Abstract
Recollection of personal past events differs across the lifespan. Older individuals recall fewer episodic details and convey more semantic information than young. Here we examine how gray matter volumes in temporal lobe regions integral to episodic and semantic memory (hippocampus and temporal poles, respectively) are related to age differences in autobiographical recollection. Gray matter volumes were obtained in healthy young (n = 158) and old (n = 105) adults. The temporal pole was demarcated and hippocampus segmented into anterior and posterior regions to test for volume differences between age groups. The Autobiographical Interview was administered to measure episodic and semantic autobiographical memory. Volume associations with episodic and semantic autobiographical memory were then assessed. Brain volumes were smaller for older adults in the posterior hippocampus. Autobiographical memory was less episodic and more semanticized for older versus younger adults. Older adults also showed positive associations between temporal pole volumes and episodic autobiographical recall; in the young, temporal pole volume was positively associated with performance on standard laboratory measures of semantic memory. Exploratory analyses revealed that age-related episodic autobiographical memory associations with anterior hippocampal volumes depended on sex. These findings suggest that age differences in brain structures implicated in episodic and semantic memory may portend reorganization of neural circuits to support autobiographical memory in later life.
Keywords: Autobiographical interview, episodic, semantic, anterior hippocampus, posterior hippocampus, aging
1. Introduction
Autobiographical memory (AM) differs across the adult lifespan, often presenting as a shift from richly episodic to more semanticized recollections from younger to older adulthood (e.g., Levine et al., 2002). This shift in AM with age is coincident with age-related brain changes to the hippocampus and temporal poles, which have been implicated in episodic and semantic memory respectively (e.g., Eslinger, 1998; Herfurth et al., 2010). However, associations between age-related changes in AM detail generation and these temporal lobe structures have not yet been directly examined. This is partially attributable to considerable population variability, both in the shifting nature of AM recollection (e.g., Wilson et al., 2002) as well as changes in regional grey matter volumes (e.g., Sele et al., 2020) that occur with normal aging. Well-powered samples are thus needed to examine how shifts in AM and volume covary. Here we collected structural imaging and Autobiographical Interview (Levine et al., 2002) data from a large sample of healthy younger and older adults to test for age differences in hippocampal and temporal pole volumes and relationships to episodic and semantic AM.
1.1. Hippocampal Specialization, Autobiographical Memory, and Volume Changes with Age
The hippocampus plays a central role in AM retrieval, particularly with respect to episodic details (Svoboda et al., 2006). Prominent theories suggest that the hippocampus binds together details that are processed across a number of cortical systems (e.g., Moscovitch et al., 2016) to invoke a sense of personal re-experiencing (e.g., Thakral et al., 2020). However, there is growing evidence of functional heterogeneity in how the hippocampus supports AM along its longitudinal axis (Poppenk et al., 2013; Strange et al., 2014; Brunec et al., 2018). Anterior and posterior segments have been functionally related to different aspects of the autobiographical retrieval process (Moscovitch et al., 2016; Sheldon & Levine, 2016). Anterior hippocampus is associated with generalized or gist-based representations of past events (semantic AM) while the posterior hippocampus is associated with more fine-grained recollections (episodic AM; see Sheldon et al., 2019).
This long-axis functional specialization has been related to structural differences between segments of the hippocampus (e.g., Poppenk & Moscovitch, 2011; Maguire et al., 2006; Brunec et al., 2019). Age-related differences in whole hippocampal volumes may preferentially affect one region of the hippocampus more than the other and alter specialization. However, there is mixed evidence on the specific nature of hippocampal structural changes, rendering it difficult to draw firm conclusions (see Ta et al., 2011 and Bettio et al., 2017 for reviews). Cross-sectional findings comparing younger and older adults have found smaller volumes in both anterior (e.g., Rajah et al., 2010) and posterior (e.g., Driscoll et al., 2003; Malykhin et al., 2008; Stark et al., 2021) portions of the hippocampus. Mixed findings have been observed with age-volume relationships in adult lifespan samples, with reports of larger age-related changes in anterior (e.g., Jack, 1997; Hackert et al., 2002; Gordon et al., 2013) as well as posterior (e.g., Kalpouzos et al., 2009) hippocampal volumes. Longitudinal aging studies report more convergent evidence for age-related volume loss in the anterior hippocampus (e.g., Chen et al., 2010). It has also been suggested that variability in hippocampal volumes does not differ across age groups and that atrophy may be a heritable trait (Lupien et al., 2006). This lack of consistency across studies suggests that testing age-related volume differences in the hippocampus is highly dependent on the experimental method, the study sample and the individuals, or group of individuals, carrying the variance (Buckner, 2004). Targeted within-subject volume changes are the focus of some studies while larger, cross-sectional cohort studies may be necessary to determine how more subtle volume-behavior relationships differ with age.
1.2. Temporal Poles, Autobiographical Memory, and Volume Changes with Age
The temporal poles are also implicated in AM (Svoboda et al., 2006; Renoult et al., 2019), particularly in the processing of schematic and personal semantic information (Graham et al., 2003; Renoult et al., 2012). Yet individuals with semantic dementia, which is associated with neurodegenerative changes to temporal polar regions (e.g., Chan et al., 2001), show impairments to both semantic and episodic AM (Irish et al., 2012). This suggests that semantic processes may be necessary for episodic AM, shaping and constraining information encoded and subsequently retrieved (Irish et al., 2012). Young adults show similar activation patterns within the anterior temporal region while viewing video clips with shared prior semantic knowledge, suggesting that amodal semantic (conceptual in this instance) representations may be evoked during encoding of naturalistic scenes (Raykov et al., 2021; Murphy et al., 2017; Patterson et al., 2007; Binder & Desai, 2011). Memory integration is also enhanced with strong prior knowledge representations (Miller-Goldwater et al., 2021). An established semantic knowledge base may therefore help to situate and organize new memories (Conway & Pleydell-Pearce, 2000). Since preservation of semantic memory is a hallmark of healthy aging (e.g., Park & Reuter-Lorenz, 2009), access to prior knowledge stores may especially facilitate older adult recollection (Umanath & Marsh, 2014; Spreng & Turner, 2019). The temporal pole may therefore show different functional associations with AM in younger and older adults, but this has yet to be tested directly.
There is mixed evidence for age-associated changes to temporal pole volumes. Stable volumes have been reported between younger and older adults (Bergfield et al., 2010). Volume changes in older age have also been reported. In a cross-sectional study of 116 older adults separated into younger and older groups, the younger elderly participants had larger temporal pole volumes than the older elderly participants (Resnick et al., 2000). Longitudinal studies also show mixed findings. Studies of annualized volume changes in the temporal poles have reported both significant reductions (Fjell et al., 2009) as well as stable volumes with advancing age (Resnick et al., 2000). A recent report with a larger sample and longer follow-up window demonstrated that temporal pole volumes more steeply decline with advancing age over a four-year period relative to other regional volumes (Sele et al., 2020). However, changes were highly variable across participants, offering a partial explanation for the variable findings reported in early studies.
1.3. Study Aims and Hypotheses
The present study directly tested whether age-related differences in hippocampus and temporal pole grey matter volumes are associated with age differences in the content of AM. Recognizing the challenges posed by inter-individual variability in detecting brain-behavior associations, we collected Autobiographical Interviews and structural MRIs in a large sample of younger and older adults. The primary study aim was to determine how individual differences in episodic and semantic AM in younger and older adults relate to hippocampal (anterior and posterior segments) and temporal pole volumes. Given the variability in previous reports we provisionally hypothesized (i) age differences in the contents of autobiographical recollection, with lower episodicity and higher semantic density predicted for older versus younger adults, (ii) age differences in grey matter volumes across the three temporal lobe brain structures, with lower volumes observed for older versus younger adults, and (iii) age differences in hippocampal and temporal pole volume associations with AM content. Specifically, we predicted that episodicity would more strongly relate to hippocampal volumes in younger adults. In contrast, given previous reports of semantic scaffolding of episodic AM (Irish et al., 2012), we predicted that both hippocampal and temporal pole volumes would be related to episodic AM in older adults.
Finally, sex is an important consideration in volumetry, particularly with respect to the hippocampus. Males and females tend to have comparable hippocampal volumes after adjusting for total brain volume (Tan et al., 2016). Young adult females have shown larger posterior hippocampal volumes than males and unique patterns of structural covariance between the posterior hippocampus and the rest of the brain (Persson et al., 2014). Rates of age-related hippocampal atrophy are also steeper in females (e.g., Fisher et al., 2018). Sex differences have also been reported in temporal pole volumes (Lotze et al., 2019). Here we investigated sex as an additional exploratory factor in volume associations with AM, recognizing the limits of statistical power in our sample to detect interaction effects.
2. Methods
2.1. Participants
Participants were 158 younger (91 female) and 105 older (57 female) healthy adults from Ithaca, New York and Toronto, Canada (Table 1). Behavioral and functional neuroimaging data from these participants have been reported elsewhere (Setton & Mwilambwe-Tshilobo, In Press; Setton et al., 2021; Spreng et al., In Press) and are summarized briefly below. This study was carried out in accordance with the Institutional Review Board at Cornell University and the Research Ethics Board at York University.
Table 1.
Sample Demographics
| Descriptive Statistics | Inferential Statistics | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Younger Adults | Older Adults | T | dof | p | 95% CI | Cohen’s d | |
|
| |||||||
| N | |||||||
| Ithaca | 131 (74 female) | 83 (47 female) | |||||
| Toronto | 27 (17 female) | 22 (11 female) | |||||
| Race | 60.39% white, 20.13% asian, 8.44% black or african american, 3.90% mixed race, .54% other, 2.60% not provided |
94.23% white, .96% asian, 1.93% black or african american, 2.88% other |
|||||
| Ethnicity | 81.17% non-hispanic or latino, 11.04% hispanic or latino, 7.79% not provided |
92.31% non-hispanic or latino, 1.92% hispanic or latino, 5.77% not provided |
|||||
| Age (years) | |||||||
| Range | 18 – 34 | 60 – 89 | |||||
| M | 22.59 | 68.19 | |||||
| SD | 3.33 | 6.29 | |||||
| Education (years)* | |||||||
| Range | 12.00 – 24.00 | 12.00 – 24.00 | −7.32 | 261 | < .001 | [−2.78, −1.60] | 0.92 |
| M | 15.18 | 17.37 | |||||
| SD | 1.94 | 2.90 | |||||
| Episodic Memory* | 15.98 | 255 | < .001 | [1.08, 1.36] | 2.22 | ||
| Range | −1.71 – 1.41 | −2.11 – 0.65 | |||||
| M | 0.48 | −0.72 | |||||
| SD | 0.47 | 0.69 | |||||
| Semantic Memory* | |||||||
| Range | −2.78 – 1.42 | −1.21 – 1.94 | −8.9 | 255 | < .001 | [−1.02, −.65] | 1.13 |
| M | −0.34 | 0.51 | |||||
| SD | 0.77 | 0.69 | |||||
Note. Episodic Memory and Semantic Memory reflect composite scores. Age group differences in NIH fluid cognition, episodic memory, and semantic memory were tested in 257 participants. Positive T values reflect higher scores in younger adults, negative values reflect higher scores in older adults. Statistical tests are nearly identical when including sex, education, site, and eWBV as covariates in an ANCOVA.
Standard inclusion and exclusion criteria were carried out to ensure that all participants were healthy and without evidence of neurological, psychiatric, or other medical illness known to impact cognition. Specifically, participants were asked about acute or chronic psychiatric illness, current or recent treatment with psychotropic medication, and significant changes to health status within three months of the eligibility interview. Individuals with the presence of any one of these were not eligible to continue.
Further exclusions were made post data collection on the basis of cognitive status and depressive symptoms. Participants with scores below 27/30 on the Mini-Mental State Examination (Mean young: 29.08; SD young: 1.23; Mean old: 28.500; SD old; 1.29; T(261)= 3.69, p < .001, [.28, .90], Cohen’s d = .47; Folstein et al., 1975) and an age-adjusted national percentile of 25 on the NIH Fluid Cognition measure (Mean young: 63.67%; SD young: 30.48; Mean old: 50.06%; SD old: 23.55; Gershon et al., 2013) were excluded. Younger and older participants were screened for depressive symptoms using the Beck Depression Inventory (Beck et al., 1996) or the Geriatric Depression Scale (Yesavage et al., 1982), respectively. Two older adults were excluded due to a rating of “moderate depression”. No differences were observed between the 214 younger and older adults with z-scored measures of depressive symptoms (T(212)= .05, p = .96, [−.26, .28], Cohen’s d = .01). All participants were right-handed with normal or corrected-to-normal vision.
Composite measures of episodic and semantic memory were derived from laboratory-based tasks to characterize the sample (Supplementary Methods). Descriptive statistics and tests for differences across groups are presented in Table 1.
2.2. Neuroimaging
T1-weighted volumetric magnetization prepared rapid gradient echo sequences were acquired at the Cornell Magnetic Resonance Imaging Facility (TR=2530ms; TE=3.4ms; 7° flip angle; 1mm isotropic voxels, 176 slices, 5m25s) with 2x acceleration and sensitivity encoding, and at the York University Neuroimaging Center (TR=1900ms; TE=2.52ms; 9° flip angle; 1mm isotropic voxels, 192 slices, 4m26s) with 2x acceleration and generalized auto calibrating partially parallel acquisition (GRAPPA) encoding at an iPAT acceleration factor of 2.
Automatic Segmentation of Hippocampal Subfields (ASHS; Yushkevitch et al., 2015) segmented each participant’s hippocampus along the longitudinal axis in native space. ASHS uses multi-atlas label fusion to segment the medial temporal lobes into subfields, and has been well-validated among manual and other automated approaches (Bussy et al., 2021). ASHS was run with the ASHS-PMC-T1 atlas (Xie et al., 2016) in all participants. Given the relatively low resolution of T1-weighted images, we limited our inspection to anterior (head) and posterior (body and tail) regions of the hippocampus (Wisse et al., 2020). All ASHS outputs were visually inspected for gross errors and to confirm the presence or absence of the uncal apex in anterior and posterior segments, respectively (Poppenk et al., 2013; see Figure 2A). No errors were observed (see Supplementary Figure 1 for examples).
Figure 2 Caption. Age Group and Sex Interactions with Internal Density in the Hippocampus.
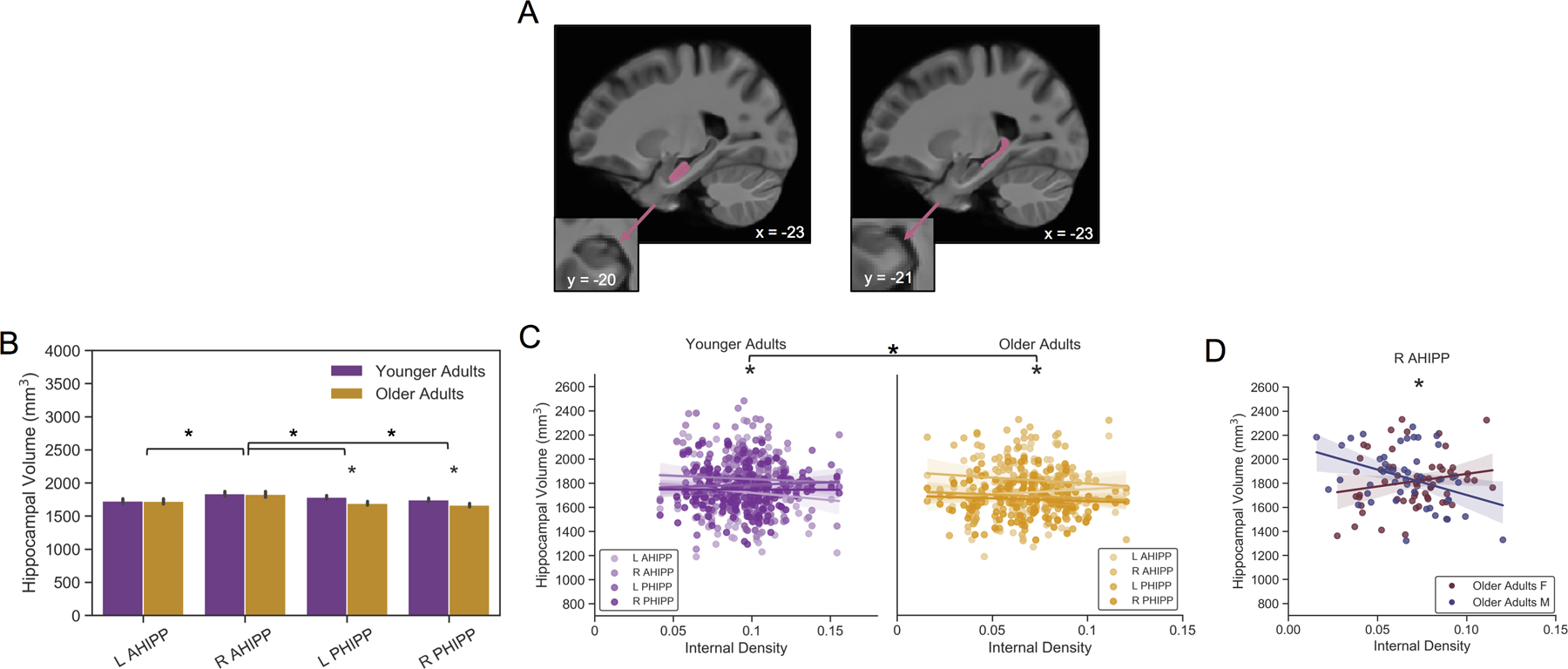
(A) Brain images show representative anterior (left) and posterior (right) hippocampal segmentations in the left hemisphere from ASHS. Zoomed-in views illustrate the presence and absence of the uncal apex in each segment. (B) Mean volumes of anterior and posterior hippocampal segments plotted by hemisphere and age group. Older adults had smaller posterior, but not anterior, hippocampus volumes than younger adults. (C) Scatterplots demonstrating a significant interaction between internal detail density and age group on hippocampal volumes. Slopes were significantly different in younger and older adults. Within each age group, relationships between internal density and hippocampal volumes (L AHIPP, R AHIPP, L PHIPP, R PHIPP) were also significantly different from one another. (D) Scatterplot demonstrating a crossover interaction in older adults between sex and internal density in the right anterior hippocampus. Older males with larger right anterior hippocampal volumes had recollections with less internal density. The opposite relationship was trending in older females: larger right anterior hippocampal volumes were associated with more internally dense recollections. Site, education, and eWBV were included as effects of no interest in all models. * denote significant effects. L ant. = left anterior; R ant. = right anterior; L post. = left posterior; R post. = right posterior. F= female; M = male.
Temporal pole volumes were extracted from cortical reconstruction and volumetric segmentation performed in FreeSurfer version 6.0.1 (Fischl et al., 2002; Reuter et al., 2012). Whole hippocampal volume was also extracted. Measurements of estimated total intracranial volume (eTIV), grey matter, and white matter volume were used for volumetric adjustment. Specifically, the residuals of a linear regression between each volume and eTIV were used to calculate an adjusted volume (Jack et al., 1989; Buckner et al., 2004; Stark et al., 2021). Compared to volumes as a proportion of eTIV (Voevodskaya et al., 2014), this approach removes the influence of head size on regional volumes, an important consideration when examining structural changes in healthy aging where age becomes a confounding variable.
All regional volumes were adjusted for head size prior to analysis (Tables S1–S2). Two younger and two older adults were excluded for outlying volume measurements after adjustment. Whole brain tissue volume is known to decrease in older age with the expansion of cerebrospinal fluid volume, even without marked change to total intracranial volume (Matsumae et al., 1996). Estimated whole brain volume (eWBV) was calculated as (grey matter + white matter)/(eTIV) and included as a covariate where indicated to narrow in on regionally specific effects (see similar approach in Schmitz et al., 2016).
2.3. Autobiographical Interview
The Autobiographical Interview (AI; Levine et al., 2002; Lockrow et al., 2021) served as our measure of AM to examine relationships to brain volume. Participants completed the interview as part of a larger set of cognitive assessments during a separate experimental session. Trained research assistants conducted the interviews, providing thorough instructions and ensuring comprehension prior to the start of each interview. Participants were asked to describe a specific episode from each of three (younger adults) or five (older adults) time periods: childhood, teenage years, young adulthood, middle adulthood, and late adulthood. For each memory, participants first described the episode in as much detail as possible (free recall). When recollection came to a natural end, participants were lightly prompted to recall any additional details (general probe). After memories were recalled from all time periods, participants went through each memory once more and were questioned with specific cues to encourage further episodic remembering (specific probe). Self-reported ratings of vividness, emotional change, significance at the time, significance now, and rehearsal were also collected for each memory. All interviews were recorded and transcribed prior to scoring.
In brief, scoring involved categorizing text into episodic-like (internal) and non-episodic (external) details. Internal details included those involving the sequence of events, location, time, sensory information, emotions, and thoughts related to the event chosen. External details included specific information about unrelated events, semantic information (general and personal), repetitions, and other non-scorable verbiage. Updated scoring protocols have recently been published (e.g., Strikwerda-Brown et al., 2017), but scoring on our high volume of interviews commenced prior to their publication. For the purpose of this study, we consider only scores from free recall and general probe cueing stages of the interview, which reflect spontaneous recollection tendencies.
The transcribed interviews were scored by two trained researchers (Inter-rater reliability internal: r(261)=.91, p < .001; external: r(261)=.82, p < .001). Counts of internal and external details were averaged across memories to provide stable measures of episodic and semantic memory. Notably, we divided detail counts by total word count to control for verbal output, which may arbitrarily underestimate or inflate detail counts if not considered. Word count was not different across groups F(1,257)= 1.03, p = .310 controlling for site, sex, and education), suggesting that verbosity did not confound density scores. Internal and external density scores were therefore our AM metrics of interest (Spreng et al., 2018; Lockrow et al., 2021).
2.4. Analyses
Our aim was to determine how individual differences in AM within younger and older cohorts relate to grey matter volume in the anterior and posterior hippocampus and temporal poles. In other words, we tested for an interaction between age group, AI detail density, and regional volume. Analyses proceeded in three parts to test for: 1) age group differences in detail density as an initial replication of prior work; 2) age group differences in hippocampal volumes and associations with detail density on the AI; 3) age group differences in temporal pole volumes and associations with detail density on the AI.
ANCOVAs were used to examine age group differences and Generalized Estimating Equations (GEE) were used to explore interactions with AM. GEEs are a semi-parametric version of the general linear model which can accommodate correlated repeated measurements (e.g., left and right volumes, anterior and posterior segments) by modeling the within-subject covariance structure and treating it as a nuisance variable (Liang & Zeger, 1986). GEEs, modeled as normal positive (gamma) distributions with exchangeable correlation matrices, were conducted with volume as the predicted variable to test for effects of age group, hemisphere, segment (anterior/posterior in the hippocampus only), detail density (internal and external separately), and their interaction (see Supplementary Results for GEEs with laboratory-based composites of episodic and semantic memory). Follow-up GEEs were carried out within age group or hemisphere subsets of the data for marginal and significant age group interactions. Finally, general linear models (GLM) were used to detect simple effects of detail density on each regional volume. Sex, site, education, and eWBV were included in each model.
2.5. Software
Statistical analyses were carried out in python 3.6.3 and R version 3.3.3. In python, descriptive statistics were tabulated with pandas and visualizations were created with seaborn. Statsmodels was used to model GEEs and GLMs. In R, ANCOVAs were run with lme4 and nlme, and emmeans was used for post-hoc paired t-tests with a Tukey HSD adjustment.
3. Results
3.1. Age Group Differences in Internal/External Density of Recollections from the Autobiographical Interview
We first tested for age group differences on the AI to replicate established findings. Age group, detail category (internal, external), sex, and the interaction between them were entered into a mixed ANCOVA on density scores. Education and site were included as covariates. Significant main effects of age group (F(1,258)= 10.22, p < .005, ηp2= .02) and detail category (F(1,261)= 1463.17, p < .001, ηp2= .74) were qualified by a significant interaction (F(1,261)= 167.57, p < .001, ηp2= .24). Compared to younger adults, older adults recalled a lower density of internal details (t(258)= 11.06, p < .001, Cohen’s d= 1.38) and a higher density of external details (t(258)= 6.32, p < .001, Cohen’s d= .79), corroborating prior work (Levine et al., 2002; Figure 1). A main effect of sex was also observed (F(1,258)= 3.98, p < .05, ηp2= .01), such that females had more internally and externally dense recollections than males overall.
Figure 1 Caption. Age Group Differences in Internal/External Density of Recollections from the Autobiographical Interview.
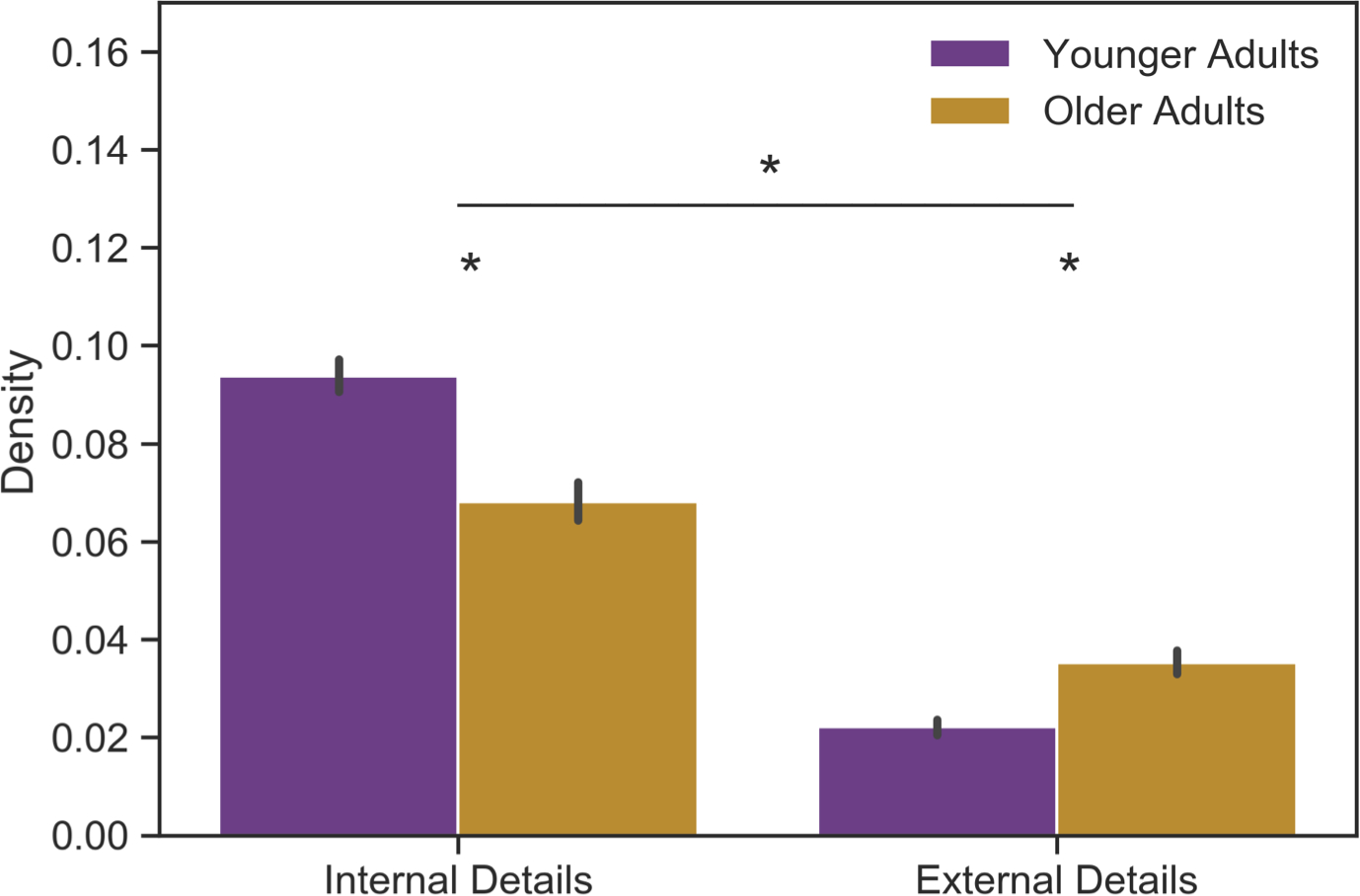
Mean internal and external detail density on the AI plotted by age group. Compared to younger adults, older adults recalled a lower density of internal details and a higher density of external details. Sex was included in the model. Site and education were included as covariates. Density = detail count / word count. * denotes significant effects.
To rule out the possibility that older adults’ density scores were disproportionately influenced by recall of temporally distant events (e.g., Linton, 1975; Rubin & Wenzel, 1996; Wagenaar, 1986), additional ANCOVAs were run testing for the influence of temporal distance on internal or external density. Age group, temporal distance (recent, remote), sex, and the interaction between them were modeled with sex and education as covariates. Recent memories were defined as the last memory recalled for each group: young adulthood for younger adults and late adulthood for older adults. Remote memories were defined as childhood memories for younger adults and young adulthood memories for older adults to equate temporal distance across groups. As above, age group differences were present for both internal (F(1,258)= 50.10, p < .001, ηp2= .12) and external density (F(1,258)= 66.19, p < .001, ηp2= .14). No interactions with temporal distance were observed. This suggests that internal and external density are stable individual difference measures with distinct age-related patterns.
3.2. Age Group Differences in Hippocampal Volumes and Associations with Internal/External Density
Next, we tested for age group differences in hippocampal volume. Older adults were found to have smaller posterior hippocampus volumes than younger adults.
Specifically, main effects of age group (F(1,258)= 4.65, p < .05, ηp2= .01), hemisphere (F(1,782)= 16.18, p < .001, ηp2= .01), and segment (F(1,782)= 27.66, p < .001, ηp2= .03) on volume were observed. These were qualified by a number of significant interactions. A hemisphere by segment interaction (F(1,782)= 59.21, p < .001, ηp2= .07) showed that right anterior segments were larger than right posterior segments (t(782)= 9.78, p < .001, Cohen’s d= .70), left anterior segments (t(782)= 8.09, p < .001, Cohen’s d= .58), and left posterior segments (t(782)= 7.32, p < .001, Cohen’s d= .52). A sex by segment interaction (F(1,782)= 11.91, p < .01, ηp2= .01) indicated that males had larger anterior compared to posterior segments (t(782)= 6.50, p < .001, Cohen’s d= .46). Segments were comparable in females. Critically, an age group by segment interaction (F(1,782)= 16.97, p < .001, ηp2= .02) showed that older adults had smaller posterior, but not anterior, hippocampus volumes compared to younger adults (t(258)= 3.71 p < .005, Cohen’s d= .46; Figure 2B), leaving older adults with larger anterior compared to posterior segments (t(782)= 6.79, p < .001, Cohen’s d= .49). Converging results were obtained when testing for differences in the ratio of posterior to anterior hippocampus volumes (see Supplemental Results). We determined that this result was not driven by the oldest of the older cohort as the same pattern was found when splitting older adults into 60–69 and 70+ age categories (Supplementary Figure 2).
In order to examine age group differences in the relationship between density scores and hippocampal volumes, we ran GEEs on hippocampal volumes modeling age group, hemisphere, segment, density score, and their interaction. Sex interaction terms with density and segment were included based on the above ANCOVAs.
The GEE with internal density yielded a significant interaction between age group, hemisphere, segment, and internal density (Wald χ2(1)= 8.53, p < .005; Figure 2C; Table S3). This suggested that the difference in hippocampal volume relationships to internal density differed as a function of age group. To decompose the interaction, follow-up models within each age group indicated hemisphere by segment by internal density interactions in both younger (Wald χ2(1)= 3.84, p = .05) and older (Wald χ2(1)= 4.94, p < .05) adults. This result suggested that there was an overall difference in slope between internal density and each of the volumes in both younger (purple in Figure 2C) and older (yellow in Figure 2C) adults. In younger adults, follow-up GLMs on each of the four hippocampal volumes showed that internal density was not significantly related to any volume (Supplementary Figure 3). In older adults, a sex by internal density interaction (Wald χ2(1)= 12.78, p < .001) was observed in the right anterior hippocampus (Figure 2D). This interaction emerged with a main effect of sex (b= .29, SE= .07, p < .001), but absent a main effect of internal density on volume (b= 1.19, SE= .73, p= .100). Internally dense recollections were associated with smaller volumes in older males (b= −2.60, SE= .81, p < .005). The association with volume was not significant in older females (b= 1.27, SE= .71, p = .075). Complementary results were obtained when the ratio of posterior to anterior hippocampus volumes was replaced as the dependent variable (see Supplemental Results & Supplementary Figure 5). Here, internal density was associated with a smaller volume ratio across sexes in the left hemisphere, but was dependent on sex in the right hemisphere.
The GEE on external density in the hippocampus showed a marginal main effect of detail density (Wald χ2(1)= 3.77, b= −2.05, SE= 1.06, p = .05; Supplementary Figure 6A), such that greater volume across all hippocampal segments was associated with less externally dense memories in all participants (see Table S4 for full results). A sex by density interaction (Wald χ2(1)= 4.36, p < .05) indicated that the negative association between external density and hippocampal volumes was driven by females (b= −3.02, SE= 1.55, p = .052; Supplementary Figure 6B). External density was not related to any of the four hippocampal volumes in males. External density was not a significant predictor of volume ratio scores (see Supplementary Results).
3.3. Age Group Differences in Temporal Pole Volumes and Associations with Internal/External Density
Turning to the temporal pole, we found that volumes were similar across age groups (Figure 3B), but males had larger volumes than females (F(1,258)= 4.18, p < .05, ηp2= .01).
Figure 3 Caption. Age Group Interaction with Density in the Temporal Poles.
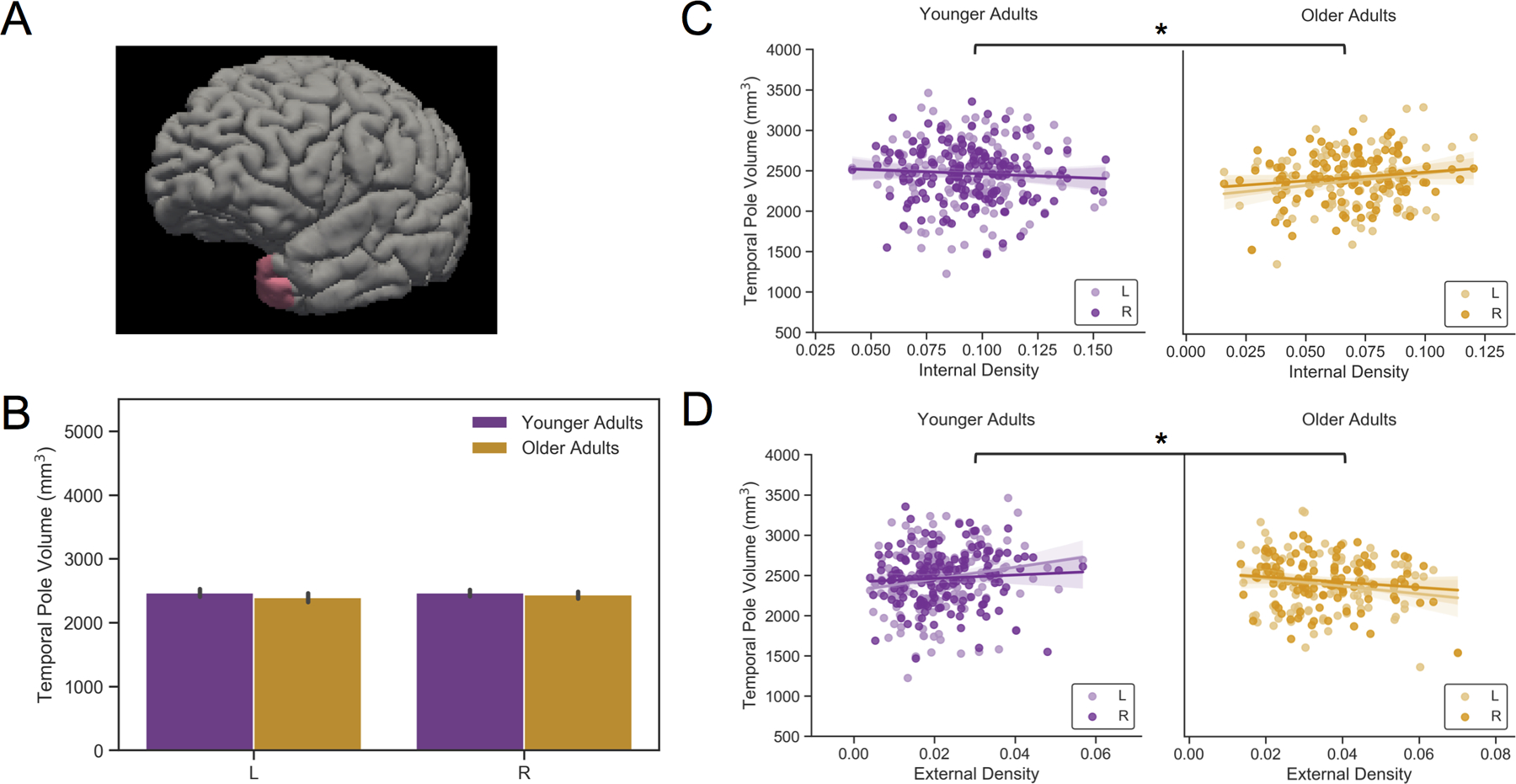
(A) A surface rendering of the left temporal pole from FreeSurfer. (B) Mean volumes of left and right temporal poles plotted by age group depict no differences. (C) Scatterplots demonstrating contrasting relationships between internal detail density and volume (top) as well as between external detail density and volume (bottom) across age groups. Older adults with more internally dense recollections had larger temporal pole volumes. All volumes were adjusted for eTIV. Sex was included in the model. Site, education, and estimated whole brain volume were included as effects of no interest in each model. * denote significant effects. L= left; R = right.
The GEE with internal density revealed a significant main effect of internal density (Wald χ2(1)= 6.18, p < .05) and an age group by internal density interaction (Wald χ2 (1)= 4.56, p < .05; see Table S5). In older adults, more internally dense recollections were related to larger temporal pole volumes bilaterally (Wald χ2(1)= 5.54, b= 2.12, SE= .90, p < .05; Figure 3C). Internal density was not related to temporal pole volumes in younger adults.
The GEE with external density on temporal pole volume showed a marginal main effect of density (Wald χ2(1)= 3.42, b= −3.02, SE= 1.633, p = .065) and a significant age group by density interaction (Wald χ2(1)= 7.91, p < .005; Table S6). Figure 3D illustrates volume-density slopes going in opposite directions for each age group. Although external density was not a significant predictor of volume within each age group alone, the slope in younger adults was highly similar to that between semantic index from laboratory-based measures and temporal pole volume (Supplementary Figure 7).
4. Discussion
We investigated age differences in AM, hippocampus and temporal pole volumes, and AM-volume associations in a large sample of healthy younger and older adults, using the Autobiographical Interview (Levine et al., 2002; Lockrow et al., 2021) to quantify the episodicity of AM recollection. Consistent with predictions, younger adult recollections were more episodic with a higher density of episodic (internal) than semantic (external) details. In contrast, older adult recollections were less episodic, marked by a higher density of semantic versus episodic information. Age differences in brain volumes were only observed for the posterior hippocampus, which was smaller in volume for older compared to younger adults (Figure 2A). Consistent with predictions, age differences in volume-AM associations were observed. Temporal pole volumes were positively associated with episodic AM in older but not younger adults (Figure 3C). In contrast, temporal pole volumes were more strongly related to laboratory-based measures of semantic memory, but not semantic AM, in younger adults (Supplementary Figure 7). We speculate that in the face of posterior hippocampal volume atrophy, episodicity of AM becomes more reliant on temporal polar cortices in older adulthood. Finally, age- and sex- dependent associations were observed between hippocampal volume and AM. Episodic density was positively correlated with anterior hippocampal volumes for older females, while a negative association was observed for males. However the limited sample sizes here urge caution in the interpretation of these sex interactions.
4.1. Younger and Older Adult Recollections Systematically Vary in Episodic and Semantic Detail Recollection
As previously reported (e.g., Levine et al., 2002), older adults included fewer episodic and more semantic details when recalling past events on the AI. Studies using the AI typically report detail counts, whereas here we report on detail density, a metric that controls for the overall verbal output of recollections. We have previously introduced density scores as a novel dependent variable from the AI (Spreng et al., 2018), and have recently found that they are more sensitive to age effects than detail counts, and provide more reliable and valid estimates of individual differences in AM (Lockrow et al., 2021). In controlling for verbosity, density scores measure the amount of all verbal output dedicated to conveying episodic or semantic details about a personal past event. Arguably, this more accurately captures the recollective process, which involves top-down control mechanisms to retrieve details relative to the event and hold them in mind (Piolino et al., 2010) while suppressing recall of non-relevant (external) details. Indeed, density scores, but not counts, associate with performance-based laboratory tasks of episodic memory and executive function (Lockrow et al., 2021). As the AI was administered to the present sample without an imposed time constraint, density scores are also less influenced by narrative length. Critical to the findings reported here, density scores for both episodic and semantic detail had significantly different distributions in younger and older adults. This suggests that the overall differences in episodic and semantic information generated during recollection cannot be explained by individual differences in recollective style, and reflects a shift as a function of older age.
4.2. Age Differences in Hippocampal Volumes Are Not Associated with Autobiographical Memory
Older adults had smaller posterior hippocampal volumes, consistent with recent longitudinal work from healthy adult lifespan samples showing greater microstructural change to the posterior hippocampus in older age (Langnes et al., 2020). Significant age-related reductions have also been observed in nearby parahippocampal white matter, which includes axons of the perforant pathway (Stoub et al., 2012). In terms of macrostructure, both anterior and posterior grey matter volumes shrink with age, but show slightly different trajectories, with the posterior region showing an earlier inflection point and steeper decline thereafter (Langnes et al., 2020; Chauveau et al., 2021). Different trajectories of atrophy may partially explain why other longitudinal findings have demonstrated more robust anterior hippocampal volume change over time in older age (Chen et al., 2010); the years sampled may disproportionately impact the rate of change observed. Similarly, the results reported here are situated among other cross-sectional studies with both converging and diverging results. Our large sample size and sensitive segmentation protocol lend confidence to our findings, which provide the necessary backdrop for comparing volume-AM associations across age groups.
Neither anterior nor posterior hippocampal volumes were related to AM in younger adults. Our study is not the first to report null relationships between hippocampal grey matter volume and hippocampal-dependent tasks including AM, imagery, and navigation in larger samples of healthy young adults (Clark et al., 2020; Weisberg et al., 2019). Using higher resolution scans to examine hippocampal subfields, such as CA2,3/DG and subiculum, may be more appropriate to capture specific relationships to hippocampal-dependent processes, such as AM (Palombo et al., 2018; Barry & Maguire, 2019). It is also possible that more extreme conditions, such as expertise (Weisberg & Ekstrom, 2021) or pathology (Van Petten, 2004) are needed to detect associations.
Older adults’ smaller posterior hippocampus volumes could speak to their recollection of fewer specific, episodic details, but no association between posterior hippocampus volumes and episodic AM was observed. According to one framework, tasks which require the recollection of specific episodic details—like the AI—recruit the posterior hippocampus to generate context and recreate the remembered scene (see Sheldon & Levine, 2016 and Sheldon et al., 2019 for reviews). Smaller volumes may impair these abilities, as suggested by findings that age-related atrophy to parahippocampal white matter volume predicts episodic memory performance in older adults (Stoub et al., 2014). Yet, our results suggest that typical aging may not sufficiently alter hippocampal volumes to allow detection of these relationships with hippocampal-dependent tasks. Consistent with this idea, strong positive associations between hippocampal volumes and episodic memory scores have been observed for individuals with mild cognitive impairment and Alzheimer’s disease, who often have more pronounced atrophy to the hippocampus than cognitively healthy older adults (Chauveau et al., 2021).
Finally, we observed an association between anterior hippocampus volumes and episodic AM in older adults that was moderated by sex. Larger right anterior hippocampus volumes were associated with less episodic AM in males and the opposite pattern was observed for females. We refrain from further interpreting this sex by age interaction here given the exploratory nature of the analysis. Future studies will be necessary to replicate this finding and directly investigate age and sex differences in the these brain and behavior associations.
4.3. Temporal Pole Volumes Relate to Episodic AM in Older Adults
We next tested for age group differences in temporal pole volumes and corresponding associations with AM. We specifically targeted the temporal pole as this region serves as a semantic processing hub (e.g., Hoffman & Morcom, 2018; Lambon Ralph, 2017) associated with prior knowledge during encoding of naturalistic stimuli (Raykov et al., 2021). Differential relationships with cognition emerged across age groups despite no discernible volume difference. Younger adult volumes were positively related to laboratory-tested semantic memory abilities, whereas older adults showed no such relationship. Rather, temporal pole volumes in older adults were positively related to episodic AM. A meta-analysis of age-related functional brain changes has demonstrated that older adults recruit the anterior temporal region more than younger adults across a number of cognitive domains including memory retrieval (Spreng et al., 2010). In fact, older adults showed coactivation of the temporal pole with anterior hippocampus during an in-scanner fMRI AM task (Addis et al., 2011). The magnitude of anterior temporal activity in older adults was positively related to episodic details recalled from a separate session.
Based on our findings in this large study sample we propose that age-related volume differences in posterior hippocampus, a region implicated in specific detail generation (Sheldon & Levine, 2016), may result in additional recruitment of areas that were age-invariant in our study, such as the anterior hippocampus and temporal poles. The anterior hippocampus forms preferential connections to the temporal pole (Kahn et al., 2008) via the uncinate fasciculus (Kier et al., 2004), laying the groundwork for greater functional connectivity at rest (Honey et al., 2009) and during semantic processing (e.g., Hoffman & Morcom, 2018). Indeed, patients with semantic dementia often demonstrate damage and altered intrinsic functional connectivity to both of these regions (e.g., Chan et al., 2001; Schwab et al., 2020). If these regions are recruited to support episodic AM recollection in older adults, such memories might necessarily be “semanticized,” or imbued with more semantic information (Spreng et al., 2018). While speculative, this view emphasizes the significance of regional integrity and supports frameworks of neurocognitive aging that describe functional reorganization in relation to structural change as a form of adaptive scaffolding (Park & Reuter-Lorenz, 2009; Spreng & Turner, 2019; Andrews-Hanna et al., 2019). Further longitudinal inquiry involving both functional and structure measures is needed to test these claims. Future work would also benefit from inspecting other lateral temporal regions, which show increased involvement during older adult cognition (Spreng et al., 2010).
4.4. Concluding Remarks
We tested associations between hippocampal and temporal pole volumes and AM, and differences with age, in a well-powered sample of healthy younger and older adults. Older adults had smaller posterior hippocampus volumes, anterior hippocampus and temporal pole volumes were age-invariant. In the context of shifts in the content of AM with age, we suggest that this pattern of volumetric changes may promote functional scaffolding among comparatively preserved regions to support AM in older age. While it is prudent to consider cohort effects when comparing younger and older adults, large cross-sectional aging investigations contrasting within- and between- group brain-behavior relationships can offer important insights into how these highly variable associations reliably differ with age. While exploratory our findings also underscore the importance of considering sex differences in lifespan developmental research. Mapping associations among often parallel age-related shifts in brain structure and behavior will be imperative to better understanding the neural mechanisms that support complex cognitive abilities, including autobiographical memory, over the course of late life development.
5. Data Availability
All data from the current report are open access and publicly available (see Spreng et al., 2022, for data descriptor). Autobiographical memory data from the present study is available through the Open Science Framework, project “Psychometrics of Autobiographical Memory” contributed by R.N.S. (https://osf.io/fzkm7/) or from the corresponding author on reasonable request. Additional demographic and behavioral data are available within the Open Science Framework project “Goal-Directed Cognition in Older and Younger Adults” (http://osf.io/yhzxe/); neuroimaging data are available on OpenNeuro (https://openneuro.org/datasets/ds003592).
Supplementary Material
Acknowledgements
This project was supported in part by NIH grant 1S10RR025145 and a Canadian Institute of Health Research grant to R. Nathan Spreng. We thank Amber Lockrow for assistance with behavioral data scoring and organization.
References
- Addis DR, Roberts RP, & Schacter DL (2011). Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia, 49(13), 3656–3669. 10.1016/j.neuropsychologia.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Grilli MD, Irish M, Andrews-Hanna JR, Grilli MD, & Irish M (2019). A Review and Reappraisal of the Default Network in Normal Aging and Dementia. Oxford Research Encyclopedia of Psychology. 10.1093/acrefore/9780190236557.013.384 [DOI] [Google Scholar]
- Andrews-Hanna JR, Saxe R, & Yarkoni T (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage, 91, 324–335. 10.1016/j.neuroimage.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DN, & Maguire EA (2019). Remote Memory and the Hippocampus: A Constructive Critique. Trends in Cognitive Sciences, 23(2), 128–142. 10.1016/j.tics.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation [Google Scholar]
- Benoit RG, & Schacter DL (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, … Alexander GE (2010). Age-related networks of regional covariance in MRI gray matter: Reproducible multivariate patterns in healthy aging. NeuroImage, 49(2), 1750–1759. 10.1016/j.neuroimage.2009.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettio LEB, Rajendran L, & Gil-Mohapel J (2017). The effects of aging in the hippocampus and cognitive decline. Neuroscience and Biobehavioral Reviews, 79(March), 66–86. 10.1016/j.neubiorev.2017.04.030 [DOI] [PubMed] [Google Scholar]
- Binder JR, & Desai RH (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. 10.1016/j.tics.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunec IK, Bellana B, Ozubko JD, Man V, Robin J, Liu ZX, … Moscovitch M (2018). Multiple Scales of Representation along the Hippocampal Anteroposterior Axis in Humans. Current Biology, 28(13), 2129–2135.e6. 10.1016/j.cub.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Brunec IK, Robin J, Patai EZ, Ozubko JD, Javadi AH, Barense MD, … Moscovitch M (2019). Cognitive mapping style relates to posterior–anterior hippocampal volume ratio. Hippocampus, 29(8), 748–754. 10.1002/hipo.23072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL (2004). Three principles for cognitive aging research. In Cabeza R, Nyberg L, & Park D(Eds.), Cognitive neuroscience of aging. New York: Oxford University Press. [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, & Snyder AZ (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage, 23(2), 724–738. 10.1016/j.neuroimage.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Bussy A, Plitman E, Patel R, Tullo S, Salaciak A, Bedford SA, … Chakravarty MM (2021). Hippocampal subfield volumes across the healthy lifespan and the effects of MR sequence on estimates. NeuroImage, 233(March). 10.1016/j.neuroimage.2021.117931 [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, … Rossor MN (2001). Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Annals of Neurology, 49(4), 433–442. 10.1002/ana.92 [DOI] [PubMed] [Google Scholar]
- Chapleau M, Montembeault M, Boukadi M, Bedetti C, Laforce R Jr, Wilson M, & Brambati SM (2019). The role of the hippocampus in the semantic variant of primary progressive aphasia: A resting-state fcMRI study. Hippocampus, 29(11), 1127–1132. 10.1002/hipo.23156 [DOI] [PubMed] [Google Scholar]
- Chauveau L, Kuhn E, Palix C, Felisatti F, Ourry V, Sayette V. D. La, … Flores R. De. (2021). Medial Temporal Lobe Subregional Atrophy in Aging and Alzheimer ‘ s Disease : A Longitudinal Study. Frontiers in Aging Neuroscience, 12. 10.3389/fnagi.2021.750154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KHM, Chuah LYM, Sim SKY, & Chee MWL (2010). Hippocampal region-specific contributions to memory performance in normal elderly. Brain and Cognition, 72(3), 400–407. 10.1016/j.bandc.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Clark IA, Monk AM, Hotchin V, Pizzamiglio G, Liefgreen A, Callaghan MF, & Maguire EA (2020). Does hippocampal volume explain performance differences on hippocampal-dependant tasks? NeuroImage, 221(July). 10.1016/j.neuroimage.2020.117211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, & Sutherland RJ (2003). The Aging Hippocampus: Cognitive, Biochemical and Structural Findings. Cerebral Cortex, 13(12), 1344–1351. 10.1093/cercor/bhg081 [DOI] [PubMed] [Google Scholar]
- Eslinger PJ (1998). Autobiographical Memory After Temporal Lobe Lesions. Neurocase, 4(6), 481–495. 10.1080/13554799808410641 [DOI] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fisher DW, Bennett DA, & Dong H (2018). Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiology of Aging, 70, 308–324. 10.1016/j.neurobiolaging.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, … Dale AM (2009). One-year brain atrophy evident in healthy aging. Journal of Neuroscience, 29(48), 15223–15231. 10.1523/JNEUROSCI.3252-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, & Nowinski CJ (2013). NIH toolbox for assessment of neurological and behavioral function. Neurology, 80(11 Suppl 3), S2–S6. 10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Blazey T, Benzinger TLS, & Head D (2013). Effects of Aging and Alzheimer’s Disease Along the Longitudinal Axis of the Hippocampus. Journal of Alzheimers Disease, 37(1). 10.3233/JAD-130011.Effects [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Lee AC, Brett M, & Patterson K (2003). The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cognitive, affective & behavioral neuroscience, 3(3), 234–254. 10.3758/cabn.3.3.234 [DOI] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, & Breteler MMB (2002). Hippocampal Head Size Associated with Verbal Memory Performance in Nondemented Elderly. NeuroImage, 17, 1365–1372. 10.1006/n [DOI] [PubMed] [Google Scholar]
- Herfurth K, Kasper B, Schwarz M, Stefan H, & Pauli E (2010). Autobiographical memory in temporal lobe epilepsy: Role of hippocampal and temporal lateral structures. Epilepsy and Behavior, 19(3), 365–371. 10.1016/j.yebeh.2010.07.012 [DOI] [PubMed] [Google Scholar]
- Hoffman P, & Morcom AM (2018). Age-related changes in the neural networks supporting semantic cognition: A meta-analysis of 47 functional neuroimaging studies. Neuroscience and biobehavioral reviews, 84, 134–150. 10.1016/j.neubiorev.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, & Hagmann P (2009). Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 2035–2040. 10.1073/pnas.0811168106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, & Piguet O (2012). Considering the role of semantic memory in episodic future thinking: Evidence from semantic dementia. Brain, 135(7), 2178–2191. 10.1093/brain/aws119 [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, … Kokmen E (1997). Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology, 49(3), 786–794. 10.1212/WNL.49.3.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, & Cascino GD (1989). Anterior temporal lobes and hippocampal formations: Normative volumetric measurements from MR images in young adults. Radiology, 172, 549–554. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, & Buckner RL (2008). Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. Journal of neurophysiology, 100(1), 129–139. 10.1152/jn.00077.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron JC, Landeau B, Mevel K, Godeau C, … Desgranges B (2009). Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiology of Aging, 30(1), 112–124. 10.1016/j.neurobiolaging.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, & Bronen RA (2004). MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. American Journal of Neuroradiology, 25(5), 677–691. [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, & Rogers TT (2016). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18(1), 42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- Langnes E, Sneve MH, Sederevicius D, Amlien IK, Walhovd KB, & Fjell AM (2020). Anterior and posterior hippocampus macro- and microstructure across the lifespan in relation to memory—A longitudinal study. Hippocampus, 30(7), 678–692. 10.1002/hipo.23189 [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, & Moscovitch M (2002). Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging, 17(4), 677–689. 10.1037//0882-7974.17.4.677 [DOI] [PubMed] [Google Scholar]
- Liang K, and Zeger SL (1986). Longitudinal data analysis using generalized linear models. Biometrika, 73:13–22. [Google Scholar]
- Lockrow AW, Setton R, Spreng KAP, Sheldon S, Turner GR, & Spreng RN (2021). Taking stock of the past: A comprehensive psychometric evaluation of the Autobiographical Interview, bioRvix: doi: 10.1101/2021.12.22.473803 [DOI] [PubMed] [Google Scholar]
- Lotze M, Domin M, Gerlach FH, Gaser C, Lueders E, Schmidt CO, & Neumann N (2019). Novel findings from 2,838 Adult Brains on Sex Differences in Gray Matter Brain Volume. Scientific Reports, 9(1), 1–7. 10.1038/s41598-018-38239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, & Pruessner JC (2007). Hippocampal volume is as variable in young as in older adults: Implications for the notion of hippocampal atrophy in humans. NeuroImage, 34(2), 479–485. 10.1016/j.neuroimage.2006.09.041 [DOI] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, & Spiers HJ (2006). London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus, 16(12), 1091–1101. 10.1002/hipo.20233 [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Camicioli R, & Coupland NJ (2008). Aging hippocampus and amygdala. NeuroReport, 19(5), 543–547. 10.1097/WNR.0b013e3282f8b18c [DOI] [PubMed] [Google Scholar]
- Matsumae M, Kikinis R, Mórocz IA, Lorenzo AV, Sándor T, Albert MS, Black PM, & Jolesz FA (1996). Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging, Journal of Neurosurgery, 84(6), 982–991. Retrieved Nov 10, 2021, from https://thejns.org/view/journals/j-neurosurg/84/6/article-p982.xml [DOI] [PubMed] [Google Scholar]
- Miller-Goldwater HE, Cronin-Golomb LM, Porter BM, & Bauer PJ (2021). Developmental differences in reactivation underlying self-derivation of new knowledge through memory integration. Cognitive Psychology, 129(July), 101413. 10.1016/j.cogpsych.2021.101413 [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, & Nadel L (2016). Episodic memory and beyond: The hippocampus and neocortex in transformation. Annual Review of Psychology, 67, 105–134. 10.1146/annurev-psych-113011-143733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Rueschemeyer SA, Watson D, Karapanagiotidis T, Smallwood J, & Jefferies E (2017). Fractionating the anterior temporal lobe: MVPA reveals differential responses to input and conceptual modality. NeuroImage, 147(August 2016), 19–31. 10.1016/j.neuroimage.2016.11.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo DJ, Bacopulos A, Amaral RSC, Olsen RK, Todd RM, Anderson AK, & Levine B (2018). Episodic autobiographical memory is associated with variation in the size of hippocampal subregions. Hippocampus, 28(2), 69–75. 10.1002/hipo.22818 [DOI] [PubMed] [Google Scholar]
- Park DC & Reuter-Lorenz P (2009). The adaptive brain : Aging and neurocognitive scaffolding. Ann Rev Psychol, 60, 173–196. 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, & Rogers TT (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci, 8(12), 976–987. 10.1038/nrn2277 [DOI] [PubMed] [Google Scholar]
- Persson J, Spreng RN, Turner G, Herlitz A, Morell A, Stening E, … Söderlund H (2014). Sex differences in volume and structural covariance of the anterior and posterior hippocampus. NeuroImage, 99, 215–225. 10.1016/j.neuroimage.2014.05.038 [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, & Eustache F (2009). Episodic autobiographical memories over the course of time: Cognitive, neuropsychological and neuroimaging findings. Neuropsychologia, 47(11), 2314–2329. 10.1016/j.neuropsychologia.2009.01.020 [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, & Nadel L (2013). Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. 10.1016/j.tics.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Poppenk J, & Moscovitch M (2011). A hippocampal marker of recollection memory ability among healthy young adults: Contributions of posterior and anterior segments. Neuron, 72(6), 931–937. 10.1016/j.neuron.2011.10.014 [DOI] [PubMed] [Google Scholar]
- Rajah MN, Kromas M, Han JE, & Pruessner JC (2010). Group differences in anterior hippocampal volume and in the retrieval of spatial and temporal context memory in healthy young versus older adults. Neuropsychologia, 48(14), 4020–4030. 10.1016/j.neuropsychologia.2010.10.010 [DOI] [PubMed] [Google Scholar]
- Ranganath C, & Ritchey M (2012). Two cortical systems for memory- guided behaviour. Nature Reviews Neuroscience, 13. 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- Raykov PP, Keidel JL, Oakhill J, & Bird CM (2021). The importance of semantic network brain regions in integrating prior knowledge with an ongoing dialogue. BioRxiv, (1), 276683. Retrieved from https://www.biorxiv.org/content/10.1101/276683v3%0Ahttps://www.biorxiv.org/content/10.1101/276683v3.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoult L, Davidson PSR, Palombo DJ, Moscovitch M, & Levine B (2012). Personal semantics: At the crossroads of semantic and episodic memory. Trends in Cognitive Sciences, 16(11), 550–558. 10.1016/j.tics.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Renoult L, Irish M, Moscovitch M, & Rugg MD (2019). From Knowing to Remembering: The Semantic–Episodic Distinction. Trends in Cognitive Sciences, 23(12), 1041–1057. 10.1016/j.tics.2019.09.008 [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, … Zonderman AB (2000). One-year age changes in MRI brain volumes in older adults. Cerebral Cortex, 10(5), 464–472. 10.1093/cercor/10.5.464 [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012). Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis. Neuroimage, 61 (4), 1402–1418. http://reuter.mit.edu/papers/reuter-long12.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, & Nathan Spreng R (2016). Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nature Communications, 7, 1–13. 10.1038/ncomms13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab S, Afyouni S, Chen Y, Han Z, Guo Q, Dierks T, … Babiloni C (2020). Functional Connectivity Alterations of the Temporal Lobe and Hippocampus in Semantic Dementia and Alzheimer’s Disease. Journal of Alzheimer’s Disease, 76(4), 1461–1475. 10.3233/JAD-191113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sele S, Liem F, Mérillat S, & Jäncke L (2020). Decline Variability of Cortical and Subcortical Regions in Aging: A Longitudinal Study. Frontiers in Human Neuroscience, 14(September), 1–16. 10.3389/fnhum.2020.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton R, Mwilambwe-Tshilobo L, Girn M Lockrow AW, Baracchini G, Lowe AJ, Cassidy BN, Li J, Bzdok D, Leahy RM, Ge T, Margulies DS, Misic B, Stevens WD, Bernhardt BC, Kundu P, De Brigard F, Turner GR, & Spreng RN (In Press). Age differences in the functional architecture of the human brain. Cerebral Cortex. 10.1093/cercor/bhac056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton R, Lockrow AW, Turner GR, & Spreng RN (2021). Troubled past: A critical psychometric assessment of the self-report Survey of Autobiographical Memory (SAM). Behavior Research Methods. 10.3758/s13428-021-01604-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Fenerci C, & Gurguryan L (2019). A neurocognitive perspective on the forms and functions of autobiographical memory retrieval. Frontiers in Systems Neuroscience, 13(January), 1–8. 10.3389/fnsys.2019.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, & Levine B (2016). The role of the hippocampus in memory and mental construction. Annals of the New York Academy of Sciences, 1369(1), 76–92. 10.1111/nyas.13006 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Lockrow AW, DuPre E, Setton R, Spreng K. a. P., & Turner GR (2018). Semanticized autobiographical memory and the default - executive coupling hypothesis of aging. Neuropsychologia, 110, 37–43. 10.1016/j.neuropsychologia.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Setton R, Alter U, Cassidy BN, Darboh B, DuPre E, Kantarovich K, Lockrow AW, Mwilambwe-Tshilobo L, Luh W, Jundu P, & Turner GR (In Press). Neurocognitive aging data release with behavioral, structural, and multi-echo functional MRI measures. Scientific Data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, & Grady CL (2010). Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neuroscience and Biobehavioral Reviews, 34(8), 1178–1194. 10.1016/j.neubiorev.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Spreng RN, & Turner GR (2019). The Shifting Architecture of Cognition and Brain Function in Older Adulthood. Perspectives on psychological science : a journal of the Association for Psychological Science, 14(4), 523–542. 10.1177/1745691619827511 [DOI] [PubMed] [Google Scholar]
- Stark SM, Frithsen A, & Stark CEL (2021). Age-related alterations in functional connectivity along the longitudinal axis of the hippocampus and its subfields. Hippocampus, 31(1), 11–27. 10.1002/hipo.23259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, Barnes CA, Shah RC, Stebbins GT, Ferrari C, & deToledo-Morrell L (2012). Age-related changes in the mesial temporal lobe: the parahippocampal white matter region. Neurobiology of aging, 33(7), 1168–1176. 10.1016/j.neurobiolaging.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, DeToledo-Morrell L, & Dickerson BC (2014). Parahippocampal white matter volume predicts Alzheimer’s disease risk in cognitively normal old adults. Neurobiology of Aging, 35(8), 1855–1861. 10.1016/j.neurobiolaging.2014.01.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, & Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), 655–669. 10.1038/nrn3785 [DOI] [PubMed] [Google Scholar]
- Strikwerda-Brown C, Mothakunnel A, Hodges JR, Piguet O, & Irish M (2018). External details revisited - A new taxonomy for coding ‘non-episodic’ content during autobiographical memory retrieval. Journal of Neuropsychology. 10.1111/jnp.12160 [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, & Levine B (2006). The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia, 44(12), 2189–2208. 10.1016/j.neuropsychologia.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta AT, Huang SE, Chiu MJ, Hua MS, Tseng WYI, Chen SHA, & Qiu A (2012). Age-related vulnerabilities along the hippocampal longitudinal axis. Human Brain Mapping, 33(10), 2415–2427. 10.1002/hbm.21364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Ma W, Vira A, Marwha D, & Eliot L (2016). The human hippocampus is not sexually-dimorphic: Meta-analysis of structural MRI volumes. NeuroImage, 124(Pt A), 350–366. 10.1016/j.neuroimage.2015.08.050 [DOI] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, & Schacter DL (2020). The core episodic simulation network dissociates as a function of subjective experience and objective content. Neuropsychologia, 136. 10.1016/j.neuropsychologia.2019.107263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umanath S, & Marsh EJ (2014). Understanding How Prior Knowledge Influences Memory in Older Adults. Perspectives on Psychological Science, 9(4), 408–426. 10.1177/1745691614535933 [DOI] [PubMed] [Google Scholar]
- Van Petten C (2004). Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia, 42(10), 1394–1413. 10.1016/j.neuropsychologia.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Voevodskaya O, Simmons A, Nordenskjöld R, Kullberg J, Håkan A, Lind L, … Initiative ADN (2014). The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Frontiers in Aging Neuroscience, 6, 1–14. 10.3389/fnagi.2014.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SM, & Ekstrom AD (2021). Hippocampal volume and navigational ability: The map(ping) is not to scale. Neuroscience and Biobehavioral Reviews, 126(November 2020), 102–112. 10.1016/j.neubiorev.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SM, Newcombe NS, Chatterjee A, 2019. Everyday taxi drivers: do better navigators have larger hippocampi. Cortex 115, 280–293. 10.1016/j.cortex.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM, Kemmler G, Deisenhammer EA, Fleischhacker WW, & Delazer M (2003). Sex differences in cognitive functions. Personality and Individual Differences, 35(4), 863–875. 10.1016/S0191-8869(02)00288-X [DOI] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, & Bennett DA (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17(2), 179–193. 10.1037/0882-7974.17.2.179 [DOI] [PubMed] [Google Scholar]
- Wisse LEM, Daugherty AM, Olsen RK, & Berron D (2017). A harmonized segmentation protocol for hippocampal and parahippocampal subregions: why do we need one and what are the key goals? Hippocampus, 27(1), 3–11. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Wisse EML, Manjon VJ Wang H, Das SR, Wolk AD, Yushkevich AP 2016. Accounting for the Confound of Meninges in Segmenting Entorhinal and Perirhinal Cortices in T1-weighted MRI. Athens, Greece. Medical Image Computing and Computer-Assisted Intervention–MICCAI 2016(pp. 564–571). Springer International Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, & Brink TL (1983). Development and validation of geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 80(1), 37–49. 10.2307/1957152 [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding SL, Gertje EC, … Wolk DA (2015). Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Human Brain Mapping, 36(1), 258–287. 10.1002/hbm.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data from the current report are open access and publicly available (see Spreng et al., 2022, for data descriptor). Autobiographical memory data from the present study is available through the Open Science Framework, project “Psychometrics of Autobiographical Memory” contributed by R.N.S. (https://osf.io/fzkm7/) or from the corresponding author on reasonable request. Additional demographic and behavioral data are available within the Open Science Framework project “Goal-Directed Cognition in Older and Younger Adults” (http://osf.io/yhzxe/); neuroimaging data are available on OpenNeuro (https://openneuro.org/datasets/ds003592).


