Abstract
Keith Reemtsma was a pioneer in xenotransplantation, the Honorary Founding President of the IXA (in 1998), and a wonderful personality. It is a privilege to be invited to give this lecture in his memory.
If he were alive today, he would be delighted to see the progress that has been made in pig organ transplantation into nonhuman primate recipients. This progress has largely resulted from two major advances – (i) the increasing availability of pigs with multiple genetic manipulations aimed at protecting the cells of the organ from the primate immune response, and (ii) the introduction of novel immunosuppressive agents that block the CD40/CD154 costimulation pathway.
There is strong evidence from numerous in vitro studies that the transplantation of a triple-knockout pig organ, particularly if expressing several human protective proteins, into a patient is likely to be significantly more successful than if that same organ is transplanted into a nonhuman primate recipient. With this fact in mind, and in view of the advances currently being made, the time has surely come when we need to consider moving from the laboratory to the clinic.
However, there are still questions we need to definitively resolve – (i) what exact genetic modifications do we need in the organ-source pig? (ii) what exact immunosuppressive regimen will we choose? (iii) how will we monitor the immune response and diagnose and treat rejection? and (iv) how do we plan to prevent or treat potential infectious complications? Furthermore, when these matters have been resolved, which patients will be offered a pig organ in the first trial? We have suggested that patients who are very unlikely to survive until a suitable deceased human donor kidney becomes available are those who should be considered for the initial trials. Assessing public attitudes to xenotransplantation is also important before embarking on a clinical trial.
I suggest that progress is much more likely to be made from a small clinical trial than if we persist in carrying out experiments in an animal model that no longer mimics the clinical situation.
Keywords: International Xenotransplantation Association, Keith Reemtsma, Nonhuman primate, Pig, Xenotransplantation
Introduction
It is a great privilege for me to be invited by the International Xenotransplantation Association (IXA) to give this lecture in memory of Keith Reemtsma (Figure1), who was a pioneer in xenotransplantation, the Honorary Founding President of the IXA (in 1998), and a wonderful personality. He had a great sense of humor, and personal interaction with him was always stimulating and enjoyable. It has often been said that, on the basis of his experience as a US Navy surgeon during the Korean War, the character of ‘Hawkeye’ in the long-running television series, ‘Mash’, was based on Keith.
Figure 1:

Keith Reemtsma (1925–2000)
Keith Reemtsma – pioneering clinical xenotransplantation
Keith is particularly remembered for the 6 kidney transplants he carried out from chimpanzees to patients in terminal renal failure in the early 1960s (1,2). He believed that this experimental approach was ethically justified because, at that time, chronic dialysis was available to only a small number of patients in the USA, and the number of deceased human donor kidneys that became available was very limited. Because of the much smaller size of the chimpanzees compared with that of the human recipients, he transplanted both kidneys from one chimpanzee into a single patient. The only effective immunosuppressive therapy available to him was azathioprine and corticosteroids.
Five of Keith’s 6 patients did not do well, dying of either rejection or infection within approximately 10 weeks. One patient, however, lived for 9 months, returning to work as a schoolteacher for a period of time, before dying rather suddenly from what was thought to be an electrolyte disturbance. At autopsy, the chimpanzee kidneys showed no features of rejection (Figure2), and the patient’s native kidneys were clearly very diseased. It is perhaps remarkable that even this one patient did so relatively well with the primitive immunosuppressive therapy available at the time. Other surgeons soon followed Keith’s lead, carrying out clinical kidney, liver, and heart transplantation from nonhuman primates (NHPs) (3,4).
Figure 2:
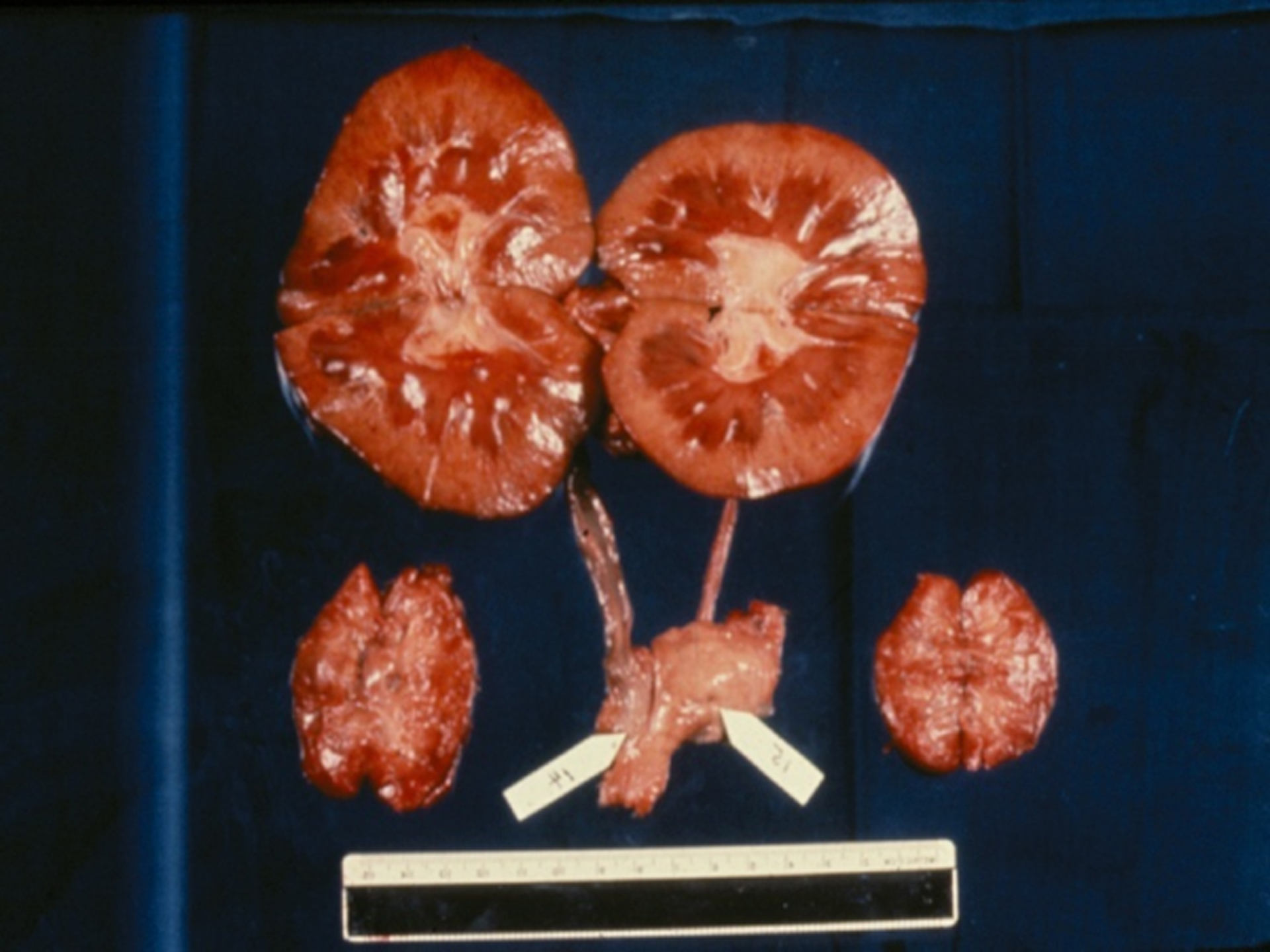
Macroscopic appearance of the two chimpanzee kidneys (top) and the two native kidneys (bottom) at necropsy 9 months after transplantation. The chimpanzee kidneys were macroscopically normal, and microscopically showed no features of rejection.
I suggest that, if we transplanted organs from chimpanzees or other NHPs into human patients today, with the greatly increased variety and potency of immunosuppressive agents available to us, some patients would survive for clinically-useful periods of time, e.g., years, rather than days or weeks.
Keith Reemtsma – later career
Keith carried out these pioneering clinical trials while on the faculty of Tulane University in New Orleans. He went on to become chairman of the department of surgery at the University of Utah (1966–1971) and subsequently at his alma mater, Columbia-Presbyterian Medical Center in New York City (1971–1994). At Columbia, he established a heart transplantation program (with his junior colleague, Eric Rose) that soon became one of the busiest programs in the world.
Although he did not continue to pursue the transplantation of organs from NHPs, Keith maintained an interest in xenotransplantation throughout his career, particularly in relation to islet xenotransplantation (2), and encouraged others to pursue the goal of introducing pig organ xenotransplantation as a form of clinical therapy. Serious exploration of the use of pigs (rather than NHPs) as sources of organs began approximately 20 years after Keith’s initial studies (5–7).
Progress in pig-to-NHP preclinical models
Although it has been a long and arduous investigation – much longer than I anticipated in the 1980s - great progress in pig organ and tissue transplantation has been made. Progress has largely resulted from two major advances – (i) the increasing availability of pigs with multiple genetic manipulations aimed at protecting the cells of the organ from the primate immune response, and (ii) the introduction of novel immunosuppressive agents that block the CD40/CD154 costimulation pathway. These advances have led to prolonged survival of pig kidney grafts in NHPs (Figure3), and today survival is being recorded in years (by the Minneapolis/Emory group) (8).
Figure 3:
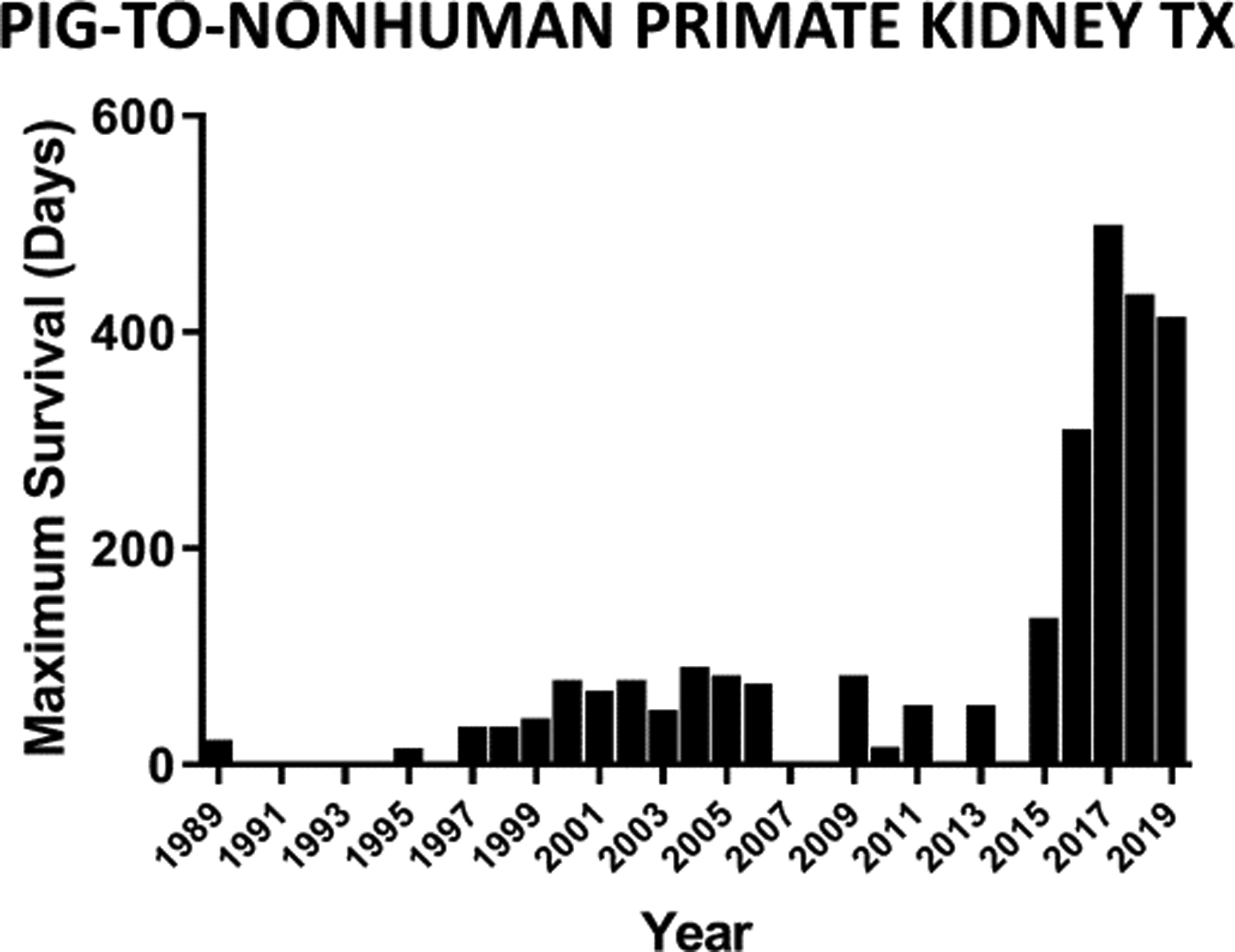
Maximal survival each year of NHPs with pig kidney grafts between 1989 and 2019. Since then, maximal survival has increased to more than 3 years (see Adams AB, reference 8).
The selection of NHP recipients with low serum anti-pig antibody levels clearly increases the likelihood of long-term graft survival (9,10), particularly if an anti-CD154 agent (rather than an anti-CD40 agent) is administered (8,11). However, despite these very encouraging results, consistent long-term success is proving difficult to achieve.
Although progress in pig liver and lung xenotransplantation in NHPs has been slow, pig heart xenotransplantation has also made considerable progress, largely through the work of Bruno Reichart and David Cleveland and their respective colleagues (12,13). Because there are some advantages in selecting pig kidneys, rather than hearts, for the first clinical trials of xenotransplantation, e.g., the availability of dialysis to support the patient if the organ fails or has to be excised (14), attention in this review will be directed largely to pig kidney transplantation.
What is the aim of preclinical studies in the pig-to-NHP model?
It is surely to prepare for clinical trials of xenotransplantation. Why else would we put so much effort into our research if we did not aim for it to advance medical therapy and thus benefit our patients? Most of us would agree that our immediate (minimum) goal is to demonstrate that relatively consistent ‘complication-free’ survival of a NHP with a life-supporting pig organ can be achieved for periods of at least 6 months in a series of at least 6 experiments. ‘Complication-free’ indicates an absence of irreversible rejection or life-threatening infection, with maintenance of satisfactory graft function. At necropsy at 6 months or later, there would need to be no significant histopathological features of rejection and no evidence of infectious or other serious complications.
Six months survival of graft and recipient may not appear long enough as preparation for a clinical trial, but most of us with experience in both the animal laboratory and clinical transplantation will confirm that it is much more difficult to manage an immunosuppressed NHP than a human patient. I believe, therefore, that, if 6 months survival can be achieved (almost) consistently, this may be sufficient to suggest longer-term survival in a clinical trial. A recent meta-analysis by Firl and Markmann suggests that this would be the case (15).
Comparison with the initial attempts at clinical kidney allotransplantation
We are far more advanced in kidney xenotransplantation than our predecessors were in regard to allotransplantation when they began their first clinical attempts. Yu Yu Voronoy, a Ukrainian, carried out the first series of clinical kidney transplants beginning in 1933 (16). Although he correctly surmised that graft loss was from an immune response, he appeared to have no concept of the effects of warm ischemia on the donor kidney, some of them being excised from the donor several hours or even days after death of the donor.
The primitive beginnings of organ allotransplantation
When I had the privilege to meet with one of the very early pioneers of kidney transplantation, French surgeon René Küss - then in his nineties - (who by the way carried out a very unsuccessful pig kidney transplant in a patient in 1966 [17]), he described to me the primitive conditions when he performed his first clinical kidney allotransplants in the 1950s. He and a colleague from another Parisian hospital would go to the local prison where a criminal was due to be guillotined, and would wait outside the execution room. The headless body would be brought out and placed on the floor. On their knees, the surgeons would open the abdomen and remove the kidneys, the surgical field illuminated by the light from a single lamp bulb. Without the use of any form of cold storage, they would each transport one kidney to their respective hospitals and transplant the kidney into the recipient. There was no form of immunosuppressive therapy – not even irradiation. It is no wonder that the results of these pioneering efforts were so poor.
Compare these efforts with what we have already learned about xenotransplantation in the experimental laboratory and from 70 years of experience with clinical kidney allotransplantation. Our first clinical attempts of pig organ transplantation should be far more successful than the first allotransplants.
Moving from the laboratory to the clinic
What do we need to do before initiating a clinical trial of xenotransplantation? I suggest there are 4 major topics we need to consider.
The genetics of the organ-source pig
Until very recently, most groups have been transplanting organs from whatever genetically-engineered pigs became available to them, but not necessarily from the pigs they would select for the first clinical trial. We have enough experience now to know what pig we believe will be optimal (at least for this early stage in the development of clinical xenotransplantation) (18). It does not need to be – indeed, will certainly not be – the ultimate or “perfect’ organ-source pig, but we need to remind ourselves that ‘perfect’ is often the enemy of ‘good’. We just need a pig that has sufficient genetic modifications to protect its tissues from the human innate immune response.
Although there are those who believe that a triple-knockout (TKO) pig, i.e., a pig in which expression of all three known carbohydrate xenoantigens has been deleted (Table1), will be sufficient as the organ-source, I would suggest that a TKO pig with 6 or more added human ‘protective’ transgenes would be preferable. These human transgenes would include one or more for complement-regulatory proteins, e.g., CD46, CD55, one or more for coagulation-regulatory proteins, e.g., thrombomodulin, endothelial protein C receptor (EPCR), one or more for ‘anti-inflammatory (apoptotic) proteins, e.g., hemeoxygenase-1, A20, and possibly for CD47 that, among other effects, may inhibit human macrophage function (18).
Table 1:
Known carbohydrate xenoantigens expressed on pig cells.
| Carbohydrate (Abbreviation) | Responsible enzyme | Gene-knockout pig |
|---|---|---|
| 1.Galactose-α1,3-galactose (Gal). | α1,3-galactosyltransferase | GTKO |
| 2.N-glycolylneuraminic acid (Neu5Gc). | CMAH | CMAH-KO |
| 3.Sda | β−1,4N-acetylgalactosaminyltransferase. | β4GalNT2-KO |
CMAH = Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH).
Although a TKO pig (+/− human transgenes) may be the optimal source of organs for transplantation into a human recipient, there is an abundance of studies indicating that it is not optimal for NHPs. To my knowledge, this observation was first made by José Estrada and his colleagues (19), who produced the first TKO pigs, and has subsequently been investigated by others, particularly by my colleagues Takayuki Yamamoto and Hidetaka Hara (20–23). I will only summarize the problem here.
The problem of the ‘4th’ xenoantigen
Knockout of CMAH (Table1), which is the enzyme responsible for the expression of N-glycolylneuraminic acid (Neu5Gc) in the pig, appears to result in exposure of another antigen – sometimes known as the 4th xenoantigen – against which all NHPs, but not all humans, have natural antibodies (Figure4). When these NHP serum antibodies bind to a pig cell, they have been found to be associated with a remarkably high level of complement-mediated cytotoxicity (Figure5). After pig-to-NHP organ transplantation, this has been demonstrated to result in a high incidence of early graft failure (24).
Figure 4:
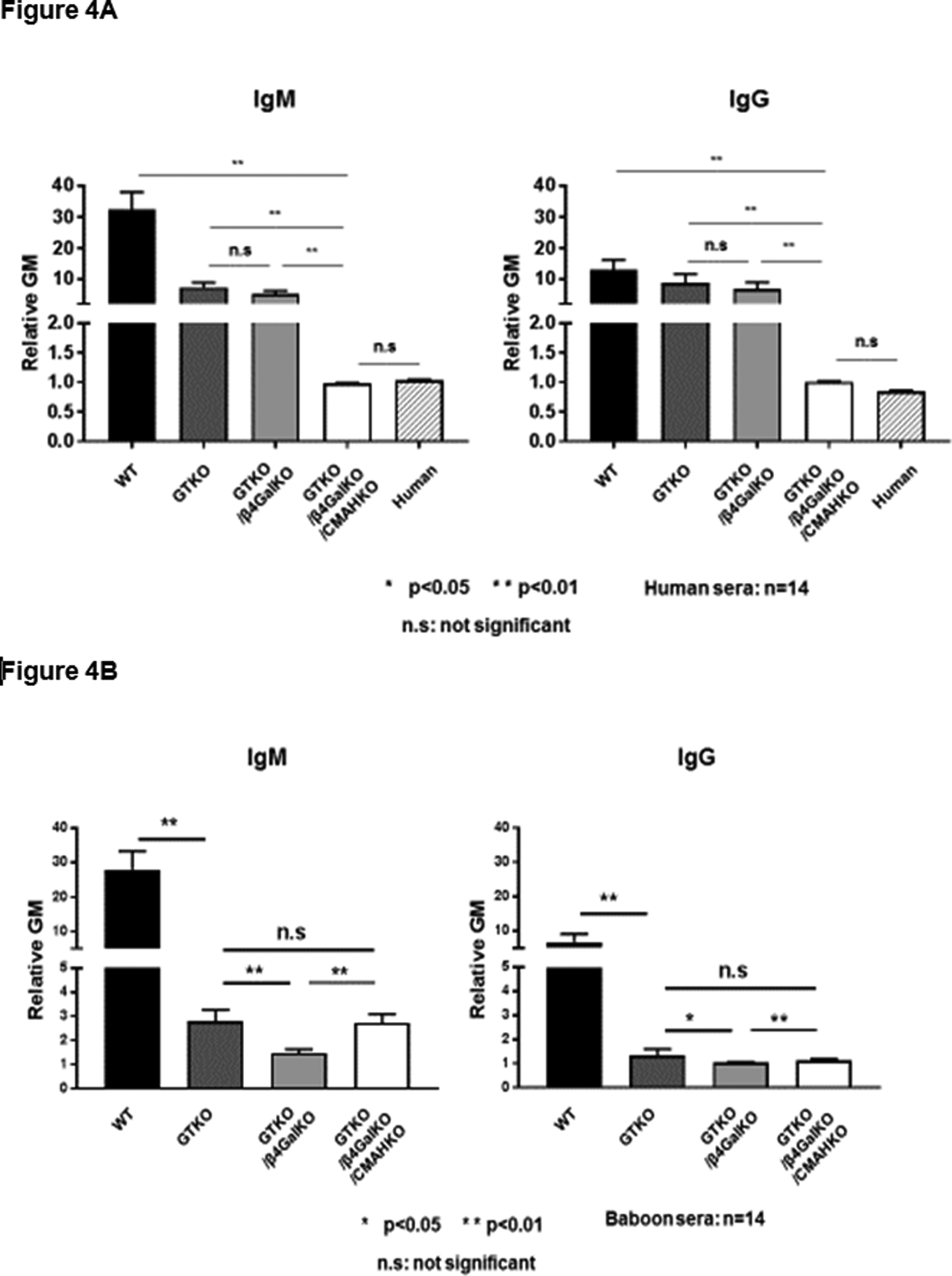
(A) Human serum (n=14) IgM (left) and IgG (right) antibody binding to wild-type (WT), GTKO, double-knockout (i.e., deletion of expression of Gal and Sda), and triple-knockout (TKO, i.e., with additional deletion of expression of Neu5Gc) pig red blood cells (RBCs). Human serum antibody binding to pRBCs was measured by flow cytometry using the relative geometric mean (rGM), which was calculated by dividing the GM value for each sample by the negative control. Negative controls were obtained by incubating the cells with secondary anti-human antibodies only (with no serum). Human IgM and IgG binding to GTKO/β4GalKO/CMAHKO (TKO) pig RBCs was almost at the level of binding to human RBCs, and there was no detectable IgM or IgG binding to TKO RBCs. Binding to TKO pig RBCs was not significantly different from human IgM and IgG binding to human RBCs of blood type O. (*p<0.05, **p<0.01; ns = not significant).
(B) Baboon (an Old World NHP, n=14) IgM and IgG antibody binding to WT, GTKO, DKO, and TKO pig RBCs. (Note that deletion of Neu5Gc [CMAH-KO] in pig cells appears to expose a fourth xenoantigen against which baboons have natural antibodies. Note also that the data support the observation that the deletion of expression of Gal has less effect in reducing antigenicity of human serum (70% reduction) (Figure1A), when compared with baboon serum (90% reduction) (*p<0.05, **p<0.01; ns = not significant).
(Reproduced in part with permission from Cooper DKC et al. Xenotransplantation 2019; Apr 15:e12516. doi: 10.1111/xen.12516, reference 18).
Figure 5:
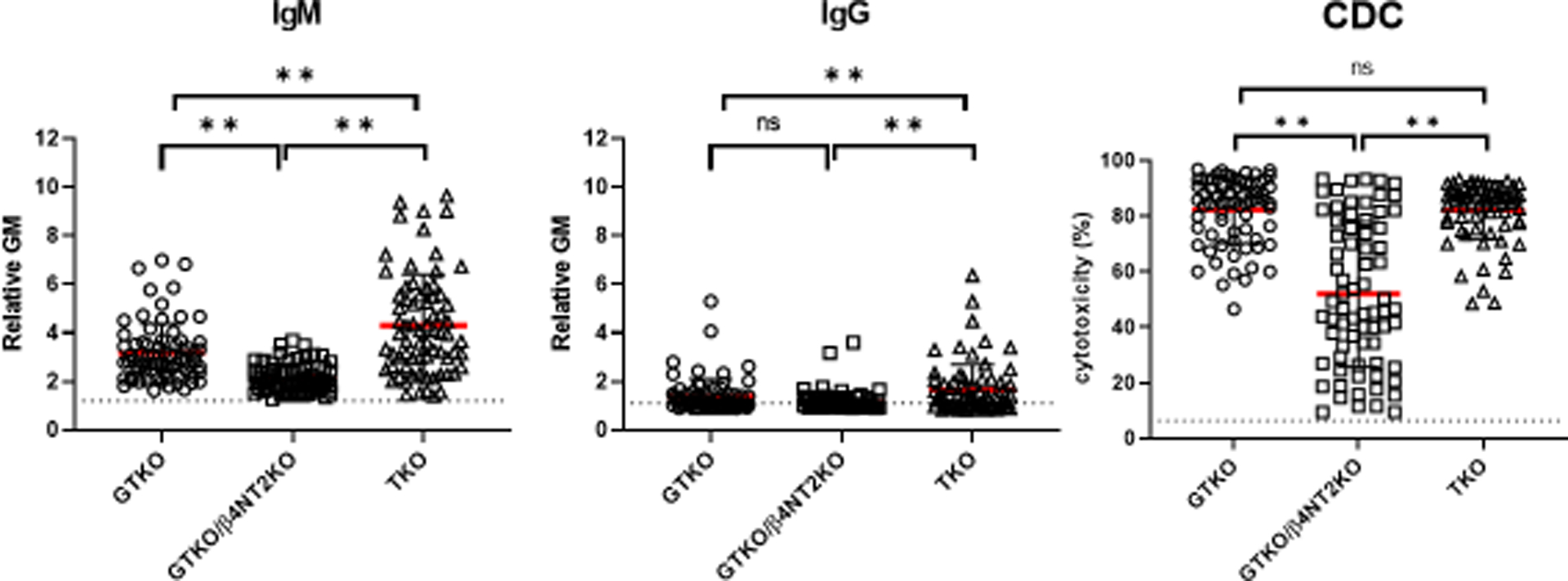
IgM (left) and IgG (middle) binding and complement-dependent cytotoxicity (right) of baboon sera to GTKO, GTKO/β4GalNT2KO (DKO), and TKO pig peripheral blood mononuclear cells (PBMCs). IgM and IgG binding and serum cytotoxicity to TKO cells were higher or comparable to binding to GTKO cells. Although mean IgM and IgG binding and mean serum cytotoxicity to DKO cells were less than to TKO cells, many baboons had a high level of cytotoxicity to DKO cells. (**p<0.01). (Reproduced with permission from Yamamoto T et al, Xenotransplantation. 2020; Jun 25:e12596, reference 21).
Although a TKO pig organ is preferred for clinical xenotransplantation, the transplantation of a GTKO pig organ may be associated with much better results in NHP recipients, and this is because galactose-α1,3-galactose (Gal) plays a much greater role as a target for the immune response in NHPs than in humans (Figure6) (25).
Figure 6:
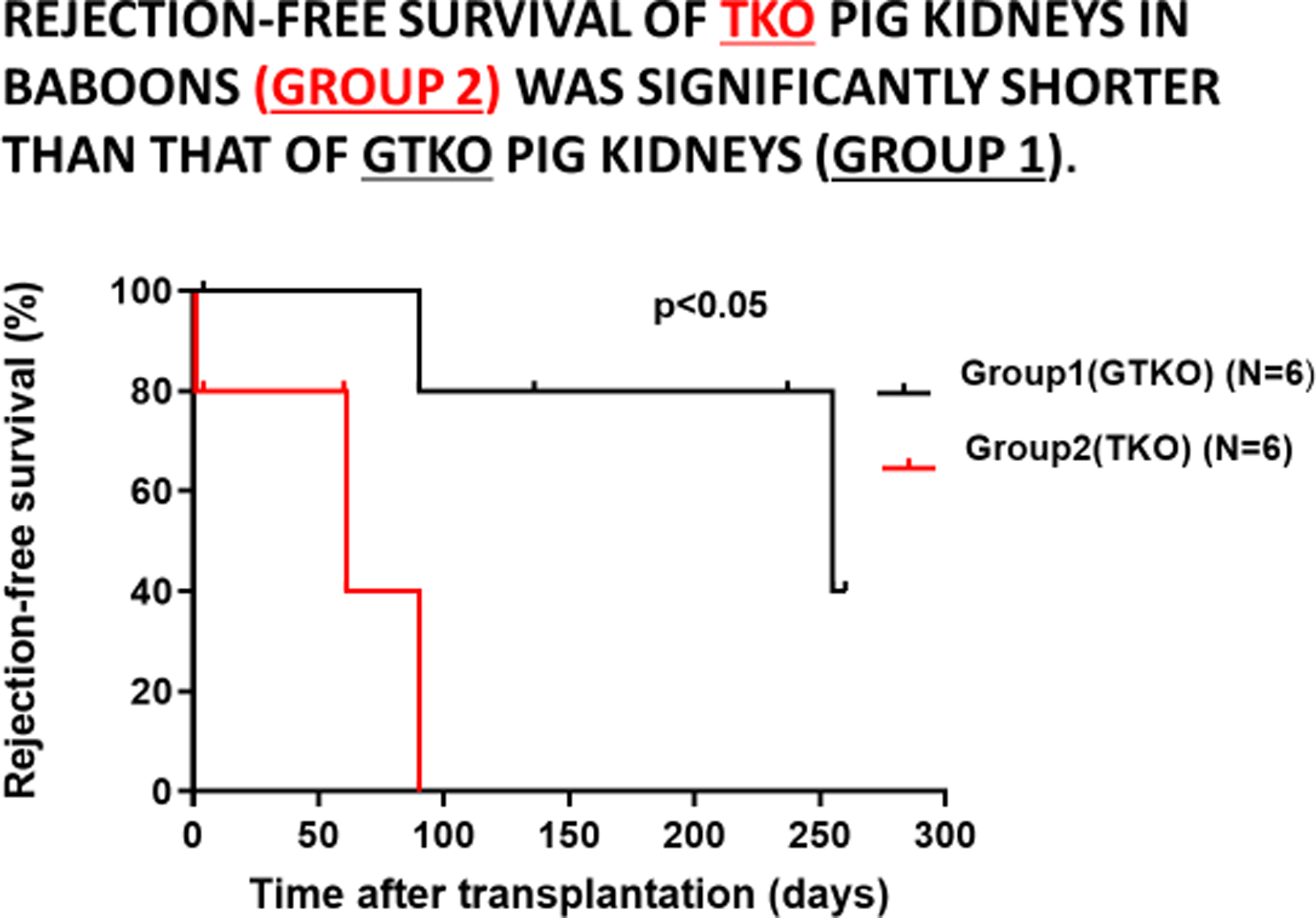
Rejection-free survival of GTKO pig kidneys in baboons (Group 1, in black) was significantly longer that that of TKO pig kidneys (Group 2, in red). (Reproduced with permission from Iwase H, et al, Xenotransplantation. 2021. 25 May. e12700, reference 24)
NHP serum antibody binding to pig cells in which only expression of Neu5Gc remains expressed (i.e., GTKO/β4GalNT2-KO [or double-knockout, DKO] pigs) is significantly reduced, but organs from these DKO pigs do not exactly mimic the results that might be achieved after TKO pig organ transplantation in humans. Despite low antibody binding to DKO pig cells, many NHPs have high levels of serum cytotoxicity to these cells (Figure5).
This suggests to me that the 4th xenoantigen expressed after deletion of Neu5Gc may not be the only remaining xenoantigen (or other aspect of the immune response) that is complicating pig-to-NHP organ transplantation. This ‘final’ barrier to consistent success in the pig-to-NHP model, therefore, remains unresolved, although it would appear that the administration of an anti-CD154 costimulation blockade agent can overcome the problem in some cases (8,11).
There are many other genetic manipulations that have been successfully achieved in pigs, several of them related to reduction of the adaptive immune response, e.g., knockdown or knockout of swine leukocyte antigens (SLA) I and/or II, but these are not essential at present because we have immunosuppressive agents that may suffice.
Accumulation of data for the regulatory authorities
The exact phenotype of the pig should be confirmed in vitro before the in vivo transplant is carried out. If in a series of experiments the organs are derived from pigs of different phenotypes, this is not contributing to the accumulation of the specific information we need to satisfy the regulatory authorities. At this stage, we should not be spending valuable resources and time on experiments that are not aimed at providing data to support a clinical trial.
The regulatory authorities will expect us to provide some justification (either from in vitro or in vivo observations) for each genetic manipulation in the pig. They will also expect us to demonstrate that expression of each transgenic protein is consistently sufficient to have the desired effect, but not excessive. Weak expression of a human transgenic protein may be detrimental to outcome, but over-expression can be equally detrimental. For example, over-expression of human coagulation-regulatory proteins can be associated with a spontaneous bleeding tendency in the pig that is detrimental to the health of the pig. Therefore, it may be that the fewer the number of genetic manipulations (as long as they are effective in protecting the organ graft from the human innate immune response), the more likely that success will follow.
Porcine endogenous retroviruses (PERVs)
Inhibition of expression of PERVs by their inactivation (26) would be an advantage by reducing the possibility of any potential complication associated with their presence, but may not be considered essential by the regulatory authorities. The observation that there has been no evidence of transmission of PERVs or of any associated complication in in vivo models in NHPs may in part be associated with difficulties in transmitting the virus to NHPs (27). A decision will therefore need to be made as to whether inhibition of the expression of PERVs is necessary for the first clinical trial.
The immunosuppressive regimen
The current evidence is that conventional therapy, as used clinically today in organ allotransplantation, e.g., tacrolimus-based, is inadequate in pig organ xenotransplantation, and that only blockade of the CD40/CD154 costimulation pathway prevents the adaptive immune response. These observations were first reported more than 20 years ago by Buhler and his colleagues (28), and have since been confirmed by others (Figure7) (29). In this respect, there is increasing evidence that an anti-CD154 agent is more effective than an anti-CD40 agent (8,30,31). Importantly, the current evidence is that blockade of the CD28/B7 pathway alone is not effective (32).
Figure 7:
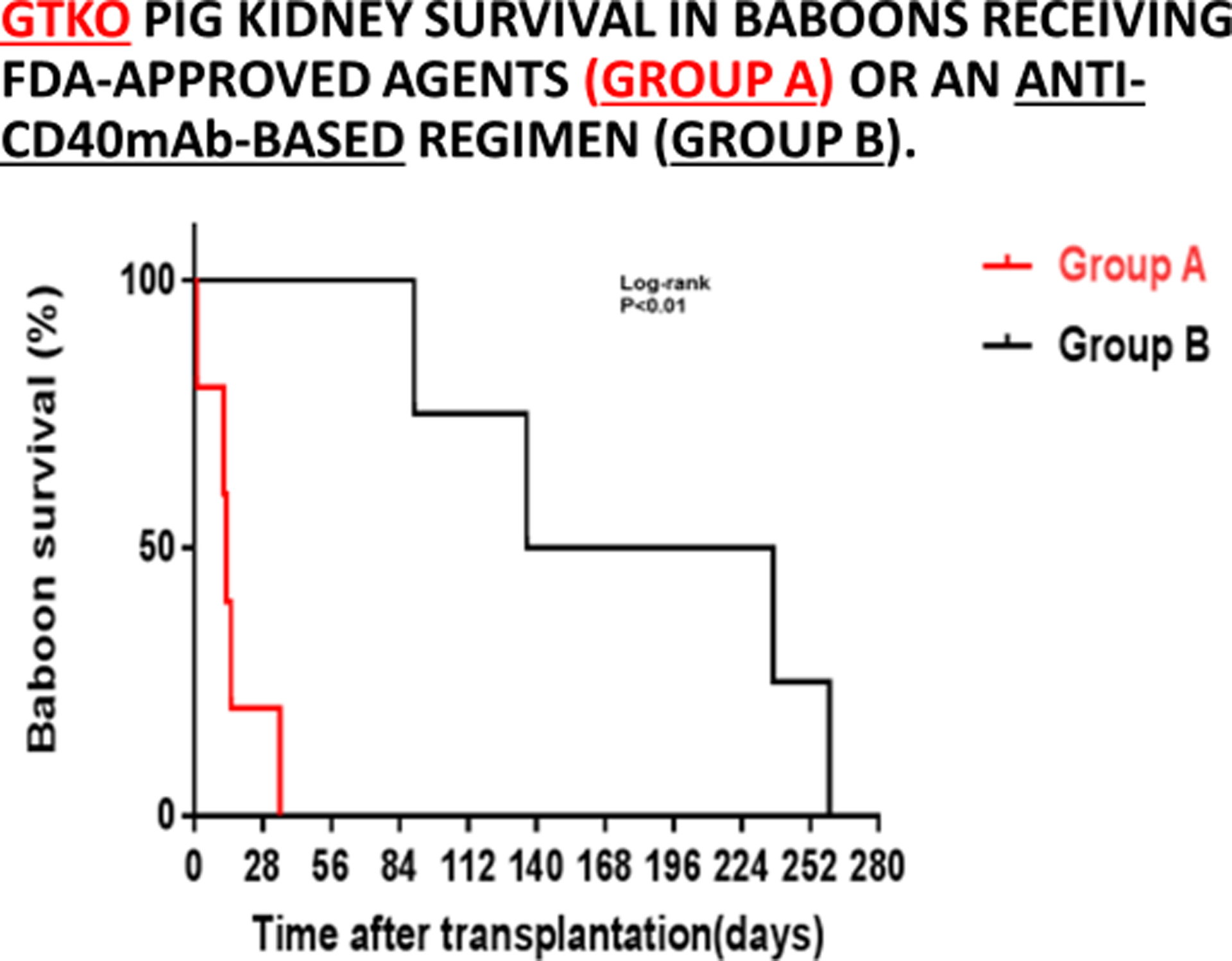
GTKO pig kidney survival in baboons receiving US FDA-approved immunosuppressive agents (Group A, in red) was much shorter than in those receiving an anti-CD40mAb-based regimen (Group B, in black). (Reproduced with permission from Yamamoto T, et al, Transplantation. 2019;103:2090–2104, reference 29)
The immunosuppressive regimen that has been followed by many groups to date (and that has proved moderately successful) has included both induction therapy and at least triple-drug maintenance therapy (Table2) (11,33). However, to my knowledge, when blockade of the CD40/CD154 pathway forms the basis of the regimen, there is no experimental study that has investigated whether either (i) induction therapy or (b) additional maintenance therapy is essential. In one of our recent experiments, monotherapy with only an anti-CD40mAb maintained excellent function of a pig kidney in a baboon for 2 months (until electively euthanized) without the need for any additional agents (34).
Table 2:
Example of the complex immunosuppressive, anti-inflammatory, and adjunctive therapy used in pig-to-baboon kidney transplantation experiments during the past several years.
| Agent | Dose (duration) |
|---|---|
| Induction | |
| Thymoglobulin (ATG) (Genzyme, Cambridge, MA) | 10 mg/kg i.v. (day −3) (to reduce the CD3+T cell count to <500/mm3) |
| Anti-CD20mAb (rituximab) (Genentech, South San Francisco, CA) | 10 mg/kg i.v. (day −2) |
|
Cobra venom factor (CVF) (Complement Technology, Tyler, TX) OR C1-esterase inhibitor (Berinert, CSL Behring, King of Prussia, PA) |
100 U/kg (days −1 and 0) 17.5 U/kg i.v. (days 0, 1, 7 and 14) |
| Maintenance | |
| Anti-CD40 monoclonal antibody (mAb) (2C10R4, a chimeric rhesus IgG4) (NIH NHP Resource Center, Boston, MA) | 50 mg/kg (days −1, 0, 4, 7, 14, and weekly) |
| Rapamycin (Rapa) (LC Laboratories, Woburn, MA) | 0.01–0.04 mg/kg i.m. ×2/d (target trough 6–10 ng/ml), beginning on day −4. |
| Methylprednisolone (Astellas, Deerfield, IL) | 5 mg/kg/d on day 0, tapering to 0.125 mg/kg/d by day 7. |
| Anti-inflammatory | |
| Etanercept (TNF-α antagonist) (Amgen, Thousand Oaks, CA) | 1 mg/kg (day 0), 0.5 mg/kg i.v. (days 3, 7, 10) |
| Adjunctive | |
| Triiodothyronine (levothyroxine) (Xgen, Big Flats, NY, USA) Aspirin (Bayer, Deland, FL) |
4μg i.v. x2/day on days 0–5. 40 mg p.o. (alternate days), beginning on day 4. |
| Low molecular weight heparin (LMWH) (Eisai, Woodcliff Lake, NJ) | 700 IU/d s.c., beginning of day 1. |
| Famotidine (APP Pharmaceuticals, Schaumburg, IL) | 0.25 mg/kg/d ×2 (days −7 to 14) |
| Erythropoietin (Amgen) | 500 U i.v. x2-3 weekly, beginning on day −4 |
| Ganciclovir (Genentech) | 5 mg/kg/d i.v., from day −4 to day 14 and when the baboon is sedated for blood draws (x2 weekly). |
| Valganciclovir (Genentech) | 15 mg/kg/d p.o., beginning on day 15 |
| Sulfamethoxazole and trimethoprim (Teva, North Wales, PA) | 10 mg/kg i.v. daily, on days 4–14 |
| Sulfamethoxazole and trimethoprim oral suspension (Akorn, Lake Forest, IL) | 75 mg/m2 p.o x2/day. x3 weekly, beginning on day 15. |
Simplifying the immunosuppressive regimen
In view of this observation and the encouraging results of Fc-modified anti-CD154 monotherapy in NHP models of organ allotransplantation (Tatsuo Kawai and Robin Pierson 2021, personal communications), this approach should be explored in a pig-to-NHP xenotransplantation model. Several anti-CD154 agents are currently in clinical trials for diseases such as rheumatoid arthritis (33). If the intensity and complexity of the immunosuppressive/adjunctive regimen could be significantly reduced, this would likely reduce the potential risk of exogenous infectious complications, and would render a clinical trial more attractive to patients, medical teams, and the regulatory authorities.
Similarly, with the improved pigs and agents we have available today, there is no definitive evidence that anti-inflammatory or anticoagulant/anti-platelet agents are essential or even beneficial.
Immune monitoring and the diagnosis and treatment of rejection
In the early days of xenotransplantation research – until pigs became available that expressed human coagulation-regulatory proteins - rejection of a pig organ graft in a NHP was always associated with rapid reductions in the platelet count and in plasma fibrinogen, which were features of the thrombotic microangiopathy and consumptive coagulopathy that developed (35–38). These features often presented before a rise in serum creatinine occurred.
Today, however, a diagnosis of rejection can be made on the same basis as that in clinical kidney allotransplantation – a rise in serum creatinine (if it is not associated with dehydration and/or hypovolemia [39]), an increase in proteinuria (which indicates rejection rather than dehydration), and a reduction in renal blood flow on ultrasound examination. The transplant nephrologist caring for the first patients with pig kidney grafts will therefore not be required to think differently from his/her previous experience, which should greatly facilitate management of the patient. However, at least in the early clinical trials, it is likely that more frequent needle biopsies of the graft may be required than in patients with kidney allotransplants today.
When it occurs, rejection of a pig kidney graft can develop rapidly, and the graft can be lost within a few days. The histopathological features are almost always those of acute antibody-mediated rejection, even if rejection is occurring some months after transplantation, with some features of thrombotic microangiopathy (40). Monitoring by immune assays, e.g., anti-pig antibody levels, is frequently non-informative because the antibodies may be absorbed on to the graft (41). It may only be when a rejected graft is excised (if this is possible, e.g., in the case of a heterotopic heart transplant) that the true increase in anti-pig antibody can be measured. In our experience, during antibody-mediated rejection the number of T and B cells in the blood may remain unchanged.
To my knowledge, successful reversal of biopsy-proven severe antibody-mediated rejection of a xenograft has never been reported. Our own experience with high-dose corticosteroid therapy has been uniformly unsuccessful, though additional therapy with an anti-TNF mAb (etanercept) appeared to delay graft failure for a month in one case (24). It will therefore be important to ensure sufficient maintenance immunosuppressive therapy is administered. Histopathological evidence of predominantly T cell-mediated rejection has been rare.
Prevention or treatment of infectious complications
We also need to provide details of (i) how and where the pigs will be bred and housed (in a biosecure facility [42], with regular sentinel pig testing to assess the microbiological status of the cohort), (ii) whether the pigs will receive vaccines and, if so, which ones, and (iii) the prophylactic and treatment plans in relation to potential infectious complications in the recipients.
In this latter respect, expert opinion is that the anticipated infections will largely be those seen in immunosuppressed patients with allografts (or with immunodeficiency conditions) and, fortunately, are likely to be successfully prevented or treated in the majority of cases (43). There is even therapy available that might successfully treat an infection associated with PERVs, if this ever occurs.
We therefore have several important decisions to make, e.g., selecting the phenotype of the organ-source pig and the exact immunosuppressive regimen, before we can provide the regulatory authorities with definitive data to support a clinical trial of pig kidney xenotransplantation.
Selection of patients for the initial clinical trials
This topic has been discussed elsewhere (44) and will only be summarized here. There is a significant mortality of patients on the kidney waitlist in most countries. For example, in the USA, within 5 years approximately 45% of patients have either died or have been removed from the waitlist because they were no longer considered acceptable candidates for the procedure (Figure8). It must be borne in mind that all of these patients were considered acceptable candidates when initially added to the waitlist, and it is only the delay caused by the critical shortage of human organs for transplantation that resulted in their change in status. We, and others, have suggested that patients who are very unlikely to survive until a suitable deceased human donor kidney becomes available are those who should be considered for the initial trials of pig kidney xenotransplantation (44).
Figure 8:
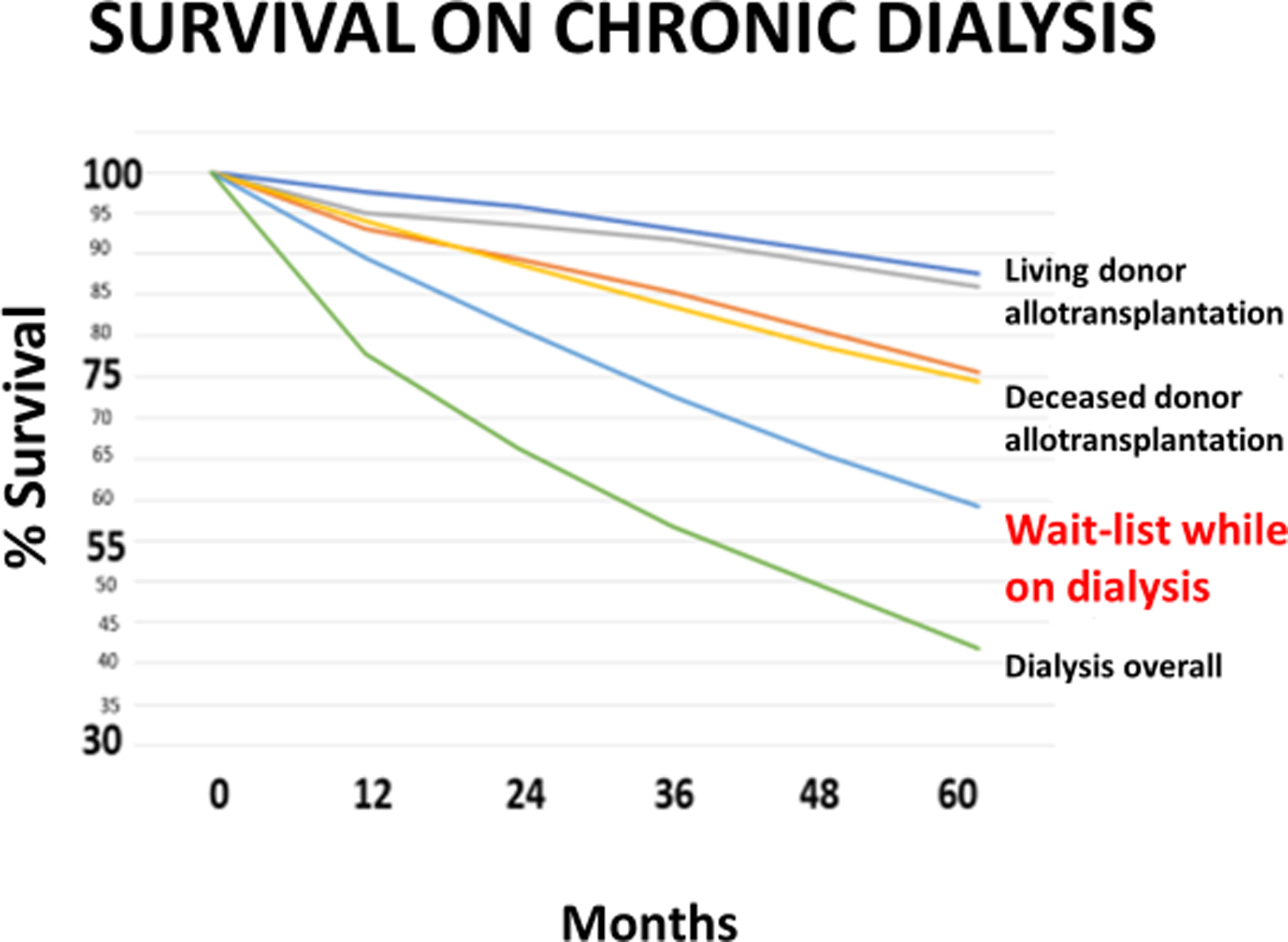
Survival of patients with a living donor kidney (top), a deceased donor kidney (second from top), on the waitlist while on dialysis (second from bottom), and while on dialysis but not waitlisted for a kidney transplant (bottom). Approximately 45% of patients receiving chronic dialysis while on the waitlist for a deceased human donor kidney either die or are removed from the waitlist (as considered no longer acceptable candidates for the procedure) within 5 years. (Reproduced with permission from Jagdale A, et al, Transplantation. 2021;105:1904–1908, reference 44)
These are largely older patients, e.g., 55–65 years-old, whose general health status is likely to deteriorate before an allograft becomes available. Younger patients are more likely to remain acceptable candidates for many years, and so perhaps should not be considered for the first clinical trials. Patients of blood groups O and B may wait longer than others and, in the USA, patients in certain geographic regions may also be at a disadvantage in this respect. There is evidence that patients with diabetes are more likely to be removed from the waitlist than others, and at a younger age, and so should be given special consideration, but this comorbidity might increase the risk of post-transplant complications. We suggest that the patients included in the first clinical trials of xenotransplantation should be fully acceptable for allotransplantation, with no serious comorbidities. Only in this way will the potential of xenotransplantation be able to be accurately assessed.
Should allosensitized patients be included in the first clinical trial?
Although allosensitized patients with high panel-reactive antibodies are likely to benefit significantly from the availability of pig organs in the future, we suggest that they should not be included in the initial clinical trials because there is some evidence that prior allosensitization may be detrimental to the outcome of xenotransplantation (45,46). This is likely a result of anti-HLA antibodies that cross-react with swine leukocyte antigens (SLA). A recent in vivo study by Jean Kwun and his colleagues in allosensitized monkeys indicated that pig graft survival was significantly reduced when compared to survival in a non-allosensitized recipient (46). However, if the pig expressed multiple human protective proteins, graft survival was significantly improved. There is hope, therefore, that selected genetic engineering of the organ-source pig may overcome this problem. In this respect, Joe Ladowski, Greg Martens, and their colleagues have demonstrated that genetic modification of the amino acids on the surface of a pig cell, e.g., by mutating an arginine to proline, can result in a reduction in antibody binding to the cell (47).
One factor to be considered in planning a clinical trial is that all of the (admittedly limited) evidence to date indicates that sensitization to pig antigens will not be detrimental to the outcome of a subsequent allotransplant, even if antibodies have developed to TKO pig antigens (48,49). Failure of a xenograft would therefore not impact the patient’s prospect of subsequent successful allotransplantation. This would enable patients on the waitlist for an allograft to maintain their status and opt for allotransplantation if and when the opportunity arose.
Assessing public attitudes to xenotransplantation
When organ allotransplantation was initiated in the 1950s and 1960s, there was considerable resistance and even antagonism from some members of society, particularly to heart transplantation, and there is likely to be as much, or even more, to pig organ xenotransplantation. Therefore, before initiating a clinical trial of xenotransplantation, it would be wise to determine the attitudes of patients, healthcare professionals, and the public to this new form of therapy.
A number of surveys and focus group interviews by Wayne Paris and his colleagues have indicated a general support for xenotransplantation - if the results are likely to be comparable to those of allotransplantation (50–57). Of course, this cannot be guaranteed and, indeed, is not likely to be achieved in the initial clinical trials. Patients awaiting a kidney transplant and their families had a more positive attitude than others in the community, though African-Americans were more cautious, probably as a result of some unethical trials to which they were exposed in the past. Pig organ xenotransplantation was acceptable to the major religious groups (Christian Jewish, Moslem), as the maintenance of human life takes precedence over other factors.
More such surveys and focus group studies need to be carried out, and reservations about clinical organ xenotransplantation discussed and resolved.
Conclusions
There is strong evidence from numerous in vitro studies that the transplantation of a TKO pig organ, particularly if expressing several human protective proteins, is likely to be significantly more successful if transplanted into a human recipient than into a NHP recipient. In view of the advances being made, the time has surely come when we need to consider moving from the laboratory to the clinic. Progress is much more likely to be made from a small clinical trial than if we persist in carrying out experiments in an animal model that no longer mimics the clinical situation. However, several important decisions need to be made before completing the preclinical studies that will provide the regulatory authorities with sufficient evidence to approve such a trial.
I leave you with the advice I received many years ago from one of my early mentors in cardiac transplantation, Christiaan Barnard, the surgeon who carried out the world’s first clinical cardiac allotransplant – “You cannot stay in the laboratory forever.” I suggest we should consider this advice today.
Acknowledgements
The author expresses his gratitude to the NIH for grant support for his studies during the past 20 years. His research is currently supported in part by NIH NIAID U19 grant AI090959, and in part by a U.S. Department of Defense grant W81XWH2010559.
Abbreviations
- IXA
International Xenotransplantation Association
- NHP
nonhuman primate
- PERV
porcine endogenous retrovirus
- TKO
triple-knockout pigs, i.e., pigs that do not express any of the 3 known carbohydrate xenoantigens
Footnotes
Conflict of interest statement
The author is a consultant to eGenesis Bio, Cambridge, MA, USA, but the opinions he has expressed in this article are his own and do not necessarily represent the views of eGenesis Bio.
References
- 1.Reemtsma K, McCracken BH, Schlegel JY, et al. Renal heterotransplantation in man. Ann Surg 1964;160:384–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy MA. The ‘Reemtsma Era’: recollections of an acolyte. In Cooper DKC (ed) Recollections of Pioneers in Xenotransplantation. Nova, New York, 2018, pp1–20 [Google Scholar]
- 3.Taniguchi S, Cooper DKC. Clinical xenotransplantation: past, present and future. Ann R Coll Surg Engl 1997;79:13–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper DKC, Ekser B, Tector AJ. A brief history of clinical xenotransplantation. Int J Surg. 2015;23(Pt B):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lexer G, Cooper DKC, Rose AG, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant 1986;5:411–418. [PubMed] [Google Scholar]
- 6.Cooper DKC, Human PA, Lexer G, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant 1988;7:238–246. [PubMed] [Google Scholar]
- 7.Alexandre GPJ, Gianello P, Latinne D, et al. Plasmapheresis and splenectomy in experimental renal xenotransplantation. In: Xenograft 25, Hardy MA (ed), Elsevier Sciences (Biomedical Division), 1989, pp. 259–266. [Google Scholar]
- 8.Adams AB. Progress in kidney xenotransplantation. Presentation at the eGenesis-sponsored symposium at the IXA/CTRMS (virtual) congress, September 2021. (https://app.ixa-ctrms2021.org) [Google Scholar]
- 9.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med 2005:11:29–31. [DOI] [PubMed] [Google Scholar]
- 10.Higginbotham L, Mathews D, Breeden CA, et al. Pre‐transplant antibody screening and anti‐CD154 costimulation blockade promote long‐term xenograft survival in a pig‐to primate kidney transplant model. Xenotransplantation 2015;22:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma D, Hirose T, Lassiter G, et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am J Transplant 2021; Jul 31. Doi: 10.1111/ajt.16780. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018. 10.1038/s41586-018-0765-z. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland DC, Jagdale A, Carlo WF, et al. The genetically engineered heart as a bridge to allotransplantation in infants: just around the corner? Ann Thorac Surg. 2021. In press. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DKC, Cleveland DC. The first clinical trial – kidney or heart? (Letter) Xenotransplantation. 2020. Dec18:e12644. doi: 10.1111/xen12644 [DOI] [PubMed] [Google Scholar]
- 15.Firl DJ, Markmann JF. Measuring success in pig to non-human-primate renal xenotransplantation: systemic review and meta-analysis of a quarter century of life sustaining NHP renal allo- and xeno-transplantation. Presentation at the eGenesis-sponsored symposium at the IXA/CTRMS (virtual) congress, September 2021. (https://app.ixa-ctrms2021.org) [Google Scholar]
- 16.Hamilton DN, Reid WA. Yu. Yu. Voronoy and the first human kidney allograft. Surg Gynecol Obstet, 1984;159:289–294. [PubMed] [Google Scholar]
- 17.Kuss R Human renal transplantation memories, 1951–1981. In: Terasaki PL (ed). History of Transplantation: Thirty-Five Recollections. Los Angeles, UCLA Tissue Typing Laboratory, 1991, pp. 37–59. [Google Scholar]
- 18.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation 2019;26:e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estrada JL, Martens G, Li P, et al. Evaluation of human and non‐human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Y, Yamamoto T, Raza SS, et al. Evidence for GTKO/β4GalNT2KO pigs as the preferred organ-source for Old World nonhuman primates as a preclinical model of xenotransplantation. Transplant Direct 2020. Jul 24;6(8):e590. doi: 10/1097/TXD1038. eCollection 2020 Aug. 2020;46:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto T, Hara H, Iwase H, et al. The final obstacle to successful preclinical xenotransplantation? Xenotransplantation. 2020; Jun 25:e12596. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Iwase H, Patel D, et al. Old World monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (triple-knockout). Sci Rep. 2020. Jun 17;10:9771: 10.1038/s41598-020-66311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto T, Hara H, Ayares D, Cooper DKC. The problem of the ‘4th xenoantigen’ after pig organ transplantation in nonhuman primates may be overcome by expression of human ‘protective’ proteins (Letter). Xenotransplantation. 2020. Nov 1;e12658. doi: 10.1111/xen.12658. [DOI] [PubMed] [Google Scholar]
- 24.Iwase H, Jagdale A, Yamamoto T, et al. Evidence suggesting that deletion of expression of N-glycolylneuraminic acid (Neu5Gc) in the organ-source pig is associated with increased antibody-mediated rejection of kidney transplants in baboons. Xenotransplantation. 2021. 25 May 2021. E12700. [DOI] [PubMed] [Google Scholar]
- 25.Cooper DKC. Clinical trials of pig heart transplantation. J Heart Lung Transplant. 2020. Aug 29:S1053–2498(20)31697–1. Doi: 10.1016/jhealun.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Niu D, Wei HJ, Lin L, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 2017;357:1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denner J Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 2018;15(1):28. doi: 10.1186/s12977-018-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bühler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation 2000;69:2296–2304. (2000a) [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Hara H, Foote J, et al. Life-supporting kidney xenotransplantation from genetically-engineered pigs in baboons: a comparison of two immunosuppressive regimens. Transplantation. 2019;103:2090–2104. [DOI] [PubMed] [Google Scholar]
- 30.Shin JS, Kim JM, Kim JS, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15:2837–2858. [DOI] [PubMed] [Google Scholar]
- 31.Shin JS, Kim JM, Min BH, et al. Pre-clinical results in pig-to-non-human primate islet xenotransplantation using anti-CD40 antibody (2C10R4)-based immunosuppression. Xenotransplantation. 2018; Jan;25(1): 10.1111/xen.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwase H, Ekser B, Satyananda V., et al. Pig-to-baboon heterotopic heart transplantation – exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 2015; 22:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bikhet MH, Iwase H, Yamamoto T, et al. What therapeutic regimen will be optimal for initial clinical trials of pig organ transplantation? Transplantation. 2021. Jan 27. Doi: 10.1097/TP.0000000000003622. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Cooper DKC., Foote JB, Javed M, et al. Initial evidence that blockade of the CD40/CD154 costimulation pathway alone is sufficient as maintenance therapy in xenotransplantation. Xenotransplantation. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bühler L, Basker M, Alwayn IPJ, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation 2000;70:1323–1331. [DOI] [PubMed] [Google Scholar]
- 36.Knosalla C, Gollackner B, Bühler L, et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am J Transplant 2003;3:1510–1519. [DOI] [PubMed] [Google Scholar]
- 37.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiologic observations in baboons with life-supporting genetically-engineered pig kidney grafts. Xenotransplantation. 2017;24: doi: 10.1111/xen.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwase H, Yamamoto T, Cooper DKC. Episodes of hypovolemia/dehydration in baboons with pig kidney transplants: A new syndrome of clinical importance? Xenotransplantation. 2018. Nov 28:e12472. doi: 10.1111/xen.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foote JB, Jagdale A, Yamamoto T, et al. Histopathology of pig kidney grafts with/without expression of the carbohydrate Neu5Gc in immunosuppressed baboons. Xenotransplantation. 2021. Oct 13. Doi: 10.1111/xen.12715. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucander ACK, Hara H, Nguyen HQ, Foote JB, Cooper DKC. Immunological selection and monitoring of patients undergoing pig kidney transplantation. Xenotransplantation. 2021. 10.1111/xen.e12686. [DOI] [PubMed] [Google Scholar]
- 42.Kraebber K, Gray E. Addressing regulatory requirements for the organ-source pig – a pragmatic approach to facility design and pathogen prevention. In: Clinical Xenotransplantation: Pathways and Progress in the Transplantation of Organs and Tissues between Species (eds: Cooper DKC, Byrne GW). Springer, New York, 2020. pp141–153. [Google Scholar]
- 43.Fishman JA. Prevention of infection in xenotransplantation: designated pathogen-free swine in the safety equation. Xenotransplantation 2020;27:e12595. doc:10/1111/xen12595. [DOI] [PubMed] [Google Scholar]
- 44.Jagdale A, Kumar V, Anderson DJ, et al. Suggested patient selection criteria for initial clinical trials of pig kidney xenotransplantation in the USA. Transplantation. 2021;105::1904–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrne GW. Does human leukocyte antigens sensitization matter for xenotransplantation? Xenotransplantation 2018;25:e12411. doi: 19.1111/xen.12411.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwun J Xenokidney transplantation in highly sensitized NHP model. Presentation at the eGenesis-sponsored symposium at the IXA/CTRMS (virtual) congress, September 2021. (https://app.ixa-ctrms2021.org) [Google Scholar]
- 47.Ladowski JM, Martens GR, Tector M, et al. Examining epitope mutagenesis as a strategy to reduce and eliminate human antibody binding to class II swine leukocyte antigens. Immunogenetics 2019;71:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Q, Hara H, Zhang Z, Breimer ME, Wang Y, Cooper DKC. Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation. 2018;25:e12393. doi: 10.1111/xen.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hara H, Nguyen H, Wang Z-Y, et al. Evidence that sensitization to triple-knockout pig cells will not be detrimental to subsequent allotransplantation. Xenotransplantation. 2021. May 30:e12701. doi: 10.1111/xen.e12701. [DOI] [PubMed] [Google Scholar]
- 50.Paris W, Seidler RJH, Fitzgerald K, Padela AI, Cozzi E, Cooper DKC. Jewish, Christian and Muslim theological perspectives about xenotransplantation. Xenotransplantation 2018. May;25:e12393. Doi: 10.1111/xen.12393. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell C, Lipps A, Padilla L, Werkheiser Z, Cooper DKC, Paris WD. Meta-analysis of public perceptions towards xenotransplantation. Xenotransplantation. 2020; Jan19:e12583. Doi: 10.1111/xen.12583. [DOI] [PubMed] [Google Scholar]
- 52.Padilla LA, Hurst DJ, Jang K, et al. Racial differences in attitudes to clinical pig organ xenotransplantation. Xenotransplantation. 2020. Oct25:e12656. [DOI] [PubMed] [Google Scholar]
- 53.Padilla LA, Hurst DJ, Lopez R, Kumar V, Cooper DKC, Paris W. Attitudes to clinical pig kidney xenotransplantation among medical providers and patients. Kidney 360. 2020;1:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padilla LA, Rhodes L, Sorabella RA, et al. Attitudes toward xenotransplantation: a survey of parents and pediatric cardiac providers. Pediatr Transplant. 2020. Oct6:e13851. Doi: 10.1111/petr.13851. [DOI] [PubMed] [Google Scholar]
- 55.Padilla LA, Sorabella RA, Carlo WF, et al. Attitudes to cardiac xenotransplantation by pediatric heart surgeons and physicians. World J Pediatr Congenit Heart Surg. 2020; 121:426–430. [DOI] [PubMed] [Google Scholar]
- 56.Hurst DJ, Padilla LA, Cooper DKC, Paris WD. Factors influencing attitudes toward xenotransplantation clinical trials: a report of focus group studies. Xenotransplantation. 2021. Mar 7:e12684. Doi: 10.1111/xen.12684. [DOI] [PubMed] [Google Scholar]
- 57.Hurst DJ, Padilla LA, Cooper DKC, Walters W, Paris WD. The attitudes of religious group leaders towards xenotransplantation: a focus group study. 2021. Submitted. [DOI] [PMC free article] [PubMed]


