With great interest, we read the review article by Agus et al, which suggested that gut microbiome alterations could affect metabolic homeostasis.1 Moreover, gut microbiome alterations in concert with metabolites perturbation could contribute to the early development of rheumatoid arthritis (RA).2 We thus conducted a three-pronged association study3 on multiomics datasets to detect the potential microbiome–metabolites–arthritis link.
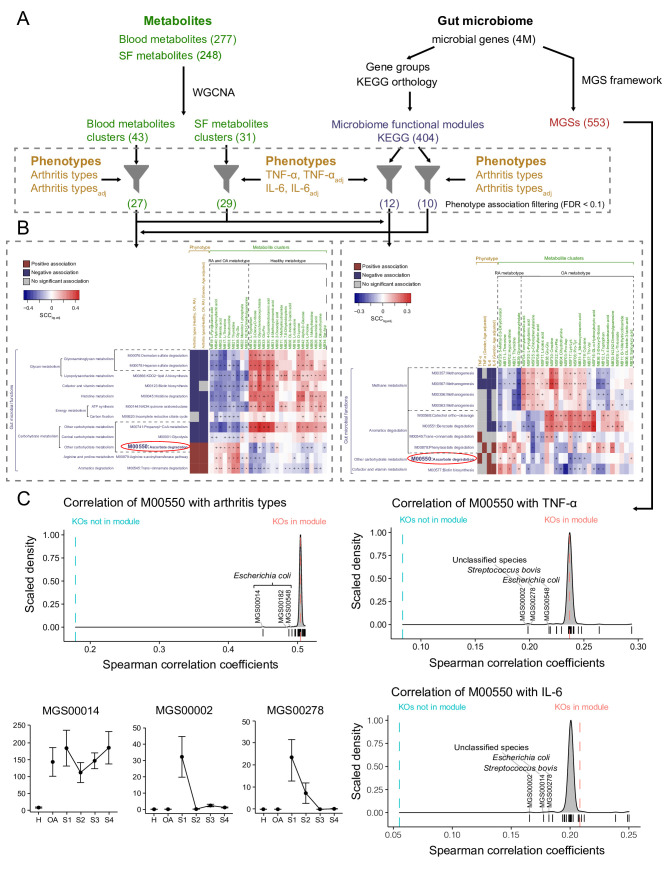
We integrated multiomics datasets including gut metagenomics, clinical phenotypes and metabolites of blood and knee-joint synovial fluid from 122 participants in the healthy group (n=27), osteoarthritis (OA) group (n=19) and RA group (n=76), using a three-pronged association framework (figure 1, online supplemental material).3 Metagenomic genes were collapsed into metagenomic species (MGS)3 4 and grouped into KEGG functional modules (figure 1A).3 Additionally, the co-abundant metabolites were categorised into metabolite clusters using WGCNA framework (figure 1A).3 The functional modules associated with clinical phenotypes (eg, types of arthritis and levels of cytokines) were further identified and the cross-domain associations between these modules and metabolite clusters were assessed (figure 1B).3 Furthermore, the leave-one-out analysis was performed to determine the MGS that particularly contributed to the observed linkage between functional modules and clinical phenotypes (figure 1C).3
gutjnl-2021-325209supp001.pdf (244.6KB, pdf)
Figure 1.
Overview of the three-pronged association framework integrating multiomics datasets. (A) Metabolites are summarised as co-abundance clusters, and microbial genes are grouped into KEGG modules and MGS, which are further filtered for statistically positive or negative associations (based on Spearman correlation) with the clinical phenotypes. The association analyses were divided by using healthy, OA and RA samples for arthritis types and using OA and RA samples for cytokine levels. The number in brackets represent the number of metabolites/metabolite clusters/microbial genes/KEGG modules/MGS in each analytical module. (B) The filtered features are further used for cross-domain association analyses. For each analysis, the left panel shows the significant associations (Mann-Whitney U test FDR<0.1) between KEGG modules and clinical phenotypes, and colour indicates significantly positive association (red), significantly negative association (blue) or insignificant association (grey). The right panel shows the associations between KEGG modules and metabolite clusters, and the colour represents the median Spearman correlation coefficient (SCC) of metabolite clusters with KEGG orthologies (KOs) in KEGG module minus those with KOs not in KEGG module. Mann-Whitney U test FDRs are denoted: +FDR<0.1; *FDR<0.01; **FDR<0.001. (C) The MGS that particularly contributed to the observed linkage between functional modules and clinical phenotypes. Three density plots: Dashed line represents the median SCC of the phenotypes with KOs in M00550 (red) and all other KOs (blue). Density plot shows the median SCC of the phenotypes with KOs in M00550, when a given MGS (indicated by short vertical lines) has been excluded from the analysis. The bottom-left dot plots show the mean±SEM of the top three driving MGS abundances among patients at each stage of disease development, with the four RA stages connected to display the variance. FDR, false discovery rate; IL-6, interleukin-6; MGS, metagenomic species; OA, osteoarthritis; RA, rheumatoid arthritis; TNF-α, tumour necrosis factor-α.
We found that gut microbial functionality in ascorbate degradation (KEGG module: M00550) was positively correlated with the types of arthritis (healthy=0, OA=1, RA=2, pWilcox=2.15×10–4) and the levels of proinflammatory cytokines TNF-α (tumour necrosis factor-α, pWilcox=6.59×10–4) and IL-6 (interleukin-6, pWilcox=1.12×10–3). Ascorbate (vitamin C) was previously reported to prevent the development of inflammatory arthritis,5 possibly through facilitating collagen synthesis, moderating autoimmune responses and ameliorating inflammation.6 Additionally, the patients with RA are usually ascorbate deficient and require high-dose supplementation to maintain an acceptable plasma level of ascorbate.7 In this study, the functional module of ascorbate degradation was observed to positively correlate with the blood metabolite cluster MB02 (pWilcox=6.90×10–3), which was represented by the level of palmitic acid (kME (eigengene-based connectivity) =0.911, kIN (intramodular connectivity) =3.46, online supplemental table 1) that acts as a proinflammatory factor, upregulating IL-6 secretion by human chondrocytes and fibroblast-like synovial cells in inflammatory arthritis.8 Furthermore, we found that Escherichia coli and Streptococcus bovis were the driving species for the observed linkage between ascorbate degradation9 and the arthritis types or the cytokines levels of TNF-α and IL-6 (figure 1C). Subsequently, we grouped patients with RA by four stages according to the comprehensive scores in rheumatoid diagnostic criteria,10 as RASI: 6–7, RASII: 8, RASIII: 9 and RASIV: 10 (online supplemental table 2). We observed that both E. coli and S. bovis were prevalent at RA stage I (RASI), while S. bovis was depleted after RASI or in the OA group. It suggested S. bovis mainly functioned at the early stage of RA, while E. coli might be crucial throughout the entire developmental stages of RA and OA. Taken together, we speculate that E. coli and S. bovis could facilitate ascorbate degradation and thus promote proinflammatory responses that facilitate the development of inflammatory arthritis.
gutjnl-2021-325209supp002.xlsx (36.4KB, xlsx)
gutjnl-2021-325209supp003.xlsx (13KB, xlsx)
Overall, we demonstrate that gut microbiota could promote RA progression via enhancing ascorbate degradation and provide a potential approach to prevent the development of arthritis through interfering gut–joint axis. The results of this study could be prospected in following contexts: First, our study provides a reservoir of the potential microbiome–metabolites–arthritis links as a reference of gut–joint axis for future studies. Second, the findings supplement the potential mechanisms related to metabolic perturbation through which gut microbiome promotes arthritis.1 2 Third, considering the inflammatory pathways of arthritis were revisited in COVID-19,11 it deserves further investigations whether microbiome–ascorbate–inflammation link of this study could contribute to the treatment of COVID-19.
Footnotes
YZ and MC contributed equally.
Correction notice: This article has been corrected since it published Online First. The figure has been replaced for clarity.
Contributors: YZ and MC designed the study, conducted the data analysis and wrote the manuscript. YZ, MC, LZ, LY, CZ, YZ and XZ collected the samples, conducted the experiments and participated in data analysis. LZ, KN and JH supervised the study and revised the manuscript.
Funding: This study was funded by National Natural Science Foundation of China (grant numbers 32071465, 82003766, 31871334, and 31671374), Academic Promotion Project of Shandong First Medical University (grant number 2019LJ001) and Ministry of Science and Technology’s National Key Research and Development Programme (grant number 2018YFC0910502).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (No. 2017-02).
References
- 1. Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021;70:1174–82. 10.1136/gutjnl-2020-323071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zaiss MM, Joyce Wu H-J, Mauro D, et al. The gut-joint axis in rheumatoid arthritis. Nat Rev Rheumatol 2021;17:224–37. 10.1038/s41584-021-00585-3 [DOI] [PubMed] [Google Scholar]
- 3. Pedersen HK, Forslund SK, Gudmundsdottir V, et al. A computational framework to integrate high-throughput '-omics' datasets for the identification of potential mechanistic links. Nat Protoc 2018;13:2781–800. 10.1038/s41596-018-0064-z [DOI] [PubMed] [Google Scholar]
- 4. Nielsen HB, Almeida M, Juncker AS, et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat Biotechnol 2014;32:822–8. 10.1038/nbt.2939 [DOI] [PubMed] [Google Scholar]
- 5. Pattison DJ, Silman AJ, Goodson NJ, et al. Vitamin C and the risk of developing inflammatory polyarthritis: prospective nested case-control study. Ann Rheum Dis 2004;63:843–7. 10.1136/ard.2003.016097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carr AC, Maggini S. Vitamin C and immune function. Nutrients 2017;9:1211. 10.3390/nu9111211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abrams E, Sandson J. Effect of ascorbic acid on rheumatoid synovial fluid. Ann Rheum Dis 1964;23:295–9. 10.1136/ard.23.4.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frommer KW, Schäffler A, Rehart S, et al. Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Ann Rheum Dis 2015;74:303–10. 10.1136/annrheumdis-2013-203755 [DOI] [PubMed] [Google Scholar]
- 9. Yew WS, Gerlt JA. Utilization of L-ascorbate by Escherichia coli K-12: assignments of functions to products of the yjf-sga and yia-sgb operons. J Bacteriol 2002;184:302–6. 10.1128/JB.184.1.302-306.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aletaha D, Neogi T, Silman AJ. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;2010:2569–81. [DOI] [PubMed] [Google Scholar]
- 11. Schett G, Manger B, Simon D, et al. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol 2020;16:465–70. 10.1038/s41584-020-0451-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-325209supp001.pdf (244.6KB, pdf)
gutjnl-2021-325209supp002.xlsx (36.4KB, xlsx)
gutjnl-2021-325209supp003.xlsx (13KB, xlsx)