Based on the positive outcomes of five randomised controlled trials [1–4], bronchoscopic lung volume reduction (BLVR) using endobronchial valves (Zephyr EBVs; PulmonX Corp., Redwood City, CA, USA) is now a treatment option for a subset of patients with severe emphysema [5]. Very few data are available regarding the relevance of a sequential, bilateral treatment using EBVs in patients with severe emphysema. Fiorelli et al. [6] reported in 2016 their experience with 14 patients treated contralaterally after loss of benefit (18 months median delay between both sides) and demonstrated that patients can recover similar functional parameters compared to outcomes obtained after the initial intervention. The same year, Trudzinski et al.
Short abstract
Bilateral endobronchial valves treatment can lead to additional significant benefits in patients who respond to the first procedure and have a clear target lobe on both lungs but increases the rate of complications https://bit.ly/3J0mZ8h
To the Editor:
Based on the positive outcomes of five randomised controlled trials [1–4], bronchoscopic lung volume reduction (BLVR) using endobronchial valves (Zephyr EBVs; PulmonX Corp., Redwood City, CA, USA) is now a treatment option for a subset of patients with severe emphysema [5]. Very few data are available regarding the relevance of a sequential, bilateral treatment using EBVs in patients with severe emphysema. Fiorelli et al. [6] reported in 2016 their experience with 14 patients treated contralaterally after loss of benefit (18 months median delay between both sides) and demonstrated that patients can recover similar functional parameters compared to outcomes obtained after the initial intervention. The same year, Trudzinski et al. [7] reported outcomes regarding 16 patients treated bilaterally in an early manner (128 days after the first side), but the patients derived no additional benefits from the second treatment despite a significant 670 mL residual volume (RV) loss (6% gain in forced expiratory volume measured over 1 s (FEV1), 24 m loss in 6-min walking distance (6MWD)). In analogy with the bilateral surgical approach [8], we hypothesised that a subset of patients might benefit from an early contralateral treatment, provided that they: 1) have a high baseline static hyperinflation (RV >200% predicted value (pred)); 2) respond to the first treatment (target lung volume reduction (TLVR) >350 ml, FEV1 gain >12%); and 3) have a clear target lobe on both sides, on computed tomography (CT) scan, StratX (PulmonX Corp.) and on perfusion tomoscintigraphy (less than 15% of total perfusion), with no collateral ventilation. We herein report outcomes in patients treated with a sequential bilateral approach.
In this two-centre study, we retrospectively reviewed 3-month outcomes for all cases being treated bilaterally between January 2018 and July 2021 in Limoges and Toulouse University Hospitals. All patients met the inclusion criteria established by the BLVR expert panel [9] before both procedures (smoking cessation, significant static hyperinflation RV ≥200% pred, modified Medical Research Council dyspnoea score (mMRC) ≥2 despite optimal medical treatment, no collateral ventilation (StratX followed by Chartis (PulmonX Corp.)); except for FEV1 that exceeded 50% pred in four cases (52%, 53%, 54% and 66% pred) before the contralateral treatment. The target lobes were selected based on StratX report and on perfusion tomoscintigraphy.
All procedures were performed under general anaesthesia and through a laryngeal mask by using a flexible bronchoscope. EBVs were inserted in segments or subsegments of the target lobe for complete occlusion. Approximately 6 months (median 199 days) after the first treatment, patients underwent a second, contralateral one. For each procedure, patients were hospitalised for at least 3 days. All patients had a complete evaluation before treatment and 3 months after each procedure: dyspnoea, nature and rate of complications, 6MWD, body plethysmography, CT scan with measurement of the target lobe, echocardiography and tomoscintigraphy (the last two only pre-treatment).
We analysed the median TLVR after each procedure and data regarding FEV1, RV, 6MWD, mMRC, BODE score (Body mass index, Obstruction (FEV1), Dyspnoea (mMRC), Exercise capacity (6MWD)) and the rate and nature of complications. Median differences between baseline, 3 months after the first procedure and 3 months after the second were calculated by using the paired Wilcoxon test R-studio software version 4.0.3 (10 October 2020).
This study was conducted in accordance with French ethics requirements and the National Commission for Data Protection and Liberties (CNIL number: 2206723 v 0).
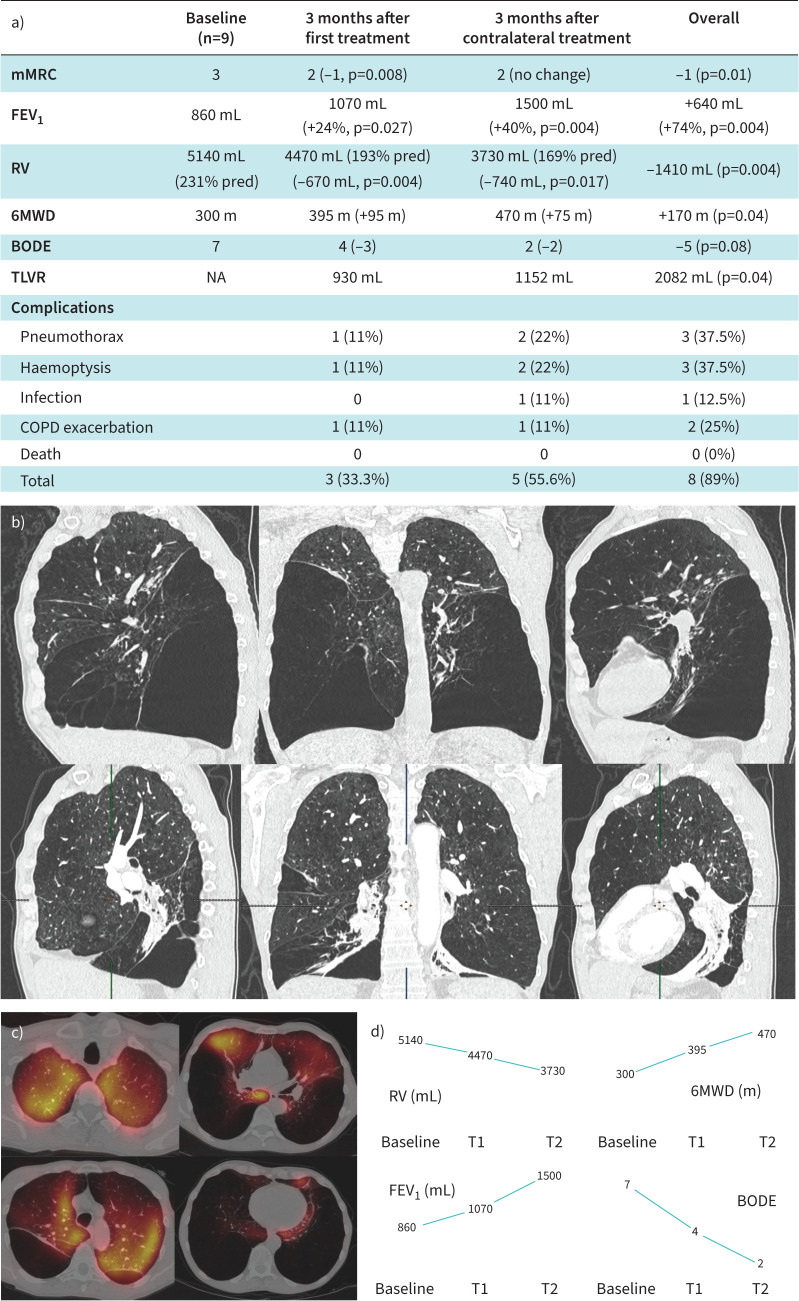
Nine patients had been treated bilaterally (five in Limoges, four in Toulouse). Median FEV1 and RV were 860 mL (30% pred) and 5140 mL (231% pred), respectively, before any procedure. The first and second target lobes counted for 9% and 8.1% of the total lung perfusion, respectively. Median number of valves (Zephyr EBV; PulmonX Corp.) was six for both procedures (total 12). All patients responded to the first treatment (TLVR >350 mL, FEV1 gain >12%). Median TLV and RV reductions were 930 and 670 mL after the first procedure, and 1152 and 740 mL after the second, respectively, resulting in an overall 2082 mL TLVR and a 1410 mL RV decrease. Median FEV1 gain was 24% (860 to 1070 mL, p=0.027) after the first procedure, 40% after the second (1070 to 1500 mL, p=0.004) for an overall 74% gain (+640 mL, p=0.004). 6MWD was improved by 170 m (95 m after the first procedure, 75 m after the second, p=0.04). Median BODE score decreased from 7 to 4 after the first treatment to 2 after the full treatment (overall decrease 5 points, p=0.08). Three complications occurred after the first treatment, five after the second. Of note, two pneumothoraxes occurred in a same patient, one requiring three additional days of hospitalisation but no chest tube or valve removal, the second a chest tube insertion for 72 h but no valve removal. The third pneumothorax occurred after the second treatment and required a chest tube insertion for 48 h with no valve removal. Valves had to be removed on one side (right lower lobe) for this last patient later on, after two additional episodes requiring chest tube insertions 8 months after the second treatment and this patient lost the benefit of the second treatment. Another patient had all EBVs removed for repeat pneumonia 3 months after the second procedure and lost all benefits. Main outcomes and complications are reported in figure 1a. Three haemoptysis were seen, two mild (haemoptoic sputum) and one moderate, all of them occurring immediately after a procedure and none of them requiring any intervention.
FIGURE 1.
a) Main outcomes after each procedure and combined outcomes. b) Computed tomography scan and c) perfusion tomoscintigraphy showing the example of a patient with an extremely heterogeneous emphysema who experienced dramatic improvements after both interventions: forced expiratory volume in 1 s (FEV1) increase by 73% after a first right lower lobe EBV treatment (460 mL to 800 mL) and 74% more after a subsequent left lower lobe treatment (800 mL to 1380 mL), after an overall 2950 mL residual volume (RV) decrease (7840 to 4890 mL). d) Changes in the main parameters: RV, FEV1, 6-min walking distance (6MWD) and BODE (body mass index, obstruction (FEV1), dyspnoea (modified Medical Research Council dyspnoea score (mMRC)), exercise capacity (6MWD)) score (median) after the first procedure (T1) and after contralateral treatment (T2). TLVR: target lung volume reduction; NA: not applicable.
Within these limited series, we report, for the first time, significant additional benefits with a contralateral EBV treatment in very severe emphysema patients. Interestingly, we even observed a deeper magnitude of response after the contralateral treatment (figure 1a). This is, to our knowledge, the first report of such an additional benefit of a contralateral procedure. Trudzinski et al. [7] demonstrated technical success of the contralateral treatment with an additional significant lung volume reduction (640 mL RV decrease). However, this showed no improvements in terms of FEV1 or 6MWD [7]. Fiorelli et al. [6] proposed a more reasonable delayed approach where a contralateral treatment is offered in cases of loss of benefits and showed patients can benefit similarly from this second treatment. Our results demonstrate that this second contralateral procedure can be offered earlier and lead to dramatic changes in RV, FEV1 and 6MWD provided that: 1) hyperinflation at baseline is marked (median RV 231% pred herein); 2) two very clear targets are identified (median perfusions below 10% for both sides herein); and 3) the first treatment is efficient (24% FEV1 gain, 95 m 6MWD improvement) but the patient remains within the main criteria for BLVR (RV >175% pred, mMRC ≥2). We observed an increase in complication rates (overall 89%) compared to what was reported in randomised controlled trials [1–4] and this should be added to the discussion. Also, EBVs removal was due to repeat infections in one case and it seems not to be directly linked to the bilateral approach; in another patient, valves were removed on one side, 8 months after the second treatment for repeat (three) pneumothoraxes. This extremely heterogeneous patient (included in figure 1b) demonstrated unusually dramatic responses after both procedures: FEV1 increase by 73% after the first procedure (460 mL to 800 mL) and 74% more after the second (800 mL to 1380 mL), after an overall 2950 mL RV decrease (7840 to 4890 mL). This extreme LVR has certainly favoured multiple episodes of pneumothoraxes and afterwards, given the significant improvements seen in this patient after the first treatment, the risk–benefit balance for a contralateral procedure was not favourable. The complete and partial loss of benefit regarding these two patients may certainly negatively impact long-term outcomes of this small sample of patients. However, even after anticipating this fact, seven out of nine patients derived great and durable benefits from this approach and median changes between both procedures for all parameters should remain within the minimal clinically important differences. The main limitation of our study is thus the lack of long-term outcomes that could have led to a more accurate comparison with the more delayed contralateral treatment after (and in case of) loss of benefit [6]. Whether the second treatment should be offered immediately after, or after loss of benefits of the first intervention remains unclear and should be carefully discussed with the patient. Nonetheless, the improvements observed herein after the second treatment strongly suggest that a bilateral treatment should be considered for a subset of patients with a contralateral target before the loss of benefit from a first BLVR.
In conclusion, in highly selected patients who respond after a first procedure but remain in the criteria for BLVR (mMRC ≥2, RV >175% pred) and have a very clear contralateral target, a bilateral treatment can lead to clinically significant additional benefits and to overall dramatic outcomes but increases the risk of complications.
Footnotes
Provenance: Submitted article, peer reviewed.
Data availability: Data will be available beginning 3 months and ending 5 years following publication to researchers who provide a methodologically sound proposal.
Conflict of interest: N. Guibert reports receiving personal fees for participation on a data safety monitoring board or advisory board for PulmonX, outside the submitted work.
Conflict of interest: R. Fumat has nothing to disclose.
Conflict of interest: M. Dupuis has nothing to disclose.
Conflict of interest: M. Dusselier has nothing to disclose.
Conflict of interest: F. Favard has nothing to disclose.
Conflict of interest: V. Heluain has nothing to disclose.
Conflict of interest: S. Mallah has nothing to disclose.
Conflict of interest: R. Barthes has nothing to disclose.
Conflict of interest: Y. Simonneau has nothing to disclose.
Conflict of interest: T. Egenod reports receiving personal fees for participation on a data safety monitoring board or advisory board for PulmonX, outside the submitted work.
References
- 1.Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015; 386: 1066–1073. doi: 10.1016/S0140-6736(15)60001-0 [DOI] [PubMed] [Google Scholar]
- 2.Klooster K, ten Hacken NHT, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015; 373: 2325–2335. doi: 10.1056/NEJMoa1507807 [DOI] [PubMed] [Google Scholar]
- 3.Valipour A, Slebos D-J, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. results from the IMPACT study. Am J Respir Crit Care Med 2016; 194: 1073–1082. doi: 10.1164/rccm.201607-1383OC [DOI] [PubMed] [Google Scholar]
- 4.Kemp SV, Slebos D-J, Kirk A, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017; 196: 1535–1543. doi: 10.1164/rccm.201707-1327OC [DOI] [PubMed] [Google Scholar]
- 5.Labarca G, Uribe JP, Pacheco C, et al. Bronchoscopic lung volume reduction with endobronchial zephyr valves for severe emphysema: a systematic review and meta-analysis. Respiration 2019; 98: 268–278. doi: 10.1159/000499508 [DOI] [PubMed] [Google Scholar]
- 6.Fiorelli A, D'Andrilli A, Anile M, et al. Sequential bilateral bronchoscopic lung volume reduction with one-way valves for heterogeneous emphysema. Ann Thorac Surg 2016; 102: 287–294. doi: 10.1016/j.athoracsur.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 7.Trudzinski FC, Lepper PM, Leppert D, et al. Bilateral endoscopic lung volume reduction in patients with severe emphysema. Respiration 2016; 92: 356–358. doi: 10.1159/000450758 [DOI] [PubMed] [Google Scholar]
- 8.Cooper JD, Trulock EP, Triantafillou AN, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995; 109: 106–116. doi: 10.1016/S0022-5223(95)70426-4 [DOI] [PubMed] [Google Scholar]
- 9.Herth FJF, Slebos D-J, Criner GJ, et al. Endoscopic lung volume reduction: an expert panel recommendation - Update 2019. Respiration 2019; 97: 548–557. doi: 10.1159/000496122 [DOI] [PubMed] [Google Scholar]