Summary
Background
Vulnerable dialysis and kidney transplant patients show impaired seroconversion rates compared to medical personnel eight weeks after SARS-CoV-2mRNA vaccination.
Methods
We evaluated six months follow up data in our observational Dia-Vacc study exploring specific cellular (interferon-γ release assay) or/and humoral immune responses after 2x SARS-CoV-2mRNA vaccination in 1205 participants including medical personnel (125 MP), dialysis patients (970 DP) and kidney transplant recipients (110 KTR) with seroconversion (de novo IgA or IgG antibody positivity by ELISA) after eight weeks.
Findings
Six months after vaccination, seroconversion remained positive in 98% of MP, but 91%/87% of DP/KTR (p = 0·005), respectively. Receptor binding domain-IgG (RBD-IgG) antibodies were positive in 98% of MP, but only 68%/57% of DP/KTR (p < 0·001), respectively. Compared to MP, DP and KTR were at risk for a strong IgG or RBD-IgG decline (p < 0·001). Within the DP but not KTR group male gender, peritoneal dialysis, short time on dialysis, BNT162b2mRNA vaccine, immunosuppressive drug use and diabetes mellitus were independent risk factors for a strong decline of IgG or RBD antibodies. The percentage of cellular immunity decline was similar in all groups.
Interpretation
Both vulnerable DP and KTR groups are at risk for a strong decline for IgG and RBD antibodies. In KTR, antibody titres peak at a markedly lower level and accelerated antibody decline is mixed with a delayed/increasing IgG, RBD-IgG, or cellular immune response in a 16% fraction of patients. In both populations, immune monitoring should be used for early timing of additional booster vaccinations.
Funding
This study was funded by the Else Kröner Fresenius Stiftung, Bad Homburg v. d. H., grant number Fördervertrag EKFS 2021_EKSE.27.
Keywords: SARS-CoV-2mRNA vaccination, Antibody fading, Medical personnel, Dialysis patients, Kidney transplant recipients
Research in context.
Evidence before this study
The vulnerable dialysis patients (DP) and kidney transplant recipients (KTR) experience a markedly increased COVID-19 disease related mortality rate compared to normal population. The most powerful mRNA vaccines BNT162b2mRNA or mRNA-1273 reach seroconversion and efficacy rates of about 95% in the general population. Immunocompromised DP unexpectedly showed vaccine-related seroconversion rates close to general population, but successful seroconversion in immunosuppressed KTR varies between 30% and 50% only. Even in successfully vaccinated persons, breakthrough COVID-19 infections and disease are possible and apparently play a substantial role in the pandemia. While COVID-19 breakthrough infections may be dependent on vaccination-related immune response quality and quantity, little information is known regarding humoral and/or cellular immunity fading in risk populations such as DP and KTR. To assess the availability of the data, results from database source National Library of Medicine(https://pubmed.ncbi.nlm.nih.gov) have been searched. For our search, following terms have been used: COVID-19, vaccination, SARS-CoV-2, BNT162b2, mRNA-1273, immunosuppression, antibody, humoral, T cells.
Added value of this study
Six months follow up data of our prospective Dia-Vacc study exploring specific cellular (interferon-γ release assay) and humoral immune responses after SARS-CoV-2mRNA boost vaccination in 1205 participants including medical personnel (MP, 125), dialysis patients (970) and kidney transplant recipients (110) with successful de novo seroconversion after eight weeks were studied. Six months after vaccination start, seroconversion remained positive in 98% of MP and 91%/87% of DP/KTR, respectively. Receptor binding domain (RBD-IgG-IgG) antibodies were positive in 98% of MP, but only 68%/57% of DP/KTR, respectively. Using 20% as a margin, only 41%/24% of MP but 68%/68% of DP, and 57%/55% of KTR showed decreased anti S1 IgG or RBD-IgG-IgG antibody titres between two and six months, respectively. 0%/1% of MP/KTR but 12-16% of transplant recipients showed IgG or RBD-IgG-IgG antibody increases up to six months. Similar results were found for the time course of cellular immunity measurements by the interferon-gamma release assay. DP and KTR were at high risk for a strong IgG or RBD-IgG-IgG decline. Within the DP but not KTR group male gender, peritoneal dialysis, BNT162b2mRNA vaccine, short time on dialysis, immunosuppressive drug use and diabetes mellitus were independent risk factors for a strong decline of IgG or RBD-IgG antibodies. Nevertheless, in all three study groups IgG and RBD-IgG antibody titres at six months were higher in patients immunized with 2x mRNA-1273 compared to 2x BNT162b2mRNA. The positive effect of individual monitor guided early reboostering following a weak seroconversion response could be demonstrated in our KTRboost group (n = 20), where almost all patients with extremely low titre seroconversion reacted with a marked antibody increase (instead of a decline) in both IgG and RBD-IgG-IgG antibodies up to six months.
Implications of all the available evidence
After successful SARS-CoV-2 mRNA vaccination, DP and KTR compared to MP are at specific risk for a strong antibody decline. In DP but not KTR, this humoral response is accelerated by male gender, diabetes mellitus as comorbidity, peritoneal dialysis, short time on dialysis, immunosuppressive drug use, and BNT162b2mRNA compared to mRNA-1273. In contrast, despite a delayed immune response with rising levels in a 16% patient fraction, successfully vaccinated KTR reach on average immunity levels markedly below MP and DP throughout the monitoring period of six months after first mRNA vaccination, but can be successfully boostered by a third vaccination when titres are quite low. This data indicate the value of immune monitoring especially in high-risk populations such as DP and KTR to define the best strategy and (early) timing of additional booster vaccinations even after positive seroconversion.
Alt-text: Unlabelled box
Introduction
COVID-19 continues to pose an unprecedented challenge to global public health. Immunity conferred by vaccination can limit breakout of any infection and is an efficacious way to fight COVID-19 pandemic. Successful vaccination is in particular critical for high risk populations such as dialysis patients (DP) and kidney transplant recipients (KTR) experiencing COVID-19 disease related increased mortality rates compared to normal population.1 The mRNA vaccines BNT162b2mRNA (Pfizer/BioNTech) or mRNA-1273 (Moderna) reach seroconversion and efficacy rates of about 95% in the general population. Immunocompromised DP unexpectedly showed vaccine-related seroconversion rates close to general population, but successful seroconversion in immunosuppressed KTR varies between 30% and 50% only.2, 3, 4, 5
Even in successfully vaccinated persons, breakthrough COVID-19 infections and disease are possible and apparently play a substantial role in the pandemic. While COVID-19 breakthrough infections may be dependent on vaccination-related immune response quality and quantity,6 little information is known regarding immunity fading in risk populations such as DP and KTR. We hypothesized that DP and KTR after seroconversion are at risk for a strong immunity decline compared to medical personnel.
The multicentre, investigator-driven, prospective observational Dia-Vacc study investigates the SARS-CoV-2-specific humoral as well as cellular immune response in DP and KTR and medical personnel at defined intervals after appropriate vaccination using 2x BNT162b2mRNA or 2x mRNA-1273 as basic vaccination.4 Here, we report the time course of cellular and/or humoral immunity measures in seroconverted study participants up to 6 months after vaccination.
Methods
Study design
The investigator-driven, multicentre, non-interventional, prospective, observational Dia-Vacc study (NCT number: 04799808) started with SARS-CoV-2 vaccination using either 2x BNT162b2mRNA or 2 × 1273-mRNA in 26 nephrology centres from January 15th to February 24th, exploring the time course of a specific cellular or/and humoral immune response to disease and/or SARS-CoV-2 vaccination in MP, DP, and KTR.4 Of all 36 dialysis centres in Saxony, being incorporated in a COVID-19 network since march 2020 as described,4 the first 26 committing dialysis centres providing 3101 study participants were accepted for the DIA-Vacc study. Later requests could not be considered due to funding restrictions. Study start (T0) was immediately before first vaccination. Further monitoring time points were three (BNT162b2mRNA) to four (mRNA-1273) weeks later before second vaccination (T1), about eight weeks after study start (T2; five to four weeks after the second vaccination respectively) and six months after study start (T3). By vaccine availability during January (BNT162b2mRNA) and February (mRNA-1273) 2021 only the first four dialysis centres being assigned to the vaccination campaign, received BNT162b2mRNA, while all other following dialysis centres received mRNA-1273 vaccine for both vaccinations. Neither any dialysis centre nor any participant nor the study centre (Dresden) had a choice or influence regarding the type of vaccine, which was assigned in the order of contacting the central vaccination institute in Saxony. Information to all dialysis centres about the start of the vaccination campaign was distributed by the central vaccination institute via email at the same time.4
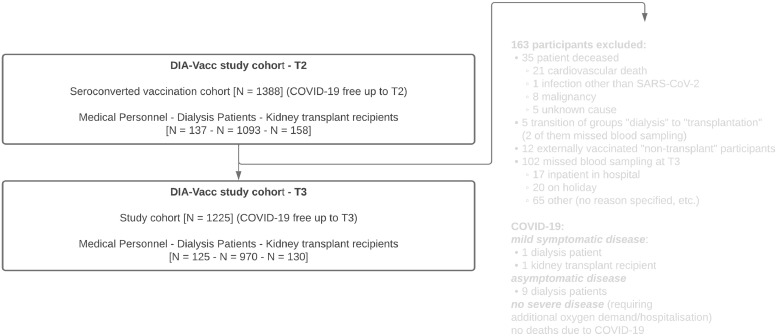
For the six months (T3) study results reported here, the “pure vaccination cohort” with a positive seroconversion eight weeks (T2) (as defined by de novo IgA or IgG antibody positivity by ELISA) after 2 x vaccination were followed up to six months after vaccination start without any additional boostering in a total of 1205 participants including 125 MP, 970 DP, and 110 KTR. In addition, a subgroup of 20 seroconverted (at T2) KTR was evaluated, in which by individual decision an additional mRNA vaccine booster was given between T2 and T3 (KTRboost). For clinical outcome assessment all PCR-positive, symptomatically and asymptomatically (NCP seroconversion) COVID-19 diseased participants between T2 and T3 were counted. On the basis of immunity monitoring, all symptomatically and asymptomatically (NCP seroconversion) COVID-19 diseased participants before, during and after vaccination (up to T3) were excluded to assess a purely vaccination-related immune response. Patients were tested for SARS-CoV-2 infection by RT-PCR, in the dialysis centres, if they presented one of the classic symptoms (fever, cough, shortness of breath, myalgias, diarrhea, or other symptoms consistent with such an infection) or if they were in contact with a person with RT-PCR-confirmed disease. Routine PCR screening without a cause was not part of good medical practice of the dialysis centres.
In all study participants (eligibility if > 18 years old and signed informed consent) at T0 (vaccination start), T2, and T3, SARS-CoV-2 specific IgG- or IgA-antibody reactions (Euroimmun7, 8, 9, 10) against the Spike protein subunit S1 and IgG-antibodies against the nucleocapsid protein subunit (NCP) were analysed. In addition, the receptor binding domain (RBD-IgG) antibody formation suggesting neutralising activity against the SARS-CoV-2 virus was also examined at T2 and T3.11 For all antibody measurements Euroimmun ELISAs on Euroimmun analysers were used.
To provide characterisation of the cellular SARS-CoV-2 immune response, a SARS-CoV-2 specific interferon-γ release assay (IGRA12) was performed at T0, T2, and T3 in representative subgroups.4 Further details on procedures and analysis are found elsewhere.4
Ethic declaration
According to the professional code of conduct for doctors (§15) the clinical study was submitted to the ethical institutional review boards at Technische Universität Dresden (TU Dresden) responsible for the coordinating investigator (BO-EK-45012021), as well as at the University of Leipzig (046/21-lk) and Saxon Medical Association (Sächsische Landesärztekammer – EK-BR-10/21-1) responsible for further participating study sites.
End points
The primary end point is the positive humoral immune response after vaccination as defined by de novo positivity of either IgG- or IgA- anti-SpikeS1 antibodies without development of virus-specific NCP antibodies. Secondary end points were the development of vaccination-induced de novo T-cellular immunity, the clinical outcome (COVID-19 disease), as well as serological and cellular immune response parameters and titres.
To investigate the time course (T2 to T3) of established vaccination – related de novo immunity reaction for the different tests, a 20% margin for (increased/equal/decreased) antibody and IGRA titre/value development was used and the percentage of patients within each margin was calculated for each group and each time point.
In addition, the time course was analysed on the interval scale. The detectable ranges of anti-S1-IgG and RBD-IgG antibody values were categorised into five intervals, labeled from 0 to 4 (referred to as “levels” in the data analysis), and the change in levels, varying from -4 to +4, was calculated for each patient. Patients whose level decreased from T2 to T3 by more than one (or ≥ two) unit were defined as a “strong decline” (Supplementary methods, S1 Figure).
Statistical analysis
In the descriptive analysis of main study endpoints, categorical variables were summarised as absolute frequencies or percentages, and continuous variables were summarised using mean and standard deviation or median and interquartile range (IQR). Time trends in IgG and RBD-IgG responses as well as between-group differences were analysed either by the Wilcoxon signed-rank test, Mann-Whitney U test, or the chi-squared test, as appropriate. The analysis of risk factors of patients with a strong antibody decline was carried out using multiple logistic regression. First, we fitted a logistic regression model to each study group separately. Because, as was observed in a number of studies,13,14 a substantial difference in seroconversion response may occur after administering different vaccines, in each logistic regression model, we included the vaccine type as a risk factor of a strong antibody decline. Other potential risk factors, common to all study groups, were gender, age, and body mass index (BMI).2 The age distribution differed considerably between study groups (see Table 1), and, therefore, in order to reduce a possible confounding effect, adjusting for age in all models was especially important. While no additional risk factors were considered for the MP group, the models for DP and KTR contained the effects of immunosuppression, hepatitis B vaccination failure, diabetes mellitus diagnosis, as well as group-specific effects: time on transplantation (KTR) and time on dialysis (DP). Because the immunosuppression is prevalent in KTR, with many patients taking several IS medications, the number of IS drugs was used as a covariate in the logistic-regression model for KTR. On the other hand, the IS prevalence in DP is relatively low, and, therefore, only an indicator of presence of IS was included in the logistic regression for DP. In order to estimate the group effect, we fitted a logistic regression model for all groups jointly, where only common risk factors were included. To investigate the effect of dialysis type on strong decline rates, we carried out a propensity score matching procedure for PD and HD patients (1:4 ratio, without resampling, logistic regression scores), based on the primary vaccine type and antibody values (IgG or RBD for the corresponding analyses) at T2. Also notable is a comparison between boostered and unboostered KTR was also implemented via propensity score matching (1:1 ratio, without resampling, logistic regression scores, based on IgG value at T2), see Table 6. For hypothesis testing, the significance level of 5% (two-sided) was chosen. A Bonferroni correction was applied during posthoc testing of group effects.
Table 1.
Baseline characteristics of SARS-CoV-2 unexposed, but at T2 seroconverted persons / patients of the DIA-Vacc pure vaccination cohort.
| Variable | Category | MP | DP | KTR |
|---|---|---|---|---|
| Number | evaluable | 125 | 970 | 110 |
| Age (years) | mean ± SD | 47·8 ± 11·7 | 67·5 ± 13·9 | 57·6 ± 13 |
| Male Sex | n / % | 28 / 22·4 | 636 / 65·6 | 69 / 62·7 |
| BMI (kg/m2) | mean ± SD | 26·1 ± 5·1 | 27·8 ± 5·8 | 26·5 ± 4·7 |
| Cause of end stage renal disease | n / % | n.a. | 797 / 82·2 | 70 / 63·6 |
| Diabetes-Hypertension-Vascular disease | n / % | n.a. | 481 / 49·6 | 19 / 17·3 |
| Glomerulonephritis-Interstitial nephritis | n / % | n.a. | 206 / 21·2 | 27 / 24·5 |
| Vasculitis | n / % | n.a. | 24 / 2·5 | 2 / 1·8 |
| Polycystic kidney disease | n / % | n.a. | 86 / 8·9 | 22 / 20 |
| Unknown | n / % | n.a. | 173 / 17·8 | 40 / 36·4 |
| Drug treated comorbidities | n / % | 29 / 23·2 | 933 / 96·2 | 95 / 86·4 |
| Diabetes mellitus | n / % | 4 / 3·2 | 348 / 35·9 | 24 / 21·8 |
| Cardiovascular disease | n / % | 23 / 18·4 | 903 / 93·1 | 90 / 81·8 |
| Lung disease | n / % | 5 / 4 | 58 / 6 | 6 / 5·5 |
| Liver cirrhosis | n / % | 0 / 0 | 13 / 1·3 | 1 / 0·9 |
| Cancer | n / % | 0 / 0 | 38 / 3·9 | 5 / 4·5 |
| None | n / % | 96 / 76·8 | 37 / 3·8 | 15 / 13·6 |
| Type of dialysis | n.a. | 970 / 100 | n.a. | |
| Hemodialysis | n / % | n.a. | 929 / 95·8 | n.a. |
| Peritonealdialysis | n / % | n.a. | 41 / 4·2 | n.a. |
| Time on dialysis (years) | mean ± SD | n.a. | 5·8 ± 5·8 | 5·9 ± 5·9 |
| On transplant waiting list | n / % | n.a. | 118 / 12·2 | n.a. |
| Time on transplantation (years) | mean ± SD | n.a. | n.a. | 11·7 ± 7·8 |
| Previous transplantation | n / % | n.a. | 68 / 7 | 19 / 17·3 |
| Hepatitis B vaccination failure | n / % | 2 / 1·6 | 202 / 20·8 | 12 / 10·9 |
| Flu vaccination winter 2020/21 | n / % | 74 / 59·2 | 713 / 73·5 | 58 / 52· |
| On immunosuppressive therapy | n / % | 1 / 0·8 | 34 / 3·5 | 109 / 99·1 |
| Corticosteroids | n / % | 0 / 0 | 24 / 2·5 | 47 / 42·7 |
| Calcineurin-Inhibitor | n / % | 0 / 0 | 8 / 0·8 | 95 / 86·4 |
| MMF/MPA | n / % | 0 / 0 | 4 / 0·4 | 62 / 56·4 |
| mTOR-Inhibitor | n / % | 0 / 0 | 1 / 0·1 | 23 / 20·9 |
| Belatacept | n / % | 0 / 0 | 1 / 0·1 | 3 / 2·7 |
| T-cell depleting ab | n / % | 0 / 0 | 0 / 0 | 0 / 0 |
| B-cell depleting ab | n / % | 0 / 0 | 0 / 0 | 0 / 0 |
| Other | n / % | 1 / 0·8 | 3 / 0·3 | 4 / 3·6 |
| Type of vaccine | ||||
| BNT162b2 mRNA | n / % | 36 / 28·8 | 151 / 15·6 | 29 / 26·4 |
| mRNA-1273 | n / % | 89 / 71·2 | 819 / 84·4 | 81 / 73·6 |
For this evaluation all patients with asymptomatic* or documented symptomatic** COVID-19 disease before and during vaccination up to T3 (six months) were excluded. Hepatitis B vaccination failure definition - patients with unsuccessful vaccination after at least four attempts; MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; MMF-MPA = mycophenolate mofetil or mycophenolic acid;
*Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T1, T2, or T3) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive.
**Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
Table 6.
Antibody response in matched KTR cohorts.
| variable | time | KTRboost | Matched KTR (unboostered) | p-value |
|---|---|---|---|---|
| IgA-Ab Spike S1 | T2 | 1·8 (1·1–3·0) | 1·8 (0·8–3·8) | 0·766 |
| IgA-Ab Spike S1 | T3 | 2 (1·6–6) | 1·0 (0·5–2·8) | 0·019 |
| IgG-Ab Spike S1 | T2 | 13·7 (4·1–22·3) | 41·6 (6·3–61·7) | 0·022 |
| IgG-Ab Spike S1 | T3 | 290·4 (75·4–384) | 51·8 (17·2–124·6) | 0·017 |
| RBD-IgG | T2 | 8·1 (4·7–12·9) | 19·0 (16·9–28·6) | < 0·001 |
| RBD-IgG | T3 | 89·3 (28·7–98·6) | 17·8 (7·1–43·5) | 0·004 |
The KTRboost group (n = 20) is defined as a very low seroconversion group with values at T2 (eight weeks) with either IgA positivity and no IgG positivity or IgG/IgA positivity and low/negative levels of RBD-IgG antibody values stimulating the individual decision for an additional boostering between T2 and T3 (six months). Propensity scores were used to match the KTRboost group with unboostered KTR (1:1 ratio). Vaccination-related antibody titre levels were compared in both groups at seroconversion (T2) and after six months (T3).
To check the robustness of our main results against missing data, we applied a multiple imputation procedure, namely, multiple imputation with chained equations (MICE),15 to impute IgG antibody values of 102 patients (Figure 1) who missed blood sampling at T3. For each cohort separately and for all participants pulled together, we generated ten data sets of the corresponding cohort size, that is, 40 data sets in total. The variables that were considered as risk factors for antibody decline, see Tables 4A–4D were used for imputation of missing IgG values at T3. Table S1 displays the proportions of patients by cohort whose IgG values remained at the same level/decreased/ increased with respect to 20% margin. We then proceeded with categorization of IgG values according to our interval scale and identified patients who experienced a strong decline in IgG. After that, to each imputed data set we applied exactly the same multiple logistic regression model as the one used for the complete data in the corresponding analysis cohort (MP, DP, KTR, all patients together). For each cohort, the ten effect estimates for each risk factor were averaged on the log scale and then converted to odds ratios. The resulting estimates, with a 95% confidence interval, are provided along with the summary obtained from complete data, see Tables 4A–4D. Antibody values for patients who died between T2 and T3 time points were not included in imputations, because within our study scope, it is not possible to estimate a confounding effect of a particular death reason, while ignoring such an effect could be a potential source of bias.
Figure 1.
Study flow chart.
Table 4A.
Multiple logistic regression analysis of a strong decline of vaccination-specific anti-S1 IgG antibodies in seroconverted participants of the DIA-Vacc pure vaccination cohort between T2 and T3.
Table 4A. Strong IgG decline for all participants (n = 1205).
| Risk factor | OR | 95% CI | p-value | OR, 95%CI (MICE) |
|---|---|---|---|---|
| Age | 1·007 | [0·998,1·016] | 0·142 | 1.008[1.007,1.009] |
| Sex (Ref. = female) | 1·412 | [1·095,1·820] | 0·008 | 1.421[1.383,1.460] |
| Vaccine type (Ref. = mRNA-1273) | 2·546 | [1·855,3·496] | <0·001 | 2.535[2.483,2.587] |
| DP (Ref. = MP) | 3·237 | [1·897,5·525] | <0·001 | 2.953[2.766,3.153] |
| KTR (Ref. = MP) | 1·926 | [1·018,3·644] | 0·044 | 1.702[1.615,1.793] |
| Diabetes mellitus | 1·304 | [1·005,1·691] | 0·045 | 1.289[1.268,1.310] |
MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; Ref. = reference category; a “strong IgG strong fading response antibody decline” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. Comparator is the MP cohort. MICE means multiple imputation with chained equations.
Model fit: AIC = 1556.70; BIC = 1592.35.
Table 4D.
Strong IgG antibody decline for kidney transplant recipients (n = 110).
| Risk factor | OR | 95% CI | p-value | OR, 95%CI (MICE) |
|---|---|---|---|---|
| Age | 1·038 | [0·997,1·080] | 0·069 | 1.034[1.027,1.041] |
| Sex (Ref. = female) | 1·008 | [0·403,2·523] | 0·987 | 0.882[0.815,0.955] |
| Time after transplantation | 0·987 | [0·925,1·052] | 0·686 | 1.000[0.994,1.007] |
| Vaccine type (Ref. = mRNA-1273) | 1·556 | [0·585,4·133] | 0·376 | 1.361[1.247,1.485] |
| BMI | 1·049 | [0·954,1·153] | 0·327 | 1.019[1.004,1.035] |
| IS drugs number | 0·988 | [0·505,1·933] | 0·972 | 0.853[0.801,0.909] |
| Diabetes mellitus | 0·630 | [0·221,1·797] | 0·388 | 0.592[0.561,0.625] |
Ref. = reference category; a “strong IgG antibody declinestrong fading response” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. IS means immunosuppressive drug; BMI means body mass index. MICE means multiple imputation with chained equations.
Model fit: AIC = 144.18; BIC = 168.06
Data analysis was implemented in the R Environment for Statistical Computing,16 version 4.0.4. R script can be provided upon request.
Results
Basic study cohort characteristics (Table 1)
Of more than 3100 original study participants, a total of 1225 participants fulfilled all “pure vaccination cohort” requirements combined with a positive de novo seroconversion at eight weeks, of whom 125 MP, 970 DP, 110 KTR, and 20 KTRboost were monitored up to six months after vaccination. A study flow chart indicates very few group changes and reasons for patient exclusions between T2 and T3 (Figure 1). Patient characteristics of the three large subgroups are shown at Table 1. For the following results it needs to be considered that de novo seroconversion definition at T2 included either IgA or IgG positivity and also a different proportion of RBD-IgG/IGRA positivity within the groups (100%/86% of MP, 95%/78% of DP but only 67%/30% of KTR), respectively.4 IgA antibody levels are calculated by a ratio and only represent semiquantitative values. The results of subgroup KTRboost are summarised separately.
Study end points
Immune response rates six months after vaccination (T3) in the seroconverted pure vaccination cohort (Table 2)
Table 2.
Immune response rates six months after vaccination (T3) in the seroconverted pure vaccination cohort.
| Variable | Category | Medical personnel | Dialysis patients | Kidney transplant recipients | p-value(chi-squared test) |
|---|---|---|---|---|---|
| Patient number | n | 125 | 970 | 110 | |
| Humoral responses | n of total n (%) | ||||
| IgG-Ab or IgA-Ab Spike S1 positive | n of total n (%) | 123 / 125 (98·4%) | 879 / 970 (90·6%) | 96 / 110 (87·3%) | 0·005 |
| IgA-Ab Spike S1 positive | n of total n (%) | 96 / 125 (76·8%) | 537 / 938 (57·2%) | 65 / 110 (59·1%) | < 0·001 |
| IgA-Ab Spike S1 increasing | n of total n (%) | 1 / 125 (0·8%) | 20 / 970 (2·1%) | 4 / 110 (3·6%) | 0·313 |
| IgA-Ab Spike S1 equal | n of total n (%) | 2 / 125 (1·6%) | 11 / 970 (1·1%) | 2 / 110 (1·8%) | 0·771 |
| IgA-Ab Spike S1 decreasing | n of total n (%) | 122 / 125 (97·6%) | 939 / 970 (96·8%) | 104 / 110 (94·5%) | 0·379 |
| De novo IgA-Ab positivity (T2 negative, T3 positive) | n of total n (%) | 0 / 125 (0%) | 5 / 970 (0·5%) | 2 / 110 (1·8%) | 0·156 |
| IgG-Ab Spike S1 positive | n of total n (%) | 123 / 125 (98·4%) | 865 / 970 (89·2%) | 92 / 110 (83·6%) | 0·001 |
| IgG-Ab Spike S1 increasing | n of total n (%) | 0 / 125 (0%) | 13 / 970 (1·3%) | 17 / 110 (15·5%) | < 0·001 |
| IgG-Ab Spike S1 equal | n of total n (%) | 74 / 125 (59·2%) | 297 / 970 (30·6%) | 30 / 110 (27·3%) | < 0·001 |
| IgG-Ab Spike S1 decreasing | n of total n (%) | 51 / 125 (40·8%) | 660 / 970 (68%) | 63 / 110 (57·3%) | < 0·001 |
| De novo IgG-Ab positivity (T2 negative, T3 positive) | n of total n (%) | 0 / 125 (0%) | 1 / 970 (0·1%) | 3 / 110 (2·7%) | < 0·001 |
| RBD positive | n of total n (%) | 122 / 125 (97·6%) | 656 / 970 (67·6%) | 63 / 110 (57·3%) | < 0·001 |
| RBD increasing | n of total n (%) | 0 / 116 (0%) | 7 / 908 (0·8%) | 12 / 104 (11·5%) | < 0·001 |
| RBD equal | n of total n (%) | 88 / 116 (75·9%) | 282 / 908 (31·1%) | 35 / 104 (33·7%) | < 0·001 |
| RBD decreasing | n of total n (%) | 28 / 116 (24·1%) | 619 / 908 (68·2%) | 57 / 104 (54·8%) | < 0·001 |
| De novo RBD positive (T2 negative, T3 positive) |
n of total n (%) | 0 / 116 (0%) | 3 / 908 (0·3%) | 6 / 104 (5·8%) | < 0·001 |
| Interferon-γ release assay (IGRA)– T-cellular response | |||||
| IGRA positive | n of total n (%) | 22 / 28 (78·6%) | 72 / 116 (62·1%) | 15 / 41 (36·6%) | 0·001 |
| IGRA increasing | n of total n (%) | 1 / 29 (3·4%) | 7 / 102 (6·9%) | 12 / 34 (35·3%) | < 0·001 |
| IGRA equal | n of total n (%) | 10 / 29 (34·5 %) | 33 / 102 (32·4%%) | 1 / 34 (2·9%) | 0·002 |
| IGRA decreasing | n of total n (%) | 18 / 29 (62·1%) | 62 / 102 (60·8%) | 21 / 34 (61·8%) | 0·989 |
| De novo positivity for IGRA | n of total n (%) | 0 / 29 (0%) | 2 / 102 (2%) | 2 / 34 (5·9%) | 0·282 |
MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; Interferon-γ release assay = IGRA;
Humoral vaccination responses were assessed as positive, when de novo production of the antibody to the Spike S1 (IgA or IgG) protein or RBD (IgG) subunit was above positivity level. A positive T-cellular response to vaccination as assessed by interferon-γ release assay (IGRA) turned from a negative result on T0 to positive on T3, respectively (≥100 mIU/ml, as being recommended by the manufactures). Using 20% as a margin, the time course of antibody or IGRA titres at T3 compared to T2 time point were categorized into increased (> 20%), equal (within 20% range), and decreased (< 20%). For this evaluation, all participants with asymptomatic* or documented symptomatic** COVID-19 disease before and during vaccination up to T3 (six months) were excluded.
*Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T2 or T3) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive.
**Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
At the six month time point, seroconversion of IgG or IgA anti-S1 antibodies as our primary study endpoint remained positive in 98% of MP, 91% of DP, and 87% of KTR (p = 0·006), respectively (Table 2). Hereby, the IgG antibody positivity was with 98% of MP, 89% of DP, and 84% of KTR the predominant denominator of a sustained seroconversion reaction up to month 6 (Table 2). In contrast, only 77% of MP, 57%% of DP, and 59% of KTR still showed antibody positivity for anti-S1 IgA antibodies at this time point (p < 0·001). RBD-IgG antibodies stayed positive in 98% of MP, but only in 68%/57% of DP/KTR (p < 0·001), respectively. T-cellular immunity to vaccination as indicated by a positive IGRA response was seen in 79% of MP, 62% in DP, and 37% of KTR at the six month time point (p < 0·001) being comparable to rates at two months.
Using 20% as a margin, only 41%/24% of MP but 68%/68% of DP, and 57%/55% of KTR showed decreased anti S1 IgG or RBD-IgG antibody titres between two and six months, respectively (p < 0·001). 0%/1% of MP/DP but 12-16% of transplant recipients showed IgG or RBD-IgG antibody increases up to six months (p < 0·001). This delayed antibody response is also reflected by 6% of seroconverted KTR, who are characterised by a de novo RBD-IgG positivity between two and six months, respectively. Therefore, 59%/76% of MP, but only 31%/31% of DP and 27%/34% of KTR remained at an equal level of IgG or RBD-IgG antibodies against S1 protein between month two and six, respectively (p < 0·001). IgA anti S1 protein antibody ratios showed a stronger decline than IgG/RBD-IgG, since 98% of MP, 97% of DP and 95% of KTR demonstrated decreased values at six compared to two months. Increased or de novo IgA antibody ratios between two and six months were rare events in all three groups.
Cellular immunity monitoring via IGRA measurements showed similar rates of decreasing titres around 60% in all groups. Substantial rates (35%) of IGRA titre increases were solely seen in KTR up to six months indicating the delayed response of KTR also in regard to T-cell immunity (Table 2). Only up to 6% of KTR showed de novo positivity for IGRA measurements at six months.
The time course of anti-SpikeS1 protein IgG or RBD-IgG antibody titres of all seroconverted Dia-Vacc study participants differs between the study groups
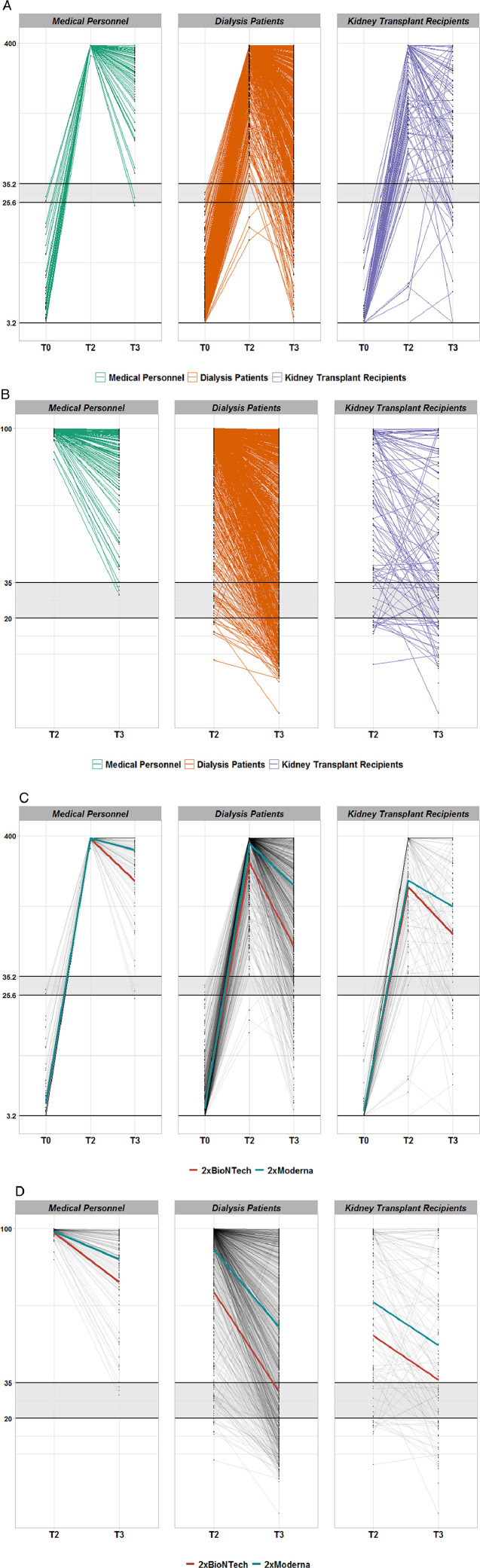
Table 3A summarises the average titre levels at two and six months (median ± interquartile range) within the different groups demonstrating largely different antibody and IGRA titres already at T2 but also different degrees of immunity fading up to T3. The higher percentage of anti-S1 IgG antibody levels below the positivity limit of 35·2 BAU/ml in the DP and KTR groups compared to the MP group can be appreciated in Figure 2A. Solely in the KTR group, antibody decline is mixed with IgG titre increases or some de novo positivity in up to 16% of patients between T2 and T3 (Figure 2A). A very similar pattern can be observed for the time course of vaccination-related RBD-IgG antibodies in the different study groups (Figure 2B).
Table 3A.
Antibody and IGRA titres two (T2) and six months (T3) after vaccination in the seroconverted pure vaccination cohort.
| Variable | Group | Category | Two months (T2) | Six months (T3) | p-value(Wilcoxon test) |
|---|---|---|---|---|---|
| Humoral responses | |||||
| IgA-Ab Spike S1 | MP | Median (interquartile range) | 9 (5·8–9) | 3·31 (1·5–5·9) | < 0·001 |
| IgA-Ab Spike S1 | DP | Median (interquartile range) | 7·87 (4·0–9) | 1·84 (0·8–4·3) | < 0·001 |
| IgA-Ab Spike S1 | KTR | Median (interquartile range) | 4·6 (2–9) | 1·8 (0·8–4·1) | < 0·001 |
| IgG-Ab Spike S1 | MP | Median (interquartile range) | 384 (384–384) | 351·1 (239–384) | < 0·001 |
| IgG-Ab Spike S1 | DP | Median (interquartile range) | 384 (384–384) | 192·9 (86–356) | < 0·001 |
| IgG-Ab Spike S1 | KTR | Median (interquartile range) | 329·8 (110·7–384) | 121·1 (51·8–295·6) | < 0·001 |
| RBD | MP | Median (interquartile range) | 99·33 (98·7–99·6) | 89 (79·2–95·9) | < 0·001 |
| RBD | DP | Median (interquartile range) | 98·1 (91·2–99·4) | 57·7 (24·8–84·9) | < 0·001 |
| RBD | KTR | Median (interquartile range) | 69·6 (38·3–95·5) | 40·1 (18·7–71·9) | < 0·001 |
| IGRA– T-cellular response | |||||
| IGRA | MP | Median (interquartile range) | 2282·1 (874·6–2487·7) | 554·6 (307·2–2461·5) | < 0·001 |
| IGRA | DP | Median (interquartile range) | 888·7 (270·4–2479) | 335·0 (79·5–1161·4) | < 0·001 |
| IGRA | KTR | Median (interquartile range) | 113·3 (14·3–326·4) | 48·0 (15·9–176·1) | 0·055 |
MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; Interferon-γ release assay = IGRA;
This table compares the average titre levels (median/interquartile range) on T3 with T2 (different columns) for the different anti-Spike S1 IgA, IgG, RBD-IgG antibodies as well as for cellular immunity via IGRA measurements. For this evaluation, all participants with asymptomatic* or documented symptomatic** COVID-19 disease before and during vaccination up to T3 (six months) were excluded.
*Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T2 or T3) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive.
**Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
Figure 2.
Time course of anti-SARS-CoV-2 IgG (A/C) or receptor binding domain (B/D) antibodies in seroconverted Dia-Vacc study participants. Figure 2A: IgG against S1 protein in different study groups. Each thin line corresponds the anti-Spike S1 protein IgG antibody values (QuantiVac, Euroimmun) of a study participant from T0 (vaccination start) via T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. All three patient groups are represented (MP-green, DP-red, KTR-blue). The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit BAU/ml. Figure 2B: Receptor binding domain antibodies against S1 protein in different study groups. Each thin line corresponds the anti-Spike S1 protein RBD-IgG antibody values (Euroimmun) of a study participant from T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. All three patient groups are represented (MP-green, DP-red, KTR-blue). The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit % inhibition. Figure 2C: IgG against S1 protein dependent on vaccine type. Each thin line corresponds the anti-Spike S1 protein IgG antibody values (QuantiVac, Euroimmun) of a study participant from T0 (vaccination start) via T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). The thicker lines represent average responses by vaccine type (red-BNT162b2mRNA and blue 1273-mRNA) in each group. Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit BAU/ml. Figure 2D: Receptor binding domain antibody against S1 protein dependent on vaccine type. Each thin line corresponds the anti-Spike S1 protein RBD-IgG antibody values (Euroimmun) of a study participant from T0 (vaccination start) via T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). The thicker lines represent average responses by vaccine type (red-BNT162b2mRNA and blue 1273-mRNA) in each group. Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit % inhibition.
Since the primary seroconversion rates in DP and KTR were markedly vaccine dependent, Figure 2 C/D as well as Table 3B shows averaged titre levels of IgG/RBD-IgG antibodies at two and six months separated by both mRNA vaccines, respectively. In MP, the average IgG levels were similar for both vaccines at two months, but remained higher at six months for mRNA-1273 compared with BNT162b2mRNA (Figure 2C, Table 3B). In DP but not KTR (only a trend) groups, mean IgG titres were higher for mRNA-1273 than for BNT162b2mRNA at two and remained even more noticeably different at six months (Figure 2C, Table 3B). A very similar vaccine-dependent pattern in regard to both levels and time courses was seen for RBD-IgG or IgA antibodies in each study group (Figure 2D, Table 3B). In contrast, IGRA levels did not differ in a vaccine dependent matter in all groups (Table 3B).
Table 3B.
Antibody and IGRA titres two (T2) and six months (T3) after vaccination in the seroconverted pure vaccination cohort dependent on vaccine type.
| Variable | Group | Time point | Category | BNT162b2 mRNA | mRNA-1273 | p-value (Mann-Whitney test) |
|---|---|---|---|---|---|---|
| Humoral responses | ||||||
| IgA-Ab Spike S1 | MP | T2 | Median (interquartile range) | 4·3 (2·3–6·8) | 9 (8·4–9) | < 0·001 |
| IgA-Ab Spike S1 | MP | T3 | Median (interquartile range) | 1·7 (1·0–3·3) | 4·2 (2·3–6) | < 0·001 |
| IgA-Ab Spike S1 | DP | T2 | Median (interquartile range) | 3·7 (1·5–7·1) | 8·8 (5·1–9) | < 0·001 |
| IgA-Ab Spike S1 | DP | T3 | Median (interquartile range) | 0·9 (0·4–1·8) | 2·2 (1·0–4·8) | < 0·001 |
| IgA-Ab Spike S1 | KTR | T2 | Median (interquartile range) | 2·9 (1·5–5·7) | 5·8 (2·6–9) | 0·007 |
| IgA-Ab Spike S1 | KTR | T3 | Median (interquartile range) | 1·6 (0·6–2·8) | 2·0 (0·9–4·7) | 0·051 |
| IgG-Ab Spike S1 | MP | T2 | Median (interquartile range) | 384 (384–384) | 384 (384–384) | 0·12 |
| IgG-Ab Spike S1 | MP | T3 | Median (interquartile range) | 241·5 (101·1–325·2) | 384 (266·7–384) | < 0·001 |
| IgG-Ab Spike S1 | DP | T2 | Median (interquartile range) | 384 (194·3–384) | 384 (384–384) | < 0·001 |
| IgG-Ab Spike S1 | DP | T3 | Median (interquartile range) | 68·6 (25·1–133·5) | 224·3 (108·7–384) | < 0·001 |
| IgG-Ab Spike S1 | KTR | T2 | Median (interquartile range) | 201·9 (90·1–384) | 384 (126·9–384) | 0·086 |
| IgG-Ab Spike S1 | KTR | T3 | Median (interquartile range) | 99·8 (31·9–184·5) | 125·0 (65·6–344·8) | 0·051 |
| RBD | MP | T2 | Median (interquartile range) | 98·7 (974–99·4) | 99·4 (99·1–99·6) | < 0·001 |
| RBD | MP | T3 | Median (interquartile range) | 82·1 (69·3–91·6) | 91·6 (83·1–97·2) | 0·001 |
| RBD | DP | T2 | Median (interquartile range) | 80·6 (57·8–95·3) | 98·4 (93·5–99·4) | < 0·001 |
| RBD | DP | T3 | Median (interquartile range) | 27·8 (9·9–47·5) | 63·8 (31·6–87·5) | < 0·001 |
| RBD | KTR | T2 | Median (interquartile range) | 56·9 (32·6–77·8) | 76·5 (40·5–97·5) | 0·018 |
| RBD | KTR | T3 | Median (interquartile range) | 35·9 (16·9–48·4) | 45·5 (21·7–81·5) | 0·072 |
| IGRA– T-cellular response | ||||||
| IGRA | MP | T2 | Median (interquartile range) | 1593·3 (740·2–2474·5) | 2413·8 (1454·7–2490·3) | 0·198 |
| IGRA | MP | T3 | Median (interquartile range) | 453·1 (200·9–1183·5) | 700·0 (481·2–2461·5) | 0·144 |
| IGRA | DP | T2 | Median (interquartile range) | 732·6 (154·5–2479·1) | 1021·17 (366·4–2477·4) | 0·364 |
| IGRA | DP | T3 | Median (interquartile range) | 256·9 (34·7–1387·1) | 428·9 (101·6–1161·4) | 0·485 |
| IGRA | KTR | T2 | Median (interquartile range) | 138·4 (14·5–326·4) | 43·8 (11·1–282·6) | 0·554 |
| IGRA | KTR | T3 | Median (interquartile range) | 75·6 (12·8–173·8) | 25·9 (16·0–244·4) | 0·627 |
MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; Interferon-γ release assay = IGRA;
This table compares the average titre levels (median/interquartile range) of 2x BNT162b2mRNA or 2×1273-mRNA (different columns) vaccinated study participants on T2 (two months) and T3 (six months) (different columns) for the different anti-Spike S1 IgA, IgG, RBD-IgG antibodies as well as for cellular immunity via IGRA measurements. For this evaluation, all participants with asymptomatic* or documented symptomatic** COVID-19 disease before and during vaccination up to T3 (six months) were excluded.
*Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T2 or T3) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive.
**Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
Multivariate analysis of risk factors of a strong antibody decline in DP and KTR cohorts compared to the MP cohort
In order to further explore risk factors of a decline in humoral response, an interval categorization of anti-S1 protein IgG and RBD antibody values was introduced. These categories, labelled from 0 to 4 and referred to as “levels” in the data analysis, where level 0 was assigned to IgG and RBD values below the positivity threshold of 35·2 BAU/ml and 35%, respectively, and the remaining values were split uniformly into four intervals of approximately equal length. Such a categorisation takes into account the truncated structure of IgG and RBD-IgG distributions and allows for a more comparable interpretation of IgG and RBD-IgG results. Under this approach, the quantification of change between T2 and T3 in IgG and RBD-IgG values can be performed on the same scale, simply by calculating the difference between the patient's level at T3 and his/her level at T2. With all changes ranging from -4 to +4, a “strong antibody decline” in either IgG or RBD-IgG was defined as a decrease by at least two levels between T2 and T3. See Supplementary Appendix for a more detailed description.
The analysis of between-group differences and potential risk factors of strong antibody decline was performed using logistic regression, applied to the study groups jointly (all seroconverted study participants) as well as to each group separately. The group effect in IgG decline was found to be highly significant ((2) = 22·258, p < 0·001). In particular, the chance of strong decline in IgG antibodies for DP or KTR was found to be about three or two times higher than for MP participants, respectively. In addition, the DP group showed a more pronounced trend towards antibody decline than the KTR group (p = 0·097, after adjustment for multiple comparison) (Table 4A).
Separate analyses by study group produced the following results: In the MP group, the vaccine type was found to be the only risk factor of strong antibody decline, with BNT162b2mRNA being associated with a five fold higher risk as compared to mRNA-1273 (p = 0·004) (Table 4B). Within the DP but not KTR group, male gender, BNT162b2mRNA (compared to mRNA-1273) vaccine, immunosuppressive drug use, diabetes mellitus as comorbidity, and a short time on dialysis were found to be independent risk factors of a strong IgG decline after vaccination (Table 4C). None of risk factors, which were included in the logistic regression analysis for the KTR group, had a statistically significant effect on strong antibody decline of IgG antibodies (Table 4D). A detailed summary of fit results is given in Tables 4A–4D. One can also notice the coherence between effect estimates obtained before and after imputation. Lower odds ratios of group effects are likely due to the imputed IgG values being closer to the average, and therefore, while a strong decline is associated to extreme cases.
Table 4B.
Strong IgG antibody decline for medical personnel (n = 125).
| Risk factor | Estimate | 95% CI | p-value | OR, 95%CI (MICE) |
|---|---|---|---|---|
| Age | 1·037 | [0·993,1·082] | 0·101 | 1.028[1.021,1.034] |
| Sex (Ref. = female) | 1·611 | [0·515,5·042] | 0·413 | 1.930[1.710,2.180] |
| Vaccine type (Ref. = mRNA-1273) | 4·567 | [1·638,12·735] | 0·004 | 5.213[4.636,5.863] |
| BMI | 0·929 | [0·819,1·052] | 0·245 | 0.925[0.911,0.939] |
Ref. = reference category; a “strong IgG antibody declinestrong fading response” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. BMI means body mass index. MICE means multiple imputation with chained equations.
Model fit: AIC = 107.44; BIC = 121.42.
Table 4C.
Strong IgG antibody decline for dialysis patients (n = 970).
| Risk factor | OR | 95% CI | p-value | OR,95%CI (MICE) |
|---|---|---|---|---|
| Age | 1·005 | [0·995,1·014] | 0·367 | 1.003[1.002,1.004] |
| Sex (Ref. = female) | 1·445 | [1·089,1·916] | 0·011 | 1.421[1.401,1.442] |
| Vaccine type (Ref. = mRNA-1273) | 2·684 | [1·836,3·923] | < 0·001 | 2.677[2.591,2.767] |
| BMI | 0·990 | [0·967,1·014] | 0·428 | 0.988[0.986,0.990] |
| IS drugs (Ref. = no drugs) | 3·052 | [1·360,6·849] | 0·007 | 3.340[3.107,3.590] |
| Time on dialysis | 0·977 | [0·953,1·001] | 0·058 | 0.977[0.975,0.979] |
| Hep B vacc failure (Ref. = no) | 1·002 | [0·723,1·388] | 0·990 | 0.965[0.945,0.986] |
| Diabetes mellitus (Ref. = none) | 1·386 | [1·041,1·845] | 0·025 | 1.389[1.356,1.424] |
Ref. = reference category; a “strong IgG antibody declinestrong fading response” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. Comparator is the MP cohort. IS means immunosuppressiveon; BMI means body mass index; Hep B vacc failure definition - patients with unsuccessful vaccination after at least four attempts. MICE means multiple imputation with chained equations.
Model fit: AIC = 1277.30; BIC = 1325.86.
The same approach was used to investigate potential risk factors of strong RBD-IgG antibody decline (Table 5). The group effect was again found to be highly significant ((2) = 40·478, p < 0·001, Table 5A). Similar to IgG, the chance of a strong decline in RBD-IgG antibodies for DP and KTR, was found to be about five or three times higher than for MP participants (p < 0·001, Table 5A), respectively. The strong antibody decline in DP was more than two times higher than in KTR (p = 0·004). Minor influences/trends for the vaccine type or age were found in MP. Within the DP but not KTR group again male gender and BNT162b2mRNA vaccine, and a short time on dialysis were risk factors of a strong RBD-IgG decline after vaccination. In contrast to IgG, immunosuppressive drug use in DP did not appear to be a risk factor of strong RBD-IgG decline. As in the IgG case for KTR, none of considered potential risk factors (including immunosuppressive drug number) of a strong RBD-IgG antibody decline were found statistically significant. A detailed summary is provided in Tables 5A, Table 5B, Table 5C, Table 5D.
Tables 5A.
Multiple logistic regression analysis of a strong decline of vaccination-specific anti-SpikeS1 receptor binding domain (RBD-IgG) antibodies in seroconverted participants of the DIA-Vacc pure vaccination cohort between T2 and T3.
Table 5A. Strong RBD-IgG antibody decline for all participants (n = 1128).
| Risk factor | OR | 95% CI | p-value |
|---|---|---|---|
| Age | 1·012 | [1·002,1·021] | 0·013 |
| Sex (Ref. = female) | 1·463 | [1·125,1·902] | 0·005 |
| Vaccine type (Ref. = mRNA-1273) | 2·147 | [1·470,3·136] | <0·001 |
| DP (Ref. = MP) | 5·435 | [2·876,10·271] | <0·001 |
| KTR (Ref. = MP) | 2·533 | [1·213,5·290] | 0·013 |
| Diabetes Mellitus | 1·214 | [0·929,1·587] | 0·155 |
MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; Ref. = reference category; a “strong RBD-IgG antibody decline” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. Comparator is the MP cohort.
Model fit: AIC = 1446.97; BIC = 1482.16
Table 5B.
Strong RBD-IgG antibody decline for medical personnel (n = 116).
| Risk factor | OR | 95% CI | p-value |
|---|---|---|---|
| Age | 1·065 | [1·006,1·129] | 0·032 |
| Sex (Ref. = female) | 1·204 | [0·299,4.853] | 0·794 |
| Vaccine type (Ref. = mRNA-1273) | 3·320 | [0·921,11·963] | 0·067 |
| BMI | 0·927 | [0·792,1·085] | 0·346 |
Ref. = reference category; a “strong RBD-IgG antibody decline” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. Comparator is the MP cohort. IS means immunosuppression; BMI means body mass index.
Model fit: AIC = 80.38; BIC = 93.97
Table 5C.
Strong RBD-IgG antibody decline for dialysis patients (n = 908).
| Risk factor | OR | 95% CI | p-value |
|---|---|---|---|
| Age | 1·009 | [0·999,1·019] | 0·087 |
| Sex (Ref. = female) | 1·441 | [1·081,1·922] | 0·013 |
| Vaccine type (Ref. = mRNA-1273) | 2·592 | [1·621,4·143] | <0·001 |
| BMI | 0·983 | [0·959,1·008] | 0·188 |
| IS drugs (Ref. = no drugs) | 0·894 | [0·398,2·008] | 0·787 |
| Time on dialysis | 0·961 | [0·937,0·986] | 0·003 |
| Hep B vacc failure | 1·185 | [0·846,1·659] | 0·323 |
| Diabetes mellitus (Ref. = none) | 1·251 | [0·934,1·676] | 0·134 |
Ref. = reference category; a “strong RBD-IgG antibody decline” ” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. Comparator is the MP cohort. IS means immunosuppressive; BMI means body mass index; Hep B vacc failure definition - patients with unsuccessful vaccination after at least four attempts.
Model fit: AIC = 1211.87; BIC = 1259.81
Table 5D.
Strong RBD-IgG antibody decline for kidney transplant recipients (n = 104).
| Risk factor | OR | 95% CI | p-value |
|---|---|---|---|
| Age | 1·017 | [0·973,1·064] | 0·458 |
| Sex (Ref. = female) | 1·559 | [0·550,4·419] | 0·403 |
| Time after transplantation | 0·987 | [0·920,1·058] | 0·703 |
| Vaccine type (Ref. = mRNA-1273) | 1·492 | [0·522,4·262] | 0·455 |
| IS number | 1·064 | [0·499,2·267] | 0·872 |
| BMI | 1·096 | [0·987,1·267] | 0·086 |
| Hep B vacc failure | 4·037 | [0·903,18·042] | 0·068 |
| Diabetes mellitus | 0·717 | [0·231,2·658] | 0·854 |
Ref. = reference category; a “strong RBD-IgG antibody decline” between T2 (two months after first vaccination) and T3 (six months) was defined as described in Results and in more detail in Supplementary material. Comparator is the MP cohort. IS means immunosuppressive drug; BMI means body mass index; Hep B vacc failure definition - patients with unsuccessful vaccination after at least four attempts.
Model fit: AIC = 127.16; BIC = 127.16
To investigate the effect of dialysis type on strong decline rates, we carried out a propensity score matching procedure for PD and HD patients. In the matched data, PD patients had three times higher chances than HD patients to experience strong decline in IgG (OR = 3·10 [1·06; 10·39], Fisher test, p = 0·024). RBD strong decline in PD group was numerically but not statistically about two times more likely, in comparison to HD (OR = 2·46 [0·68, 11·38], Fisher test, p = 0·176).
Clinical outcome between T2 and T3
Only one dialysis and one transplant patient experienced mild symptomatic and nine dialysis patients asymptomatic COVID-19 disease (NCP seroconversion) between two and six months after vaccination. No single patient had to be hospitalised or died due to COVID-19 disease. Between T2 and T3, only very few group changes took place (Figure 1). No allograft failure was reported between two and six months. 35 deaths not related to COVID-19 were noted between two and six months (Figure 1).
Results of the KTRboost group
All patients of this group showed very low seroconversion values at T2 with either IgA positivity and no IgG positivity or very low/negative levels of RBD-IgG antibody values stimulating the individual decision for an additional boostering at 4·2 ± 1 months despite seroconversion. The IgA antibody ratio (mean/interquartile range) changed from 1·8 (1·1–3·0) at T2 to 2·0 (1·6–6) at T3, the IgG titres from 13·7 BAU/ml (4·1–22·3) to 290·4 BAU/ml (75·4–384), and the RBD-IgG levels from 8·1% (4·7–12·9) to 89·3% (28·7–98·6). At six months, 90% of patients were still positive for either IgA or IgG antibodies, 85%/90%/65% were positive for IgA/IgG/RBD-IgG antibodies, respectively. In contrast to the unboostered KTR group, only 10·3% qualified for a strong antibody decline between two and six months, while 65%/85%/86% showed increased (by > 20%) IgA/IgG/RBD-IgG antibody levels for this time period. No patient developed de novo IgA, but 75% de novo IgG and 64% RBD-IgG antibodies between T2 and T3. One patient experienced asymptomatic COVID-19 disease with NCP antibody conversion. To get a closer look at the booster effect, we used propensity scores to match the KTRboost group with unboostered KTR (1:1 ratio, without resampling). Only their IgG values at T2 were used for matching, as it reflected on the actual selection procedure used by the clinics to select KTRs for booster vaccination. A summary of seroconversion markers for the matched groups is given in Table 6 and shows the marked differences in the time course of antibody titres of the KTRboost versus unboostered group.
Discussion
Comparative immunity fading data are scarce but highly relevant considering especially that DP and KTR belong to the most vulnerable patient populations exposed to markedly increased COVID-19 related mortality rates.1,4
In our Dia-Vacc study six months after vaccination start, almost all study participants of the MP group with seroconversion after two months still possessed positive anti-S1 IgG and RBD-IgG antibodies, but in the seroconverted DP or KTR groups, up to 16%/43% of patients were negative for anti-S1 IgG/RBD-IgG antibodies at six months, respectively. This indicates severe differences of antibody development and decline in the immunocompromised and immunosuppressed risk groups of DP and KTR compared to the MP group, which we further analysed in this study.
Hereby, it needs to be considered that on average the antibody titres two months after vaccination were highest in the MP, intermediate in DP, and lowest in KTR groups.4 Using different measures (20% titre decrease or a strong decline qualification – see Methods), IgG or RBD-IgG antibody decline between two and six months was smallest in MP, highest in DP and intermediate in KTR. Interestingly, almost nobody of the MP or DP groups showed IgG or RBD-IgG titres with more than a 20% increase from two to six months, but about up to 16% of KTR did. This apparently reflects a unique delayed immune response, which appears to be aggravated in a part of the immunosuppressed transplant patients and includes even some delayed de novo positivity for RBD-IgG antibodies at six months despite IgG or IgA seroconversion at two months. Similar to this finding, about 15% of KTR without a positive seroconversion two months after vaccination experience a delayed seroconversion up to six months without any additional vaccine booster.17 These results are consistent with other studies in KTR, where compared to a healthy control group a delayed humoral response specific for IgG antibody formation and secretion was seen after vaccination against influenza18 or after COVID-19 disease.19 Whether the immunosuppressive drugs belatacept and MMF/MPA being predominantly responsible for impaired seroconversion rates in KTR in our Dia-Vacc4 and other studies3 also cause a delayed humoral response with accelerated antibody decline, should be explored in future studies.
Similar to the humoral response, the T-cellular immunity response as determined by IGRA measurements was most frequent and highest in the MP, intermediate in the DP, and lowest in the KTR group at two4 but still also at six months. Between two and six months, IGRA titres decreased in about 60% of patients in all three groups. Solely in the KTR group, about a third of patients showed increased IGRA titres between two and six months, mimicking the delayed antibody response also at the T-cellular level.
Analysis of risk factors for a strong IgG or RBD-IgG antibody decline between two and six months identified the DP and also the KTR group at risk compared to the MP group.
While most differences of the mRNA vaccine types have been found regarding seroconversion rates in patient study groups exposed to therapeutics altering the immune response,3,4 in this study BNT162b2mRNA (compared to mRNA-1273) seemed to be a risk factor of strong IgG or RBD-IgG antibody decline (besides age) in the MP group. Within the KTR group, all of the considered patient characteristics, including immunosuppressive drug number, could not be identified as risk factors of a strong antibody decline.
In the DP group, several risk factors for a strong antibody decline were identified. The vaccine type BNT162b2mRNA was associated with a 2.5 fold increased risk for both IgG and RBD-IgG decline adding another reason for using mRNA-1273 as the preferred vaccine type in DP besides higher seroconversion rates and peak antibody titres.4,20 The use of immunosuppressive drugs and diabetes mellitus as a comorbidity were risk factors of IgG but not of RBD-IgG antibody decline in the DP cohort. This difference between IgG and RBD-IgG antibody decline cannot be explained but suggests differential immune response influences under/due to immunosuppression or diabetes mellitus. Male gender and short time on dialysis, but not BMI were associated with an increased risk of a strong decline in both IgG and RBD-IgG antibodies between two and six months. While it was unexpected that short time on dialysis could be identified as a risk factor for antibody decline after seroconversion, it was also associated with increased risk for seroconversion failure.4 This suggests that either the humoral immune system may adapt/recover during longer times on dialysis therapy in stable DP or that this may reflect a “survival of the fittest” effect. Consistent with hepatitis B vaccination studies,21 the dialysis type was linked to a higher risk of a strong decline in both IgG and RBD-IgG antibodies, with peritoneal dialysis being associated with a four times higher risk of strong antibody decline than hemodialysis. Whether this antibody decline difference relates to potential differences in the clearance of uremia related substances is unclear. In contrast, seroconversion rates for both SARS-CoV-222 or Hepatitis B23 vaccination are equivalent in hemodialysis and peritoneal dialysis patients.
Most importantly, between month two and six after vaccination, no single seroconverted study participant experienced any severe COVID-19 disease. While this region in Germany was exposed to the third wave pandemic with high COVID-19 incidences,24 only 12 breakthrough infections occurred in this large study population. Symptomatic breakthrough infections are known to be related to lower titre levels.6 Nevertheless, to define general antibody cut-off values for full COVID-19 protection may not be feasible considering antibody test heterogeneity,25 individual SARS-CoV-2 infectious particle load and route, different virus variants, the degree of additional T-cell immunity, and the dynamic time course of immunity.
Our study highlights great immune response variability with specific characteristics in different patient populations implicating the usefulness of antibody (and T-cell immune) monitoring especially in DP and KTR to determine timely reboostering. In contrast to our MP group only six months after the start of mRNA vaccination, two thirds of successfully seroconverted DP show a strong antibody decline, which is dependent on individual risk factors such as male gender, diabetes mellitus as comorbidity, dialysis type, immunosuppressive drug use, but also vaccine type. mRNA-1273 could be identified as the vaccine with the highest antibody levels during that follow up period. In DP, T-cell immunity fading as measured by IGRA appeared at a similar rate as in MP, but on average titre levels were bisected. In contrast, successfully vaccinated KTR are also at an increased risk for a strong antibody decline when compared with the MP group. Unique to the KTR group is the mixture of (humoral and T-cellular) immunity fading and rise, which fits to a delayed immune response in at least a 16% fraction of immunosuppressed transplant patients. Besides these two intercalating effects, KTR in general reach antibody and IGRA levels markedly below the “normal” immunocompetent or even the immunocompromised DP population during the six months monitoring period. The positive effect of individual monitor guided early reboostering following a weak seroconversion response could be demonstrated in our KTRboost group, where almost all patients with extremely low titre seroconversion reacted with a marked antibody increase (instead of a decline) in both IgG and RBD-IgG antibodies apparently providing clinical protection up to six months.
Our study has several limitations including the observational, non-randomized study character and the lack of demographic matching between different cohorts. Although the former cannot be controlled for and may lead to hidden bias, the latter was accounted for in the multivariate analyses by including demographic factors as covariates. Due to the complete lack of mRNA vaccination related immunity monitoring data in dialysis and transplant patients at study start, no sample size calculation was performed for this study. Financial resources of the study determined that only 3101 participants in 26 out of 36 available dialysis centres of the Saxonian network could be recruited as described in methods. 102 patients missed blood sampling at T3. Using a multiple imputation procedure, conclusions remained consistent across all imputed sets and to those obtained based on the pure vaccination cohort. Further limitations apply to individual determination of ethnicity, race and income/occupation as well as socio-economic position, factors known to be associated with infection risk and risk of KTR or DP (but not regarding antibody fading). In addition, only mRNA vaccines were given to all study participants excluding heterologous vaccination strategies. Many different test systems for SARS-CoV-2 related humoral or T-cellular immunity are commercially available and may not give interchangeable results to our study. Nevertheless, it can be considered a study strength that all participants have been examined centralised with the same system even on the same analysers including a complete panel of antibodies to differentiate disease and vaccination immunity. Applying de novo NCP positivity for counting and exclusion of asymptomatic COVID-19 breakthrough infections from further analysis may underestimate cases.25 Finally, the T-cell response was not measured in all study participants.
In conclusion, this study shows that after successful SARS-CoV-2 mRNA vaccination, DP and KTR compared to MP are at specific risk for a strong antibody decline. In DP but not KTR, this fading response is accelerated by male gender, diabetes mellitus as comorbidity, peritoneal dialysis, immunosuppressive drug use, and BNT162b2mRNA compared to mRNA-1273. In contrast, despite a delayed immune response with rising levels in a 16% patient fraction, successfully vaccinated KTR reach on average immunity levels markedly below MP and DP throughout the monitoring period of six months, but can be successfully boostered by a third vaccination when titres are low (as in the KTRboost group). This data indicates the value of immune monitoring especially in high risk populations such as DP and KTR to define the best strategy and (early) timing of additional booster vaccinations even after positive seroconversion.
Contributors
JS and CH contributed to study design, data collection, data interpretation, and drafting of the manuscript and contributed to data interpretation and drafting of the manuscript.
AS, FG, FK, HK, PA, JSr, KF, TT were involved in data acquisition and collection or study organization. AK, RM, XG, were involved in statistical analysis or data management of the study. JSc, TL, LA, TS, CK, JH, HM, PM, RF-W, TLa, HS, TSt, FM, AP, KA-R, KE, FP, JeS, HSe, KB, TP, SC, APa, IB were involved in patient recruitment and data collection. All authors have approved the final version for submission.
Declaration of interests
JS, JSc, TL, LA, TS, CK, JH, HM, PM, RF-W, TLa, HS, TSt, FM, AP, KA-R, KE, FP, JeS, HSe, KB, TP, SC, APa, IB, AS, FG, FK, HK, PA, JSr, KF, AK, RM, XG, and TT have no conflict of interests.
Acknowledgments
Acknowledgment
We acknowledge the support from the DIA-VACC Investigators (see full list in Appendix).
Further supporters
D. Michael Albrecht, Medizinischer Vorstand (Sprecher), Universitätsklinikum Carl Gustav Carus Dresden
Thomas Grünewald, Chemnitz, Vorsitzender Sächsische Impfkommission
Patricia Klein, Ärztliche Geschäftsführerin, Sächsische Landesärztekammer Körperschaft des öffentlichen Rechts
Marcus Strotkötter, Senior Operations Officer for Vaccination Center and Mobile Vaccination Teams at DRK Landesverband Sachsen e.V.
EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany provided antibody ELISAs and interferon-gamma release assays for this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100371.
Appendix A. *DIA-Vacc- investigators
Members in alphabetical order within the corresponding centres:
1Medizinische Klinik und Poliklinik III, Universitätsklinikum, Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
Andrea Angermann, Nicole Bunk, Susanne Dollfus, Petya Drinova, Annegret Fleischer, Chris Gebauer, Susann Graichen, Kerstin Haaser, Sabrina Heide, Gabriele Hirt, Franziska Klemt, Jens Passauer, Miriam Poser, Margrit Reinhardt, Ronny Rettig, Vladimir Todorov, Kerstin Zäumer
3Dialysezentrum Chemnitz, Chemnitz, Germany
Markus Gläser, Ulrike Richardt, Anja Richter, Kathrin Scheibner, Andreas Sohn
4Division of Nephrology, University Hospital Leipzig, Leipzig, Germany
Anette Bachmann, Jonathan Christoph de Fallois, Jan Halbritter, Claudia Kreyßig, Christof Mayer, Benjamin Sandner
5Dialysepraxis Leipzig, Leipzig, Germany
Daniel Grey
6KfH-Nierenzentrum am Klinikum Chemnitz, Krankenhaus Küchwald, Chemnitz, Germany
Angela Grzegorek, Markus Hallmann, Saskia Härtwig, Christiane Hintzen-Kruse, Frauke Neidt und Söhne, Susann Reissland, Bianca Seidel, Anett Speer, Peggy Weiß, Christine Wiech
7KfH-Nierenzentrum am Klinikum St. Georg, Leipzig, Germany
Joachim Beige, Grit Glombig, Kathrin Heipmann, Christian Schauff, Peyo Kolev Sivenov
8Nephrocare GmbH Döbeln, Döbeln, Germany
Noureddin Ibrahim
9Nephrologisches Zentrum Zwickau, Zwickau, Germany
Sebastian Höhne, Konrad Mager, Grit Müller, Ingrid Petzold, Alexander Sämann
10PHV Dialysezentrum Dresden-Johannstadt, Dresden, Germany
Steffen Bischoff, Tatjana Seitz
11Nephrologisches Zentrum Freiberg, Freiberg, Germany
Anja Sawalha, Emad Sawalha
12Dialysezentrum Annaberg, Annaberg-Buchholz, Germany
Rainer Giese
13PHV Dialysezentrum Dresden Friedrichstadt, Dresden, Germany
Roland Behnisch, Peter Grossmann, Peter Heduschka
14KfH-Nierenzentrum Bautzen, Bautzen, Germany
Norgit Meyer, Sybille Stehr, Patrice Steinert
15KfH-Nierenzentrum am Städtischen Klinikum Görlitz, Görlitz, Germany
Angela Jansen, Ellen Pirlich, Torsten Siegert
16Via medis Nierenzentrum Dresden MVZ GmbH, Dresden, Germany
Ivo Döhler, Matthias Pietzonka
17KfH-Nierenzentrum Bischofswerda, Bischofswerda, Germany
Simone Kuchinke, Eva Strakow, Valeria Uschakow
18KfH-Gesundheitszentrum Aue, Aue-Bad-Schlema, Germany
Matthias Bauer, Grit Baumgart, Anne Martin, Tino Peter Zschocke
19Dialysiszentrum Hoyerswerda, Hoyerswerda, Germany
Jan Nawka
21KfH-Nierenzentrum am Vogtland Krankenhaus Plauen, Plauen, Germany
Kristin Hartmann, Monika Horbach, Sina Koch, Mike Zahn
22Dialyse-Praxis Weißwasser, Weißwasser, Germany
Mato Nagel
23Dialyse Heidenau, Heidenau, Germany
Sebastian Heinrich, Iris Meyer, Annett Olschewski
24KfH-Nierenzentrum Grimma, Grimma, Germany
Dietmar Göhring, Michael Windgassen
25ELBLAND Dialyse Großenhain, Großenhain, Germany
Michael Dechant, Kerstin Heinze, Daniela Kschiwan
26Institut für Transfusionsmedizin Plauen, DRK-Blutspendedienst Nord-Ost gemeinnützige GmbH, Plauen, Germany
Nadine Hildebrandt, Andreas Karl
29Coordinating Centre for Clinical Trials, Dresden, Germany
Stephan Friedrich
33Nieren-, Hochdruck- und Rheuma-Zentrum Dresden MVZ mit Dialyse und Apherese, Dresden, Germany
Sven Hänsel, Falko Neumann, Catrin Palm, Doreen Reimann
Appendix B. Supplementary materials
References
- 1.Jager K.J., Kramer A., Chesnaye N.C., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupper A., Rabinowich L., Schwartz D., et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stumpf J., Siepmann T., Lindner T., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumpf J., Tonnus W., Paliege A., et al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. 2021;105(11):267–269. doi: 10.1097/TP.0000000000003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimeglio C., Herin F., Martin-Blondel G., et al. Antibody titers and protection against a SARS-CoV-2 infection. J Infect. 2021;84(2):248–288. doi: 10.1016/j.jinf.2021.09.013. S0163-4453(21)00483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padoan A., Sciacovelli L., Basso D., et al. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio-Acero R, Castelletti N, Fingerle V, et al. In search for the SARS-CoV-2 protection correlate: a head-to-head comparison of two quantitative S1 assays in a group of pre-characterized oligo-/asymptomatic patients. medRxiv 2021: 2021.02.19.21252080.
- 9.Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. medRxiv 2021: 2021.03.03.21251066.
- 10.Meyer B., Torriani G., Yerly S., et al. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin Microbiol Infect. 2020;26(10):1386–1394. doi: 10.1016/j.cmi.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EUROIMMUN Medizinische Labordiagnostika AG aPc. SARS-CoV-2 NeutraLISA. 31.03.2021 2021. https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/EI_2606_D_UK_F.pdf.
- 12.Aiello A., Najafi Fard S., Petruccioli E., et al. Spike is the most recognized antigen in the whole-blood platform in both acute and convalescent COVID-19 patients. Int J Infect Dis. 2021;106:338–347. doi: 10.1016/j.ijid.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw R.H., Stuart A., Greenland M., et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munro A.P.S., Janani L., Cornelius V., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3. S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azur M.J., Stuart E.A., Frangakis C., Leaf P.J. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team . R: A Language and Environment for Statistical Computing. version 4.0.1. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 17.Stumpf J, Schwöbel J, Siepmann T, et al. Revaccination success in kidney transplant recipients with no initial humoral response is linked to basic vaccine type. (submitted) [DOI] [PMC free article] [PubMed]
- 18.Gangappa S., Wrammert J., Wang D., et al. Kinetics of antibody response to influenza vaccination in renal transplant recipients. Transpl Immunol. 2019;53:51–60. doi: 10.1016/j.trim.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cravedi P., Ahearn P., Wang L., et al. Delayed kinetics of IgG, but not IgA, antispike antibodies in transplant recipients following SARS-CoV-2 infection. J Am Soc Nephrol. 2021;32(12):3221–3230. doi: 10.1681/ASN.2021040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumpf J., Hugo C. Humoral immunity to SARS-CoV-2 vaccination in haemodialysis patients—authors´ reply. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100244. ; Nov; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S.Y., Liu J.H., Lin C.C., et al. Comparison of hepatitis B surface antibody decay rates after vaccination between hemodialysis and peritoneal dialysis patients. Vaccine. 2011;29(21):3738–3741. doi: 10.1016/j.vaccine.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 22.Stumpf J., Klimova A., Mauer R., et al. Equivalent humoral and cellular immune response but different side effect rates following SARS-CoV-2 vaccination in peritoneal and hemodialysis patients using mRNA vaccines. Nephrol Dial Transplant. 2021;37(4):796–798. doi: 10.1093/ndt/gfab324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y.L., Kao M.T., Huang C.C. A comparison of responsiveness to hepatitis B vaccination in patients on hemodialysis and peritoneal dialysis. Vaccine. 2005;23(30):3957–3960. doi: 10.1016/j.vaccine.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 24.COVID-19-Inzidenzen. Robert Koch Institut, 2021. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Inzidenzen.html.). Accessed 4 May 2021.
- 25.Whitaker H.J., Gower C., Otter A.D., et al. Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: impact of variant, vaccination, and choice of assay cut-off. Medrxiv 2021 Oct; 10.1101/2021.10.25.21264964. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.