Abstract
Links between supine “going to sleep” position and stillbirth risk have led to campaigns regarding safe maternal sleep position. This study profiles the distribution of sleep positions overnight and relationships to sleep onset position during pregnancy, and the relationships between supine sleep, sleep-disordered breathing (SDB), and pregnancy outcomes. Data from three prospective cohort studies evaluating SDB in healthy and complicated pregnancies were pooled. All participants underwent one night of polysomnography in late pregnancy and birth outcome data were collected. 187 women underwent polysomnography at a median gestation of 34 weeks'. The left lateral position was preferred for falling asleep (52%) compared to supine (14%), but sleep onset position was the dominant sleep position overnight in only half (54%) of women. The median percentage of sleep time in the supine position was 24.2%; women who fell asleep supine spent more time supine overnight compared to those who began non-supine (48.0% (30.0,65.9) vs. 22.6% (5.7,32.2), p < .001). Women with growth-restricted fetuses were more likely to fall asleep supine than those with well-grown fetuses (36.6% vs. 7.5%, p < .001). Positional SDB was observed in 46% of those with an RDI ≥ 5. Sleep onset position was the dominant position overnight for half of the sample, suggesting that sleep onset position is not always a reliable indicator of body position overnight. Supine sleep was related to fetal growth restriction and birthweight at delivery, though causality cannot be inferred. It is critical that we pursue research into verifying the important relationship between supine sleep and increased stillbirth risk, and the mechanisms behind it.
Keywords: pregnant, sleep study, lateral, supine, birthweight, fetal health, sleep apnea, polysomnography, sleep onset
Statement of Significance.
Self-reported sleep onset position has been identified as a risk factor for stillbirth, but objective measures of this relationship are lacking. Within a large cohort of pregnant women with full polysomnography, we have demonstrated that sleep onset position is not representative of overnight sleep position for many pregnant women, and that the proportion overnight of sleep spent supine may be related to birthweight. Supine sleep may be more comfortable for those with smaller fetuses, and so this relationship may be bidirectional, and warrants further clarification. Further work is needed to validate the relationship between supine sleep and stillbirth risk and better understand the mechanisms of action, although this will be challenging given the low prevalence of stillbirth.
Introduction
Sleep position has become increasingly recognised as a risk factor for poor fetal outcomes, including stillbirth and reduced fetal growth [1–3]. A recent meta-analysis of five case-control studies revealed that retrospective self-report of going to sleep in the supine position increases the odds of late stillbirth by 2.6 times, and that adoption of a lateral sleep onset position could potentially lead to a 5.8% reduction in these tragic occurrences [1]. A proposed mechanism behind increased stillbirth risk with supine sleep is compression of the inferior vena cava by the enlarged uterus, leading to decreased maternal cardiac output [4]. Consequently, the Centre of Research Excellence in Stillbirth has recently put together a bundle of care to be implemented and evaluated across maternity services in Australia, with similar campaigns in other countries including the United Kingdom and New Zealand [5, 6]. The “Safer Baby Bundle” focuses on five care elements, including improving awareness of maternal safe sleeping position in late pregnancy [7].
Significant unavoidable limitations of the studies relating sleep-onset position to increased stillbirth risk include a lack of maternal sleep position validation and retrospective recall of sleep-onset position after the occurrence of stillbirth, potentially leading to recall bias [8]. The correlation between self-report and video-determined sleep position is only moderate [9], and position on “waking up” is poorly recalled [10]. These studies measure sleep onset position, which is the only available surrogate for overall sleep duration in a given position, yet biologically it makes sense that position- and position change- throughout the night may be a better measure of risk. A few small studies have objectively measured that more than 80% of pregnant women spend time sleeping supine [11], with the overall proportion of sleep time in the supine position ranging from 9.5% to 26.5% [11, 12]. However, we do not know how reflective “going to sleep” position is of sleep behaviour for the whole night, which is important given the physiological rationale of supine positioning causing inferior vena cava compression leading to fetal compromise.
In the non-pregnant population, supine sleep position is associated with increased severity of sleep-disordered breathing (SDB), mostly due to gravitational forces collapsing the upper airway and reduced lung volume [13]. One study has demonstrated that this is also the case during pregnancy, although the magnitude of the supine position-related increase in SDB severity was small [14]. SDB during pregnancy has been linked with a series of unfavourable outcomes for mother and baby including preeclampsia, gestational diabetes, with some studies demonstrating reduced fetal growth [15–17] but not others [18, 19]. Sleep-related breathing disorders may thus be another plausible mechanism by which supine sleep position contributes to maternal and fetal morbidity.
The polysomnography (PSG) data that we have collected from three cohort studies looking at maternal sleep and fetal wellbeing allowed a large-scale analysis of objectively measured sleep position during late pregnancy. The aim of this analysis was to examine typical sleep positions overnight during late pregnancy and how they relate to sleep onset position, with a focus on supine sleep. Secondly, this study evaluated the relationships between supine sleep position, degree of SDB, and birth outcomes in complicated and uncomplicated pregnancies.
Methods
Participants
These data are from three prospective cohort studies investigating SDB during (1) healthy pregnancy [16], (2) pregnancies complicated by fetal growth restriction with BMI- and gestation-matched controls [20], and (3) hypertensive disorders of pregnancy (including gestational hypertension, preeclampsia, and chronic hypertension) with BMI- and gestation-matched healthy controls [21], between 2009 and 2018. The protocol for all three studies contained one night of PSG conducted in the same manner in the latter stages of pregnancy. All participants were recruited from antenatal outpatient clinics or as inpatients. Multiple pregnancy, those with already diagnosed sleep disorders, and those less than 18 years old were excluded. The Human Research Ethics Committees at Austin Health, Mercy Hospital for Women and University of Melbourne approved the studies and written informed consent was obtained from all participants.
Procedures
Basic demographic and obstetric data were collected at recruitment to the studies. This included maternal age, parity, gestation, and relevant comorbidities including hypertensive disorders of pregnancy, fetal growth restriction, and gestational diabetes [22]. Hypertensive disorders of pregnancy encompassed (1) gestational hypertension, defined as the new onset of hypertension after 20 weeks gestation; (2) preeclampsia, defined as new hypertension after 20 weeks gestation accompanied by one or more of proteinuria, other maternal organ dysfunction, or uteroplacental dysfunction; (3) chronic hypertension, known before pregnancy or present in the first 20 weeks of gestation, and (4) superimposed preeclampsia, diagnosed when one or more systemic features of preeclampsia occur after 20 weeks gestation in addition to pre-existing hypertension [23]. Fetal growth restriction was defined using the ultrasound Delphi consensus criteria [24] (based on estimated fetal weight or abdominal circumference <3rd centile, or estimated fetal weight or abdominal circumference <10th centile with abnormal fetoplacental Dopplers on ultrasound). Following delivery, birth outcomes including gestation, birthweight, and birthweight centile (customised for maternal BMI, ethnicity, parity, fetal sex, and gestation) were recorded.
All participants underwent overnight full PSG. Attended overnight PSG was conducted in the Austin Health sleep laboratory using the Compumedics E series (Abbotsford, Victoria, Australia), or if preferred, unattended in the participant’s home with the Somté PSG V1 (Compumedics) portable sleep-monitoring device. Inpatients were studied using the portable device. For both attended and unattended PSG, signals recorded included electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), arterial oxygen saturation, thoracic and abdominal respiratory effort via inductance plethysmography, nasal airflow measured via nasal cannula, oronasal thermistor, leg movements, snoring and body position at the level of the chest. For attended in-laboratory studies the 4-position sensor (left, right, supine, prone) was taped centrally to the thoracic band, and position was verified via digital infrared video. For unattended studies, the patient input box (PIB) has an internal 4-position sensor, with the PIB placed on the thoracic band centrally located over the sternum. Participants were not given specific instructions regarding which body positions to sleep in as these studies predated public health messaging regarding side sleeping in pregnancy, but if asked were told to sleep as “normally” as possible. Sleep, arousals, and respiratory events were staged as per the American Academy of Sleep Medicine (AASM) criteria [25], with the number of apneas and/or hypopneas per hour of sleep calculated as the apnea/hypopnoea index (AHI). The addition of respiratory event related arousals (RERAs) per hour to the AHI was expressed as the respiratory disturbance index (RDI). The oxygen desaturation index was defined as the number of arterial oxygen desaturations of ≥3% from baseline per hour of sleep (ODI 3%). BMI at the time of the sleep study was also recorded. Sleep onset was defined as the first epoch of any stage of sleep and waking position was defined as the body position at the last sleep offset for the sleep study. Settling into bed position was defined as the position at the time of lights out, when the participant first attempted to go to sleep.
Statistical analysis
All statistical analyses were performed with Stata 16.0 (StataCorp LP, College Station, TX). Data are given in means with standard deviations (M ± SD) or median and interquartile range (Mdn (IQR)) for non-normally distributed variables. Due to total sleep time spent in the supine and prone positions being skewed, Mdn (IQR) was used for all position data for consistency. A two-sided p value of less than .05 was considered to indicate statistical significance.
Group comparisons were made with Pearson’s chi-squared test of independence for categorical variables, independent t-tests for normally distributed continuous variables, and Mann–Whitney U tests for non-normally distributed continuous variables. The percentage of sleep in the left, right, supine and prone position across the women with fetal growth restriction and appropriate for gestational age (birthweight centile > 10th centile) fetuses was compared using four separate Mann–Whitney U tests with Bonferroni adjustment for multiple comparisons (p < .05/4 = .0125 indicates statistical significance). Supplementary analyses were performed across pregnancy groups and sleep study locations with analysis of variance with post-hoc pairwise comparisons (Bonferroni) or with Kruskal–Wallis with Dunn’s Test for pairwise comparisons. Position-based comparisons for SDB indices were made with Wilcoxon signed rank sum tests, with the results displayed with box and whisker plots. Participant numbers for these comparisons were limited to those who had both supine and non-supine sleep sampled. Gestational diabetes data were only available for 176 participants, as 11 women did not have a glucose tolerance test.
Linear regression was used to assess the univariate relationships between supine sleep onset and percentage of total sleep time (%TST) in supine and right lateral sleep and birth outcomes, with multiple regression used to control for the influence of fetal growth restriction and inpatient status at the time of the sleep study. Multiple regression was also used to adjust for gestational age in the relationship between fetal growth restriction and supine sleep onset and %TST supine. To meet analysis assumptions, data were transformed prior to analysis as follows: due to positive skewness, %TST supine sleep and birthweight centile were square root transformed; due to negative skewness, birthweight was reflected and then square root transformed and delivery gestation was reflected and then log transformed. The relationship between SDB and birth outcomes was assessed with Spearman’s rank order correlations.
Results
Participants
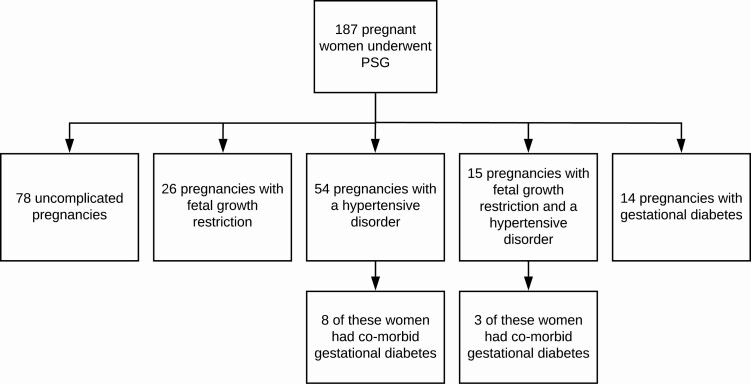
A total of 187 polysomnograms were performed at a median gestational age of 34.1 (31.0, 36.6) weeks. At the time of the sleep study, the average age of the participants was 33.1 ± 5.0 years with an average BMI of 34.7 ± 7.4 kg/m2. A breakdown of the number of participants by pregnancy type (uncomplicated, fetal growth restriction, hypertensive disorders of pregnancy, and gestational diabetes) is shown in Figure 1. Forty (21.4%) studies were performed within the sleep laboratory, 32 were performed on inpatients with complicated pregnancies (12 with fetal growth restriction, 8 with a hypertensive disorder, and 12 with both conditions), with the remaining 115 unattended at home. One participant with severe early onset fetal growth restriction had a fetal death in utero at 27 weeks’ gestation.
Figure 1.
Number of participants with uncomplicated or complicated pregnancies.
Typical sleep positions in pregnancy
The left lateral position was the preferred position for both settling into bed and falling asleep. Only 14% of the women went to sleep in the supine position. However, at waking there was an approximately even spread across supine and both lateral positions (Table 1). Forty (21.4%) of the pregnant women fell asleep in a different position to that in which they first settled to bed. More than half (59.9%) of the women woke in a different position to how they fell asleep.
Table 1.
Comparison of settling to bed, sleep onset, and final wake positions
| Position | Settling | Sleep onset | Wake |
|---|---|---|---|
| Supine | 38 (20.3%) | 26 (13.9%) | 59 (31.6%) |
| Left | 93 (49.7%) | 97 (51.9%) | 68 (36.6%) |
| Right | 52 (27.8%) | 60 (32.1%) | 57 (30.7%) |
| Prone | 4 (2.1%) | 4 (2.1%) | 3 (1.6%) |
Note. Values given a n (%).
The key sleep architecture and sleep position data are described in Table 2. The percentage of total sleep time (TST) was split evenly for the left and right lateral positions, with a median of 24.2% of TST spent in the supine position (Table 2). The percentage of time in the supine position in both sleep and wake across the whole night was slightly greater than during sleep only but not significantly so (24.9% (10.2, 39.4), p = .054), with an absolute median difference of 2.1% (0.9, 4.6) between the two measures. Body position during the night changed frequently, with an average of 9.7 body re-positionings, and most women got out of bed at least once during the night.
Table 2.
Sleep architecture and sleep position during late pregnancy
| Average | Range | |
|---|---|---|
| Total sleep time (min) | 378.9 ± 76.7 | 155.5–594.5 |
| Sleep efficiency (%) | 78.8 ± 10.4 | 40.9–96.9 |
| Sleep latency (min) | 11.0 (5.5, 20.5) | 0.5–195.5 |
| %TST N3 | 31.5 ± 12.5 | 2.0–84.9 |
| %TST REM | 15.6 ± 6.1 | 0.0–30.1 |
| %TST in position | ||
| Supine | 24.2 (7.4, 40.3) | 0.0–100.0 |
| Left | 36.1 (22.6, 48.6) | 0.0–98.9 |
| Right | 35.6 (18.2, 49.4) | 0.0–100.0 |
| Prone | 0.0 (0.0, 0.0) | 0.0–59.4 |
| Position changes | 9.7 ± 4.6 | 0–27 |
| Times out of bed | 1.0 (1.0, 2.0) | 0–9 |
| Initial time in sleep onset position before repositioning (min) | 51.5 (10.0, 72.5) | 0.5–492.0 |
| Overall %TST in sleep onset position | 44.5 ± 20.8 | (0.2–100.0) |
Note. n = 187. Values given as M ± SD, Mdn (IQR) or n (%). %TST = percentage of total sleep time, REM = rapid eye movement.
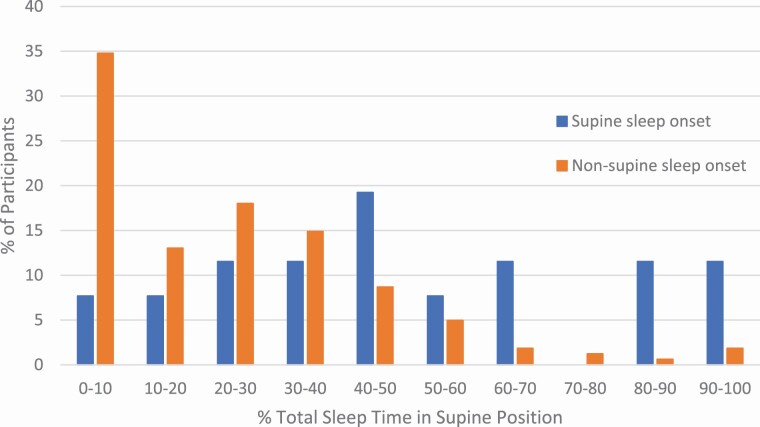
Sleep onset position was the dominant sleep position for the entire night in just over half (54%) of the sample, with women spending an average of 44.5% of their time in their sleep onset position. Once asleep, women on average spent less than an hour in their initial sleep onset position before moving. Women who began in the non-supine position spent less TST in the supine position for the rest of the night than those who fell asleep in the supine position (22.6% (5.7, 32.2) vs. 48.0% (30.0, 65.9), p < .001; Figure 2). The amount of supine sleep overnight was highly variable regardless of sleep onset position (Figure 2).
Figure 2.
Distribution of percentage of total sleep time spent in the supine position for participants with supine sleep onset (n = 26) and participants with non-supine sleep onset (n = 161). Supine sleep onset was associated with more total sleep time in the supine position (48.0% (30.0, 65.9) compared to those who fell asleep in a non-supine position (22.6% (5.7, 32.2), p < .001).
Similar to sleep onset position, pregnant women spent 45.0% (SD = 21.9) of TST in the position they awoke in at sleep offset, with wake position being the dominant sleep position for 98 (52.4%) women. For the third of women who woke up in the supine position at the end of the night, the %TST spent in the supine position was greater than those who woke in a non-supine position (32.4% (23.2, 53.6) vs. 17.3% (2.4, 31.7), p < .001).
Sleep position in high-risk pregnancies
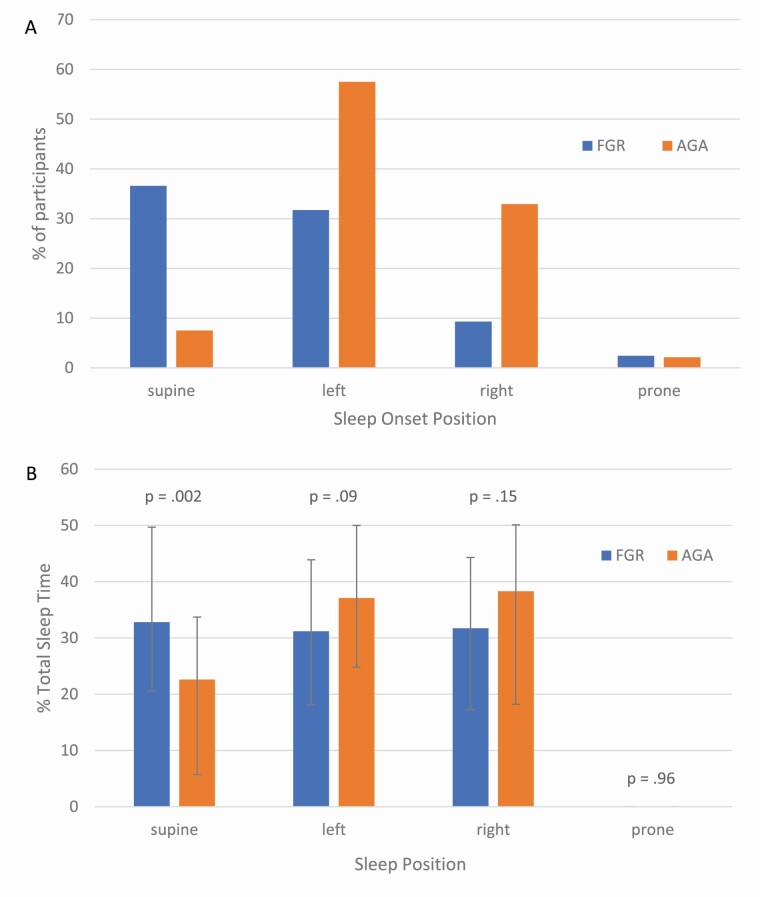
The patterns of sleep onset position, sleep architecture, and the distribution of sleep positions overnight were similar across the uncomplicated pregnancies and those with hypertensive disorders or gestational diabetes (See Supplemental Tables S1 and S2). However, women with growth-restricted fetuses (including those with a comorbid hypertensive disorder) had significantly different sleep onset positions compared to those with appropriate for gestational age fetuses (X2 (3) = 23.70, p < .001, Figure 3, a). Specifically, women with fetal growth restriction were much more likely to fall asleep in the supine position, whereas left lateral was the preferred position for women with appropriate for gestational age fetuses. The association between supine sleep onset and fetal growth restriction remained after adjusting for gestation at the time of the sleep study (p < .001). In addition, women with fetal growth restriction spent more time overnight in the supine position (%TST = 32.8% (20.5, 49.7) vs. 22.6% (5.6, 33.7), p = .002, Figure 3, b), however this was no longer significant after adjusting for gestational age at the time of the sleep study (p = .059).
Figure 3.
A comparison of pregnant women with fetal growth restriction (FGR; n = 41) and appropriate for gestational age (AGA) fetuses (n = 146) on A) sleep onset position, χ 2 (3) = 23.70, p < .001; and B) median (IQR) percentage of total sleep time in each position compared with separate Mann–Whitney U tests. The higher percentage of time spent overnight in the supine position in the FGR group was no longer significant after adjusting for gestational age at the time of the sleep study (p = .059).
Women having an inpatient PSG were more likely to go to sleep in the supine position (10/32 = 31.3%) compared to women having a home-based PSG (14/115 = 12.2%) and lab-based PSG (2/40 = 5.0%, p = .004). This was due to the pregnant women with a hypertensive disorder having a higher likelihood of supine sleep onset as an inpatient compared to at home (37.5% vs. 6.1%, p = .02) rather than the women with fetal growth restriction (supine sleep onset as an inpatient vs. at home = 29.2% vs. 53.9%, p = .14). There was also a significantly higher proportion of TST spent in the supine body position for women having their PSG as an inpatient (31.7% (21.7, 50.3)) versus those having their study in the lab (21.8% (6.5, 34.3) or at home (22.6% (6.3, 36.9), p = .03). This was again driven by more supine sleep in the inpatients with hypertensive disorders of pregnancy rather than the inpatients with fetal growth restriction (See Supplementary Table S3), suggesting that the relationship between fetal growth restriction and supine sleep is not mitigated by PSG location.
Sleep position and sleep-disordered breathing
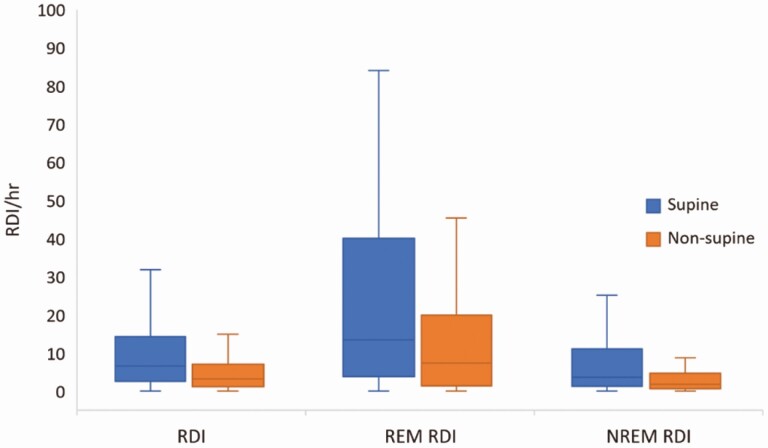
Overall, 81 (43.3%) women in the sample had an RDI ≥ 5, with an average RDI of 4.2 (2.2, 7.8). The RDI (Figure 4), AHI and ODI 3% were higher in supine compared to non-supine sleep (AHI = 5.5/h (2.0, 13.6) vs. 2.6/h (1.1, 6.3), p < .001; ODI3% = 1.2/h (0.0, 5.0) vs. 0.8/h (0.0, 3.3), p = .002). Similarly, the arousal index was higher during supine sleep (22.2/h (15.2, 33.4) compared to non-supine sleep (16.4/h (12.3, 23.2); p < .001). After controlling for the proportion of supine sleep that occurred during REM sleep (when respiratory events are more likely), the overall RDI significantly increased with higher amounts of supine sleep (β = 0.22, t(184) = 2.61, p = .01). Of those who had an RDI ≥ 5 overall, almost half had supine based SDB (43.2%, defined as a supine RDI > 2x non-supine RDI) [26, 27].
Figure 4.
Respiratory disturbance index (RDI per hour) overall (n = 174) and in REM (n = 116) and NREM (n = 174) sleep for supine and non-supine body position. Note: extreme outliers have been removed.
Supine sleep was also related with a higher RDI compared to non-supine sleep within the uncomplicated pregnancies, the fetal growth restriction with or without a hypertensive disorder group, and the hypertensive disorders of pregnancy group, but not for women with gestational diabetes (Supplementary Figure S1). The prevalence of supine based SDB for those with an RDI ≥ 5 overall was similar across all groups (uncomplicated pregnancies = 44.8%, fetal growth restriction with or without a hypertensive disorder group = 45.0%, hypertensive disorders of pregnancy group = 42.3%, gestational diabetes group = 33.3%; p = .96).
Sleep position and birth outcomes
Supine sleep onset was significantly related to birthweight (p = .017) and birthweight centile (p = .001), but not gestational age at birth (p = .28). These outcomes are known to be closely related to the diagnosis of fetal growth restriction; and these women were more likely to have supine sleep onset. After controlling for fetal growth restriction, supine sleep onset was not related to any birth outcome (all p > .40).
Women with higher levels of supine %TST on the sleep study delivered their babies earlier (p = .005), had smaller birthweights (p < .001) and lower birthweight centiles (p = .004; Table 3). After controlling for fetal growth restriction and inpatient status (women who may be more likely to have adverse outcomes), the relationships between supine sleep and delivery gestation, and supine sleep and birthweight centile were no longer significant (p = .23 and p = .16 respectively), however increased levels of supine sleep were still associated with lower absolute birthweights (p = .02). The percentage of sleep time in the right lateral position overnight was not related to any birth outcome (all p > .15). In terms of SDB, there were no significant correlations between RDI and birth outcomes among all participants (all p > .13) nor within the pregnancies affected by fetal growth restriction (all p > .3; see Supplementary Table S4).
Table 3.
Relationship between percentage of supine sleep and birth outcomes, with and without adjustment for fetal growth restriction and inpatient status
| Delivery gestation | Birthweight | Birthweight centile | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||
| B (SE) | β | B (SE) | β | B (SE) | β | B (SE) | Β | B (SE) | β | B (SE) | β | |
| %TST supine | .05 (.02) | .20** | .02 (.01) | .07 | 1.30 (.34) | .27*** | .60 (.25) | .13* | −.26 (.09) | −.22** | −.10 (.07) | −.08 |
| FGR | .52 (.09) | .40*** | 14.73 (1.8) | .52*** | −3.82 (.51) | −.53*** | ||||||
| Inpatient | .48 (.10) | .34*** | 7.09 (1.97) | .23*** | −1.20 (.56) | −.15* | ||||||
| F | 7.83** | 48.76*** | 14.50*** | 63.37*** | 8.91** | 42.66*** | ||||||
| R2 (%) | 4.13 | 44.83 | 7.53 | 51.93 | 4.79 | 42.24 |
*p < .05, **p < .01, ***p < .001. Model 1 = predictor of %TST supine only; Model 2 = Model 1 with adjustment for FGR and hospital inpatient at time of sleep study.
Note. Delivery gestation reflected and log transformed, birthweight reflected and square root transformed, birthweight centile square root transformed, %TST supine square root transformed. %TST = percentage of total sleep time, FGR = fetal growth restriction.
Discussion
This is the largest study to date that has utilised objective measures to describe sleep position during pregnancy. Most pregnant women settle in bed and fall asleep in a lateral position, with the left side preferred. However, 14% of women were supine at sleep onset and a quarter of sleep time across the whole sample was in the supine position. Body position at sleep onset was the dominant position across the entire night for only half of the sample, meaning that sleep onset position is not representative of overnight sleep behaviour for almost half of pregnant women. However, it is noteworthy that those who settled non-supine had less total sleep time in the supine position. Women with a growth restricted fetus were much more likely to fall asleep in a supine position and stay in that position overnight compared to women with a healthy pregnancy. Furthermore, a preference for supine sleep was associated with lower infant birthweight. Supine sleep positioning resulted in higher indices of SDB, with positional SDB common during pregnancy.
The recent research demonstrating a relationship between self-reported supine sleep onset position and increased stillbirth risk has hugely important clinical implications [28–31]. Following these studies, an educational awareness campaign on healthy maternal sleep positioning during pregnancy (amongst other practices to reduce late stillbirth such as awareness of decreased fetal movements and smoking cessation) is currently being implemented and evaluated in maternity services across Australia [7, 32].
Despite this, further reinforcement of this relationship and research into the mechanisms of action should be pursued, as many questions remain [8]. Our results showed that sleep onset position is not representative of overnight sleeping position for a large proportion of pregnant women, challenging the argument made by many that the position in which women first falls asleep has the longest duration overnight [1, 3, 33]. This assumption is based on a study of 30 pregnant women with overnight video recording, who maintained their position at sleep onset for a median of 73.5 minutes [10]. At an average of 37 weeks gestation, these women may have had greater difficulty shifting positions. Our results demonstrated wide variability in how long sleep onset position was maintained, averaging 52 minutes but ranging from 30 s to 8 h, with only 44.5% of the night spent in the sleep onset position on average. We also found that 20% of women settled into bed supine with 14% of women initiating sleep in the supine position, compared to only 3.2% of women with livebirths recalling a supine sleep onset position in the meta-analysis of sleep onset position and stillbirth risk, which may reflect recall bias in this retrospective self-reported data [1]. Nevertheless, it is reassuring that non-supine sleep onset was associated with significantly less supine sleep overnight.
Interestingly, one-fifth of women settled to bed in one position but actually fell asleep in another, questioning which position pregnant women recall as their “sleep-onset” position. Few studies have validated perception of sleep onset position in the general population, however one study showed that 30% of the cohort incorrectly reported their body position at the time of lights out, suggesting that uncertainty exists even when recollecting position during wake [34]. Within clinical sleep populations, perception of supine sleep overnight has been investigated given its importance in the diagnosis and treatment of position-related OSA, with patient estimates of supine sleep duration being largely inaccurate [35] or frequently underestimated [36]. During pregnancy there are also large individual differences in reporting accuracy of left-sided sleep position [9]. Only one study has looked at the accuracy of self-reported sleep onset position in pregnancy, with 22 out of 30 women accurately recalling the position they went to sleep in and only 12 out of 30 accurately recalling wake position [10].
Potential discrepancies between “lights out” and “sleep onset” body position also leads to the question of whether it is supine position in sleep, or supine position during both sleep and wake overnight that matters in terms of proposed fetal risk. The former would indicate that some sleep specific physiology such as cardiovascular or upper airway changes are important, whereas the latter would indicate that mechanical issues such as inferior vena cava compression have the most impact. In this study, we showed minimal difference between total sleep time and total recording time in the supine position overnight (24.2% vs. 24.9%) so this question may be difficult to answer.
The presence of fetal growth restriction is among the highest risk factors for stillbirth [37, 38]. In this study, women with a growth restricted fetus were much more likely to fall asleep in the supine position compared to women with a healthy fetus, however the increased proportion of supine sleep overnight in this group was tempered by earlier gestational age. In turn, we found that women with a propensity to sleep supine were more likely to have smaller babies born at an earlier gestation. Even after controlling for fetal growth restriction and hospital admission for pregnancy complications, supine sleep was associated with lower birthweight. This may be an important finding given that up to half of late stillbirths are unexplained [39], in that a specific cause (such as fetal growth restriction or SGA – small for gestational age; birthweight centile < 10th centile) cannot be identified. Similarly, a study of Ghanaian women and a meta-analysis of studies from Australia, New Zealand, and the United Kingdom found that the newborns of women who reported supine sleep or supine sleep onset during pregnancy were at increased risk of low birthweight and stillbirth [2, 3]. Conversely, two prospective cohort studies (n = 8706 and n = 148) of both self-reported and objectively measured sleep position found no relationship between body position and perinatal outcomes including birthweight and stillbirth [14, 40]. A limitation of these studies- and our own- is the inability to ascertain directionality to this relationship. The rationale for reduced fetal growth due to supine sleep relates to changes in maternal cardiovascular and cardiorespiratory parameters, placental blood flow, and fetal oxygenation [41]. Specifically, the inferior vena cava becomes compressed by the gravid uterus in the supine position, leading to a reduction in cardiac output and a 44%–85% reduction in blood flow through the inferior vena cava [4]. Conversely, a growth restricted fetus with a smaller uterine size may cause less nocturnal discomfort for the mother and therefore encourage more frequent adoption of a supine sleep position. Hence, while the relationship with growth restriction and supine sleep is intriguing given the apparent protection of side sleeping for stillbirth, causality cannot be inferred based on this and other studies.
There is also debate as to whether lying on the right side is less protective than the left in regards to stillbirth risk [31]. Due to anatomical positioning, the enlarged uterus can exert greater compression on the inferior vena cava and abdominal aorta when the mother lies either supine or right lateral, which can inhibit venous return and decrease uterine blood flow [42]. Further, cardiac autonomic nervous activity can be significantly altered in the right versus left lateral position [43]. However, our results did not reveal any relationship between right-sided sleep and pregnancy complications or birth outcomes.
Both supine sleep onset and supine waking position were related to a higher proportion of supine sleep overnight. This raises the question of why a consistent relationship exists between increased stillbirth risk and reported supine sleep onset, but not supine position on waking [31, 44]. It may be that maternal body position at the start of the night matters more, or simply that recollection of sleep onset position is more reliable than after arousal from sleep [10]. Stone et al. [45] demonstrated that periods of high fetal heart rate activity were more likely in the early part of the night whereas a stable fetal heart rate indicative of quiet sleep was more likely later in the night, and suggested that maternal position change may affect circadian patterns of fetal heart rate overnight. Supine sleep alone as a risk factor for stillbirth is clearly neither necessary, nor sufficient: many women in this, and other studies, sleep supinely during late pregnancy with no ill consequences. This study was not powered to detect an increase in the risk of such a rare event as stillbirth. However, it may be one important factor in the “triple risk” model for stillbirth proposed by Warland et al. [46], which suggests that an interplay of maternal factors, placental and/or fetal factors, and a stressor (such as supine sleep) is needed to cause unexplained stillbirth.
Unsurprisingly, all indices of SDB during pregnancy were significantly higher in the supine position compared to non-supine, with an association between time spent in the supine position and overall RDI. Almost half of the women with SDB had positional SDB, regardless of pregnancy complication, a similar proportion to that which has been demonstrated in the general population [26, 47]. Supine SDB is likely attributable to unfavourable airway mechanics, reduced lung volume, and an inability of airway dilator muscles to adequately compensate as the airway collapses [13]. Physiological and respiratory system changes in pregnancy further impact on airway size and lung capacity, promoting breathing difficulties during supine sleep. Nocturnal arterial oxygen saturation is already lowered in pregnancy [48]; more severe upper airway compromise in the supine position could lead to acute and repetitive hypoxic insults – having a detrimental effect on placental perfusion and oxygenation. Although mild degrees of SDB were not related to pregnancy outcomes in this study, the co-existence of position-related SDB may be a double blow for pregnant women and the health of their fetus given that SDB has been associated with various obstetric and perinatal complications [41] and represents a further reason to advocate for lateral sleeping positions during the later stages of pregnancy.
Strengths and weaknesses
This study presents the largest cohort of pregnant women to undergo full polysomnographic analysis of sleep position and SDB. Given the current imperative to identify risk factors to reduce preventable stillbirth this study provides comprehensive baseline data on the sleep behaviour of pregnant women. We found that the average percentage of supine sleep during the third trimester of pregnancy was 24%, with 14% of women falling asleep on their back. The data in this study were collected before the Safer Baby Bundle campaign was introduced into maternity care and can therefore be used as a baseline to measure the impact of the public health messaging on sleep behaviour.
The use of full PSG compared to other studies using abbreviated devices [14, 40] enabled us to compare the magnitude of position-related SDB differences across REM and NREM sleep, and include more subtle markers of SDB associated with sleep fragmentation, such as RERAs. Despite a preference for unattended home PSG by the participants, we can be confident that the position sensor located within the patient input box at the sternum was accurate as there were no significant differences in position statistics to the video-confirmed in-laboratory studies. It is also acknowledged that like previous objective studies of sleep position, these findings need to be interpreted with caution given the limitations of only one night of sleep data, and the potential impact on sleep architecture of the measuring devices themselves. Data have shown that patients undergoing PSG may spend more time in the supine position than whilst otherwise unencumbered [49], whereas more recent data showed no difference between full PSG and a less intrusive watch-like sleep monitoring device [50], and in fact less supine sleep for women undergoing PSG. Furthermore, our results are not dissimilar from previous studies quantifying supine sleep during pregnancy, whether measurement was made with polygraphy or video recording [9, 10].
The sample in this study had a high prevalence of diabetes and hypertension. It is possible that women with these disorders may be more susceptible to the potential hemodynamic consequences of supine sleep due to their vascular pathology at baseline. Hence the perinatal outcomes in this study that are linked to supine sleep may not be generalizable to the general low risk pregnant population.
Unfortunately, the studies from which these data were collected did not include a self-report component for sleep position. Given the time intensive nature of conducting PSG during pregnancy, gathering more information on the accuracy of self-reported sleep position during pregnancy could help guide further protocol development in this area.
Conclusion
There is wide variation in sleep position during pregnancy, with body position changing frequently overnight. While lateral sleep position was preferred, supine sleep was still common in this study which predates the current public health messages advocating side sleeping in pregnancy. Sleep onset position was the dominant position throughout the night for just over half of the sample, suggesting that sleep onset position may not be a reliable indicator for the rest of the night. Reassuringly though, non-supine sleep onset was associated with less overall supine sleep. Supine sleep onset was frequent in growth restricted pregnancies, with higher proportions of supine sleep overnight related to birthweight at delivery in adjusted analyses, although the potential bidirectional nature of this relationship means causality cannot be implied. While the association between reported supine sleep onset and increased stillbirth risk has important clinical implications, it is critical that we continue to pursue research into clarifying the nature of this relationship and the mechanisms behind it.
Supplementary Material
Acknowledgments
Thank you to the antenatal outpatient clinics at the Mercy Hospital for Women and the Sleep Laboratory at Austin Health for their support of these projects. In particular, thank you to Pavlina Toman for polysomnographic analysis.
Contributor Information
Danielle L Wilson, Institute for Breathing and Sleep, Austin Health, Heidelberg, Victoria, Australia; Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, Victoria, Australia.
Alison M Fung, Mercy Perinatal, Mercy Hospital for Women, Heidelberg, Victoria, Australia.
Gabrielle Pell, Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, Victoria, Australia.
Hannah Skrzypek, Mercy Perinatal, Mercy Hospital for Women, Heidelberg, Victoria, Australia.
Maree Barnes, Institute for Breathing and Sleep, Austin Health, Heidelberg, Victoria, Australia; Department of Medicine, University of Melbourne, Parkville, Victoria, Australia.
Ghada Bourjeily, Department of Medicine, The Miriam Hospital, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Susan P Walker, Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, Victoria, Australia; Mercy Perinatal, Mercy Hospital for Women, Heidelberg, Victoria, Australia.
Mark E Howard, Institute for Breathing and Sleep, Austin Health, Heidelberg, Victoria, Australia; Department of Medicine, University of Melbourne, Parkville, Victoria, Australia.
Disclosures
This research was supported by a National Health and Medical Research Council (NHMRC) postgraduate scholarship for DW, National Health and Medical Research Council (NHMRC) project grant 1065854, the Austin Medical Research Foundation and the Norman Beischer Medical Research Foundation.
Financial Disclosure. Mark E. Howard received research equipment from Philips Respironics, and Ghada Bourjeily is funded by the National Institutes of Health HL-130702 and HL-130701-4S1.
Non-financial Disclosure. None.
Data Availability
The data underlying this article are available in Figshare, at https://dx.doi.org/10.6084/m9.figshare.15089898.
References
- 1. Cronin RS, et al. An individual participant data meta-analysis of maternal going-to-sleep position, interactions with fetal vulnerability, and the risk of late stillbirth. EClinicalMedicine. 2019;10:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owusu JT, et al. Association of maternal sleep practices with pre-eclampsia, low birth weight, and stillbirth among Ghanaian women. Int J Gynaecol Obstet. 2013;121:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson NH, et al. Association of supine going-to-sleep position in late pregnancy with reduced birth weight: a secondary analysis of an individual participant data meta-analysis. JAMA Netw Open. 2019;2(10):e1912614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Humphries A, et al. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med. 2019;32(23):3923–3930. [DOI] [PubMed] [Google Scholar]
- 5. Cure Kids. Sleep on side when baby’s inside from 28 weeks of pregnancy.2018; cited 30 Dec 2021. Available from: https://www.sleeponside.org.nz/
- 6. Tommy’s. Sleep on side - a pregnancy campaign.2017; cited on 30 Dec 2021. Available from: https://www.tommys.org/pregnancy-information/im-pregnant/sleep-side/sleep-side-pregnancy-campaign.
- 7. Centre of Research Excellence in Stillbirth. Safer baby bundle: a bundle of care to improve mothers’ and babies’ health [Internet].South Brisbane QLD: Centre for Research Excellence in Stillbirth; 2019; cited 29 August 2019. Available from: https://www.stillbirthcre.org.au/resources/bundle-of-care/. [Google Scholar]
- 8. Simoes M, et al. Sleep position and stillbirth - is it time to change sleep practices? Eur Respir Pulm Dis. 2019;5(1):14–15. [Google Scholar]
- 9. Warland J, et al. Accuracy of self-reported sleep position in late pregnancy. PLoS One. 2014;9(12):e115760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McIntyre JP, et al. A description of sleep behaviour in healthy late pregnancy, and the accuracy of self-reports. BMC Pregnancy Childbirth. 2016;16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Brien LM, et al. Typical sleep positions in pregnant women. Early Hum Dev. 2014;90(6):315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warland J, et al. Modifying maternal sleep position in late pregnancy through positional therapy: a feasibility study. J Clin Sleep Med. 2018;14(8):1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joosten SA, et al. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18(1):7–17. [DOI] [PubMed] [Google Scholar]
- 14. Dunietz GL, et al. Sleep position and breathing in late pregnancy and perinatal outcomes. J Clin Sleep Med. 2020;16(6):955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Facco FL, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fung AM, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013;8(7):e68057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pamidi S, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 2016;71:719–725. [DOI] [PubMed] [Google Scholar]
- 18. Bourjeily G, et al. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2020;66:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson DL, et al. The presence of coexisting sleep-disordered breathing among women with hypertensive disorders of pregnancy does not worsen perinatal outcome. PLoS One. 2020;15(2):e0229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skrzypek H, et al. Fetal heart rate monitoring during sleep in fetal growth restriction. E-Poster. Aust N Z J Obstet Gynaecol. 2021;61:10–11. https://obgyn.onlinelibrary.wiley.com/doi/epdf/10.1111/ajo.13345 [Google Scholar]
- 21. Wilson DL, et al. Sleep-disordered breathing in hypertensive disorders of pregnancy: a BMI-matched study. J Sleep Res. 2018;27(5):e12656. [DOI] [PubMed] [Google Scholar]
- 22. Nankervis A, et al. ADIPS Consensus Guidelines for the Testing and Diagnosis of Hyperglycaemia in Pregnancy in Australia and New Zealand. Australasian Diabetes in Pregnancy Society; 2014. https://www.adips.org/downloads/2014ADIPSGDMGuidelinesV18.11.2014_000.pdf [Google Scholar]
- 23. Brown MA, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. [DOI] [PubMed] [Google Scholar]
- 24. Gordijn SJ, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333–339. [DOI] [PubMed] [Google Scholar]
- 25. Berry RB, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.3. Darien, Illinois: American Academy of Sleep Medicine; 2016. [Google Scholar]
- 26. Oksenberg A, et al. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112(3):629–639. [DOI] [PubMed] [Google Scholar]
- 27. Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. doi: 10.1093/sleep/7.2.110 [DOI] [PubMed] [Google Scholar]
- 28. Gordon A, et al. Sleep position, fetal growth restriction, and late-pregnancy stillbirth: the Sydney stillbirth study. Obstet Gynecol. 2015;125(2):347–355. [DOI] [PubMed] [Google Scholar]
- 29. Heazell A, et al. Association between maternal sleep practices and late stillbirth - findings from a stillbirth case-control study. BJOG. 2018;125(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCowan LME, et al. Going to sleep in the supine position is a modifiable risk factor for late pregnancy stillbirth; Findings from the New Zealand multicentre stillbirth case-control study. PLoS One. 2017;12(6):e0179396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stacey T, et al. Association between maternal sleep practices and risk of late stillbirth: a case-control study. Br Med J. 2011;342:d3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andrews CJ, et al. Implementation and evaluation of a quality improvement initiative to reduce late gestation stillbirths in Australia: Safer Baby Bundle study protocol. BMC Pregnancy Childbirth. 2020;20(1):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horne R. Sleep your baby supine, but mums-to-be should sleep on their side. J Physiol. 2021;599(6):1725–1726. [DOI] [PubMed] [Google Scholar]
- 34. Russo K, et al. How reliable is self-reported body position during sleep? J Clin Sleep Med. 2016;12(1):127–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wallbridge PD, et al. Accuracy of patient perception of supine sleep. J Clin Sleep Med. 2018;14(7):1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sorscher AJ, et al. Patient-predicted sleep position vs. HST data: a tendency to underestimate supine sleep. Sleep Breath. 2018;22(3):625–630. [DOI] [PubMed] [Google Scholar]
- 37. Gardosi J, et al. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flenady V, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331–1340. [DOI] [PubMed] [Google Scholar]
- 39. Silver RM, et al. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196(5):433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silver RM, et al. Prospective evaluation of maternal sleep position through 30 weeks of gestation and adverse pregnancy outcomes. Obstet Gynecol. 2019;134(4):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robertson NT, et al. Pathophysiological changes associated with sleep disordered breathing and supine sleep position in pregnancy. Sleep Med Rev. 2019;46:1–8. [DOI] [PubMed] [Google Scholar]
- 42. Milsom I, et al. Factors influencing aortocaval compression in late pregnancy. Am J Obstet Gynecol. 1984;148(6):764–771. [DOI] [PubMed] [Google Scholar]
- 43. Kuo CD, et al. The effect of position on autonomic nervous activity in late pregnancy. Anaesthesia. 1997;52(12):1161–1165. [DOI] [PubMed] [Google Scholar]
- 44. Silver RM. Maternal going to sleep position and late stillbirth: time to act but with care. EClinicalMedicine. 2019;10:6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stone PR, et al. An investigation of fetal behavioural states during maternal sleep in healthy late gestation pregnancy: an observational study. J Physiol. 2017;595(24):7441–7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Warland J, et al. A triple risk model for unexplained late stillbirth. BMC Pregnancy and Childbirth. 2014;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Joosten SA, et al. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17(1):99–107. [DOI] [PubMed] [Google Scholar]
- 48. Bourne T, et al. Nocturnal hypoxaemia in late pregnancy. Br J Anaesth. 1995;75:678–682. [DOI] [PubMed] [Google Scholar]
- 49. Metersky ML, et al. The effect of polysomnography on sleep position: possible implications on the diagnosis of positional obstructive sleep apnea. Respiration. 1996;63(5):283–287. [DOI] [PubMed] [Google Scholar]
- 50. Kukwa W, et al. The effect of in-lab polysomnography and home sleep polygraphy on sleep position. Sleep Breath. 2021;25(1):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in Figshare, at https://dx.doi.org/10.6084/m9.figshare.15089898.