Abstract
Emtricitabine [(−)FTC] [(−)-β-2′,3′-dideoxy-5-fluoro-3′-thiacytidine] has been shown to be an effective inhibitor of hepatitis B virus (HBV) in cell culture, with a potency and selectivity that are essentially identical to those of lamivudine. The antiviral activity of oral administration of (−)FTC against WHV replication in chronically infected woodchucks, an established and predictive model for antiviral therapy against HBV, was examined in a placebo-controlled study. (−)FTC significantly reduced viremia and intrahepatic WHV replication in a dose-dependent manner that was comparable to the antiviral activity of lamivudine observed in previous studies conducted by our laboratories. No effect on the levels of hepatic WHV RNA or the levels of woodchuck hepatitis surface antigen or anti-woodchuck hepatitis surface and core antibodies in the serum of the treated animals was observed. No evidence of drug-related toxicity was observed in any of the animals treated.
(−)-β-2′,3′-dideoxy-5-fluoro-3′-thiacytidine [(−)FTC] has been shown to be an effective inhibitor of human immunodeficiency virus types 1 and 2, hepatitis B virus (HBV), and woodchuck hepatitis virus (WHV) replication in cell culture and WHV DNA polymerase in in vitro assays with a potency and selectivity that are essentially identical to those of lamivudine (3TC) (4, 5, 8, 13, 17). WHV and its natural host, the Eastern woodchuck (Marmota monax), constitute a useful model of HBV-induced disease, including primary hepatocellular carcinoma (HCC) (7, 18). Published studies have shown that antiviral activities of several agents used in anti-HBV clinical trials have comparable anti-WHV activities in chronically infected woodchucks (see reference 10 for a review). In a previously published report, (−)FTC was shown to be moderately effective against WHV replication in chronically infected woodchucks when delivered by intraperitoneal injection (3). The pharmacokinetic profile of (−)FTC given orally is favorable to antiviral therapy and is similar to, or in some aspects superior to, that observed for lamivudine [(−)3TC] both in vitro and in vivo (1, 13, 16). The pharmacokinetic profile of lamivudine delivered orally in woodchucks is similar to that observed in humans, and lamivudine is an effective antiviral agent against chronic WHV infection (9, 12, 15). In this report, the antiviral activity of (−)FTC delivered orally, once daily, against WHV replication in chronically infected woodchucks is examined in a placebo-controlled study.
The woodchucks used in these studies were born to WHV-negative females in a breeding colony and were inoculated at 3 days of age to induce chronic infections (20). Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (13a) and were reviewed and approved by the Cornell University Institutional Animal Care and Use Committee. Six groups of four adult chronic carrier woodchucks were used in this study. One group served as placebo controls. The remaining five groups were treated with (−)FTC at one of five doses: 0.3, 1.0, 3.0, 10, or 30 mg/kg of body weight. (−)FTC was solubilized in isotonic saline solution and was administered orally in a liquid diet once daily for 28 days (19). The liquid diet was also administered daily to the control group of animals. Serum samples were taken for analysis on the first day of treatment prior to drug delivery (week 0); at 1, 2, 3, and 4 weeks of treatment; and at 1, 2, 3, 4, 6, 8, and 12 weeks following the end of therapy. Needle liver biopsies were obtained at the time of the first serum specimen (week 0), at the end of the treatment period (week 4), and at 4 and 12 weeks posttreatment as previously described (10, 19). The general health of the woodchucks was assessed by daily observation at the time they received food and water, at the time of drug (or placebo) administration, and at the times they were anesthetized (19). Any abnormalities in behavior, appearance, or food and water intake were recorded. Body weights were monitored weekly when serum and/or liver specimens were taken. Hematologic and routine blood chemistry analyses were performed at the beginning and end of the experimental periods, at the end of the treatment period, and at 4 weeks following the termination of therapy as previously described (19). WHV viremia in serum samples was assessed by dot blot hybridization (four 10-μl replicates per sample) or PCR-based analysis (two 5-μl replicates per sample) as previously described (10, 11, 19). Levels of woodchuck hepatitis surface antigen (WHsAg) and the presence of anti-woodchuck hepatitis surface (WHs) and anti-woodchuck hepatitis core (WHc) antibodies in serum samples were assessed as previously described with WHV-specific enzyme immunoassays (2, 14). Levels of intrahepatic WHV nucleic acids were quantitatively determined by Southern or Northern blot hybridization as previously described (10, 11, 19).
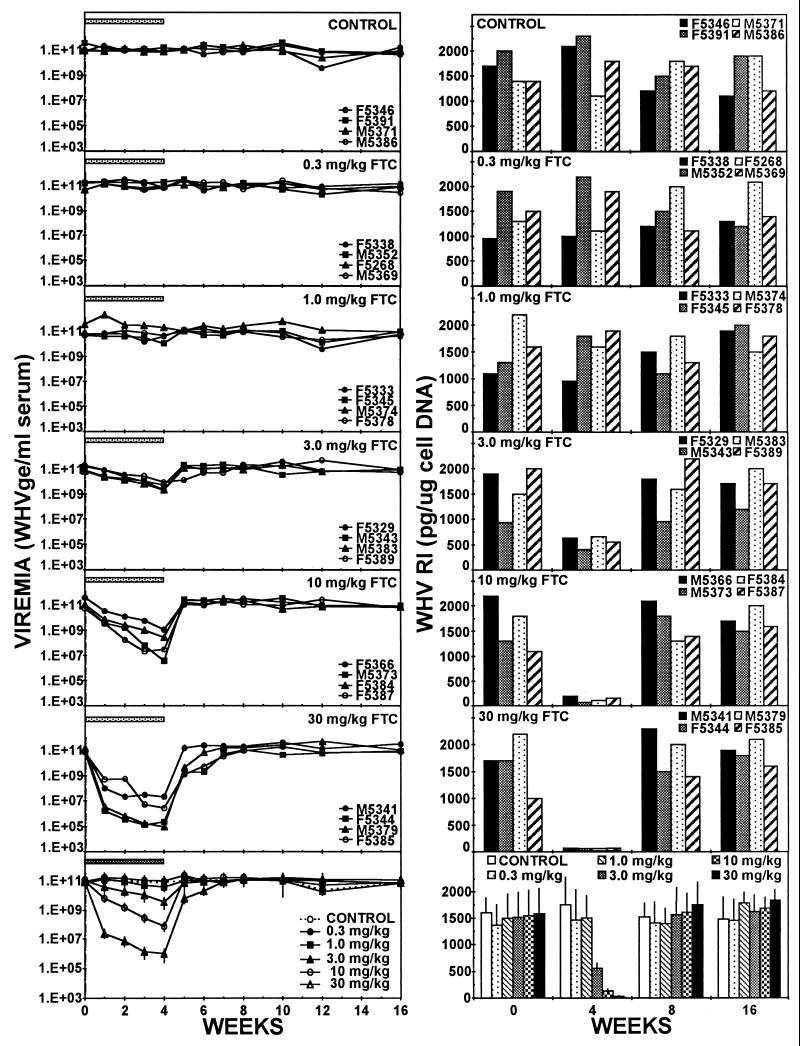
Treatment of chronic carrier woodchucks reduced WHV viremia and intrahepatic WHV replication in a dose-dependent manner in this study (Fig. 1 and Table 1). No significant antiviral effect was observed following therapy with 0.3 or 1.0 mg/kg. Therapy with 3.0 mg of (−)FTC per kg induced a gradual and progressive reduction in viremia of approximately 10-fold by the end of the treatment period. A dose of 10 mg/kg induced a more rapid decline in viremia that was 100-fold greater than that observed for the 3.0-mg/kg dose. Treatment with 30 mg of (−)FTC per kg produced the greatest antiviral effect, inducing a rapid loss of viremia after 1 week, followed by a much slower rate of decline during the next 3 weeks of therapy. Viremia returned to pretreatment levels within 1 to 2 weeks following the end of treatment in all treated animals.
FIG. 1.
Effect of antiviral treatments on WHV replication in chronic WHV carrier woodchucks. Horizontal bars denote the treatment period. Values for individual animals in each treatment group are displayed in the top 12 panels. Mean values for all of the experimental groups are compared in the bottom two panels (vertical lines denote standard deviations). WHVge, WHV genomic equivalents (virion or WHV DNA-containing virus particles); WHV RI, hepatic WHV DNA RI. Levels of hepatic cellular DNA were quantified by hybridization to a commercial β-actin gene probe (Oncor, Inc., Gaithersburg, Md.) by Southern or dot blot hybridization techniques as described in the text.
TABLE 1.
Antiviral activity of (−)FTC against WHV in chronically infected woodchucks
| Time point for group | Level of WHVa:
|
||
|---|---|---|---|
| Viremia (log10 WHVge/ml of serum) | RI (pg/μg of cell DNA) | RNA (pg/μg of total cell RNA) | |
| Control (placebo) | |||
| Pretreatment (wk 0) | 11.1 ± 0.3 (10.9–11.5) | 1,607 ± 287 (1,400–1,700) | 59 ± 13 (49–78) |
| End of treatment (wk 4) | 10.9 ± 0.2 (10.8–11.1) | 1,759 ± 525 (1,100–2,300) | 61 ± 14 (50–80) |
| 4 wk posttreatment (wk 8) | 11.0 ± 0.2 (10.8–11.3) | 1,532 ± 265 (1,200–1,800) | 62 ± 15 (42–78) |
| 12 wk posttreatment (wk 16) | 10.9 ± 0.2 (10.7–11.2) | 1,477 ± 435 (1,100–1,900) | 66 ± 7 (50–70) |
| FTC | |||
| 0.3 mg/kg | |||
| Pretreatment (wk −1) | 11.0 ± 0.3 (10.7–11.3) | 1,370 ± 397 (950–1,900) | 55 ± 6 (55–62) |
| End of treatment (wk 4) | 11.0 ± 0.2 (10.8–11.3) | 1,464 ± 592 (1,000–2,200) | 57 ± 16 (49–78) |
| 4 wk posttreatment (wk 8) | 11.1 ± 0.2 (10.8–11.2) | 1,411 ± 404 (1,100–2,000) | 69 ± 2 (66–71) |
| 12 wk posttreatment (wk 16) | 10.9 ± 0.3 (10.5–11.2) | 1,463 ± 408 (1,200–2,100) | 59 ± 8 (52–68) |
| 1.0 mg/kg | |||
| Pretreatment (wk 0) | 10.9 ± 0.4 (10.6–11.5) | 1,498 ± 480 (1,100–2,200) | 58 ± 13 (46–77) |
| End of treatment (wk 4) | 10.6 ± 0.5 (10.0–11.3) | 1,510 ± 427 (950–1,900) | 62 ± 11 (49–74) |
| 4 wk posttreatment (wk 8) | 11.1 ± 0.2 (10.9–11.4) | 1,402 ± 299 (1,100–1,800) | 58 ± 6 (50–63) |
| 12 wk posttreatment (wk 16) | 10.8 ± 0.2 (10.6–11.0) | 1,790 ± 216 (1,500–2,000) | 60 ± 7 (53–69) |
| 3.0 mg/kg | |||
| Pretreatment (wk 0) | 11.0 ± 0.2 (10.8–11.3) | 1,517 ± 486 (930–2,000) | 57 ± 14 (43–77) |
| End of treatment (wk 4) | 9.6 ± 0.3 (9.3–9.9)†§ | 553 ± 112 (410–660)*‡ | 63 ± 8 (55–70) |
| 4 wk posttreatment (wk 8) | 11.1 ± 0.2 (10.9–11.3) | 1,566 ± 522 (950–2,200) | 52 ± 9 (44–66) |
| 12 wk posttreatment (wk 16) | 10.9 ± 0.1 (10.7–10.9) | 1,623 ± 332 (1,200–2,000) | 61 ± 10 (48–71) |
| 10 mg/kg | |||
| Pretreatment (wk 0) | 11.0 ± 0.4 (10.7–11.6) | 1,543 ± 497 (1,100–2,200) | 63 ± 7 (56–73) |
| End of treatment (wk 4) | 7.8 ± 1.1 (6.6–9.0)†§ | 122 ± 53 (69–190)†§ | 54 ± 4 (50–60) |
| 4 wk posttreatment (wk 8) | 11.2 ± 0.2 (11.0–11.5) | 1,620 ± 370 (1,300–2,100) | 59 ± 2 (57–62) |
| 12 wk posttreatment (wk 12) | 10.8 ± 0.1 (10.7–10.9) | 1,690 ± 216 (1,500–2,000) | 62 ± 10 (47–69) |
| 30 mg/kg | |||
| Pretreatment (wk 0) | 10.9 ± 0.2 (10.8–11.2) | 1,588 ± 493 (1,000–2,200) | 62 ± 12 (49–78) |
| End of treatment (wk 4) | 6.0 ± 1.1 (5.0–7.3)†§ | 20 ± 7 (15–31)†§ | 60 ± 10 (50–73) |
| 4 wk posttreatment (wk 8) | 11.1 ± 0.2 (11.0–11.3) | 1,763 ± 424 (1,400–2,300) | 66 ± 5 (60–72) |
| 12 wk posttreatment (wk 12) | 11.1 ± 0.3 (10.9–11.5) | 1,841 ± 208 (1,600–2,100) | 59 ± 8 (50–69) |
Values are means ± standard deviations, and overall ranges of values for WHV nucleic acids are presented in parentheses. WHVge, WHV genome equivalents. Levels of intrahepatic intracellular WHV DNA RI and total WHV RNA were normalized for total cellular DNA or RNA by using the relative levels of β-actin DNA or RNA (β-actin hybridization probe purchased from Oncor, Inc., Gaithersburg, Md.). * and †, statistically different from placebo control at week 4: *, P < 0.05; †, P < 0.01. ‡ and §, statistically different from pretreatment values: ‡, P < 0.05; §, P < 0.01.
Consistent with the observed effect on viremia, intrahepatic WHV replication was significantly reduced by doses of 3.0 mg of (−)FTC per kg and higher after 4 weeks of therapy (Fig. 1 and Table 1). Hepadnaviral DNA replication intermediates in liver tissue are comprised of a heterogeneous population of single-stranded and partially double-stranded viral DNA molecules that migrate as a distinctive smear with an apparent molecular size of 0.2 to 3.0 kb in Southern blot hybridization analyses (6). Progressively larger reductions in hepatic WHV replication were observed as the dose of (−)FTC increased (3-fold to 80-fold). No significant effect on the levels of intrahepatic WHV RNA were observed (Table 1). No changes in the levels of WHsAg or antibodies to WHsAg (anti-WHs) or WHcAg (anti-WHc) in the serum of the treated animals were observed (data not shown).
No significant changes in the levels of viremia, WHV replication intermediates (RI), hepatic WHV RNA (Fig. 1 and Table 1), WHsAg, anti-WHs, or anti-WHc (data not shown) were observed in the control (placebo treated) group of animals during the course of the study. No obvious treatment-related clinical (e.g., body weight loss), hematologic, or serologic indications of toxicity were observed in any of the treated animals.
The relative activity of (−)FTC in this study was greater than that previously reported for treatments of WHV chronic carriers (3). While the reasons for this difference are not readily apparent at this time, one possible explanation could be the different methods of delivery used in the two studies. In the previous study, (−)FTC was delivered by intraperitoneal injection, while in the current study, the more clinically relevant mode of oral delivery was chosen.
The rapid rebound of WHV replication following the termination of therapy, as well as the lack of an effect on hepatic WHV RNA and viral antigen expression in this study, is most likely due to a failure to lower WHV DNA replication to levels low enough to reduce the levels of covalently closed circular WHV DNA (cccDNA) in the liver. Hepadnaviral cccDNA genomes are the template for viral RNA transcription (6). In studies with primary woodchuck hepatocytes, both lamivudine and (−)FTC were ineffective in reducing WHV cccDNA levels following more than 5 weeks of a treatment that induced a substantial reduction in WHV replication (13).
In summary, (−)FTC is an effective inhibitor of WHV replication in vivo, consistent with previously reported activities against HBV and WHV replication in vitro and in vivo (3–5, 13, 17). The relative antiviral activity of (−)FTC was comparable to that observed by our laboratories for (−)3TC against WHV replication in chronically infected woodchucks (9, 10).
Acknowledgments
This work was supported by contract N01-AI-45179 between the National Institute of Allergy and Infectious Diseases (NIAID) and Georgetown University and contract N01-AI-35164 between the NIAID and the College of Veterinary Medicine of Cornell University.
The technical assistance of Frances Wells, Tracy Franklin, and Kristine Farrar of Georgetown University and Betty Baldwin, Mary Ascenzi, and Lou Ann Graham of Cornell University is gratefully acknowledged.
REFERENCES
- 1.Abobo C V, Ni L, Schinazi R F, Liotta D C, Boudinot F D. Pharmacokinetics of 2′,3′-dideoxy-5-fluoro-3′-thiacytidine in rats. J Pharm Sci. 1994;83:96–99. doi: 10.1002/jps.2600830122. [DOI] [PubMed] [Google Scholar]
- 2.Cote P J, Roneker C, Cass K, Schodel F, Peterson D, Tennant B, DeNoronha F, Gerin J L. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 1993;6:161–169. doi: 10.1089/vim.1993.6.161. [DOI] [PubMed] [Google Scholar]
- 3.Cullen J M, Smith S L, Davis M G, Dunn S E, Botteron C, Cecchi A, Linsey D, Linzey D, Frick L, Paff M T, Goulding A, Biron K. In vivo antiviral activity and pharmacokinetics of (−)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob Agents Chemother. 1997;41:2076–2082. doi: 10.1128/aac.41.10.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis M G, Jansen R W. Inhibition of hepatitis B virus in tissue culture by alpha interferon. Antimicrob Agents Chemother. 1994;38:2921–2924. doi: 10.1128/aac.38.12.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furman P A, Davis M, Liotta D C, Paff M, Frick L W, Nelson D J, Dornsife R E, Wurster J A, Wilson L J, Fyfe J A, Tuttle J V, Miller W H, Condreny L, Averett D R, Schinazi R F, Painter G R. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganem D. Hepadnaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2703–2737. [Google Scholar]
- 7.Gerin J L. Experimental WHV infection of woodchucks: an animal model of hepadnavirus-induced liver cancer. Gastroenterol Jpn. 1990;25(Suppl.):38–42. doi: 10.1007/BF02779926. [DOI] [PubMed] [Google Scholar]
- 8.Gosselin G, Schinazi R F, Sommadossi J-P, Mathé C, Bergogne M-C, Aubertin A-M, Kirn A, Imbach J-L. Anti-human immunodeficiency virus activities of the β-l enantiomer of 2′,3′-dideoxycytidine and its 5-fluoro derivative in vitro. Antimicrob Agents Chemother. 1994;38:1292–1297. doi: 10.1128/aac.38.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurwitz S J, Tennant B C, Korba B E, Gerin J L, Schinazi R F. Viral pharmacodynamics of (−)-β-2′,3′-dideoxy-3′-thiacytidine in chronically virus-infected woodchucks compared to its pharmacodynamics in humans. Antimicrob Agents Chemother. 1998;42:2804–2809. doi: 10.1128/aac.42.11.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korba, B., B. Baldwin, W. Hornbuckle, P. Cote, B. Tennant, and J. Gerin. Treatment of chronic WHV infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in man. Hepatology, in press. [DOI] [PubMed]
- 11.Korba B E, Wells F, Tennant B C, Yoakum G H, Purcell R H, Gerin J L. Hepadnavirus infection of peripheral blood lymphocytes in vivo: woodchuck and chimpanzee models of viral hepatitis. J Virol. 1986;58:1–8. doi: 10.1128/jvi.58.1.1-8.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason W S, Cullen J, Moraleda G, Saputelli J, Aldrich C E, Miller D S, Tennant B, Frick L, Averett D, Condreay L D, Jilbert A R. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 13.Moraleda G, Saputelli J, Aldrich C E, Averett D, Condreay L, Mason W S. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.National Academy of Sciences. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 14.Ponzetto A, Cote P J, Ford E C, Purcell R H, Gerin J L. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984;52:70–76. doi: 10.1128/jvi.52.1.70-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopalan P, Boudinot F D, Chu C K, Tennant B C, Baldwin B H, Schinazi R F. Pharmacokinetics of (−)-2′-3′-dideoxy-3′-thiacytidine in woodchucks. Antimicrob Agents Chemother. 1996;40:642–645. doi: 10.1128/aac.40.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schinazi R F, Boudinot F D, Ibrahim S S, Manning C, McClure H M, Liotta D C. Pharmacokinetics and metabolism of racemic 2′,3′-dideoxy-5-fluoro-3′-thiacytidine in rhesus monkeys. Antimicrob Agents Chemother. 1992;36:2432–2438. doi: 10.1128/aac.36.11.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schinazi R F, Gosselin G, Faraj A, Korba B E, Liotta D C, Chu C K, Mathé C, Imbach J-L, Sommadossi J-P. Pure nucleoside enantiomers of β-2′,3′-dideoxycytidine analogs are selective inhibitors of hepatitis B virus in vitro. Antimicrob Agents Chemother. 1994;38:2172–2174. doi: 10.1128/aac.38.9.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tennant B, Gerin J. The woodchuck model of hepatitis B infection. In: Arias I, Boyer J, Fausto N, Jacoby W, Schachter D, Shafritz D, editors. The liver: biology and pathobiology. New York, N.Y: Raven Press; 1994. pp. 1455–1466. [Google Scholar]
- 19.Tennant B C, Baldwin B H, Graham L A, Ascenzi M A, Hornbuckle W E, Rowland P H, Tochkov I A, Yeager A E, Erb H N, Colacino J M, Lopez C, Engelhardt J A, Bowsher R R, Richardson F C, Lewis W, Cote P J, Korba B E, Gerin J L. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology. 1998;28:179–191. doi: 10.1002/hep.510280124. [DOI] [PubMed] [Google Scholar]
- 20.Tennant B C, Hornbuckle W E, Baldwin B H, King J M, Cote P, Popper H, Purcell R H, Gerin J L. Influence of age on the response to experimental woodchuck hepatitis virus infection. In: Zuckerman A J, editor. Viral hepatitis liver disease. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 462–464. [Google Scholar]