Abstract
Purpose
This study aims to identify the genetic causes of 12 women with primary infertility characterized by primarily oocyte maturation abnormality and consequent early embryonic arrest.
Methods
Genomic DNA was isolated from peripheral blood samples. Whole-exome sequencing was performed on the probands, and the identified variants were confirmed by Sanger sequencing. The pathogenicity of the identified variants on the protein was accessed in silico. And we used qRT-PCR to detect the possible effects of the novel mutation on the mRNA level of NLRP5.
Results
A novel homozygous frameshift variant (p.V429Efs*30) in NLRP5 and compound heterozygous variants with a novel frameshift variant (p.A297Efs*20) and a recurrent variant (c. 223-14_223-2delCCCTCCTGTTCCA) in PATL2 were identified in two unrelated affected individuals. qRT-PCR showed an obvious decrease of the mutant NLRP5 mRNA. In addition, the truncated proteins of NLRP5 and PATL2 were predicted to be non-functional due to the deletion of the most or the whole region of the critical functional domain(s) respectively.
Conclusions
This study identified novel mutations in NLRP5 and PATL2, further expanding the mutational and phenotypic spectrum of both genes. This is the first report of the NLRP5 mutations that associates with oocyte maturation abnormality in humans.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02412-4.
Keywords: Female infertility, Oocyte maturation abnormality, Early embryonic arrest, NLRP5, PATL2
Introduction
During in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI), it is common for a small proportion (8.6–15.2%) of oocytes to arrest at germinal vesicle (GV) stage or metaphase I (MI) stage [1]. When the proportion of immature oocytes was more than 25%, the rates of fertilization, blastocyst production, and clinical pregnancy were markedly reduced. When the proportion of immature oocytes exceeded 40% including at least one maturation-resistant oocyte, the pregnancy would be hardly achieved and the oocytes exhibited abnormal nuclear maturation [2]. In some severe but extremely rare cases, the collected oocytes were repeatedly all immature [3]; or a majority of the retrieved oocytes were immature, together with a very small proportion of oocytes showing an appearance of first polar body (PB1) extrusion but actually having abnormal nuclear maturation, consequently resulting in fertilization failure or early embryonic arrest [4]; the underlying reason for these conditions is largely unknown.
Maternal components stored in oocyte are essential for oocyte maturation, and they are also the main factor that determines the embryo development and implantation potential [5]. These maternal components in oocytes were encoded by maternal-effect genes. If such a gene was impaired, it would bring bad effects on the maturation of oocyte, and subsequently the development of early-stage embryo [6]. Recently, numerous studies have identified that mutations in genes required for human oocyte maturation, including TUBB8 (MIM: 616,768) [7] and PATL2 (MIM:614,661) [8–11], TRIP13 (MIM: 604,507) [12], are primarily responsible for oocyte maturation arrest. However, different mutations in these genes can result in variability in the clinical phenotypes, in terms of oocyte maturation arrest, fertilization failure, or early embryonic arrest [13]. For mutations in such genes encoding a protein as a member of subcortical maternal complex (SCMC), including TLE6 (MIM: 612,399) [14], PADI6 (MIM: 610,363) [15], KHDC3L (MIM: 610,363) [16], NLRP2 (MIM:609,364), and NLRP5 (MIM: 609,658) [17], they have been shown to mainly cause early embryonic arrest. NLRP5, as one of the maternal-effect genes, is highly expressed in oocytes. Initially, its biallelic mutations had been identified to cause multiple pregnancy loss or birth of offspring with multi-locus imprinting disturbances (MLID) or healthy offspring [18, 19]. Subsequently, biallelic NLRP5 mutations had been also linked to human female infertility with embryonic arrest [17, 20, 21]. More recently, two studies had identified biallelic NLRP5 mutations were causative for female infertility with recurrent fertilization failure [22, 23]. So far, no mutations in NLRP5 have been reported in human in the context of oocyte maturation abnormality.
In this study, we used whole-exome sequencing (WES) to identify genetic causes of 12 primary infertile women with primarily oocyte maturation abnormality and consequent early embryonic arrest. We discovered novel mutations in NLRP5 and PATL2 in two affected women respectively, thereby expanding the mutational and phenotypic spectrum of both genes. This is the first study that associated biallelic NLRP5 mutations with human oocyte maturation abnormality.
Materials and methods
Subjects and clinical investigation
A total of 12 women with primary infertility characterized by primarily oocyte maturation abnormality (> 60% of oocytes were immature) and consequent early embryonic arrest (embryos were arrested before 6-cell stage (grade III)) on day 3 were recruited during July 2014 and November 2021. All probands had normal karyotypes (46, XX). Peripheral blood samples were obtained from all probands and their available family members. Written informed consent was obtained from all participants.
Evaluation of oocyte and embryo phenotypes
The morphological evaluation of oocyte maturity, fertilization, and embryonic development was performed under a light microscope. Metaphase II (MII) oocytes were observed with the BP1 extruded (Fig. 1a). GV oocytes had an intact germinal vesicle (Fig. 1a). Metaphase I (MI) oocytes had no GV or polar body. GV oocytes and MI oocytes were considered to be immature. The fertilization assessment was performed 17 ± 1 h post-insemination. The embryos were cultured and incubated to day 5 or day 6.
Fig. 1.
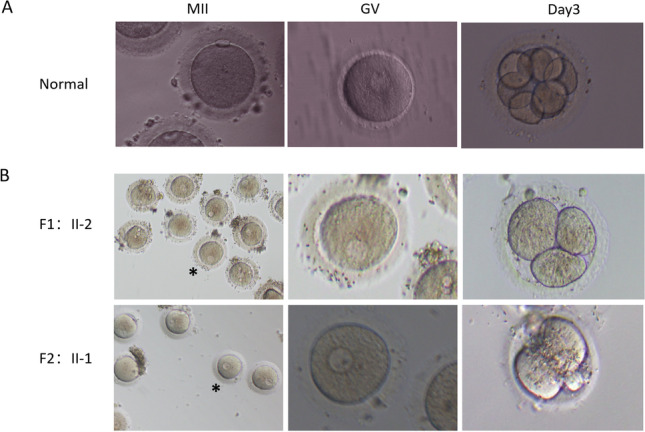
Phenotypes of oocytes and embryos from a normal individual and two infertile women with primarily oocyte maturation abnormality and consequent early embryonic arrest. a Morphology of an MII oocyte, a GV oocyte, and an embryo of day 3 from a normal individual. Left panel, an MII oocyte. Middle panel, a GV oocyte. Right panel, an embryo of day 3. b Morphology of the oocytes and embryos from the two affected women with biallelic NLRP5 and PATL2 mutations respectively. Left panels, part of the oocytes retrieved. Middle panels, the oocyte marked with a star on the left panel was magnified. Right panels, embryos of day 3. Upper panels, the proband from family 1 (F1:II-2), the second cycle of an IVF attempt. Lower panels, the proband from family 2 (F2:II-1). Scale bars in all panels: 50 μm. MII = metaphase II; GV = germinal vesicle
Whole-exome sequencing
WES was performed using the DNA samples of the probands. Genomic DNA library was prepared using the Agilent Human SureSelect All Exon V6 kit and exome sequencing was performed on an Illumina NovaSeq 6000 platform. Clean sequencing reads were aligned to the human reference sequence (hg19). Sequence variants including single-nucleotide variants (SNVs) and small insertions or deletions (indels) were annotated by ANNOVAR pipeline. Common variants (defined as a minor allele frequency (MAF) above 1% in public databases: 1000 genome, dbSNP, ESP6500, gnomAD, or ExAC) were excluded. SNVs and indels were classified by position as intergenic, 5′ UTR, 3′UTR, intronic, splicing, or exonic. Exonic variants were then classified by predicted amino acid change as a stopgain, missense, synonymous, or frameshift variant, inframe insertion or deletion, or possible splicing mutation. For coding or potential splicing mutations, the conservation at the variant site and the predicted effect on protein function were evaluated with in silico tools: SIFT, PolyPhen-2, MutationTaster, and NNSplice.
Sanger sequencing
The coding regions flanking the variants in the NLRP5 and PATL2 gene were amplified by PCR using the specific primers respectively (Table S1). The PCR products were directly Sanger sequenced in both forward and reverse directions, using an ABI 3100 DNA analyzer (Applied Biosystem, Foster City, CA, USA). The variants were validated by Sanger sequencing in the probands and all their available family members.
Quantitative reverse transcription PCR
Total RNA was extracted from the peripheral blood leukocytes of the proband from family 1 and from a normal fertile female by the Trizol Reagent (Thermo Fisher Scientific, Inc.). cDNA was synthesized using reverse transcriptase (TaKaRa PrimeScript reagent kit) and amplified by PCR with Taq polymerase using the specific primers spanned exons 13–15 of NLRP5 shown in Table S2. The PCR products were analyzed by electrophoresis and further sequenced using the ABI 3100 DNA analyzer (Applied Biosystem, Foster City, CA, USA). Quantitative reverse transcription PCR (qRT-PCR) was performed using StepOnePlus Real-time PCR system (Applied Biosystems, Foster City, CA). qRT-PCR reactions contain iTaq Sybrgreen (Biorad) and specific primers. GAPDH was used as an internal control. The 2−∆∆CT method [24] is used to analyze the relative expression of the NLRP5 mRNA in the proband from family 1 (F1:II-2) with c.1286_1289del mutation and in a normal control. Three independent experiments were performed. P value was calculated using Student’s unpaired, two-tailed t-test.
Results
Clinical phenotypes of the patients
Two out of twelve unrelated probands with primary infertility due to primarily oocyte maturation abnormality and consequent early embryonic arrest had been found to have a genetic cause for their clinical phenotypes. Their spouses had normal semen volume, sperm concentration, motility, and sperm morphology.
The 34-year-old proband in family 1 (F1:II-2) from a consanguineous family had a 1-year history of primary infertility. She had undergone two trials of failed intrauterine insemination (IUI) with her ex-husband. She remarried to a man who had a child with his ex-wife. She had regular menses. Hysterosalpingography indicated incomplete patency of the bilateral fallopian tubes. The proband had two failed cycles of IVF attempts (Table 1). In the first cycle, a gonadotropin-releasing hormone (GnRH) antagonist protocol was performed and 30 oocytes were retrieved but all arrested at GV stage, even after culture of in vitro maturation (IVM). In the second cycle, a progestin-primed ovarian stimulation (PPOS) protocol was utilized and 17 out of 24 oocytes remained immature even after IVM. A total of 4 of the other 7 oocytes with PB1 were fertilized by IVF but resulted in early embryonic arrest at the 2–3-cell stage (grade IV) on day 3 (Table 1) (Fig. 1b). Her sister (II-1) had a son and a daughter through natural pregnancy.
Table 1.
Oocyte and embryo characteristics of IVF and ICSI attempts of three probands
| Individual | Age (years) | Duration of infertility (years) | IVF/ICSI attempts | Stages of oocytes | No. of fertilized oocytes | No. of usable D3 embryos | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GV | MI | PB1 | Degenerated | 2PN | 1PN | Multiple PN | ||||||
| F1:II-2 | 34 | 1 | 1st IVF | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | / |
| 2nd IVF | 15 | 2 | 7 | 0 | 4 | 0 | 0 | 0 | All embryos were arrested at the 2–3-cell stage on day 3 | |||
| F2:II-1 | 41 | 10 | 1st IVF | 11 | 0 | 2 | 1 | 1 | 0 | 1 | 0 | The embryo was arrested at the 2-cell stage on day 3 |
IVF in vitro fertilization; ICSI intracytoplasmic sperm injection; GV germinal vesicle; MI metaphase I; PB1 the first polar body; PN pronucleus/pronuclei
The 41-year-old proband in family 2 (F2:II-1) was from a non-consanguineous family with a 10-year history of primary infertility. She had regular menstrual cycles. Due to the venerable age of the patient and the long period of infertility, the couples accepted the IVF treatment. In the cycle of IVF attempt, a long protocol was performed and 14 oocytes were retrieved; among them, 11 oocytes were arrested at GV stage, one oocyte appeared degraded, one oocytes with PB1was abnormally fertilized resulting in a zygote with multiple pronuclei (multiple PN), and one oocyte was normally fertilized (2PN) but resulting in an unusable embryo arrested at the 2-cell stage (grade IV) on day 3 (Table 1) (Fig. 1b). Her sister (II-2) had two children through natural pregnancy.
Identification of novel mutations in NLRP5 and PATL2
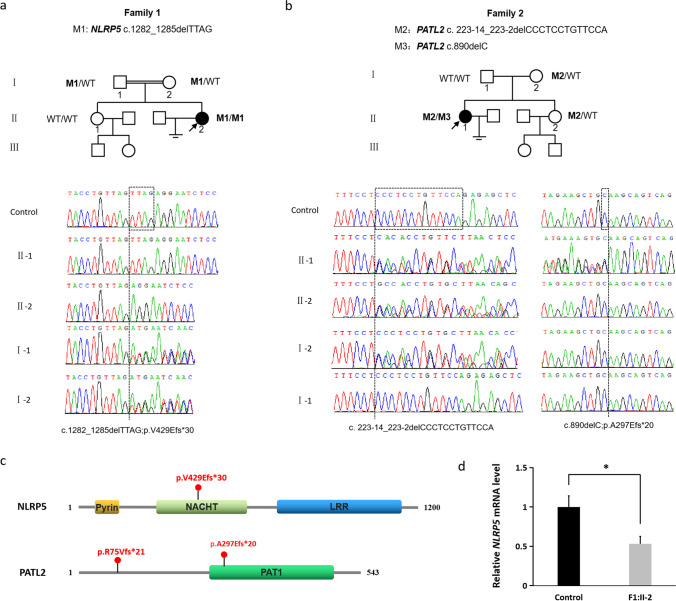
In family 1, the proband (F1:II-2) was identified with a novel homozygous frameshift mutation in NLRP5 (NM_153447; c.1286_1289del; p.V429Efs*30). Her consanguineous parents were heterozygous for this mutation and her fertile sister was not found the mutation by Sanger sequencing (Fig. 2a). In family 2, the proband (F1:II-1) was identified with compound heterozygous mutations in PATL2 (NM_001145112.1), including a recurrent splicing mutation (c. 223-14_223-2delCCCTCCTGTTCCA) (eventually causing a frameshift p.R75Vfs*21) and a novel frameshift mutation (c.890delC; p.A297Efs*20). Her mother and her fertile sister were heterozygous for the splicing mutation, but her father was found no mutations in PATL2. Five very rare homozygous SNPs (Table S3) in the proband were used for paternity test. Sanger sequencing showed that all these SNPs were heterozygous in her mother and father (Fig. S1), which demonstrated a very high possibility of paternity. Since the heterozygous frameshift mutation (p.A297Efs*20) was not inherited from her mother nor her father; it might be a de novo mutation in the patient (Fig. 2b).
Fig. 2.
Identification of novel mutations in NLRP5 and PATL2 in two unrelated primary infertile patients with primarily oocyte maturation abnormality and consequent early embryonic arrest. a Pedigree analysis of the family 1 with NLRP5 mutations and chromatograms of partial NLRP5 sequences in family members. b Pedigree analysis of the family 1 with PATL2 mutations and chromatograms of partial PATL2 sequences in family members. For a and b, a black circle with an arrow presents the proband. The individual with wild type is marked as a clear square or square. The double line is consanguinity. The chromatograms were the results of Sanger sequencing. The dashed lines indicate the positions of variants. c The positions of the mutations in the structure of NLRP5 and PATL2 protein. d Relative expression of NLRP5 mRNA in the peripheral blood leukocytes of the proband in family 1 (F1:II-2) and a normal control. The relative expression was measured by qRT-PCR and normalized to GAPDH. The bars show the mean of three independent experiments and error bars denote standard deviations. *, p < 0.001
The c.1286_1289del (p.V429Efs*30) mutation is recorded as rs769276313 in dbSNP (v153) and has a low allele frequency in the gnomAD browser (14/280438). The mutation c.890delC (p.A297Efs*20) was not recorded in dbSNP (v153) or the gnomAD browser (Table 2).
Table 2.
Effects of novel NLRP5 and PATL2 mutations predicted using in silico tools
| Position on chromosome | cDNA alteration | Amino acid alteration | Exon | Mutation | gnomAD allele frequency | gnomAD homozygote frequency | dbSNP (v153) | SIFT | Polyphen-2 | MutationTaster | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1:II-2 | NLRP5 Chr19: 56538881_56538884delTTAG | c.1286_1289del | p.V429Efs*30 | 7 | Frameshift | 14/280438 | 0/280438 | rs769276313 | - | - | 1 (D) |
| F2:II-1 | PATL2 Chr15:44961748delG | c.890delC | p.A297Efs*20 | 10 | Frameshift | Not found | Not found | - | - | - | 1 (D) |
a. Mutation assessment by SIFT, PolyPhen-2, and MutationTaster
D damaging; P probably damaging
Prediction of the effects of the novel mutations on the protein
The frameshift mutations c.1286_1289del (p.V429Efs*30) and c.890delC (p.A297Efs*20) were predicted to produce a premature termination code (PTC) in the mRNA of NLRP5 and PATL2 respectively. If the aberrant mRNAs were translated, the truncated protein of NLRP5 would lack large part of NACHT (NAIP, CIITA, HET-E, and TP1) domain and the whole leucine-rich repeats (LRR) domain; and the truncated protein of PATL2 would lack nearly the whole PAT1 domain (Fig. 2c).
Effect of a novel mutation on the expression of NLRP5 mRNA
qRT-PCR was performed to explore the effect of the novel homozygous frameshift mutation on the expression of NLRP5 mRNA. The results indicated that the relative expression of NLRP5 mRNA was significantly reduced in the proband (F1:II-2) with c.1286_1289del mutation compared with a wild-type normal control (Fig. 2d).
Discussion
In this study, we identified a novel homozygous frameshift NLRP5 mutation and compound heterozygous mutations of PATL2 including a recurrent splicing mutation and a novel frameshift mutation in two out of twelve affected women with primary infertility due to primarily oocyte maturation abnormality and consequent early embryonic arrest. Their typical clinical phenotypes indicated by IVF/ICSI procedures were that the whole or most oocytes were immature and only a small part of oocytes were fertilized but embryos were arrested at an early stage of development.
The novel frameshift mutations c.1286_1289del (p.V429Efs*30) and c.890delC (p.A297Efs*20) producing a PTC in the mRNA of NLRP5 and PATL2 respectively were predicted to trigger the rapid degradation by the pathway of nonsense-mediated decay (NMD) [25], resulting in no translation of protein. qRT-PCR results showed an obvious decrease of the mutant NLRP5 mRNA (Fig. 2d), supporting that the mutant NLRP5 mRNA expression was under the regulation of NMD. Even if the mutant NLRP5 mRNAs were not all degraded, a significantly reduced amount of the truncated protein, lacking a large part of NACHT domain and the entire LRR domain in the C-terminus (Fig. 2c), could be translated but would be non-functional. Similarly, if the aberrant PALT2 mRNAs were not all degraded by NMD, the truncated protein lacking nearly the whole part of PAT1 domain in the C-terminus (Fig. 2c) would lose its main function.
So far, only five studies have linked biallelic NLRP5 mutations to female infertility [17, 20–23]. The first study identified two pairs of compound heterozygous NLRP5 mutations (p.Gln98* and p.Thr694Ile, p.Gly289Glu and p.Thr1107Ile) in two unrelated primary infertile females with early embryonic arrest [17]. The second study found a homozygous missense NLRP5 mutation (p.P354L) in one primary infertile woman from a consanguineous family with early embryonic arrest [20]. The third study reported three affected individuals with primary fertility characterized by early embryonic arrest with biallelic NLRP5 mutations, including a homozygous missense mutation (p.Arg635Cys), compound heterozygous missense mutations (p.Ser893Thr and p.Leu1116Trp) in two unrelated affected individuals respectively and two homozygous missense mutations (p.Arg143Pro and p.Arg462Cys) in another affected individual [21]. More recently, one study has identified a homozygous frameshift NLRP5 mutation c.2274_2275del (Trp759Aspfs*4) in an affected woman [22] and another study has identified compound heterozygous missense mutations c.1598G > C (p.Arg533Pro) and c.1919 T > G (p.Leu640Arg) in another affected woman with primary infertility and total fertilization failure [23]. These two studies at least suggested that NLRP5 may exert its function before fertilization. To date, no mutations in the gene of NLRP5, which is highly expressed in oocytes, have been reported to be associated with human oocyte maturation abnormality.
Here, we identified the second homozygous frameshift NLRP5 mutation c.1286_1289del (p.V429Efs*30) in a patient from a consanguineous family with primary infertility. This patient experienced two failed IVF attempts that all retrieved 30 oocytes were arrested at GV in the first cycle even after IVM, and in the second cycle, the majority oocyte (17/24) were immature (oocyte maturity was only 29.17%) and a small part of oocytes (4/24) were fertilized but embryos were all arrested at an early stage. As far as we know, this is the first study that linked the biallelic NLRP5 mutations to oocyte maturation abnormality. In five previous studies, phenotypes of seriously decreased oocyte maturity were not demonstrated in those affected individuals with biallelic NLRP5 mutations [15, 18–21]. We supposed that the novel homozygous frameshift mutation p.V429Efs*30, if not degraded by NMD, lacking a larger fragment in the C-terminus of protein, may cause more serious effect on the protein function of NLRP5, compared with the homozygous frameshift mutation p.Trp759Aspfs*4 and other currently identified homozygous or compound heterozygous missense mutations. It requires further studies to illustrate whether the protein of NLRP5 plays a role in regulating or modifying the maturation of oocyte.
In addition, we also identified a novel mutation of PATL2 in a primary infertile patient with a large proportion of oocyte maturation abnormality and a small number of oocytes fertilized resulting in early embryonic arrest. So far, numerous studies have shown that biallelic mutations in PATL2 are mainly responsible for human oocyte germinal vesicle-stage arrest [10, 11] and that different mutations may result in a wide range of phenotypes in oocyte maturation arrest, fertilization failure, or embryonic developmental arrest [13, 26]. At present, one additional novel PATL2 mutation was identified, which further supported the evidence that PATL2 plays a critical role in oocyte maturation and early embryonic development.
In conclusion, this study identified novel mutations in the maternal-effect genes NLRP5 and PATL2, expanding the spectrum of genetic causes of female infertility with primarily oocyte maturation abnormality and consequent early embryonic arrest. As far as we know, this is the first study that associated biallelic NLRP5 mutations with the phenotype of oocyte maturation abnormality. The genetic analysis of larger cohorts of infertile patients due to oocyte maturation abnormality and/or consequent early embryonic arrest is needed to formulate more accurate genotype and phenotype correlations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all families for participating in this study.
Author contribution
L.H., R.J., and X.T. conceived and designed the experiments. L.H., F.L., Q.J, J.J., L.L., R.J., and X.T. collected the samples. L.H. and Y.W. performed the experiments. L.H., Y.W., and G.S. analyzed the data. L.H. wrote the paper. All authors approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81801440 to L.H.), the Natural Science Foundation of Anhui Province (1808085MH241 to L.H.), the Fundamental Research Funds for the Central Universities (WK9110000028 to L.H.), and the National Key R&D Program of China (2018YFC1003900 to X.T.).
Declarations
Ethics approval
The study was approved by the biomedical research ethics committee of the University of Science and Technology of China.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lingli Huang, Email: huangll163@163.com.
Rentao Jin, Email: jrtao2001@163.com.
Xianhong Tong, Email: tong68xianhong@163.com.
References
- 1.Bar-Ami S, Zlotkin E, Brandes JM, Itskovitz-Eldor J. Failure of meiotic competence in human oocytes. Biol Reprod. 1994;50:1100–1107. doi: 10.1095/biolreprod50.5.1100. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y, Ferrer-Buitrago M, Popovic M, Neupane J, De Vos WH, Lierman S, et al. Patients with a high proportion of immature and meiotically resistant oocytes experience defective nuclear oocyte maturation patterns and impaired pregnancy outcomes. Reprod Biomed Online. 2018;36:396–407. doi: 10.1016/j.rbmo.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril. 2010;94:2507–2513. doi: 10.1016/j.fertnstert.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hourvitz A, Maman E, Brengauz M, Machtinger R, Dor J. In vitro maturation for patients with repeated in vitro fertilization failure due to “oocyte maturation abnormalities”. Fertil Steril. 2010;94:496–501. doi: 10.1016/j.fertnstert.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Yanez LZ, Han J, Behr BB, Pera RAR, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun. 2016;7:10809. doi: 10.1038/ncomms10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859–870. doi: 10.1242/dev.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374:223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, et al. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101:609–615. doi: 10.1016/j.ajhg.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddirevula S, Coskun S, Alhassan S, Elnour A, Alsaif HS, Ibrahim N, et al. Female infertility caused by mutations in the oocyte-specific translational repressor PATL2. Am J Hum Genet. 2017;101:603–608. doi: 10.1016/j.ajhg.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Tong X, Wang F, Luo L, Jin R, Fu Y, et al. Novel mutations in PATL2 cause female infertility with oocyte germinal vesicle arrest. Hum Reprod. 2018;33:1183–1190. doi: 10.1093/humrep/dey100. [DOI] [PubMed] [Google Scholar]
- 11.Christou-Kent M, Kherraf ZE, Amiri-Yekta A, Le Blevec E, Karaouzene T, Conne B, et al. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med. 2018;10:e8515. doi: 10.15252/emmm.201708515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Li B, Fu J, Li R, Diao F, Li C, et al. Bi-allelic missense pathogenic variants in TRIP13 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2020;107:15–23. doi: 10.1016/j.ajhg.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sang Q, Zhou Z, Mu J, Wang L. Genetic factors as potential molecular markers of human oocyte and embryo quality. J Assist Reprod Genet. 2021;38:993–1002. doi: 10.1007/s10815-021-02196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alazami AM, Awad SM, Coskun S, Al-Hassan S, Hijazi H, Abdulwahab FM, et al. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol. 2015;16:240. doi: 10.1186/s13059-015-0792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, et al. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet. 2016;99:744–752. doi: 10.1016/j.ajhg.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry DA, Logan CV, Hayward BE, Shires M, Landolsi H, Diggle C, et al. Mutations causing familial biparental hydatidiform mole implicate c6orf221 as a possible regulator of genomic imprinting in the human oocyte. Am J Hum Genet. 2011;89:451–458. doi: 10.1016/j.ajhg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu J, Wang W, Chen B, Wu L, Li B, Mao X, et al. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J Med Genet. 2019;56:471–480. doi: 10.1136/jmedgenet-2018-105936. [DOI] [PubMed] [Google Scholar]
- 18.Docherty LE, Rezwan FI, Poole RL, Turner CL, Kivuva E, Maher ER, et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat Commun. 2015;6:8086. doi: 10.1038/ncomms9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparago A, Verma A, Patricelli MG, Pignata L, Russo S, Calzari L, et al. The phenotypic variations of multi-locus imprinting disturbances associated with maternal-effect variants of NLRP5 range from overt imprinting disorder to apparently healthy phenotype. Clin Epigenetics. 2019;11:190. doi: 10.1186/s13148-019-0760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Qian Y, Liu Y, Wang Q, Wang R, Zhou Y, et al. A novel homozygous variant in NLRP5 is associate with human early embryonic arrest in a consanguineous Chinese family. Clin Genet. 2020;98:69–73. doi: 10.1111/cge.13744. [DOI] [PubMed] [Google Scholar]
- 21.Zheng W, Hu H, Dai J, Zhang S, Gu Y, Dai C, et al. Expanding the genetic and phenotypic spectrum of the subcortical maternal complex genes in recurrent preimplantation embryonic arrest. Clin Genet. 2021;99:286–291. doi: 10.1111/cge.13858. [DOI] [PubMed] [Google Scholar]
- 22.Maddirevula S, Awartani K, Coskun S, AlNaim LF, Ibrahim N, Abdulwahab F, et al. A genomics approach to females with infertility and recurrent pregnancy loss. Hum Genet. 2020;139:605–613. doi: 10.1007/s00439-020-02143-5. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Jia M, Zhao X, Shi R, Xue X. A new NLRP5 mutation causes female infertility and total fertilization failure. Gynecol Endocrinol. 2021;37:283–284. doi: 10.1080/09513590.2020.1832069. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2021;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Chen H, Li D, Song D, Chen B, Yan Z, et al. Novel mutations in PATL2: expanding the mutational spectrum and corresponding phenotypic variability associated with female infertility. J Hum Genet. 2019;64:379–385. doi: 10.1038/s10038-019-0568-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.