Abstract
Background
Data on safety and immunogenicity of coronavirus disease 2019 (COVID-19) vaccination in patients with compensated (C-cirrhosis) and decompensated cirrhosis (D-cirrhosis) are limited.
Methods
In this prospective multicenter study, adult participants with C-cirrhosis and D-cirrhosis were enrolled and received two doses of inactivated whole-virion COVID-19 vaccines. Adverse events were recorded within 14 days after any dose of vaccination, and serum samples of enrolled patients were collected and tested for SARS-CoV-2 neutralizing antibodies at least 14 days after the second dose. Risk factors for negative neutralizing antibody were analyzed.
Results
In total, 553 patients were enrolled from 15 centers in China, including 388 and 165 patients with C-cirrhosis and D-cirrhosis. The vaccines were well tolerated, most adverse reactions were mild and transient, and injection site pain (23/388 [5.9%] vs 9/165 [5.5%]) and fatigue (5/388 [1.3%] vs 3/165 [1.8%]) were the most frequently local and systemic adverse events in both the C-cirrhosis and D-cirrhosis groups. Overall, 4.4% (16/363) and 0.3% (1/363) of patients were reported Grades 2 and 3 alanine aminotransferase (ALT) elevations (defined as ALT > 2 upper limit of normal [ULN] but ≤ 5 ULN, and ALT > 5 ULN, respectively). The positive rates of COVID-19 neutralizing antibodies were 71.6% (278/388) and 66.1% (109/165) in C-cirrhosis and D-cirrhosis groups. Notably, Child–Pugh score of B and C levels was an independent risk factor of negative neutralizing antibody.
Conclusions
Inactivated COVID-19 vaccinations are safe with acceptable immunogenicity in cirrhotic patients, and Child–Pugh score of B and C levels is associated with hyporesponsive to COVID-19 vaccination.
Keywords: Child–Pugh, Compensated cirrhosis, Coronavirus disease 2019, Decompensated cirrhosis, Hepatitis B virus, Immunogenicity, Safety, Severe acute respiratory syndrome coronavirus 2, Vaccine, Vaccination
Introduction
Over the past 2 years, the pandemic of coronavirus disease 2019 (COVID-19), which caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has severely disrupted the global order and resulted in millions of fatalities worldwide [1, 2]. Numerous studies have shown that people with comorbidities, including liver comorbidities such as cirrhosis, are more susceptible to be infected with SARS-CoV-2 [1, 3]. Meanwhile, SARS-CoV-2 infection in turn translates into increased severity and mortality in patients with cirrhosis [1, 3]. It is known that COVID-19 vaccination is one of the most effective ways to prevent SARS-CoV-2 infection and decrease the risk of severity and mortality for general populations [4–6].
Previous studies indicated that individuals with cirrhosis and immunosuppressed status are frequently hyporesponsive to previously licensed vaccines [1, 3]. Recently, two studies have demonstrated relatively impaired or poor responses of mRNA-based COVID-19 vaccines in patients with chronic liver diseases (CLD), compensated and decompensated cirrhosis, and liver transplantation [7–9]. Moreover, our previous study indicated that patients with CLD had lower immunological response to inactivated COVID-19 vaccines than healthy populations [10]. However, data concerning the safety and immunogenicity of inactivated COVID-19 vaccines in patients with cirrhosis, especially decompensated cirrhosis, are limited, and the understanding of safety and effectiveness of COVID-19 vaccines in these patients is urgently needed. This study aimed to investigate the safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis.
Patients and methods
Study design and participants
In this multicenter, prospective, open-label study, adult participants with compensated cirrhosis and decompensated cirrhosis were enrolled from the network of Portal Hypertension Alliance in China (CHESS) and National Medical Center for Infectious Diseases (NMCID) in China. All participants received two doses of inactivated SARS-CoV-2 vaccines (CoronaVac, BBIBP-CorV, or WIBP-CorV). The time interval between the first and second SARS-CoV-2 vaccine doses was 3–8 weeks, according to the guidance of SARS-CoV-2 vaccination enacted by National Health Commission of the People’s Republic of China [10].
Inclusion and exclusion criteria
The eligibility criteria included participants with compensated and decompensated cirrhosis aged 18 years or older who were willing to comply with the study procedures and provide written informed consent. The exclusion criteria contain pregnancy, lactation, active or known history of SARS-CoV-2 infection, hepatocellular carcinoma (HCC), liver transplantation, immunosuppressive or immunodeficient state including confirmed human immunodeficiency virus infection, and history of receiving systemic immunosuppressants, systemic immunoglobulins or immunopotentiators within 3 months prior to the day of screening.
The previous history of SARS-CoV-2 infection was confirmed by reverse-transcription polymerase chain reaction, and antigen or antibody tests mainly using naso-oropharyngeal swabs. Cirrhosis was confirmed in all participants using clinical or biochemical evidence (splenomegaly, platelet count, ascites, hepatic encephalopathy, and/or variceal bleeding), FibroScan®, liver imaging, endoscopy or liver biopsy [8, 11–14]. The splenomegaly was defined as the largest dimension of more than 11 cm. The cut-off values of platelet count and liver stiffness measurement were defined as < 150 × 109/L and > 15 kPa to indicate the cirrhosis [15, 16]. Ascites was defined by compatible signs on examination and was confirmed by ultrasonography or paracentesis. Hepatic encephalopathy was defined as West-Haven grade 3–4 determined by specialist or requiring admission [17]. It is important to point out that the diagnosis of cirrhosis in current study does not rely on a single parameter, but on a comprehensive analysis of multiple parameters, including the symptoms and signs. The decompensated cirrhosis was defined as cirrhosis with at least one episode of ascites, jaundice, hepatic encephalopathy, or variceal bleeding, or with a Child–Pugh score of B or C levels [10, 13, 14]. The diagnosis of HCC can be established by non-invasive imaging criteria and/or pathology according to the Chinese HCC guideline: for patients with chronic hepatitis B/C or cirrhosis of any etiology, nodules > 2 cm in diameter can be diagnosed as HCC based on the typical features on one imaging technique, whereas nodules ≤ 2 cm need confirmation by two imaging modalities [18].
Demographical and clinical data collection
Basic characteristics (age, sex, body mass index), etiology of liver diseases, liver diseases severities (compensated or compensated cirrhosis), comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, coronary artery disease, chronic kidney diseases, malignancy, arrhythmia and asthma, smoking history and ongoing alcoholism) and concomitant medications, and related laboratory testing results were collected at the enrollment and during the study.
Safety assessment
The primary safety outcome is the adverse events of participants injected with inactivated SARS-CoV-2 vaccines. After receiving the vaccine, the patient were monitored at the vaccination site for more than 30 min. If an adverse reaction occurs that the patient cannot handle on his or her own, the patient will be evaluated at a nearby tertiary hospital in accordance with the SARS-CoV-2 vaccination emergency management plan. All the related adverse effects after vaccinations were collected by using the pre-designed excel database where investigators and participants were required to record the injection-site and systemic reactions.
The following side events were reported: local injection site reaction including pain, swelling, redness, induration, erythema, pruritus, axillary swelling and tenderness on vaccination arm, and systemic reactions including fatigue, headache, vertigo, myalgia, chills, arthralgia, fever, cough, hypersensitivity, anorexia, dyspnea, syncope, nausea, vomiting, and pharyngalgia. Patients can also report any other unreported adverse reaction that not mentioned above. Liver function abnormalities were monitored after the whole schedule of SARS-CoV-2 vaccination.
Abnormality in liver function was defined as any of the following parameter increased over upper limit of normal (ULN) including alanine aminotransferase (ALT, 0–40 U/L), aspartate aminotransferase (AST, 0–40 U/L), γ-glutamyl transpeptidase (GGT, 0–50 U/L), alkaline phosphatase (ALP, 0–150 U/L), albumin (> 35 g/L), and total bilirubin (TBIL, 0–17.1 μmol/L) and direct bilirubin (DBIL, 0–6 μmol/L).
Immunogenicity evaluation
The primary immunoresponsive outcome is the immunogenicity of inactivated COVID-19 vaccines. Serum samples of enrolled participants were taken at least 14 days after the second dose to detect neutralizing antibody to SARS-CoV-2, with a maximum time limit of 90 days. The competitive binding immuoenzymatic capture chemiluminescence immunoassays (CLIA) SARS-CoV-2 neutralizing antibody assay (Shenzhen Mindray Bio-Medical Electronics Co., Ltd, China) was used to quantitatively detect neutralizing antibodies to the ancestral SARS-CoV-2 according to the manufacture instructions. The measuring range are 2.0–400.0 AU/ml, and results over 10 AU/mL was considered as evidence of immune response and below 2.0 U/mL as undetectable, according to the instruction book. We defined results over 10.0 AU/mL as positive, and results below 10.0 AU/mL as negative.
Statistical analysis
Continuous variables are summarized as the medians and interquartile ranges (IQR). The percentage of patients in each category was calculated for categorical variables. The percentages were compared between the two groups using the chi-square test. The Mann–Whitney U test was performed to compare continuous variables between the two groups. Paired-samples t tests or related-samples Wilcoxon signed rank tests were performed to compare continuous variables between various time points within one group, as appropriate. We fitted binary logistic regression models for univariate and multivariate analysis of factors related to the serological responses. When comparing immunogenic outcomes, we adjusted for factors that were substantially different between various subgroups, or among patients within subgroups, using logistic regression or analysis of covariance. A two-sided p < 0.05 was considered significant. The analyses were performed using SPSS software 25.0 for Windows (SPSS Inc. Chicago, IL, USA).
Ethical concerns
Written informed consents were obtained before the enrollment. The study protocol and informed consent form were approved by the involved Ethics Committees. This study is registered at ClinicalTrials.gov (NCT04883177).
Results
Participant’s characteristics
A total of 553 participants were included from 15 centers in China between January 2021 and December, 2021, including 388 and 165 patients with compensated and decompensated cirrhosis, with the median age of 53 and 54 years old, respectively (Table 1). Among these patients, 340, 151, and 62 received CoronaVac, BBIBP-CorV, and WIBP-CorV, respectively. Notably, hepatitis B virus (HBV) infection accounted for 87.1% and 77.6% of etiology in groups of compensated and decompensated cirrhosis, and 73.2% (341/466) of those were receiving anti-HBV treatment. The most common comorbidity is the hypertension, which presented in 13.8% and 14.3% of these two groups, followed by diabetes, arrhythmia, asthma, and coronary artery disease (Table 1). Liver function parameters were generally normal or stable, and with regard to the Child–Pugh score, B and C levels appeared in 2.8% and 31.5% of patients in groups of compensated and decompensated cirrhosis, respectively.
Table 1.
Baseline characteristics of cirrhotic participants
| Characteristics | Total (n = 553) | C-cirrhosis (n = 388) | D-cirrhosis (n = 165) | p value |
|---|---|---|---|---|
| Age, years | 54 (45–61) | 53 (45–64) | 54 (44–60) | 0.184 |
| Sex, male | 374 (67.63) | 261 (67.27) | 113 (68.48) | 0.843 |
| Body mass index | 24 (22.2–26.0) | 23.8 (22.0–25.4) | 24 (22.5–26.3) | 0.059 |
| Overweight | 223 (40.33) | 157 (40.46) | 66 (40.0) | 0.999 |
| Etiology | ||||
| HBV | 466 (84.27) | 338 (87.11) | 128 (77.58) | 0.007 |
| HCV | 36 (6.69) | 22 (5.9) | 14 (8.48) | 0.267 |
| Alcoholic | 40 (7.45) | 24 (6.45) | 16 (9.7) | 0.213 |
| NAFLD | 17 (3.07) | 15 (3.87) | 2 (1.21) | 0.113 |
| AIH/PBC/PSC | 13 (2.42) | 9 (2.41) | 4 (2.42) | 0.999 |
| Others | 23 (4.16) | 14 (3.61) | 9 (5.45) | 0.353 |
| Chronic hepatitis B | ||||
| HBeAg positive | 99 (20.54) | 79 (23.58) | 20 (13.61) | 0.014 |
| HBV DNA positive | 122 (22.18) | 87 (22.48) | 35 (21.47) | 0.823 |
| Antiviral therapy | 351 (63.47) | 273 (70.36) | 78 (47.27) | < 0.001 |
| Comorbidities | ||||
| Any | 112 (20.25) | 73 (18.81) | 39 (23.64) | 0.205 |
| Hypertension | 70 (13.97) | 47 (13.82) | 23 (14.29) | 0.891 |
| Diabetes | 54 (10.82) | 35 (10.36) | 19 (11.8) | 0.645 |
| Arrhythmia | 13 (2.78) | 9 (2.69) | 4 (3.03) | 0.765 |
| Asthma | 1 (0.2) | 1 (0.3) | 0 (0) | 0.514 |
| CAD | 10 (2.01) | 6 (1.79) | 4 (2.48) | 0.734 |
| Liver function | ||||
| ALT, U/L | 27.0 (18.0–39.0) | 26.0 (18.0–37.2) | 28.3 (19.9–45) | 0.104 |
| AST, U/L | 27.8 (21.4–39.0) | 27.0 (21.0–35.0) | 31.0 (23.0–55.4) | < 0.001 |
| Albumin, g/L | 45.3 (41.0–48.2) | 45.7 (42.3–48.5) | 43.0 (34.8–47.2) | < 0.001 |
| TBIL, μmol/L | 18.3 (13.7–25.1) | 16.8 (12.8–22.4) | 23.4 (16.0–35.1) | < 0.001 |
| DBIL, μmol/L | 4.4 (2.9–7.5) | 3.8 (2.6–6.2) | 6.7 (3.8–11.5) | < 0.001 |
| GGT, U/L | 30.0 (18.8–55) | 28.0 (19.0–48) | 35.0 (20.7–68.0) | 0.014 |
| ALP, U/L | 77.6 (63.0–98.5) | 75.4 (63.0–92.0) | 89.0 (64.0–113.0) | 0.002 |
| Child–Pugh | < 0.001 | |||
| A | 490 (88.61) | 377 (97.16) | 113 (68.48) | |
| B + C | 63 (11.39) | 11 (2.84) | 52 (31.52) |
Data are displayed as median (interquartile range) and n (%). AIH, autoimmune hepatitis; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CAD, Coronary artery disease; C-cirrhosis, compensated cirrhosis; DBIL, direct bilirubin; D-cirrhosis, decompensated cirrhosis; GGT, γ-glutamyl transpeptidase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; TBIL, total bilirubin
COVID-19 vaccination safety
The inactivated COVID-19 vaccinations were generally well tolerated in patients with compensated and decompensated cirrhosis. A total of 43 (11.1%) and 18 (11.0%) patients with compensated and decompensated cirrhosis reported at least one adverse reaction within 14 days of either dose of COVID-19 vaccination, and no significant difference in adverse reactions was found between two groups (Table 2). The most common local adverse reaction was pain, which was reported in 5.9% (23/388) and 5.5% (9/165) of patients with compensated and decompensated cirrhosis, respectively (p = 0.316), followed by the induration, erythema, swelling, and so on (Table 2). Notably, all these local adverse reactions can be resolved spontaneously. Meanwhile, the most commonly reported systematic adverse reaction was fatigue, which was reported in 1.3% (5/388) and 1.8% (3/165) of patients with compensated and decompensated cirrhosis, respectively (p = 0.442). Among 363 patients with available laboratory follow-up data, there were 6/240 (2.5%) and 10/123 (8.1%) patients reporting Grade 2 ALT elevation (defined as ALT > 2 ULN but ≤ 5 ULN), and 1/240 (0.4%) and 0/123 (0%) patient reporting Grade 3 ALT elevation (defined as ALT > 5 ULN) in compensated and decompensated cirrhosis groups, respectively. Meanwhile, 6/240 (2.5%) and 9/123 (7.3%) patients were reported Grade 2 AST elevation, and 0/240 (0%) and 1/123 (0.8%) patient reporting Grade 3 AST elevation in compensated and decompensated cirrhosis groups, respectively.
Table 2.
Adverse reactions after either dose of SARS-CoV-2 vaccines
| Characteristics | C-cirrhosis (n = 388) | D-cirrhosis (n = 165) | p value |
|---|---|---|---|
| Local adverse reactions | |||
| Any | 29 (7.47) | 11 (6.67) | 0.737 |
| Pain | 0.316 | ||
| Grade 1 | 23 (5.93) | 8 (4.85) | |
| Grade 2 | 0 (0) | 1 (0.61) | |
| Induration | 0.999 | ||
| Grade 1 | 1 (0.26) | 0 (0) | |
| Erythema | 0.508 | ||
| Grade 1 | 1 (0.26) | 1 (0.61) | |
| Swelling | 0.329 | ||
| Grade 1 | 5 (1.29) | 0 (0) | |
| Pruritus | 0.298 | ||
| Grade 1 | 0 (0) | 1 (0.61) | |
| Systematic adverse events | |||
| Any | 19 (4.9) | 11 (6.67) | 0.401 |
| Fatigue | 0.442 | ||
| Grade 1 | 5 (1.29) | 2 (1.21) | |
| Grade 2 | 0 (0) | 1 (0.61) | |
| Vertigo | 0.332 | ||
| Grade 1 | 5 (1.29) | 1 (0.61) | |
| Somnolence | 0.732 | ||
| Grade 1 | 4 (1.0) | 2 (1.21) | |
| Grade 2 | 0 (0) | 1 (0.61) | |
| Nausea | 0.586 | ||
| Grade 1 | 2 (0.52) | 2 (1.21) | |
| Fever | 0.113 | ||
| Grade 1 | 2 (0.52) | 1 (0.61) | |
| Ventosity | 0.089 | ||
| Grade 2 | 0 (0) | 2 (1.21) | |
| Diarrhea | 0.508 | ||
| Grade 1 | 1 (0.26) | 1 (0.61) | |
| Edema of lower limbs | 0.508 | ||
| Grade 1 | 1 (0.26) | 1 (0.61) | |
| Cough | 0.508 | ||
| Grade 1 | 1 (0.26) | 1 (0.61) | |
| Arthralgia | 0.999 | ||
| Grade 1 | 1 (0.26) | 0 (0) | |
| Xerostomia | 0.298 | ||
| Grade 1 | 0 (0) | 1 (0.61) | |
| Albiduria | 1.000 | ||
| Grade 1 | 1 (0.26) | 0 (0) | |
| Hearing loss | 1.000 | ||
| Grade 1 | 1 (0.26) | 0 (0) | |
| Myalgia | 0.298 | ||
| Grade 1 | 0 (0) | 1 (0.61) | |
| Anorexia | 0.089 | ||
| Grade 1 | 0 (0) | 1 (0.61) | |
| Grade 2 | 0 (0) | 1 (0.61) |
Data are displayed as n (%). C-cirrhosis, compensated cirrhosis; D-cirrhosis, decompensated cirrhosis
COVID-19 vaccination immunogenicity
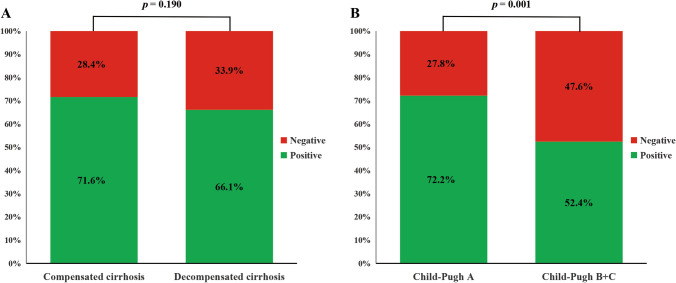
A total of 70.0% (387/553) of patients had positive neutralizing antibody (Table 3), with the median level of 15 AU/mL at 34 days (IQR: 19–61) after the second dose of vaccinations. Among patients with compensated cirrhosis, the neutralizing antibodies were detected at 34.5 days (IQR: 21–60) after the second dose of vaccinations, the neutralizing antibody positive rate was 71.6% (278/388, Fig. 1A), with a median level of neutralizing antibody of 15.2 AU/mL; thereinto, the median levels were 22.7 AU/mL and 7.4 AU/mL in neutralizing antibody positive and negative subgroups, respectively. Of patients with decompensated cirrhosis, the neutralizing antibodies were detected at 34 days (IQR: 14–70) after the second dose of vaccinations, the neutralizing antibody positive rate was 66.1% (109/165, Fig. 1A), with a median level of neutralizing antibody of 16 AU/mL; thereinto, the median levels were 21.4 AU/mL and 6.3 AU/mL in neutralizing antibody positive and negative subgroups, respectively. Notably, the neutralizing antibody positive rates were 72.2% and 52.4% after dividing the patients into Child A group and Child B + C group, respectively (Fig. 1B, p = 0.001). The immunogenicity of three types of vaccines distributed in two groups is presented in Table 3.
Table 3.
Immunogenicity of three types of inactivated vaccines
| Parameters | C-cirrhosis (n = 388) | D-cirrhosis (n = 165) | p value |
|---|---|---|---|
| CoronaVac | 234/388 (60.3) | 106/165 (64.2) | 0.615 |
| Neutralizing antibody positive | 181/234 (77.4) | 76/106 (71.7) | 0.277 |
| > 14–30 days | 78/100 (78.0) | 36/54 (66.7) | 0.847 |
| > 30–60 days | 67/82 (81.7) | 18/22 (81.8) | 0.998 |
| > 60 days | 36/52 (69.2) | 22/30 (73.3) | 0.824 |
| Neutralizing antibody level, AU/mL | 17.1 (10.5–31.4) | 17.1 (9.5–27) | 0.494 |
| > 14–30 days | 19.4 (10.2–38.8) | 15.9 (9–25.1) | 0.306 |
| > 30–60 days | 18.6 (11.6–30.4) | 22.7 (13.6–38.2) | 0.412 |
| > 60 days | 13.5 (8.7–22.8) | 15.4 (10.4–20.3) | 0.751 |
| BBIBP-CorV | 104/388 (26.8) | 47/165 (28.5) | 0.315 |
| Neutralizing antibody positive | 58/104 (56.9) | 24/47 (51.1) | 0.601 |
| > 14–30 days | 34/43 (79.1) | 13/18 (72.2) | 0.739 |
| > 30–60 days | 15/31 (48.4) | 5/11 (45.4) | 0.999 |
| > 60 days | 9/30 (30.0) | 6/18 (33.3) | 0.998 |
| Neutralizing antibody level, AU/mL | 11.6 (8–19.3) | 10.6 (5.6–17.7) | 0.100 |
| > 14–30 days | 17.5 (11.1–27.6) | 19.4 (9.9–26.6) | 0.942 |
| > 30–60 days | 9.8 (7.9–17.2) | 9.5 (5.6–18.4) | 0.741 |
| > 60 days | 8.6 (5.8–11.2) | 6.8 (5.1–11) | 0.320 |
| WIBP-CorV | 50/388 (12.9) | 12/165 (7.3) | 0.944 |
| Neutralizing antibody positive | 39/50 (78.0) | 9/12 (75.0) | 0.176 |
| > 14–30 days | 17/22 (77.3) | 3/4 (75.0) | 0.998 |
| > 30–60 days | 19/22 (86.4) | 6/7 (85.7) | 0.997 |
| > 60 days | 3/6 (50.0) | 0/1 (0) | 0.650 |
| Neutralizing antibody level, AU/mL | 22.5 (12–79.9) | 30.5 (9–73.1) | 0.950 |
| > 14–30 days | 18.6 (9.9–197.9) | 72.3 (51.6–113.9) | 0.413 |
| > 30–60 days | 88 (25.1–128.4) | 34.4 (25–66.4) | 0.559 |
| > 60 days | 11.08 (4.94–47.22) | - | - |
Data are presented as median (interquartile range), n (%), or n/N (%), where N is the total number of patients of a subgroup or during a specific period. C-cirrhosis, compensated cirrhosis; D-cirrhosis, decompensated cirrhosis
Fig. 1.
Positive rate of neutralizing antibody to inactivated coronavirus disease 2019 vaccines in compensated and decompensated cirrhotic patients (A) and in cirrhotic patients with Child–Pugh scores of A and B + C (B)
Factors associated with vaccination responses
Univariate and multivariate analysis of factors that potentially associated with the serological response of COVID-19 vaccines were conducted in all patients (Table 4). In univariate analysis, male, hepatitis B e antigen positive status, HBV DNA detectable, albumin, and Child–Pugh score of B and C levels were identified as the risk factors of nonresponse to COVID-19 vaccinations. However, in multivariate analysis, Child–Pugh score of B and C levels was suggested to be the only one independent risk factor for negative serological response to COVID-19 vaccination (1.430 [1.094–4.478], p = 0.027) after taking into considerations of all other risk factors involved in the univariate model.
Table 4.
Factors associated with negative neutralizing antibody to COVID-19 vaccinations in patients with cirrhosis
| Characteristics | Positive (n = 387) |
Negative (n = 166) |
Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| OR | p value | OR | p value | |||
| Age | 56 (47.8–61) | 53 (43–62) | 0.999 (0.984–1.016) | 0.947 | ||
| Sex, male | 250 (64.6) | 124 (74.7) | 1.618 (1.083–2.450) | 0.021 | 1.292 (0.971–2.658) | 0.07 |
| Body mass index | 24.2 (22.4–25.9) | 23.9 (22.1–26) | 1.056 (0.988–1.128) | 0.11 | ||
| Overweight | 148 (38.24) | 75 (45.18) | 1.331 (0.92–1.923) | 0.128 | ||
| Etiology | ||||||
| HBV | 326 (84.24) | 140 (84.34) | 1.008 (0.617–1.684) | 0.976 | ||
| HCV | 25 (6.65) | 11 (6.79) | 1.023 (0.472–2.082) | 0.952 | ||
| Alcoholic | 31 (8.27) | 9 (5.56) | 0.653 (0.287–1.351) | 0.275 | ||
| NAFLD | 10 (2.58) | 7 (4.22) | 1.66 (0.593–4.399) | 0.313 | ||
| AIH/PBC/PSC | 11 (2.93) | 2 (1.23) | 0.415 (0.064–1.567) | 0.256 | ||
| Others | 18 (4.65) | 5 (3.01) | 0.637 (0.207–1.627) | 0.38 | ||
| Cirrhosis | 1.298 (0.876–1.915) | 0.19 | ||||
| Compensated | 278 (71.83) | 110 (66.27) | ||||
| Decompensated | 109 (28.17) | 56 (33.73) | ||||
| Chronic Hepatitis B | ||||||
| HBeAg positive | 54 (15.98) | 45 (31.25) | 2.391 (1.511–3.776) | 0 | 1.355 (0.885–2.928) | 0.115 |
| HBV DNA positive | 74 (19.27) | 48 (28.92) | 1.704 (1.115–2.59) | 0.013 | 1.377 (0.491–1.731) | 0.824 |
| Antiviral therapy | 254 (65.63) | 97 (58.43) | 0.736 (0.507–1.071) | 0.108 | ||
| Comorbidities | ||||||
| Any | 80 (20.67) | 32 (19.28) | 0.916 (0.574–1.437) | 0.708 | ||
| Hypertension | 47 (13.43) | 23 (15.23) | 1.158 (0.666–1.969) | 0.593 | ||
| Diabetes | 40 (11.46) | 14 (9.33) | 0.795 (0.406–1.477) | 0.484 | ||
| Arrhythmia | 9 (2.7) | 4 (2.99) | 1.108 (0.296–3.466) | 0.867 | ||
| Asthma | 1 (0.29) | 0 (0) | 1 (0.998–1.002) | 0.981 | ||
| CAD | 7 (2.01) | 3 (2.01) | 1.001 (0.213–3.655) | 0.999 | ||
| Liver function | ||||||
| ALT | 29 (18–39) | 26 (17–37) | 1 (0.997–1.003) | 0.993 | ||
| AST | 27 (21–38.2) | 26.9 (21–37) | 1 (0.996–1.003) | 0.956 | ||
| Albumin | 45.9 (40.6–49) | 45.8 (42.2–48.1) | 0.957 (0.929–0.986) | 0.004 | 1.020 (0.934–1.01) | 0.149 |
| TBIL | 19.8 (13.3–29.1) | 17.9 (13.9–24.3) | 1.002 (0.997–1.006) | 0.495 | ||
| DBIL | 4 (2.6–7.6) | 4.1 (2.7–6.9) | 1.002 (0.994–1.009) | 0.649 | ||
| GGT | 32 (19.8–48.2) | 27 (18–54) | 1.002 (0.999–1.005) | 0.215 | ||
| ALP | 83.5 (63–107.2) | 77 (63–95) | 1 (0.996–1.001) | 0.678 | ||
| Child–Pugh | 2.366 (1.385–4.033) | 0.002 | 1.430 (1.094–4.478) | 0.027 | ||
| A | 354 (91.47) | 136 (81.93) | ||||
| B + C | 33 (8.53) | 30 (18.07) | ||||
Data are displayed as median (interquartile range) and n (%). AIH, autoimmune hepatitis; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CAD, Coronary artery disease; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; TBIL, total bilirubin
Discussion
Commonly, patients with CLD, especially the compensated and decompensated cirrhosis, are considered immunocompromised and are frequently hyporesponsive to licensed vaccines [1, 3]. As a special population, CLD patients with various severities have been concerned by the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) timely [1, 3]. The EASL and AASLD recommended that providers advocate for prioritizing patients with compensated and decompensated cirrhosis for COVID-19 vaccination based upon local health policies, protocols, and vaccine availability [1, 3]. However, related data concerning safety and efficacy/effectiveness/immunogenicity of COVID-19 vaccination are limited in patients with cirrhosis, especially in patients with decompensated cirrhosis [4–7, 19–21].
In this multicenter prospective study, we found that inactivated COVID-19 vaccines are safe and well tolerated, and the local and systemic adverse reactions were mostly mild in severity in patients with compensated and decompensated cirrhosis. Notably, the current study indicated that inactivated COVID-19 vaccines can induce a neutralizing antibody positive rate of 70% (387/553) in those cirrhotic patients. Additionally, Child–Pugh score of B and C levels is the independent risk factor of negative neutralizing antibody after vaccinations.
For the mRNA COVID-19 vaccines, two large-scale phase III trials only included 0.6% (217) of 37,706 participants in the BNT162b2 mRNA vaccine trial and 0.6% (196) of 30,351 participants in the mRNA-1273 vaccine trial [4, 6]. Recent study included 62 liver transplantation patients, 79 cirrhotic patients, and 92 CLD patients receiving mRNA COVID-19 vaccines has indicated that 61% of liver transplant recipients and 24% of patients with CLD had poor antibody responses, however, only 10 of 79 cirrhotic patients with decompensated states were enrolled in this study [8]. In another retrospective study using mRNA COVID-19 vaccines, a total of 16,895 and 3142 patients with compensated and decompensated cirrhosis were included, which was the largest sample size study concerning mRNA COVID-19 vaccinations in patients with cirrhosis, and found mRNA vaccination was associated with a delayed but modest reduction in COVID-19 infection [7]. Most recently, John and colleagues found that cirrhotic patients can develop breakthrough COVID-19 after full or partial vaccination of the BNT162b2 mRNA or 1273-mRNA vaccines, and these infections are associated with reduced mortality [9].
For the inactivated COVID-19 vaccines, our previous study indicated the favorable safety and immunogenicity of inactivated COVID-19 vaccination in 381 patients with nonalcoholic fatty liver disease, unfortunately, no patient with decompensated cirrhosis was included in this study [11]. In another our previous study, we included 284 non-cirrhotic CLD patients, 123 compensated cirrhotic patients, and only 30 decompensated cirrhotic patients, although this study found that the tolerance of inactivated COVID-19 vaccination is well and the immunogenicity is lower in patients with non-cirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis than that in healthy participants, the sample size is relatively small, especially for decompensated cirrhotic patients [10]. Recently, another small sample size study found a favorable safety profile and efficient immunogenicity in patients with chronic HBV infection in real-world setting, however, only ten patients with HBV-related cirrhosis were included, and no liver decompensation was found in this study [22].
This study included 553 patients with cirrhosis, and there into, 165 patients were decompensated. To the best of our knowledge, this study is the largest sample size study concerning cirrhotic patients administered with inactivated COVID-19 vaccines, and found that the positive rate of neutralizing antibody in decompensated cirrhosis was lower than compensated cirrhosis (66.1% [109/165] vs 71.6% [278/388]), although the difference is not significant (p = 0.190), and Child–Pugh score of B and C levels is associated with negative neutralizing antibody (p = 0.015). Given that the neutralizing antibody is likely to be the key antibody of protection for COVID-19 [23–26], we therefore introduced the neutralizing antibody other than immunoglobulin G antibody against SARS-CoV-2 spike protein for detection and analysis in current study, which is as same as our previous study [10]. Compared with healthy participants in our previous study, the overall positive rate of neutralizing antibody in cirrhotic patients in this study was significantly lower as mentioned above (70% vs 90.3%, p < 0.001) [10]. These results further confirm the EASL and AASLD’s concerns about low responsiveness to COVID-19 vaccine in patients with cirrhosis [1, 3].
Although the head-to-head comparisons of the mRNA COVID-19 vaccines and the inactivated COVID-19 vaccines are limited, it is believed that the potential lack of a T-cell-associated immune response to inactivated whole virus vaccine as compared to spike mRNA-lipid formulations may attribute to a lower protective response [27, 28]. However, the role of the adjuvant used in inactivated vaccine as potential contributor for T- and B-cell may help in stimulation of immune system to many virus proteins in context to C- or D-cirrhosis [29, 30]. Notably, as shown in Table 3, the antibody titers and response against inactivated antigens diminish with time, and as a result, the inactivated COVID-19 vaccines may require periodic supplemental doses to increase or “boost” the antibody titers.
We acknowledge that our study has limitations. First, no real-world effectiveness data exist. Because the strict prevention and management strategies in China, especially the Chinese government has been implementing the “ZERO COVID-19” policy for more than 2 years, the COVID-19 epidemic situation in China has been mild, only few imported and domestic cases were reported intermittently, therefore, patients who have been vaccinated against COVID-19 in our study are less likely to be exposed to sources of infection. Second, no healthy control groups have been included in the present study. However, multiple previous studies, including our study, have revealed the well tolerance and antibody seropositive rates of about 70–90% to vaccination in healthy populations [10, 19, 22, 31]. Regardless of these limitations, the current study indicated that favorable safety and acceptable immunogenicity of inactivated COVID-19 vaccines in a large sample size of patients with compensated and decompensated cirrhosis.
In conclusion, given that the favorable safety and acceptable immunogenicity of the inactivated COVID-19 vaccines observed in the current study and the potential benefits may outweigh the risks, we suggest patients with compensated and decompensated cirrhosis to be vaccinated against COVID-19. Nonetheless, given the continuing emergence of novel SARS-CoV-2 variants, the hepatologists should provide appropriate guidance regarding continued social distancing, masking, and frequent hand washing and follow other exposure-mitigating behaviors to these compensated and decompensated cirrhotic patients who have received vaccinations against SARS-CoV-2 infection.
Acknowledgements
The authors sincerely thank all the involved participants for their cooperation regarding the clinical monitoring and evaluations.
Abbreviations
- AIH
Autoimmune hepatitis
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- CAD
Coronary artery disease
- COVID-19
Coronavirus disease 2019
- DBIL
Direct bilirubin
- GGT
γ-Glutamyl transpeptidase
- HBeAg
Hepatitis B e antigen
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- NAFLD
Nonalcoholic fatty liver disease
- PBC
Primary biliary cholangitis
- PSC
Primary sclerosing cholangitis
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TBIL
Total bilirubin
Author contributions
XQ and WZ contributed to the concept, design, supervision, administration, technology, and material support of the study; all authors contributed to the data acquisition and interpretation; Q-LZ and JW contributed to the drafting of the manuscript; XQ and Q-LZ contributed to the critical revision of the manuscript for important intellectual content; and Q-LZ and JW contributed to the statistical analysis. All authors have seen and approved the final version of the manuscript.
Funding
None.
Data availability
All the data generated and analyzed during this study are included in our manuscript. Any additional data is available from the corresponding author upon request.
Declarations
Conflict of interest
Jitao Wang,Qiran Zhang,Jingwen Ai,Dengxiang Liu,Chuan Liu,Huiling Xiang,Ye Gu,Ying Guo,Jiaojian Lv,Yifei Huang,Yanna Liu,Dan Xu,Shubo Chen,Jinlong Li,Qianqian Li,Jing Liang,Li Bian,Zhen Zhang,Xiaoqing Guo,Yinong Feng,Luxiang Liu,Xuying Zhang,Yanliang Zhang,Faren Xie,Shujun Jiang,Wei Qin,Xiaodong Wang,Wei Rao,Qun Zhang,Qiuju Tian,Ying Zhu,Qingwei Cong,Juan Xu,Zhiyun Hou,Nina Zhang,Aiguo Zhang,Hongmei Zu,Yun Wang,Zhaolan Yan,Xiufang Du,Aifang Hou,Yan Yan,Yuanwang Qiu,Hangyuan Wu,Shengjuan Hu, Yanhong Deng,Jiansong Ji,Jie Yang,Jiansheng Huang, Zhongwei Zhao, Shengqiang Zou, Hailei Ji, Guohong Ge, Li Zhong, Song He, Xiaosong Yan, Bian Ba Yangzhen, Ci Qu, Liting Zhang,Shiying Yang,Xiaoqin Gao, Muhan Lv,Qingliang Zhu,Xinxin Xu,Qing‑Lei Zeng,Xiaolong Qi,Wenhong Zhang these authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Ethical approval
The study protocol was approved by each participating institution’s review board or ethics committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Consent to participate
Written informed consent was obtained from all patients for being included in the study.
Consent for publication
Informed consent for publication was obtained from all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jitao Wang, Qiran Zhang, Jingwen Ai, Dengxiang Liu, Chuan Liu, Huiling Xiang and Ye Gu contributed equally as co-first authors.
Contributor Information
Qing-Lei Zeng, Email: zengqinglei2009@163.com.
Xiaolong Qi, Email: qixiaolong@vip.163.com.
Wenhong Zhang, Email: zhangwenhong@fudan.edu.cn.
References
- 1.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74(4):944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marjot T, Webb GJ, Barritt AS, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fix OK, Blumberg EA, Chang KM, et al. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74(2):1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John BV, Deng Y, Scheinberg A, et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med. 2021;181(10):1306–1314. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75(6):1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John BV, Deng Y, Schwartz KB, et al. Post‐Vaccination COVID‐19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology 2022:In press. [DOI] [PMC free article] [PubMed]
- 10.Ai J, Wang J, Liu D, et al. Safety and immunogenicity of SARS-CoV-2 vaccines in patients with chronic liver diseases (CHESS-NMCID 2101): a multicenter study. Clin Gastroenterol Hepatol 2021: In press. [DOI] [PMC free article] [PubMed]
- 11.Wang J, Hou Z, Liu J, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021;75(2):439–441. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75(3):659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Augustin S, Pons M, Maurice JB, et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66(6):1980–1988. doi: 10.1002/hep.29363. [DOI] [PubMed] [Google Scholar]
- 17.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 18.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang T, Liang B, Wang H, et al. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell Mol Immunol. 2021;18(12):2679–2681. doi: 10.1038/s41423-021-00795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau EHY, Tsang OTY, Hui DSC, et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat Commun. 2021;12(1):63. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller L, Andree M, Moskorz W, et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin Infect Dis. 2021;73(11):2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malipiero G, D’Agaro P, Segat L, Moratto A, Villalta D. Long-term decay of anti-RBD IgG titers after BNT162b2 vaccination is not mirrored by loss of neutralizing bioactivity against SARS-CoV-2. Clin Chim Acta. 2022;524:11–17. doi: 10.1016/j.cca.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erasmus JH, Khandhar AP, O’Connor MA, et al. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci Transl Med 2020;12(555):eabc9396. [DOI] [PMC free article] [PubMed]
- 28.Pilkington EH, Suys EJA, Trevaskis NL, et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 30.Ikewaki N, Iwasaki M, Kurosawa G, et al. beta-glucans: wide-spectrum immune-balancing food-supplement-based enteric (beta-WIFE) vaccine adjuvant approach to COVID-19. Hum Vaccin Immunother. 2021;17(8):2808–2813. doi: 10.1080/21645515.2021.1880210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated and analyzed during this study are included in our manuscript. Any additional data is available from the corresponding author upon request.