Abstract
Background
The pentadecapeptide BPC-157 has been shown to have anti-inflammatory and wound healing effects on multiple target tissues and organs. Peptides have potent anti-inflammatory effects on periodontal tissues in rats with periodontitis. Few studies have investigated the effect of BPC-157 on pain after dental procedures or oral surgeries. The purpose of the present study was to investigate the antinociceptive effects of BPC-157 on postoperative incisional pain in rats.
Methods
Sprague–Dawley rats were randomly divided into five groups: control (saline with the same volume), BPC10 (10 µg/kg of BPC-157), BPC20 (20 µg/kg of BPC-157), BPC40 (40 µg/kg of BPC-157), and morphine (5 mg/kg of morphine). A 1-cm longitudinal incision was made through the skin, fascia, and muscle of the plantar aspect of the hind paw in isoflurane-anesthetised rats. Withdrawal responses were measured using von Frey filaments at 0, 2, 6 h and 4, 7 d after incision. The formalin test was also performed to differentiate its anti-nociceptive effect from an inflammatory reaction or central sensitization. Pain behavior was quantified periodically in phases 1 and 2 by counting the number of flinches in the ipsilateral paw after injection with 30 µL of 5% formalin.
Results
The threshold of mechanical allodynia was significantly increased in the BPC10, BPC20, BPC40 and morphine groups compared with that in the control group at 2 h. These increasing thresholds then returned to the levels of the control group. The BPC-157 group showed a much higher threshold at 4 days after incision than the control group. The thresholds of the BPC groups, except the morphine group, were normalized 7 days after incision.
The flinching numbers of the BPC10, BPC20, BPC40 and morphine groups were significantly decreased in phase 1, but there was no decrease in the BPC-157 groups except the morphine group in phase 2.
Conclusions
BPC-157 was effective only for a short period after incision. It was also effective during phase 1 but not during phase 2, as determined by the formalin test. BPC-157 might have a short antinociceptive effect, even though it has anti-inflammatory and wound healing effects.
Keywords: BPC-157, Formalin Test, Incisional Pain Model, Mechanical Allodynia
INTRODUCTION
Most patients who visit dental clinics worry of pain after dental procedures or surgery. Although analgesics such as NSAIDs are widely used for pain control, they cause some adverse reactions such as upper gastrointestinal bleeding [1,2,3]. Recently, there has been a trial to use peptides for muscle healing and muscle pain control during body building and fitness.
Pentadecapeptide body protection compound (BPC)-157 is composed of 15 amino acids and is a partial sequence of the BPC discovered in human gastric juice [4]. Several experiments have demonstrated that it accelerates the healing process of tissues, including transected rat Achilles tendon [5]. BPC-157 has also been shown to exert anti-inflammatory effects. These effects on inflammation and bone resorption have been demonstrated in rats with experimental periodontitis. BPC-157 increased gingival blood flow, and periodontitis produced by a silk ligature placed around the molar was improved after treatment with BPC-157 [6]. This study suggests that BPC-157 may represent a new peptide candidate for the treatment of periodontal disease. This anti-inflammatory capability of BPC-157 may be used to improve the inflammatory pain response after dental procedures and surgery in clinical practice.
However, there is not enough information about the antinociceptive effect of BPC-157 on postoperative pain. There are two commonly used experimental methods to investigate the characteristics of pain in rodents. Undoubtedly, experiments on the Brennan rodent paw incision model have contributed significantly to the existing knowledge on postoperative pain [7,8]. An incisional pain model was used to establish experimental conditions, such as gingival incision in dental clinics. Injection of formalin into the hind paws of rats can be used to evaluate pain due to inflammation or central sensitization. Formalin induces transient biphasic pain, including the first and second phases. The first phase is the direct stimulation of sensory nerve endings by formalin, which causes acute pain as an inflammatory response, and the second phase causes persistent pain by central sensitization. This subcutaneous chemical irritant activates the peripheral nerves, leading to the activation of dorsal horn neurons. It is widely accepted that the initial wave of C-fiber activity in phase 1 leads to phase 2, which depends on the inflammatory response and central sensitization. This model is well-suited for reproducing inflammatory pain [9]. This study aimed to investigate the effects of BPC-157 in reducing postoperative incisional pain in rats using an incisional pain model and a formalin test.
METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Pusan National University Hospital (approval number: PNUH-2019-157). All experiments followed the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (National Institute of Health Publication No. 85–23, updated 2011). Fifty-four male Sprague–Dawley rats weighing 225–300 g (Hana Laboratories) were used in this study. Rats were housed in a humidity- (50 ± 5%) and temperature-controlled (23 ± 2℃) room, under a 12/12 h light (200 lux)/dark cycle, with rodent standard chow and water available ad libitum. All the drugs were purchased from Sigma Chemical Co. (St. Louis, MO, USA). BPC-157, formalin and morphine were prepared in physiological saline (0.9% weight/volume NaCl). All drugs were prepared immediately before use.
Animals were randomly divided into five groups: control, BPC10, BPC20, BPC40 and morphine. The patients were divided into two groups. One was for the incisional pain experiment and the other was for the formalin test experiment. The drug dosage and number in each group are shown in Table 1. The experimental groups received 5 mg/kg morphine; the BPC-157 group received 10, 20, or 40 µg/kg BPC-157; and the control group received normal saline (all the same volume). The drug administration methods were intraperitoneal (i.p.) injections 60 min before incision or formalin injection. These pretreatment doses and times were selected based on studies by Dickenson and Sullivan [10].
Table 1. Characteristics and number of each group in experiments.
| Group | Weight (g) | Drug & dosage to use | Number in each experiment | |
|---|---|---|---|---|
| Incisional pain | Formalin test | |||
| Control | 281.4 ± 0.9 | Saline as same volume | 8 | 7 |
| BPC10 | 266.7 ± 12.9 | BPC-157, 10 μg/kg ip | 4 | 6 |
| BPC20 | 261.8 ± 12.5 | BPC-157, 20 μg/kg ip | 4 | 6 |
| BPC40 | 272.3 ± 10.3 | BPC-157, 40 μg/kg ip | 4 | 6 |
| Morphine | 281.0 ± 2.9 | Morphine, 5 mg/kg ip | 4 | 5 |
BPC, body protection compound.
1. Incisional pain experiment
We used a rat model of incisional pain to evaluate postoperative pain. An incision was made under 2% isoflurane anesthesia delivered via a nose cone, as described by Brennan et al. [11]. Briefly, the plantar aspect of the left hind paw was prepared, and a 1-cm longitudinal incision was made through the skin, fascia, and muscle. In the present study, the incision was started 12 mm distal to the heel edge. The skin was closed with two 5-0 nylon sutures and the wound was covered with an antibiotic ointment. Withdrawal responses were measured using a von Frey filament around the wound before surgery and for the next seven days. A cumulative pain score based on the weight-bearing behavior of the animals was also utilized.
2. Formalin-induced nociceptive behavioral test (formalin test)
All experiments were prepared as in the previous formalin model study described by Dubuisson and Dennis [9]. The number of flinches was counted for a 1-min period at each minute interval from 1 min to 10 min (phase 1) and at 10-min intervals from 10 min to 60 min (phase 2) after formalin injection.
Rats in both experiments were euthanized in a CO2 chamber at the end of the experiment.
All data are presented as mean ± SEM. Statistical analysis between groups was performed using Mann–Whitney and Kruskal–Wallis tests. Statistical significance was set at P < 0.05.
RESULTS
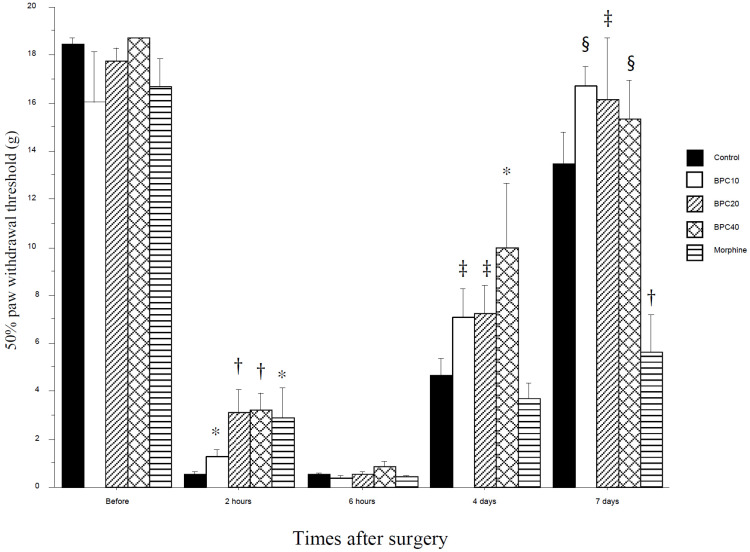
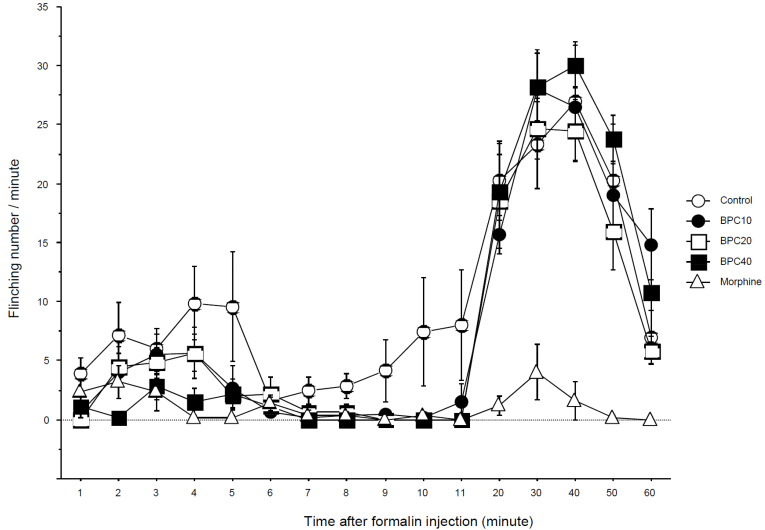
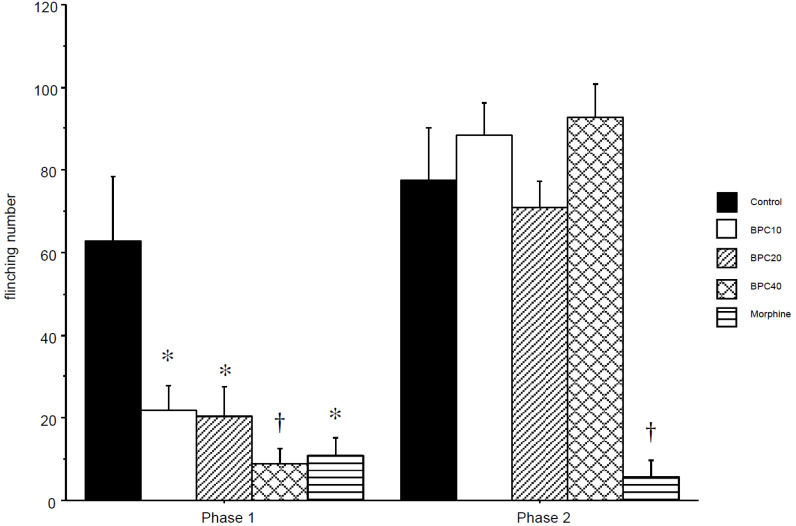
The 50% paw withdrawal threshold from the von Frey filament dramatically decreased 2 h after hindpaw incision (Fig. 1). This decreased threshold was significantly attenuated in the BPC10, BPC20, BPC40 and morphine groups at the same time. This is a dose-dependent increase in the threshold. The attenuating effects of BPC-157 and morphine rapidly disappeared 6 h after incision. This effect of BPC40 reappeared 4 days after the incision (P < 0.05). The thresholds of BPC10 and BPC20 compared with the morphine group were significantly increased 4 days after incision (P < 0.05). Seven days after incision, the threshold of the morphine group was significantly lower than that of the control, BPC10, BPC40 (P < 0.01) and BPC20 (P < 0.05) groups. In the formalin test, the flinching numbers of the BPC10, BPC20, BPC40, and morphine groups were significantly decreased in phase 1, but there was no decrease in the BPC groups, except for the morphine group in phase 2 (Fig. 2). The total flinching numbers of the BPC-157 and morphine groups were significantly decreased in phase 1, but those of the BPC-157 groups, except the morphine group, were not different from the control group (Fig. 3).
Fig. 1. Withdrawal threshold (g) after incision. BPC-157 attenuated the decreased threshold in an early time (2 h after incision) but this effect disappeared thereafter. *P < 0.05 vs. control group, †P < 0.01 vs. control group, ‡P < 0.05 vs. morphine group, §P < 0.01 vs. morphine group. Control: control group with saline as same volume; BPC10: BPC10 group with 10 µg/kg of BPC-157; BPC20: BPC20 group with 20 µg/kg of BPC-157; BPC40: BPC40 group with 40 µg/kg of BPC-157; morphine: morphine group with 5 mg/kg of morphine. BPC, body protection compound.
Fig. 2. Time-effect curve of the formalin test. Data are presented as the number of flinching. BPC-157 suppressed flinches in a dose-dependent manner in phase 1 but not in phase 2. Morphine suppressed flinches in both phases. Morphine group. Control: control group with saline at the same volume; BPC10: BPC10 group with 10 µg/kg of BPC-157; BPC20: BPC20 group with 20 µg/kg of BPC-157; BPC40: BPC40 group with 40 µg/kg of BPC-157; morphine: morphine group with 5 mg/kg of morphine. BPC, body protection compound.
Fig. 3. Cumulated number of flinching in phase 1 and 2 after formalin injection. BPC-157 suppressed flinches significantly in a dose-dependent manner in phase 1 but not in phase 2. Morphine suppressed flinches significantly in both phases. *P < 0.05 vs. control group, †P < 0.01 vs. control group. Control: control group with saline at the same volume; BPC10: BPC10 group with 10 µg/kg of BPC-157; BPC20: BPC20 group with 20 µg/kg of BPC-157; BPC40: BPC40 group with 40 µg/kg of BPC-157; morphine: morphine group with 5 mg/kg of morphine. BPC, body protection compound.
Based on these results, BPC-157 was only effective for a short period after incision. It was also effective during phase 1 but not during phase 2, as determined by the formalin test. BPC-157 might have a short antinociceptive effect, even though it has anti-inflammatory and wound healing effects.
DISCUSSION
Pain is known to spontaneously occur after tissue injury. It is also caused by non-noxious stimuli such as light touch (allodynia) or exaggerated extent of mildly noxious stimuli (hyperalgesia). In clinical practice, people who visit the dental clinic fear direct nerve stimulating pain and postoperative pain during and after the procedure and operation. We attempted to identify other safe analgesic materials to reduce pain and side effects.
The pentadecapeptide BPC-157 has been shown to have anti-inflammatory and wound-healing effects on multiple target tissues and organs. Keremi et al. [6] investigated the effects of BPC-157 on inflammation and bone resorption in rats with experimental periodontitis. They studied the acute effect of BPC on gingival blood flow and periodontitis induced by a silk ligature placed around the lower left first molar. BPC-157 treatment significantly reduced plasma extravasation, histological alterations, and alveolar bone resorption. They concluded that systemic application of BPC-157 did not alter blood circulation in healthy gingiva, and chronic application of the peptide had potent anti-inflammatory effects on periodontal tissues in ligature-induced periodontitis in rats. This study was based on the potent anti-inflammatory effects of BPC-157. BPC-157 can also influence inflammatory pain through its wound healing effect.
Incision in the rat hind paw is a widely used rodent model of postoperative pain. Experimental injury, a surgical incision in the plantar aspect of the rat hind paw, leads to a remarkable reduction in paw withdrawal threshold to mechanical stimuli for several days, representing mechanical hyperalgesia [10]. Pain behaviors in these rats and the time course of mechanical hyperalgesia are similar to patients’ pain reports in the postoperative period [12]. From this incision, non-evoked pain behavior (guarding) lasts relatively short (up to 3–4 days) and evoked behaviors are present for a week or more [7]. Previous studies have reported an increase in the levels of nerve growth factor [13], lactate [14], and interleukin (IL)-1 at the incision site [15]. Hyperalgesia is an increased response to noxious stimuli following tissue injury and inflammation. Primary hyperalgesia after tissue injury is suggested to result from sensitization of primary afferent fibers, but sensitization to mechanical stimuli has been difficult to demonstrate. We measured the withdrawal threshold at 2 and 6 h and 4 and 7 days after the incision. Whether the early response after incision is mechanical sensitization remains controversial. Pogatzki el at. [16] reported that sensitization of mechano-responsive A- and C-fibers did not explain pain behavior 45 min after an incision in the rat hind paw. Responsiveness to monofilaments was significantly enhanced in A-fibers 1 d after incision. Therefore, sensitization of the A- and C-fibers was apparent one day after incision. Because sensitization of afferent fibers to mechanical stimuli is correlated with behavioral results, sensitization may contribute to the reduced withdrawal threshold after incision. Non-evoked pain behavior might be accounted for by spontaneous activity in the A and C fibers. Mechanical hyperalgesia may also be caused by centrally amplifying responses. This early response might be due to mechano-insensitive afferents (MIAs). Some afferent fibers with very high response thresholds before the experimental incision reduced their response threshold 1 h after the incision [17]. Handwerker et al. [18] and Meyer et al. [19] suggested that these fibers, termed as MIAs, likely contribute to mechanical hyperalgesia. This corresponds with recent studies demonstrating that MIAs can become responsive to mechanical stimuli under conditions of inflammation. Schmidt et al. [20] further support the suggestion that MIAs may play a role in inflammation-induced mechanical hyperalgesia. In the present study, the withdrawal threshold at 2 h after incision was significantly decreased, and this decrease was attenuated in the BPC-157 and morphine groups. However, these effects disappeared after 6h to 4days. It is possible that the anti-nociceptive effects of BPC-157 and morphine were sustained shortly. The withdrawal thresholds of the BPC-157 groups significantly increased four days after incision. This late increase may be due to wound healing and anti-inflammatory actions. The present formalin injection experiment supports this speculation.
The behavioral response of the rats to the injection of a 5% formalin solution into the hind paw was used to assess pain and analgesia [9]. Phase 1 corresponds to a high level of activity in the primary afferent as a result of C-fiber activation due to peripheral stimulation, whereas phase 2 results from a continuous low-level small afferent input, together with a facilitated state of spinal processing [21]. BPC-157 showed a dose-dependent effect compared to the control during phase 1 but showed little effect during phase 2 in the present study. BPC-157 may have little effect on the component of the response evoked by persistent small afferent inputs. Thus, the anti-inflammatory action of BPC-157 might contribute partly to reversing acute thresholds or motor function induced by local tissue injury of inflammatory cytokines [22].
The delayed effects of BPC-157 in the present study might have been due to its healing effect. BPC-157 has a beneficial effect on muscle healing, with a suggested role in the regeneration of damaged intramuscular nerve branches [23]. BPC-157 also influences the healing of transected rat sciatic nerve [24]. Rivot et al. [25] demonstrated that inflammatory processes in the paw correlate with a spinal increase in nitric oxide (NO) production. BPC-157 interacts with the NO system and modulates NO synthesis [26]. BPC-157 can also inhibit other inflammatory mediators such as myeloperoxidase and thromboxane B2 [27]. This action might be used in the treatment of periodontitis and inflammatory bowel diseases such as ulcerative colitis [28,29,30]. BPC-157 also counteracted NSAID-mediated lesions and arthritis [2,31,32].
One unexpected result was the threshold level of the morphine group 7 days after incision. The antinociceptive effect of morphine was shown for a short period, but morphine might have contributed to wound healing. Rook et al. [33] found that the topical application of morphine delayed wound closure on days 0–3 post-wounding; however, no significant delays in closure were observed on day 4 post-wounding. During wound closure, neurokinin-1 and neurokinin-2 receptor expression in the vasculature of the healing wound decreased after topical morphine application on days 3 and 8 [34]. This might have resulted in an insufficient return to the threshold, as in the present study. Chronic morphine use significantly delays and reduces neutrophil and macrophage recruitment to the wound site. There was also significant suppression of angiogenesis and myofibroblast recruitment. These delays in the recruitment of cellular events can be attributed to wound healing [35]. Although we investigated only 7 days after incision, it might be suggested that the analgesic effect of morphine was shown in the early period; however, the delayed wound closing effect might appear in a manner of decreased threshold in the late period.
The important points for the control of postoperative pain, such as incisional pain, are both antinociceptive action and wound healing. Anti-nociceptive mechanisms provide an analgesic effect directly against incision, while anti-inflammatory mechanisms for wound healing can also provide an analgesic effect. Based on the present results, BPC-157 might have an anti-inflammatory and wound healing effect and not a centrally mediated analgesic effect. This demonstrated the possibility of pain control in BPC-157 and could not prove how BPC-157 could function as an anti-nociceptor. Its mechanism needs to be investigated further.
The synthetically produced peptide BPC-157 is currently not approved for use as a human drug. A few human trials have investigated the anti-nociceptive effects of BCP-157 [36]. The U.S. Anti-Doping Agency states that it is an experimental compound that has been investigated for inflammatory bowel disease and soft tissue healing. As of January 1, 2022, the experimental peptide BPC-157 is prohibited under the World Anti-Doping Agency Prohibited List. Furthermore, this substance is not approved for human clinical use by any global regulatory authority and may lead to negative health effects.
ACKNOWLEDGEMENT
The authors declare no conflict of interest relevant to this article. The study was funded by the Basic Research Support Project of Pusan National University.
Footnotes
- Young-Hoon Jung: Visualization, Writing – original draft.
- Haekyu Kim: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing.
- HyaeJin Kim: Methodology, Resources, Software.
- Eunsoo Kim: Conceptualization, Writing – original draft, Writing – review & editing.
- Jiseok Baik: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources.
- Hyunjong Kang: Data curation, Resources, Visualization, Writing – original draft.
DECLARATION OF INTEREST: The authors declare that they have no conflicts of interest.
References
- 1.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76–83. doi: 10.2174/13816128130111. [DOI] [PubMed] [Google Scholar]
- 2.Sikiric P, Seiwerth S, Grabarevic Z, Rucman R, Petek M, Jagic V, et al. Pentadecapeptide BPC 157 positively affects both non-steroidal anti-inflammatory agent-induced gastrointestinal lesions and adjuvant arthritis in rats. J Physiol Paris. 1997;91:113–122. doi: 10.1016/s0928-4257(97)89474-0. [DOI] [PubMed] [Google Scholar]
- 3.Ilic S, Drmic D, Zarkovic K, Kolenc D, Brcic L, Radic B, et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol. 2011;667:322–329. doi: 10.1016/j.ejphar.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Sikirić P, Petek M, Rucman R, Seiwerth S, Grabarević Z, Rotkvić I, et al. A new gastric peptide BPC. An overview of stomach — organoprotection hypothesis and beneficial effect of BPC. J Physiol. 1993;87:313–327. doi: 10.1016/0928-4257(93)90038-u. [DOI] [PubMed] [Google Scholar]
- 5.Staresinic M, Sebecic B, Patrlj L, Jadrijevic S, Suknaic S, Perovic D, et al. Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth. Orthop Res. 2003;21:976–983. doi: 10.1016/S0736-0266(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 6.Keremi B, Lohinai Z, Komora P, Duhaj S, Borsi K, Jobbagy-Ovari G, et al. Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. J Physiol Pharmacol. 2009;60(Suppl 7):115–122. [PubMed] [Google Scholar]
- 7.Xu J, Brennan TJ. The pathophysiology of acute pain: animal models. Curr Opin Anaesthesiol. 2011;24:508–514. doi: 10.1097/ACO.0b013e32834a50d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011;152(3 Suppl):S33–S40. doi: 10.1016/j.pain.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 10.Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurons in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 11.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–502. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 12.Tverskoy M, Oz Y, Isakson A, Finger J, Bradley EL, Kissin I. Preemptive effect of fentanyl and ketamine on postoperative pain and wound hyperalgesia. Anesth Analg. 1994;78:205–209. doi: 10.1213/00000539-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–135. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 14.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Gautam M, Prasoon P, Kumar R, Reeta KH, Kaler S, Ray SB. Role of neurokinin type 1 receptor in nociception at the periphery and the spinal level in the rat. Spinal Cord. 2016;54:172–182. doi: 10.1038/sc.2015.206. [DOI] [PubMed] [Google Scholar]
- 16.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of A- and C-fibers innervating the plantar rat hindpaw one day after an Incision. J Neurophysiol. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 17.Hämäläinen MM, Gehart GF, Brennan TJ. Acute effect of an incision on mechanosensitive afferents in the plantar rat hindpaw. J Neurophysiol. 2002;87:712–720. doi: 10.1152/jn.00207.2001. [DOI] [PubMed] [Google Scholar]
- 18.Handwerker HO, Kilo S, Reeh PW. Unresponsive afferent nerve fibres in the sural nerve of the rat. J Physiol. 1991;435:229–242. doi: 10.1113/jphysiol.1991.sp018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res. 1991;561:252–261. doi: 10.1016/0006-8993(91)91601-v. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt R, Schmelz M, Torebjork HE, Handwerker HO. Mechanoinsensitive nociceptors encode pain evoked by tonic pressure to human skin. Neuroscience. 2000;98:793–800. doi: 10.1016/s0306-4522(00)00189-5. [DOI] [PubMed] [Google Scholar]
- 21.McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett. 1996;208:45–48. doi: 10.1016/0304-3940(96)12552-0. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Choi SR, Kim JH, Lee SC, Jeong SY, Jeong JH, et al. Antinociceptive effect of BPC-157 in the formalin-induced pain model. Kosin Med J. 2021;36:1–13. [Google Scholar]
- 23.Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drimic D, et al. Brain-gut axis and pentadecapeptide BPC 157: theoretical and practical implications. Curr Neuropharmacol. 2016;14:857–865. doi: 10.2174/1570159X13666160502153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gjurasin M, Miklic P, Zupancic B, Perovic D, Zarkovic K, Brcic L, et al. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul Pept. 2010;160:33–41. doi: 10.1016/j.regpep.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Rivot JP, Montagne-Clavel J, Besson JM. Subcutaneous formalin and intraplantar carrageenan increase nitric oxide release as measured by in vivo voltammetry in the spinal cord. Eur J Pain. 2002;6:25–34. doi: 10.1053/eujp.2001.0268. [DOI] [PubMed] [Google Scholar]
- 26.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126–1135. doi: 10.2174/13816128113190990411. [DOI] [PubMed] [Google Scholar]
- 27.Veljaca M, Lesch CA, Pllana R, Sanchez B, Chan K, Guglietta A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1995;272:417–422. [PubMed] [Google Scholar]
- 28.Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, et al. BPC 157 and blood vessels. Curr Pharm Des. 2014;20:1121–1125. doi: 10.2174/13816128113199990421. [DOI] [PubMed] [Google Scholar]
- 29.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19:126–132. doi: 10.2174/092986712803414015. [DOI] [PubMed] [Google Scholar]
- 30.Sikiric P, Seiwerth S, Brcic L, Blagaic AB, Zoricic I, Sever M, et al. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach and vascular response. Inflammopharmacology. 2006;14:214–221. doi: 10.1007/s10787-006-1531-7. [DOI] [PubMed] [Google Scholar]
- 31.Ilic S, Drmic D, Franjic S, Kolenc D, Coric M, Brcic L, et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011;88:535–542. doi: 10.1016/j.lfs.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Ilic S, Drmic D, Zarkovic K, Kolenc D, Coric M, Brcic L, et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736) J Physiol Pharmacol. 2010;61:241–250. [PubMed] [Google Scholar]
- 33.Rook JM, Hasan W, McCarson KE. Temporal effects of topical morphine application on cutaneous wound healing. Anesthesiology. 2008;109:130–136. doi: 10.1097/ALN.0b013e31817b5ac3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rook JM, Hasan W, McCarson KE. Morphine-induced early delays in wound closure: involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochem Pharmacol. 2009;77:1747–1755. doi: 10.1016/j.bcp.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am J Pathol. 2010;176:786–799. doi: 10.2353/ajpath.2010.090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee E, Padgett B. Intra-articular injection of BPC 157 for multiple types of knee pain. Altern Ther Health Med. 2021;27:8–13. [PubMed] [Google Scholar]