Abstract
Background:
Immune checkpoint inhibitors (ICI) improve overall survival (OS) in patients with locally advanced, unresectable, or metastatic urothelial carcinoma (aUC), but response rates can be modest. We compared outcomes between patients with and without prior intravesical Bacillus Calmette-Guerin (BCG), who received ICI for aUC, hypothesizing that prior intravesical BCG would be associated with worse outcomes.
Patients and Methods:
We performed a retrospective cohort study across 25 institutions in US and Europe. We compared observed response rate (ORR) using logistic regression; progression-free survival (PFS) and OS using Kaplan-Meier and Cox proportional hazards. Analyses were stratified by treatment line (first line / salvage) and included multivariable models adjusting for known prognostic factors.
Results:
1,026 patients with aUC were identified; 614, 617, and 638 were included in ORR, OS, PFS analyses, respectively. Overall, 150 pts had history of prior intravesical BCG treatment. ORR to ICI was similar between those with and without prior intravesical BCG exposure in both first line and salvage settings (adjusted odds radios 0.55 [p=0.08] and 1.65 [p=0.12]). OS (adjusted hazard ratios 1.05 [p=0.79] and 1.13 [p=0.49]) and PFS (adjusted hazard ratios 1.12 [p=0.55] and 0.87 [p=0.39]) were similar between those with and without intravesical BCG exposure in first line and salvage settings.
Conclusions:
Prior intravesical BCG was not associated with differences in response and survival in patients with aUC treated with ICI. Limitations include retrospective nature, lack of randomization, presence of selection and confounding biases. This study provides important preliminary data that prior intravesical BCG exposure may not impact ICI efficacy in aUC.
Keywords: Bladder Cancer, Bacillus Calmette-Guerin, BCG, Immune Checkpoint Inhibitors, Urothelial Carcinoma
Microabstract:
Immune checkpoint inhibitors improve overall survival in advanced urothelial carcinoma, but response rates remain modest. We performed a multi-institutional retrospective cohort study comparing outcomes (observed response rate, progression-free and overall survival) between patients with and without prior intravesical BCG exposure. All outcomes were similar between the two populations. This study provides preliminary data that prior intravesical BCG exposure does not impact checkpoint inhibitor efficacy.
Introduction
Most patients with bladder cancer, around 75%, initially present with non-muscle-invasive disease (1). Intravesical bacillus Calmette-Guerin (BCG), a live attenuated strain of Mycobacterium bovis, initially used for vaccination against tuberculosis, has been shown to induce a durable and effective anti-tumor response with favorable outcomes based on the SWOG 8216 and SWOG 8507 trials and is the standard-of-care for treatment-naïve intermediate or high-risk non-muscle invasive bladder cancer (NMIBC) (2–3). However, a significant proportion of patients with NMIBC treated with intravesical BCG experience recurrence (about 40–50%) and progression to muscle-invasive and advanced urothelial carcinoma (aUC) (4).
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) are FDA-approved for aUC and were shown to prolong overall survival (OS) after platinum-based chemotherapy, either as switch maintenance or as salvage therapy (5,6). Pembrolizumab, an anti-PD-1 agent, is also approved for treatment of BCG-unresponsive, high-risk NMIBC in patients who either cannot undergo or refuse radical cystectomy based on the Keynote-057 trial (7). Despite FDA approvals and broad use, overall response rates (ORR) and progression-free survival (PFS) with ICIs for aUC remain modest, while immune related adverse events can cause clinically relevant sequelae.
Little is known regarding response and clinical outcomes with ICIs for patients with aUC previously treated with intravesical BCG, a different type of immunotherapy. While the exact mechanism of action remains unknown, intravesical BCG induces a robust innate immune response leading to long-lasting adaptive immunity in bladder cancer (8). However, the systemic effects and potential long-term implications of prior BCG therapy on systemic immune response are less clear. Patients who had relapse or progression on/after intravesical BCG may have differential expression of inflammatory components (e.g., IL-1β), and lower expression of IFN-γ, HMOX-1 and GNLY, when compared to those with durable response to intravesical BCG (9). Another proposed mechanism is that there may be a lower frequency of DNA damage response (DDR) gene alterations, such as ERCC2, in patients who progress to MIBC and aUC after prior intravesical BCG compared to those without prior BCG, which may impair response to subsequent ICI therapy (10).
Given that intravesical BCG may alter the systemic immune response as discussed earlier, it is possible that progression on/after intravesical BCG may impact response to systemically administered ICI. Subset analyses from clinical trials of ICIs in aUC regarding this question have been relatively limited. Therefore, we conducted a retrospective cohort study comparing outcomes (ORR, PFS and OS) between those with and without prior intravesical BCG exposure. We hypothesized that prior intravesical BCG would be associated with lower ORR shorter PFS and OS in patients with aUC treated with ICI.
Materials and Methods
Patient Selection and Data Collection
Institutional review board approval was attained, and the study was conducted in concordance with the Declaration of Helsinki principles. We used a previously collected cohort of patients, which included patients from 25 institutions in the United States and Europe. Consecutive patients at each institution were identified using a combination of provider-driven and electronic health record search algorithms.
For data collection and storage, we used web-based, secure, and standardized REDCap capture tools hosted at the Institute of Translational Sciences at University of Washington (11–12). Data collected included demographics, clinicopathological factors, including intravesical BCG exposure, ICI treatment and outcomes [ORR, PFS, OS]. Timing of imaging and designation of response and progression were investigator designated; although RECIST v1.1 criteria principles were used for the evaluation of best response, that endpoint was determined by the chart abstractor based on best available information in clinical notes and radiographic studies without central radiology review. Patients with pure non-UC histology, those with multiple ICI treatment lines and those with upper tract UC were excluded. Patients who received ICIs on as combinations or on clinical trials were also excluded due to the heterogeneity of trials and combinations with other chemotherapy and targeted therapies. Due to sample size considerations and overall study feasibility, we included patients regardless of prior documented history of NMIBC, including those with de novo metastatic UC, but we performed several adjustments for confounding prognostic factors in multivariable analyses, as discussed below.
Statistical Analysis
Baseline characteristics were summarized with descriptive statistics and compared with chi-square test and Student’s t-test for categorical and continuous variables, respectively. ORR was calculated as the sum of patients with investigator-determined complete or partial response divided by the total number of patients with available data. OS was measured from the date of ICI initiation until the date of death and PFS was measured from the date of ICI initiation until the date of investigator determined radiographic and/or clinical progression, or death. Patients without event (OS or PFS) were censored at the date of last follow-up visit. To assess the follow-up, we used the reverse Kaplan-Meier method.
For all outcomes (ORR, PFS and OS), patients were stratified by treatment line (first line [1L] and subsequent line [2+L]) and compared between those with and without prior intravesical BCG exposure. Multivariable logistic regression was used to estimate the odds ratio (OR) and 95% confidence intervals (CI) for ORR. Kaplan–Meier method was used for survival curves and to estimate median (m)OS and median (m)PFS. Cox regression was used to determine the effect of intravesical BCG exposure on OS and PFS; differences between groups were expressed as hazard ratios (HRs) and 95% CIs. For the multivariable analysis, models were adjusted based on calculated risk scores: an internally developed risk score (Khaki risk factors), which includes ECOG performance status, liver metastases, baseline neutrophil to lymphocyte ratio (NLR) and albumin (13) was used for 1L, and Bellmunt prognostic risk factor score (14), which includes ECOG performance status, liver metastases and hemoglobin, for 2+L (salvage) analysis. Statistical significance was set at P < 0.05; all P values were two-tailed. All statistical analyses were performed using Stata IC 16.0 (StataCorp LLC, College Station, TX, USA).
Results
Patient Selection and Characteristics
Out of a total of 1,026 patients with aUC treated with an ICI between 2013 and 2020 across 25 different institutions, 639 patients were included in the study population with 614, 638, and 617 patients ultimately in the ORR, PFS and OS analyses, respectively (Figure 1). The median follow-up time by the reverse Kaplan-Meier method was 21 months.
Figure 1.
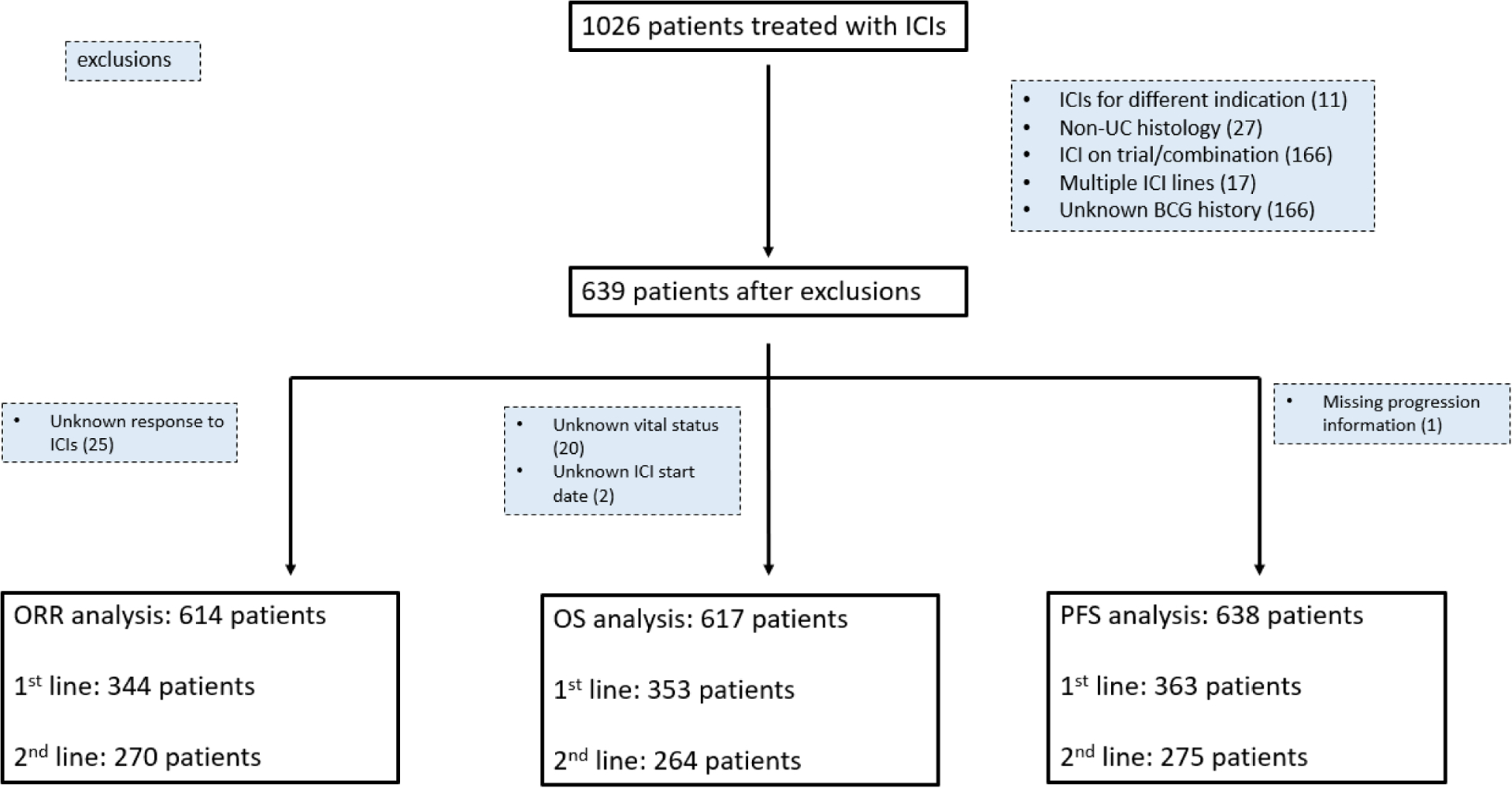
CONSORT diagram: patient selection & exclusion rationale
Table 1 shows the baseline characteristics for patients with and without history of intravesical BCG, stratified by line of therapy [1L and salvage (2+L)]. Among 363 and 276 patients treated with 1L and 2+L ICI, 77 (21%) and 73 (26%) had history of prior intravesical BCG treatment, respectively. In both the 1L and 2+L setting, there was a significantly higher prevalence of men in patients previously been treated with intravesical BCG. For those treated with ICIs in the 2+L setting, patients with history of intravesical BCG were also older (median age 77 vs 66 years).
Table 1.
Baseline characteristics for patients with advanced urothelial carcinoma treated with immune checkpoint inhibitors, stratified by treatment line and prior history of BCG
| First line ICI | Second or later line ICI | |||||
|---|---|---|---|---|---|---|
| History of BCG | No | Yes | P | No | Yes | P |
| Number of Patients | 287 | 78 | 205 | 75 | ||
| Age at ICI initiation, median (IQR) | 70 (67–82) | 69 (49–79) | 0.22 | 66 (59–80) | 77 (60–82) | <0.001 |
| Sex, N (%) | ||||||
| Male | 206 (72) | 63 (81) | 0.11 | 154 (75) | 66 (88) | 0.02 |
| Female | 81 (28) | 15 (20) | 51 (25) | 9 (12) | ||
| Smoking History, N (%) | ||||||
| Ever Smoker | 204 (72) | 49 (63) | 0.12 | 148 (72) | 51 (68) | 0.49 |
| Never Smoker | 80 (28) | 29 (37) | 57 (28) | 24 (32) | ||
| Race, N (%) | ||||||
| White race | 216 (75) | 61 (78) | 0.85 | 151 (74) | 57 (76) | 0.04 |
| Hispanic | 31 (11) | 6 (8) | 31 (15) | 4 (5) | ||
| African American | 16 (6) | 4 (5) | 12 (6) | 5 (7) | ||
| Asian | 8 (3) | 4 (5) | 8 (4) | 3 (4) | ||
| Histology, N (%) | ||||||
| Pure UC | 188 (66) | 56 (72) | 0.31 | 157 (77) | 57 (76) | 0.87 |
| Mixed UC | 98 (34) | 22 (28) | 47 (23) | 18 (24) | ||
| Non-UC | 1 (0) | 0 (0) | 1 (0) | 0 (0) | ||
| Prior platinum chemotherapy, N (%) | ||||||
| No | 148 (52) | 52 (67) | 0.018 | 14 (7) | 3 (4) | 0.38 |
| Yes | 139 (48) | 26 (33) | 191 (93) | 72 (96) | ||
| Albumin<3.5 g/dL at ICI initiation, N (%) | ||||||
| No | 96 (33) | 24 (31) | 0.48 | 63 (31) | 32 (43) | 0.07 |
| Yes | 171 (60) | 52 (67) | 131 (64) | 40 (53) | ||
| Hgb<10 g/dL at ICI initiation, N (%) | ||||||
| No | 212 (74) | 53 (68) | 0.29 | 144 (70) | 60 (80) | 0.14 |
| Yes | 68 (24) | 23 (30) | 55 (27) | 14 (19) | ||
| Liver Metastasis at ICI initiation, N (%) | ||||||
| No | 249 (87) | 63 (81) | 0.18 | 156 (76) | 57 (76) | 0.99 |
| Yes | 38 (13) | 15 (19) | 49 (24) | 18 (24) | ||
| ECOG Performance Status, N (%)a | ||||||
| 0 | 57 (20) | 18 (23) | 0.67 | 36 (18) | 18 (24) | 0.58 |
| 1 | 131 (46) | 32 (41) | 119 (58) | 40 (53) | ||
| 2 | 69 (24) | 18 (23) | 24 (12) | 11 (15) | ||
| 3 | 6 (2) | 3 (4) | 5 (2) | 1 (1) | ||
| 4 | 1 (0) | 1 (1) | 0 (0) | 0 (0) | ||
| Missing | 23 (8) | 6 (8) | 21 (10) | 5 (7) | ||
| ICI Received, N (%) | ||||||
| Atezolizumab | 113 (39) | 30 (39) | 0.37 | 110 (54) | 44 (60) | 0.18 |
| Avelumab | 1 (0) | 0 (0) | 1 (<1) | 0 (0) | ||
| Durvalumab | 7 (2) | 4 (5) | 6 (3) | 1 (1) | ||
| Nivolumab | 16 (6) | 1 (1) | 26 (13) | 4 (6) | ||
| Pembrolizumab | 147 (51) | 42 (54) | 60 (30) | 24 (33) | ||
| Risk Scoreb | ||||||
| 0 | 112 (39) | 24 (31) | 0.62 | 27 (13) | 11 (15) | 0.27 |
| 1 | 83 (29) | 22 (28) | 83 (41) | 38 (51) | ||
| 2 | 37 (13) | 12 (15) | 59 (29) | 19 (25) | ||
| 3c | 26 (9) | 7 (10) | 12 (6) | 1 (1) | ||
| Missing | 28 (10) | 12 (16) | 24 (11) | 6 (8) | ||
At time of ICI initiation
First-line: Internally developed risk score, Ali risk model (ECOG PS, liver mets, NLR, albumin) [13]; Second or later line: Bellmunt risk score (ECOG, liver mets, Hgb) [14]
First-line risk score includes four factors thus score of 3 is ≥3
Observed Response Rate
A total of 614 patients were included in ORR analysis; 344 and 270 patients were treated with ICI in the 1L and 2+L setting, respectively. In the 1L, those with prior intravesical BCG exposure had ORR 23% (95% CI 14–34) and those without had ORR 31% (95% CI 26–37; Table 2). Similarly, among those treated with ICIs in 2+L, ORR was 31% (95% CI 21–42) and 24% (95% CI 15–28) for those with and without prior intravesical BCG, respectively. In both 1L and 2+L, the odds of response to ICI were not significantly different among those with and without prior intravesical BCG exposure.
Table 2.
Observed response rate (ORR), Overall Survival (mOS) and Progression-Free Survival (mPFS) according to prior history of intravesical BCG treatment, stratified by treatment line
| Treatment Line | History of BCG treatment? | ORR (%) (95% CI) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| First Line | No (n = 273) | 31 (26–37) | Reference | 0.08 |
| Yes (n = 71) | 23 (14–34) | 0.55 (0.28–1.06) | ||
| Subsequent Line | No (n = 198) | 24 (18–30) | Reference | 0.12 |
| Yes (n = 72) | 31 (21–42) | 1.65 (0.95–2.58) | ||
| Median Overall Survival (mOS) | ||||
| History of BCG treatment? | mOS, months (95% CI) | Adjusted HR (95% CI) | p-value | |
| First Line | No (n = 279) | 11 (8–14) | Reference | 0.79 |
| Yes (n = 74) | 11 (6–15) | 1.05 (0.71–1.56) | ||
| Subsequent Line | No (n = 191) | 10 (8–12) | Reference | 0.49 |
| Yes (n = 73) | 7 (5–12) | 1.13 (0.79–1.63) | ||
| Median Progression-Free Survival (mPFS) | ||||
| History of BCG treatment? | mPFS, months (95% CI) | Adjusted HR (95% CI) | p-value | |
| First Line | No (n = 286) | 4 (4–6) | Reference | 0.55 |
| Yes (n = 77) | 3 (2–7) | 1.12 (0.77–1.62) | ||
| Subsequent Line | No (n = 203) | 4 (3–4) | Reference | 0.39 |
| Yes (n = 72) | 4 (3–7) | 0.87 (0.63–1.19) | ||
Progression Free Survival
A total of 638 patients were included in the PFS analysis; 363 and 275 were treated with ICI in the 1L and 2+L, respectively. Median PFS for those with and without prior intravesical BCG exposure was 4 (95% CI 4–6) and 3 months (95% CI 2–7) in 1L, and 4 (95% CI 3–4) and 4 months (95% CI 3–7) in 2+L, respectively. No significant difference in PFS in multivariable analysis was noted among patients with vs without prior intravesical BCG exposure in either 1L or 2+L setting (Table 2).
Overall Survival
A total of 617 patients were included in the OS analysis: 353 with 1L ICI and 264 with 2+L ICI. In the 1L, median OS for patients with vs without prior intravesical BCG exposure was 11 (95% CI 6–15) vs 11 months (95% CI 8–14; Figure 1), respectively. In the 2+L subgroup, patients with prior intravesical BCG had median OS of 7 months [95% CI 5–12] vs 10 months [95% CI 8–12]), which was not significantly different on Cox Regression (HR 1.13 [95% CI 0.79–1.63], p=0.49).
Discussion
In this retrospective multi-institution cohort study of patients with aUC treated with ICIs, prior intravesical BCG exposure was not associated with significant difference in ORR, PFS or OS. This hypothesis-generating study provides relevant data suggesting that prior intravesical BCG may not need to be a stratification factor in ICI trials in aUC or impact clinical decision making.
Few prior studies have investigated outcomes with ICI therapy among patients with and without prior intravesical BCG exposure. The DANUBE trial, a phase III trial investigating durvalumab with or without tremelimumab vs platinum-based chemotherapy in patients with aUC, showed in a subset analysis in patients treated with durvalumab and tremelimumab, those patients with prior intravesical BCG exposure did not have significant difference in OS compared to those without prior intravesical BCG exposure (median 17.7 vs 15 months, respectively (15). The ABACUS phase II trial investigated the efficacy of two doses of neoadjuvant atezolizumab prior to radical cystectomy in cisplatin-unfit patients with operable localized MIBC, presented a subset analysis showing that patients who were previously treated with intravesical BCG had similar pathologic complete response rate compared to the entire cohort, 30% (95% CI: 7–65%) vs 31% (95% CI: 21–41%) (16). These findings align with ours which also did not show significant difference in ORR, PFS, or OS based on prior intravesical BCG exposure in patients treated with ICIs with aUC.
Although BCG and ICIs are both immunotherapy modalities, they have different mechanisms of action and potentially of resistance. The exact mechanism of BCG action is unclear, however in vivo studies have shown that internalization of BCG into bladder tumor cells can lead to cell apoptosis and necrosis and can induce cytokines causing an immune cascade that alters the tumor immune microenvironment and facilitates a robust host immune response leading to tumor cell death (17). Just as its exact mechanism of action is not fully elucidated, the same is true for mechanisms of resistance. It has been postulated that increased upregulation of PD-L1 may lead to immune silencing and reduced BCG efficacy (17). If this were the case, we would expect possibly higher ORR in patients with prior intravesical BCG exposure, which we did not note.
We originally hypothesized that patients with aUC with prior exposure to intravesical BCG would have diminished response to ICI. We thought that, given intravesical BCG can induce a systemic immune response and that those who progressed on/after BCG may have an altered immunomodulatory systemic landscape, this might negatively impact response to future systemic ICI. A retrospective study showed that patients with primary MIBC (without exposure to prior intravesical BCG) had higher frequency of DDR gene alterations, such as ERCC2, compared to secondary MIBC (with possible prior exposure to BCG) (10). This may subsequently make the former cohort of patients potentially more responsive to subsequent ICI therapy. However, our data shows similar response and outcomes to ICI for aUC for those with and without prior intravesical BCG exposure suggests that if there were systemic changes, these may not affect ICI outcomes.
Notably, there are currently multiple trials investigating combining intravesical BCG with ICI across the spectrum of NMIBC (18–20). It is important to highlight that these trials are distinct from our retrospective study, which investigated outcomes with ICI in a population who received prior intravesical BCG. Intravesical BCG remains the standard of care for high risk NMIBC and ICIs have shown very promising data for BCG-unresponsive NMIBC, so BCG and ICI combination warrants further investigation in that setting.
Strengths of our study include the use of real-world data and the large sample size from multiple institutions across the United States and Europe. Inherent limitations of our study include the retrospective study design, lack of randomization, possible selection bias and unmeasured confounding. For example, patients with MIBC or de novo metastatic disease who did not present with NMIBC may have different tumor biology, therapy and clinical course compared to those who initially had NMIBC and may have received intravesical BCG. We also were not able to collect the extent and duration of prior intravesical BCG therapy, whether BCG was administered in the induction and/or maintenance setting, response to BCG, and the time elapsed between last BCG dose and ICI initiation, all of which may modify the relationship between prior intravesical BCG and ICI outcomes. In addition, given the multi-institution retrospective study design, we could not conduct central radiology or pathology review, while there may have been practice-related variability in therapy administration, disease monitoring and follow up periods, which could affect ascertainment of response and progression. We also did not assess toxicity to intravesical BCG and ICIs, prior BCG vaccination against tuberculosis, or molecular biomarkers. Despite these limitations, this analysis provides important preliminary data that selection for ICI treatment for aUC as well as clinical trial eligibility and stratification should not be impacted by prior exposure to intravesical BCG.
Conclusion
In conclusion, our hypothesis-generating study did not demonstrate significant differences in ORR, PFS or OS with ICI for patients with aUC based on prior intravesical BCG exposure. Further clinical and molecular biomarker exploration is needed to refine patient selection for ICI and trial designs in UC.
Supplementary Material
Figure 2.
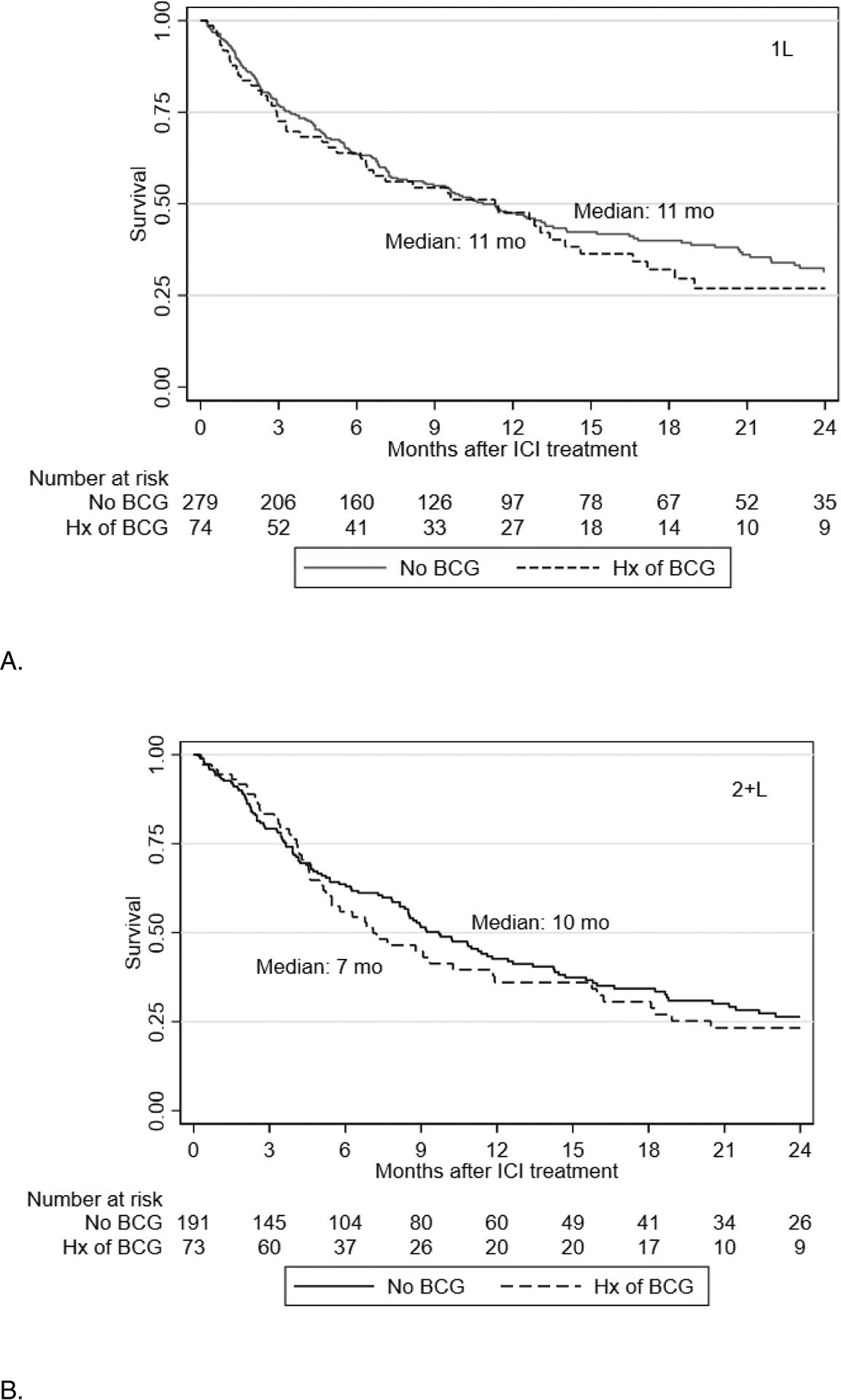
Kaplan Meier curves for overall survival with checkpoint inhibitors in first line (A) and salvage (B) setting.
Figure 3.
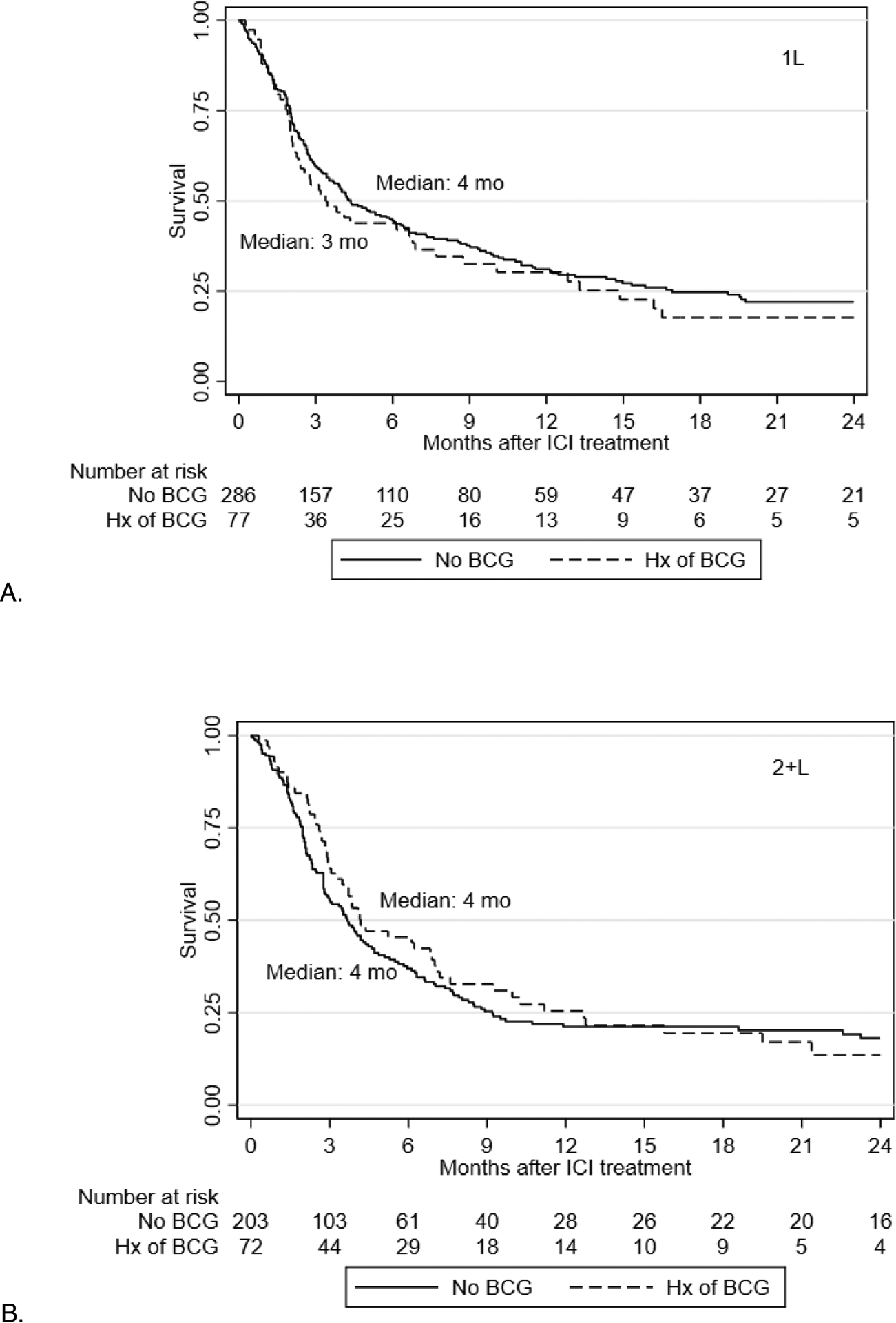
Kaplan Meier curves for progression-free survival with checkpoint inhibitors in first line (A) and salvage (B) setting.
Clinical Practice Points.
While immune checkpoint inhibitors (ICIs) improve survival in locally advanced unresectable and metastatic urothelial carcinoma (aUC), little is known regarding outcomes in patients with prior exposure to intravesical BCG
We compared observed response rate, progression-free and overall survival between patients with and without prior intravesical BCG exposure who were treated with ICIs for aUC
Our findings provide important preliminary data that prior intravesical BCG exposure may not impact ICI efficacy in aUC.
COI:
In the last 3 years, Petros Grivas’ institution has received grants from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm, EMD Serono, GlaxoSmithKline, Immunomedics/Gilead, Merck & Co., Mirati Therapeutics, Pfizer, QED Therapeutics. Dr. Grivas has received consulting fees from AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Clovis Oncology, Dyania Health, Driver, EMD Serono, Exelixis, Foundation Medicine, Genentech/Roche, Genzyme, GlaxoSmithKline, Guardant Health, Heron Therapeutics, Immunomedics/Gilead, Infinity Pharmaceuticals, Janssen, Merck & Co., Mirati Therapeutics, Pfizer, QED Therapeutics, Regeneron Pharmaceuticals, Seattle Genetics, 4D Pharma PLC, UroGen.
Abbreviations
- aUC
advanced urothelial carcinoma
- BCG
Bacillus Calmette-Guerin
- ICI
Immune checkpoint inhibitors
- NLR
neutrophil to lymphocyte ratio
- NMIBC
non-muscle invasive bladder cancer
- MIBC
muscle invasive bladder cancer
- ORR
observed response rate
- OS
overall survival
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- PFS
progression-free survival
- SWOG
Southwest Oncology Group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement
Rafee Talukder MD: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing- Original Draft, Writing- Review and Editing. Dimitrios Makrakis MD: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing- Original Draft, Writing- Review and Editing. Leonidas N. Diamantopoulos MD: Investigation, Data Curation, Writing- Review and Editing. Lucia Carril-Ajuria MD: Investigation, Writing- Review and Editing. Daniel Castellano MD: Investigation, Writing- Review and Editing. Ivan De Kouchkovsky MD: Investigation, Writing- Review and Editing. Vadim S. Koshkin MD: Investigation, Writing- Review and Editing. Joseph J. Park MD: Investigation, Writing- Review and Editing. Ajjai Alva MD: Investigation, Writing- Review and Editing. Mehmet A. Bilen MD: Investigation, Writing- Review and Editing. Tyler F. Stewart MD: Investigation, Writing- Review and Editing. Rana R. McKay MD: Investigation, Writing- Review and Editing. Victor S. Santos MD: Investigation, Writing- Review and Editing. Neeraj Agarwal MD: Investigation, Writing- Review and Editing. Jayanshu Jain MD: Investigation, Writing- Review and Editing. Yousef Zakharia MD: Investigation, Writing- Review and Editing. Rafael Morales-Barrera MD: Investigation, Writing- Review and Editing. Michael E. Devitt MD: Investigation, Writing- Review and Editing. Michael Grant MBBS: Investigation, Writing- Review and Editing. Mark P. Lythgoe MBBS: Investigation, Writing- Review and Editing. David J. Pinato MD, PhD: Investigation, Writing- Review and Editing. Ariel Nelson MD: Investigation, Writing- Review and Editing. Christopher J. Hoimes DO: Investigation, Writing- Review and Editing. Evan Shreck MD: Investigation, Writing- Review and Editing. Benjamin A. Gartrell MD: Investigation, Writing- Review and Editing. Alex Sankin MD: Investigation, Writing- Review and Editing. Abhishek Tripathi MD: Investigation, Writing- Review and Editing. Roubini Zakopoulou MD, PhD: Investigation, Writing- Review and Editing. Aristotelis Bamias MD, PhD: Investigation, Writing- Review and Editing. Jure Murgic MD PhD: Investigation, Writing- Review and Editing. Ana Fröbe MD, PhD: Investigation, Writing- Review and Editing. Alejo Rodriguez-Vida MD, PhD: Investigation, Writing- Review and Editing. Alexandra Drakaki MD: Investigation, Writing- Review and Editing. Sandy Liu MD: Investigation, Writing- Review and Editing. Vivek Kumar MD: Investigation, Writing- Review and Editing. Giuseppe Di Lorenzo MD: Investigation, Writing- Review and Editing. Monika Joshi MD: Investigation, Writing- Review and Editing. Pedro Isaacsson Velho MD: Investigation, Writing- Review and Editing. Lucia Alonso Buznego MD: Investigation, Writing- Review and Editing. Ignacio Duran MD: Investigation, Writing- Review and Editing. Marcus Moses MS: Investigation. Pedro Barata MD, MSc: Investigation, Writing- Review and Editing. Guru Sonpavde MD: Investigation, Writing- Review and Editing. Evan Y. Yu MD: Investigation, Writing- Review and Editing. Jonathan L. Wright MD: Investigation, Writing- Review and Editing. Petros Grivas MD PhD: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing- Original Draft, Writing- Review and Editing, Supervision. Ali Raza Khaki MD MS: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing- Original Draft, Writing- Review and Editing, Supervision.
References
- 1.Burger M, Catto JWF, Dalbagni G, et al. Epidemiology and Risk Factors of Urothelial Bladder Cancer. Eur Urol. 2013;63(2):234–241. [DOI] [PubMed] [Google Scholar]
- 2.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med 1991;325:1205–9. [DOI] [PubMed] [Google Scholar]
- 3.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9. [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006; 49:465–466.475–477. [DOI] [PubMed] [Google Scholar]
- 5.Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383:1218–1230. [DOI] [PubMed] [Google Scholar]
- 6.Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30(6):970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balar AV, Kulkarni GS, Uchio EM, et al. Keynote 057: Phase II trial of pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guérin (BCG). J Clin Oncol 2019; 37: (7_Suppl): 350.30557524 [Google Scholar]
- 8.Larsen ES, Joensen UN, Poulsen AM, et al. Bacillus Calmette–Guérin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. APMIS. 2020;128(2):92–103. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi K, Koga S, Nishikido M, et al. Systemic immune response after intravesical instillation of bacille Calmette-Guérin (BCG) for superficial bladder cancer. Clin Exp Immunol. 1999. Jan;115(1):131–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietzak EJ, Zabor EC, Bagrodia A, et al. Genomic Differences Between “Primary” and “Secondary” Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy. Eur Urol. 2019. Feb;75(2):231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaki AR, Li A, Diamantopoulos LN, et al. A New Prognostic Model in Patients with Advanced Urothelial Carcinoma Treated with First-line Immune Checkpoint Inhibitors. Eur Urol Oncol. 2021;S2588–9311(20)30214–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial [published correction appears in Lancet Oncol. 2021 Jan;22(1):e5]. Lancet Oncol. 2020;21(12):1574–1588. [DOI] [PubMed] [Google Scholar]
- 16.Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial [published correction appears in Nat Med. 2020 Jun;26(6):983]. Nat Med. 2019;25(11):1706–1714. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Gu X, Li Y, Wu Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed Pharmacother. 2020. Sep;129:110393. [DOI] [PubMed] [Google Scholar]
- 18.Kamat AM, Shore N, Hahn N, et al. KEYNOTE-676: Phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Future Oncol. 2020;16(10):507–516. [DOI] [PubMed] [Google Scholar]
- 19.Albisinni S, Martinez Chanza N, Aoun F, et al. Immune checkpoint inhibitors for BCG-resistant NMIBC: the dawn of a new era [published online ahead of print, 2021 Mar 29]. Minerva Urol Nephrol. 2021. [DOI] [PubMed] [Google Scholar]
- 20.Santis MD; Abdrashitov R; Hegele A et al. A phase III, randomized, open-label, multicenter, global study of durvalumab and bacillus calmette-guérin (BCG) versus BCG alone in high-risk, BCG-naïve non-muscle-invasive bladder cancer (NMIBC) patients (POTOMAC). J. Clin. Oncol 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


