto-immune diseases. Arthritis Rheumatol. 2021 doi: 10.1002/art.42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madelon N., Lauper K., Breville G., Sabater Royo I., Goldstein R., Andrey D.O., Grifoni A., Sette A., Kaiser L., Siegrist C.A., Finckh A., Lalive P.H., Didierlaurent A.M., Eberhardt C.S. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin. Infect. Dis. 2021:ciab954. doi: 10.1093/cid/ciab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 24.Kundu R., Narean J.S., Wang L., Fenn J., Pillay T., Fernandez N.D., Conibear E., Koycheva A., Davies M., Tolosa-Wright M., Hakki S., Varro R., McDermott E., Hammett S., Cutajar J., Thwaites R.S., Parker E., Rosadas C., McClure M., Tedder R., Taylor G.P., Dunning J., Lalvani A. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022;13:80. doi: 10.1038/s41467-021-27674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., Demissie E.G., El-Qunni A.A., Haile A., Huang K., Kinnett B., Liebeskind M.J., Liu Z., McMorrow L.E., Paez D., Pawar N., Perantie D.C., Schriefer R.E., Sides S.E., Thapa M., Gergely M., Abushamma S., Akuse S., Klebert M., Mitchell L., Nix D., Graf J., Taylor K.E., Chahin S., Ciorba M.A., Katz P., Matloubian M., O'Halloran J.A., Presti R.M., Wu G.F., Whelan S.P.J., Buchser W.J., Gensler L.S., Nakamura M.C., Ellebedy A.H., J Kim A.H. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann. Intern. Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picchianti-Diamanti A., Aiello A., Laganà B., Agrati C., Castilletti C., Meschi S., Farroni C., Lapa D., Najafi Fard S., Cuzzi G., Cimini E., Grassi G., Vanini V., Di Rosa R., Salemi S., Nalli G., Salmi A., Repele F., Altera A.M.G., Maffongelli G., Palazzolo C., Vita S., Leone S., Puro V., Capobianchi M.R., Ippolito G., Nicastri E., Goletti D. Immunosuppressive therapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front. Immunol. 2021;212:740249. doi: 10.3389/fimmu.2021.740249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landewé R.B.M., Kroon F.P.B., Alunno A., Najm A., Bijlsma J.W., Burmester G.R., Caporali R., Combe B., Conway R., Curtis J.R., Elkayam O., Gossec L., Heijstek M.W., Haupt L., Iagnocco A., Isaacs J.D., Juhász I.Á., Makri S., Mariette X., McInnes I.B., Mehta P., Mueller-Ladner U., Schulze-Koops H., Smolen J.S., Wiek W., Winthrop K.L., Navarro-Compán V., Machado P.M. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann. Rheum. Dis. 2022 doi: 10.1136/annrheumdis-2021-222006. annrheumdis-2021-222006. [DOI] [PubMed] [Google Scholar]
- 28.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., Garonzik-Wang J.M. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher F., Mrdenovic N., Scheicht D., Pons-Kühnemann J., Scheibelhut C., Strunk J. Humoral immunogenicity of COVID-19 vaccines in patients with inflammatory rheumatic diseases under treatment with Rituximab: a case-control study (COVID-19VacRTX) Rheumatology. 2022 doi: 10.1093/rheumatology/keac036. keac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jyssum I., Kared H., Tran T.T., Tveter A.T., Provan S.A., Sexton J., Jørgensen K.K., Jahnsen J., Kro G.B., Warren D.J., Vaage E.B., Kvien T.K., Nissen-Meyer L.H., Anderson A.M., Grødeland G., Haavardsholm E.A., Vaage J.T., Mjaaland S., Syversen S.W., Lund-Johansen F., Munthe L.A., Goll G.L. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.